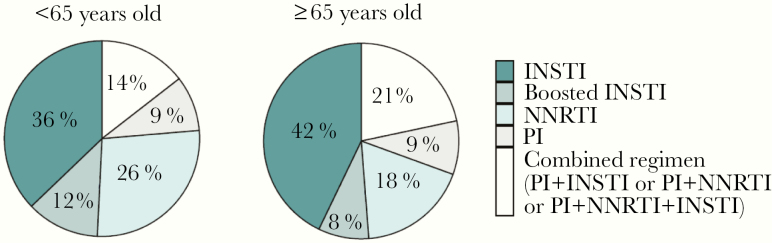Abstract
Background
Antiretroviral therapy has transformed HIV infection from a deadly into a chronic condition. Aging people with HIV (PWH) are at higher risk of polypharmacy, potential drug–drug interactions (DDIs), and potentially inappropriate medications (PIMs). This study aims to compare prescribed drugs, polypharmacy, and potential DDIs between young (<65 years old) and elderly (≥65 years old) PWH. The prevalence of PIMs was assessed in elderly.
Methods
PWH from 2 centers within the Swiss HIV Cohort Study were asked to fill in a form with all their current medications. Polypharmacy was defined as being on ≥5 non-HIV drugs. PIMs were evaluated using Beers criteria. Potential DDIs for the most prescribed therapeutic classes were screened with the Liverpool interaction database.
Results
Among the 996 PWH included, 122 were ≥65 years old. Polypharmacy was more frequent in the elderly group (44% vs 12%). Medications and potential DDIs differed according to the age group: cardiovascular drugs and related potential DDIs were more common in the elderly group (73% of forms included ≥1 cardiovascular drug; 11% of cardiovascular drugs involved potential DDIs), whereas central nervous system drugs were more prescribed and involved in potential DDIs in younger PWH (26%, 11%). Potential DDIs were mostly managed through dosage adjustments. PIMs were found in 31% of the elderly group.
Conclusions
Potential DDIs remain common, and PIMs constitute an additional burden for the elderly. It is important that prescribers develop and maintain a proactive approach for the recognition and management of DDIs and other prescribing issues frequently encountered in geriatric medicine.
Keywords: aging, drug–drug interactions, elderly, HIV, inappropriate drugs, polypharmacy
Antiretroviral treatments (ARTs) have transformed HIV infection from a deadly disease into a chronic condition. As a consequence, people with HIV (PWH) are getting older, living long enough to develop age-related chronic conditions and consequently to receive significant polymedication in addition to their ARTs [1–4]. Furthermore, aging is characterized by physiological changes known to affect the exposure or response to drugs [5]. Thus, all together, elderly PWH are at increased risk of having polypharmacy, drug–drug interactions, and potentially inappropriate medications (PIMs) [6–10].
ARTs are among the therapeutic agents with the highest potential for DDIs, either as perpetrators (ART impacting a non-ART drug) or victims (ART being impacted by a non-ART drug). Pharmacokinetic interactions can occur at the level of absorption (eg, complexation with divalent cations, pH modification) [11, 12], distribution, metabolism, or elimination (induction/inhibition of cytochrome [CYP] isoforms, glucuronidation enzymes or transporters) [13–15]. Interactions have the potential to lead to substantial risks of either toxicity or decreased therapeutic efficacy for either ARTs or non-ARTs. For these reasons, the prevention, identification, and management of DDIs are crucial in PWH.
Little is known about PIMs in older PWH, which may harm this vulnerable population. To the best of our knowledge, only 3 studies have raised this issue, showing that 52% to 66% of older PWH had at least 1 medication-related problem [7, 16, 17].
The aim of this study was to compare prescribed medications, polypharmacy, and potential DDIs between young and elderly PWH included in 2 centers of the Swiss HIV Cohort Study (SHCS). The prevalence of PIMs was assessed in the elderly group. In addition, dosage adjustment was evaluated for comedications for which official dosing recommendations are available in order to assess the management of DDIs in real life.
METHODS
Study Design
The SHCS, a multicenter prospective cohort study, has been continuously enrolling PWH since its establishment in 1988 [18]. Approximately 75% of PWH receiving ART in Switzerland agree to be followed within the SHCS network. Within the framework of SHCS project 815, we have launched a comprehensive analysis of relevant DDIs between ARTs and commonly prescribed comedications from January 2017 to December 2018 in the HIV clinics at the University Hospitals of Lausanne and Basel. PWH were contacted by post 1 week before their biannual SHCS appointment and invited to report all their current medications, the respective dosage, and date/time of last drug intake before SHCS visit in a dedicated form, which they would bring back during their routine SHCS visit. Clinical nurses were responsible for gathering and checking the completeness of the medication forms. PWH were classified as “elderly” if they were ≥65 years old. This age cutoff was adopted as it represents the definition of elderly age in most developed world countries.
