Abstract
In renal tumors, suspicious for renal cell carcinoma, where there is any doubt and discrepancy between morphology and immune profile, we recommend performing further immunohistochemical staining for pan‐cytokeratin, S100, NSE, and inhibin‐alpha. Thus, follow‐up overtreatment can be avoided in cases of benign kidney tumors.
Keywords: carcinoma, case report, hemangioblastoma, kidney, renal cell, von Hippel‐Lindau disease
In renal tumors, suspicious for renal cell carcinoma, where there is any doubt and discrepancy between morphology and immune profile, we recommend performing further immunohistochemical staining for pan‐cytokeratin, S100, NSE, and inhibin‐alpha. Thus, follow‐up overtreatment can be avoided in cases of benign kidney tumors.
1. BACKGROUND
Hemangioblastoma is a benign tumor of mesenchymal cell proliferation and normally occurs in the central nervous system (CNS), mainly in the cerebellum. Most of these tumors emerge sporadically, while approximately 20%‐25% are associated with the von Hippel‐Lindau (VHL) disease.1, 2 VHL disease is a rare genetic disorder with mutations of the VHL tumor suppressor gene and can cause various benign and malignant tumors, particularly in the CNS but also in the internal organs.3
In some cases, hemangioblastomas are located externally usually combined with VHL disease. The VHL gene is located on chromosome 3p25 and encodes for a tumor suppressor protein. Mutations result in a loss of function of the protein complex, which causes an accumulation of hypoxia‐inducible factors. As a result, the transcription of hypoxia‐responsive genes involving cell proliferation, angiogenesis, erythropoiesis, and other proangiogenetic factors are initiated. These factors often cause the development of vascular tumors. 4
However, sporadic renal hemangioblastoma (RH) without VHL disease is very rare. The morphological character is more less the same as hemangioblastomas occurring in the CNS as they show both oval and polygonal cells with pale or eosinophilic cytoplasm. Typically, prominent vascularity with thin‐walled and thick‐walled blood vessels is seen. Hemangioblastomas can be easily misdiagnosed for a renal cell carcinoma because of similar histological and immunohistochemical features.5
So far, only 14 cases of RH have been reported. Compared to the World Health Organization (WHO) World Cancer Report 2014, kidney cancer is the ninth most common cancer in men and 14th most common cancer in woman with approximately 330 000 cases in 2012.6, 7 The differences in adjuvant treatment and prognosis make it important to be able to differentiate between the rarely occurring RH and the much more frequent renal cell carcinoma.
We present one of (very) few cases of an isolated RH, admitted to our Department of Urology, identifying its pathological features and discussing a review of the literature.
2. CASE PRESENTATION
In April 2016, a 72‐year‐old woman, with a renal mass in her left kidney was admitted to our department for further examination. An abdominal computed tomography (CT) scan showed a 4.2 × 3.6 × 4.3 cm large tumor in the lower pole of the kidney, with 20‐160 Hounsfield units with heterogeneous contrast enhancement, except for a hypodense region in the center (Figure 1). These findings aroused the suspicion of a renal cell carcinoma. A chest CT scan showed no evidence of metastasis.
Figure 1.
