Abstract
Questions remain regarding the long-term impact of combat concussive blast exposure. While efforts have begun to highlight the clinical impact, less is known about neuroimaging trajectories that may inform underlying pathophysiological changes post-injury. Through collaborative efforts in combat, following medical evacuation, and at universities in the USA, this study followed service members both with and without blast concussion from the sub-acute to 1-year and 5-year outcomes with quantitative neuroimaging. The following two primary and two exploratory groups were examined: combat-deployed controls without blast exposure history ‘non-blast control’ and concussive blast patients (primary) and combat concussion arising not from blast ‘non-blast concussion’ and combat-deployed controls with blast exposure history ‘blast control’ (exploratory). A total of 575 subjects were prospectively enrolled and imaged; 347 subjects completed further neuroimaging examination at 1 year and 342 subjects completed further neuroimaging examination at 5 years post-injury. At each time point, MRI scans were completed that included high-resolution structural as well as diffusion tensor imaging acquisitions processed for quantitative volumetric and diffusion tensor imaging changes. Longitudinal evaluation of the number of abnormal diffusion tensor imaging and volumetric regions in patients with blast concussion revealed distinct trends by imaging modality. While the presence of abnormal volumetric regions remained quite stable comparing our two primary groups of non-blast control to blast concussion, the diffusion tensor imaging abnormalities were observed to have varying trajectories. Most striking was the fractional anisotropy ‘U-shaped’ curve observed for a proportion of those that, if we had only followed them to 1 year, would look like trajectories of recovery. However, by continuing the follow-up to 5 years in these very same patients, a secondary increase in the number of reduced fractional anisotropy regions was identified. Comparing non-blast controls to blast concussion at each time point revealed significant differences in the number of regions with reduced fractional anisotropy at both the sub-acute and 5-year time points, which held after adjustment for age, education, gender, scanner and subsequent head injury exposure followed by correction for multiple comparisons. The secondary increase identified in patients with blast concussion may be the earliest indications of microstructural changes underlying the ‘accelerated brain aging’ theory recently reported from chronic, cross-sectional studies of veterans following brain injury. These varying trajectories also inform potential prognostic neuroimaging biomarkers of progression and recovery.
Keywords: blast, concussion, imaging, longitudinal outcome
Patients with and without blast concussion were evaluated at sub-acute, 1-year and 5-year outcomes to investigate long-term neuroimaging blast sequelae. Analysis revealed significant differences in the number of reduced fractional anisotropy regions at sub-acute and 5-year outcomes. The secondary increase may provide insight into changes underlying the ‘accelerated brain aging’ theory.
Graphical Abstract
Graphical Abstract.
Introduction
While considerable research has begun to shed light on the early neuroimaging alterations associated with mild blast traumatic brain injury (TBI), the link to potential long-term brain changes remains less clear. Previous work has examined quantitative neuroimaging outcomes in combat-deployed veterans using both diffusion tensor imaging (DTI) and quantitative volumetrics, but most studies have been limited by single time point evaluations with exposure history based largely on self-report (Levin et al., 2010; Jorge et al., 2012; Matthews et al., 2012; Bazarian et al., 2013; Lindemer et al., 2013; Morey et al., 2013; Sorg et al., 2014; Tate et al., 2014; Yeh et al., 2014; Hayes et al., 2015; Taber et al., 2015; Trotter et al., 2015; Govindarajan et al., 2016; Miller et al., 2016; Ware et al., 2016). Questions regarding how these neuroimaging findings evolve or resolve following mild blast TBI treated in theatre remain. It is also unclear whether these imaging trajectories would vary by injury mechanism be it blast exposure or blunt head trauma in combat. Longitudinal efforts in civilian TBI have been largely focused on acute to early chronic time points with reports suggesting alterations in white matter microstructure evidenced by reductions in fractional anisotropy (FA) on DTI (Stokum et al., 2015; Edlow et al., 2016). One civilian study did follow a small number of patients with TBI over a 4-year period and found further degeneration in reductions in FA on DTI (Farbota et al., 2012), although it is unclear how translatable this may be to mild brain injury exposures sustained in combat. Through collaborative efforts at Kandahar Airfield, Camp Leatherneck, Landstuhl Regional Medical Center, and academic universities in the USA, we have been provided the unique opportunity to follow, and image with quantitative MRI, the very same patients from the point of injury in theatre (Adam et al., 2015) to both 1-year (Mac Donald et al., 2011) and 5-year outcomes. The primary objective of the current study was to analyse the quantitative DTI and volumetric neuroimaging trajectory in patients with blast concussion compared with service members deployed to combat who did not sustain any form of head injury exposure. A secondary exploratory objective was to investigate whether the trajectory of patients with blast concussion was similar or different to that of service members who sustained a concussion in combat not associated with a blast exposure for hypothesis generating purposes regarding the role mechanism may play on brain white matter microstructure over long-term outcome.
Materials and methods
Participants in this study were originally enrolled into one of the four previous cohorts between 2008 and 2013 (Mac Donald et al., 2011, 2014a, b, 2016, 2017; Adam et al., 2015). This is the 5-year evaluation in a continued prospective, observational, longitudinal research study. In this study, we report the longitudinal imaging outcomes in our two primary and two exploratory subject groups: combat-deployed controls without history of blast exposure ‘non-blast control’ and concussive blast TBI ‘blast TBI’ (primary) and combat concussion arising not from blast ‘non-blast TBI’ and combat-deployed controls with history of blast exposure ‘blast control’ (exploratory). Inclusion criteria have been reported elsewhere (Mac Donald et al., 2011, 2016; Adam et al., 2015). Briefly, participants were service members, deployed to the combat theatre, between 2008 and 2013, in which original enrollment was completed either directly in Afghanistan (Adam et al., 2015; Mac Donald et al., 2015) or following medical evacuation to Landstuhl Regional Medical Center in Germany (Mac Donald et al., 2011, 2016, 2017). Diagnosis of head injury was determined by trained medical personnel working in the TBI clinics in Afghanistan or Germany. For the concussive blast TBI group, all available clinical histories indicated blast exposure plus another mechanism of head injury, such as a fall, motor vehicle crash, and being struck by a blunt object. None suffered an isolated blast injury. All concussive blast and non-blast TBI subjects met the Department of Defense definition for mild, uncomplicated TBI (Management of Concussion/mTBI Working Group, 2009). All combat-deployed controls were clinically evaluated to be free of signs and symptoms of head injury for both the ‘non-blast’ and ‘blast’ control groups and additionally no history of blast exposure for the ‘non-blast control’ group. Prior psychiatric and TBI diagnoses were exclusions for all groups.
