Abstract
Ketamine significantly increases the locomotor activity of rodents, however this effect varies according to the sex and age of the animal being tested. To determine the role monoamine systems play in ketamine’s locomotor activating effects: (a) male and female preweanling, adolescent, and adult rats were pretreated with vehicle or the monoamine depleting agent reserpine (1 or 5 mg/kg), and (b) the behavioral actions of ketamine (20 or 40 mg/kg) were then compared to D-amphetamine (2 mg/kg) and cocaine (10 or 15 mg/kg). The ability of reserpine to deplete dorsal striatal dopamine (DA) and serotonin (5-HT) in male and female rats was determined using HPLC. Ketamine caused substantial increases in the locomotion of preweanling rats and older female rats (adolescents and adults), but had only small stimulatory effects on adolescent and adult male rats. When compared to cocaine and D-amphetamine, ketamine was especially sensitive to the locomotor-inhibiting effects of monoamine depletion. Ketamine-induced locomotion is at least partially mediated by monoamine systems, since depleting DA and 5-HT levels by 87–96% significantly attenuated the locomotor activating effects of ketamine in male and female rats from all three age groups. When administered to reserpine-pretreated rats, ketamine produced a different pattern of behavioral effects than either psychostimulant, suggesting that ketamine does not stimulate locomotor activity via actions at the presynaptic terminal. Instead, our results are consistent with the hypothesis that ketamine increases locomotor activity through a down-stream mechanism, possibly involving ascending DA and/or 5-HT projection neurons.
Keywords: Ketamine, cocaine, D-amphetamine, reserpine, ontogeny, locomotor activity
1. Introduction
Ketamine is an NMDA receptor channel blocker that is used by humans for both licit (e.g., as an anesthetic and a quick-acting treatment for major depression) [1,2] and illicit purposes [3]. In animal studies, ketamine produces anesthetic effects at high doses, while stimulating locomotor activity at lower doses [4,5]. For example, rats and mice injected with a moderate dose of ketamine often exhibit an initial period of hypoactivity, lasting for up to 30 min, and then a prolonged period of hyperactivity [6]. Importantly, ketamine’s actions vary greatly depending on both the sex and age of the animal being tested. In adolescent and adult rats, ketamine induces substantially more locomotor activity in females than in males [7–10]. During the preweanling period, ketamine also increases locomotor activity, but no sex differences are apparent [9,10]. Independent of these sex effects, the overall pattern of ketamine-induced locomotor activity differs among preweanling, adolescent, and adult rats [9,10,11–13].
Although ketamine-induced anesthesia has been linked to actions at the NMDA receptor [14], the neural mechanisms underlying ketamine’s locomotor activating effects remain uncertain. A likely possibility is that ketamine stimulates locomotor activity by directly or indirectly enhancing monoamine neurotransmission. Indeed, selective antagonism of either serotonin (5-HT) 2A receptors or dopamine (DA) D1 or D2 receptors significantly reduces the hyperactivity produced by both ketamine [6,15–18] and the noncompetitive NMDA receptor antagonist MK-801 [19–24]. Consistent with these results, ketamine has also been reported to dose-dependently increase DA release and block DA reuptake in several brain regions, including the dorsal striatum [4,25], nucleus accumbens [26,27], and prefrontal cortex [28,29]. Similarly, ketamine increases 5-HT release and blocks 5-HT reuptake in the prefrontal cortex and dorsal striatum [25,30–32]. The mechanisms by which ketamine alters monoamine concentrations are in dispute [33]. For example, ketamine may act as an indirect agonist (i.e., like amphetamine or cocaine) at the presynaptic terminal [6,26,34], or ketamine may indirectly enhance monoamine neurotransmission by altering the firing rate of afferent projection neurons [27,32,35,36].
The purpose of the present study was two-fold: first, to determine whether monoamine neurotransmission plays a critical role in the ketamine-induced locomotor activity of male and female preweanling, adolescent, and adult rats; and, second, to determine whether ketamine produces the same pattern of locomotor effects as prototypical psychostimulant compounds. To accomplish these goals, we compared the locomotor activating effects of ketamine with the actions of cocaine and D-amphetamine in rats pretreated with vehicle or reserpine (a monoamine depleting agent). D-Amphetamine was used because this compound has an approximately 100-fold greater affinity for DA transporters than 5-HT transporters, while cocaine has a high affinity for both transporter types [37]. Reserpine was chosen because it significantly reduces DA [38–40], 5-HT [39,41–43], and norepinephrine (NE) [44,45] content in brain by depleting vesicular stores [46,47], while differentially affecting cocaine- and amphetamine-induced locomotor activity [44,48–51]. Although seldom used in combination with ketamine, Uchihashi et al. [15] previously reported that 1 mg/kg reserpine did not attenuate the ketamine-induced locomotor activity of adult male mice. The present study extends those findings by: (a) testing multiple doses of reserpine (1 and 5 mg/kg), (b) assessing both male and female rats, (c) examining these drug effects during three ontogenetic periods (i.e., the preweanling period, adolescence, and adulthood), and (d) comparing the actions of ketamine to cocaine (10 and 15 mg/kg) and D-amphetamine (2 mg/kg). These doses of cocaine and D-amphetamine were chosen because they produce substantial locomotor activity in all age groups tested [52–55]. In the companion paper to this study [56], the locomotor activating effects of ketamine, cocaine, and D-amphetamine were assessed in male and female rats pretreated with selective DA and 5-HT synthesis inhibitors.
2. Materials and Methods
2.1. Subjects
Subjects were preweanling (male, N = 122; female, N = 122), adolescent (male, N = 138; female, N = 138), and adult (male, N = 53; female, N = 58) Sprague-Dawley rats that were PD 21, PD 41, and PD 81 on the day of behavioral testing. Adult rats were purchased from Charles River (Hollister, CA), whereas preweanling and adolescent rats were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3. Preweanling rats were kept with the dam and littermates, whereas adolescent rats were weaned at PD 21 and group housed with same-sex littermates. All rats were housed in large polycarbonate maternity cages (30.5 × 43 × 19 cm) on ventilated racks. Food and water were freely available. The colony room was maintained at 22–23 °C and kept under a 12:12 light-dark cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” [57] under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
2.2. Apparatus
Behavioral testing was done in activity monitoring chambers that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Whitehall, PA). In order to equate for differences in body size [58,59], preweanling rats were tested in smaller chambers (26 × 26 × 41 cm) than adolescent and adult rats (41 × 41 × 41 cm). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that had a photobeam resolution of either 0.76 cm (small chambers) or 1.27 cm (large chambers). The position of each rat was determined every 100 ms, thus allowing for a precise measure of how much distance (cm) the rat traveled (a measure of locomotor activity).
2.3. Drugs
(±)-Ketamine hydrochloride, (−)-cocaine hydrochloride, and D-amphetamine hemisulfate salt were dissolved in saline, whereas reserpine was dissolved in a minimal amount of glacial acetic acid and diluted with saline. Drugs were injected intraperitoneally (ip) at a volume of 2.5 ml/kg (preweanling rats) or 1 ml/kg (adolescent and adult rats). Ketamine was purchased from Spectrum Chemicals (New Brunswick, NJ), while all other compounds were purchased from Sigma-Aldrich (St. Louis, MO).
2.4. Experiment 1a: Effects of low-dose reserpine treatment on the ketamine-induced locomotor activity of male and female preweanling rats
On PD 20, rats were injected with saline and habituated to activity chambers for 30 min. On PD 21, rats were pretreated with vehicle or 1 mg/kg reserpine and returned to their home cages. After 4 h, rats (n = 8 per group) were injected with saline or ketamine (5, 10, 20, or 40 mg/kg, ip) and immediately placed in activity chambers where distance traveled was measured for 120 min. This injection protocol (i.e., administering 1 mg/kg reserpine 4 h before ketamine treatment) was similar to the procedure described by Uchihashi and colleagues [15].
2.5. Experiment 1b: Effects of low-dose reserpine treatment on the D-amphetamine- and cocaine-induced locomotor activity of male and female preweanling rats
Male and female preweanling rats were habituated to the testing chamber and injected with reserpine in the same manner as described in Experiment 1a. On PD 21, rats (n = 8 per group) were injected (ip) with saline, 2 mg/kg D-amphetamine, or 10 mg/kg cocaine and immediately placed in activity chambers where distance traveled was measured for 120 min.