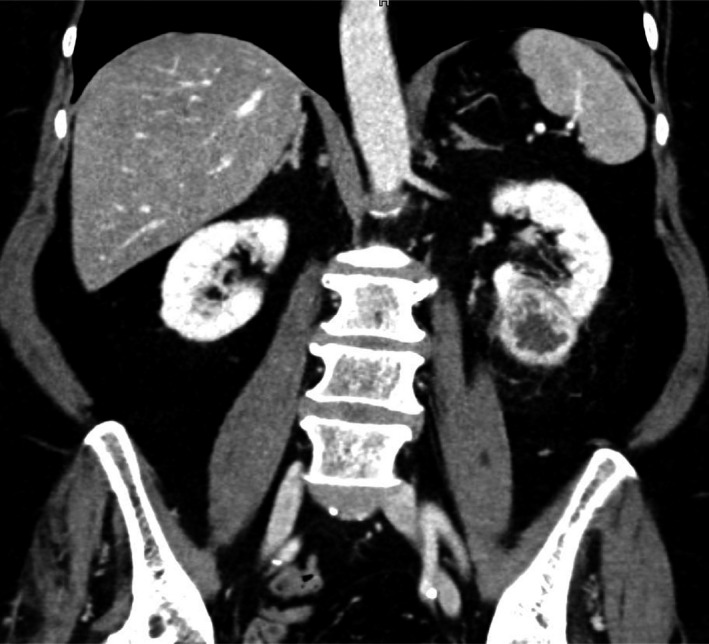
CT scan, preoperative. A 4.2 × 3.6 × 4.3 cm large mass in the lower pole of the left kidney
The patient was asymptomatic and did not show urinary symptoms, such as microhematuria or abdominal pain. Laboratory examination revealed normal findings. The patient's family history included two sisters with breast cancer and a father with lung cancer. Otherwise, there was no history of neoplastic disease for over four generations. In addition, renal scintigraphy was performed and revealed a general loss of renal function, with a calculated clearance of 75 mL/min, but no evidence of mechanical obstruction of the upper urinary tract.
Later on, the patient underwent a laparoscopic partial nephrectomy under general anesthesia. In warm ischemia, the tumor was excised within 25 minutes. After its removal, the specimen was put in a box with a mixture of water and formaldehyde and was send to our department for pathology. Next, gross examination and histology were performed (Figure 2). Paraffin blocks, H&E sections and further stainings for immunohistochemistry were sliced and pathological findings led us finally to diagnose a hemangioblastoma.
Figure 2.
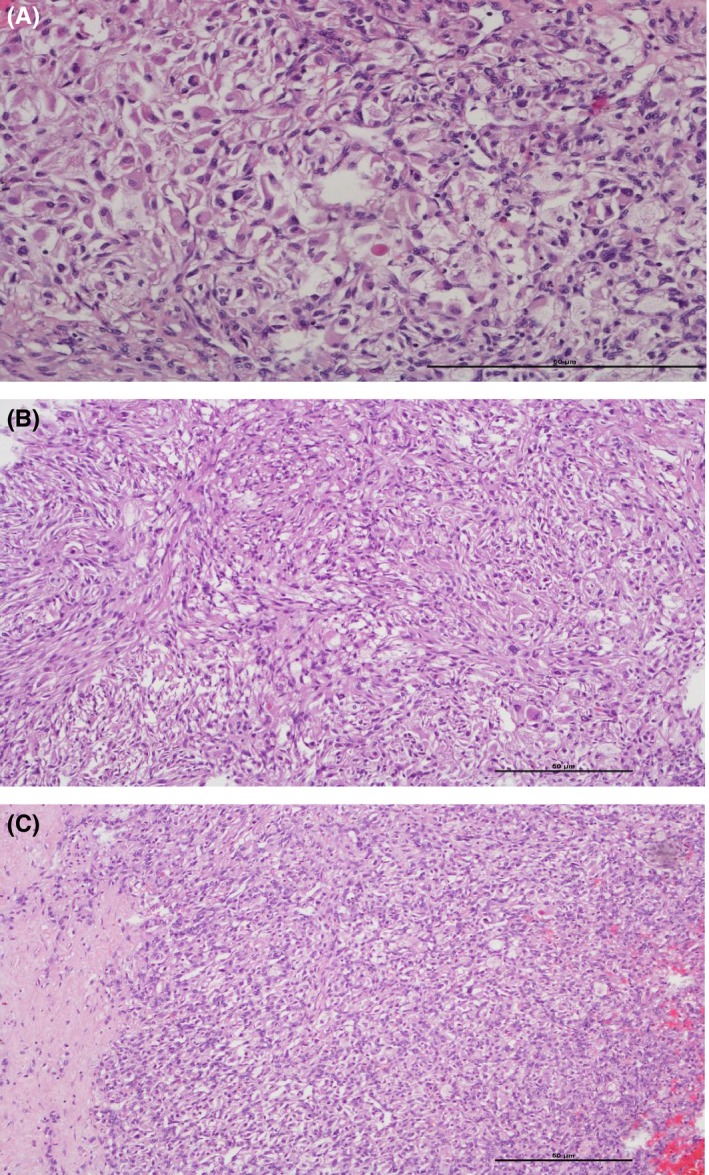
Histological examination demonstrated a renal hemangioblastoma with (A) rhabdoid morphology and clear cytoplasma, H&E stain, 200×. In addition the tumour showed (B) areas of spindle cells, H&E stain, 100×, as well as (C) a prominent vascularity and sharp demarcation, H&E stain, 100×
The hospital stay of the patient was uncomplicated and her laboratory findings were stable. After a good recovery, the patient was discharged on the fifth postoperative day. A follow‐up CT scan 6 months after partial nephrectomy revealed no evidence of recurrence.
