Breast cancer is the most commonly diagnosed cancer among women. It is estimated that there will be 252,710 new breast cancer diagnoses and 40,610 deaths in 2017 in the United States.1 In China, 15% of all new cancer diagnoses in women are breast cancer, and the disease is the leading cause of cancer deaths in women younger than 45 years of age.2 Globally, with the application of tamoxifen, the breast cancer recurrence and mortality rates were decreased by 41% and 34% respectively.3 Third-generation aromatase inhibitors (AIs), including anastrozole, letrozole, and exemestane, are associated with significant improvement in disease-free and overall survival for post-menopausal women with breast cancer.4,5 Therefore, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines of Breast Cancer (version 2.2017) recommend that women with early stage, hormone receptor positive breast cancer receive at least 5 years of endocrine therapy generally consisting of tamoxifen for pre-menopausal women and an aromatase inhibitor (AI) for post-menopausal women.6 Additionally, except for immediately life-threatening cases, endocrine therapy alone or in combination has been recommended as an initial treatment for women with hormone receptor positive metastatic breast cancer by the American Society of Clinical Oncology.7
While endocrine therapy significantly improves the overall and disease-free survival in women with breast cancer, this treatment is associated with multiple symptoms that may have a detrimental impact on medication adherence, functional status and quality of life.8–10 Co-occurring symptoms associated with endocrine therapy were reported as one of the most common reasons for treatment discontinuation (66.7% of AI discontinuers and 59.1% of tamoxifen discontinuers).8 Moreover, endocrine therapy-related symptoms are more likely to be neglected by both health care providers and patients due to less frequent follow-up visits, compared to follow-ups for other forms of adjuvant therapy, such as chemotherapy and radiation therapy.11
Although assessment of adverse events is essential in clinical trials of endocrine therapy development mainly for the purpose of safety, evidence now suggests that endocrine therapy associated symptoms were underestimated. Ruhstaller et al. reported that hot flashes/sweats (70% vs. 38–40% in clinical trials), low energy (45% vs. 9–15% in clinical trials), fluid retention (22% vs. 7% in clinical trials), and vaginal dryness (30% vs. 3% in clinical trials) were significantly underrated in clinical trials of endocrine therapy.12 Therefore, having a comprehensive understanding of the symptom experience associated with endocrine therapy is urgently needed, as it will serve as the bases for development of interventions to manage those symptoms. The purpose of this scoping review is to map the occurrence (frequency), intensity, and distress of symptoms during endocrine therapy for breast cancer.
Methods
This scoping review was conducted under the framework proposed by Khalil et al. and the Joanna Briggs Institute methods of evidence synthesis as detailed bellow.13
Step 1 Identify the Research Question
The research question for this scoping review was: what is the symptom(s) experience during endocrine therapy for breast cancer that has been reported? The Joanna Briggs Institute suggests using PCC (population, concept, and context) to construct a clear and meaningful scoping review. Therefore, we further defined the PCC of this scoping review as follows.
Population
Participants in the included studies in this scoping review are adult females (18 years or older), who were diagnosed with breast cancer and receiving oral endocrine therapy. Both observational studies describing the symptom(s) experience and experimental studies comparing the symptom experience among different types of endocrine therapies were eligible. Studies with samples that were undividable from other types of cancer or other types of treatment were excluded from this review because they precluded the ability to discern symptoms specifically related to endocrine therapy.
Concept
Endocrine therapy and symptom experience are two key concepts in this scoping review. Endocrine therapy refers to oral adjuvant endocrine therapy currently recommended by the NCCN Guideline for Breast Cancer, including selective estrogen receptor modulators (SERMS) such as Tamoxifen (Nolvadex and Soltamox), and aromatase inhibitors including Anastrozole (Arimidex), Letrozole (Femara), and Exemestane (Aromasin). Symptom experience is defined as the “perception of the frequency, intensity, distress, and meaning of symptoms as they are produced and expressed” in accordance with the Symptom Experience Model (SEM).14
Context
In this scoping review, the symptom(s) experience is determined within the context of endocrine therapy for breast cancer in clinical studies. Excluded are clinical trials or studies using endocrine therapy to prevent breast cancer or chemoprevention.
Step 2 Identify Relevant Studies
Studies published in English and Chinese language before February 2017 were comprehensively searched. A three-step search strategy was utilized. An initial scoping search was conducted in PubMed and China Science Periodical Databases (CSPD) to identify key terms. Then, comprehensive searches were performed in the following databases: PubMed, CINAHL®, and CSPD. The following search terms were combined: breast, neoplasm, endocrine therapy, hormonal therapy, antineoplastic agents, aromatase inhibitor, tamoxifen, symptom, and adverse effects. The search string in PubMed is: ((((“Antineoplastic Agents, Hormonal/adverse effects”[Majr]) OR “Aromatase Inhibitors/adverse effects”[Majr]) OR “Tamoxifen/adverse effects”[Majr])) AND “Breast Neoplasms”[Mesh:NoExp]. Lastly, additional pertinent studies were identified by reviewing the bibliographies of included studies.
Step 3 Study Selection
The initial search revealed 2,551 references (PubMed=1,489, CINAL=822, CSPD=236, other recourses=4). After removal of 70 duplicated references, 2,481 (2,245 English and 236 Chinese) were screened by title and abstract for eligibility. Figure 1 summarizes the details of study selection. Studies were reviewed by two researchers for determination of eligibility. A third researcher adjudicated situations in which there was a disagreement. Eventually, 53 clinical studies were identified from 57 articles (54 in English, 3 in Chinese) and were included in this scoping review (Table 1)
Figure 1.
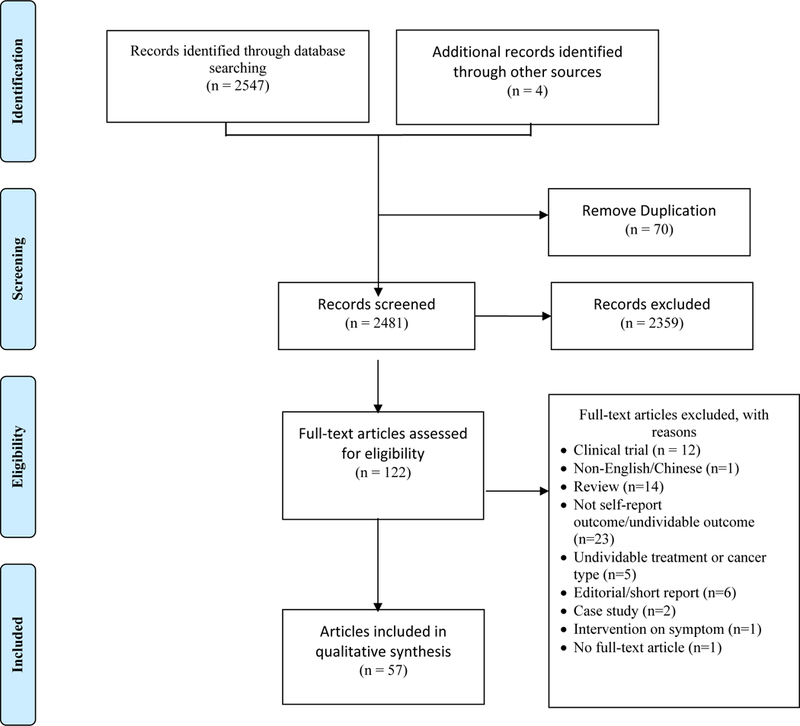
The Process of Selecting Studies
Table 1.