2.6. Experiment 1c: Effects of high-dose reserpine treatment on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female preweanling rats
On PD 20, rats were injected with saline and habituated to activity chambers for 30 min. Immediately after habituation, rats were injected with vehicle or 5 mg/kg reserpine and returned to their home cages. After 24 h, rats (n = 8 per group) were injected with saline, ketamine (20 or 40 mg/kg, ip), 2 mg/kg D-amphetamine, or 15 mg/kg cocaine (a higher dose of cocaine was used in this experiment in order to increase basal rates of locomotion). Rats were then placed in activity chambers where distance traveled was measured for 120 min. This injection protocol (i.e., administering 5 mg/kg reserpine 24 h before ketamine treatment) has been frequently employed [38, 39, 60], and was used to avoid the acute locomotor-debilitating effects of high-dose reserpine treatment.
2.7. Experiment 2: Effects of low- and high-dose reserpine treatment on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
On PD 40, male and female adolescent rats were habituated to the testing chamber. Vehicle and reserpine (1 or 5 mg/kg) were injected in the same manner as described for Experiments 1a and 1c. On PD 41, rats (n = 8 per group) were injected with saline, ketamine (20 or 40 mg/kg, ip), D-amphetamine (2 mg/kg, ip), or cocaine (15 mg/kg, ip) and immediately placed in activity chambers where distance traveled was measured for 120 min.
2.8. Experiment 3: Effects of low- and high-dose reserpine treatment on the ketamine-induced locomotor activity of male and female adult rats
Male and female adult rats were injected with saline and habituated to the testing chamber for 30 min on PD 80. On PD 81 (24 h after administering 5 mg/kg reserpine and 4 h after administering 1 mg/kg reserpine), male (n = 5–6 per group) and female (n = 6–7 per group) rats were injected with saline or 40 mg/kg ketamine and immediately placed in activity chambers where distance traveled was measured for 120 min.
2.9. Experiment 4: Effects of low- and high-dose reserpine treatment on monoamine content in the dorsal striatum of male and female preweanling, adolescent, and adult rats
Male and female rats were pretreated with vehicle or reserpine (1 or 5 mg/kg) as described above. On PD 21, PD 41, and PD 81, male and female preweanling, adolescent, and adult rats (n = 6 male and female rats per group at each age) were decapitated and dorsal striatal sections were dissected bilaterally on an ice-cold dissection plate and stored at −80 °C.
To measure DA and 5-HT content, frozen striatal sections were sonicated in 10 volumes of 0.1 N HClO4 and centrifuged at 20, 000 × g for 20 min at 4 °C. The supernatant was then filtered through a 0.22 μm centrifugation unit at 2, 000 × g for 5 min at 4 °C. Twenty microliters of the resulting extract was assayed for DA and 5-HT using high performance liquid chromatography (HPLC) with electrochemical detection [Hypersil ODS column (150 × 3 mm) and Coulochem III detector; Thermo Fisher Scientific, Waltham, MA]. The mobile phase consisted of 75 mM NaH2PO4, 1.4 mM 1-octane sulfonic acid, 10 mM EDTA, and 10% acetonitrile (pH 3.1) and was pumped at a rate of 0.5 ml/min.
2.10. Data analysis
Multifactor analyses of variance (ANOVAs) were used to statistically analyze distance traveled data as well as DA and 5-HT content (analyzed and presented as percent of same age vehicle controls). When ANOVAs included a repeated measures factor (i.e., the behavioral experiments), the Huynh-Feldt epsilon statistic was used to adjust degrees of freedom if the assumption of sphericity was violated [61]. Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a” in the parenthetical statistical reports. When further analyzing statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for Tukey calculations were based on separate one- or two-way ANOVAs at each time block. To minimize litter effects, no more than one subject per litter was assigned to a given group [62].
3. Results
3.1. Experiments 1–3: Habituation
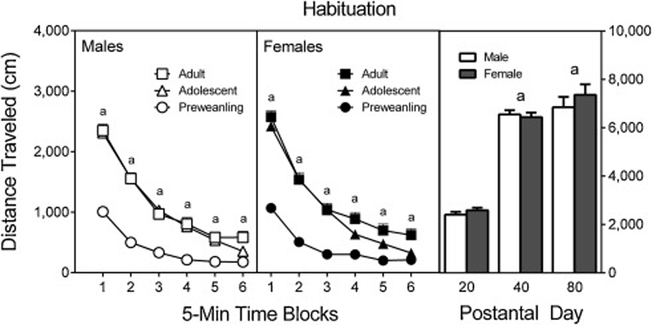
During the habituation phase (Figure 1), adolescent and adult rats exhibited more locomotor activity than preweanling rats on time blocks 1–5 and time blocks 1–6, respectively [Age main effect, F (2, 556) = 324.97, p < 0.001; aAge × Time Block interaction, F (8, 2179) = 101.40, p < 0.001; and Tukey tests, p < 0.05]. The main effect and interactions involving the sex variable were not statistically significant.
Figure 1.
Mean distance traveled scores (±SEM) of male and female preweanling, adolescent, and adult rats during the habituation phase. The right panel represents total distance traveled collapsed across the testing session. a = Significantly different from preweanling (PD 20) rats.
3.2. Effects of low-dose reserpine treatment on the ketamine-induced locomotor activity of male and female preweanling rats
Overall, ketamine (10–40 mg/kg) significantly increased the locomotor activity of male and female preweanling rats [Treatment main effect, F (4, 70) = 62.70, p < 0.001; and Tukey tests, p < 0.05], while 1 mg/kg reserpine suppressed locomotion [Pretreatment main effect, F (1, 70) =27.07, p < 0.001] (Figure 2, right panels). The latter effect was most apparent after treatment with the greater dose of ketamine, as reserpine (1 mg/kg) significantly reduced the distance traveled of rats given 40 mg/kg, but not 20 mg/kg, ketamine [Pretreatment × Treatment interaction, F (4, 70) = 18.39, p < 0.001; and Tukey tests, p < 0.05]. Tukey tests (p > 0.05) suggested that reserpine did not reduce basal locomotor activity; however, a separate ANOVA, including only saline-treated rats, indicated that 1 mg/kg reserpine reduced distance traveled scores relative to vehicle controls [Pretreatment main effect, F (1, 14) = 16.16, p < 0.001].
Figure 2.
Mean distance traveled scores (±SEM) of male and female preweanling rats pretreated with vehicle (upper graph) or 1 mg/kg reserpine (lower graph) and then tested after saline or ketamine (5–40 mg/kg) treatment on PD 21. The right panels represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same dose of ketamine.
Analysis of the Pretreatment × Treatment × Time Block interaction [aF (19, 334) = 7.06, p < 0.001] indicated that reserpine (1 mg/kg) altered the pattern of ketamine-induced locomotion. When compared to saline controls, 40 mg/kg ketamine significantly increased the distance traveled scores of vehicle-pretreated rats on time blocks 3–12 (Figure 2, upper graph); whereas, reserpinized rats treated with 40 mg/kg ketamine only showed elevated distance traveled scores on time blocks 8–12 (Figure 2, lower graph) [Tukey tests, p < 0.05]. Similarly, vehicle-pretreated rats injected with 20 mg/kg ketamine had greater distance traveled scores than their saline controls on time blocks 1–3, while reserpinized rats given 20 mg/kg ketamine did not show elevated distance traveled scores until later in the testing session (i.e., time blocks 3–6) [Tukey tests, p < 0.05]. Distance traveled scores of the preweanling rats did not vary according to sex (for presentation purposes, data were collapsed across the sex variable).