This study was approved by the University of Washington Institutional Review Board with additional approval from the US Army Medical Research and Materiel Command Institutional Review Board and carried out in accordance with the approved protocol. Reconsent for each follow-up evaluation was provided by all participants according to the Declaration of Helsinki; no surrogate consent was allowed. Active-duty military subjects were not paid for participation per government guidelines, though travel expenses to the follow-up evaluations were covered.
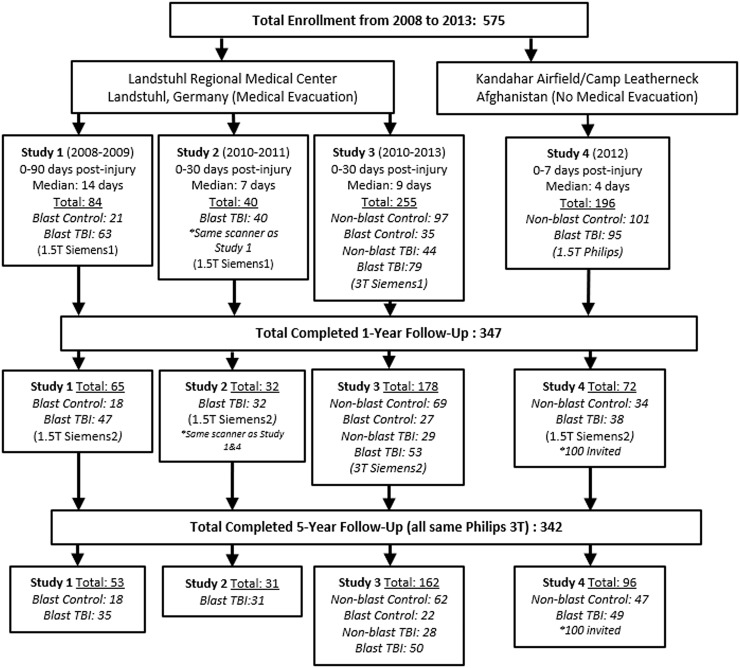
Through these efforts, 575 subjects have been prospectively enrolled and assessed with neuroimaging at the acute (0–7 days, median 4) and sub-acute (0–30 days, median 7–9; 0–90 days, median 14) time points; 347 of whom completed further neuroimaging examination at 1 year post-injury and 342 at 5 years post-injury. Figure 1 shows the enrollment flow diagram summarizing enrollment including details of the specific groups evaluated. Note that, due to funding restrictions, only a subset of Study 4 could be followed. Over the course of the studies, a variety of different MRI scanners have been used for evaluation. We have been at the mercy of whatever scanner was present in combat (Phillips 1.5 T—Study 4), following medical evacuation (Siemens 1.5 T—Studies 1 and 2; Siemens 3 T—Study 3), at 1-year follow-up (Siemens 1.5 T—Studies 1, 2 and 4; Siemens 3 T—Study 3), at 5-year follow-up (all on same Philips 3 T). For this reason, we strategically enrolled control participants to be scanned on every MRI scanner in which patients with TBI were to be scanned such that the quantitative imaging methods could be compared by scanner. These control participants were also invited back for follow-up as indicated in Fig. 1 to again be able to compare by scanner but also over time. Each MRI scan included a 1-mm isotropic MPRAGE and two diffusion-weighted imaging (DWI) acquisitions that were used for this analysis. At both the first and second time points, all studies acquired two 25-direction DWI acquisitions (Mac Donald et al., 2011; Studies 1–3) or two 15-direction DWI acquisitions (Adam et al., 2015; Study 4) on their respective scanners. At the third time point, all participants were imaged on the same scanner, which allowed for 32-direciton DWI acquisition to be collected with reverse polarity (A–P, P–A; Mac Donald et al., 2017). While the specifics of the protocols varied by scanner each also included a T2, FLAIR and T2*/GRE or SWI. At the first two time points, Studies 1 and 2 were acquired with 2D sequences for T2, FLAIR and T2*/GRE/SWI, while Studies 3 and 4 were acquired with 3D sequences for these structural acquisitions. At the third time point, all were acquired as 3D acquisitions and all were on the same scanner. At every time point, all scans were evaluated by a board-certified neuroradiologist and determined to be unremarkable for signs of brain injury pathology consistent with the radiological interpretations previously identified in these subjects (Mac Donald et al., 2011, 2017; Adam et al., 2015).
Figure 1.
Consort diagram of enrollment and longitudinal evaluation by scanner.