Studies Included in This Scoping Review
| Author, Year |
Country | Agent | Design | Instrument | Recall period | Domain | Symptoms |
|---|---|---|---|---|---|---|---|
| Ashraf 200915 |
India | TAM | Retrospective review of case records (n=3000) | Medical records | - | Occurrence Intensity |
TAM-related side effects |
| Baumgart 201116 |
Sweden | ET | Cross-sectional (n=97) |
FACT-ES IIQ-7 UDI-6 |
- | Occurrence Intensity |
Urogenital symptoms ET related symptoms |
| Baumgart 201317 |
Sweden | ET | Cross-sectional (n=97) |
Standardized questionnaire | Past 12 months | Occurrence Frequency Distress |
Sexual dysfunction symptoms |
| Baxter 201418 |
Canada | TAM | Cross-sectional (n=132) |
Survey | Past 7 days | Occurrence Frequency Intensity |
Hot flashes and TAM-related symptoms |
| Boehm 200919 |
Germany | TAM | Cross-sectional (n=136) |
Questionnaire | Past 7 days | Occurrence Frequency |
AI related symptoms |
| Boonstra 201320 |
Netherlands | AI | Cross-sectional (n=57) |
Rheumatoid Arthritis Disease Activity Index, FACT-ES | Past 7 days | Occurrence Intensity |
Arthralgia/stiffness/AI related symptoms |
| Bowles 20128 |
USA | ET | Cross-sectional (n=538) |
Survey | At any point in ET | Occurrence | Adverse effects of ET |
| Brown 201421 |
USA | AI | Cross-sectional (n=300) |
WOMAC M-SACRAH Quick DASH |
- | Occurrence Intensity |
Musculoskeletal symptoms |
| Castel 201322 |
USA | AI | Longitudinal (pre- and 2, 4, 6, 8, 12, 52 weeks post-AI, n=91) | FACT-ES PRAI |
- | Occurrence Intensity |
Arthralgia ET related symptoms |
| Chim 201323 |
USA | AI | Cross-sectional (n=437) |
BPI | Past 24 hours | Occurrence Intensity Distress |
Joint Pain |
| Chin 200924 |
Canada | ET | Cross-sectional (n=251) |
FACT-ES Sexual activity questionnaire |
Past 7 days | Occurrence Intensity |
Vulvovaginal and urinary symptoms ET related symptoms |
| Crew 200725 |
USA | AI | Cross-sectional (n=200) |
Questionnaire adapted from BPI-SF | Past 7 days | Occurrence, Intensity | Joint symptoms |
| Desai 201326 |
USA | AI | Cross-sectional (n=413) |
Insomnia severity index (ISI) | Current | Occurrence Intensity Distress |
Insomnia |
| Dizdar 200927 |
Turkey | AI | Cross-sectional (n=92) |
Patient Interview | Recently | Occurrence | Arthralgia |
| Egawa 201628 |
Japan | AI | Longitudinal (pre- and 3, 6, 9, 12 months post-AI, n=391) | Questionnaire | - | Frequency Distress |
Joint Symptoms |
| Frechette 201329 |
Canada | ET | Longitudinal (pre- and 6 months post-ET, n=66) | Female sexual function index, FSDS-R, FACT-ES | Past 30 days (FSDS-R)/7 days (FACT-ES) | Occurrence Intensity Distress |
Sexual dysfunction, ET related symptoms |
| Gallicchio 201230, 31 |
USA | AI | Longitudinal (pre- and 3, 6 months post-AI, n=95) | VAS Symptom checklist of 20 menopausal-type symptoms |
Past 4 weeks | Occurrence Intensity Distress |
Musculoskeletal pain Menopausal-type symptoms |
| Gallicchio 201332 |
USA | ET | Survey (n=851) |
Hospital registry-based survey | Past 4 weeks | Distress | Hair loss & hair thinning |
| Garreau 200633 |
USA | ET | Cross-sectional (n=452) |
Questionnaire | - | Occurrence | ET related symptoms |
| Hadji 201434 |
Germany | AI | Longitudinal (pre- and 3, 6, 9 months post-study n=1916) | RASQ | - | Occurrence Intensity |
Arthralgia |
| Horimoto 200935 |
Japan | AI | Retrospective (n=329) |
Chart Review | - | Occurrence | Arthralgia |
| Hu 201636 |
China | ET | Retrospective review of case records (n=160) | Chart review | - | Occurrence | ET-related symptoms |
| Huang 201037 |
China | ET | Cross-sectional (n=315) |
VAS | - | Occurrence Intensity |
Cancer related fatigue |
| Inglis 201538 |
Australia | AI | Cross-sectional (n=93) |
OSDI FACT-ES |
Past 2 weeks | Occurrence Intensity Distress |
Dry eye syndrome ET related symptoms |
| Kanti 201639 |
Germany | TAM | Longitudinal (1 day pre-therapy to up to 28 weeks post-therapy) (n=17) |
Diary, questionnaire modified of the hairdex and the SF-MPQ | Past day | Occurrence Distress |
Trichodynia (hair pain) |
| Kyvernitakis 201440 |
Germany | AI | Longitudinal (pre- and 12, 24 months post-AI, n=174) | MRS | - | Occurrence Intensity |
Menopausal symptoms |
| Laroche 201441 |
France | AI | Longitudinal (pre- and 1, 3, 6, 12 months post-AI, n=135) | VAS, McGill Pain Questionnaire, BPI | - | Occurrence, Intensity, Distress | Pain |
| Lintermans 201442, 43 |
Belgium | ET | Longitudinal (pre- and 3, 6, 12 months post-ET, n=292) | NSABP symptom checklist VAS Musculoskeletal questionnaire |
Past 7 days | Occurrence intensity |
Musculoskeletal pain |
| Lu 201144 |
China | AI | Retrospective review of case records (n=271) | Telephone interview | - | Occurrence | ET-related symptoms |
| Mao 200945 |
USA | AI | Cross-sectional (n=300) |
Questionnaire | Past 7 days | Occurrence Intensity |
Arthralgia |
| Mao 201146 |
USA | AI | Cross-sectional (n=390) |
Self-reported Arthralgia | - | Occurrence | Arthralgia |
| Menas 201247 |
USA | AI | Cross-sectional (n=206) |
Retrospective chart review | - | Occurrence | Arthralgia |
| Mortimer 199948 |
USA | TAM | Cross-sectional (n=57) |
BCPT Symptom Checklist | Past 4 weeks | Occurrence Distress |
ET related symptoms |
| Mortimer 200849 |
USA | TAM | Baseline data of RCT (n=864) | “Thoughts and Feelings” questionnaire | Past 4 weeks | Occurrence Intensity |
Physical/psychological symptoms |
| Napoli 201050 |
USA | AI | Cross-sectional (n=145) |
Modified Leuven questionnaire | - | Occurrence Intensity |
Musculoskeletal symptoms |
| Oberguggenberger 201151 |
Australia | AI | Cross-sectional (n=280) |
FACT-ES | Past 7 days | Occurrence Intensity |
ET related symptoms |
| Ochayon 201452 |
Israel | ET | Cross-sectional (n=210) |
MDASI BCPT Symptom Checklist |
Past 24 hours Past 4 weeks |
Occurrence Intensity Distress |
ET related symptoms |
| Ohsako 200654 |
Japan | ANA | Longitudinal (n=53) |
CTCAEver3.0 | - | Occurrence Intensity |
Musculoskeletal symptom |
| Olufade 201510 |
USA | AI | Cross-sectional (n=68) |
VAS | Past 4 weeks | Occurrence Intensity |
Musculoskeletal pain |
| Presant 200754 |