Description of Medications
The drugs reported in the forms included ARTs, prescription medications, and over-the-counter remedies. Comedications were classified according to the anatomical therapeutic chemical classification (ATC), as recommended by the World Health Organization [19], taking into account up to 3 digits. If a medication contained 2 or more pharmacologically active agents, each substance was counted individually in the analysis. Polypharmacy was defined as the concurrent administration of 5 or more comedications in addition to ART, which represents a rather conservative criterion, as the overall number of 5 drugs is commonly used to define polypharmacy [20]. As ARTs or comedications can be modified during the follow-up visit, all medication forms collected during the study period were considered for this analysis. Number and type of comedications were visually compared in multiple age groups.
PIMs were assessed using the most recent version of the classical Beers criteria and included, for instance, drugs with anticholinergic properties or benzodiazepines, which can impair cognition and consequently increase the risk of falls in elderly persons [3, 21]. Proton pump inhibitors were not considered a PIM, as our study did not capture treatment duration and only proton pump inhibitor treatment for longer than 8 weeks is considered inappropriate according to Beers criteria. Dosage of comedications was not taken into account in the analysis of PIMs. Anticholinergic burden was measured by means of the validated Anticholinergic Risk Scale, assigning drug points from 0 to 3, the latest corresponding to higher anticholinergic potential [22, 23]. If an elderly PWH received several drugs with anticholinergic properties, the total anticholinergic score was calculated by summing up each individual medication score.
Identification of Potential DDIs
We focused on 2 therapeutic classes, that is, cardiovascular and central nervous system (CNS), due to the fact that these therapeutic classes are largely utilized in PWH, as indicated by a previous analysis of the SHCS [24], and due to their potential for clinically relevant DDIs with ARTs. All medication forms containing at least 1 cardiovascular or CNS drug were included in the analysis. Potential DDIs between ARTs and these comedications were screened using the University of Liverpool HIV drug interaction checker [25]. These charts rank the clinical significance of an interaction from “no interaction” (green flag interaction) to “interaction of weak intensity not requiring additional action” (yellow flag interaction), “potentially clinically relevant DDI requiring either dose adaptation or close clinical monitoring” (amber flag interaction), or “contraindicated” (red flag interaction). Interactions within ARTs or within non-HIV medications were excluded from this analysis. Potential DDIs involving comedications not listed in the Liverpool drug interaction database were checked using Up-to-Date (https://www.uptodate.com/drug-interactions/#di-druglist). When a comedication was involved in several potential DDIs as a victim, the most severe potential DDI was retained.
Dosage adjustments of comedications were evaluated to assess how DDIs are managed in real life. This evaluation was performed only for comedications whose label provides dosing recommendations to overcome given DDIs. Both European and American dosing guidelines were considered [26–28].
Statistical Analyses
Statistical and graphical analyses were performed in R, using the packages tableone and ggplot2 [29]. In the descriptive analysis, continuous variables were described by their medians and interquartile ranges (IQRs) and compared between groups using the Mann-Whitney U or Wilcoxon tests. Categorical variables were described by proportions and compared with the χ 2 test. Repeated-measures analyses were not performed considering the time interval between 2 follow-up visits, during which both ARTs and comedications could have been changed. Medication forms fulfilled more than once by a patient were therefore considered independent measures. In addition, the proportion of patients reporting multiple medication forms was a priori expected to be similar in younger and elderly PWH, as all patients had medical appointments on a biannual basis.
RESULTS
Study Population and Medication Use
In total, 996 PWH, mostly male (69%), were included in the study. Of those, 874 (88%) were <65 years old (median [IQR], 49 [40–55] years), and 122 (12%) were ≥65 years old (median [IQR], 71 [67–74] years). Elderly PWH tended to have longer duration of HIV infection and thereby HIV treatment. Furthermore, elderly individuals tended to have more complex ARTs and more comedications. The demographic and clinical characteristics of the study population at their first recorded cohort visit, stratified by age, are summarized in Table 1. Medication forms were completed 1, 2, or 3 times by 41% (n = 403), 57% (n = 570), and 2% (n = 23) of participants, respectively.
Table 1.
