Abstract
This case highlights the first reported association of doxorubicin with takotsubo cardiomyopathy (TC) presenting as cardiogenic shock during the first continuous infusion in a patient with adult T‐cell leukemia/lymphoma. We aim to raise awareness to recognize and distinguish between irreversible doxorubicin‐associated cardiomyopathy and reversible doxorubicin‐associated TC in patients with cancer.
Keywords: cancer, chemotherapy, doxorubicin, takotsubo cardiomyopathy
This case highlights the first reported association of doxorubicin with takotsubo cardiomyopathy (TC) presenting as cardiogenic shock during the first continuous infusion in a patient with adult T‐cell leukemia/lymphoma. We aim to raise awareness to recognize and distinguish between irreversible doxorubicin‐associated cardiomyopathy and reversible doxorubicin‐associated TC in patients with cancer.
1. INTRODUCTION
Stress‐induced cardiomyopathy, also known as takotsubo cardiomyopathy (TC), is a transient systolic dysfunction triggered by emotional or physical stress. Presentation is similar to myocardial infarction, however, with the absence of obstructive coronary artery disease (CAD). The most common presenting symptom is chest pain, although heart failure can be the initial presentation in some patients and approximately 10% of these patients may develop cardiogenic shock.1 TC was first described in Japan in 1990 and has been increasingly recognized over the last 25 years.2 A review of the International Takotsubo Registry shows that the disease is more common in women (~90%) with a mean age of 67, and triggers can be physical (~35%), emotional (27%‐37%), or unknown (~25%).1, 3 Due to uncertainty in diagnosis, the Mayo Clinic has proposed four diagnostic criteria that need to be met to confirm a diagnosis of TC. These criteria include the following: transient regional left ventricle systolic dysfunction (hypokinesis, akinesis, or dyskinesis), absence of obstructive CAD, new electrocardiography (EKG) findings, or modest elevation in cardiac troponin and absence of pheochromocytoma or myocarditis.4 Recently, TC has been more frequently recognized in the field of oncology, not only due to the theoretical association with the emotional and physical burden of the disease, but a few case reports have also identified some chemotherapy agents as potential triggers of TC, most reports implicating fluorouracil.5
Doxorubicin is an intravenous chemotherapeutic agent of the anthracycline group, and its associated cardiotoxicity is well described in the literature.6, 7 The toxic effect on the myocardium is dependent on the doxorubicin cumulative dose (from 300 to 500 mg/m2) and most commonly causes dilated cardiomyopathy, which is irreversible and excludes patients from further treatment with anthracyclines.
To our knowledge, doxorubicin‐associated TC has not been reported in the literature and we herein describe the first case.
2. CASE PRESENTATION
A healthy 53‐year‐old Afro‐Caribbean woman presented with altered mental status, 20‐pound weight loss due to anorexia, and fatigue over 3 weeks. She reported no fever, headaches, chest pain, dyspnea, palpitations, or edema. In the emergency room, her blood pressure was 92/52 mm Hg, and her heart rate was 129 beats/min. Physical examination revealed lethargy and confusion without any focal neurological deficit. Her heart and lungs examinations were unremarkable. Examination of the lymph nodes revealed painless, small supraclavicular, and bilateral inguinal nodes. There was no rash or organomegaly. Her initial EKG showed sinus tachycardia (105 beats/min) and left ventricular hypertrophy with nonspecific T‐wave abnormalities in anterolateral leads (Figure 1A). Her initial laboratory studies were significant for leukocytosis (white blood count 88 000/μL [4500‐11 000 cell/μL]), renal failure (creatinine 4.8 mg/dL [0.5‐1.2 mg/dL]), and severe hypercalcemia (corrected calcium of 21 mg/dL [8.5‐10.2 mg/dL]). Peripheral blood smear was remarkable for increased white blood cells, predominantly atypical lymphocytes. Other laboratory findings were as follows: hemoglobin 15.3 g/dL (12.0‐16.0 g/dL), platelets 240 000 cells/μL (150 000‐450 000/μL), lactate 4.8 mmol/L (0.5‐1 mmol/L), parathyroid hormone level 6.0 ng/L (10‐65 ng/L), uric acid 18.0 mg/dL (2.4‐6.0 mg/dL), phosphorus 5.0 mg/dL (2.5‐4.5 mg/dL), potassium of 4.3 mg/dL (3.5‐5.5 mg/dL), and lactate dehydrogenase 2050 U/L (100‐250 U/L). Laboratory findings were consistent with tumor lysis syndrome (TLS). Computed tomography scans of the head, neck, chest, abdomen, and pelvis showed diffuse lytic lesions involving the skull, sternum and clavicles, and bilateral enlarged axillary, para‐aortic, and inguinal lymph nodes with the largest being 1.1 cm.
