Abstract
This paper reviews theory, measurements, and computer simulations of scattering from cancellous bone reported by many laboratories. Three theoretical models (Binary Mixture, Faran Cylinder, and Weak Scattering) for scattering from cancellous bone have demonstrated some consistency with measurements of backscatter. Backscatter is moderately correlated with bone mineral density in human calcaneus in vitro (r2 = 0.66 – 0.68). Backscatter varies approximately as frequency cubed and trabecular thickness cubed in human calcaneus and femur in vitro. Backscatter from human calcaneus and bovine tibia exhibits substantial anisotropy. So far, backscatter has demonstrated only modest clinical utility. Computer simulation models have helped to elucidate mechanisms underlying scattering from cancellous bones.
I. INTRODUCTION
Prospective [1–8] and retrospective [9–19] clinical trials have demonstrated that broadband ultrasound attenuation (BUA) (the slope of attenuation coefficient vs. frequency) and speed of sound (SOS) in calcaneus are very useful for diagnosis of osteoporosis. This clinical utility is supported by abundant pre-clinical data [20–57].
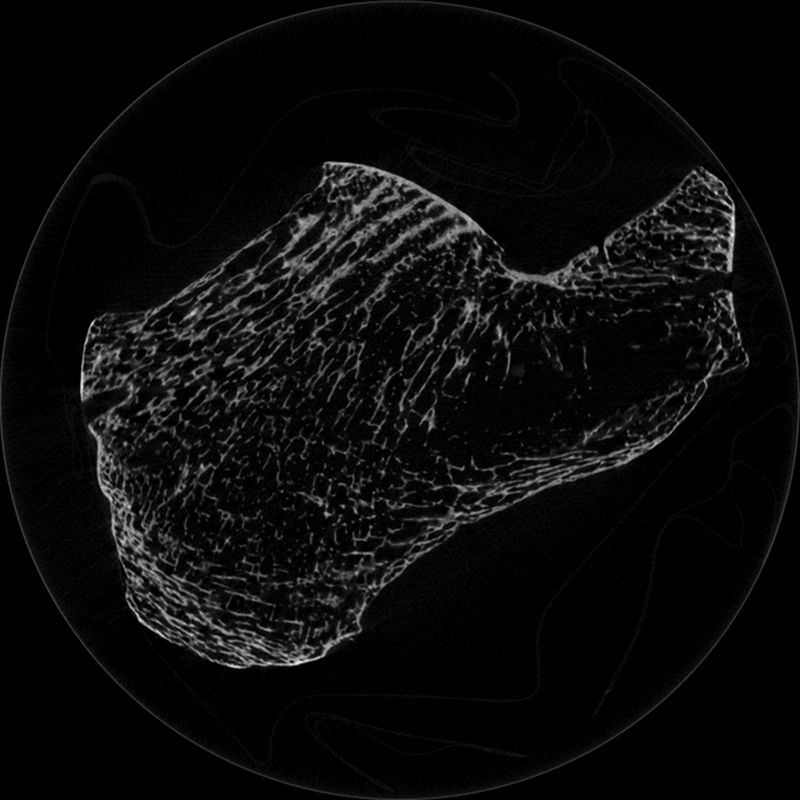
Ultrasonic scattering from bone has received less attention than BUA and SOS. However, the study of scattering is important for two reasons. First, it can elucidate mechanisms responsible for BUA, which is the combined result of absorption and scattering. (Scattering in general may include both longitudinal-to-longitudinal and longitudinal-to-transverse components.) Second, scattering measurements have shown diagnostic promise in their own right in studies in vitro [58–87] and in vivo [88–93]. Since measurement of backscatter may be performed with a single transducer in pulse-echo mode, it has the potential to be measured at sites other than the calcaneus, such as the hip and spine. Backscatter is known to provide information regarding size, shape, number density, and elastic properties of scatterers [94–99]. Cancellous bone contains many approximately cylindrically-shaped scatterers (trabeculae) and plate-like structures arrayed in a mesh. See Figure 1. The spaces between the trabeculae are filled with marrow (in vivo) or water (in vitro). Trabeculae are likely candidates for scattering sites due to the disparity in acoustic properties between mineralized trabeculae and the fluid filler. See Table I.
1.
Micro computed tomogram of calcaneus. Some trabeculae appear to terminate as they move into and out of the imaging plane. Image acquired by Andres Laib, Scanco Medical AG, Brüttisellen, Switzerland.
Table I.
Material properties of bone and bone-like substances. The parameters listed are longitudinal sound velocity (cl), shear velocity (cs), density (ρ) and Poisson’s ratio (ν). Note that the values for cancellous bone refer to only to the trabecular component, not to the composite medium consisting of the trabecular matrix filled with either marrow (in vivo) or water (in vitro).
| Reference | Substance | cl (m/s) | cs (m/s) | ρ (g/cc) | υ |
|---|---|---|---|---|---|
| Hosokawa & Otani, [40] | Bovine cancellous bone | 3800 | 2000 | 1.95 | 0.32 |
| Anderson et al., [102] | Hydroxyapatite | 6790 | 3.22 | 0.28 | |
| Luo et al., [45] | Cortical bone | 2900 | 1303 | 1.85 | |
| Gong et al., [103] | Bovine cancellous bone | 1.93 | |||
| Lang et al., [104] | Bovine cortical bone | 1.96 | 0.32 | ||
| Williams, [105] | Bovine cancellous bone | 3800 * | |||
| Rho et al., [106] | Bovine cancellous bone | 2898±85 | |||
| Ashman et al., [107] | Human cancellous bone | 2639−2754 | 1.73−1.80 | ||
| - | Bovine cancellous bone | 2501 | 1.74 | ||
| Grenoble et al., [99] † | Powdered human femur | 3917 | 2020 | 0.33 | |
| - | Fresh bovine femur | 4890 | 2495 | 0.34 | |
| - | Hydroxyapatite (mineral) | 5565 | 4765 | 0.27 | |
| - | Hydroxyapatite (synth.) | 5628 | 4818 | 0.28 |
Extrapolated (to zero porosity) from bulk measurements over a range of porosities (where porosity is the volume fraction of marrow). See (J. L. Williams, 1992), figure 2.
Assuming ρ = 1.96 gm/cc.
II. THEORETICAL MODELS
As shown in Figure 1, cancellous bone has an extremely complicated structure. Existing theoretical models of ultrasonic scattering from cancellous bone therefore rely on many simplistic assumptions in order to make the problem tractable. In spite of their simplicity, however, these models have shown considerable success in predicting aspects of scattering from cancellous bone. Scattering measurements from cancellous bone are often in the low ka regime where k = 2π / λ, λ = wavelength, a = radius = Tb.Th/2, and Tb.Th = trabecular thickness. For example, for interrogation of calcaneus (with mean Tb.Th of 0.127 mm [100]) at a typical diagnostic frequency of 500 kHz, ka = 0.13.
A. Binary Mixture Model
Strelitzki et al. proposed the Binary Mixture model in which the scattering coefficient is proportional to mean fluctuations in velocity and density [59, 65]. This approach has shown success in soft tissues [94]. Following the tradition of the application of the Binary Mixture model to soft tissues, Strelitzki et al. neglect density fluctuations. The predictions of the Binary Mixture model are consistent with the reported nonlinear dependence of BUA on porosity in cancellous bone [35]. The Binary Mixture model also predicts a quasi-linear dependence of attenuation due to scattering with frequency over a limited bandwidth for sufficiently small scatterers [65].
B. Faran Cylinder Model
Wear proposed the Faran Cylinder model, which regards trabeculae as solid cylinders embedded in fluid (marrow in vivo or water in vitro) [60]. See Figure 1. The cylinders (trabeculae) are assumed to be longer than the beam width. Faran’s theory of scattering from cylinders is then used to predict scattering from trabeculae [95]. The model assumes that the trabeculae are positioned sufficiently randomly that the incoherent contribution to scattering dominates the coherent contribution. (In other words, the phase difference between scattered signals from pairs of cylinders is assumed to be uniformly distributed between 0 and 2π). The model also ignores multiples scattering. When the cylinder diameter is much smaller than the wavelength, the Faran Cylinder model predicts that backscatter (and total scatter) should be approximately proportional to frequency cubed [60]. (The mean trabecular thickness in human calcaneus, about 127 microns [100], is much smaller than the wavelength at the typical diagnostic frequency of 500 kHz, about 3 mm, in the surrounding fluid.) The model also predicts that backscatter should be approximately proportional to trabecular thickness cubed [74].