Diffusion tensor imaging postprocessing and analysis
The DTI postprocessing pipeline utilized for this study (Mac Donald et al., 2017) employed the analytical methods constructed by Dr. Carlo Pierpaoli and colleagues at the NIH called TORTOISE (Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble; Pierpaoli et al., 2010). Each DWI acquisition was initially run through DiffPrep (Rohde et al., 2004; Wu et al., 2008) in TORTOISE for susceptibility distortion correction, motion correction, eddy current correction and registration to a 3D high-resolution structural image. For EPI distortion correction, the diffusion images were registered to the 1-mm isotropic T2 image using non-linear b-splines. Eddy current and motion distortions were corrected using standard affine and quadratic transformations, followed by re-orientation of the b-matrix for the rotational aspect of the rigid body motion. When reverse polarity data were collected (Scan 3), the output images from both the A–P and P–A DWI acquisitions were then sent through Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging (DR-BUDDI; Irfanoglu et al., 2015) in TORTOISE for further EPI distortion and eddy current correction that can be completed with diffusion data that has been collected with reverse polarity. This step combines the reverse polarity imaging data to create a single, cleaned, DWI data set. Following this step, or in the absence of reverse polarity data, the output of Diffprep/DRBUDDI is then sent through DiffCalc in TORTOISE (Basser et al., 1994; Mangin et al., 2002; Chang et al., 2005, 2012; Rohde et al., 2005; Koay et al., 2006, 2009; Pierpaoli et al., 2010). This step completes the tensor estimation (Hoy et al., 2014) using the robust estimation of tensors by outlier rejection (RESTORE) approach (Chang et al., 2005). Following tensor estimation, a variety of DTI metrics are derived. For this study, FA was the primary DTI metric for analysis, but we secondarily examined other DTI metrics including mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
Following this postprocessing in TORTOISE, 3D image stacks for MD and FA were introduced into DTIstudio (Oishi et al., 2009; Zhang et al., 2010) for segmentation of the DTI atlas (Oishi et al., 2011) onto each participants DTI data set in ‘participant space’ through the Diffeomap programme in DTIstudio using both linear and non-linear transformations. This is a semi-automated process that allows for the extraction of DTI metrics within each 3D atlas-based region of interest providing a comprehensive sampling throughout the entire brain into 189 regions including ventricular space. For this study, selection of regions focused on regions of white matter, as the main hypothesis regarding DTI was that there would be reductions in white matter integrity observed with FA related to concussion. This reduces the number of regions used for further analysis to 78. To select only white matter, FA images were then threshold at 0.2 or greater and the data within each of the 78 regions of interest were extracted in ROIEditor on the FA or other coregistered DTI metric (MD, AD, and RD) image for further analysis (Mac Donald et al., 2017). All DTI images were inspected following postprocessing for any misalignment and image or signal artefacts by an imaging scientist, blinded to the clinical status of the subject, with expertise in DTI data processing and neuroanatomy. In addition, noise root mean squared values extracted from TORTOISE were assessed for image quality. These are known to vary by scanner so the advised approach is to examine the noise root mean squared values for the study groups of interest by MRI scanner to confirm that there is no systematic shift in image quality in particular to the patient group where concern over patient stability during the imaging session is heightened. Noise root mean squared values by group, by machine, at each time point, were compared, and no significant differences were identified (Table 1).
Table 1.
Noise root mean squared values by group for image quality comparison
| Scanner noted in consort diagram (Fig. 1) | Non-blast controls noise RMS |
Blast TBI noise RMS |
Blast control noise RMS |
Non-blast TBI noise RMS |
Unadjusted P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 1.5 T Siemens 1 | 10.2 | 0.5 | 10.5 | 1.7 | 0.3 | ||||
| 3 T Siemens 1 | 11.3 | 1 | 11.6 | 1.4 | 11.3 | 1 | 11.6 | 1 | *0.08 - 0.8 |
| 1.5 T Philips | 17 | 10.8 | 16.2 | 6.7 | 0.8 | ||||
| 1.5 T Siemens 2 | 11 | 0.5 | 10.6 | 0.8 | 10.3 | 0.9 | *0.07-0.2 | ||
| 3 T Siemens 2 | 12.8 | 1.2 | 12.4 | 1.3 | 12.8 | 1.2 | 13.3 | 1.2 | *0.1-0.8 |
| 3 T Phillips | 2 | 1.2 | 2.2 | 3.1 | 1.8 | 0.3 | 2.5 | 2.5 | *0.5-0.9 |
RMS, root mean squared.
All groups compared with non-blast controls.
Quantitative volumetric postprocessing and segmentation
Postprocessing of the 3D T1-weighted high-resolution structural MPRAGE image was completed using Freesurfer (Fischl et al., 2002) v5.3 for volumetric segmentation. Freesurfer is a semi-automatic segmentation programme for analysing volumetric data using a high-resolution structural T1-weighted image. This process is divided into two primary parts. The first part consists of sub-cortical/white matter surface creation and segmentation of the individual structures. The second part provides reconstruction of the cortical surface, created from the underlying white matter surface followed by parcellation of the cortical areas. Image processing steps include initial removal of extraneous, non-cortex/non-white matter tissue, motion correction and alignment with the MNI305 template. Spatial registration of the brain mask with the subsequent creation of surfaces in native Freesurfer RAS space is completed prior to the elimination of topological defects. After this, generation of the ‘pial/cortical’ and ‘white matter’ tessellated surfaces is completed. Quality assurance of the image processing included review of QA/QC measures including noise values with the cutoff of 15 for white matter SNR per Freesurfer guidelines (Kaufman, 2016), null values and fit measures. In addition, complete visual inspection in each orthogonal plane of the segmentation was done with the application of control points where needed to assure correct surface generation via the automated Freesurfer programme and to correct for small erroneous inclusions of other anatomy, such as blood vessels, dura and any white matter lesions. This was completed by an imaging scientist, blinded to the clinical status of the participant, who has been trained in neuroanatomy and is an expert using the Freesurfer processing software. A total of 167 subcortical and cortical regions in addition to total brain volume were examined for further analysis (Mac Donald et al., 2017).
Strategy for longitudinal neuroimaging
Given the known MRI scanner differences that often confound quantitative imaging studies, we intentionally enrolled and scanned controls at each site and followed them over time along with the TBI groups to help account for scanner variance impacting our quantitative imaging results. To appreciate any imaging changes over time, we have carefully computed standard scores for each DTI and volumetric regions of interest for each participant completed by scanner, at each time point, using the controls on the same scanner for normative data (mean and standard deviation). To compute standard scores for each region for the controls, we employed a ‘leave-one-out’ approach in which the control subject (n) is removed and the new control group (total excluding subject n) mean and standard deviation for each DTI and volumetric regional parameter is recomputed and then compared with the ‘left out’ control to obtain the standard scores for that specific control. This was done for every control by scanner at every time point. The standard scores for each DTI and volumetric region were flagged as regions more likely to be an unusual occurrence in a non-injured population, and suspicious for an abnormality, if the standard score was >2 SD away from mean control in the hypothesized direction. For FA and volume, this was below mean control while for MD, AD, and RD, this was above mean control, given the hypothesized directionality of brain injury pathology over time on these measures as reported in both preclinical and clinical brain injury studies (Mac Donald et al., 2007a, b; Niogi and Mukherjee, 2010). By computing standard scores and counting the number of abnormalities for each participant at each time point, we can more directly compare imaging findings over time in the very same subject and across scanners. This approach is also flexible to the heterogeneity of brain injury as it does not assume that all patients will be injured in the same brain region, a common critique of quantitative neuroimaging analysis in TBI studies that examine group differences by brain region. Controls used for each scanner were the non-blast controls. There is one exception which was for Scan 1 in Studies 1 and 2, only blast controls were scanned on that MRI scanner, so the standard scores were calculated for the blast TBI groups relative to the blast controls. Thus, the number of abnormalities for those patients with blast TBI at Scan 1 for these two groups may be somewhat underestimated.