Figure 1.
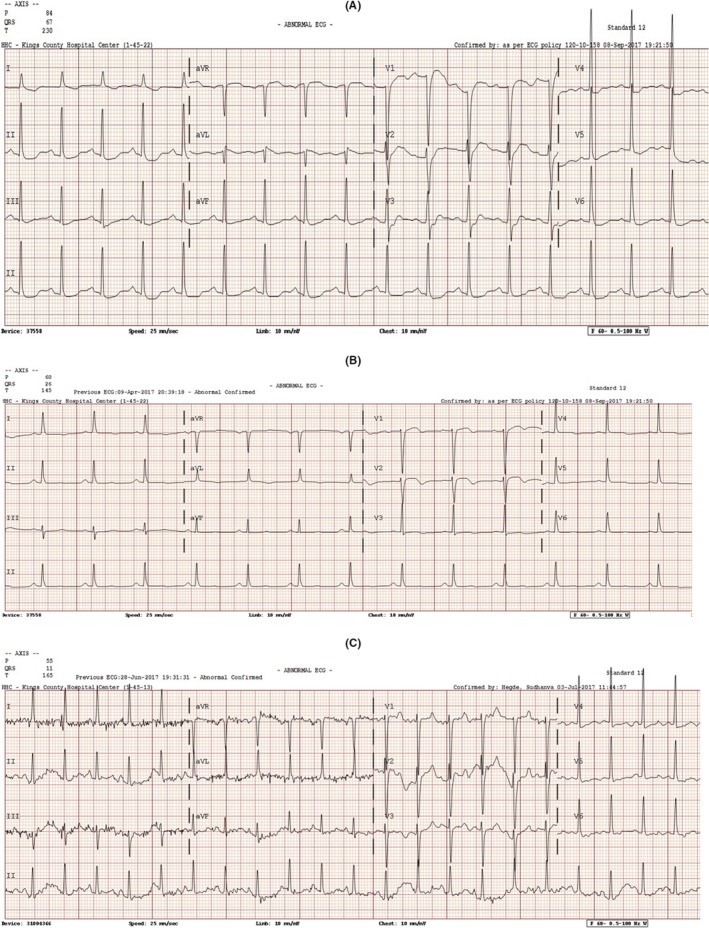
EKG on admission (A), after chemotherapy (B), and at recovery 3 mo later (C)
The patient was admitted to the intensive care unit for management of severe TLS and hypercalcemia while diagnostic testing was ongoing to confirm a malignant diagnosis. Bone marrow biopsy revealed hypercellular marrow with 20% atypical lymphoid cells. Immunohistochemical stains were positive for CD3, CD5, CD4, and CD25 cells and negative for CD2, CD7, CD8, and FOXP3. Concurrent flow cytometry showed abnormal CD4 positive T‐cell population confirming a T‐cell clonal neoplasm. Peripheral blood serology was positive for human T‐lymphotropic virus 1 (HTLV‐1), and a diagnosis of adult T‐cell leukemia/lymphoma (ATLL)‐acute type was made.
Baseline transthoracic echocardiogram (TTE) was normal with left ventricular ejection fraction (LVEF) 65%. The patient's clinical status improved after a week of treatment for TLS, with resolution of hypotension, tachycardia, hypercalcemia, renal failure, and hyperuricemia after aggressive hydration, calcitriol, denosumab, hemodialysis, and rasburicase. Due to medical stabilization and the aggressive course of ATLL and its associated dim prognosis, the decision was made to initiate chemotherapy inpatient.