C. Weak Scattering Model
Chaffai et al. and Jenson et al. proposed the Weak Scattering model, which is based on a random description of the scattering tissue [64]. Like the Binary Mixture model, the Weak Scattering model has shown much prior success in modeling scattering from soft tissues [96]. Structural autocorrelation functions may be measured from cancellous bone samples (e.g. using micro computed tomography, microCT) and used as inputs for the calculation of predicted backscatter coefficients. The weak scattering model requires small contrasts in acoustic properties (sound speed and density) between scatterers (i.e. trabeculae) and the fluid filler (marrow in vivo or water in vitro) [97]. As with the Faran Cylinder model, the Weak Scattering model considers only the incoherent component of scattering [76]. Deligianni and Apostolopoulos extended the Weak Scattering model to a two-component form [98].
D. Comparison of Models
All three models are simplistic. All three ignore multiple and coherent scattering. It is instructive to consider the disadvantages and the advantages of the Faran Cylinder model compared with the other two. The main disadvantage of the Faran Cylinder model is that it assumes an idealized cylindrical geometry for cancellous bone. In reality, trabeculae can be somewhat jagged and curved, which means that they deviate from a true cylindrical shape. (However, as can be seen in Fig. 1, the extent of curvature is often minimal on the scale of an ultrasonic beam width—typically about 1 cm at 500 kHz.) In addition, cancellous bone can contain small plate-like structures and cross-struts between trabeculae. Scattering contributions from point-like (on the scale of an ultrasonic wavelength) plates and short cross-struts may explain measurements of backscatter coefficient that vary with frequency more rapidly than the cubic dependence predicted by the Faran Cylinder model (since scattering from structures small compared with the wavelength are proportional to frequency to the fourth power). The Binary Mixture and Weak Scattering models, with their statistical characterization of the cancellous bone, are more flexible in their ability to accommodate complex microstructure.
Advantages of the Faran Cylinder model include the following. 1) Unlike the Binary Mixture and Weak Scattering models, the Faran Cylinder model does not require that the acoustic properties (density and sound speed) within the two-component trabecular bone medium deviate only slightly from their mean values (spatial mean throughout the entire scattering volume). Although the true density and sound speed of trabeculae are not known with great certainty, they probably deviate substantially from density and sound speed of the fluid filler. See Table I, and note that the density and longitudinal sound speed of water and marrow are approximately 1 g/cm3 and 1480 m/s. Jenson et al. have acknowledged this limitation of the Weak Scattering model [76]. The Binary Mixture model actually neglects density fluctuations altogether. 2) Unlike the Binary Mixture and Weak Scattering models, the Faran Cylinder model allows for the propagation of shear waves within trabeculae. Shear wave propagation has been measured in cortical bone [99] and is therefore plausible within trabeculae. Simulation studies suggest, however, that shear waves within the trabecular network may play a negligible role in backscattering properties of cancellous bone [79]. 3) Unlike the Binary Mixture [59,65] and the Weak Scattering [76] models, the Faran Cylinder model does not require isotropy. Cancellous bone, particularly in weight-bearing bones like calcaneus, however, does exhibit some anisotropy (see Section III.E). Jenson et al. have also acknowledged this limitation of the Weak Scattering model [76].
III. EXPERIMENTAL RESULTS
A. Dependence of Backscatter on Bone Mineral Density
In the first investigation of ultrasonic backscatter from cancellous bone, Roberjot et al. found that integrated (average) backscatter coefficient exhibits a moderate correlation (r2 = 0.68) with bone mineral density (BMD) in vitro (human calcaneus, 200 – 600 kHz) [58]. Subsequently, Wear and Armstrong found that that backscatter coefficient at 500 kHz exhibits a similar correlation in vitro (r2 = 0.66) (human calcaneus) [62]. Hoffmeister et al. found apparent (not compensated for attenuation) integrated backscatter to decrease gradually (but with statistical significance) with BMD (bovine cancellous tibia, 1–3 MHz) [66]. The rate of decrease is even steeper at higher frequencies (bovine cancellous tibia, 2.5–7.5 MHz), resulting in higher magnitudes of correlation with BMD (r2 = 0.82 in transverse orientation and r2 = 0.49 in longitudinal orientation) [87].
B. Dependence of Backscatter on Frequency
Frequency-dependent backscatter may be measured by interrogating cancellous bone samples using broadband transducers. The average frequency-dependent backscatter coefficient, η(f), may be fit to a power law form, η(f) = Afn, and the exponent n (which is an index of frequency dependence) may be compared with theoretical models. The power law model for backscatter coefficient has intuitive appeal because the theoretical value for the exponent, n, is known in some simple limiting cases. For example, n = 4 for scatterers that are much smaller than a wavelength, and n = 3 for long cylinders that are much narrower than a wavelength (under some conditions). Generally speaking, n decreases as scatterer size increases (unless resonances complicate the situation).
Wear reported an average value of n = 3.26 ± 0.20 (standard error) in human calcaneus (300–700 kHz), which is a little higher than the value of approximately 3 predicted by the Faran Cylinder model for thin cylinders over this frequency range [60, 73]. (As discussed in section V.A. and II.D., multiple scattering and scattering from small plates and cross-struts may explain this slightly elevated exponent). Subsequently, Chaffai et al. measured 3.38 ± 0.31 (standard error) in human calcaneus (0.4–1.2 MHz), which is close to the value of 3.48 predicted by the Weak Scattering model [64] for the samples in question over this frequency range. Padilla et al. measured 3.1 ± 1.09 (standard deviation) in human femur (0.4–1.2 MHz) [81], which is reasonably close to these values, given the accuracy and precision of the measurement. Deligianni and Apostolopoulos [98] achieved good agreement between theoretical predictions (based on a two-component Weak Scattering model) and experimental measurements of frequency-dependent backscatter in bovine cancellous bone but did not report their results in power-law form.
For the frequency ranges and mean trabecular thicknesses (Tb.Th) considered above, scattering may be considered to be primarily in the low ka regime where k = 2π / λ, λ = wavelength, and radius a = Tb.Th/2. Assuming a mean trabecular thickness of 0.127 mm in calcaneus [101], the frequency range from 300–700 kHz (λ in the surrounding fluid: 5.0 – 2.1 mm) corresponds to a ka range of 0.08 – 0.19 and the frequency range from 0.4 – 1.2 MHz (λ in the surrounding fluid: 3.8 – 1.3 mm) corresponds to a ka range of 0.11 – 0.32. Assuming a mean trabecular thickness of 0.172 mm in femur [101], the frequency range from 0.4 – 1.2 MHz corresponds to a ka range of 0.14 – 0.43.