3.3. Experiment 1b: Effects of low-dose reserpine treatment on the D-amphetamine- and cocaine-induced locomotor activity of male and female preweanling rats
Overall, D-amphetamine (2 mg/kg), and to a lesser extent cocaine (10 mg/kg), significantly increased the locomotor activity of male and female preweanling rats (Figure 3, right panels) [Treatment main effect, F (2, 42) = 135.15, p < 0.001; and Tukey tests, p < 0.05]. Relative to vehicle-pretreated saline controls (Figure 3, upper left panel), D-amphetamine increased distance traveled on time blocks 2–12, while cocaine increased distance traveled on time blocks 1, 2, 11, and 12 [aPretreatment × Treatment × Time Block interaction, F (9, 191) = 9.92, p < 0.001; and Tukey tests, p < 0.05]. Reserpine (1 mg/kg) modestly reduced the locomotor activating effects of D-amphetamine [Pretreatment × Treatment interaction, F (2, 42) = 5.60, p < 0.01; and Tukey tests, p < 0.05], as rats in the Vehicle-Amphetamine group had greater distance traveled scores than rats in the Reserpine-Amphetamine group on time blocks 5–12 (Figure 3, left panels) [aPretreatment × Treatment × Time Block interaction; and Tukey tests, p < 0.05]. Reserpine also attenuated the locomotor activating effects of 10 mg/kg cocaine, because the Vehicle-Cocaine group had greater distance traveled scores than the Reserpine-Cocaine group on 8 out of the 12 time blocks [Tukey tests, p < 0.05]. Like in Experiment 1a, a separate statistical analysis showed that 1 mg/kg reserpine reduced the basal locomotor activity of saline-treated rats [Pretreatment main effect, F (1, 14) = 19.80, p < 0.001]. Distance traveled scores did not differ according to sex.
Figure 3.
Mean distance traveled scores (±SEM) of male and female preweanling rats pretreated with vehicle (upper graph) or 1 mg/kg reserpine (lower graph) and then tested after saline, cocaine (10 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 21. The right panels represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same agonist.
3.4. Experiment 1c: Effects of high-dose reserpine treatment on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female preweanling rats
Among the vehicle-pretreated groups, ketamine (20 and 40 mg/kg), cocaine (15 mg/kg), and D-amphetamine (2 mg/kg) significantly increased the distance traveled scores of male and female preweanling rats (Figure 4, upper right panel) [Pretreatment × Treatment interaction, F (1, 70) = 20.03, p < 0.001; and Tukey tests, p < 0.05]. Significant differences between these treatment groups and their saline controls were apparent at various points across the testing session (20 mg/kg ketamine, time blocks 1–5; 40 mg/kg ketamine, time blocks 3–10; 15 mg/kg cocaine, time blocks 1–9; 2 mg/kg D-amphetamine, time blocks 2–12) (Figure 4, upper panels) [aPretreatment × Treatment × Time Block interaction, F (19, 340) = 6.48, p < 0.001; and Tukey tests, p < 0.05]. High-dose reserpine (5 mg/kg) treatment fully attenuated ketamine-induced locomotion, partially attenuated cocaine-induced locomotion, and left D-amphetamine-induced locomotor activity unaffected (Figure 4, right panels) [Pretreatment × Treatment interaction; and Tukey tests, p < 0.05]. Among reserpinized rats, ketamine did not increase distance traveled scores on any of the time blocks (Figure 4, left bottom panel); whereas, reserpinized rats given amphetamine had elevated distance traveled scores, relative to their saline controls, on time blocks 1–12 (Figure 4, middle bottom panel) [aPretreatment × Treatment × Time Block interaction; and Tukey tests, p < 0.05]. Cocaine also increased locomotor activity after high-dose reserpine pretreatment, as rats given 5 mg/kg reserpine and 15 mg/kg cocaine had greater distance traveled scores on time blocks 2–7 than rats injected with reserpine and saline [Tukey tests, p < 0.05]. Even so, reserpine did depress cocaine-induced locomotion, since vehicle-pretreated rats given cocaine exhibited elevated distance traveled scores on time blocks 1, 2, and 8 when compared to rats pretreated with reserpine and tested with cocaine [Tukey tests, p < 0.05]. Once again, distance traveled scores did not differ according to sex.
Figure 4.
Mean distance traveled scores (±SEM) of male and female preweanling rats pretreated with vehicle (upper graph) or 5 mg/kg reserpine (lower graph) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 21. The right panels represent total distance traveled collapsed across the testing session, a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same agonist.
3.5. Experiment 2: Effects of low- and high-dose reserpine treatment on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
3.5.1. Male adolescent rats
Overall, cocaine and D-amphetamine increased the distance traveled of male adolescent rats (Figure 5, right panels) [Treatment main effect, F (4, 105) = 53.73, p < 0.001; and Tukey tests, p < 0.05]. Although the omnibus ANOVA suggested that ketamine (20 or 40 mg/kg) was without behavioral effect, a separate ANOVA comparing only the saline- and ketamine-treated rats showed that the high dose of ketamine (40 mg/kg) significantly increased the distance traveled of male adolescent rats [Treatment main effect, F (2, 69) = 15.89, p < 0.001; and Tukey tests, p < 0.05]. A low dose of reserpine (1 mg/kg) caused a small, but significant, reduction in D-amphetamine-induced locomotion, whereas 5 mg/kg reserpine decreased the distance traveled scores of saline-and ketamine-treated rats [Pretreatment × Treatment interaction, F (8, 105) = 3.40, p <0.01; and Tukey tests, p < 0.05]. The cocaine-induced locomotor activity of male adolescents was not affected by reserpine (1 or 5 mg/kg).
Figure 5.
Mean distance traveled scores (±SEM) of male adolescent rats pretreated with vehicle (upper graphs), 1 mg/kg (middle graphs), or 5 mg/kg reserpine (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right panels represent total distance traveled collapsed across the testing session, a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from vehicle-pretreated rats tested with the same drug.
A finer grain analysis involving time blocks showed that 40 mg/kg ketamine (time block 2), 15 mg/kg cocaine (time blocks 2–6 and 8), and 2 mg/kg D-amphetamine (time blocks 3–12) increased the distance traveled scores of vehicle-pretreated male adolescent rats (Figure 5, upper panels) [aPretreatment × Treatment × Time Block interaction, F (27, 366) = 5.10, p < 0.001; and Tukey tests, p < 0.05]. A low dose of reserpine (1 mg/kg) had minimal actions, as it only reduced the distance traveled scores of D-amphetamine-treated male rats on time blocks 4, 5, and 8–12 (Fig. 4, middle panels) [Tukey tests, p < 0.05]. A high dose of reserpine (5 mg/kg) decreased ketamine-induced locomotion on time blocks 1–3, D-amphetamine-induced locomotion on time blocks 8–12, and cocaine-induced locomotion on time blocks 5 and 6 (Figure 5, lower panels) [Tukey tests, p < 0.05]. Oddly, reserpine (5 mg/kg) potentiated the distance traveled scores of D-amphetamine-treated rats at the start of the testing session (time blocks 2 and 3), which is a phenomenon reported before [48,63].
3.5.2. Female adolescent rats
Ketamine (40 mg/kg), D-amphetamine, and cocaine significantly increased the distance traveled scores of vehicle-pretreated female adolescent rats (Figure 6, upper right panel) [Pretreatment × Treatment interaction, F (8, 105) = 3.68, p < 0.001; and Tukey tests, p < 0.05]. A low dose of reserpine (1 mg/kg) marginally depressed the distance traveled of rats given 40 mg/kg ketamine and 2 mg/kg D-amphetamine [Tukey tests, p < 0.05]. In contrast, 5 mg/kg reserpine caused a substantial reduction in the distance traveled scores of female adolescent rats given ketamine (20 and 40 mg/kg) [Tukey tests, p < 0.05], while not affecting D-amphetamine- or cocaine-induced locomotion. A separate statistical analysis indicated that neither dose of reserpine affected the basal locomotor activity of female adolescent rats.
Figure 6.
Mean distance traveled scores (±SEM) of female adolescent rats pretreated with vehicle (upper graphs), 1 mg/kg (middle graphs), or 5 mg/kg reserpine (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right panels represent total distance traveled collapsed across the testing session, a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from vehicle-pretreated rats tested with the same drug, c = Significantly different from vehicle-pretreated male rats given 40 mg/kg ketamine.