Treatment with EPOCH chemotherapy regimen was initiated, consisting of etoposide 50 mg/m2/d, vincristine 0.4 mg/m2/d, doxorubicin 10 mg/m2/d (equivalent of 19.5 mg/d), all three given as continuous infusion over 4 days, with cyclophosphamide 750 mg/m2 given as single dose on day 5, and prednisone 100 mg on days 1‐5. Ten hours into the infusion of doxorubicin, etoposide, and vincristine, the patient developed hypoxia (70% on room air), tachycardia (152/min), and tachypnea (40/min) warranting airway protection and intubation. Chemotherapy infusion was discontinued. EKG showed new nonspecific T‐wave inversions in the V2‐V3 leads (Figure 1B). A bedside echocardiogram showed ballooning of the left ventricle and severe hypokinesis of the anterior and the anteroseptal walls, the other walls were mildly hypokinetic, and the estimated LVEF was 20%. Chest X‐ray showed fluid overload predominantly in the lower lungs with enlarged cardiac silhouette. N‐terminal pro‐brain natriuretic peptide was 7000 pg/mL (normal < 125 pg/mL), and peak troponins were mildly elevated at 0.7 ng/mL (normal < 0.01 ng/mL).
Patient was started on the vasopressor dobutamine and discontinued in <24 hours. She was managed for her heart failure with furosemide, as needed, hydralazine/isosorbide dinitrate, metoprolol, and lisinopril, which were both added 3 days later (all continued throughout her course). Due to the acuity of her heart failure, typical TTE findings, and temporal association with chemotherapy administration, the patient was diagnosed with TC secondary to chemotherapy. Invasive diagnostic testing for acute coronary syndrome was not pursued due to the patient's low cardiovascular risk, medical instability, rapid improvement with medical management, deteriorating kidney function, and clinical picture consistent with TC. A repeat EKG showed changes similar to the baseline EKG (Figure 1C). Due to its known cardiotoxic effects, doxorubicin was considered the likely cause of her TC and hence discontinued from her chemotherapy regimen. Chemotherapy without doxorubicin was resumed, and the patient completed the first cycle of chemotherapy 4 days later without complications. Of note, a repeat TTE 3 months later showed improvement of the patient's LVEF to 45% and resolution of the regional wall abnormalities. In total, she completed 4 cycles of chemotherapy over a 12‐week period (without doxorubicin) with improvement in her leukocytosis and clinical status. Her treatment course was later complicated by recurrent neutropenic fever and sepsis leading to the decision to manage supportively without chemotherapy. The patient eventually expired after developing septic shock.
3. DISCUSSION
To our knowledge, this is the first reported case of TC associated with EPOCH chemotherapy regimen, specifically doxorubicin, presenting as cardiogenic shock hours after the first continuous infusion in a previously healthy woman who was diagnosed with ATLL.
Takotsubo cardiomyopathy is a transient systolic dysfunction triggered by emotional or physical stress. It typically presents similar to myocardial infarction with chest pain and dyspnea in the absence of obstructive CAD. TC can also present as heart failure, and approximately 10% of patients develop cardiogenic shock.1
We acknowledge that formal exclusion of obstructive CAD as per the Mayo Clinic criteria was not done in our case.4 However, invasive angiography was not performed due to the patient's medical instability and rapid improvement. Furthermore, although our patient is 53 years old and of Caribbean ethnicity, which can theoretically put her at some level of cardiovascular risk, this does not make our diagnosis of TC less likely, not only for all the aforementioned reasons, but also given that it is well described that many patients with TC can have concurrent CAD (15% in some studies). Additionally, the significant and transient clinical manifestations and regional echocardiographic abnormalities extending beyond the distribution of a single epicardial coronary artery (severe systolic dysfunction with left ventricular ejection fraction [LVEF] 20% and akinetic anterior and anteroseptal walls) are out of proportion to the degree of elevation in her troponin level, which makes obstructive CAD less likely.
Furthermore, there were no diagnostic findings suggestive of other etiologies to explain the cardiogenic shock in our patient. Clinical presentation did not correlate with the diagnosis of pheochromocytoma; therefore, a dedicated workup was not pursued for that. Although acute myocarditis is also among the differential of TC and cardiac MRI could help distinguish both entities, this was not available at our institution at the time and we think that myocarditis was unlikely in our case for many reasons. The mildly detected troponin level, the echocardiographic regional wall abnormalities, the rapid clinical improvement of heart failure, the gradual improvement in systolic function, as well as resolution of EKG changes and of regional wall abnormalities on follow‐up TTE, all support the diagnosis of TC and are not consistent with myocarditis. The fact that her LVEF did not completely recover after 3 months (LVEF 45%) is not unusual in TC and doesn't decrease its likelihood as it can take up to 6 months for many cases of TC to normalize their LVEF, especially the severe forms such as our patient who developed cardiogenic shock requiring dobutamine and intubation.