In addition, a genetic examination for VHL disease was performed. It revealed normal findings for the DNA sequencing of the VHL‐coding exons and normal findings on the multiplex ligation‐dependent probe amplification analysis of the VHL gene. Therefore, there was no evidence of VHL disease.
2.1. Pathological findings
The histological findings showed a well‐demarcated tumor with polygonal and spindle cells with occasionally enlarged and bizarre nuclei. The cytoplasm was pale partly clear and in some regions, eosinophilic. Some tumor cells showed small eosinophilic globules and vacuoles in their cytoplasm. Rhabdoid features, necrosis, calcification, and hemorrhagic areas were also seen. The proliferation was accompanied by prominent vascularity. The proliferation rate of the tumor, as measured with Ki‐67, was low (5%). No mitotic activity was observed. In immunohistochemical findings, the tumor cells diffusely expressed S100, inhibin‐alpha, neuron‐specific‐enolase (NSE), CD10, and vimentin. Only focally isolated cells showed positivity with EMA and a weak but definite expression for PAX8 and WT1. SMA showed only focal staining in scattered tumor cells beside the expression of vessels, which was also demonstrated for CD34. Negative staining results were found for AE1/AE3, HMB‐45, melan A, myogenin, RCC, CK818, TFE, and desmin. Immunohistochemical data and general characteristics are summarized in Tables 1, 2, and 3.
Table 1.
Immunohistochemical markers
| Case report | CD10 | S100 | Vimentin | Inhibin | PAX8 | WT1 | Pan‐cytokeratin | NSE | EMA | Necrosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Present Case | + | + | + | + | + | + | − | + | focal + | + |
| Wang et al | n.i. | + | n.i. | + | n.i. | n.i. | n.i. | + | n.i. | + |
| Kurado et al | − | + | + | + | + | n.i. | − | n.i. | n.i. | n.i. |
| Doyle et al | n.i. | + | n.i. | + | + | n.i. | − | + | n.i. | − |
| Nonaka et al | n.i. | + | + | + | n.i. | − | − | n.i. | − | − |
| Verine et al | − | + | + | + | n.i. | n.i. | − | + | focal + | − |
| Ip et al | n.i. | + | n.i. | + | n.i. | n.i. | − | + | n.i. | − |
| Wang et al | n.i. | + | n.i. | + | n.i. | n.i. | n.i. | + | − | n.i. |
| Yin et al | + | + | + | + | n.i. | n.i. | − | + | focal + | + |
| Liu et al | n.i. | + | n.i. | + | n.i. | n.i. | − | + | − | − |
| Jiang et al | + | + | n.i. | + | n.i. | n.i. | − | + | n.i. | − |
| Zhao et al | + | + | + | + | + | n.i. | focal + | + | focal + | − |
Abbreviation: n.i., no indication.