USA | AI | Semi-structured interview (n=56) | A linear analogue pain scale, location, character and treatment | - | Occurrence Intensity |
Arthralgia |
| Ribi 200755 Ruhstaller 200956 |
Switzerland | ET | Cross-sectional (n=373) |
Checklist for Patients with Endocrine Therapy (C-PET) | - | Occurrence Frequency |
ET-related symptoms |
| Rosenberg 201557 |
USA | ET | Cross-sectional (n=2086) |
BCPT Symptom Checklist | Past 4 weeks | Occurrence Distress |
ET related symptoms |
| Sagara 201058 |
Japan | AI | Longitudinal (n=656) |
Symptom were collected retrospectively (no detail mentioned) | - | Occurrence | ANA related adverse events |
| Schover 201459 |
USA | AI | Cross-sectional (n=129) |
FSFI, MSIQ, FSDS-R, BESS | Past 4 weeks | Occurrence Distress |
Sexual Function |
| Servitja 201260 |
Spain | AI | Longitudina (pre- and 3 months post-AI, n=343) | VAS | - | Occurrence Intensity |
Arthralgia |
| Shi 201361 |
USA | AI | Longitudinal (pre- and biweekly for 1 year, n=47) | BPI MDASI Joint Pain Assessment (JPA) |
Past 24 hours Past 7 days |
Occurrence Intensity Distress |
Arthralgia ET related symptoms |
| Singer 201262 |
USA | AI | Longitudinal (pre- and 3, 6 months post-AI, n=52) | FACT-ES Global pain AUSCAN |
Past 7 days | Occurrence Intensity |
Arthralgia ET related symptoms |
| Su 201063 |
USA | AI | Cross-sectional (n=300) |
Questionnaire | Past 7 days | Occurrence Frequency Intensity |
Hot flashes Weight Gain |
| Swenson 201364 |
USA | AI | Longitudinal (pre- and 1, 3, 6 months post-AI, n=122) | BCPT Symptom Checklist AUSCAN WOMAC BPI QuickDASH |
Past 24 hours Past 4 weeks |
Occurrence Intensity Distress |
Musculoskeletal symptoms |
| Waltman 200965 |
USA | AI | Cross-sectional (n=29) |
The Aromatase Inhibitor Questionnaire | Past 7 days | Occurrence Intensity Distress |
Musculoskeletal symptoms |
| Wang 201366 |
China | AI | Cross-sectional (n=436) |
CTCAEver 3.0 WOMAC M-SACRAH BPI-SF |
Past 7 days | Occurrence Intensity Distress |
Musculoskeletal symptoms |
| Xu 201467 |
China | ET | Cross-sectional (n=122) |
Retrospective telephone interview | - | Occurrence | Musculoskeletal symptoms |
| Zhan 200768 |
USA | TAM | Cross-sectional (n=138) |
Symptom checklist | - | Occurrence | TAM-related side effects |
| Zhou 201169 |
China | ET | Retrospective review of case records (n= 50) | CTCAEver3.0 | - | Occurrence | ET-related symptoms |
Abbreviations: AI, Aromatase Inhibitor; AUSCAN, Australian/Canadian Hand Osteoarthritis Index; ANA, Anastrozole; BCPT Symptom Checklist, Breast Cancer Prevention Trial Symptom Checklist; BESS, Breast Cancer Prevention Trial Eight Symptoms Scale; BPI, Brief Pain Inventory; CTCAEver3.0, Common Terminology Criteria for Adverse Events v3.0; ET, Endocrine Therapy; FACT-ES, Functional Assessment of Cancer; Therapy-Endocrine Symptom; FSDS-R, Female Sexual Distress S-Revised; FSFI, Female Sexual Function Index; IIQ-7, Incontinence Impact Questionnaire; MDASI, The M. D. Anderson Symptom Inventory; MRS, Menopause Rating Scale; M-SACRAH, Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands; MSIQ, Menopausal Sexual Interest Questionnaire; NSABP symptom checklist, National Surgical Adjuvant Breast and Bowel Project Symptom Checklist; OSDI, Ocular Surface Disease Index; PRAI, Patient-reported Arthralgia Inventory; Quick DASH, Quick Disabilities of the Arm, Shoulder and Hand Questionnaire; SF-MPQ, Short Form of the McGill Pain Questionnaire; TAM, Tamoxifen; UDI-6, Urogenital Distress Inventory-6; VAS, Visual Analog Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Step 4 Charting the Data
Data charting includes the process of data extraction and describing the data both narratively and in tabular form. The SEM model was used to guide the data charting, the process of data extraction. We synthesized the symptom experience based on each domain (frequency, intensity, distress). Since most studies reported symptom occurrence (a dichotomized variable of frequency), we integrated occurrence into a frequency domain. Due to the heterogeneity of study design (e.g., cross-sectional or longitudinal) and characteristics of participant across studies (e.g., tamoxifen or aromatase inhibitor users, Caucasian or African American), data on each characteristic at every time point were charted as an independent report if they were available in the original articles, to facilitate comparison across studies. For example, a 6-month longitudinal study on the occurrence of joint pain could have 12 occurrence reports based on the combination of time point (0, 3, 6 months), agent (tamoxifen or aromatase inhibitor) and ethnicity (Caucasian or African American). In terms of intensity and distress, some studies reported the percentage of people who experience different levels of symptoms among the entire sample, while some reported the percentage of participants who developed symptoms. To make the data comparable, we recalculated the percentage for the latter situation so that the percentages were referring to proportion among the all participants. For example, when a study reported 30% of 50 patients out of 100 research participants had severe pain, we recalculated the percentage of patients who had severe pain as 50*30%/100=15%.
Step 5 Collating, Summarizing and Reporting the Results
Symptom data from individual studies were collated after being extracted. Summary and interpretation of data were demonstrated in the results session. The implications of the findings for clinical practice and future research were further detailed in the discussion session.
Results
Since 2006, the number of studies on symptoms associated with endocrine therapy fluctuated with an increasing trend, reaching its peak in 2013 (n=11), and dropped dramatically in 2015 and 2016 (Figure 2). More studies focused on aromatase inhibitors than tamoxifen (34 vs 7). The sample sizes varied considerably ranging from 17 to 3000. Most of the studies used a cross-sectional design (n=33). The longest follow-up period for symptom assessment in the longitudinal studies was 24 months (see Table 1).
Figure 2.