In recent years, TC has been increasingly recognized in patients with cancer; the prevalence of malignancy was first reported to be 18% when compared to matched cohorts with acute myocardial infarction and left ventricular dysfunction.8, 9 Both physical and emotional stressors have been identified as triggers for TC, with surgery being the most common.1, 3 Undoubtedly, a diagnosis of cancer can be a major stressful event in an individual's life, but the exact mechanism of TC in such cases remains poorly defined. The plausible factors discussed in the literature include a combination of stress‐induced catecholamine excess, associated paraneoplastic syndromes, and the use of chemotherapeutic agents.10 In our case, the emotional stress of receiving the diagnosis of an aggressive incurable cancer such as ATLL and the psychological burden of initiating chemotherapy urgently may have played a role in the pathophysiology of TC in our patient. As in any cancer patient with TC, it is always difficult to ascertain whether the development of TC is due to the underlying malignancy, a direct adverse effect of chemotherapy, catecholamine surge from either an emotional or physical stressors or combination of all. However, it is important to note the following pertinent observations in our case: Our patient was treated with chemotherapy more than a week and not immediately after she received her cancer diagnosis, she developed her cardiac event within hours of doxorubicin infusion, her LVEF improved after discontinuing doxorubicin, her heart failure resolved with medical treatment, and she was able to tolerate reinitiation of the rest of her chemotherapy regimen (doxorubicin excluded) without complications. All of these factors further support our hypothesis that the triggering factor for her TC was most likely doxorubicin. Since the etiology and pathophysiology of TC remain incompletely understood, we hope that this report and others will contribute to a better understanding of this disorder.
Our patient presented with TLS and hypercalcemia, which required hydration and could have played an additional role in her TC. However, our patient's baseline echocardiogram—which was normal—was done at a time when TLS was still active, her TLS had completely resolved, and she was medically stable and euvolemic at the time of chemotherapy administration.
Among chemotherapy agents, fluorouracil (5‐FU) has been identified as the most common agent associated with TC5, 11, 12, 13 and has been reported in at least 15 patients. Capecitabine, the oral prodrug of 5FU, has been reported in 5 other cases.14 Other described chemotherapies include paclitaxel,11 cisplatin,15 and high‐dose cytarabine.16 Additionally, recent reports have implicated some novel therapies as causative agents of TC. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), has been associated with TC in 2 patients.17 One case was also reported with axitinib (an oral inhibitor of multiple tyrosine kinases including VEGF receptor 18). Two other cases were reported with the widely used agent trastuzumab (monoclonal antibody against HER2 receptor) that is used mainly in breast cancer and is known to cause reversible cardiomyopathy.19, 20 The recent report of a TC case in a Japanese patient with ATLL associated with mogamulizumab (a novel monoclonal antibody against the chemokine receptor CCR4) raises the question about the possible though unlikely implication of the underlying disease increasing the risk of developing TC as in our case.21 Nonetheless, this highlights the need for more research and awareness from clinicians, especially cardiologists and oncologists.
Unlike doxorubicin‐associated TC, doxorubicin and anthracycline associated cardiotoxicity is well known. It generally consists of irreversible and dose‐dependent dilated cardiomyopathy,7, 22 and, less commonly, arrhythmias such as tachycardia and heart block.23, 24 The underlying mechanism of the cardiomyopathy involves reactive oxygen species formation due to the redox cycling ability of doxorubicin.25, 26, 27 Doxorubicin has also been shown to induce apoptosis, DNA damage, and inhibit protein synthesis, therefore leading to cardiac dysfunction, and dilated cardiomyopathy.28, 29, 30 In contrast, the mechanism of doxorubicin and other chemotherapy associated TC remains unknown, and we can only postulate that the emotional stress and increased sympathetic flow are contributors. Additionally, the fact that most reported cases of chemotherapy‐associated TC involve 5‐FU suggests a potential role for vasospasm in the pathophysiology of TC.
