Abstract
Two phase 3 trials reported a prolonged survival in the third‐line setting of colorectal cancer patients treated with regorafenib with the longest duration of treatment of 16 months. Herein, we reported a unique case of a patient refractory to conventional chemotherapy who showed a prolonged stable disease with regorafenib.
Keywords: colorectal cancer, long‐term survival, regorafenib
Two phase 3 trials reported a prolonged survival in the third‐line setting of colorectal cancer patients treated with regorafenib with the longest duration of treatment of 16 months. Herein, we reported a unique case of a patient refractory to conventional chemotherapy who showed a prolonged stable disease with regorafenib.
1. INTRODUCTION
Regorafenib (Stivarga®) is a tyrosin kinase inhibitor (TKI) impairing angiogenesis through the block of both vascular endothelial growth factor receptors (VEGFR) 1 and 3 (VEGFR3) and tyrosine kinase with immunoglobulin‐like and EGF‐like domains 2 (TIE2). Moreover, it targets tumor microenvironment through the inhibition of platelet‐derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR).1, 2, 3
Actually, this drug represents a therapeutic option in the third‐line setting of metastatic colorectal cancer (mCRC) patients according to the results of two phase III randomized trials (CORRECT and CONCUR) which showed a significant improvement both in terms of progression‐free survival (PFS) and overall survival (OS) compared to best supportive care (BSC)4, 5 alone. Median overall survivals (mOS)s were 6.4 and 8.8 months, for Western4 and Asiatic trials,5 respectively, with the longest duration of treatment of 16 months in CORRECT trial.
Herein, we report a unique case of a patient refractory to oxaliplatin‐based and irinotecan‐based chemotherapy combined with bevacizumab who showed a prolonged response (25 months) to regorafenib.
2. CASE PRESENTATION
A 57‐year‐old caucasian woman underwent right hemicolectomy for a poorly differentiated mucinous CRC. Presurgical radiological staging deemed negative for distant metastases. Pathological stage was pT3N2M0 (stage III). Subsequently, she received adjuvant systemic therapy with the combination of capecitabine plus oxaliplatin for eight cycles.
A computed tomography (CT) scan carried out at the end of this treatment revealed a bulky left ovarian mass (70 × 67 mm) associated with high serum levels of CA19.9 (289 µ/mL) and CEA (48 ng/mL). Therefore, the patient underwent an exploratory laparotomy with evidence of multiple peritoneal nodules. Thus, debulking surgery was performed with left ovariectomy and excision of two peritoneal metastases. Histological examination revealed a localization of well‐differentiated mucinous CRC (CDX2 positive, CK7, and CK20 partially positive; KRAS‐codon 12 mutation and BRAF wild‐type) associated with peritoneal carcinosis. RAS and RAF determinations had been performed on metastatic site since it had been demonstrated the high concordance between RAS and RAF between primary and metastatic CRC.6
The CT scan performed 2 months after surgery showed controlateral ovaric mass (100 × 80 mm) and multiple peritoneal nodules (maximum diameter of 70 mm in recto‐uterine pouch). High levels of tumor markers (CA19.9 302 µ/mL, CEA 27 ng/mL) were observed.
First‐line therapy according to FOLFIRI regimen in combination with bevacizumab was started. Nevertheless, 2 months after starting of therapy a CT scan showed an increase in ovaric mass (140 × 130 mm) and peritoneal involvement and the appearance of two hepatic lesions (largest diameters of 12 and 10 mm in V and II hepatic segments, respectively). She was enrolled in a clinical trial by another referral cancer center. Nonetheless, a CT scan documented an early progression with the appearance of right ovarian metastases (largest diameter of 30 cm) associated with omolateral hydroureteronephrosis and bowel subocclusion. A second debulking cytoreductive surgery was performed with histological confirmation of moderately differentiated mucinous metastases from CRC.
Subsequently CT scan confirmed multiple peritoneal and omental implants up to 95 mm and liver metastases (Figure 1). Serum levels of CA19.9 and CEA were 434 ng/mL and 32 µ/mL, respectively.
Figure 1.