Statistical analysis
Data analysis was completed in February 2019. Demographic characteristics among the four study cohorts were assessed for statistically significant differences using Kruskal–Wallis and Fisher’s exact tests as appropriate. Differences in the number of abnormalities among the cohorts at each time point were assessed using mixed-effects regression modelling. Due to the skewed distribution of number of abnormalities, a non-parametric approach was used to determine statistical significance in which the actual abnormality counts were replaced with their within-scan percentile, calculated as the rank within scan divided by the number of cases analysed for that scan time and multiplied by 100.
For the primary analysis comparing FA and volumetric abnormalities between the controls and blast TBI’s at each scan time, a single mixed-effects regression model for each metric was constructed using the data from all three scans from just these cohorts. Within-subject correlations were modelled using a random intercept and unstructured correlation matrix. Scan was modelled as a categorical effect, and a group-by-scan interaction was included in our model to account for group differences across scans. Since statistically significant demographic differences were observed among the four cohorts, our model adjusted for age, sex and education; however, since education was not consistently collected on all subjects, military rank (officer or enlisted) was used as a surrogate. Our model also adjusted for the number of subsequent head injury exposures since enrollment that was captured at 5-year follow-up. Although differences due to the specific scanner were already addressed by the conversion of raw values to standard scores as described above, a scanner effect was nevertheless included in our model as a precaution. We defined contrasts to test the significance of between-group differences at each scan. Lastly, the resulting P-values at the three scan times were interpreted in the context of multiple comparisons per Benjamini–Hochberg (Benjamini and Hochberg, 1995) using a false discovery rate of 5%.
The model equation is described as follows:
where is the outcome value for subject at scan (i.e. time point) . The term represents the main effect of scan relative to the reference Scan 1. The terms represent the effect of within-patient covariates that remain constant over scan (indicator variable group with the reference category as non-blast controls, sex, rank), while the terms represent within-patient covariates that can vary over time (participant age, number of head injury exposures after study entry, specific scanner used). The term represents the interaction between scan and group. The and terms represent the unobserved error terms for person and scan-within-person. Age and number of head injury exposures after study entry were modelled as linear effects; sex and rank were dichotomous; and scan time point and scanner were multi-level categorical variables with scan time point described by its two indicator variables for clarity. Although group would usually be considered a multi-level categorical variable, in these analyses, it was reduced to dichotomous since every model was based exclusively on data coming from the two groups being compared. All models utilized an unstructured covariance matrix.
All exploratory analyses carried out on the other FA and volumetric comparisons by groups, and secondary analysis of the other DTI measures were assessed with an approach and methodology identical to that of the primary analysis. In all cases, each regression model consisted of all three scans for just the two cohorts being compared, adjusting for all covariates described above regardless of significance, and the P-values for each of the three scans interpreted in the context of multiple comparisons.
Data availability
The data used for the analysis in this study are available through a data use agreement with the corresponding author.
Results
Evaluation of enrollment demographics across both the primary and exploratory subject groups did reveal significant differences in age, gender and education, which in addition to scanner, were adjusted for in all of the analyses prior to correction for multiple comparisons (Table 2). At 5-year follow-up, subsequent head injury exposure, defined as any exposure occurring after the point of enrollment, was collected and was used for statistical adjustment in addition to the four other parameters to determine group differences. For the two primary groups, the following total numbers of scans passed QA/QC for use in this analysis: 191 non-blast controls and 270 blast TBI (sub-acute), 96 non-blast controls and 167 blast TBI (1-year follow-up) and 103 non-blast controls and 156 blast TBI (5-year follow-up) with 47% of non-blast controls and 57% of blast TBI completing all three scans.
Table 2.
Participant characteristics at enrollment
| Characteristic | Non-blast CTL (n = 198) | Concussive blast TBI (n = 277) | Blast-exposed CTL (n = 56) | Non-blast TBI (n = 44) | P-Value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 30.1 ± 7.4 | 26.3 ± 6.3 | 32.3 ± 8.2 | 29.5 ± 9.2 | <0.001 |
| Education in years, mean (SD) | 14.9 ± 2.9 | 13.0 ± 1.7 | 13.6 ± 2.0 | 13.5 ± 2.0 | <0.001 |
| Gender, n (%) | |||||
| Male | 166 (84) | 268 (97) | 51 (91) | 40 (91) | <0.001 |
| Female | 32 (16) | 9 (3) | 5 (9) | 4 (9) | |
| Race/ethnicity, n (%) | |||||
| White | 140 (71) | 207 (75) | 42 (75) | 30 (68) | 0.145 |
| African American | 37 (18) | 25 (9) | 8 (14) | 9 (20) | |
| Hispanic/Latino | 20 (10) | 38 (14) | 4 (7) | 3 (7) | |
| Others | 1 (1) | 7 (2) | 2 (4) | 2 (5) | |
| Branch of service, n (%) | |||||
| US Army | 118 (59) | 232 (84) | 48 (86) | 36 (82) | <0.001 |
| US Air Force | 25 (13) | 3 (1) | 3 (5) | 3 (7) | |
| US Marine Corps | 14 (7) | 38 (14) | 5 (9) | 4 (9) | |
| US Navy | 41 (21) | 4 (1) | 0 (0) | 1 (2) | |
| Military rank, n (%) | |||||
| *Education surrogate | |||||
| Enlisted | 167 (84) | 267 (97) | 51 (91) | 42 (95) | <0.001 |
| Officer | 31 (16) | 10 (3) | 5 (9) | 2 (5) | |
Statistical significance by Kruskal–Wallis and Fisher’s exact tests as appropriate.