Among vehicle-pretreated rats (Figure 6, upper panels), 2 mg/kg D-amphetamine (time blocks 2–12), 15 mg/kg cocaine (time blocks 2–7), 20 mg/kg ketamine (time blocks 1 and 2), and 40 mg/kg ketamine (time blocks 3–10) significantly increased distance traveled scores [aPretreatment × Treatment × Time Block interaction, F (32, 414) = 3.90, p < 0.001; and Tukey tests, p < 0.05]. The locomotion induced by 40 mg/kg ketamine was significantly reduced by both 1 mg/kg (time blocks 6–11) and 5 mg/kg reserpine (time blocks 3–11) [Tukey tests, p < 0.05]. Reserpine did not alter cocaine-induced locomotor activity, while only reducing D-amphetamine-induced distance traveled scores on a few time blocks (1 mg/kg reserpine, time blocks 7 and 8; 5 mg/kg reserpine, time blocks 11 and 12) [Tukey tests, p < 0.05].
3.5.3. Cross-sex comparisons
Overall, female adolescent rats exhibited more locomotion than male rats (Figure 7) [Sex main effect, F (1, 210) = 34.78, p < 0.001]. Although most interactions involving the sex variable were nonsignificant, sex did interact with the treatment variable to affect locomotion. Specifically, female adolescent rats injected with 40 mg/kg ketamine, 2 mg/kg amphetamine, or 15 mg/kg cocaine had greater distance traveled scores than male adolescent rats given the same drug treatments [Sex × Treatment interaction, F (4, 210) = 2.49, p < 0.05; and Tukey tests, p < 0.05]. Interestingly, the cocaine-induced sex differences were only apparent in reserpine-pretreated rats (Figure 7, upper graph).
Figure 7.
Total distance traveled (mean, ±SEM) of male and female adolescent rats pretreated with vehicle (upper graph), 1 mg/kg (middle graph), or 5 mg/kg reserpine (lower graph) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. a = Significantly different from male rats given the same drug treatment.
3.6. Experiment 3: Effects of low- and high-dose reserpine treatment on the ketamine-induced locomotor activity of male and female adult rats
Ketamine increased distance traveled scores of male and female adult rats (Figure 8) [Treatment main effect, F (1, 63) = 57.84, p < 0.001; and Tukey tests, p < 0.05]. A low dose of reserpine (1 mg/kg) did not modify ketamine’s actions; however, 5 mg/kg reserpine significantly reduced ketamine-induced locomotion in adult rats [Pretreatment × Treatment interaction, F (2, 63) = 6.98, p < 0.001; and Tukey tests, p < 0.05]. Performance of adult rats varied according to sex [Sex main effect, F (1, 63) = 20.46, p < 0.001], as 40 mg/kg ketamine caused significantly larger distance traveled scores in females than males [Treatment × Sex interaction, F (1, 63) =11.16, p < 0.01; and Tukey tests, p < 0.05]. A separate ANOVA assessing only saline-treated rats indicated that 5 mg/kg reserpine, but not 1 mg/kg reserpine, reduced the basal locomotor activity of adult rats [Pretreatment main effect, F (2, 32) = 16.23, p < 0.001].
Figure 8.
Mean distance traveled scores (±SEM) of male and female adult rats pretreated with vehicle (upper graphs), 1 mg/kg (middle graphs), or 5 mg/kg reserpine (lower graphs) and then tested after saline or ketamine (40 mg/kg) treatment on PD 81. The right panels represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated same-sex rats from the same pretreatment condition; b = Significantly different from same-sex rats pretreated with 5 mg/kg reserpine; c = Significantly different from ketamine-treated male rats from the same pretreatment condition.
Further analysis of the significant Pretreatment × Treatment × Sex Pretreatment × Time Block interaction [aF (10, 316) = 1.98, p < 0.05; and Tukey tests, p < 0.05] showed that ketamine stimulated greater distance traveled scores in vehicle-pretreated females rats than vehicle-pretreated male rats on time blocks 4–12 (Figure 8, upper left panel). Although 1 mg/kg reserpine did not significantly affect performance, 5 mg/kg reserpine decreased the distance traveled scores of ketamine-treated adult female rats on time blocks 5–8, 10 and 11 (Figure 8, compare upper and lower left panels) [Tukey tests, p < 0.05]. High-dose treatment with reserpine also decreased the ketamine-induced locomotion of adult male rats on time blocks 1 and 4–6 [Tukey tests, p < 0.05].
3.7. Experiment 4: Effects of low- and high-dose reserpine treatment on monoamine content in the dorsal striatum of male and female preweanling, adolescent, and adult rats
3.7.1. DA content
Overall, reserpine reduced DA content in the dorsal striatum [Pretreatment main effect, F (2, 99) = 661.00, p < 0.001; and Tukey tests, p < 0.05], with this effect being more pronounced in the youngest age group [Age main effect, F (2, 99) = 57.14, p < 0.001; and Tukey tests, p < 0.05] (Figure 9, upper graph). In preweanling rats, both doses of reserpine caused near maximal DA depletion [Pretreatment × Age interaction, F(4, 99)= 68.34, p < 0.001; and Tukey tests, p < 0.05]. In contrast, 1 mg/kg reserpine did not significantly lower the DA levels of adolescent and adult rats (13.6% and 14.8% reductions, respectively); however, 5 mg/kg reserpine caused significant reductions in the dorsal striatal DA levels of adolescent and adult rats (89.1% and 96.0% reductions, respectively) [Tukey tests, p < 0.05]. The magnitude of reserpine-induced DA depletion did not differ according to sex at any age.
Figure 9.
Mean dorsal striatal DA and 5-HT content (±SEM) of male and female preweanling (PD 21), adolescent (PD 41), and adult (PD 81) rats pretreated with reserpine (1 or 5 mg/kg) or vehicle (represented by the dashed line). Data are expressed as percent of same age vehicle controls. Males and females did not differ, so data are collapsed over the sex variable, a = Significantly different from same-age vehicle-pretreated rats; b = Significantly different from adolescent and adult rats pretreated with 1 mg/kg reserpine.
3.7.2. 5-HT content
Reserpine affected 5-HT levels in an almost identical manner to DA (Figure 9, bottom graph). Specifically, 1 mg/kg reserpine only caused a significant reduction in the dorsal striatal 5-HT content of preweanling rats, but not adolescent or adult rats [Pretreatment × Age interaction, F (4, 99)= 15.50, p < 0.001; and Tukey tests, p) < 0.05]. Conversely, 5 mg/kg reserpine reduced the 5-HT levels of preweanling, adolescent, and adult rats by 87.4% to 89.6% [Tukey tests, p < 0.05]. Dorsal striatal 5-HT levels did not differ according to sex.
4. Discussion
In the present study, ketamine-induced locomotor activity varied greatly depending on the age and sex of the rats being tested. More specifically, robust dose-dependent increases in locomotor activity were evident when adolescent and adult female rats, as well as prepubertal preweanling rats, were injected with ketamine. In contrast, ketamine produced only a minor augmentation in the locomotor activity of adolescent and adult male rats. Sex-dependent differences in ketamine pharmacokinetics are primarily responsible for these behavioral effects, because both peak ketamine levels and drug availability in brain (area under the curve) are substantially greater in postpubertal female rats than male rats [10,64]. Other non-competitive NMD A receptor antagonists share similar properties, as adult female rats metabolize phencyclidine (PCP) at a slower rate than adult male rats and, consequently, exhibit more locomotor activity [65–67]. Estrogens are responsible for this effect, because estrogens reduce cytochrome P450 enzymes in the liver resulting in elevated concentrations of PCP [65,66]. Although similar studies have not been undertaken using ketamine, it has been established that low-dose ketamine treatment has more pronounced hedonic and antidepressant effects in female rats than male rats, and that these actions are mediated by estrogens [68, 69].