Characteristics of the 996 PWH at Their First Visit With Fulfilled Medication Form, by Age Group
| Characteristics | <65 Years Old (n = 874) | ≥65 Years Old (n = 122) |
|---|---|---|
| Age, median [IQR], y | 48.8 [40.4–55.5] | 71.0 [67.3–74.0] |
| Male sex, No. (%) | 580 (66.4) | 105 (86.8) |
| Weight, median [IQR], kg | 73.0 [64.0–83.0] | 73.0 [67.0–85.0] |
| Ethnicity, No. (%) | ||
| White | 611 (70.1) | 114 (94.2) |
| Black | 199 (22.8) | 3 (2.5) |
| Hispano-American | 30 (3.4) | 2 (1.7) |
| Asian | 31 (3.6) | 2 (1.7) |
| CD4, median [IQR], cells/mm3 | 691.5 [527.0–919.0] | 616.0 [413.0–821.0] |
| HIV RNA <50 copies/mL, No. (%) | 845 (97.6) | 114 (94.2) |
| Date of HIV diagnosis, No. (%) | ||
| <1990 | 99 (13) | 17 (17) |
| 1990–1999 | 156 (20) | 35 (34) |
| 2000–2009 | 299 (38) | 34 (33) |
| ≥2010 | 228 (29) | 17 (17) |
| ART start date, No. (%) | ||
| <1990 | 1 (0.1) | 0 |
| 1990–1999 | 235 (27) | 55 (46) |
| 2000–2009 | 338 (39) | 45 (37) |
| ≥2010 | 298 (34) | 21 (17) |
| Non-NRTI ARTs, No. (%) | ||
| Integrase inhibitor | 413 (47.3) | 57 (46.7) |
| Combined regimen | 136 (15.6) | 29 (23.8) |
| NNRTI | 238 (27.2) | 23 (18.9) |
| Protease inhibitor | 85 (9.7) | 13 (10.7) |
| NRTIs (backbone), No. (%) | ||
| ABC/3TC | 317 (36.3) | 57 (46.7) |
| TDF/FTC | 330 (37.8) | 20 (16.4) |
| TAF/FTC | 151 (17.3) | 18 (14.8) |
| Others | 45 (5.1) | 17 (13.9) |
| No backbone | 31 (3.5) | 10 (8.2) |
| Number of comedications, No. (%) | ||
| 0 | 382 (43.7) | 14 (11.5) |
| 1 | 121 (13.8) | 8 (6.6) |
| 2 | 118 (13.5) | 10 (8.2) |
| 3 | 86 (9.8) | 20 (16.4) |
| 4 | 61 (7) | 18 (14.8) |
| ≥5 | 106 (12.1) | 52 (42.6) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; FTC, emtricitabine; IQR, interquartile range; NRTI, nucleoside reverse transcriptase inhibitor; PWH, people with HIV; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Taking into account all the 1610 collected forms, integrase strand transfer inhibitor (INSTI)–containing regimens were the most prescribed, accounting for ~50% of overall ARTs in both age groups (Figure 1). Of interest, combined ARTs (ie, boosted protease inhibitor [PI] + INSTI or boosted PI + non-nucleoside reverse transcriptase inhibitor [NNRTI] or boosted PI + INSTI + NNRTI), representing complex ARTs characterized by a higher potential to cause DDIs, were used more in elderly PWH (21% vs 14%). The most frequently administered boosted PI was ritonavir-boosted darunavir (68% of all boosted PIs), whereas efavirenz was the most prescribed NNRTI (38% of all NNRTIs).
Figure 1.