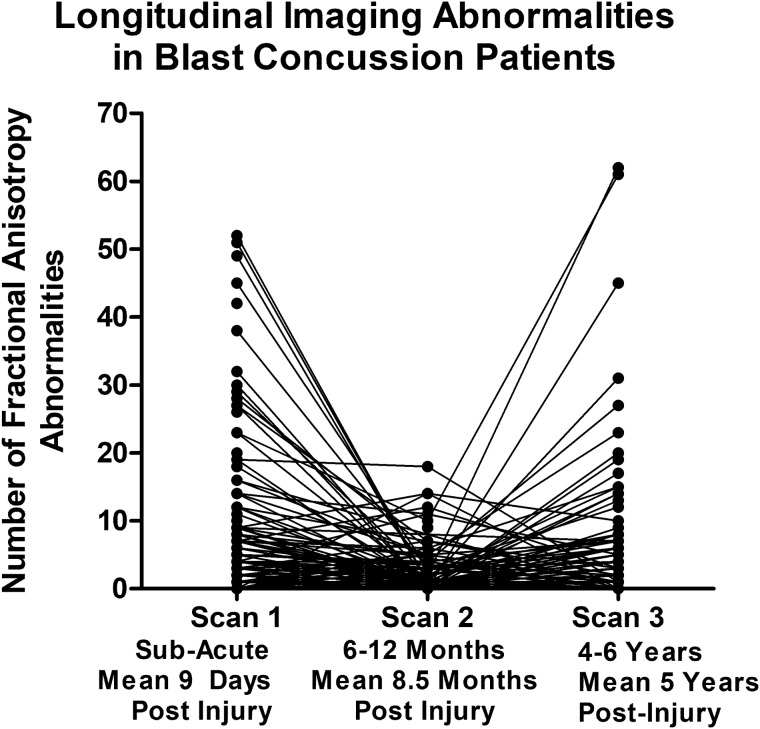
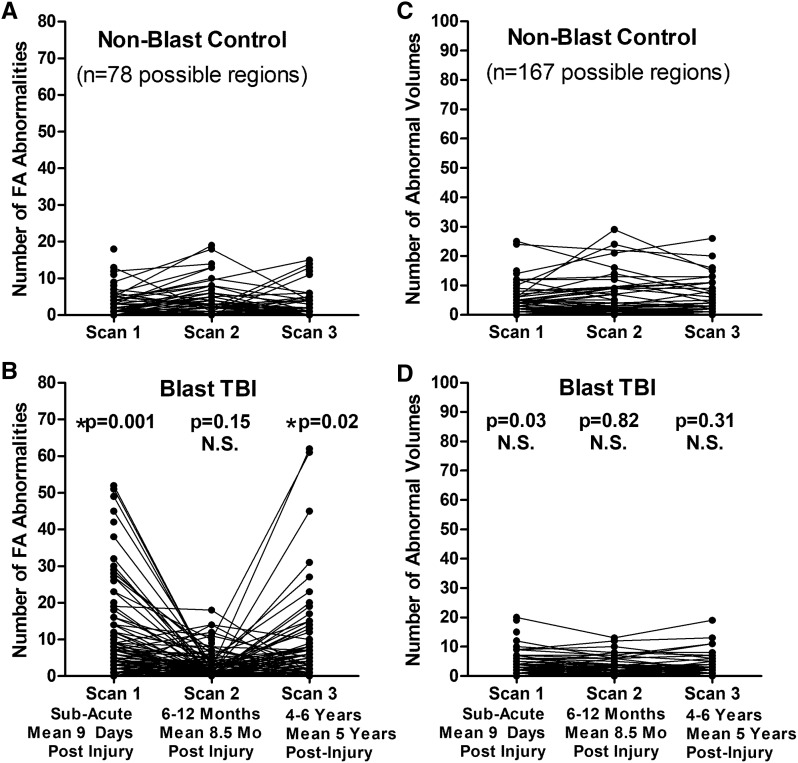
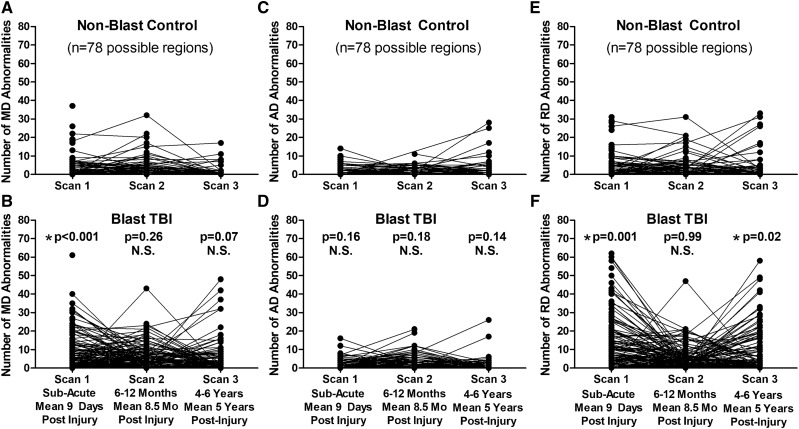
Longitudinal evaluation of the number of abnormal FA and volumetric regions in patients with blast concussion revealed distinct trends by imaging modality (Fig. 2). While the presence of abnormal volumetric regions remained quite stable comparing our two primary groups of non-blast control with blast TBI, the FA abnormalities were observed to have varying trajectories. Most striking was the ‘u-shaped’ curve observed for a proportion of those that, if we had only followed them to 1 year, would look like trajectories of recovery, but only by continuing follow-up in the very same participant to 5 years, a secondary increase in the number of reduced FA regions is identified. Overall, 23% of the abnormal regions at Scan 2 for non-blast controls were also abnormal at Scan 3. In blast TBI, 39% of the abnormal regions at Scan 2 were also abnormal at Scan 3. From the 1- to 5-year scans, the number of abnormal regions remained stable in 84% of non-blast controls with 10% showing a decrease of 5 or more abnormal regions and 6% of controls showing an increase of 5 or more abnormal regions. In contrast, from the 1- to 5-year scans, the number of abnormal regions remained stable in 76% of blast TBI while 4% had a decrease of 5 or more abnormal regions and 20% had an increase of 5 or more abnormal regions, which was significantly different than the non-blast controls (chi-squared P = 0.008). Comparing non-blast controls with blast TBI at each time point revealed significant differences in the number of regions with reduced FA at both the sub-acute and 5-year time points, which held after adjustment for age, education, gender, scanner and subsequent head injury exposure (5 years only) followed by correction for multiple comparisons. Further examination of the additional DTI metrics (MD, AD and RD) comparing our two primary groups of non-blast control with blast TBI revealed that the reductions in FA at the sub-acute and 5-year time points appeared to be mostly driven by increases in RD. There were significant differences in the number of regions with increased RD at both the sub-acute and 5-year time points in patients with blast TBI, which held after adjustment and correction (Fig. 3).