Cocaine (10 and 15 mg/kg) and D-amphetamine (2 mg/kg) also increased the locomotor activity of preweanling and adolescent rats, but sex-dependent behavioral effects were only evident during adolescence. Specifically, both D-amphetamine and cocaine caused significantly more locomotor activity in adolescent females than males; however, sex differences involving cocaine were only apparent in rats pretreated with reserpine. This pattern of results was not entirely expected, since cocaine typically causes a more pronounced locomotor response in normosensitive female rats than male rats [54,70,71]. A possible explanation for the latter result is that hormonal cycling was not accounted for in the present study, and it is known that adult females exhibit enhanced sensitivity to cocaine and D-amphetamine during proestrous and estrous (i.e., periods of high estrogen levels) [72–74], Therefore, leaving the estrous cycle uncontrolled could have masked a cocaine-induced sex effect. Unlike with PCP, estrogen probably does not cause hyperresponsiveness in postpubertal female rats by altering the pharmacokinetics of D-amphetamine and cocaine [71,75,76]. Instead, an estrogen-induced increase in DA release, possibly via actions affecting the D2 autoreceptor, may be responsible for the enhanced locomotor response exhibited by psychostimulant-treated females [71,77]. Not surprisingly, psychostimulants do not usually produce sex-dependent differences in the locomotor activity of prepubertal male and female rats [54,78,79]. Consistent with these past findings, the cocaine- and D-amphetamine-induced locomotor activity of preweanling rats did not differ according to sex.
4.1. Effects of reserpine on ketamine-induced locomotor activity and dorsal striatal monoamine levels
Pretreating rats with a monoamine depleting agent differentially affected ketamine-induced locomotor activity, with behavior varying according to the dose of reserpine administered, and the age and sex of the rats being tested. For example, 1 mg/kg reserpine caused a moderate decline in the locomotor activity of both adolescent female rats and preweanling rats treated with 40 mg/kg ketamine. In the case of preweanling rats, 1 mg/kg reserpine appeared to “delay” the onset of locomotion produced by 40 mg/kg ketamine (Figure 2, bottom left panel). In contrast, the ketamine-induced locomotion of male adolescent rats, as well as female and male adult rats, was unaffected by 1 mg/kg reserpine. It is noteworthy that the latter result is consistent with the findings of Uchihashi and colleagues [15], who reported that 1 mg/kg reserpine did not reduce the “ambling” of ketamine-treated adult male mice. In contrast, 5 mg/kg reserpine, a dose not tested by Uchihashi et al. [15], caused major reductions (56–93%) in the locomotor activity of rats treated with 20 or 40 mg/kg ketamine. This high-dose reserpine effect was evident in both male and female rats and at all ages tested (i.e., PD 21, PD 41, and PD 81). It is important to note that 5 mg/kg reserpine had actions of its own, as this dose of reserpine reduced the basal locomotor activity of adolescent and adult male rats as well as adult female rats. Nonetheless, reserpine did not simply prevent rats from engaging in motor movement, because 5 mg/kg reserpine had minimal effects on D-amphetamine-induced locomotion. When these results are considered together, it is clear that monoamine depletion attenuates the ketamine-induced locomotor activity of rats, and this effect is evident in both sexes and across much of ontogeny (i.e., during the preweanling period, adolescence, and adulthood).
Reserpine’s dose and age-dependent behavioral effects appear to be a function of the amount of DA and/or 5-HT depletion. Specifically, 1 mg/kg reserpine caused both a substantial reduction in the dorsal striatal DA and 5-HT content of preweanling rats [80] and a significant decline in ketamine-induced locomotion. Even so, DA/5-HT depletion does not readily explain the “delayed” onset of locomotion in preweanling rats treated with 40 mg/kg ketamine, especially since this effect was not evident after treatment with lower doses of ketamine or when 5 mg/kg reserpine was administered. In contrast to preweanling rats, 1 mg/kg reserpine produced only a modest reduction in the DA content (13.6% and 14.3%) and 5-HT content (8.2% and 13.6%) of adolescent and adult rats [40,81], while ketamine-induced locomotor activity was left largely unaffected. The higher dose of reserpine (5 mg/kg) produced large reductions in the dorsal striatal DA content (89.0% to 96.0% declines) and 5-HT content (87.4% to 89.6% declines) of all age groups [38,39,43] and significantly decreased ketamine-induced locomotor activity. In addition to showing that preweanling rats are more sensitive to the monoamine depleting effects of reserpine than older animals, these results indicate that modest reductions in DA and/or 5-HT content (~15%) are insufficient to fully attenuate ketamine-induced locomotor activity. Conversely, near maximal reductions of monoamine levels (~85–95%) are sufficient to disrupt the ketamine-induced locomotor activity of preweanling, adolescent, and adult rats.
4.2. Effects of reserpine on cocaine- and D-amphetamine-induced locomotor activity
In terms of psychostimulant compounds, monoamine depletion differentially affected the behavioral effects of cocaine and D-amphetamine, with age-dependent differences being prominent. Most noticeably, reserpine (1 and 5 mg/kg) did not affect the cocaine-induced locomotor activity of male and female adolescent rats, while both doses of reserpine reduced the locomotion of cocaine-treated preweanling rats. Adult rats respond like preweanling rats, as reserpine (1–5 mg/kg) was reported to decrease the locomotor activity of cocaine-treated adult rats and mice [45,48,49]. This pattern of results suggests that the reserpine-induced attenuation of cocaine’s behavioral effects is blunted during adolescence relative to either earlier or later developmental periods.
In contrast to these cocaine effects, the D-amphetamine-induced locomotor activity of preweanling and adolescent rats was not affected by pretreatment with 5 mg/kg reserpine, although administering 1 mg/kg reserpine 4 h before testing, did cause small, but significant, reductions in D-amphetamine locomotion. Even though results are not completely uniform across studies, it is generally reported that reserpine does not attenuate amphetamine locomotion in adult rats and mice [48–51,63; but see 45]. The disparate actions of D-amphetamine and cocaine are a consequence of reserpine’s mechanism of action, as reserpine depletes monoamines located in vesicular storage pools [47]. Therefore, D-amphetamine, which primarily releases newly synthesized DA from cytosolic pools [82], is less sensitive to the effects of reserpine than cocaine, which relies on vesicular stores [45,46]. Regardless of mechanism, past and present results combine to show that reserpine has minimal effects on the D-amphetamine-induced locomotor activity of male and female rats across ontogeny.
Somewhat surprisingly, 1 mg/kg reserpine seemed to cause greater reductions in the psychostimulant-induced locomotor activity of preweanling rats than did the higher dose of reserpine (5 mg/kg). Although of lesser magnitude, the same pattern of psychopharmacological effects was evident in adolescent rats. Low and high doses of reserpine produced similar amounts of monoamine depletion in preweanling rats, so this reserpine dose effect is probably due to the acute motoric debilitating actions of the drug (i.e., in an attempt to replicate Uchihashi et al. [15], the lower dose of reserpine was administered 4 h prior to behavioral testing rather than 24 h). This explanation should be tempered by the observation that a similar reserpine dose effect was not apparent when ketamine was tested instead of a psychostimulant.
4.3. Comparing the locomotor activating effects of ketamine to cocaine and D-amphetamine in reserpinized rats: possible neural mechanisms
A main goal of the present study was to compare the behavioral effects of ketamine with those of cocaine and D-amphetamine in reserpinized rats. What is most clear is that at all ages tested, and especially in female rats, monoamine depletion substantially reduced ketamine-induced locomotor activity. It is also clear that ketamine is more sensitive to reserpine’s actions than cocaine or D-amphetamine, and that different behavior patterns are evident after reserpine-drug co-administration (i.e., reserpine-ketamine vs. reserpine-amphetamine or reserpine-cocaine). The dissociation between the actions of ketamine and the two psychostimulants suggests that ketamine is not affecting locomotor activity by acting at presynaptic monoamine terminals. Hanania and Zahniser [83], comparing the behavioral effects of various DA transport inhibitors and noncompetitive NMDA receptor antagonists, came to a similar conclusion. Indeed, Can et al. [33] recently reported that ketamine has minimal affinity for the DA transporter and does not affect the magnitude of evoked DA release.