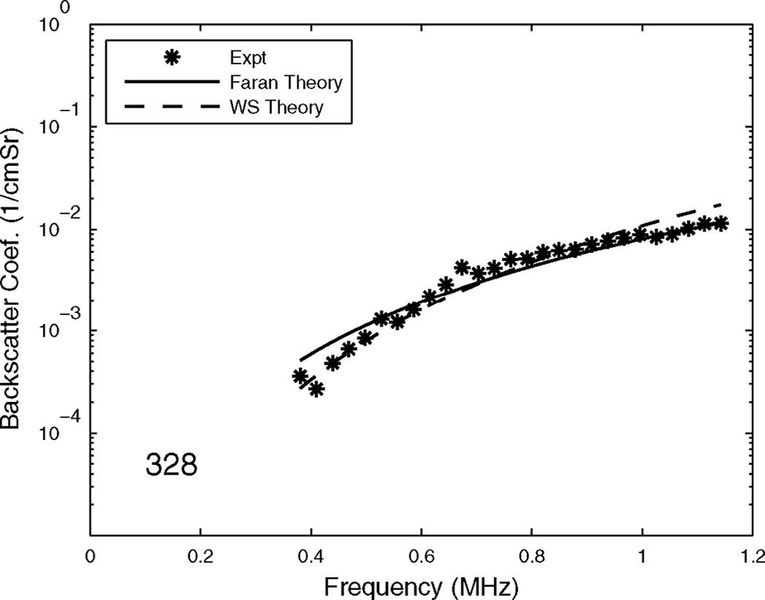
The measurements of backscattering power law exponent suggest that the Faran Cylinder and Weak Scattering models both predict the observed dependence of backscatter on frequency fairly well. (The Binary Mixture model has not been directly compared with experimental backscatter measurements). Because of 1) the limited accuracy and precision of experimental backscatter measurements (see Section III.F.), and 2) the uncertainty of the values for material properties of cancellous bone (see Table I), it is difficult, based on existing data, to conclude that either model performs superior to the other in this regard. Moreover, both models are sufficiently simplistic in their assumptions that neither one can be expected to model the physics extremely well. The accuracy and precision of experimental measurements are limited by many factors, including small sample size, inhomogeneous samples, speckle noise, phase cancellation, coherent effects, and multiple scattering. Therefore, the uncertainty of measurements of exponents in cancellous bone samples is probably at least a few tenths. The main conclusion to draw from the theory and data is that the true exponent for cancellous bone is probably near the 3.0 – 3.5 range. Both models are in reasonable agreement with this. Frequency-dependent backscatter coefficient model predictions and measurements for a human femoral cancellous bone sample (porosity = 94% and mean trabecular thickness = 134 microns, measured by microCT) are shown in Figure 2.
2.
Frequency-dependent backscatter coefficient measurements from human femoral cancellous bone specimen. Model predictions are also shown. Experimental measurements and the Weak Scattering model predictions were provided by Pascal Laugier and Frederic Padilla from the University of Paris.
C. Dependence of Backscatter on Trabecular Thickness
The dependence of backscatter on trabecular thickness (Tb.Th) has potential diagnostic importance because a reduction in trabecular thickness would be expected to be associated with increased fracture risk and reduced backscatter coefficient. The backscatter coefficient at a particular frequency, η(f0, Tb.Th), may be fit to a power law form, η(f0, Tb.Th) = BTb.Thm, and the exponent m may be compared with theoretical models. The Faran cylinder model predicts the exponent m to be 2.9 [74]. Wear and Laib reported an average measurement of m of 2.80 (95% confidence interval: 1.7 −3.9) (human calcaneus, 500 kHz) [74], where Tb.Th was assessed using microCT. The square of the correlation coefficient of a linear regression of log backscatter coefficient vs. log Tb.Th was 0.40, suggesting that 40% of variations in backscatter may be attributed to variations in Tb.Th [74].
The Weak Scattering model predicts scattering based on the autocorrelation function of the scattering medium. The width of the autocorrelation function is related to Tb.Th. Jenson et al. found the Weak Scattering model to yield good agreement between estimates of Tb.Th obtained directly from microCT (130 ± 6.5 μm) and indirectly from ultrasound backscattering measurements (138 ± 6.5 μm) (human calcaneus, 0.4–1.2 MHz) [76]. Subsequently, Padilla et al. found good agreement (microCT: 132 ± 12 μm; ultrasound: 134 ± 15 μm) (human femur, 0.4–1.2 MHz) [81, 86]. The square of the correlation coefficient of microCT-based and ultrasound-based estimates of Tb.Th was 0.44 [80, 84], a value very similar to that obtained in Faran-cylinder-model based study mentioned above (0.40) [74].
D. Dependence of Backscatter on Fluid Filler (Marrow or Water)
Most in vitro studies of the ultrasonic properties of cancellous bone involve defatted bone specimens immersed in water tanks. In these experiments, water in vitro substitutes for marrow in vivo as the fluid filler within the porous trabecular matrix. Nicholson and Bouxsein found a small but statistically significant 2.1 dB decrease in backscatter coefficient when water is substituted for marrow (water: −30.9 ± 6.3 dB; marrow: −28.8 ± 4.7 dB; human calcaneus, 600 kHz) [49]. (The statistical significance arose from the fact that 46 samples were measured in this study so that the standard errors were lower than the standard deviations by a factor of approximately seven.) Hoffmeister et al. found a small but statistically significant decrease of approximately 0.5 dB in apparent integrated backscatter when water is substituted for marrow (bovine tibia, 2.25 MHz) [71]. The magnitudes of these differences are fairly small, however, relative to the variations in backscatter associated with other factors such as BMD, frequency, and trabecular thickness. Therefore, experiments that substitute water for marrow are relevant to in vivo scattering.
E. Anisotropy of Backscatter
Although commercial bone sonometers interrogate calcaneus in the mediolateral (ML) orientation, calcaneus samples may be interrogated in multiple orientations in vitro. Wear found average backscatter coefficient to be 50% (1.8 dB) higher in the ML direction than in the anteroposterior (AP) direction (human calcaneus, 500 kHz) [61]. Backscatter coefficient in both orientations approximately conformed to a cubic dependence on frequency [61]. In the ML orientation, the ultrasound propagation direction is approximately perpendicular to the trabecular axes. In the AP orientation, a wide range of angles between the ultrasound propagation direction and trabecular axes is encountered. The higher backscatter in the ML direction may be due to the fact that the trabeculae are oriented in such a way to present the maximum cross sectional area available to intercept (and therefore, scatter) the incident ultrasound beam.
Hoffmeister found apparent integrated backscatter to be slightly higher in the ML direction (−33.9 dB) than in the AP (−34.3 dB) or superoinferior (SI) (−34.6 dB) directions (bovine tibia, 1–3 MHz) [66].
F. Precision of Backscatter Measurements
In order to appreciate the limitations of comparisons between theoretical predictions and experimental measurements of scattering from cancellous bone, it is important to understand measurement precision. Cancellous bone, like soft tissues, contains a complicated arrangement of densely-packed scatterers (trabeculae) that produce highly variable scattered signals, depending on the extent to which echoes from individual scatterers interfere constructively or destructively. The resulting interference pattern, which gives rise to speckle in medical ultrasound images, produces wide fluctuations in scattering measurements. For example, Chaffai et al. reported a wide variation of measurements of the exponent, n, of frequency dependence of power law fit (see Section III.B) from individual human calcaneus samples (ranging from 2.76 to 3.86) [64]. However, Chaffai et al. could not be certain whether the variation was attributable to micro-architectural variations or to random interference effects [64].
In order to investigate the relative roles of micro-architectural variations and random interference effects, Wear adapted a model developed by Lizzi et al. [108] to predict statistical fluctuations in midband backscatter coefficient and n in human calcaneus [67]. The model of Lizzi et al. is appropriate for scattering media (such as cancellous bone) that contain many scatterers per resolution cell. It accounts for the random interference of echoes (speckle) from individual scatterers (e.g. trabeculae) as they are summed at the receiver. Wear showed that while random interference effects only partially explain measured variations in the midband (or average) backscatter coefficient, they are virtually entirely responsible for observed variations in n (human calcaneus, 500 kHz) [67]. Consequently, measurements of n in individual human calcanea at diagnostic frequencies provide little or no diagnostic information. (In other words, it is far more difficult to estimate n from noisy backscatter measurements on individual calcanea than from average backscatter measurements obtained from large numbers of samples as discussed in Section III.B.)
G. Clinical Findings
Backscatter is interesting from a clinical standpoint because it can be measured with a single transducer and therefore may be potentially extended beyond the calcaneus to other sites in the skeleton such as the hip and spine. So far, only modest clinical success has been reported for backscatter, however. This may be due to high variability of backscatter measurements due to speckle noise [67] and the distorting effects of rough cortical bone surfaces.