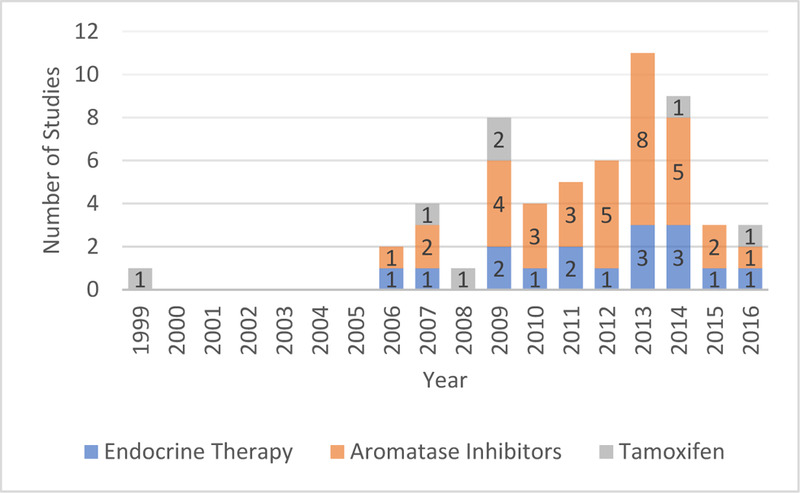
Number of studies on endocrine therapy for breast cancer over time
Most studies assessed symptoms by using self-report questionnaires or symptom checklists. Retrospective medical record reviews or telephone interviews was adopted in 8 studies.15, 35, 36, 44, 47, 58, 67, 69 Two studies conducted retrospective semi-structured interviews54 and patient interviews27. The recall period ranged from 24 hours to 12 months, with recall for the past 7 days and 4 weeks most commonly adopted. Twenty-three studies did not report recall period (see Table 1).
The mostly used symptom assessments used were the Breast Cancer Prevention Trial (BCPT) Symptom Checklist, Functional Assessment of Cancer Therapy (FACT-ES), and the M.D. Anderson Symptom Inventory (MDASI). Symptom intensity and distress were quantified using Likert scales. Investigator-developed symptom questionnaires and checklists adapted from Visual Analog Scales (VAS) were commonly used as well (see Table 1).
In this scoping review, individual symptoms identified were categorized into cognitive, musculoskeletal, vasomotor, gastrointestinal, urogenital, mood-related, sleep-related, and sexual symptoms, adapted from the subscales of BCPT Symptom Checklist. Symptoms which did not fall into these categories were grouped into a separate category labeled “others”. Symptom occurrence, intensity, and distress reported by each study are exhibited in the Supplemental Table 1.
Mostly Studied Symptoms
Based on the numbers of studies which reports of symptom occurrence, the 16 mostly studied symptoms were joint/muscle pain, hot flashes, vaginal dryness/insufficient lubrication, sleep disorder/insomnia, fatigue/lack of energy, nausea, vaginal bleeding or spotting, headaches/migraines, irritability, joint/muscle stiffness, weight gain, vaginal discharge, depression/depressive mood, low sexual interest/desire, difficulty breathing/short of breath, and dizziness/faintness (see Figure 3). Far fewer studies reported symptom intensity and distress. In the 16 mostly reported symptoms, results related to the occurrence, intensity, and distress domains were only reported for eight symptoms including joint/muscle pain, hot flashes, vaginal dryness/insufficient lubrication, sleep disorder/insomnia, fatigue/lack of energy, irritability, joint/muscle stiffness, and depression/depressive mood. Intensity was reported from more than one study on only 10 symptoms: joint/muscle pain (10), hot flashes (4), vaginal dryness/insufficient lubrication (4), vaginal discharge (3), joint/muscle stiffness (2), genital itching/irritation (2), vaginal bleeding/spotting (2), incontinence (2), sleep disorder/insomnia (2), and fatigue/lack of energy (2). Distress was reported from more than one study on only 10 symptoms: joint/muscle pain (5), hot flashes (3), pain with intercourse (3), forgetfulness (2), general aches and pains (2), joint/muscle stiffness (2), unhappy with the appearance of body (2), irritability (2), headaches/migraines (2), and loss of hair/hair thinning (2).
Figure 3.
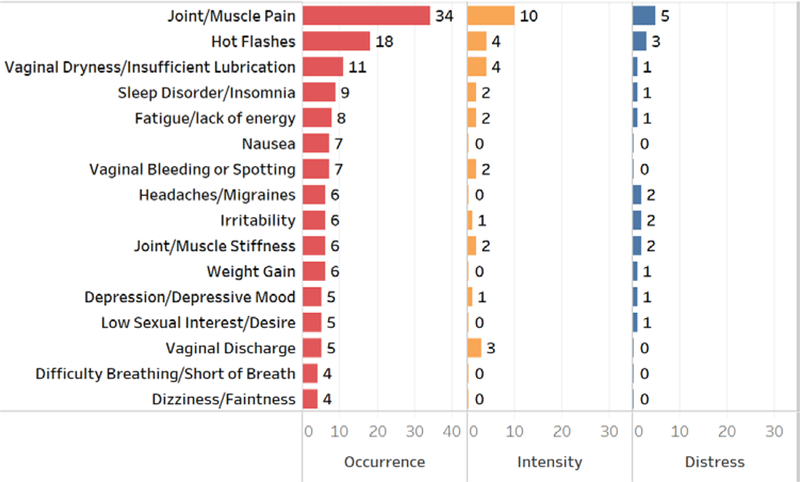
Number of studies on the occurrence, intensity, distress of symptoms during endocrine therapy
Symptoms with Highest Occurrence, Intensity, and Distress
After extracting the symptom occurrences (the percentage of people who reported the symptom) from included studies (see Supplemental Table 1), we sorted the occurrences from low to high for each symptom and identified the median occurrence for each symptom. Based on the median of occurrence of individual symptom, we identified 15 symptoms with highest occurrence (most prevalent symptoms). From high to low, these 15 most prevalent symptoms include cramps, hot flashes, fatigue/lack of energy, eye irritation, heart discomfort, joint/muscle pain, night sweats, sexual arousal problem/orgasmic dysfunction, anxiety, dyspareunia, low sexual interest/desire, joint/muscle stiffness, urinary urgency, numbness or tingling, and dry eye syndrome (see Figure 4). Notably, 6 of these 15 symptoms (including cramps, eye irritation, heart discomfort, anxiety, dyspareunia, urinary urgency, numbness and tingling, and dry eye syndrome) were reported by only one study. Sexual arousal problem/orgasmic dysfunction were reported by only two studies.
Figure 4.