Even so, there is substantial evidence that ketamine stimulates locomotion through a monoaminergic mechanism. For example, D1 and D2 receptor antagonists as well as 6-OHDA-induced lesions of the nigrostriatal tract are reported to attenuate the ketamine-induced locomotor activity of adult male rodents [6,15,17,18]. Consistent with these findings, DA receptor antagonists and catecholamine-depleting agents reduce the locomotor activity produced by the NMDA receptor antagonists MK-801 and PCP [16,19–21,23,84,85]. Despite this wealth of studies, there is compelling evidence that the locomotor activating effects of MK-801 and PCP are at least partially independent of dopaminergic mediation [86–88]. For example, there is a clear discord between MK-801- and PCP-induced hyperactivity and ventral striatal DA efflux [86,88]. Therefore, a likely possibility is that two or more neurotransmitter systems interact complexly or independently to mediate the locomotor activating effects of NMDA receptor antagonists.
GABA is a possible candidate system, because there is evidence that NMDA receptor blockade causes disinhibition of GABAergic inputs to excitatory fibers projecting to cortical and subcortical motor areas [89–91]. Even so, GABAergic projections from the nucleus accumbens to the ventral pallidum are not involved in this effect, because ibotenic acid lesions and muscimol injections in pallidal regions block amphetamine-, but not MK-801-induced locomotor activity [87]. 5-HT is another candidate system, because ketamine increases 5-HT release in both the dorsal striatum and prefrontal cortex [25,30–32], Moreover, 5-HT2A receptor antagonists decrease the MK-801-induced hyperactivity of adult mice [22–24], NE is a less likely candidate, because various α- and β-adrenergic antagonists do not block ketamine- and MK-801-induced locomotor activity [6,19].
The various mechanisms by which NMDA receptor antagonists modulate monoamine system functioning are not fully understood, but it is well established that ketamine increases the firing rate and burst firing of DA projection neurons in the VTA [27,35,92], These findings are not surprising, since DA neurons in the VTA express NMDA receptors, as do cortical and subcortical neurons projecting to the VTA [93], In addition, NMDA receptors modulate the firing rate of 5-HT neurons in the median and dorsal raphe [94], These serotonergic neurons project to a variety of motor structures (VTA, substantia nigra, nucleus accumbens, and dorsal striatum) [95], where they modulate locomotion [96,97], If ketamine does stimulate locomotor activity through a down-stream dopaminergic and/or serotonergic mechanism, it would explain why the behavioral effects of ketamine, when compared to those of cocaine and D-amphetamine, are particularly susceptible to monoamine depletion. Lastly, both the DA and 5-HT systems show dramatic maturational changes across ontogeny [98–101], thus potentially accounting for some of the age-dependent psychopharmacological effects observed in the present study.
4.4. Conclusions
Ketamine (20 and 40 mg/kg) dramatically increased the locomotor activity of male preweanling rats and female rats of all ages; whereas, the locomotion of older male rats (adolescents and adults) was only modestly affected by ketamine. Ketamine-induced locomotion is at least partially mediated by monoamine systems, since DA and 5-HT depletion of around 87–96% significantly attenuated ketamine’s locomotor activating effects in male and female rats from all three developmental periods. It remains possible that other non-monoaminergic systems also mediate ketamine-induced locomotor activity [87,88]. In reserpinized rats, ketamine’s effects were unlike those of cocaine and D-amphetamine, thus our results are consistent with the hypothesis that ketamine enhances locomotion through indirect actions (e.g., by altering the firing rate of ascending monoamine projection neurons), rather than direct actions at the presynaptic terminal.
Highlights.
Ketamine increased the locomotor activity of female rats across ontogeny, but only minimally enhanced the locomotion of male adolescent and adult rats
Ketamine’s locomotor stimulating effects are mediated through a monoaminergic mechanism
By comparing the locomotor activating effects of ketamine to cocaine and amphetamine in reserpinized rats, it appears that ketamine enhances locomotor activity through indirect actions rather than having direct effects at the presynaptic terminal
Acknowledgements
This work was supported by the National Institute of General Medical Sciences [grant number GM083883] and the National Institute of Drug Abuse [grant number DA033877].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bergman SA, Ketamine: review of its pharmacology and its use in pediatric anesthesia, Anesth. Prog 46 (1999) 10–20. [PMC free article] [PubMed] [Google Scholar]
- [2].aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ, Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression, Biol. Psychiatry 67 (2010) 139–145. [DOI] [PubMed] [Google Scholar]
- [3].Jansen KLR, A review of the nonmedical use of ketamine: use, users and consequences, J. Psychoactive Drugs 32 (2000) 419–433. [DOI] [PubMed] [Google Scholar]
- [4].Usun Y, Eybrard S, Meyer F, Louilot A, Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex, Behav. Brain Res 256 (2013) 229–237. [DOI] [PubMed] [Google Scholar]
- [5].Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H, Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase, Neurosci. Lett 610 (2016) 48–53. [DOI] [PubMed] [Google Scholar]
- [6].Irifune M, Shimizu T, Nomoto M, Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice, Pharmacol. Biochem. Behav 40 (1991) 399–407. [DOI] [PubMed] [Google Scholar]
- [7].Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, Sumner J, Naloxone increases ketamine-induced hyperactivity in the open field in female rats, Pharmacol. Biochem. Behav 81 (2005) 530–534. [DOI] [PubMed] [Google Scholar]
- [8].Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A, Effects of age and sex on ketamine-induced hyperactivity in rats, Physiol. Behav 91 (2007) 202–207. [DOI] [PubMed] [Google Scholar]
- [9].McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V, Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose, Psychopharmacology 234 (2017) 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McDougall SA, Park GI, Ramirez GI, Gomez V, Adame BC, Crawford CA, Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats, Eur. Neuropsychopharmacol 29 (2019) 740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiley JL, Evans RL, Grainger DB, Nicholson KL, Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug, Pharmacol. Rep 63 (2011) 1085–1092. [DOI] [PubMed] [Google Scholar]
- [12].Rocha A, Hart N, Trujillo KA, Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration, Pharmacol. Biochem. Behav 157 (2017) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bates MLS, Trujillo KA, Long-lasting effects of repeated ketamine administration in adult and adolescent rats, Behav. Brain Res 369 (2019) 111928. doi: 10.1016/j.bbr.2019.111928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kohrs R, Durieux ME, Ketamine: teaching an old drug new tricks, Anesth. Analg 87 (1998) 1186–1193. [DOI] [PubMed] [Google Scholar]
- [15].Uchihashi Y, Kuribara H, Tadokoro S, Assessment of the ambulation-increasing effect of ketamine by coadministration with central-acting drugs in mice, Jpn. J. Pharmacol 60 (1992) 25–31. [DOI] [PubMed] [Google Scholar]
- [16].Lapin IP, Rogawski MA, Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice, Behav. Brain Res 70 (1995) 145–151. [DOI] [PubMed] [Google Scholar]
- [17].Yamamoto M, Mizuki Y, Suetsugi M, Ozawa Y, Ooyama M, Suzuki M, Effects of dopamine antagonists on changes in spontaneous EEG and locomotor activity in ketamine-treated rats, Pharmacol. Biochem. Behav 57 (1997) 361–365. [DOI] [PubMed] [Google Scholar]
- [18].Matulewicz P, Kasicki S, Hunt MJ, The effect of dopamine receptor blockade in the rodent nucleus accumbens on local field potential oscillations and motor activity in response to ketamine, Brain Res 1366 (2010) 226–232. [DOI] [PubMed] [Google Scholar]
- [19].Maj J, Rogóz Z, Skuza G, Locomotor hyperactivity induced by MK-801 in rats, Pol. J. Pharmacol. Pharm 43 (1991) 449–458. [PubMed] [Google Scholar]