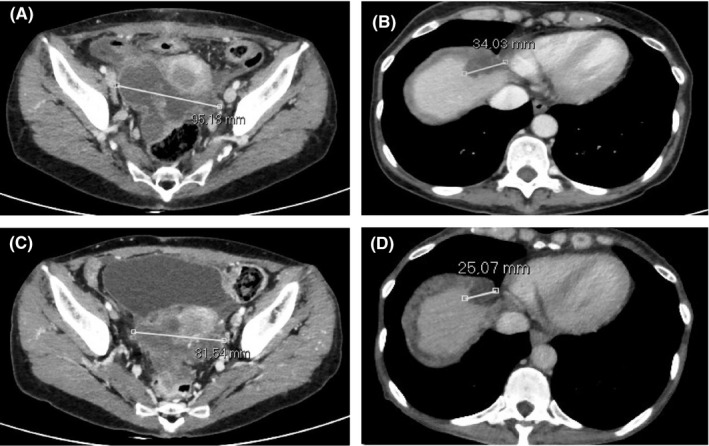
Abdominal CT scan at the beginning of therapy with regorafenib showed: (A) peritoneal mass localized in recto‐uterine pouch (largest diameter of 95 mm); (B) liver metastasis at segment VIII (largest diameter of 34 mm) (B). Abdominal CT scan performed 6 mo after the beginning of therapy (best response) showed the reduction of peritoneal and liver lesions (largest diameters: 81 and 25 mm, respectively) (C‐D)
A third‐line treatment with regorafenib (160 mg po day for 3 weeks and 1‐week rest) was started. Remarkably, this therapeutic approach allowed to obtain a prolonged modest reduction of the dimensions of both peritoneal nodules and liver metastases associated with the decrease in serum levels of CA19.9 and CEA (up to 113 ng/mL and 13 µ/mL, respectively). (Figure 1) The most frequent observed regorafenib‐related grade 1‐2 adverse events were hypertension, hand‐foot syndrome, stomatitis, and hoarseness. Occasionally, grade 3 diarrhea and fatigue required dosage modulations.
After 25 months of treatment with regorafenib, a CT scan revealed a peritoneal and liver metastases progression in combination with a performance status decline. After few weeks of BSC, the patient's exitus was registered.
3. DISCUSSION
Regorafenib improved mOS in patients with CRC who were pretreated with conventional chemotherapies. In particular, mOSs were 6.4 and 5 months in the regorafenib group and placebo group, respectively (hazard ratio 0.77; 95% CI 0.64‐0.94; one‐sided P = .0052), in CORRECT trial.4 This phase III trial randomized pretreated patients with CRC to receive regorafenib or placebo. Additionally, CONCUR phase III trial on pretreated Asian CRC patients investigated the same randomization.5 This trial demonstrated a mOS improvement with regorafenib than placebo (hazard ratio 0.55, 95% CI 0.40‐0.77, one‐sided P = .00016; 8.8 vs 6.3 months, respectively). The longest duration of regorafenib treatment was 16 months.
Only few reports described patients who received this molecule for a prolonged period (Table 1). Rosati et al reported an OS of 13 months after administration of regorafenib.7
Table 1.
Review of literature of long‐term survivor mCRC patient treated with regorafenib
| Patient's characteristics | Pretretments | Regorafenib‐OS | Months of regorafenib treatment | References |
|---|---|---|---|---|
| Caucasian male, 67 y |
Cetuximab plus irinotecan‐based bevacizumab plus oxaliplatin and 5‐FU Rechallenge with panitumumab rechallenge of an oxaliplatin‐based CT |
13 mo | 13 mo | Rosati7 |
| Caucasian male, 59 y |
Folfox‐Bevacizumab Folfiri‐Bevacizumab Folfox‐Cetuximab |
24 mo | 17 mo | Callebout8 |
| Asiatic male, 54 y |
FOLFIRI Oral FU plus LV XELOX‐bevacizumab FOLFIRI‐panitumumab mFOLFOX6‐panitumumabmab |
24 mo+ | 24 mo+ | Yoshino9 |
| Caucasian male, 54 y |
FOLFOX‐4 FOLFIRI‐aflibercept |
36 mo (interspersed by RT) | 36 mo+ | Roberto10 |
| Caucasian male, 54 y |
FOLFOX‐4 FOLFIRI‐aflibercept |
30 mo (interspersed by RT) | 25 mo | Korphaisarn11 |
| Caucasian male, 63 y |
FOLFOX Folfiri‐Bevacizumab |
25 mo | 25 mo | Amram22 |
| Caucasian female, 57 y |
Xelox Folfiri‐Bevacizumab |
25 mo | 25 mo | Present report |
Abbreviations: CT, chemotherapy; FU, fluorouracil; LV, leucovorin; OS, overall survival; RT, radiotherapy; ys, years.