Wear and Garra found a moderate correlation (r2 = 0.76) between ultrasonic backscatter and bone mineral density (BMD) assessed using x-ray computed tomography (CT) (normal human volunteers, calcaneus, 2.25 MHz) [88, 91]. In the most definitive clinical investigation (the only one measuring fracture risk) involving backscatter, Roux et al. found broadband ultrasonic backscatter to be moderately predictive of fracture (odds ratio: 1.58; 95% CI: 1.14–2.19) (210 women, calcaneus, 200–600 kHz) [92]. Wear and Armstrong found modest correlations between average backscatter coefficient, BUA, SOS, and age (women, calcaneus, 1 MHz) [93]. Wear et al. reported a modest correlation (r2 = 0.37) between the backscatter spectral centroid shift and BMD (human spine, 2.5 MHz) [72].
IV. SIMULATION RESULTS
Simulations have been useful for complementing theoretical and experimental approaches in the investigation of scattering of ultrasound by cancellous bone. Luo et al. implemented a 2D simulation of ultrasound propagation that solved the 2D visco-elastic wave equation [46]. In an extension of this work, Kaufman et al. [83]—using slices from digitized 3D micro-tomographs of a human cancellous calcaneus sample to represent the 2D distribution of material properties in a simulation—analyzed two cases for a through-transmission measurement over the range from 300 to 900 kHz: 1) with full accounting for viscous absorption losses, and 2) with all viscous loss terms set to zero. They found attenuation to be similar in both cases, and argued that scattering may therefore be the main source of attenuation for simple 2D situations.
Bossy et al. [79] subsequently implemented a finite-difference time-domain (FDTD) algorithm to compute a numerical solution for 3D linear elastic wave propagation through cancellous bone specimens immersed in water. They used digitized 3D synchrotron micro-tomographs of cancellous bone samples (31 human femur specimens) to represent 3D distributions of material properties in their simulations. Their algorithm accounted for reflection, refraction, and mode conversion at bone interfaces. Although their algorithm did not take absorption into account, their simulations reproduced major phenomena consistent with widely-accepted experimental findings: 1) the quasi-linear variation of attenuation with frequency, 2) the monotonic increase of BUA and SOS with bone volume fraction, and 3) negative velocity dispersion [79]. Subsequent extensions of this work by Padilla et al. [84] and Haïat et al. [85] reinforced the initial findings of Bossy et al. [79]. In another recent extension, Bossy et al. provided evidence that for human cadaver femur cancellous bone specimens, absorption may be the primary source of attenuation at low frequencies (below approximately 600 kHz for average specimens) while scattering may be the primary source of attenuation at high frequencies (above approximately 600 kHz) [109]. This would suggest that absorption is the primary source of attenuation throughout most of the diagnostic range (300 – 700 kHz). They further argued that the importance of absorption may increase with volume fraction. Bossy et al. suggested that mode-conversion from longitudinal to shear could be a significant form of scattering in these simulations [79].
V. DISCUSSION
A. Multiple Scattering
Multiple scattering, if present, would have the effect of applying additional high-pass filtering to scattered waves (since scattering coefficient increases rapidly with frequency). This would increase the measured exponent (n) of frequency dependence of scattering. Since measurements of n only slightly exceed the single-scattering Faran Cylinder model prediction, multiple scattering does not seem to play a big role in typical scattering measurements [60]. The reasons for this may be as follows. The magnitude of the scattering coefficient from individual trabeculae and the number of trabeculae per unit volume may be sufficiently low to marginalize the importance of multiple scattering. In addition, multiple-scattered waves, if present, would experience greater attenuation than singly scattered waves because 1) attenuation coefficient is directly proportional to frequency and multiple-scattered wave spectra tend to have higher frequency content (due to having experienced multiple high-pass filters) than single-scattered waves, and 2) attenuation increases with path length and multiple-scattered waves tend to traverse longer paths than single-scattered waves [60]. Chaffai et al. [64] and Jenson et al. [75], based on their Weak Scattering theory and experimental data, agreed that multiple-scattered waves are probably negligible in typical scattering measurements. Recent work by Haϊat et al., however, suggests that multiple scattering effects can help explain negative velocity dispersion in cancellous bone [110].
B. Absorption and Scattering
Attenuation is the combined result of absorption and scattering. While attenuation in cancellous bone is approximately proportional to frequency to the first power [20–56], backscatter coefficient is approximately proportional to frequency to the third power (in the diagnostic frequency range, 300 – 700 kHz) [60]. Moreover, the Faran Cylinder model predicts that total scatter, not just backscatter, should be approximately proportional to frequency to the third power [60]. Even without the Faran Cylinder model, it is difficult to imagine a circumstance that would lead to cubic backscatter (scattering at 180 degrees) and linear total scatter (the integral of scattering coefficient over all solid angles). This inconsistency of frequency dependencies for attenuation and scattering led Wear to suggest that scattering may be only a minor contribution to attenuation at diagnostic frequencies [60]. Chaffai et al. subsequently agreed with this [64]. Wear further suggested [60] (and Chaffai et al. agreed [64]) that this inconsistency of frequency dependencies implied that absorption may be the primary source of attenuation. Padilla and Laugier subsequently proposed a weak scattering / poroelasticity model that also suggested that absorption was the primary source of attenuation [68]. However, since scattering measurements were limited to longitudinal-to-longitudinal scattering, this conclusion may have been somewhat premature since the relative importance of longitudinal-to-shear conversion was not fully appreciated at that time. Recent simulation work has suggested that the conversion of incident longitudinal waves into scattered shear waves may be significant [79, 84, 85, 109], but would only exceed absorption loss at high frequencies (above approximately 600 kHz) [109] (see Section IV), which are at the high end of the diagnostic range (300 to 700 kHz). Measurements of attenuation from 1 to 10 MHz in phantoms consisting of graphite fibers suspended in gelatin have shown the combined result of classical viscous loss within the gelatin and scattered evanescent shear waves to produce an approximately linear frequency dependence of attenuation [111] (consistent with theoretical predications [112]) parallel to the fibers and a somewhat higher than linear frequency dependence of attenuation perpendicular to the fibers (which is orientation in which scattering measurements on cancellous bone have been performed). See Ref. [111], Figure 8.
Recent simulations [79, 83–85, 109] modeled through-transmission experiments and did not explore the immediate fate of shear wave energy scattered from trabeculae. Regardless of whether the redirected shear wave energy persists as an ultrasound wave or is absorbed by the cancellous bone medium, it would elude the receiving transducer in simulated through-transmission experiments. Although shear waves may arise from mode conversion at trabecular interfaces, they may be extremely transient. For example, Hosokawa estimated shear attenuation coefficients in bovine cancellous bone to be approximately 17 dB/mm (at 1 MHz) [113], implying that shear wave power is reduced by approximately 98% for each mm of propagation. Similarly, Mottley and Miller described shear waves generated from graphite particles suspended in gelatin as “evanescent” [111]. Like the multiple-scattered wave discussed above, the mode-converted shear wave may have transient significance in the immediate proximity of the scatterer, but due to high attenuation it is rapidly extinguished. If longitudinal-to-shear mode conversion is significant, then the relative roles of absorption and scattering in cancellous bone (even at high frequencies, above 600 kHz) will depend somewhat on the relative roles of absorption and scattering of mode-converted shear waves. If the rapid attenuation of mode-converted shear waves is primarily due to absorption, then absorption would be the dominant loss mechanism even at high frequencies, albeit with the caveat that the ultrasonic energy briefly takes the form of a very short-lived shear wave prior to absorption. More research is needed in this area.
VI. CONCLUSION
Pulse-echo data has been acquired from cancellous bone samples in vitro to probe the dependence of ultrasonic backscatter on bone mineral density, frequency, mean trabecular thickness, fluid filler (marrow or water), and direction of ultrasound propagation relative to the predominant trabecular orientation. Three theoretical models for scattering from cancellous bone have demonstrated some consistency with experimental observations. So far, backscatter has demonstrated only modest clinical utility. Computer simulation models have helped to elucidate mechanisms underlying scattering from cancellous bones.
The Binary Mixture, Faran Cylinder, and Weak Scattering models are useful for prediction of scattering properties of cancellous bone. Because of 1) the limited accuracy and precision of experimental measurements of ultrasound backscatter and 2) the uncertainty of the values for material properties of cancellous bone, it is difficult, based on existing data, to conclude that one model performs superior to the others. Moreover, all three models are sufficiently simplistic in their assumptions that none can be expected to model the physics extremely well. The accuracy and precision of experimental measurements are limited by many factors, including small sample size, inhomogeneous samples, speckle noise, phase cancellation, coherent effects, and multiple scattering.