Figure 2.
Longitudinal FA and volumetric abnormalities in concussive blast TBI compared with non-blast control. (A and B) Comparison of the number of abnormal FA regions by time point identified significant differences in blast TBI at the sub-acute and 5-year, but not 1-year, evaluations compared with non-blast controls. (C and D) In contrast, there were no significant differences in the number of abnormal volumetric regions at any time point comparing the two groups. P-values reported are adjusted for age, education, gender and scanner at each time point. Subsequent head injury exposure was also collected at the 5-year evaluation and included with the other covariates for Scan 3 statistical adjustment to determine significance. Asterisk indicates P-values that remained significant after Benjamini–Hochberg correction for multiple comparisons.
Figure 3.
Longitudinal MD, AD and RD abnormalities in concussive blast TBI compared with non-blast control. (A and B) Comparison of the number of abnormal MD regions by time point identified a significant difference at the sub-acute, but not 1-year or 5-year, follow-up evaluations. (C and D) In contrast, there were no significant differences in the number of abnormal AD regions at any time point comparing the two groups. (E and F) Evaluation of abnormal RD regions over time revealed significant differences at the sub-acute and 5-year, but not 1-year, evaluations for blast TBI compared with non-blast controls parallel to the pattern observed with FA. P-values reported are adjusted for age, education, gender and scanner at each time point. Subsequent head injury exposure was also collected at the 5-year evaluation and included with the other covariates for Scan 3 statistical adjustment to determine significance. Asterisk indicates P-values that remained significant after Benjamini–Hochberg correction for multiple comparisons.
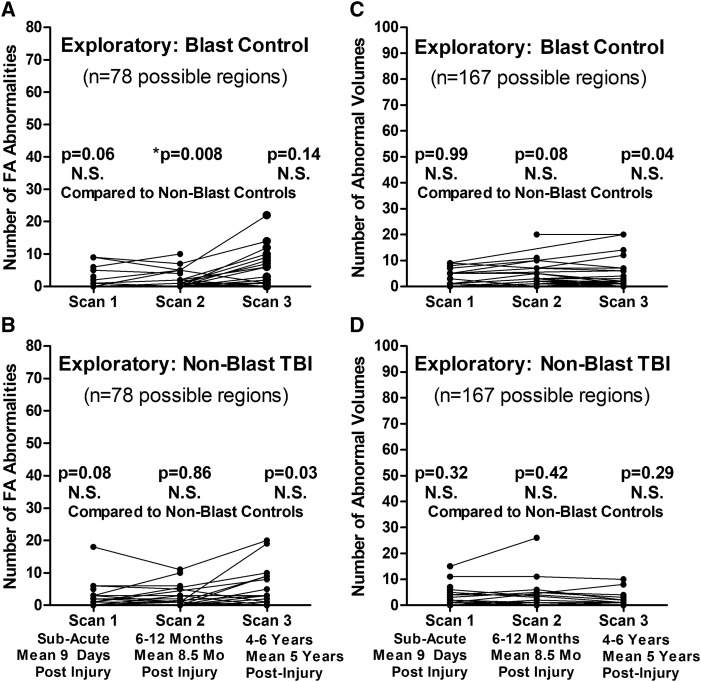
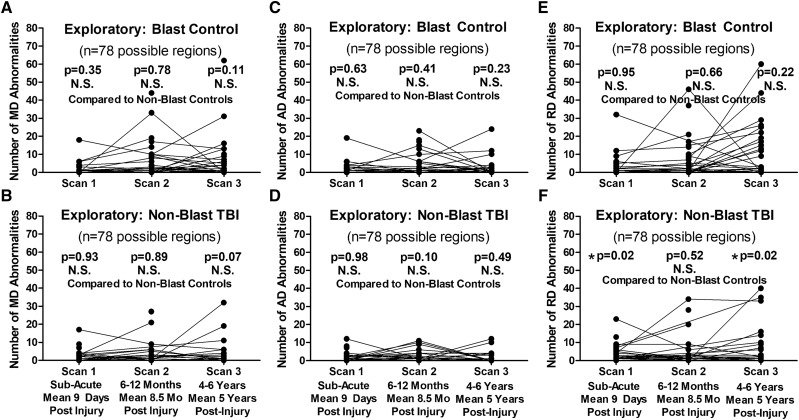
Exploratory analysis comparing both blast control and non-blast TBI participants with the non-blast control group as the main control group only identified a significant difference in the number of reduced FA regions at 1-year follow-up in the blast controls (Fig. 4). For the two exploratory groups, the following total numbers of scans passed QA/QC for use in this analysis: 33 blast controls and 42 non-blast TBI (sub-acute), 27 blast controls and 28 non-blast TBI (1-year follow-up) and 37 blast controls and 25 non-blast TBI (5-year follow-up) with 60% of blast controls and 52% of non-blast TBI completing all three scans. Furthermore, examination of the additional DTI metrics (MD, AD and RD) did not identify any significant differences for blast controls or non-blast TBI at any of the time points for MD and AD that held after adjustment and correction (Fig. 5). Only significant differences were observed in the number of regions with increased RD at the sub-acute and 5-year follow-ups but not 1-year follow-up in non-blast TBI paralleling the pattern observed in the blast TBI group. Caution is warranted in interpreting these results as we consider these findings hypothesis generating rather than definitive given the small group sizes of these two exploratory groups.
