Abstract
BACKGROUND
Oocyte aging has significant clinical consequences, and yet no treatment exists to address the age-related decline in oocyte quality. The lack of progress in the treatment of oocyte aging is due to the fact that the underlying molecular mechanisms are not sufficiently understood. BRCA1 and 2 are involved in homologous DNA recombination and play essential roles in ataxia telangiectasia mutated (ATM)-mediated DNA double-strand break (DSB) repair. A growing body of laboratory, translational and clinical evidence has emerged within the past decade indicating a role for BRCA function and ATM-mediated DNA DSB repair in ovarian aging.
OBJECTIVE AND RATIONALE
Although there are several competing or complementary theories, given the growing evidence tying BRCA function and ATM-mediated DNA DSB repair mechanisms in general to ovarian aging, we performed this review encompassing basic, translational and clinical work to assess the current state of knowledge on the topic. A clear understanding of the mechanisms underlying oocyte aging may result in targeted treatments to preserve ovarian reserve and improve oocyte quality.
SEARCH METHODS
We searched for published articles in the PubMed database containing key words, BRCA, BRCA1, BRCA2, Mutations, Fertility, Ovarian Reserve, Infertility, Mechanisms of Ovarian Aging, Oocyte or Oocyte DNA Repair, in the English-language literature until May 2019. We did not include abstracts or conference proceedings, with the exception of our own.
OUTCOMES
Laboratory studies provided robust and reproducible evidence that BRCA1 function and ATM-mediated DNA DSB repair, in general, weakens with age in oocytes of multiple species including human. In both women with BRCA mutations and BRCA-mutant mice, primordial follicle numbers are reduced and there is accelerated accumulation of DNA DSBs in oocytes. In general, women with BRCA1 mutations have lower ovarian reserves and experience earlier menopause. Laboratory evidence also supports critical role for BRCA1 and other ATM-mediated DNA DSB repair pathway members in meiotic function. When laboratory, translational and clinical evidence is considered together, BRCA-related ATM-mediated DNA DSB repair function emerges as a likely regulator of ovarian aging. Moreover, DNA damage and repair appear to be key features in chemotherapy-induced ovarian aging.
WIDER IMPLICATIONS
The existing data suggest that the BRCA-related ATM-mediated DNA repair pathway is a strong candidate to be a regulator of oocyte aging, and the age-related decline of this pathway likely impairs oocyte health. This knowledge may create an opportunity to develop targeted treatments to reverse or prevent physiological or chemotherapy-induced oocyte aging. On the immediate practical side, women with BRCA or similar mutations may need to be specially counselled for fertility preservation.
Keywords: BRCA, BRCA1/2, ovarian aging, mutations, oocyte, DNA repair, ovarian reserve, chemotherapy, ovarian response, anti-Mullerian hormone
Introduction
Women are born with a finite number of quiescent primordial follicles that first form in foetal ovaries around the end of the first trimester of pregnancy (Pelosi et al., 2015). These follicles are considered as the source of oocytes for entire female reproductive life, and menopause occurs when nearly all follicles are depleted (Faddy et al., 1992). During this protracted period, the majority of the primordial oocytes remain arrested in the first meiotic prophase and may be subjected to various endogenous and exogenous insults that may cause DNA damage (Roos and Kaina, 2013). DNA damage can occur in the form of both single- and double-strand breaks (DSBs) (Lips and Kaina, 2001). DNA DSBs represent the most deleterious and complex type of DNA damage, which may result in chromosomal instability and failed rearrangements (Vilenchik and Knudson, 2003). If DNA DSBs are not promptly and accurately repaired, they may cause lethal consequences for cells, including severe mutagenesis, carcinogenesis and apoptotic cell death (Roos and Kaina, 2006; Cohen et al., 2015). There are two main mechanisms of DNA DSB repair: nonhomologous end joining (NHEJ) and homologous recombination (HR) repair (Petersen and Cote, 2004). HR is based on using genetic information from a corresponding undamaged region on homologous chromosomes to replace the deleted information on the damaged strand, whereas two broken ends of the chromosome are ‘glued’ back together in NHEJ (Hustedt and Durocher, 2016). Because NHEJ cannot always replace the lost genetic information as it typically does not have access to a homologous strand for replication, with few exceptions, it is considered the error-prone repair mechanism for DNA DSBs (Cannan and Pederson, 2016). Although NHEJ is the main mode of DSB repair in mitotic cells and those that are in