Table 2.
Immunohistochemical markers, present case
|
Immunohistochemical panel Present Case | |
|---|---|
| Positive | Negative |
| CD10 (Mako) | RCC (Cell marque) |
| S100 (Ventana) | Pan‐cytokeratin (Mako) |
| Vimentin (Ventana) | CD34 (Cell marque) |
| Aktin (Cell marque) | Pan Melanoma (Bio care) |
| PAX8 (Cell marque) | Myogenin (Cell marque) |
| Inhibin (Cell marque) | CK8/18 (Cell marque) |
| WT1 (Cell marque) | Melan A (Ventana) |
| EMA (Ventana) | HMB45 (Ventana) |
| CK7 (Ventana) | |
| Desmin (Ventana) | |
| TFE3 (Cell marque) | |
Table 3.
General characteristics
| Case report | Age (y)/gender | Chief complaint | Size (cm) | VHL (yes/no) | Follow‐up (mo)/prognosis |
|---|---|---|---|---|---|
| Wang et al | 61/male | Asymptomatic |
6.5 |
no | 24/no recurrence |
| Kurado et al | 37/male | Asymptomatic | 3.6 | no | n.i. |
| Doyle et al |
3 cases |
Hematuria Fever and weight loss Asymptomatic |
n.i. n.i. n.i. |
n.i. n.i. n.i. |
n.i. n.i. n.i. |
| Nonaka et al | 71/female | Asymptomatic | 6.8 | no | 108/no recurrence |
| Verine et al | 64/male | Other disease |
3.2 |
no |
12/no recurrence |
| Ip et al |
58/male 55/female |
Hematuria Low back pain |
5.5 3.5 |
no no |
24/n.i. 48/n.i. |
| Wang et al | 29/male | Other disease |
2.7 |
no |
20/no recurrence |
| Yin et al | 61/male | Asymptomatic | 5.3 | no | 12/n.i. |
| Liu et al | 16/female | Hematuria |
1.2 |
no |
6/no recurrence |
| Jiang et al | 57/female | Asymptomatic | 3 | no | 6/no recurrence |
| Zhao et al | 51/female | Right‐sided lumbar abdominal pain |
5.5 |
no | 12/no recurrence |
Abbreviation: n.i., no indication.
3. DISCUSSION
The present case showed a tumor with polygonal and spindle cells with enlarged nuclei and an eosinophilic but also clear cytoplasm. Some tumor cells demonstrated eosinophilic globules and vacuolization. Other areas with rhabdoid features and necrosis were also observed. Prominent vascularity was identified. Immunohistochemistry showed positivity of the tumor cells for CD10, PAX8, and WT1, which is a master control gene that is essential for kidney development, suggesting a tumor of renal origin.8 After negative staining for pan‐cytokeratin and RCC, which is a monoclonal antibody against renal tubule antigen that is positive in the majority of clear cell and papillary renal cell carcinomas, renal cell carcinoma was excluded.9 Additional immunhistochemical analysis was carried out and strong expression for S100, NSE, and inhibin‐alpha were observed. The immunoprofile in conjunction with the morphology and low proliferation rate led us to exclude a renal cell carcinoma and to diagnose a RH, which is the 15th reported case to date.