Figure 4.
Exploratory evaluation of longitudinal FA and volumetric abnormalities in blast-exposed controls and concussive non-blast TBI compared with non-blast control. Comparison of the number of abnormal FA regions by time point identified only a significant difference at the 1-year follow-up for blast-exposed controls (A) and no differences at any time point for non-blast TBI (B) when compared with non-blast control. There were also no significant differences in the number of abnormal volumetric regions at any time point for either the blast-exposed control (C) or non-blast TBI (D) compared with non-blast controls. P-values reported are adjusted for age, education, gender and scanner at each time point. Subsequent head injury exposure was also collected at the 5-year evaluation and included with the other covariates for Scan 3 statistical adjustment to determine significance. Asterisk indicates P-values that remained significant after Benjamini–Hochberg correction for multiple comparisons.
Figure 5.
Exploratory evaluation of longitudinal MD, AD and RD abnormalities in blast-exposed controls and concussive non-blast TBI compared with non-blast controls. Comparison of the number of abnormal MD regions by time point did not identify any significant differences for blast-exposed controls (A) or non-blast TBI (B) compared with non-blast controls. There were also no significant differences in the number of abnormal AD regions at any time point for either the blast-exposed controls (C) or non-blast TBI (D) compared with non-blast controls. For the number of abnormal RD regions, there were no differences for blast-exposed control (E), while there were significant differences at the sub-acute and 5-year time points but not 1-year time point for non-blast TBI (F) compared with non-blast controls. P-values reported are adjusted for age, education, gender and scanner at each time point. Subsequent head injury exposure was also collected at the 5-year evaluation and included with the other covariates for Scan 3 statistical adjustment to determine significance. Asterisk indicates P-values that remained significant after Benjamini–Hochberg correction for multiple comparisons.
Discussion
In summary, distinct time course patterns of imaging abnormalities were observed in concussive blast TBI compared with non-blast controls. While the volumetric abnormalities were quite stable, there were significant differences in the number of regions with reduced FA both at the sub-acute and 5-year, but not 1-year, follow-ups. This secondary increase may be the earliest indications of the microstructural changes underlying the ‘accelerated brain aging’ theory recently reported from chronic, cross-sectional studies of veterans following brain injury (Savjani et al., 2017). These findings are also in line with prior studies in veterans reporting reductions in FA by DTI using different analytical methods (Jorge et al., 2012; Morey et al., 2013; Hayes et al., 2015; Trotter et al., 2015; Miller et al., 2016). They extend and expand upon the excellent work of others to provide new insights into the trajectory of brain imaging changes from the sub-acute time period following injury in combat to 5-year outcome.
Strengths of the study include the longitudinal evaluation of both combat-related concussive blast patients and combat-deployed controls enrolled and followed since the point of injury and deployment; the additional exploratory groups of service members enrolled including those with combat concussions arising not from blast, as well as those with blast exposure but clinically evaluated to be free of signs and symptoms of head injury; the evaluation at the sub-acute, 1-year and 5-year time points; the employment of analytical methods that allowed for comparisons of quantitative neuroimaging over time with an approach flexible to the heterogeneity of brain injury; collection and adjustment of subsequent head injury exposures that could confound results; and the relatively large sample size of the two primary groups of comparison concussive blast TBI and combat-deployed controls at 100–200+ at every time point.
Limitations of the study include the modest sample size of the exploratory groups, the inability to obtain neuroimaging consistently on the same scanner for every participant over the period of longitudinal evaluation, the demographic imbalance that required statistical adjustment of the findings and unmeasured factors that could influence these neuroimaging results in ways not accounted for in the statistical analysis. In addition, it should be noted that the description of rank as officer or enlisted military status has limitations as a surrogate for education since some enlisted may go on to obtain a college degree as part of an extended career in the military.
In general, the strategy of identifying an occurrence that would be unusual in a non-injured population was employed here, as there is no gold standard approach identified in the neuroimaging community to address the comparison of quantitative neuroimaging data across MRI scanners in the absence of a priori sequence harmonization. It is well recognized in the field that the post hoc harmonization of such quantitative data is met with many challenges to address all forms of systematic and machine-dependent variances. Our own strategy was to focus on collecting control participants on each machine and carefully follow them over time along with the patients to provide a sense of normative data on each machine that could be used for comparison. Then, we chose to focus on these unusual occurrences compared across machines over time as it would be inappropriate to directly compare DTI metrics, such as FA, given the machine differences. Summing up the number of these unusual occurrences in each patient provided a representative metric of brain alternations in white matter microstructure that we believe is more robust to hardware differences than direct DTI metric comparison. We acknowledge that the approach we have employed in this study is merely one of many proposed strategies, each of which comes with limitations. As the field advances, there may be newer methods developed that will better address these challenges. There is inherent selection bias that we cannot overlook given the somewhat arbitrary nature of our criterion for how we defined an unusual occurrence in addition to the selection criterion for white matter thresholding. While we tried to use cutoffs that would reduce the erroneous inclusion of normal variation in the data and be more likely to identify a true unusual occurrence, we cannot rule out the possible influence of type I errors in this approach that focuses on individual scan data. To this end, we cannot say for certain that an alternative strategy would glean the same conclusions. We further recognize that the complex logistical nature of the study, literally following patients around the world, did not allow for the utilization of a single site scanner scenario, or a priori sequence harmonization and is more in line with the reality of complex longitudinal patient care. This is not to excuse any limitation in our study design but to merely acknowledge the limitation it introduces in examination via neuroimaging in such a patient population. Hopefully, these efforts will motivate further funding initiatives and large-scale research consortia that can better address these logistical hurdles in future studies.
The identification of neuroimaging changes after 1-year evaluation in a proportion of service members underscores the importance of serially evaluating these patients and emphasizes the evolution not resolution of brain imaging changes in a subset of these cases. Only by following the very same individual over time were these differences observed. These findings also provide support for the potential prognostic utility of this quantitative neuroimaging approach and highlight the role these imaging changes could play in potential stratification for clinical trial intervention. Further work is needed to better understand and validate whether this approach would be sensitive to identifying a therapeutic response as well as the implication of these findings as they pertain to brain aging and the potential link to downstream dementia.