the G0–G1 phase of the cell cycle, HR plays the predominant role in cells that are in S-G2/M phase of the cell cycle and is likely to be the main pathway of choice responsible for DNA damage repair in oocytes (Kujjio et al., 2010). As HR uses an intact sister chromatid for regenerating the lost information in DNA and is tightly regulated by the Ataxia telangiectasia mutated (ATM)-mediated signalling pathways, it is a high fidelity, ‘error-free’ repair mechanism. Because it requires the availability of sister chromatids for repair and because primordial follicles are arrested at the G2/M phase of the cell cycle, and because error-free repair of genetic information is critical in germ cells, HR is hypothesised to be the physiologically dominant DNA repair mechanism in oocytes. However, recent data suggest that the NHEJ and HR pathways may not be entirely exclusive as BRCA may also be involved in the NHEJ type repair (Wu et al., 2010; Saha and Davis, 2016). Although it is not likely to be a main mechanism of repair in oocytes, further investigation is warranted on the role of NHEJ in ovarian aging.
BRCA1 and 2 are involved in homologous DNA recombination and play essential roles in ATM-mediated regulation of the DNA DSB repair (Venkitaraman, 2012). Numerous mutations in BRCA genes are associated with increased susceptibility to breast, ovarian and other cancer types (Lowery et al., 2018; Rao et al., 2018). While developing an ovarian stimulation protocol with aromatase inhibitors to reduce oestrogen exposure in women with breast cancer undergoing in vitro fertilisation for fertility preservation, we observed that women with BRCA mutations produced fewer oocytes and proposed that altered DNA DSB repair may be responsible for accelerated ovarian aging in these women (Oktay et al., 2010). Subsequent to these observations, we and others have completed numerous basic, translational and clinical studies investigating that hypothesis. In this manuscript, we will systematically review the evidence originating from those studies and summarise the current understanding of the role of DNA repair and BRCA mutations in human reproduction.
Methods
We searched for published articles in the PubMed database containing key words, BRCA, BRCA1, BRCA2, Mutations, Fertility, Ovarian Reserve, Infertility, Mechanisms of Ovarian Aging, Oocyte and Oocyte DNA Repair, in the English-language literature until May 2019. We did not include abstracts or conference proceedings because the data are usually difficult to assess. Out of 2972 articles identified initially, by cross-referencing 96 were found to be relevant and were evaluated carefully. Of these articles, 69 were laboratory studies, 26 were clinical studies and 1 was translational, including both laboratory and clinical data (Titus et al., 2013) (Fig. 1).
Figure 1.
Flow chart of study selection.
Overview of ATM-Mediated DNA DSB Repair via HR
While a detailed review of the ATM-mediated DNA DSB repair does not belong to this manuscript, we provide a brief explanation of the current understanding of this pathway to enhance our interpretation of the translational and clinical data (Fig. 2). As the focus of this review is human reproduction, we also highlighted the aspects of the pathway relevant to oocytes and meiotic cells in this brief description. DSBs represent a type of DNA damage in which two complementary strands of the double helix of DNA are damaged due to physical, chemical and biological stressors (Ashwood-Smith and Edwards, 1996). These severe brakes are first sensed by the MRN complex (MRE11, Rad50 and NBS1 complex) which not only activates ATM, the subsequent orchestrator of the HR repair pathway, but also serves as a platform for DNA repair (Lamarche et al., 2010; Dinkelmann et al., 2009; Titus et al., 2013). At the same time, Histone H2AX protein is phosphorylated (and is now called γ-H2AX) and attracted to DNA DSB sites to facilitate repair. This binding is on a one-to-one basis, and as a result, DNA DSBs can be quantitated by visualisation of γ-H2AX foci by immunohistochemical methods and this serves as a reliable method of DNA DSB assessment (Lowndes and Toh, 2005). γ-H2AX also has an activating effect on ATM which in turn activates other repair proteins, such as the BRCA1 and BRCA2 as well as check point regulators in the pathway. A partner in sensing DNA DSBs is 53BP1 which is also attracted to DNA DSB sites and activates ATM (Jackson and Bartek, 2009). BRCA1 has a more complex role in the pathway, compared to BRCA2, as it plays roles in damage sensing and HR as well as check point activation, while BRCA2 is mainly involved in the HR repair. This physiological distinction is important because, as will be seen later in the discussion, BRCA1 dysfunction appears to have more prominent and earlier impact on reproductive aging than BRCA2 dysfunction.