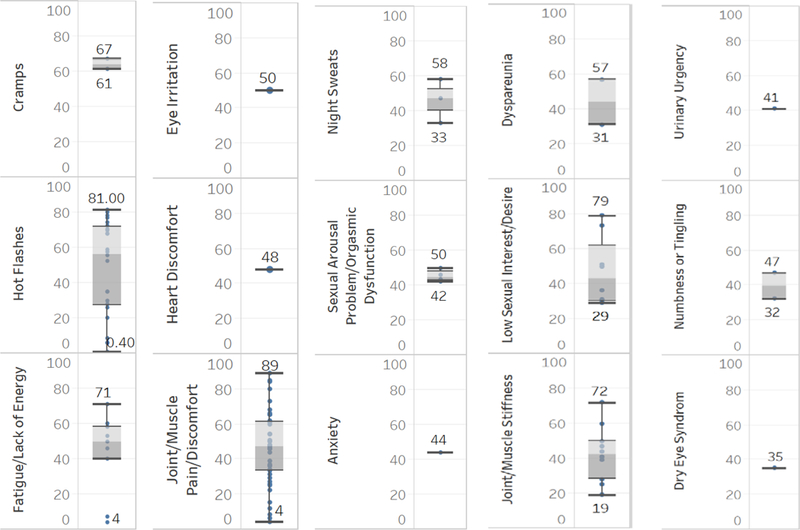
Top 15 Prevalent Symptoms (based on median) Reported by Current Studies on Endocrine Therapy for Breast Cancer
Five out of the 15 most prevalent symptoms overlapped with the most studied symptoms, including joint/muscle pain, hot flashes, low sexual interest/desire, joint/muscle stiffness, and fatigue/lack of energy (see Figures 3 and 4). Interestingly, these five symptoms had the top 5 highest maximum symptom occurrences, suggesting that these five symptoms are particularly relevant to women receiving endocrine therapy for breast cancer.
Intensity and distress were assessed using visual analog scales (VAS) in several studies. The proportion of participants who rated symptoms as mild, moderate, severe or extremely severe and distressful was also reported (see Supplemental Table 1). Intensity of only four symptoms (joint/muscle pain, hot flashes, vaginal dryness/insufficient lubrication, and vaginal discharge) were reported by more than two studies. Moderate to severe joint/muscle pain was reported by 31.5% to 46% of participants.23, 25, 45, 54 The range of mean intensity scores for joint/muscle pain was 4.9 to 5.4 out of 10.20, 40, 61 Reports ranging from 19.7% to 53% of participants reported moderate to severe hot flashes.16, 18, 40, 63 There were 20.6% to 32.8% of participants reported moderate to severe vaginal dryness/insufficient lubrication.16, 24, 40 Moderate to severe vaginal discharge was reported by 4% to 17.6% of participants across studies.16, 24 Only 3 symptoms have more than two studies reporting distress including joint/muscle pain, hot flashes, and pain with intercourse. Moderate or greater distress associated with joint/muscle pain was reported by 36% of participants.28 The mean distress with joint/muscle pain was 3.29 out of 10.61 The mean distress of hot flashes was 1.41 out of 4 and of pain with intercourse was 1.17 out of 4.52
Discussion
Symptoms are increasingly important self-reported outcomes during cancer treatment. Symptom science and self-management are listed as research priorities of both the National Institutes of Health (NIH) and National Institute of Nursing Research (NINR). However, the science of the symptom experience during endocrine therapy remains underdeveloped. Heterogeneity across symptom assessment instruments and methodological limitations across completed studies underscore the inability to integrate the evidence and better understand the phenomenon of symptom experience during endocrine therapy for breast cancer.
Instrumentations for Symptoms during Endocrine Therapy
In this scoping review, considerable methodological heterogeneity was identified across the included 57 articles, including variance in study design, symptom assessment instruments, symptom measurement recall period, data collection procedures and sample characteristics (e.g., ethnicity, menopause status, previous treatments, cancer stage, etc.). The biggest barrier to the comparison of results across studies is the heterogeneity of symptom assessment instruments. Given the consensus related to the experience of multiple co-occurring symptoms, a self-reported symptom questionnaire/checklist is a plausible approach to efficient assessment of concurrent symptoms. Unfortunately, although the three most commonly used self-reported symptom questionnaires/checklists (BCPT, FACT-ES, and MDASI) have been reported to be reliable and valid,70–72 none of them assesses symptoms associated with endocrine therapy comprehensively in terms of the types of symptoms experienced and the occurrence, intensity and distress associated with those symptoms. Table 2 shows the coverage of the 16 most commonly studied symptoms among these three commonly used symptoms questionnaires/checklists. The FACT-ES covers 14/16 symptoms, the BCPT symptom checklist covers 12/16 symptoms and the MDASI covers 5/16 symptoms. Table 3 shows the coverage of the 15 symptoms with the highest occurrences among the three symptom checklists. The BCPT and FACT-ES both cover 6/15 symptoms. The MDASI covers 2/15 symptoms. Compared to the BCPT and FACT-ES, the MDASI covers far fewer symptoms as illustrated in Tables 3 and 4. This is most probably due to the fact that the MADSI is not an endocrine therapy specific symptom assessment. However, only the MDASI comprehensively assesses the three domains of symptoms. The BCPT assesses symptom distress, the FACT-ES assesses occurrence and intensity, and the MDASI assesses occurrence, intensity and distress. In addition, (Table 3) six symptoms with high occurrence rates in women receiving endocrine therapy are not included in any of the three instruments, including eye irritation,19 heart discomfort,40 sexual arousal problem/orgasmic dysfunction,17, 29 dyspareunia,17 urinary urgency,24 and dry eye syndrome.38 Interestingly, each of these six non-included symptoms were reported by only one or two studies. Given the high occurrence rates, future studies should insure the inclusion of these six symptoms. In addition, more studies are needed to confirm the robustness of the high occurrence of these six symptoms.
Table 2.
Coverage of 16 Mostly Studied Symptoms in BCPT Symptom Checklist, FACT-ES, and MDASI
| BCPT | FACT-ES | MDASI | |
|---|---|---|---|
| 1. Joint/Muscle Pain | √ | √ | - |
| 2. Hot Flashes | √ | √ | - |
| 3. Vaginal Dryness/Insufficient Lubrication | √ | √ | - |
| 4. Sleep Disorder/Insomnia | - | √ | √ |
| 5. Fatigue/Lack of Energy | - | √ | √ |
| 6. Nausea | √ | √ | √ |
| 7. Vaginal Bleeding or Spotting | √ | √ | - |
| 8. Headaches/Migraines | √ | √ | - |
| 9. Irritability | √ | √ | - |
| 10. Joint/Muscle Stiffness | √ | - | - |
| 11. Weight Gain | √ | √ | - |
| 12. Vaginal Discharge | √ | √ | - |
| 13. Depression/Depressive Mood | - | √ | √ |
| 14. Low Sexual Interest/Desire | - | √ | - |
| 15. Difficulty Breathing/Short of Breath | √ | - | √ |
| 16. Dizziness/Faintness | √ | √ | - |
Included in the instrumentation
Not included in the instrumentation
Abbreviations: BCPT Symptom Checklist, Breast Cancer Prevention Trial Symptom Checklist; FACT-ES, Functional Assessment of Cancer; MDASI, The M. D. Anderson Symptom Inventory.
Table 3.