- [20].Kuribara H, Asami T, Ida I, Tadokoro S, Characteristics of the ambulation-increasing effect of the noncompetitive NMDA antagonist MK-801 in mice: assessment by the coadministration with central-acting drugs, Jpn. J. Pharmacol 58 (1992) 11–18. [DOI] [PubMed] [Google Scholar]
- [21].Martin P, Svensson A, Carlsson A, Carlsson ML, On the roles of dopamine D-1 vs. D-2 receptors for the hyperactivity response elicited by MK-801, J. Neural Transm. Gen. Sect 95 (1994) 113–121. [DOI] [PubMed] [Google Scholar]
- [22].Martin P, Waters N, Waters S, Carlsson A, Carlsson ML, MK-801-induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride, Eur. J. Pharmacol 335 (1997) 107–116. [DOI] [PubMed] [Google Scholar]
- [23].Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML, Rodent data and general hypothesis: antipsychotic action exerted through 5-HT2A receptor antagonism is dependent on increased serotonergic tone, J. Neural Transm 105 (1998) 365–396. [DOI] [PubMed] [Google Scholar]
- [24].Carlsson ML, Martin P, Nilsson M, Sorensen SM, Carlsson A, Waters S, Waters N, The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice, J. Neural Transm 106 (1999) 123–129. [DOI] [PubMed] [Google Scholar]
- [25].Tso MM, Blatchford KL, Callado LF, McLaughlin DP, Stamford JA, Stereoselective effects of ketamine on dopamine, serotonin and noradrenaline release and uptake in rat brain slices, Neurochem. Int 44 (2004) 1–7. [DOI] [PubMed] [Google Scholar]
- [26].Hancock PJ, Stamford JA, Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens, Br. J. Anaesth 82 (1999) 603–608. [DOI] [PubMed] [Google Scholar]
- [27].Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM, The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits, J. Pharmacol. Exp. Ther 358 (2016) 71–82. [DOI] [PubMed] [Google Scholar]
- [28].Lindefors N, Barati S, O’Connor WT, Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex, Brain Res 759 (1997) 205–212. [DOI] [PubMed] [Google Scholar]
- [29].Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA, Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268, Neuroscience 117 (2003) 697–706. [DOI] [PubMed] [Google Scholar]
- [30].Martin LL, Bouchal RL, Smith DJ, Ketamine inhibits serotonin uptake in vivo, Neuropharmacology 21 (1982) 113–118. [DOI] [PubMed] [Google Scholar]
- [31].Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM, Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice, Neuropharmacology 112 (2017) 198–209. [DOI] [PubMed] [Google Scholar]
- [32].Kinoshita H, Nishitani N, Nagai Y, Andoh C, Asaoka N, Kawai H, Shibui N, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S, Ketamine-induced prefrontal serotonin release is mediated by cholinergic neurons in the pedunculopontine tegmental nucleus, Int. J. Neuropsychopharmacol 21 (2018) 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD, Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters, J. Pharmacol. Exp. Ther 359(2016) 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M, Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells, Anesthesiology 88 (1998) 768–774. [DOI] [PubMed] [Google Scholar]
- [35].Belujon P, Grace AA, Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity, Biol. Psychiatry 76 (2014) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T, Kaneko S, Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex, Int. J. Neuropsychopharmacol 17 (2014)1321–1326. [DOI] [PubMed] [Google Scholar]
- [37].Howell LL, Kimmel H, Monoamine transporters and psychostimulant addiction, Biochem. Pharmacol 75 (2008) 196–217. [DOI] [PubMed] [Google Scholar]
- [38].Niddam R, Arbilla S, Scatton B, Dennis T, Langer SZ, Amphetamine induced release of endogenous dopamine in vitro is not reduced following pretreatment with reserpine, Naunyn-Schmied. Arch. Pharmacol 329 (1985) 123–127. [DOI] [PubMed] [Google Scholar]
- [39].Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA, Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity, J. Neurochem 80 (2002) 960–969. [DOI] [PubMed] [Google Scholar]
- [40].Dluzen DE, Bhatt S, McDermott JL, Differences in reserpine-induced striatal dopamine output and content between female and male mice: implications for sex differences in vesicular monoamine transporter 2 function, Neuroscience 154 (2008) 1488–1496. [DOI] [PubMed] [Google Scholar]
- [41].Adell A, Sarna GS, Hutson PH, Curzon G, An in vivo dialysis and behavioural study of the release of 5-HT by p-chloroamphetamine in reserpine-treated rats, Br. J. Pharmacol 97 (1989) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Martín F, Artigas F, Simultaneous effects of p-chloroamphetamine, d-fenfluramine, and reserpine on free and stored 5-hydroxytryptamine in brain and blood, J. Neurochem 59 (1992) 1138–1144. [DOI] [PubMed] [Google Scholar]
- [43].Heslop KE, Curzon G, Depletion and repletion of cortical tissue and dialysate 5-HT after reserpine, Neuropharmacology 33 (1994) 567–573. [DOI] [PubMed] [Google Scholar]
- [44].Finn IB, Iuvone PM, Holtzman SG, Depletion of catecholamines in the brain of rats differentially affects stimulation of locomotor activity by caffeine, D-amphetamine, and methylphenidate, Neuropharmacology 29 (1990) 625–631. [DOI] [PubMed] [Google Scholar]
- [45].Florin SM, Kuczenski R, Segal DS, Effects of reserpine on extracellular caudate dopamine and hippocampus norepinephrine responses to amphetamine and cocaine: mechanistic and behavioral considerations, J. Pharmacol. Exp. Ther 274 (1995) 231–341. [PubMed] [Google Scholar]
- [46].Parker EM, Cubeddu LX, Effects of d-amphetamine and dopamine synthesis inhibitors on dopamine and acetylcholine neurotransmission in the striatum. I. Release in the absence of vesicular transmitter stores, J. Pharmacol. Exp. Ther 237 (1986) 179–192. [PubMed] [Google Scholar]
- [47].Callaway CW, Kuczenski R, Segal DS, Reserpine enhances amphetamine stereotypies without increasing amphetamine-induced changes in striatal dialysate dopamine, Brain Res 505 (1989) 83–90. [DOI] [PubMed] [Google Scholar]
- [48].Smith CB, Enhancement by reserpine and α-methyl dopa of the effects of d-amphetamine upon the locomotor activity of mice, J. Pharmacol. Exp. Ther 142 (1963) 343–350. [PubMed] [Google Scholar]
- [49].van Rossum JM, Hurkmans JATM, Mechanism of action of psychomotor stimulant drugs: significance of dopamine in locomotor stimulant action, Int. J. Neuropharmacol 3 (1964) 227–239. [DOI] [PubMed] [Google Scholar]
- [50].Fessler RG, Sturgeon RD, Meltzer HY, Effects of phencyclidine and methylphenidate on d-amphetamine-induced behaviors in reserpine pretreated rats, Pharmacol. Biochem. Behav 13 (1980) 835–842. [DOI] [PubMed] [Google Scholar]
- [51].Hong M, Jenner P, Marsden CD, Comparison of the acute actions of amine-depleting drugs and dopamine receptor antagonists on dopamine function in the brain in rats, Neuropharmacology 26 (1987) 237–245. [DOI] [PubMed] [Google Scholar]
- [52].McDougall SA, Kozanian OO, Greenfield VY, Horn LR, Gutierrez A, Mohd-Yusof A, Castellanos KA, One-trial behavioral sensitization in preweanling rats: differential effects of cocaine, methamphetamine, methylphenidate, and D-amphetamine, Psychopharmacology 217(2011)559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McDougall SA, Nuqui CM, Quiroz AT, Martinez CM, Early ontogeny of D-amphetamine-induced one-trial behavioral sensitization, Pharmacol. Biochem. Behav 104 (2013) 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA, Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2High receptors, Psychopharmacology 232 (2015) 2287–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McDougall SA, Apodaca MG, Mohd-Yusof A, Mendez AD, Katz CG, Teran A, Garcia-Carachure I, Quiroz AT, Crawford CA, Ontogeny of cocaine-induced behaviors and cocaine pharmacokinetics in male and female neonatal, preweanling, and adult rats, Psychopharmacology 235 (2018) 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McDougall SA, Rios JW, Apodaca MG, Park GI, R Montejano N, Taylor JA, Moran AE, Robinson JAM, Baum TJ, Teran A, Crawford CA, Effects of dopamine and serotonin synthesis inhibitors on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of preweanling and adolescent rats: sex differences, Behav. Brain Res. under editorial review [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].National Research Council, Guide for the Care and Use of Laboratory Animals, eighth ed., National Academies Press, Washington, 2010. [Google Scholar]