We acknowledge that in addition to doxorubicin, our patient also received vincristine and etoposide prior to the cardiac event. An argument could be made that a combination of the drugs was the inciting factor. However, unlike doxorubicin, vincristine, and cyclophosphamide have no reported cardiac side effects. We hypothesize that chemotherapeutic agents that are known to cause cardiotoxicity such as 5‐FU,31 which could trigger myocardial ischemia, have a higher risk of causing TC. It has been established that doxorubicin‐associated cardiomyopathy precludes further use of any cardiotoxic agent. However, the risks of resuming doxorubicin in patients with resolved cardiac toxicity such as TC is not known. A case report has been published of a patient with diffuse large B‐cell lymphoma who developed TC 5 hours after liposomal doxorubicin and was able to resume chemotherapy (including liposomal doxorubicin) with no recurrent cardiac issues.32 In contrast, our patient had an incurable disease (ATL) and a severe form of TC (cardiogenic shock). Therefore, given the severity of the event in our case, the risks/benefits balance, the lack of data to guide management, and our palliative treatment goals, we decided against re‐exposing our patient to doxorubicin. The other noncardiotoxic chemotherapy agents were resumed without recurrent or new cardiac events. We also acknowledge that the supportive medical treatment including angiotensin‐converting enzyme inhibitor and beta‐blockers may have contributed to the improvement of the patient's cardiac symptoms and her LVEF. However, these agents are indicated in severe forms of TC.
4. CONCLUSIONS
In conclusion, this represents the first reported case of TC occurring as cardiogenic shock hours after infusion of doxorubicin. The clinical presentation, transient EKG and echocardiogram regional wall abnormalities and rapid improvement with doxorubicin discontinuation and medical management make TC the most likely diagnosis. The temporal association with doxorubicin infusion and reintroduction of the rest of her chemotherapy regimen without doxorubicin further support our hypothesis that the most likely trigger of her TC was doxorubicin.
We hope that this case will raise awareness to recognize and distinguish between irreversible doxorubicin‐associated cardiomyopathy and reversible doxorubicin‐associated TC in patients with cancer. The development of doxorubicin‐associated cardiomyopathy requires permanent cessation of the drug while treatment can at least theoretically be resumed after recovery of cardiac function in chemotherapy‐associated TC in patients in whom it is potentially lifesaving. We also hope that our case will shed more light into the pathophysiology of TC in cancer patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHOR CONTRIBUTIONS
GM and MH: participated in collection of images and literature review and drafted the initial manuscript. BS: critically reviewed and edited the manuscript. CL and ET: critically reviewed and approved the final version of the manuscript.
Mubarak G, Haddadin M, Samra B, Luhrs C, Taiwo E. Doxorubicin‐associated takotsubo cardiomyopathy in a patient with adult T‐cell leukemia/lymphoma. Clin Case Rep. 2019;7:2466–2471. 10.1002/ccr3.2504
REFERENCES
- 1. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929. [DOI] [PubMed] [Google Scholar]
- 2. Sato H, Taiteishi H, Uchida T. Takotsubo‐type cardiomyopathy due to multivessel spasm In: Kodama K, Haze K, Hon M. eds. Clinical aspect of myocardial injury: From ischemia to heart failure. Tokyo, Japan: Kagakuhyouronsha; 1990:56. [Google Scholar]
- 3. Pelliccia F, Parodi G, Greco C, et al. Comorbidities frequency in Takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med. 2015;128(654):e11. [DOI] [PubMed] [Google Scholar]
- 4. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858. [DOI] [PubMed] [Google Scholar]
- 5. Grunwald MR, Howie L, Diaz LA Jr. Takotsubo cardiomyopathy and Fluorouracil: case report and review of the literature. J Clin Oncol. 2012;30(2):e11‐e14. [DOI] [PubMed] [Google Scholar]
- 6. Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981‐1988. [DOI] [PubMed] [Google Scholar]
- 7. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin‐induced congestive heart failure. Ann Intern Med. 1979;91:710. [DOI] [PubMed] [Google Scholar]
- 8. Burgdorf C, Kurowski V, Bonnemeier H, et al. Long‐term prognosis of the transient left ventricular dysfunction syndrome (Tako‐Tsubo cardiomyopathy): focus on malignancies. Eur J Heart Fail. 2008;10:1015‐1019. [DOI] [PubMed] [Google Scholar]