Distribution of the most prescribed antiretroviral regimens for the entire study period, stratified by age group. Abbreviations: INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
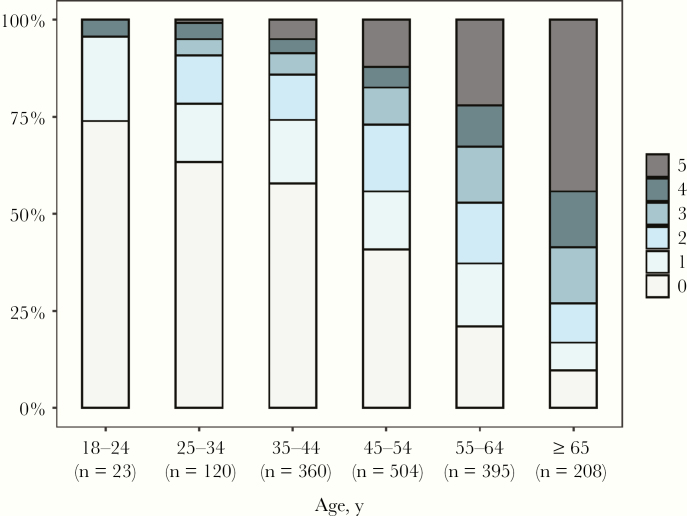
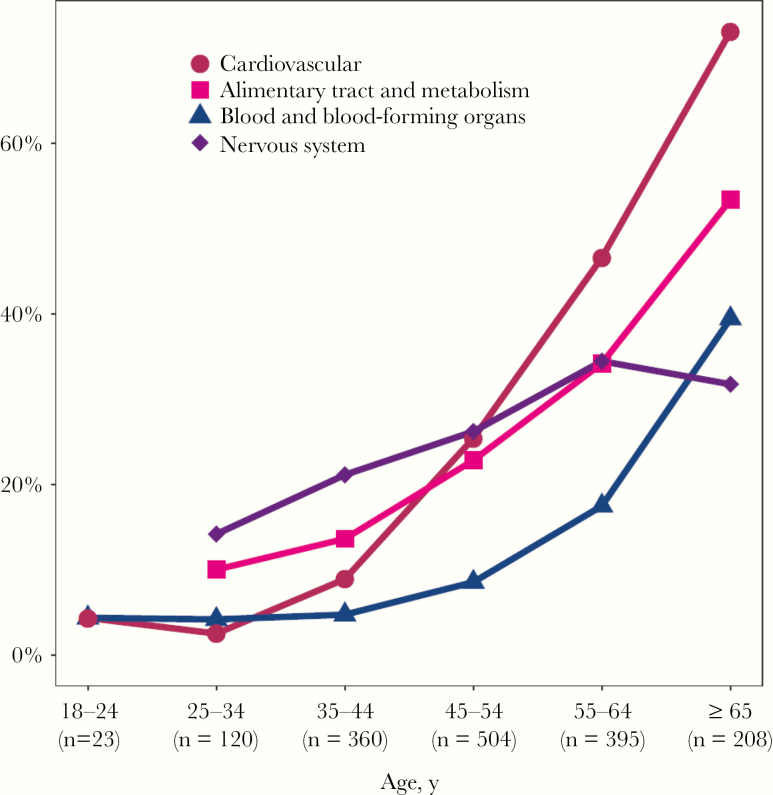
As expected, the number of prescribed comedications increased with age (Figure 2). Considering all the 1610 collected forms, elderly PWH tended to use a higher number of comedications (median [range], 4 [0–22]) compared with younger PWH (median [range], 1 [0–14]; P < .001). Ninety percent (n = 188) of the medication forms completed by elderly PWH included at least 1 comedication. Polypharmacy was more prevalent in PWH ≥65 years (44%) compared with the younger group (12%). As indicated in Figure 3, drugs belonging to the cardiovascular class were the most utilized in the elderly group (73% of medication forms of elderly PWH included at least 1 cardiovascular drug), whereas CNS drugs were most commonly prescribed in younger PWH (26% of forms of younger PWH included at least 1 CNS medication). Calcium/vitamin D3 and acetylsalicylic acid (prescribed as an antithrombotic agent) were the most prescribed medications in their respective therapeutic classes (33% and 52%, respectively). With the exception of CNS drugs, the use of the most prescribed therapeutic classes (ie, cardiovascular, alimentary tract and metabolism, blood and blood-forming organs) increased in an exponential way with increasing age.
Figure 2.
Overall distribution of the number of prescribed comedications for the entire study period, stratified by age group.
Figure 3.
Percentage of people with HIV treated with at least 1 comedication of the 4 most prescribed therapeutic classes for the entire study period, stratified by age group.
Thirty-eight elderly patients (31%) had a least 1 PIM, mostly benzodiazepines and hypnotics (n = 19, 27% of PIM). Three PWH received drugs characterized by a high anticholinergic burden (anticholinergic risk scale = 3), that is, dimenhydrinate, quetiapine, and trimipramine.