Coverage of 15 Most Prevalent Symptoms in BCPT Symptom Checklist, FACT-ES, and MDASI
| BCPT | FACT-ES | MDASI | |
|---|---|---|---|
| 1. Cramps | √ | - | - |
| 2. Hot Flashes | √ | √ | - |
| 3. Fatigue/Lack of Energy | - | √ | √ |
| 4. Eye Irritation | - | - | - |
| 5. Heart Discomfort | - | - | - |
| 6. Joint/Muscle Pain | √ | √ | - |
| 7. Night Sweats | √ | √ | - |
| 8. Sexual Arousal Problem/Orgasmic Dysfunction | - | - | - |
| 9. Anxiety | - | √ | - |
| 10. Dyspareunia | - | - | - |
| 11. Low Sexual Interest/Desire | - | √ | - |
| 12. Joint/Muscle Stiffness | √ | - | - |
| 13. Urinary Urgency | - | - | - |
| 14. Numbness or Tingling | √ | - | √ |
| 15. Dry Eye Syndrome | - | - | - |
Included in the instrumentation
Not included in the instrumentation
Abbreviations: BCPT Symptom Checklist, Breast Cancer Prevention Trial Symptom Checklist; FACT-ES, Functional Assessment of Cancer; MDASI, The M. D. Anderson Symptom Inventory.
Methodological Limitations in the Current Studies
In this scoping review, we identified several methodological issues that preclude comprehensively understanding of symptoms during endocrine therapy for breast cancer.
Firstly, most of the current studies used cross-sectional designs. Cross-sectional design precludes the possibility of examining causal relationships related to factors that may be associated with symptoms during endocrine therapy. Moreover, the onset time and shape of trajectories of symptoms remained understudied due to the lack of longitudinal studies. In addition, the few longitudinal studies that have been reported only included follow-up periods up to 24 months,40 a relatively short time frame relative to the 5–10 years of endocrine therapy typically recommended by the NCCN Guideline. Due to the relative short follow-up time, the trajectories of symptoms during the course of endocrine therapy are not fully described.
Secondly, there is a considerable variance in recall period. The recall period can affect the accuracy and comparability of symptom outcomes. However, the optimal recall period of symptoms is still under controversy; a shorter recall period (e.g., 3 days in children and 4 days in adults) may help assess symptoms occurrence accurately73 but may underestimate symptoms distress when symptoms have diurnal fluctuation.74
Thirdly, there is a lack of definitions of symptoms in the current studies. The wording of one symptom vary among different studies. For example, lack of energy, low energy, feeling tired, physical and mental exhaustion, and fatigue are used by different studies.8, 18, 34, 37, 40, 44, 51, 55, 56 Without a clear definition, it is not rigorous to treat them as one symptom. Moreover, it also remains arguable that whether or not the outcomes from one item of a symptom checklist and a series of items of a questionnaire for one symptom are equivalent. In addition, the definitions of the extents of intensity/distress (e.g., mild, moderate, severe, very severe) are not defined in most of the current studies, especially in the studies using symptoms checklist to assess multiple concurrent symptoms.
Lastly, there is a lack of theoretical guidance for the symptom related studies during endocrine therapy. Theoretical frameworks established for examining symptoms (e.g., the UCSF Symptom Management Theory (SMT), Symptom Experience Model (SEM), NIH Symptoms Science Model, etc.) should be encouraged in future studies.14, 75, 76
Other Gaps of Current Studies
None of the included studies adopted common data elements (CDE). The National Institute of Nursing Research (NINR) recommended six symptoms (pain, fatigue, sleep disturbance, mood, anxiety, and cognitive disturbance) as common data elements for symptoms studies.77 However, these symptoms were not well assessed and reported in the studies on symptoms during endocrine therapy. This impedes further comparison of symptoms results both among studies for endocrine therapy, but also among different types of cancer treatments.
Symptom cluster and trajectory patterns are understudied in symptoms during endocrine therapy for breast cancer. The numbers of research on symptom clusters in cancer patients are exponentially increasing.78 However, the symptom cluster in endocrine therapy for breast cancer is poorly studied. None of the identified studies in this scoping review is aiming to identify symptom clusters. The vast majority of current studies on symptom clusters are focusing on cancer patients in the period of surgery, chemotherapy and radiation therapy. Patients under endocrine therapy are rarely included. It is the same situation in the research focusing on trajectory patterns and high-risk subgroup membership. With an insufficient understanding of phenotypic characteristics of symptoms during endocrine therapy, there is a lack of studies further exploring underlying mechanisms of symptom clusters and phenotypic variant associated with endocrine therapy.
Strengths and Limitations
To the authors’ knowledge, this is the first scoping review to map the multiple symptoms experienced during endocrine therapy for breast cancer. Furthermore, the methodology and data charting process were both framework-guided, which assured the rigorousness of this scoping review. In addition, including both English and Chinese language published articles facilitates broadening the scope of the results of related studies.
However, this review should be taken within the context of several limitations. The limitations include: 1) studies published in languages other than English and Chinese and unpublished studies were not included; 2) by only focusing on quantitative research, the meaning domain of the symptom experience was not included and discussed in this scoping review.
Implication for Clinical Practice and Future Research
For the implications for clinical practice, this scoping review identified 5 well-studied and highly prevalent symptoms which should be assessed in women with breast cancer receiving endocrine therapy. These five symptoms are joint/muscle pain, hot flashes, low sexual interest/desire, joint/muscle stiffness, and fatigue/lack of energy. Moreover, some rarely studied but highly prevalent symptoms should also be assessed, including cramps, eye irritation, heart discomfort, anxiety, dyspareunia, urinary urgency, numbness and tingling, and dry eye syndrome. When assessing symptoms, nurses should evaluate the frequency of occurrence, intensity, and distress of key symptoms to have a clear and comprehensive understanding of the symptom experience during endocrine therapy in women with breast cancer. Nurses should also assess the influence of symptoms experienced on the quality of life and functional ability of women receiving endocrine therapy.
Given the state of the science related to symptoms experienced by women with breast cancer receiving endocrine therapy, there are several implications for future research. Firstly, compared to the occurrence domain, there is a dearth of research addressing the intensity and distress domains of symptoms. There is a considerable need for studies to comprehensively determine the frequency of occurrence, intensity of symptoms, symptom distress and the impact of symptoms on functional ability and quality of life. Secondly, since the heterogeneity of instruments significantly affects the comparison of results across studies, a symptoms questionnaire/checklist encompassing multiple domains of endocrine therapy specific symptoms is urgently needed. Meanwhile, use of common data elements should be encouraged in future studies on symptoms during endocrine therapy. An optimal recall period and clear definitions of symptoms should be studied and standardized in the future studies. Thirdly, this scoping review indicates that more research is needed investigating rarely studied but highly prevalent symptoms, such as cramps, eye irritation, heart discomfort, anxiety, dyspareunia, urinary urgency, numbness and tingling, and dry eye syndrome, to confirm the robustness of the current evidence. Lastly, more studies are needed to determine the symptoms clusters that occur in women receiving endocrine therapy and the trajectory patterns of symptoms (such as joint pain) during endocrine therapy. In addition, more studies to determine the mechanisms underlying the symptoms/symptom clusters, and phenotypic variance should be conducted to gain a deeper understanding of symptoms during endocrine therapy as the basis for the development of symptom management interventions.
Conclusions
In this scoping review, five key symptoms associated with endocrine therapy were identified, including joint/muscle pain, hot flashes, low sexual interest/desire, joint/muscle stiffness, and fatigue/lack of energy. These symptoms should be included in clinical practice and future studies of endocrine therapy for breast cancer. There remain substantial gaps in the science related to the symptom experience during endocrine therapy for breast cancer, especially for the domains of symptom intensity and distress, specific understudied symptoms and for symptom clusters. Investigations examining rarely studied but highly prevalent symptoms (e.g., cramps, eye irritation, heart discomfort, anxiety, dyspareunia, urinary urgency, numbness and tingling, and dry eye syndrome) are needed. Future studies on symptom clusters, individual variants of certain symptoms, and focused symptom assessment instruments are urgently needed.