ACKNOWLEDGEMENTS
The author thanks 1) the FDA Office of Women’s Health for providing funding for this research, 2) Andres Laib, Scanco Medical AG, Brüttisellen, Switzerland, for providing the micro computed tomogram in Figure 1, and 3) Pascal Laugier and Frederic Padilla from Université Pierre et Marie Curie-Paris 6 for providing measurements of frequency-dependent backscatter and Weak Scattering model predictions for a cancellous bone specimen shown in Figure 2.
Biography
Keith A. Wear graduated from the University of California at San Diego with a B.A. in Applied Physics in 1980. He received his M.S. and Ph.D. in Applied Physics with a Ph.D. minor in Electrical Engineering from Stanford University in 1982 and 1987.
He was a post-doctoral research fellow with the Physics department at Washington University, St. Louis from 1987–1989. He has been a research physicist at the FDA Center for Devices and Radiological Health since 1989. His research has included measurements of ultrasonic scattering properties from tissues, high-resolution spectral estimation, magnetic resonance spectroscopic image reconstruction methods, analysis of statistical properties of ultrasonic echoes from tissues, and improved measurement methodology in bone sonometry.
He is an adjunct professor of Radiology at Georgetown University. He is a Fellow of the American Institute for Medical and Biological Engineering (AIMBE) and the American Institute of Ultrasound in Medicine (AIUM). He is a senior member of IEEE. He is a member of the Acoustical Society of America, IEEE Ultrasonics Society, the National Osteoporosis Foundation and the AIUM Technical Standards Committee. He served as Vice-Chairman (2002–2004) and Chairman (2004–2006) of the AIUM Basic Science and Instrumentation Section. He is an associate editor of IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control.
REFERENCES
- [1].Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, Delmas PD, Pouilles JM, Breart G, and Meunier PJ. “Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study,” Lancet, 348, pp. 511–514, 1996. [DOI] [PubMed] [Google Scholar]
- [2].Bauer DC, Glüer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, and Black DM. “Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women,” Arch. Intern, Med 157, pp. 629–634 1997. [PubMed] [Google Scholar]
- [3].Miller PD, Siris ES, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Chen Y, Berger ML, Santora AC, and Sherwood LM, “Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the national osteoporosis risk assessment,” J. Bone & Miner. Res, 17, pp. 2222–2230, 2002. [DOI] [PubMed] [Google Scholar]
- [4].Hans D, Schott AM, Duboeuf F, Durosier C, and Meunier PJ, “Does follow-up duration influence the ultrasound and DXA prediction of hip fracture? The EPIDOS prospective study,” Bone, 35, 357–363, 2004. [DOI] [PubMed] [Google Scholar]
- [5].Huopio J, Kroger H, Honkanen R, Jurvelin J, Saarikoski S, and Alhava E, “Calcaneal ultrasound predicts early postmenopausal fractures as well as axial BMD. A prospective study of 422 women,” Osteo. Int, 15, pp. 190–195, 2004. [DOI] [PubMed] [Google Scholar]
- [6].Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N, Oakes S, and Day N, “Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study,” Lancet, 363, 197–202, 2004. [DOI] [PubMed] [Google Scholar]
- [7].Schott AM, Hans D, Duboeuf F, Dargent-Molina P, Hajri T, Breart G, and Meunier PJ, “Quantitative ultrasound parameters as well as bone mineral density are better predictors of trochanteric than cervical hip fractures in elderly women. Results from the EPIDOS study,” Bone, 37, 858–863, 2005. [DOI] [PubMed] [Google Scholar]
- [8].Krieg M, Cornuz J, Ruffieux C, Melle GV, Buche D, Dambacher MA, Hans D, Hartl F, Hauselmann HJ, Kraenzlin M, Lippuner K, Neff M, Pancaldi P, Rizzoli R, Tanzi F, Theiler R, Tyndall A, Wimpfheimer C, and Burckhardt P., “Prediction of hip fracture risk by quantitative ultrasound in more than 7000 Swiss women ≥ 70 years of age: comparison of three technologically different bone ultrasound devices in the SEMOF study,” J. Bone. Miner. Res, 21, 1456–1463, 2006. [DOI] [PubMed] [Google Scholar]
- [9].Schott M, Weill-Engerer S, Hans D, Duboeuf F, Delmas PD, and Meunier PJ, “Ultrasound discriminates patients with hip fracture equally well as dual energy X-ray absorptiometry and independently of bone mineral density,” J. Bone Min. Res, 10, pp. 243–249 1995. [DOI] [PubMed] [Google Scholar]
- [10].Turner CH, Peacock M, Timmerman L, Neal JM, and Johnston CC Jr., “Calcaneal ultrasonic measurements discriminate hip fracture independently of bone mass,” Osteo. International, 5, pp. 130–135 1995. [DOI] [PubMed] [Google Scholar]
- [11].Mautalen C, Vega E, Gonzalez D, Carrilero P, Otano A, and Silberman F, “Ultrasound and dual x-ray absorptiometry densitometry in women with hop fracture,” Calcif. Tissue Int, 57, 441–449, 1995. [DOI] [PubMed] [Google Scholar]
- [12].Glüer CC, Cummings SR, Bauer DC, Stone K, Pressman A, Mathur A, and Genant HK. “Osteoporosis: Association of recent fractures with quantitative US findings”, Radiology, 199, pp. 725–732, 1996. [DOI] [PubMed] [Google Scholar]
- [13].Thompson P, Taylor J, Fisher A, and Oliver R, “Quantitative heel ultrasound in 3180 women between 45 and 75 years of age: compliance, normal ranges and relationship to fracture history,” Osteo. Int’l, 8, pp. 211–214, 1998. [DOI] [PubMed] [Google Scholar]
- [14].Frost ML, Blake GM, and Fogelman I, “Contact quantitative ultrasound: an evaluation of precision, fracture discrimination, age-related bone loss and applicability of the WHO criteris,” Osteo. Int, 10, 441–449, 1999. [DOI] [PubMed] [Google Scholar]
- [15].Njeh CF, Hans D, Li J, Fan B, Fuerst T, He YQ, Tsuda-Futami E, Lu Y, Wu CY, and Genant HK, “Comparison of six calcaneal quantitative ultrasound devices: precision and hip fracture discrimination,” 11, Osteo. Int, pp. 1051–1062, 2000. [DOI] [PubMed] [Google Scholar]
- [16].Krieg MA, Cornuz J, Ruffieux C, Sandini L, Buche D, Dambacher MA, Hartl F, Hauselmann HJ, Kraenzlin M, Lippuner K, Neff M, Pancaldi P, Rizzoli R, Tanzi F, Theiler R, Tyndall A, Wimpfheimer C, and Burckhardt P, “Comparison of three bone ultrasounds for the discrimination of subjects with and without osteoporotic fractures among 7562 elderly women,” J. Bone Miner. Res 18, 1261–1266, 2003. [DOI] [PubMed] [Google Scholar]
- [17].Glüer CC, Eastell R, Reid DM, Felsenberg D, Roux C, Barkmann R, Timm W, Blenk T, Armbrecht G, Stewart A, Clowes J, Thomasius FE, and Kolta S, “Association of five quantitative ultrasound devices and bone densitometry with osteoporotic vertebral fractures in a population-based sample: the OPUS study,” J. Bone & Miner. Res, 19, pp. 782–793, 2004. [DOI] [PubMed] [Google Scholar]
- [18].Welch A, Camus J, Dalzell N, Oakes S, Reeve J, and Khaw KT, “Broadband ultrasound attenuation (BUA) of the heel bone and its correlates in men and women in the EPIC-Norfolk cohort: a cross-sectional population-based study,” Osteo. Int, 15, 217–225, 2004. [DOI] [PubMed] [Google Scholar]