Characteristics and Effect of the Identified Potential Drug–Drug Interactions With Cardiovascular and CNS Drugs
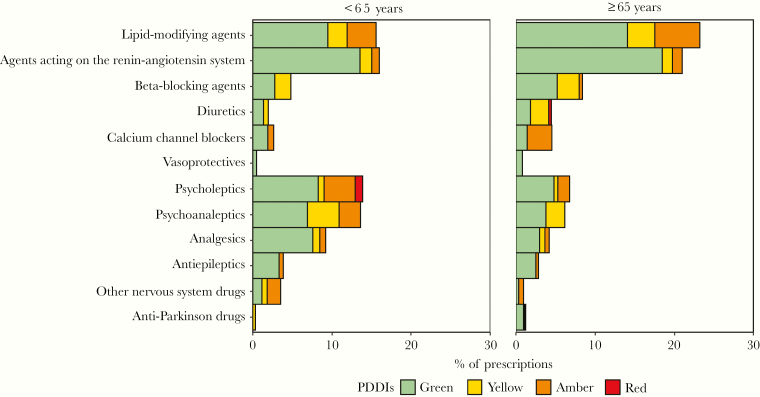
A total of 767 medications forms comprising at least 1 cardiovascular or CNS drug were collected in 500 PWH and were included in the analysis of potential DDIs. Of those, 417 prescriptions (54%) did not contain any potential DDIs. For the remainder of prescriptions, 23% (n = 178), 28% (n = 215), and 2% (n = 17) had at least 1 yellow, amber, and red flag potential DDI, respectively. These proportions were not statistically different between the 2 age groups (P = 1, .22, and .50 for yellow, amber, and red flag potential DDIs, respectively). However, most potential DDIs in elderly PWH were between ARTs and cardiovascular drugs, whereas in younger PWH, potential DDIs were mainly with CNS drugs. The frequency of potential DDIs with cardiovascular and CNS drugs, stratified by age, are depicted in Figure 4. Amber flag potential DDIs involved mainly zolpidem (n = 36, 12%) and rosuvastatin (n = 32, 11%), whereas red flag interactions involved predominantly the coadministration of quetiapine with boosted PIs (n = 12, 71%). Ritonavir-boosted darunavir was the most common ART involved in amber flag (n = 93, 32%) and red flag potential DDIs (n = 7, 41%). Potential pharmacodynamic DDIs resulting in potentially additive adverse effects (mostly QT prolongation interval or additive risk of nephrotoxicity) were found in 5% (n = 43) of the prescriptions.
Figure 4.
Percentage of prescriptions (n = 767) with at least 1 drug of the corresponding therapeutic class for the entire study period. Potential DDIs between ART and non-ART drugs are represented with different shades according to the severity of potential DDIs: red flag (deleterious), amber flag (potential clinical relevance, manageable by performing dosage adjustment or close clinical monitoring), and yellow flag (weak clinical relevance). The green flag corresponds to the absence of potential DDIs. Abbreviations: ART, antiretroviral therapy; DDI, drug–drug interaction; PDDI, potential drug–drug interaction.
Of note, apart from cardiovascular and CNS drugs, 5 patients (4 young and 1 elderly PWH) were treated with boosted PIs and clopidogrel, resulting in a red flag DDI.
Management of Potential DDIs With Cardiovascular and CNS Drugs
The maximum daily dose of atorvastatin recommended in the presence of boosted darunavir is 20 mg (US product label) and 40 mg (European product label). These dosing recommendations were respected in all prescriptions. Although coadministration of ritonavir-boosted atazanavir and atorvastatin is not recommended by both US and European guidelines, 1 patient (2 cohort visits) was concomitantly receiving both drugs. However, the atorvastatin dosage was limited to 10 mg once daily, which is in line with the recommendations of the University of Liverpool database [25].
Concerning rosuvastatin, the maximum daily dose is 20 mg in the presence of boosted darunavir (US product label). This recommendation was applied in all prescriptions. The Food and Drug Administration (FDA) recommends that the dosage of rosuvastatin should not exceed 10 mg when coadministered with boosted atazanavir [28], whereas the European AIDS Clinical Society suggests that rosuvastatin use is generally safe if started at a low dose, not exceeding 20 mg daily [30]. In our study, 1 patient received 20 mg of rosuvastatin daily, exceeding the maximum dose recommended by the FDA.
Finally, there are clear dosage recommendations for quetiapine when used together with boosted PIs. US prescribing information recommends that the dosage of quetiapine be reduced to one-sixth of the original dose [31]. In our study, this recommendation was followed for 11 out of the 14 patients, whereas the other 3 received quetiapine extended-release at a dosage ranging from 50 mg to 200 mg once daily.
Discussion
Our findings provide evidence that a high proportion of aging PWH are polymedicated and that the overall burden of medications has shifted from ARTs to treatments for other comorbidities. Our result demonstrating that 44% of elderly PWH are polymedicated is in line with recent studies reporting a rate of 37% [32, 33]. Cardiovascular and CNS drugs were the most represented therapeutic classes in older and younger PWH, respectively. This is in agreement with a previous [24] and a more recent analysis of the SHCS [34], showing that cardiovascular disease is the first cause of comedication prescription, followed by depression. In addition to a higher number of comedications, elderly PWH received more complex ART regimens, characterized by a higher propensity to cause DDIs, than younger patients, thus further complicating their treatment. These associations of multiple ART drugs are likely to result from of a longer history of HIV infection, with the acquisition of drug resistance leading to the need for more complex ARTs. Indeed, most of the younger PWH (39%) started their ARTs between 2000 and 2009, whereas half of the elderly PWH received their first ARTs between 1990 and 1999.