Acknowledgements
We would like to thank the service members, their families, commanding officers and clinical providers for making this study possible. We are grateful for the assistance of the University of Washington Diagnostic Imaging Sciences team including Serena Bennett, Kris McKown and Liza Young for their support with the 5-year imaging acquisition and logistical planning. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the US Government, Department of Defense, or the US Department of Veterans Affairs, and no official endorsement should be inferred.
Funding
Funding for this longitudinal analysis and 5-year follow-up wave has been provided by a National Institute of Health RO1 grant from the National Institute of Neurological Disorders and Stroke (1R01NS091618-01) and Department of Defense grant through the Chronic Effects of Neurotrauma Consortium (W81XWH-13-2-0095) both awarded to C.M.D.
Competing interests
The authors report no competing interests.
Glossary
- AD
axial diffusivity
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- RD
radial diffusivity
- TBI
traumatic brain injury
References
- Adam O, Mac Donald CL, Rivet D, Ritter J, May T, Barefield M, et al. Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology 2015; 85: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D.. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994; 103: 247–54. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J.. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehab 2013; 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57: 289–300. [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C.. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 2005; 53: 1088–95. [DOI] [PubMed] [Google Scholar]
- Chang LC, Walker L, Pierpaoli C.. Informed RESTORE: a method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn Reson Med 2012; 68: 1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Copen WA, Izzy S, Bakhadirov K, van der Kouwe A, Glenn MB, et al. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: a longitudinal analysis. BMC Neurol 2016; 16: 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbota KD, Bendlin BB, Alexander AL, Rowley HA, Dempsey RJ, Johnson SC.. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci 2012; 6: 160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Govindarajan KA, Narayana PA, Hasan KM, Wilde EA, Levin HS, Hunter JV, et al. Cortical thickness in mild traumatic brain injury. J Neurotrauma 2016; 33: 1809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M.. The nature of white matter abnormalities in blast-related mild traumatic brain injury. Neuroimage Clin 2015; 8: 148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AR, Koay CG, Kecskemeti SR, Alexander AL.. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage 2014; 103: 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C.. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage 2015; 106: 284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry 2012; 169: 1284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman Z. wm-anat-snr-help; 2016. Available from: http://ftp.nmr.mgh.harvard.edu/pub/dist/freesurfer/dev_binaries/centos7_x86_64/wm-anat-snr (cited 20 May 2019).
- Koay CG, Chang LC, Carew JD, Pierpaoli C, Basser PJ.. A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J Magn Reson 2006; 182: 115–25. [DOI] [PubMed] [Google Scholar]
- Koay CG, Ozarslan E, Basser PJ.. A signal transformational framework for breaking the noise floor and its applications in MRI. J Magn Reson 2009; 197: 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma 2010; 27: 683–94. [DOI] [PubMed] [Google Scholar]
- Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP.. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin 2013; 2: 601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Adam OR, Johnson AM, Nelson EC, Werner NJ, Rivet DJ, et al. Acute post-traumatic stress symptoms and age predict outcome in military blast concussion. Brain 2015; 138: 1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Andre J, Evans N, Panks C, Sun S, et al. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clin 2017; 14: 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D.. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007a; 27: 11869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL.. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol 2007b; 205: 116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011; 364: 2091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol 2014a; 71: 994. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Nelson EC, Werner NJ, Fang R, Flaherty SF, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma 2014b; 31: 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline: management of concussion/mild traumatic brain injury. J Rehabil Res Dev 2009; 46: CP1–68. [PubMed] [Google Scholar]
- Mangin JF, Poupon C, Clark C, Le Bihan D, Bloch I.. Distortion correction and robust tensor estimation for MR diffusion imaging. Med Image Anal 2002; 6: 191–8. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Spadoni AD, Lohr JB, Strigo IA, Simmons AN.. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in Operations Enduring and Iraqi Freedom. Psychiatry Res 2012; 204: 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M.. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum Brain Mapp 2016; 37: 220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, et al. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp 2013; 34: 2986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P.. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehab 2010; 25: 241–55. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 2009; 46: 486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Van Zijl PC, Mori S, MRI atlas of human white matter. 2nd edn Oxford, UK: Elsevier; 2011. [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett AS, Basser P, Chang L-C, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. In: 18th annual meeting on International Society for Magnetic Resonance in Medicine; 2010.
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C.. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med 2004; 51: 103–14. [DOI] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Pierpaoli C.. Estimating intensity variance due to noise in registered images: applications to diffusion tensor MRI. Neuroimage 2005; 26: 673–84. [DOI] [PubMed] [Google Scholar]
- Savjani RR, Taylor BA, Acion L, Wilde EA, Jorge RE.. Accelerated changes in cortical thickness measurements with age in military service members with traumatic brain injury. J Neurotrauma 2017; 34: 3107–16. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Delano-Wood L, Luc N, Schiehser DM, Hanson KL, Nation DA, et al. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehab 2014; 29: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Sours C, Zhuo J, Kane R, Shanmuganathan K, Gullapalli RP.. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Injury 2015; 29: 47–57. [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, et al. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehab 2015; 30: E15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, York GE, Reid MW, Cooper DB, Jones L, Robin DA, et al. Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav 2014; 8: 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter BB, Robinson ME, Milberg WP, McGlinchey RE, Salat DH.. Military blast exposure, ageing and white matter integrity. Brain 2015; 138: 2278–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JB, Biester RC, Whipple E, Robinson KM, Ross RJ, Nucifora PG.. Combat-related mild traumatic brain injury: association between baseline diffusion-tensor imaging findings and long-term outcomes. Radiology 2016; 280: 212–9. [DOI] [PubMed] [Google Scholar]
- Wu M, Chang LC, Walker L, Lemaitre H, Barnett AS, Marenco S, et al. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. Med Image Comput Comput Assist Interv 2008; 11: 321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Wang B, Oakes TR, French LM, Pan H, Graner J, et al. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp 2014; 35: 2652–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 2010; 52: 1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the analysis in this study are available through a data use agreement with the corresponding author.