- [19].Maggi S, Naole M, Giannini S, Adami S, Defeo D, Isaia G, Sinigaglia L, Filipponi P, and Crepaldi G., “Quantitative heel ultrasound in a population-based study in Italy and its relationship with fracture history: the ESOPO study,” Osteo. Int, 17, 237–244, 2006. [DOI] [PubMed] [Google Scholar]
- [20].Langton CM, Palmer SB, and Porter RW. “The measurement of broadband ultrasonic attenuation in cancellous bone.” Eng. in Med 13, pp. 89–91, 1984. [DOI] [PubMed] [Google Scholar]
- [21].Rossman P, Zagzebski J, Mesina C, Sorenson J, and Mazess R, “Comparison of Speed of Sound and Ultrasound Attenuation in the Os Calcis to Bone Density of the Radius, Femur and Lumbar Spine,” Clin. Phys. Physiol. Meas, 10, pp. 353–360, 1989. [DOI] [PubMed] [Google Scholar]
- [22].Tavakoli MB and Evans JA. “Dependence of the velocity and attenuation of ultrasound in bone on the mineral content.” Phys. Med. Biol, 36, pp. 1529–1537, 1991. [DOI] [PubMed] [Google Scholar]
- [23].Zagzebski JA, Rossman PJ, Mesina C, Mazess RB, and Madsen EL, “Ultrasound transmission measurements through the os calcis,” Calcif. Tissue Int’l, 49, pp. 107–111, 1991. [DOI] [PubMed] [Google Scholar]
- [24].Kaufman JJ and Einhorn TA, “Perspectives: Ultrasound Assessment of Bone”, J. Bone. Min. Res, 8, pp. 517–525, 1993. [DOI] [PubMed] [Google Scholar]
- [25].Laugier P, Giat P, Droin P, Saied A, and Berger G, “Ultrasound images of the os calcis: a new method of assessment of bone status,” Proc. IEEE Ultrason. Symp¸Baltimore, MD, pp. 989–992, 1993. [Google Scholar]
- [26].Langton CM, Njeh CF, Hodgskinson R, and Carrey JD, “Prediction of Mechanical Properties of the Human Calcaneus by Broadband Ultrasonic Attenuation,” Bone, 18, pp. 495–503, 1996. [DOI] [PubMed] [Google Scholar]
- [27].Alves JM, Xu W, Lin D, Siffert RS, Ryaby JT, and Kaufman JJ, “Ultrasonic assessment of human and bovine trabecular bone: a comparison study,” IEEE Trans. Biomed. Eng, 43, 249–258, 1996. [DOI] [PubMed] [Google Scholar]
- [28].Alves JM, Ryaby JT, Kaufman JJ, Magee FP, and Siffert RS, “Influence of marrow on ultrasonic velocity and attenuation in bovine trabecular bone,” Calcif. Tissue Int, 58, 362–367, 1996. [DOI] [PubMed] [Google Scholar]
- [29].Njeh CF, Hodgskinson R, Currey JD, and Langton CM. “Orthogonal relationships between ultrasonic velocity and material properties of bovine cancellous bone.” Med. Eng. Phys, 18, pp. 373–381, 1996. [DOI] [PubMed] [Google Scholar]
- [30].Strelitzki R and Evans JA, “On the measurement of the velocity of ultrasound in the os calcis using short pulses,” Euro. J. Ultrasound, 4, 205–213, 1996. [Google Scholar]
- [31].Strelitzki R, Clarke AJ, and Evans JA, “The measurement of the velocity of ultrasound in fixed trabecular bone using broadband pulses and single-frequency tone bursts,” Phys. Med. Biol, 41, 743–753, 1996. [DOI] [PubMed] [Google Scholar]
- [32].Nicholson PHF, Lowet G, Langton CM, Dequeker J, and Van der Perre G, “A comparison of time-domain and frequency-domain approaches to ultrasonic velocity measurement in trabecular bone,” Phys. Med. Biol, 41, 2421–2435, 1996. [DOI] [PubMed] [Google Scholar]
- [33].Nicholson PHF, Lowet G, Cheng XG, Boonen S, Van der Perre G, and Dequeker J, “Assessment of the strength of the proximal femur in vitro: relationship with ultrasonic measurements of the calcaneus,” Bone, 20, 219–224, 1997. [DOI] [PubMed] [Google Scholar]
- [34].Njeh CF, Hodgskinson R, Currey JD, and Langton CM, “Orthogonal relationships between ultrasonic velocity and material properties of bovine cancellous bone,” Med. Eng. Phys, 18, 373–381, 1996. [DOI] [PubMed] [Google Scholar]
- [35].Serpe L and Rho JY, “The nonlinear transition period of broadband ultrasound attenuation as bone density varies,” J. Biomech, 29, pp. 963–966, 1996. [DOI] [PubMed] [Google Scholar]
- [36].Chappard C, Laugier P, Fournier B, Roux C, and Berger G, “Assessment of the relationship between broadband ultrasound attenuation and bone mineral density at the calcaneus using BUA Imaging and DXA,” Osteo. Int, 7, pp. 316–322, 1997. [DOI] [PubMed] [Google Scholar]
- [37].Njeh CF and Langton CM, “The effect of cortical endplates on ultrasound velocity through the calcaneus: an in vitro study,” Brit. J. Radiol, 70, 504–510, 1997. [DOI] [PubMed] [Google Scholar]
- [38].Njeh CF, Kuo CW, Langton CM, Atrah HI, and Boivin CM, “Prediction of human femoral bone strength using ultrasound velocity and BMD: an in vitro study,” Osteo. Int, 7, 471–477, 1997. [DOI] [PubMed] [Google Scholar]
- [39].Bouxsein ML and Radloff SE. “Quantitative ultrasound of the calcaneus reflects the mechanical properties of calcaneal trabecular bone,” J. Bone Miner. Res 12, pp. 839–846, 1997. [DOI] [PubMed] [Google Scholar]
- [40].Laugier P, Droin P, Laval-Jeantet AM, and Berger G. “In vitro assessment of the relationship between acoustic properties and bone mass density of the calcaneus by comparison of ultrasound parametric imaging and quantitative computed tomography.” Bone, 20, pp. 157–165, 1997. [DOI] [PubMed] [Google Scholar]
- [41].Hosokawa A and Otani T “Ultrasonic wave propagation in bovine cancellous bone,”, J. Acoust. Soc. Am, 23, pp. 405–418, 1997. [DOI] [PubMed] [Google Scholar]
- [42].Nicholson PHF, Muller R, Lowet G, Cheng XG, Hildebrand T, Ruegsegger P, Van Der Perre G, Dequeker J, and Boonen S. “Do quantitative ultrasound measurements reflect structure independently of density in human vertebral cancellous bone?” Bone. 23, pp. 425–431, 1998. [DOI] [PubMed] [Google Scholar]
- [43].Droin P, Berger G, and Laugier P, “Velocity dispersion of acoustic waves in cancellous bone,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 45, 581–592, 1998. [DOI] [PubMed] [Google Scholar]
- [44].Hans D, Wu C, Njeh CF, Zhao S, Augat P, Newitt D, Link T, Lu Y, Majumdar S, and Genant HK. “Ultrasound velocity of trabecular cubes reflects mainly bone density and elasticity.” Calcif. Tissue Intl 64, pp. 18–23, 1999. [DOI] [PubMed] [Google Scholar]
- [45].Trebacz H, and Natali A. “Ultrasound velocity and attenuation in cancellous bone samples from lumbar vertebra and calcaneus.” Osteo. Int’l, 9, pp. 99–105, 1999. [DOI] [PubMed] [Google Scholar]
- [46].Luo G, Kaufman JJ, Chiabrera A, Bianco B, Kinney JH, Haupt D, Ryaby JT, and Siffert RS, “Computational methods for ultrasonic bone assessment,” Ultrasound Med. Biol, 25, 823–830, 1999. [DOI] [PubMed] [Google Scholar]
- [47].Wear KA, “The effects of frequency-dependent attenuation and dispersion on sound speed measurements: applications in human trabecular bone,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 47, 265–273, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wear KA, “Measurements of phase velocity and group velocity in human calcaneus,” Ultrasound Med. Biol, 26, 641–646., 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nicholson PHF and Bouxsein ML, “Bone marrow influences quantitative ultrasound measurements in human cancellous bone,” Ultrasound Med. & Biol, 28, 369–375, 2002. [DOI] [PubMed] [Google Scholar]