Complex ARTs’ associations with comedications would be expected to lead to an increased risk for DDIs in elderly PWH. Remarkably, our results did not demonstrate a higher frequency of potential DDIs in elderly PWH compared with younger patients. This observation could be explained by the fact that HIV clinicians of SHCS are well aware of the DDI potential of ART and therefore prescribe comedications devoid of interaction potential, particularly in the elderly. Of interest, the rate of red flag potential DDIs was 3%, similar to the previous value of 2% reported in an analysis of the SHCS performed in 2010 [24], whereas the rate of amber flag potential DDIs was significantly lower, likely due to a larger proportion of patients shifted to unboosted INSTIs, now recommended as firstline therapy and characterized by more favorable DDI profiles than boosted PIs or NNRTIs [35, 36]. Nevertheless, red flag potential DDIs remain clinically significant, particularly in cases involving boosted PIs coadministered with clopidogrel. It has been demonstrated that clopidogrel’s active metabolite exposure was significantly reduced in PWH receiving boosted regimens, leading to insufficient inhibition of platelet aggregation in 44% of the patients [37]. Although prasugrel’s active metabolite exposure was decreased to a similar extent by boosted regimens, this has no negative effect on prasugrel’s pharmacodynamics, likely explained by its higher potency. Thus, prasugrel should be preferred in the presence of boosted regimens unless its use is contraindicated, in which case an alternative antiplatelet agent or ART should be considered.
Although the prevalence of potential DDIs remains important in PWH, our results demonstrated that potential DDIs notably with statins were generally managed correctly in real life through dosage adjustments, thereby reducing the probability of adverse events such as myalgia or even rhabdomyolysis. Due to our study design, it was not possible to assess the management of potential DDIs in an exhaustive manner, as for several drugs, like CNS drugs, a large range of drug doses is authorized, and dosage is adjusted mainly based on the clinical response and side effects. Finally, the prevalence of pharmacodynamic interactions was particularly low in our study due to the increasing use of tenofovir alafenamide, characterized by a lower nephrotoxicity compared with tenofovir disoproxil fumarate (TDF) [38].
PIMs were found in 31% of elderly PWH. This rate is lower than the rates reported in other studies, varying from 52% to 66% [7, 16, 17]. This difference may be explained by the fact that our study could not include all criteria defining inappropriate prescribing, for example, drugs prescribed without clinical indication, drugs administered beyond the recommended treatment duration, drugs not adjusted to the renal function of the patient, or prescribing omission. In addition, the prevalence of drugs with anticholinergic risk scale ≥3 was very low compared with the value of 17% reported by Greene et al. in PWH, even lower than the value of 4% that was reported in HIV-negative individuals in the same study [7]. This could possibly be explained by the lower number of prescribed comedications in our study (median, 4) compared with the publication of Green et al. (median, 6). In addition, differences in prescribing patterns between the United States and Europe could also explain this difference. In our study, inappropriate prescribing mainly resulted from benzodiazepines and hypnotics, which are associated with an increased risk of falls, impaired cognition, loss of independence, and hospitalization in the elderly [39]. Although clinicians might be aware of the risks associated with benzodiazepines or hypnotics in the elderly, they might not be able to stop such treatments, as patients become dependent.
Some limitations of our study should be acknowledged. First, although we focused on 2 therapeutic classes of interest, potential DDIs may also have occurred with other drug classes. Moreover, we did not assess the interactions between non-HIV comedications, resulting in an underestimation of the actual number of potential DDIs. Another limitation, common to all studies of this type, relies upon the fact that potential DDIs are assessed only between 2 compounds, which poorly accounts for the complexity of multiple and mutual DDIs encountered in polymedicated patients, not to mention pharmacogenetic issues. Finally, the lack of data about plasma drug concentrations and clinical outcomes arising from these potential DDIs prevented us from adequately evaluating their management. This was especially true for CNS drugs with a wide range of possible dosages and dosage adjustments mainly based on clinical situation.