Hemangioblastoma is a rare benign mesenchymal tumor. It normally occurs in the CNS and is characterized by neoplastic stromal cells with a prominent vascularity.10 Doyle et al reported the pathological findings of 22 cases of peripheral hemangioblastomas outside the CNS (spinal nerve roots, soft tissues, kidneys, intestines, peritoneum, and orbit). All the tumors showed spindle and microvacuolated cells with eosinophilic or clear cytoplasmic contents, and all of them were characterized by intense vascularization. On immunohistochemical examinations, a considerable number of these hemangioblastomas positively expressed inhibin‐alpha (95%), neuron‐specific enolase (79%), and S100 (65%). In addition, markers detected in a few cases included weak expression for PAX8 and focal expression for EMA, desmin, and SMA. CD 31 and CD34 were only identified in endothelium of the capillary network but not in tumor cells.11
To date, 14 cases of a sporadic RH have been reported.2, 5, 11, 12, 13, 14, 15, 16, 17, 18, 19 In terms of the immunohistochemical findings, significant markers indicating diagnosis of RH include S100, inhibin‐alpha, and NSE. In all the reported cases, S100 and inhibin‐alpha expression were examined, and positivity was seen in 100% of the cases. NSE staining was performed in 12 of 14 cases of RH, and all of these tumors expressed it (Table 1). Most authors examined pan‐cytokeratin for exclusion of the major differential diagnosis, RCC. Staining for CD10, which is a marker expressed in the proximal tubular cells of the kidney and in the majority of clear cell and papillary renal cell carcinomas, was performed in 6 cases of RH and showed positive results in 50% of the cases. Also, PAX8, which is a transcription marker expressed in the renal tubular epithelial cells and in most renal cell carcinomas, was examined in 5 cases and showed positive results in 60% of the cases.20 For central and most peripheral hemangioblastomas outside the kidney no CD10 or PAX8 has been reported.21, 22 This supports the hypothesis that depending on the site of origin, hemangioblastomas have the capacity to express variable lines of differentiation.16, 18 An overview is shown in Table 1.
Interestingly, Montironi et al discussed whether the previously reported RHs are true RHs, or a diffuse hemangioblastoma‐like change in a clear cell renal cell carcinoma. The group of Montironi reported two cases of renal cell carcinomas each with two tumor components. The first part of the tumor consisting of hemangioblastoma‐like features and the second with morphology of a renal cell carcinoma. They described an acquired expression of inhibin‐alpha and S100 in the hemangioblastoma‐like part but not in the carcinoma cells. PAX8, CD10, and RCC were demonstrated in both components of the tumor. They believed that the hemangioblastoma‐like pattern could have a favorable prognostic significance.23 In the present case, inhibin‐alpha and S100 expression was consistently positive throughout the tumor and differed not between various regions.
In addition, foci with necrosis and cell atypia were found in the present case. In previous reports, necrosis in RH was observed in 18% (2/11) of the cases (see Table 1). In different types of tumors, necrosis, in combination with cellular atypia, can be indicative of malignancy.24, 25 In addition, necrosis is associated with aggressive behavior in some types of malignant tumors, especially in RCCs.26 In this context, it is important not to misjudge the dignity of the present tumor based only on these morphological features.
4. CONCLUSION
Renal hemangioblastoma is a very rare benign tumor, and a diagnosis cannot be made with radiographic techniques alone, such as CT or magnetic resonance imaging. Surgical excision of the tumor is needed to perform a precise and comprehensive pathological analysis. Since the tumor cells in renal cell carcinoma and RH share some morphological features and both express markers such as CD10 and PAX8, some of these renal tumors are difficult to classify.
When diagnosing a renal cell carcinoma, a strict follow‐up plan over years is necessary. Depending on the risk factors; based on pathological stage, comorbidities, and relapse location; post‐treatment surveillance has to be carried out. In intermediate‐ and high‐risk tumors, the European Association of Urology‐panel recommends computed tomography examination of chest and abdomen once a year for 3 years followed by computed tomography once every 2 years. And even in low‐risk tumors, a computed tomography should be performed at least every 2 years.27 The mean effective dose for whole body CT is about 14 mSv, which means a significant radiation exposure over the time of follow‐up for each patient. Calandrino and co‐workers evaluated an absolute additional risk of developing second cancer due to CT‐scans of follow‐up protocols between 0, 1%, and 10%. Major risk determinants were tumor pathology and age at exposure.28 However, a totally excised benign tumor needs no more surveillance and further radiation exposure can be avoided.