Figure 2.
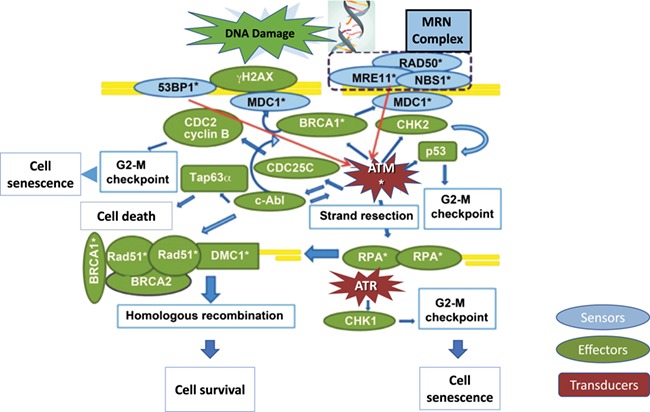
ATM-mediated DNA DSB repair pathway. DNA damage is sensed by the MRN complex (sensor of DSBs, consisting of MRE11, RAD50 and NBS1) and 53BP1 (sensor of changes in chromatin structure) which consequently activate ATM (red arrows). MDC1 binds to γH2AX via BRCA1 and is involved in the retention of the MRN complex to chromatin and accumulation of ATM, as well as mediation of the interaction between ATM and γH2AX. ATM phosphorylates γH2AX and activates downstream pathways leading to cell cycle arrest via CHK2 and inhibition of CDC2 (at the G2/M checkpoint, as applicable to oocyte), DNA repair (via activation of DNA strand resection which leads to homologous recombination) and/or apoptosis (via c-abl and TAp63α). DNA strand resection is necessary to invade in the homologous DNA strand. The resulting single-strand (ss) DNA is coated with RPA which in turn activates ATR and leads to cell cycle arrest (via CHK1). In germ cells, RPA is eventually replaced by Rad51 and DMC1 (the latter being germ-cell-specific) through a BRCA2-mediated process, which results in the initiation of homologous recombination. DSB sensor proteins are shown in blue, effectors are shown in green and transducers are shown in red. Molecules also involved in meiotic recombination are denoted with asterisk.
The activation of the ATM-mediated DNA DSB Repair Pathway leads to three potential outcomes. First and most desirable is the successful repair of the DSBs, as explained in Fig. 2. However, when the DNA damage is beyond repair, two other mechanisms have evolved to prevent cells with severe mutagenic information propagating themselves. One of these outcomes is c-ABL and/or TAP 63-alpha mediated activation of apoptotic pathways which result in the elimination of the damaged cells (Kerr et al., 2012; Hutt et al., 2013), although the role of ABL1 and ABL2 in activating apoptosis in response to cisplatinum treatment in oocytes has been challenged (Kim et al., 2018). However, commonly used gonadotoxic drugs in cancer treatment are cyclophosphamide and doxorubicin, and it is possible that different drugs may elicit different repair and damage responses. Regardless, this outcome may represent the main mechanism of age- and chemotherapy-induced accelerated primordial follicle loss, as will be discussed later. Another potential outcome is the prevention of these cells from progressing in the cell cycle by activation of cell cycle checkpoint proteins such as the CHK1. The latter results in what is called the cell senescence. This may represent the few primordial follicles that remain in the post-menopausal ovary and which never activate to result in follicle growth or ovulation.