Supplementary Material
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romera JL, Hernández TD, Fernández IP, et al. Update on adjuvant hormonal treatment of early breast cancer. Adv Ther. 2011;28(S6):1–18. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines of Breast Cancer (Version 2.2017). https://www.nccn.org/professionals/physician_gls/PDF/breast.pdf [Google Scholar]
- 7.Rugo HS, Rumble RB, Burstein HJ. Endocrine therapy for hormone receptor positive metastatic breast cancer: American Society of Clinical Oncology guideline summary. J Oncol Pract. 2016;12(6):583–587. [DOI] [PubMed] [Google Scholar]
- 8.Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012;8(6):e149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell KM, Harte SE, Hayes DF, et al. Patient‐reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120(16):2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olufade T, Gallicchio L, MacDonald R, Helzlsouer KJ. Musculoskeletal pain and health-related quality of life among breast cancer patients treated with aromatase inhibitors. Support Care Cancer. 2015;23(2):447–455. [DOI] [PubMed] [Google Scholar]
- 11.Fallowfield L, Atkins L, Catt S, et al. Patients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol. 2005;17(2):205–210. [DOI] [PubMed] [Google Scholar]
- 12.Ruhstaller T, Von Moos R, Rufibach K, et al. Breast cancer patients on endocrine therapy reveal more symptoms when self-reporting than in pivotal trials: an outcome research study. Oncology. 2009;76(2):142–148. [DOI] [PubMed] [Google Scholar]
- 13.Khalil H, Peters M, Godfrey CM, McInerney P, Soares CB, Parker D. An evidence-based approach to scoping reviews. Worldviews Evid Based Nurs. 2016;13(2):118–123. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong TS. Symptoms experience: a concept analysis. Oncol Nurs Forum. 2003; 30(4), 601–606. [DOI] [PubMed] [Google Scholar]
- 15.Ashraf M, Biswas J, Majumdar S, et al. Tamoxifen use in Indian women--adverse effects revisited. Asian Pac J Cancer Prev. 2009;10(4):609–612. [PubMed] [Google Scholar]
- 16.Baumgart J, Nilsson K, Stavreus-Evers A, et al. Urogenital disorders in women with adjuvant endocrine therapy after early breast cancer. Am J Obstet Gynecol. 2011;204(1):26 e21–27. [DOI] [PubMed] [Google Scholar]
- 17.Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20(2):162–168. [DOI] [PubMed] [Google Scholar]
- 18.Baxter SD, Teft WA, Choi YH, Winquist E, Kim RB. Tamoxifen-associated hot flash severity is inversely correlated with endoxifen concentration and CYP3A4* 22. Breast Cancer Res Treat. 2014;145(2):419–428. [DOI] [PubMed] [Google Scholar]
- 19.Boehm DU, Lebrecht A, Eckhardt T, et al. Quality of life and adjuvant tamoxifen treatment in breast cancer patients. Eur J Cancer Care. 2009;18(5):500–506. [DOI] [PubMed] [Google Scholar]
- 20.Boonstra A, van Zadelhoff J, Timmer-Bonte A, Ottevanger PB, Beurskens CH, van Laarhoven HW. Arthralgia during aromatase inhibitor treatment in early breast cancer patients: prevalence, impact, and recognition by healthcare providers. Cancer Nurs. 2013;36(1):52–59. [DOI] [PubMed] [Google Scholar]
- 21.Brown JC, Mao JJ, Stricker C, Hwang WT, Tan KS, Schmitz KH. Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast J. 2014;20(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castel LD, Hartmann KE, Mayer IA, et al. Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer. 2013;119(13):2375–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer. 2009;9(2):108–117. [DOI] [PubMed] [Google Scholar]
- 25.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877–3883. [DOI] [PubMed] [Google Scholar]
- 26.Desai K, Mao JJ, Su I, et al. Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support Care Cancer. 2013;21(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dizdar O, Özçakar L, Malas FÜ,et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor–related arthralgia. J Clin Oncol. 2009;27(30):4955–4960. [DOI] [PubMed] [Google Scholar]
- 28.Egawa C, Hirokaga K, Takao S, et al. Risk factors for joint symptoms in postmenopausal Japanese breast cancer patients treated with anastrozole: a prospective multicenter cohort study of patient-reported outcomes. Int J Clin Oncol. 2016;21(2):262–269. [DOI] [PubMed] [Google Scholar]
- 29.Frechette D, Paquet L, Verma S, et al. The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: encouraging results from a prospective study. Breast Cancer Res Treat. 2013;141(1):111–117. [DOI] [PubMed] [Google Scholar]
- 30.Gallicchio L, MacDonald R, Wood B, Rushovich E, Fedarko NS, Helzlsouer KJ. Changes in bone biomarker concentrations and musculoskeletal symptoms among breast cancer patients initiating aromatase inhibitor therapy and women without a history of cancer. J Bone Miner Res. 2012;27(9):1959–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallicchio L, MacDonald R, Wood B, Rushovich E, Helzlsouer KJ. Menopausal-type symptoms among breast cancer patients on aromatase inhibitor therapy. Climacteric. 2012;15(4):339–349. [DOI] [PubMed] [Google Scholar]
- 32.Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast Cancer Res Treat. 2013;142(2):435–443. [DOI] [PubMed] [Google Scholar]
- 33.Garreau JR, DeLaMelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192(4):496–498. [DOI] [PubMed] [Google Scholar]
- 34.Hadji P, Jackisch C, Bolten W, et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann Oncol. 2013;25(2):372–377. [DOI] [PubMed] [Google Scholar]
- 35.Horimoto Y, Saito M, Kasumi F. Arthralgia in 329 patients taking aromatase inhibitors. Breast Care. 2009;4(5):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu G, Zhan H, Hu R. Side effects associated with endocrine therapy in premenopausal patients with breast cancer-related research outcomes. Shanxi Medical Journal. 2016;45(9), 1007–1010. [Google Scholar]
- 37.Huang X, Zhang Q, Kang X, Song Y, Zhao W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: a cross-sectional study. BMC Cancer. 2010;10(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inglis H, Boyle FM, Friedlander ML, et al. Dry eyes and AIs: If you don’t ask you won’t find out. Breast. 2015;24(6), 694–698. [DOI] [PubMed] [Google Scholar]
- 39.Kanti V, Nuwayhid R, Lindner J, et al. Evaluation of trichodynia (hair pain) during chemotherapy or tamoxifen treatment in breast cancer patients. J Eur Acad Dermatol Venereol. 2016;30(1):112–118. [DOI] [PubMed] [Google Scholar]
- 40.Kyvernitakis I, Ziller V, Hars O, Bauer M, Kalder M, Hadji P. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer. Climacteric. 2014;17(3):252–259. [DOI] [PubMed] [Google Scholar]
- 41.Laroche F, Coste J, Medkour T, et al. Classification of and risk factors for estrogen deprivation pain syndromes related to aromatase inhibitor treatments in women with breast cancer: a prospective multicenter cohort study. J Pain. 2014;15(3):293–303. [DOI] [PubMed] [Google Scholar]
- 42.Lintermans A, Van Asten K, Wildiers H, et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Res Treat. 2014;146(1):109–116. [DOI] [PubMed] [Google Scholar]