Although renal cell carcinoma is much more common, where there is any doubt, we recommend immunohistochemical stainings for pan‐cytokeratin, S100, NSE, and inhibin‐alpha to avoid the potential for misdiagnosis and to keep in mind a hemangioblastoma as differential diagnosis.
CONFLICT OF INTEREST
Lukas Oberhammer, Lukas Lusuardi, Thomas Kunit, Martina Hager, Martin Drerup, Daniela Colleselli, Hubert Grießner, and Michael Mitterberger declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
LO: collected data, wrote the manuscript, and involved in project development. LL, TK, DC, and MD: wrote the manuscript. MH: collected data and wrote the manuscript. HG: collected data. MM: wrote the manuscript and involved in project development.
ETHICAL APPROVAL
Not applicable.
CONSENT TO PUBLISH
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
ACKNOWLEDGMENT
The authors express their sincere appreciation for the language editing assistance provided by Tom Broughton.
Oberhammer L, Mitterberger MJ, Lusuardi L, et al. Sporadic renal hemangioblastoma: A case report of a rare benign renal tumor. Clin Case Rep. 2019;7:2321–2326. 10.1002/ccr3.2466
DATA AVAILABILITY STATEMENT
Pathological findings were evaluated in the Department of Pathology, Uniklinikum Salzburg, Austria. The CT scan was performed in the Department of Radiology, Uniklinikum Salzburg, Austria. On demand, all the data can be delivered.
REFERENCES
- 1. Pschyrembel, klinisches Wörterbuch. 260. Auflage. Berlin. de Gruyter. 2010;699.
- 2. Wang Y, Wei C, Mou L, et al. Sporadic renal hemangioblastoma: case report and review of the literature. Oncol Lett. 2013;5:360‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su JH, Choong‐kun L, Kyu YH, et al. A Case of von Hippel‐Lindau Disease with colorectal adenocarcinoma, renal cell carcinoma and hemangioblastomas. Cancer Res Treat. 2016;48(1):409‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez‐Rodriguez B, Villar Gómez de las Heras K, Aguirre DT, et al. Evaluation of the safety and effectiveness of oral propranolol in patients with von Hippel‐Lindau disease and retinal hemangioblastomas: phase III clinical trial. BMJ Open Ophthalmol. 2019;4(1):e000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yong W, Tao W, Pei‐Pei Z, Xiaoqun Y, Jian W, Chao‐Fu W. Extraneural hemangioblastoma of the kidney: the challenge for clinicopathological diagnosis. J Clin Pathol. 2015;68:1020‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurado N, Agatsuma Y, Tamura M, Martinek P, Hes O, Michal M. Sporadic renal hemangioblastoma with CA9, PAX2 and PAX8 expression: diagnostic pitfall in the differential diagnosis from clear cell renal cell carcinoma. Int J Clin and ExpPathol. 2015;8(2):2131‐2138. [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart BW, Wild CP. World Cancer Report 2014. Lyon: International Agency for Research on Cancer; 2014:437. [Google Scholar]
- 8. Kreidberg J. WT1 and kidney progenitor cells. Organogenesis. 2010;6(2):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perna AG, Ostler DA, Ivan D, et al. Renal cell carcinoma marker (RCC‐Ma) is specific for cutaneous metastasis of renal cell carcinoma. J Cutan Pathol. 2007;34(5):381‐385. [DOI] [PubMed] [Google Scholar]
- 10. Jalikis FG, Hoch BL, Bakthavatsalam R, Montenovo MI. Sporadic retroperitoneal hemangioblastoma: report of a case and review of the literature. Case Rep Pathol. 2017;1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyle LA, Fletcher CD. Peripheral hemangioblastoma: clinicopathologic characterization in a series of 22 cases. Am J Surg Pathol. 2014;38(1):119‐127. [DOI] [PubMed] [Google Scholar]