Some strengths of our study should be emphasized nevertheless. The multicenter and prospective design provides valuable data about potential DDIs, as it reflects the general prescribing patterns and documents at best an individual’s complete drug regimen. Our large sample of PWH gives to our observations a fair degree of representativeness. To our knowledge, this is the first study prospectively analyzing prescriptions filled out by PWH.
In conclusion, high rates of polypharmacy and the consequent DDI potential suggest that particular attention is needed when prescribing treatments to elderly PWH. Although the use of unboosted INSTIs is growing, one-fourth of elderly PWH had complex ARTs acting as perpetrator of DDIs. The acknowledgment that some medications may be inappropriate for aged patients constitutes an additional burden in health care provision to elderly PWH. Thus with the aging HIV population, education on geriatric medicine principles and periodic review of medicines is warranted to limit the risk of inappropriate prescribing in this vulnerable population. Clinicians should maintain a proactive approach for the recognition and management of potential DDIs, as well as for other prescribing issues traditionally encountered in geriatric medicine.
Acknowledgments
We would like to thank the PWH who agreed to participate in this study and the following persons for their invaluable help with forms and blood sample collection as study coordinators and study nurses: Alexandra Mitouassiwou-Samba, Deolinda Alves, Rachel Ribeiro, Valérie Sormani, Vreneli Waelti Da Costa (Lausanne); and Kerstin Asal, Rebekka Plattner, Vreni Werder, Reinhild Harant, Irena Ferati (Basel).
Members of the Swiss HIV Cohort Study. Anagnostopoulos A, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Rudin C, Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
Financial support. This work was supported by 2 Swiss National Science Foundation, grant number 324730-165956 (Lausanne) and 324730-166204 (Basel), and by the OPO and the Isaac Dreyfus Foundations (Basel). This study has received the 2017 SHCS AbbVie Award (given to L.A.D. and C.M.).
Potential conflicts of interest. P.C., F.L., M.G., M.B., T.B., S.A.S., C.C., and L.A.D. have no conflicts of interest to declare. M.C. has received through his institution research grants from ViiV and Gilead and offered expert testimony for AbbVie, MSD, Gilead, and Sandoz. M.S. has received fees for advisory board participation from Gilead, ViiV, MSD, Sandoz, and Mepha, as well as grants for conferences from Gilead and MSD, unrelated to the present study. C.M. received a research grant from Gilead and speaker honoraria for her institution from MSD.
Author contributions. Study design: P.C., C.M., L.A.D. Recruitment of participants: M.B., M.C., M.S. Recording in a database: P.C., S.A.S. Analysis and interpretation of data: P.C., C.M., F.L., M.G., C.C. Manuscript draft: P.C., C.M., F.L. Critical review and approval of the manuscript: all authors.
Contributor Information
Swiss HIV Cohort Study:
A Anagnostopoulos, M Battegay, E Bernasconi, J Böni, D L Braun, H C Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, C A Fux, H F Günthard, D Haerry, B Hasse, H H Hirsch, M Hoffmann, I Hösli, M Huber, C R Kahlert, L Kaiser, O Keiser, T Klimkait, R D Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, C Marzolini, K J Metzner, N Müller, D Nicca, P Paioni, G Pantaleo, M Perreau, A Rauch, C Rudin, A U Scherrer, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, P Vernazza, G Wandeler, R Weber, and S Yerly
References
- 1. Hasse B, Ledergerber B, Furrer H, et al. ; Swiss HIV Cohort Study Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 2. Smit M, Brinkman K, Geerlings S, et al. ; ATHENA observational cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Womack JA, Murphy TE, Rentsch CT, et al. Polypharmacy, hazardous alcohol and illicit substance use, and serious falls among PLWH and uninfected comparators. J Acquir Immune Defic Syndr 2019; 82:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marzolini C, Livio F. Prescribing issues in elderly individuals living with HIV. Expert Rev Clin Pharmacol 2019; 12:643–59. [DOI] [PubMed] [Google Scholar]
- 6. Edelman EJ, Gordon KS, Glover J, et al. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging 2013; 30:613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc 2014; 62:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marzolini C, Back D, Weber R, et al. ; Swiss HIV Cohort Study Members Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 2011; 66:2107–11. [DOI] [PubMed] [Google Scholar]
- 9. Morillo-Verdugo R, Blanco Ramos JR, Abdel-Kader Martín L, Álvarez de Sotomayor M. The challenge of aging and pharmacoterapeutic complexity in the HIV + patient. Farm Hosp 2018; 42:120–7. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Centeno B, Badenes-Olmedo C, Mataix-Sanjuan A, et al. Polypharmacy and drug-drug interactions in HIV-infected subjects in the region of Madrid, Spain: a population-based study. Clin Infect Dis. 2019;. ciz811. [DOI] [PubMed] [Google Scholar]