Preclinical Assessment
Declining DNA DSB repair and accumulation of DNA DSBs in aging oocytes
Recent evidence, including our own results, suggests that the DNA DSBs accumulate with age, possibly due to reduced DNA repair capacity with age in the oocytes of humans and mice (Titus et al., 2013; Govindaraj et al., 2015; Oktay et al., 2015; Govindaraj et al., 2017). Following our initial observations of low ovarian response to stimulation in women with BRCA mutations (Oktay et al., 2010), we hypothesised that declining DNA DSB repair deficiency may be a factor responsible for age-induced accelerated primordial follicle loss and aging. To investigate this hypothesis, we took a combined laboratory and clinical approach (Titus et al., 2013). First, we showed that an increased percentage of primordial follicle oocytes were stained for γ-H2AX in ovaries of older females. Second, by single-cell real-time quantitative PCR, we showed that the expression of key ATM-mediated DNA DSB repair pathway members, such as BRCA1, ATM, MRE11 and RAD51, declined with age in human germinal vesicle (GV) oocytes, particularly after age 37. The latter finding correlates with the accelerated loss of human primordial follicles after age 37 (Faddy et al., 1992) as well as the sharp decline of fecundity and oocyte quality after that age (Oktay et al., 2015). Interestingly, even though we did not detect an overall significant decline in the expression of BRCA2 in GV oocytes with age in this group of women aged 23–41, (r-squared: 0.0253, slope −0.0171, P = 0.75), further analysis of the data revealed that BRCA2 expression showed a trend for decline after age 36 (r-squared 0.4201, slope −0.0161, P = 0.08), i.e. in the terminal years of reproductive life and later than that of BRCA1. This difference may explain the seemingly lesser impact of BRCA2 dysfunction on ovarian aging. While the above findings do not prove that the decline in the function of ATM-mediated BRCA-related HR repair is a vital mechanism of oocyte aging, our subsequent gene manipulation studies supported this notion. When key members of the ATM-mediated DNA repair pathway were knocked down, oocytes have become more susceptible to genotoxic stress, as will be discussed later (Titus et al. 2013).
Our finding of age-induced accumulation of DNA DSBs and reduced expression of DNA DSB repair genes has further been confirmed in several in vivo and in vitro studies in various species (Govindaraj et al., 2017; He et al., 2018). Govindaraj et al. recently showed considerable differences in the expression patterns of over 1000 genes involved in the network of oocyte meiosis, chromatin stability, chromosome segregation, spindle formation, DNA repair, gene transcription and apoptosis between immature and aged rat primordial follicles (Govindaraj et al., 2017). They also observed a significant age-related decline in the expression of mRNA for BRCA1 in primordial follicles of rats (Govindaraj et al., 2015). The same group extended their studies to water buffalo and studied the expression of BRCA1 related DNA repair genes in primordial follicles of young and adult buffaloes and showed a significant decrease in mRNA levels of BRCA1 in adult primordial follicles as compared to the young, which presumably resulted in inefficient DNA DSB repair (Govindaraj et al., 2016). While decreased expression does not directly prove that protein function is decreased, it is a highly probable explanation for age-related increase in DSBs in primordial follicles. Should the DNA DSB repair capacity have remained steady throughout life, one would expect steady levels of DSBs in primordial follicles throughout life due to constant repair. Furthermore, it may be questioned why the presence of DNA DSBs in primordial follicles does not immediately trigger apoptosis. Since we are looking at a static picture in tissue sections, we might be capturing the population immediately before apoptosis or, alternatively, there is likely a threshold of DSBs that has to be crossed before oocytes trigger apoptotic mechanisms and give up on repair. In fact, recent studies have suggested that, to enable repair to succeed, cells can tolerate a large number of DNA DSBs before triggering cell death mechanisms (Qiao et al., 2018).
In humans, microarray analysis of young and old metaphase II oocytes donated by patients undergoing ovarian stimulation has shown significant changes in the genes related to spindle checkpoint regulation, DNA stability and DNA repair. Moreover, it has been shown that responses to DNA damage and chromosome segregation are significantly affected by age (Grondahl et al. 2010). Others have shown that DNA damage in oocytes may result in a delay in meiosis resumption, the activation of spindle assembly checkpoint or germ cell apoptosis (Suh et al., 2006; Kerr et al., 2012; Marangos et al., 2015).