- 9. Desai R, Abbas SA, Goyal H, et al. Frequency of takotsubo cardiomyopathy in adult patients receiving chemotherapy (from a 5‐Year nationwide inpatient study). Am J Cardiol. 2019;123(4):667‐673. [DOI] [PubMed] [Google Scholar]
- 10. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539. [DOI] [PubMed] [Google Scholar]
- 11. Giza DE, Lopez‐Mattei J, Vejpongsa P, et al. Stress‐induced cardiomyopathy in cancer patients. Am J Cardiol. 2017;120(12):2284‐2288. [DOI] [PubMed] [Google Scholar]
- 12. Coen M, Rigamonti F, Roth A, Koessler T. Chemotherapy‐induced Takotsubo cardiomyopathy, a case report and review of the literature. BMC Cancer. 2017;17(1):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schweizer MT, Mehta R, Salgia R, Villaflor VM. Takotsubo cardiomyopathy in a patient with squamous cell esophageal carcinoma. J Clin Oncol. 2011;29(20):e598‐e600. [DOI] [PubMed] [Google Scholar]
- 14. Qasem A, Bin Abdulhak AA, Aly A, Moormeier J. Capecitabine‐induced takotsubo cardiomyopathy: a case report and literature review. Am J Ther. 2016;23(5):e1188‐e1192. [DOI] [PubMed] [Google Scholar]
- 15. Toyooka S, Akagi S, Furukawa M, et al. Takotsubo cardiomyopathy associated with pulmonary resections after induction chemoradiotherapy for non‐small cell lung cancer. Gen Thorac Cardiovasc Surg. 2012;60(9):599‐602. [DOI] [PubMed] [Google Scholar]
- 16. Baumann S, Huseynov A, Goranova D, et al. Takotsubo cardiomyopathy after systemic consolidation therapy with high‐dose intravenous cytarabine in a patient with acute myeloid leukemia. Oncol Res Treat. 2014;37(9):487‐490. [DOI] [PubMed] [Google Scholar]
- 17. Franco TH, Khan A, Joshi V, et al. Takotsubo cardiomyopathy in two men receiving bevacizumab for metastatic cancer. Ther Clin Risk Manag. 2008;4(6):1367‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ovadia D, Esquenazi Y, Bucay M, Bachier CR. Association between takotsubo cardiomyopathy and axitinib: case report and review of the literature. J Clin Oncol. 2015;33(1):e1‐3. [DOI] [PubMed] [Google Scholar]
- 19. Burgy M, Brossat H, Barthelemy P, et al. First report of trastuzumab treatment after postoperative Takotsubo cardiomyopathy. Anticancer Res. 2014;34(7):3579‐3582. [PubMed] [Google Scholar]
- 20. Khanji M, Nolan S, Gwynne S, et al. Tako‐Tsubo syndrome after trastuzumab ‐ an unusual complication of chemotherapy for breast cancer. Clin Oncol. 2013;25(5):329. [DOI] [PubMed] [Google Scholar]
- 21. Yamanaka S, Nakayama K, Tamai H, et al. Adult T‐cell leukemia‐lymphoma complicated by Takotsubo cardiomyopathy and HTLV‐1‐associated myelopathy after treatment with the anti‐CCR4 antibody mogamulizumab. Rinsho Ketsueki. 2017;58(4):309‐314. [DOI] [PubMed] [Google Scholar]
- 22. Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy‐induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749‐2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg JS, Cohen AJ, Wasserman AG, et al. Acute arrhythmogenicity of doxorubicin administration. Cancer. 1987;60(6):1213‐1218. [DOI] [PubMed] [Google Scholar]
- 24. Kilickap S, Akgul E, Aksoy S, et al. Doxorubicin‐induced second degree and complete atrioventricular block. Europace. 2005;7(3):227‐230. [DOI] [PubMed] [Google Scholar]
- 25. Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987;19(8):817. [DOI] [PubMed] [Google Scholar]
- 26. Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2‐mediated induction of cyclooxygenase‐2. J Biol Chem. 1999;274(8):5038. [DOI] [PubMed] [Google Scholar]
- 27. Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11(12):931. [DOI] [PubMed] [Google Scholar]
- 28. Zhu H, Sarkar S, Scott L, et al. Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress. React Oxyg Species. 2016;1(3):189‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double‐strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839. [DOI] [PubMed] [Google Scholar]
- 30. Capranico G, Tinelli S, Austin CA, et al. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132(1):43. [DOI] [PubMed] [Google Scholar]
- 31. Steger F, Hautman M, Kolbl O. 5‐FU‐induced cardiac toxicity ‐ an underestimated problem in radiooncology? Radiat Oncol. 2012;7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandez SF, Basra M, Canty JM Jr. Takotsubo cardiomyopathy following initial chemotherapy presenting with syncope and cardiogenic shock–a case report and literature review. J Clinic Experiment Cardiol. 2:124 10.4172/2155-9880.1000124 [DOI] [Google Scholar]