- [50].Lee KL, Roh H, and Yoon SW, “Correlations between acoustic properties and bone density in bovine cancellous bone from 0.5 to 2 MHz,” J. Acoust. Soc. Am, 113, 2933–2938, 2003. [DOI] [PubMed] [Google Scholar]
- [51].Lee KL, Rho H, and Yoon SW, “Acoustic wave propagation in bovine cancellous bone: application of the modified Biot-Attenborough model,” J. Acoust. Soc. Am, 114, 2284–2293, 2003. [DOI] [PubMed] [Google Scholar]
- [52].Hakulinen M, Day JS, Töyräs J, Timonen M, Kröger K, Weinans H, Kiviranta I, and Jurvelin JS, “Prediction of density and mechanical properties of human trabecular bone in vitro by using ultrasound transmission and backscattering measurements at 0.2–6.7 MHz frequency range,” Phys. Med. Biol, 50, pp. 1629–1642, 2005. [DOI] [PubMed] [Google Scholar]
- [53].Chen P, Chen T, Lu M, and Yao W, “The measurements of ultrasound parameters on calcaneus by two-sided interrogation techniques,” Meas. Sci. Technol, 16, 1349–1354, 2005. [Google Scholar]
- [54].Haϊat G, Padilla F, Barkmann R, Kolta S, Latremouille C, Glüer CC, and Laugier P, “In vitro speed of sound measurement at intact human femur specimens,” Ultrasound in Med. & Biol, 31, 987–996, 2005. [DOI] [PubMed] [Google Scholar]
- [55].Haϊat G, Padilla F, Cleveland RO, and Laugier P, “Effects of frequency-dependent attenuation and velocity dispersion on in vitro ultrasound velocity measurements in intact human femur specimens,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 53, 39–51, 2006. [DOI] [PubMed] [Google Scholar]
- [56].Yamoto Y, Matsukawa M, Otani T, Yamazaki K, and Nagano A, “Distribution of longitudinal wave properties in bovine cortical bone in vitro,” Ultrasonics, 44, e233–e237, 2006. [DOI] [PubMed] [Google Scholar]
- [57].Xia Y, Lin W, and Qin Y, “Bone surface topology mapping and its role in trabecular bone quality assessment using scanning confocal ultrasound,” Osteo. Int, 18, 905–913, 2007. [DOI] [PubMed] [Google Scholar]
- [58].Roberjot V, Laugier P, Droin P, Giat P, and Berger G, “Meaurement of integrated backscatter coefficient of trabecular bone. Proc. 1996 IEEE Ultrason. Symp Vol. 2, pp. 1123–1126, 1996. [Google Scholar]
- [59].Strelitzki R, Nicholson PHF, and Paech V, “A model for ultrasonic scattering in cancellous bone based on velocity fluctuations in a binary mixture,” Physiol. Meas, 19, 189–196, 1998. [DOI] [PubMed] [Google Scholar]
- [60].Wear KA. “Frequency dependence of ultrasonic backscatter from human trabecular bone: theory and experiment.” J. Acoust. Soc. Am 106, pp. 3659–3664, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wear KA. “Anisotropy of ultrasonic backscatter and attenuation from human calcaneus: Implications for relative roles of absorption and scattering in determining attenuation.” J. Acoust. Soc. Am, 107, pp. 3474–3479, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wear KA and Armstrong DW, “The relationship between ultrasonic backscatter and bone mineral density in human calcaneus,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 47, pp. 777–780, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wear KA, Stuber AP, and Reynolds JC, “Relationships of ultrasonic backscatter with ultrasonic attenuation, sound speed, and bone mineral density in human calcaneus,” Ultrason. Med. Biol, 26, 1311–1316, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chaffai S, Roberjot V, Peyrin F, Berger G, and Laugier P. “Frequency dependence of ultrasonic backscattering in cancellous bone: Autocorrelation model and experimental results.” J. Acoust. Soc. Am 108, pp. 2403–2411, 2000. [DOI] [PubMed] [Google Scholar]
- [65].Nicholson PHF, Strelitzki R, Cleveland RO, and Bouxsein ML, “Scattering of ultrasound in cancellous bone: predictions from a theoretical model,” J. Biomech 33, pp. 503–506, 2000. [DOI] [PubMed] [Google Scholar]
- [66].Hoffmeister BK, Whitten SA, and Rho JY, “Low megahertz ultrasonic properties of bovine cancellous bone,” Bone, 26, pp. 635–642, 2000. [DOI] [PubMed] [Google Scholar]
- [67].Wear KA “Fundamental precision limitations for measurements of frequency dependence of backscatter: applications in tissue-mimicking phantoms and trabecular bone,” J. Acoust. Soc. Am 110(6), pp. 3275–3282, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Padilla F and Laugier P, “Prediction of ultrasound attenuation in cancellous bones using poroelasticity and scattering theories,” Proc. IEEE Ultrason. Symp, 1201–1204, 2001. [Google Scholar]
- [69].Laugier P, Padilla F, and Jenson F, “Ultrasonic scattering models for cancellous bone,” 143rd meeting of the Acoustical Society of America, J. Acoust. Soc. Am, 111(5), May, p. 2412, 2002, Pittsburgh, PA. [Google Scholar]
- [70].Hoffmeister BK, Whitten SA, Kaste SC, and Rho JY, Effect of collagen and mineral content on the high frequency ultrasonic properties of human cancellous bone, Bone, Osteo. Int, 13:26–32, 2002. [DOI] [PubMed] [Google Scholar]
- [71].Hoffmeister BK, Auwarter JA, and Rho JY, “Effect of marrow on the high frequency ultrasonic properties of cancellous bone,” Phys. Med. Biol, 47, 3419–3427, 2002. [DOI] [PubMed] [Google Scholar]
- [72].Wear KA, “Characterization of Trabecular Bone Using the Backscattered Spectral Centroid Shift,” IEEE Trans. Ultrason., Ferro. Freq. Cont, 50, 402–407, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wear KA, “The effect of trabecular material properties on the frequency dependence of backscatter from cancellous bone,” J. Acoust. Soc. Am, 113, 62–65, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wear KA and Laib A, “The Relationship Between Ultrasonic Scattering and Micro-architecture in Human Calcaneus,” IEEE Trans. Ultrason., Ferro. Freq. Cont, 50, 979–986, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Padilla F, Peyrin F, and Laugier P, “Prediction of backscatter coefficient in trabecular bones using a numerical model of three-dimensional microstructure,” J. Acoust. Soc. Am, 113, 1122–1129, 2003. [DOI] [PubMed] [Google Scholar]
- [76].Jenson F, Padilla F, and Laugier P, “Prediction of frequency-dependent ultrasonic backscatter in cancellous bone using statistical weak scattering model,” Ultrasound. Med. & Biol, 29, 455–464, 2003. [DOI] [PubMed] [Google Scholar]
- [77].Wear KA, “Measurement of frequency dependence of scattering from cylinders using focused transducers—with applications in trabecular bone,” J. Acoust. Soc. Am, 115, 66–72, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Periera WCA, Bridal SL, Coron A, and Laugier P, “Singular spectrum analysis applied to backscattered ultrasound signals from in vitro human cancellous bone specimens,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 51, 302–312, 2004. [PubMed] [Google Scholar]
- [79].Bossy E, Padilla F, Peyrin F, and Laugier P, “Three-dimensional simulation of ultrasound propagation through trabecular bone structures measured by synchrotron micro-tomography,” Phys. Med. Biol, 50, 5545–5556, 2005. [DOI] [PubMed] [Google Scholar]