- 43.Lintermans A, Vanderschueren D, Verhaeghe J, et al. Arthralgia induced by endocrine treatment for breast cancer: a prospective study of serum levels of insulin like growth factor-I, its binding protein and oestrogens. Eur J Cancer. 2014;50(17):2925–2931. [DOI] [PubMed] [Google Scholar]
- 44.Lu H, Li Q, Xu B, et al. Effects of anastrozole on lipid metabolism in Chinese postmenopausal women with breast cancer. Chinese J Oncol. 2011;33(7), 520–525. [PubMed] [Google Scholar]
- 45.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115(16):3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao JJ, Su HI, Feng R, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res Treat. 2011;13(1):R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menas P, Merkel D, Hui W, Lawton J, Harper A, Carro G. Incidence and management of arthralgias in breast cancer patients treated with aromatase inhibitors in an outpatient oncology clinic. J Oncol Pharm Pract. 2012;18(4):387–393. [DOI] [PubMed] [Google Scholar]
- 48.Mortimer JE, Boucher L, Baty J, Knapp DL, Ryan E, Rowland JH. Effect of tamoxifen on sexual functioning in patients with breast cancer. J Clin Oncol. 1999;17(5):1488. [DOI] [PubMed] [Google Scholar]
- 49.Mortimer JE, Flatt SW, Parker BA, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108(3):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Napoli N, Vattikuti S, Ma C, et al. High prevalence of low vitamin D and musculoskeletal complaints in women with breast cancer. Breast J. 2010;16(6):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberguggenberger A, Hubalek M, Sztankay M, et al. Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat. 2011;128(2):553–561. [DOI] [PubMed] [Google Scholar]
- 52.Ochayon L, Tunin R, Yoselis A, Kadmon I. Symptoms of hormonal therapy and social support: is there a connection? Comparison of symptom severity, symptom interference and social support among breast cancer patients receiving and not receiving adjuvant hormonal treatment. Eur J Oncol Nurs. 2015;19(3):260–267. [DOI] [PubMed] [Google Scholar]
- 53.Ohsako T, Inoue K, Nagamoto N, Yoshida Y, Nakahara O, Sakamoto N. Joint symptoms: a practical problem of anastrozole. Breast Cancer. 2006;13(3):284–288. [DOI] [PubMed] [Google Scholar]
- 54.Presant CA, Bosserman L, Young T, et al. Aromatase inhibitor–associated arthralgia and/or bone pain: frequency and characterization in non–clinical trial patients. Clin Breast Cancer. 2007;7(10):775–778. [DOI] [PubMed] [Google Scholar]
- 55.Ribi K, Bernhard J, Rufibach K, et al. Endocrine symptom assessment in women with breast cancer: what a simple “yes” means. Support Care Cancer. 2007;15(12):1349–1356. [DOI] [PubMed] [Google Scholar]
- 56.Ruhstaller T, Von Moos R, Rufibach K, et al. Breast cancer patients on endocrine therapy reveal more symptoms when self-reporting than in pivotal trials: an outcome research study. Oncology. 2009;76(2):142–148. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist. 2015;20(6):598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sagara Y, Kosha S, Baba S, et al. Adverse events and bone health during anastrozole therapy in postmenopausal Japanese breast cancer patients. Breast Cancer. 2010;17(3):212–217. [DOI] [PubMed] [Google Scholar]
- 59.Schover LR, Baum GP, Fuson LA, Brewster A, Melhem‐Bertrandt A. Sexual problems during the first 2 years of adjuvant treatment with aromatase inhibitors. J Sex Med. 2014;11(12):3102–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Servitja S, Nogués X, Prieto-Alhambra D, et al. Bone health in a prospective cohort of postmenopausal women receiving aromatase inhibitors for early breast cancer. Breast. 2012;21(1):95–101. [DOI] [PubMed] [Google Scholar]
- 61.Shi Q, Giordano SH, Lu H, Saleeba AK, Malveaux D, Cleeland CS. Anastrozole-associated joint pain and other symptoms in patients with breast cancer. J Pain. 2013;14(3):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singer O, Cigler T, Moore AB, et al. Defining the aromatase inhibitor musculoskeletal syndrome: a prospective study. Arthritis Care Res. 2012;64(12):1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su HI, Sammel MD, Springer E, Freeman EW, DeMichele A, Mao JJ. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res Treat. 2010;124(1):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swenson KK, Nissen MJ, Henly SJ, et al. Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors. Oncol Nurs Forum. 2013;40(6), 549–557. [DOI] [PubMed] [Google Scholar]
- 65.Waltman NL, Ott CD, Twiss JJ, Gross GJ, Lindsey AM. Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nurs. 2009;32(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Lu K, Song Y, et al. Indications of clinical and genetic predictors for aromatase inhibitors related musculoskeletal adverse events in Chinese Han women with breast cancer. PloS One. 2013;8(7):e68798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L, Wang J, Xue DD, He W. Aromatase inhibitors associated musculoskeletal disorders and bone fractures in postmenopausal breast cancer patients: a result from Chinese population. Medical Oncology. 2014;31(9):128. [DOI] [PubMed] [Google Scholar]
- 68.Zhan M, Flaws JA, Gallicchio L, Tkaczuk K, Lewis LM, Royak-Schaler R. Profiles of tamoxifen-related side effects by race and smoking status in women with breast cancer. Cancer Detect Prev. 2007;31(5):384–390. [DOI] [PubMed] [Google Scholar]
- 69.Zhou LH, Liu GY, Di GH, Lu JS, Shen ZZ, Shao ZM. Safety of sequential exemestane therapy after 2–3 years’ tamoxifen treatment in postmenopausal women with hormone sensitive breast cancer. Tumor. 2011;31(4):354–358. [Google Scholar]
- 70.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 71.Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: Validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):187–197. [DOI] [PubMed] [Google Scholar]
- 72.Terhorst L, Blair-Belansky H, Moore PJ, Bender C. Evaluation of the psychometric properties of the BCPT Symptom Checklist with a sample of breast cancer patients before and after adjuvant therapy. Psycho-oncology. 2011;20(9):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39(2):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norquist JM, Girman C, Fehnel S, DeMuro-Mercon C, Santanello N. Choice of recall period for patient-reported outcome (PRO) measures: criteria for consideration. Qual Life Res. 2012;21(6):1013–1020. [DOI] [PubMed] [Google Scholar]
- 75.Cashion AK, Grady PA. The National institutes of health/national institutes of nursing research intramural research program and the development of The National institutes of health symptom science model. Nurs Outlook. 2015;63(4):484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. [DOI] [PubMed] [Google Scholar]
- 77.Redeker NS, Anderson R, Bakken S, et al. Advancing symptom science through use of common data elements. J Nurs Scholarsh. 2015;47(5):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miaskowski C Future directions in symptom cluster research. Seminars Oncol Nurs. 2016; 32(4), 405–415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