- [58].Campbell BA, Lytle LD, Fibiger HC, Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat, Science 166 (1969) 635–637. [DOI] [PubMed] [Google Scholar]
- [59].Shalaby IA, Spear LP, Psychopharmacological effects of low and high doses of apomorphine during ontogeny, Eur. J. Pharmacol 67 (1980) 451–459. [DOI] [PubMed] [Google Scholar]
- [60].Thomas KL, Rose S, Jenner P, Marsden CD, Acute reserpine treatment induces down regulation of D-1 dopamine receptor associated adenylyl cyclase activity in rat striatum, Biochem. Pharmacol 44 (1992) 83–91. [DOI] [PubMed] [Google Scholar]
- [61].Huynh H, Feldt LS, Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs, J. Educ. Stat 1 (1976) 69–82. [Google Scholar]
- [62].Holson RR, Pearce B, Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species, Neurotoxicol. Teratol 14 (1992) 221–228. [DOI] [PubMed] [Google Scholar]
- [63].Stolk JM, Rech RH, Enhanced stimulant effects of d-amphetamine on the spontaneous locomotor activity of rats treated with reserpine, J. Pharmacol. Exp. Ther 158 (1967) 140–149. [PubMed] [Google Scholar]
- [64].Saland SK, Kabbaj M, Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats, J. Pharmacol. Exp. Ther 367 (2018) 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nabeshima T, Yamaguchi K, Furukawa H, Kameyama T, Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats, Eur. J. Pharmacol 105 (1984) 197–206. [DOI] [PubMed] [Google Scholar]
- [66].Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T, Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats, Eur. J. Pharmacol 98 (1984) 217–227. [DOI] [PubMed] [Google Scholar]
- [67].Shelnutt SR, Gunnell M, Owens SM, Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats, J. Pharmacol. Exp. Ther 290 (1999) 1292–1298. [PubMed] [Google Scholar]
- [68].Carrier N, Kabbaj M, Sex differences in the antidepressant-like effects of ketamine, Neuropharmacology 70 (2013) 27–34. [DOI] [PubMed] [Google Scholar]
- [69].Saland SK, Schoepfer KJ, Kabbaj M, Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner, Sci. Rep (2016) 6:21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schindler CW, Carmona GN, Effects of dopamine agonists and antagonists on locomotor activity in male and female rats, Pharmacol. Biochem. Behav 72 (2002) 857–863. [DOI] [PubMed] [Google Scholar]
- [71].Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V, Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels, Neuropharmacology 46 (2004) 672–687. [DOI] [PubMed] [Google Scholar]
- [72].Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ, Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats, Behav. Brain Res 101 (1999) 15–20. [DOI] [PubMed] [Google Scholar]
- [73].Sell SL, Scalzitti JM, Thomas ML, Cunningham KA, Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats, J. Pharmacol. Exp. Ther 293 (2000) 879–886. [PubMed] [Google Scholar]
- [74].Becker JB, Molenda H, Hummer DL, Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse, Ann. N.Y. Acad. Sci 937 (2001) 172–187. [DOI] [PubMed] [Google Scholar]
- [75].van Harren F, Garcea M, Anderson KG, Tebbett IR, Cocaine and benzoylecgonine in serum microsamples of intact and gonadectomized male and female Wistar rats, Pharmacol. Biochem. Behav 58 (1997) 421–424. [DOI] [PubMed] [Google Scholar]
- [76].Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM, Effects of sex and gonadectomy on cocaine metabolism in the rat, J. Pharmacol. Exp. Ther 290 (1999) 1316–1323. [PubMed] [Google Scholar]
- [77].Becker JB, Gender differences in dopaminergic function in striatum and nucleus accumbens, Pharmacol. Biochem. Behav 64 (1999) 803–812. [DOI] [PubMed] [Google Scholar]
- [78].Snyder KJ, Katovic NM, Spear LP, Longevity of the expression of behavioral sensitization to cocaine in preweanling rats, Pharmacol. Biochem. Behav 60 (1998) 909–914. [DOI] [PubMed] [Google Scholar]
- [79].Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA, Ontogeny of methamphetamine- and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats, Behav. Pharmacol 23 (2012) 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wacan JJ, Reichel CM, Farley CM, McDougall SA, The partial dopamine D2-like receptor agonist terguride functions as an agonist in preweanling rats after a 5-day reserpine regimen, Psychopharmacology 185 (2006) 104–111. [DOI] [PubMed] [Google Scholar]
- [81].Ji J, McDermott JL, Dluzen DE, Sex differences in K+-evoked striatal dopamine output from superfused striatal tissue fragments of reserpine-treated CD-1 mice, J. Neuroendocrinol 19 (2007) 725–731. [DOI] [PubMed] [Google Scholar]
- [82].Kuczenski R, Biochemical actions of amphetamine and other stimulants, in Creese I (Ed.), Stimulants: Neurochemical, Behavioral and Clinical Perspectives, Raven Press, New York, 1983, pp. 31–61. [Google Scholar]
- [83].Hanania T, Zahniser NR, Locomotor activity induced by noncompetitive NMDA receptor antagonists versus dopamine transporter inhibitors: opposite strain differences in inbred long-sleep and short-sleep mice, Alcohol Clin. Exp. Res 26 (2002) 431–440. [PubMed] [Google Scholar]
- [84].Yamaguchi K, Nabeshima T, Kameyama T, Role of dopaminergic and GABAergic mechanisms in discrete brain areas in phencyclidine-induced locomotor stimulation and turning behavior, J. Pharmacobiodyn 9 (1986) 975–986. [DOI] [PubMed] [Google Scholar]
- [85].Narayanan S, Willins D, Dalia A, Wallace L, Uretsky N, Role of dopaminergic mechanisms in the stimulatory effects of MK-801 injected into the ventral tegmental area and the nucleus accumbens, Pharmacol. Biochem. Behav 54 (1996) 565–573. [DOI] [PubMed] [Google Scholar]
- [86].Mele A, Fontana D, Pert A, Alterations in striatal dopamine overflow during rotational behavior induced by amphetamine, phencyclidine, and MK-801, Synapse 26 (1997) 218–224. [DOI] [PubMed] [Google Scholar]
- [87].Mele A, Thomas DN, Pert A, Different neural mechanisms underlie dizocilpine maleate-and dopamine agonist-induced locomotor activity, Neuroscience 82 (1998) 43–58. [DOI] [PubMed] [Google Scholar]
- [88].Adams B, Moghaddam B, Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine, J. Neurosci 18 (1998) 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moghaddam B, Adams B, Verma A, Daly D, Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex, J. Neurosci 17(1997) 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S, Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci 24 (2001) 330–334. [DOI] [PubMed] [Google Scholar]
- [91].Lopez Hill X, Scorza MC, Role of the anterior thalamic nucleus in the motor hyperactivity induced by systemic MK-801 administration in rats, Neuropharmacology 62 (2012) 2440–2446. [DOI] [PubMed] [Google Scholar]
- [92].French ED, Ceci A, Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons, Neurosci. Lett 119 (1990) 159–162. [DOI] [PubMed] [Google Scholar]
- [93].Morikawa H, Paladini CA, Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms, Neuroscience 198 (2011) 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ, AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei, Eur. J. Neurosci 25 (2007) 3001–3008. [DOI] [PubMed] [Google Scholar]
- [95].Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P, Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem, Brain Struct. Funct 221 (2016) 535–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Geyer MA, Serotonergic functions in arousal and motor activity, Behav. Brain Res 73 (1996)31–35. [DOI] [PubMed] [Google Scholar]
- [97].Mylecharane EJ, Ventral tegmental area 5-HT receptors: mesolimbic dopamine release and behavioural studies, Behav. Brain Res 73 (1996) 1–5. [DOI] [PubMed] [Google Scholar]
- [98].Andersen SL, Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev 27 (2003) 3–18. [DOI] [PubMed] [Google Scholar]
- [99].Andersen SL, Stimulants and the developing brain, Trends Pharmacol. Sci 26 (2005) 237–243. [DOI] [PubMed] [Google Scholar]
- [100].Teissier A, Soiza-Reilly M, Gaspar P, Refining the role of 5-HT in postnatal development of brain circuits, Front. Cell. Neurosci 23 (2017) 11:139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Galineau L, Kodas E, Guilloteau D, Vilar MP, Chalon S, Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study, Neurosci. Lett 363 (2004) 266–271. [DOI] [PubMed] [Google Scholar]