- [80].Wear KA, “Fred Lizzi’s statistical framework and the interpretation of ultrasound backscatter from bone,” Ultrasonic Imaging, 27, 41–42, 2006. [DOI] [PubMed] [Google Scholar]
- [81].Padilla F, Jenson F, and Laugier P, “Estimation of trabecular thickness using ultrasonic backscatter,” Ultrasonic Imaging, 28, 3–22, 2006. [DOI] [PubMed] [Google Scholar]
- [82].Jenson F, Padilla F, Bousson V, Bergot C, Laredo JD, and Laugier P, “In vitro ultrasonic characterization of human cancellous femoral bone using transmission and backscatter measurements: relationships to bone mineral density,” J. Acoust. Soc. Am, 119, 654–663, 2006. [DOI] [PubMed] [Google Scholar]
- [83].Kaufman JJ, Luo G, and Siffert RS, “On the relative contributions of absorption and scattering to ultrasound attenuation in trabecular bone: a simulation study,” Proc. 2003 IEEE Ultrason. Symp, Honolulu, HI, 1519–1523, 2003. [Google Scholar]
- [84].Padilla F, Bossy E, Haϊat G, Jenson F, and Laugier P, “Numerical simulation of wave propagation in cancellous bone,” Ultrasonics, 44, e239–e243, 2006. [DOI] [PubMed] [Google Scholar]
- [85].Haϊat G, Padilla F, Barkmann R, Glüer CC, and Laugier P, “Numerical simulation of the dependence of quantitative ultrasonic parameters on trabecular bone microarchitecture and elastic constants,” Ultrasonics, 44, e289–e294, 2006. [DOI] [PubMed] [Google Scholar]
- [86].Padilla F, Jenson F, and Laugier P, “Influence of the precision of spectral backscatter measurements on the estimation of scatterers size in cancellous bone,” Ultrasonics, 44, e57–e60, 2006. [DOI] [PubMed] [Google Scholar]
- [87].Hoffmeister BK, Jones III CI, Caldwell GJ, and Kaste SC, “Ultrasonic characterization of cancellous bone using apparent integrated backscatter,” Phys. Med. Biol, 51, 2715–2727. 2006. [DOI] [PubMed] [Google Scholar]
- [88].Wear KA and Garra BS. “Assessment of bone density using broadband ultrasonic backscatter,” Proc. 22nd Int. Symp. Ultrason. Imag. and Tissue Char, Washington, DC., p. 14 (Abstract), 1997. [Google Scholar]
- [89].Giat P, Chappard C, Roux C, Laugier P, and Berger G. Preliminary clinical assessment of the backscatter coefficient in osteoporosis, Proc. 22nd Int. Symp Ultrason. Imag. and Tissue Char, Washington, DC, p. 16 (Abstract), 1997. [Google Scholar]
- [90].Laugier P, Giat C, Chappard C, Roux C, and Berger G, “Clinical assessment of the backscatter coefficient in osteoporosis,” Proc. 1997 IEEE Ultrason. Symp, pp. 1104–1105, October, 1997. [Google Scholar]
- [91].Wear KA and Garra BS. Assessment of bone density using ultrasonic backscatter. Ultrason. Med. & Biol 24, pp. 689–695, 1998. [DOI] [PubMed] [Google Scholar]
- [92].Roux C, Roberjot V, Paorcher R, Kolta S, Dougados M, and Laugier P, “Ultrasonic backscatter and transmission parameters at the os calcis in postmenopausal osteoporosis,” J. Bone & Mineral Res, 16, pp. 1353–1362, 2001. [DOI] [PubMed] [Google Scholar]
- [93].Wear KA and Armstrong DW, “Relationships among calcaneal backscatter, anttenuation, sound speed, hip bone mineral density, and age in normal adult women.” J. Acoust. Soc. Am, 110, 573–578, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sehgal CM, “Quantitative relationship between tissue composition and scattering of ultrasound,” J. Acoust. Soc. Am, 94, pp. 1944–1952, 1993. [DOI] [PubMed] [Google Scholar]
- [95].Faran JJ, “Sound scattering by solid cylinders and spheres,” J. Acoust. Soc. Am, 23, 405–418, 1951. [Google Scholar]
- [96].Insana MF and Brown DG, “Acoustic scattering theory applied to soft biological tissues,” in Ultrasonic Scattering in Biological Tissues, Shung KK and Thieme GA ed.s, CRC Press, Boca Raton, FL, 75–124., 1993. [Google Scholar]
- [97].Ueda M and Ichikawa H, “Analysis of an echo signal reflected from a weakly scattering volume by a discrete model of the medium,” J. Acoust. Soc. Am, 70, 1768–1775, 1981. [Google Scholar]
- [98].Deligianni DD and Apostolopoulos KN, “Characterization of dense bovine cancellous bone tissue microstructure by ultrasonic backscattering using weak scattering models,” J. Acoust. Soc. Am, 122 1180.-, 2007. [DOI] [PubMed] [Google Scholar]
- [99].Grenoble DE, Katz JL, Dunn KL, Gilmore RS, and Murty KL, “The elastic properties of hard tissues and apatites,” J. Biomed. Mater. Res, 6, pp. 221–233, 1972. [DOI] [PubMed] [Google Scholar]
- [100].Sehgal CM and Greenleaf JF, “Scattering of ultrasound by tissues,” Ultrason. Imag, 6, pp. 60–80, 1984. [DOI] [PubMed] [Google Scholar]
- [101].Ulrich D, van Rietbergen B, Laib A, and Ruegsegger P, “The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone,” Bone, 25, 55–60, 1999. [DOI] [PubMed] [Google Scholar]
- [102].Anderson ME, Soo SC, and Trahey GE, “Microcalcifications as elastic scatterers under ultrasound,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 45, pp. 925–934, 1998. [DOI] [PubMed] [Google Scholar]
- [103].Gong JK, Arnold JS, and Cohn SH, “Composition of trabecular and cortical bone,” Anat. Rec, 149, pp. 325–332, 1964. [DOI] [PubMed] [Google Scholar]
- [104].Lang S, “Ultrasonic method for measuring elastic coefficients of bone and results on fresh and dried bovine bones,” IEEE Trans. Biomed. Eng, BME-17, pp. 101–105, 1970. [DOI] [PubMed] [Google Scholar]
- [105].Williams JL, “Ultrasonic wave propagation in cancellous and cortical bone: predictions of some experimental results by Biot’s theory,” J. Acoust. Soc. Am, 92, pp. 1106–1112, 1992. [DOI] [PubMed] [Google Scholar]
- [106].Rho JY, Ashman RB, and Turner CH, “Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements,” J. Biomech 26, pp. 111–119, 1993. [DOI] [PubMed] [Google Scholar]
- [107].Ashman RB and Rho JY, “Elastic modulus of trabecular bone material,” J. Biomech, 21, pp. 177–181, 1988. [DOI] [PubMed] [Google Scholar]
- [108].Lizzi FL, Feleppa EJ, Astor M, and Kalisz A, “Statistics of ultrasonic spectral parameters for prostate and liver examinations,” IEEE Trans. Ultrason. Ferro. Freq. Cont, 44, 935–942, 1997. [Google Scholar]
- [109].Bossy E, Laugier P, Peyrin F, and Padilla F, “Attenuation in trabecular bone: a face-to-face comparison between numerical simulation and experimental results,” J. Acoust. Soc. Am, 122, 2469–2475, 2007. [DOI] [PubMed] [Google Scholar]
- [110].Haϊat G, Padilla F, Lonne S, Lhémery A, Laugier P, and Naili S, “Modeling of velocity dispersion in trabecular bone: effect of multiple scattering and viscous absorption,” Proc. Euro. Symp. Ultrason. Char. Bone, page 11, 2007. [Google Scholar]
- [111].Mottley JG and Miller JG, “Anisotropy of the ultrasonic attenuation in soft tissues: measurements in vitro,” J. Acoust. Soc. Am, 88, 1203–1210, 1990. Appendix. [DOI] [PubMed] [Google Scholar]
- [112].Ahuja AS and Hendee WR, “Effects of particle shape and orientation on propagation of sound in suspension,” J. Acoust. Soc. Am, 63, 1074–1080, 1978. Section II. B. [Google Scholar]
- [113].Hosokawa A, “Simulation of ultrasound propagation through bovine cancellous bone using elastic and Biot’s finite-difference time-domain methods,” J. Acoust. Soc. Am, 118, 1782–1789, 2005. [DOI] [PubMed] [Google Scholar]