- 12. Ip YT, Yuan JQ, Cheung H, Chan JK. Sporadic hemangioblastoma of the kidney: an underrecognized pseudomalignant tumour? Am J Surg Pathol. 2010;34(11):1695‐1700. [DOI] [PubMed] [Google Scholar]
- 13. Wang CC1, Wang SM, Liau JY. Sporadic hemangioblastoma of the kidney in a 29‐year‐old man. Int J Surg Pathol. 2012;20(5):519‐522. [DOI] [PubMed] [Google Scholar]
- 14. Yin W, Li J, Chan J. Sporadic hemangioblastoma of the kidney with rhabdoid features and focal CD10 expression: report of a case and literature review. Diagn Pathol. 2012;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Qiu E, Wang E. Sporadic Hemangioblastoma of the Kidney: a rare renal tumour. Diagn Pathol. 2012;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang J, Rao Q, Xia QY, et al. Sporadic hemangioblastoma of the kidney with PAX2 and focal CD10 expression: report of a case. Int J Clin Exp Pathol. 2013;6(9):1953‐1956. [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao M, Williamson SR, Yu J, et al. PAX8 expression in sporadic hemangioblastoma of the kidney supports a primary renal cell lineage: implications for differential diagnosis. Hum Pathol. 2013;44(10):2247‐2255. [DOI] [PubMed] [Google Scholar]
- 18. Nonaka D, Rodriguez J, Rosai J. Extraneural hemangioblastoma: a report of 5 cases. Am J Surg Pathol. 2007;31(10):1545‐1551. [DOI] [PubMed] [Google Scholar]
- 19. Verine J, Sandid W, Miquel C, Vignaud JM, Mongiat‐Artus P. Sporadic hemangioblastoma of the kidney: an underrecognized pseudomalignant tumour? Am J Surg Pathol. 2011;35(4):623‐624. [DOI] [PubMed] [Google Scholar]
- 20. Truong L, Shen S. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92‐109. [DOI] [PubMed] [Google Scholar]
- 21. Carney EM, Banerjee P, Ellis CL, et al. PAX2(‐)/PAX8(‐)/inhibin A(+) immunoprofile in hemangioblastoma: A helpful combination in the differential diagnosis with metastatic clear cell renal cell carcinoma to the central nervous system. Am J Surg Pathol. 2011;35(2):262‐267. [DOI] [PubMed] [Google Scholar]
- 22. Jung SM, Kuo TT. Immunoreactivity of CD10 and inhibin alpha in differentiating hemangioblastoma of central nervous system from metastatic clear cell renal cell carcinoma. Mod Pathol. 2005;18(6):788‐794. [DOI] [PubMed] [Google Scholar]
- 23. Montironi R, Lopez‐Beltran A, Cheng L, Galosi AB, Montorsi F, Scarpelli M. Clear cell renal cell carcinoma (ccRCC) with hemangioblastoma‐like features: a previously unreported pattern of ccRCC with possible clinical significance. Eur Urol. 2014;66(5):806‐810. [DOI] [PubMed] [Google Scholar]
- 24. Bredholt G, Mannelqvist M, Stefansson I, et al. Tumour necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015;6(37):39676‐39691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Väyrynen SA, Väyrynen JP, Klintrup K, et al. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer. 2016;114(12):1334‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumour necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;1;104(3):511‐520. [DOI] [PubMed] [Google Scholar]
- 27. European Association of Urology Guidelines. 2019 edition. Renal Cell Carcinoma. 43‐45.
- 28. Calandrino R, Ardu V, Corletto D, et al. Evaluation of second cancer induction risk by CT follow‐up in oncological long‐surviving patients. Health Phys. 2013;104(1):1‐8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pathological findings were evaluated in the Department of Pathology, Uniklinikum Salzburg, Austria. The CT scan was performed in the Department of Radiology, Uniklinikum Salzburg, Austria. On demand, all the data can be delivered.