Of note, Callebout et al reported an OS of 25 months.8 Nonetheless, in this report the therapeutic program was discontinuous due to radiotherapy combinatorial approach. Conversely, our case achieved the same OS with uninterrupt ed medical treatment. Intriguingly, the histology of Callebout and colleagues’ report was a mucinous colorectal cancer likewise our patient. As these authors reported, mucinous histology shows highly epithelial‐mesenchimal transition signature, which, given FGFR and PDGFR inhibition, could represent a regorafenib target. Also Yoshino et al described a case of a 2‐year survival with regorafenib treatment.9 These authors reported a case of a CRC patient with RAS‐RAF WT and a sustained OS of over 9 years. Roberto et al illustrated the case of a CRC patient with a regorafenib‐related OS of 36 months, even if his oligometastatic disease was controlled with stereotactic radiotherapy.10 Also in this report, the patient's OS resulted in about 6 years. Similarly, Korphaisarnet al. described the case of a chemo‐resistant rectal cancer with a prolonged response to regorafenib and locoregional progression which underwent a RT control.11
These patients shared common features, such as their prolonged response to previous lines of therapy. It is reasonable to speculate the presence of biological features of these tumors related to their chemo‐responsiveness.12, 13 Two peculiar aspects of our patient are represented by the prolonged response to regorafenib combined to the lack of response to oxaliplatin‐based and irinotecan‐based chemotherapy. Furthermore, our patient underwent two debulking surgeries before the beginning of therapy with regorafenib.
In order to better understand the predictive role and the clinical activity of regorafenib in CRC, a retrospective, exploratory analysis of circulating DNA and protein biomarkers had been carried out in patients enrolled in the CORRECT trial. Several biomarkers have been evaluated. In particular, it was demonstrated that regorafenib had a greater impact on OS of patients with high concentration of TIE‐1 than in those with a low concentration14 as emerges from the study a post hoc analysis. In fact, the great response to regorafenib should be improved to sensible activation of pathways conventionally inhibited by regorafenib,15 namely angiogenesis and vasculogenesis, which in mucinous mCRC result hyperactivated due to hypoxic microenvironment.16
Our patient displayed several regorafenib‐related adverse events (ie, hand‐foot syndrome, stomatitis, hypertension, and hoarseness), even if only of grades 1‐2 with the exception of grade 3 diarrhea and fatigue which required temporary dose modifications according to summary of product characteristics. Also, this aspect is relevant in our case due to the frequent correlation between the length of treatment and the appearance of AE which sometimes could require hospitalization.17, 18
Histopatological and clinical features of this tumor associated with its chemorefractory to previous lines of chemotherapy support its poor prognosis. Conversely, the prolonged stable disease to regorafenib in combination with its good toxicity profile supports the potential therapeutic role of this drug.
Conclusively, we believe that only the knowledge of the molecular aspects of primary tumor and of its metastases could have helped in the deeper comprehension of the unique history of this patient. In particular, the analysis of clinical, laboratoristic, and biological features s uch as for other anti‐angiogenic drug 19, 20, 21 might provide novel insights explaining the long‐term survival.
CONFLICT OF INTEREST
The authors declare the absence of conflicts of interest.
AUTHOR CONTRIBUTIONS
OB, LP, and AGS: conceptualized the study. AC and AA: involved in methodology. OB, AC, and LP: formally analyzed the data. AA, OB, and AGS: investigated the study. OB: involved in data curation. AA and OB: involved in writing—original draft preparation. OB: involved in writing—review and editing; AA and OB: provided resources. OB and AGS: supervised the study; AGS: acquired funding.