- 11. Béïque L, Giguère P, la Porte C, Angel J. Interactions between protease inhibitors and acid-reducing agents: a systematic review. HIV Med 2007; 8:335–45. [DOI] [PubMed] [Google Scholar]
- 12. Moss DM, Siccardi M, Murphy M, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother 2012; 56:3020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barry M, Mulcahy F, Merry C, et al. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet 1999; 36:289–304. [DOI] [PubMed] [Google Scholar]
- 14. Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther 2005; 106:97–132. [DOI] [PubMed] [Google Scholar]
- 15. Marzolini C, Battegay M, Back D. Mechanisms of drug interactions II: transport proteins. In: Piscitelli CS, Rodvold AK, Pai PM, eds. Drug Interactions in Infectious Diseases. Totowa, NJ: Humana Press; 2011:43–72. [Google Scholar]
- 16. Livio F, Rrustemi F, Moffa G et al. Polypharmacy, drug-drug interactions and potentially inappropriate prescribing in elderly patients of the Swiss HIV Cohort Study. Paper presented at: 19th International Workshop on Clinical Pharmacology of Antiviral Therapy; 22–24 May 2018; Baltimore, MD. [Google Scholar]
- 17. McNicholl IR, Gandhi M, Hare CB, et al. A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy 2017; 37:1498–506. [DOI] [PubMed] [Google Scholar]
- 18. Swiss HIV Cohort Study. Available at: http://www.shcs.ch/. Accessed 07 August 2017.
- 19. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2017. Oslo: 2017. [Google Scholar]
- 20. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017; 17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019; 67: 674–94. [DOI] [PubMed] [Google Scholar]
- 22. Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–13. [DOI] [PubMed] [Google Scholar]
- 23. Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Heatlh 2008; 4: 311–20. [Google Scholar]
- 24. Marzolini C, Elzi L, Gibbons S, et al. ; Swiss HIV Cohort Study Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther 2010; 15:413–23. [DOI] [PubMed] [Google Scholar]
- 25. Liverpool HIV drug interactions website. Available at: http://www.hiv-druginteractions.org/. Accessed 20 December 2017.
- 26. European AIDS Clinical Society. Guidelines, version 9.0 2017. Available at: http://www.eacsociety.org/files/guidelines_9.0-english.pdf. Accessed 11 January 2018.
- 27. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV.Department of Health and Human; Services; Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 11 January 2018. [Google Scholar]
- 28. Food and Drug Administration. FDA Drug Safety Communication: Interactions between certain HIV or hepatitis C drugs and cholesterol-lowering statin drugs can increase the risk of muscle injury [news release]. Food and Drug Administration; March 1, 2012. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-interactions-between-certain-hiv-or-hepatitis-c-drugs-and-cholesterol. Accessed 20 December 2017. [Google Scholar]
- 29. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 30. European AIDS Clinical Society. Guidelines, version 9.0 2017 Available at: https://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 11 January 2018.
- 31. Food and Drug Administration. PREZISTA (Darunavir) Label. Food and Drug Administration; 2006. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021976s021lbl.pdf. Accessed 20 April 2018. [Google Scholar]
- 32. Guaraldi G, Malagoli A, Calcagno A, et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65 - 74 years and more than 75 years. BMC Geriatr 2018; 18:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nozza S, Malagoli A, Maia L, et al. ; GEPPO Study Group Antiretroviral therapy in geriatric HIV patients: the GEPPO cohort study. J Antimicrob Chemother 2017; 72:2879–86. [DOI] [PubMed] [Google Scholar]
- 34. Kamal S, Bugnon O, Cavassini M, Schneider MP. HIV-infected patients’ beliefs about their chronic co-treatments in comparison with their combined antiretroviral therapy. HIV Med 2018; 19:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baecke C, Gyssens IC, Decoutere L, et al. Prevalence of drug-drug interactions in the era of HIV integrase inhibitors: a retrospective clinical study. Neth J Med 2017; 75:235–40. [PubMed] [Google Scholar]
- 36. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA Panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsousi N, Samer CF, Fontana P, et al. Coadministration of ticagrelor and ritonavir: toward prospective dose adjustment to maintain an optimal platelet inhibition using the PBPK approach. Clin Pharmacol Ther 2016; 100:295–304. [DOI] [PubMed] [Google Scholar]
- 38. Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine 2016; 95:e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3:335–48. [DOI] [PubMed] [Google Scholar]