ACKNOWLEDGMENTS
This research project was supported in part by the Apulian Regional Project “Medicina di Precisione”
Brunetti O, Calabrese A, Palermo L, Solimando AG, Argentiero A. Long‐term survival of an advanced colorectal cancer patient treated with Regorafenib: Case report and literature review. Clin Case Rep. 2019;7:2379–2383. 10.1002/ccr3.2496
REFERENCES
- 1. Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73–4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mross K, Frost A, Steinbild S, et al. A phase I dose‐escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658‐2667. [DOI] [PubMed] [Google Scholar]
- 3. Quatrale AE, Porcelli L, Silvestris N, et al. GFR tyrosine kinases inhibitors in cancer treatment: In vitro and in vivo evidence. Front Biosci. 2011;16:1962‐1972. [DOI] [PubMed] [Google Scholar]
- 4. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013;381:303‐312. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, doubleblind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2015;16:619‐629. [DOI] [PubMed] [Google Scholar]
- 6. Santini D, Spoto C, Loupakis F, et al. High concordance of BRAF status between primary colorectal tumours and related metastatic sites: implications for clinical practice. Ann Oncol. 2010;21:1565. [DOI] [PubMed] [Google Scholar]
- 7. Rosati G, Del Gaudio N, Scarano E, et al. Unexpected and durable response with regorafenib in a metastatic colorectal cancer patient without KDR mutation. Medicine. 2018;97:e11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callebout E, Ribeiro SM, Laurent S, et al. Long term response on regorafenib in non‐V600E BRAF mutated colon cancer: a case report. BMC Cancer. 2019;19:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshino K, Manaka D, Kudo R, et al. Metastatic colorectal cancer responsive to regorafenib for 2 years: a case report. J Med Case Rep. 2017;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberto M, Falcone R, Mazzuca F, et al. The role of stereotactic body radiation therapy in oligometastatic colorectal cancer: clinical case report of a long‐responder patient treated with regorafenib beyond progression. Medicine. 2017;96:e9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korphaisarn K, Loree JM, Nguyen V, et al. Genomic analysis of exceptional responder to regorafenib intreatment‐refractory metastatic rectal cancer: a case report and review of the literature. Oncotarget. 2017;8:57882‐57888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakayama I, Shinozaki E, Matsushima T, et al. Retrospective study of RAS/PIK3CA/BRAF tumor mutations as predictors of response to first‐line chemotherapy with bevacizumab in metastatic colorectal cancer patients. BMC Cancer. 2017;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russo A, Rizzo S, Bronte G, et al. The long and winding road to useful predictive factors for anti‐egfr therapy in metastatic colorectal carcinoma: the KRAS/BRAF pathway. Oncology. 2009;77:57‐68. [DOI] [PubMed] [Google Scholar]
- 14. Tabernero J, Lenz HJ, Siena S, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan Y, Grothey A. Molecular profiling in the treatment of colorectal cancer: focus on regorafenib. Onco Targets Ther. 2015;8:2949‐2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malfettone A, Silvestris N, Paradiso A, et al. Overexpression of nuclear NHERF1 in advanced colorectal cancer: association with hypoxic microenvironment and tumor invasive phenotype. Exp Mol Pathol. 2012;92:296‐303. [DOI] [PubMed] [Google Scholar]
- 17. Numico G, Longo V, Courthod G, et al. Cancer survivorship: long‐term side‐effects of anticancer treatments of gastrointestinal cancer. Curr Opin Oncol. 2015;27:351‐357. [DOI] [PubMed] [Google Scholar]
- 18. Numico G, Cristofano A, Mozzicafreddo A, et al. Hospital admission of cancer patients: avoidable practice or necessary care? PLoS ONE. 2015;10:e0120827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silvestris N, Scartozzi M, Graziano G, et al. Basal and bevacizumab‐based therapy‐induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther. 2015;15:155‐162. [DOI] [PubMed] [Google Scholar]
- 20. Azzariti A, Porcelli L, Brunetti O, et al. Total and not bevacizumab‐bound vascular endothelial growth factor as potential predictive factors to bevacizumab‐based chemotherapy in colorectal cancer. World J Gastroenterol. 2016;22:6287‐6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silvestris N, Marech I, Brunetti AE, et al. Predictive factors to targeted treatment in gastrointestinal carcinomas. Cancer Biomark. 2014;14:151‐162. [DOI] [PubMed] [Google Scholar]
- 22. Amram ML, Montet X, Roth AD. Long‐term survival with regorafenib in KRAS‐mutated metastatic rectal cancer. Case Rep Oncol. 2017;10:1029‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]