The importance of intact ATM-mediated DNA DSB repair via HR is apparent from many clinical syndromes. In syndromes resulting from mutations of the members of the ATM-mediated DNA DSB repair pathway, such as the Fanconi anemia (FA), ATM and Bloom (Uhrhammer et al., 1998; Thompson and Schild, 2002; Taniguchi T et al., 2006), females experience premature ovarian insufficiency and early menopause. Ovaries of mice with the Fanconi-gene mutation were found to be hypoplastic and the numbers of primordial follicles were reduced (Luo et al., 2004). Interestingly, FANCD1, one of the genes responsible for FA, is identical to BRCA2 and it was demonstrated that other proteins that are involved in the same syndrome, specifically FANCA and FANCD2, interact with BRCA1 (Garcia-Higuera et al., 2001). However, there has not been a mechanistic investigation in these groups of patients to determine whether they are born with lower endowment of primordial follicles or have accelerated follicle loss, or both.
However, genome-wide associations studies (GWAS) investigating the genetic determinants of age at natural menopause have provided further proof for the role of DNA repair in ovarian aging. Multiple GWAS have already identified several DNA repair genes as potential susceptibility genes of early natural menopause (Choi et al., 2007; Steuerwald et al., 2007; Sharov et al., 2008; Chowdhury et al., 2009). A large GWAS in ~70 000 women found genes related to DNA DSB repair, particularly BRCA1, to be critical in determining age at natural menopause (Day et al., 2015). This study also supported a common link between reproductive aging and breast cancer susceptibility.
A novel key component of the HR repair pathway, namely oocyte-expressed protein (Ooep), is required for efficient ATM kinase activation and Rad51 recombinase focal accumulation at DNA DSB locations. Ooep also plays a role in ATM activation and cell cycle checkpoint regulation (Xu et al., 2015; Zhu et al., 2015). It was recently found that Ooep-null GV oocytes are defective in DNA DSB repair, which results in increased susceptibility to apoptosis and delayed meiotic maturation upon exposure to DNA damaging insults (He et al., 2018). Notably, mRNA expression of Ooep in mouse oocytes was found to be reduced with advanced maternal age (He et al., 2018), consistent with the finding of an age-related decline in the expression of ATM-mediated DNA repair pathway members in women (Titus et al., 2013).
Some groups have also studied age-induced changes in DNA damage and repair in granulosa cells, although they are mitotic renewable somatic cells. A recent study found of γ-H2AX expression that there is age-induced accumulation of DNA DSBs in cumulus cells collected during oocyte retrievals (Sun et al., 2018). Aging cumulus cells showed a more frequent occurrence of early apoptosis and shortened telomere length than young cumulus cells (Sun et al., 2018). Likewise, Zhang et al. showed increased DSBs and decreased DNA repair efficiency in rhesus monkey granulosa cells due to ovarian aging (Zhang et al., 2015). However, in that study, the expression of BRCA1 did not change, suggesting that the function of the BRCA1 in granulosa cells is intact in middle-aged monkeys (Zhang et al., 2015). While the clinical significance of these findings remains to be understood, we have not found an increase in DNA damage in human primordial follicle pregranulosa cells, although we did not study DNA damage in granulosa or cumulus cells (Titus et al., 2013). It is, however, physiologically conceivable that the age-induced increased DNA damage in granulosa cells will contribute to declining oocyte health (Almeida et al., 2018).
Accelerated ovarian aging in BRCA-mutant mice
Other evidence on the role of BRCA function and DNA DSB repair efficiency in ovarian aging comes from transgenic mice deficient for BRCA1 or BRCA2 (Titus et al., 2013). In one study performed by us (Titus et al., 2013), the BRCA1-mutant mouse carried a deletion of 330 bp in intron 10 plus 407 bp in exon 11 (the largest exon) of the BRCA1 gene, which was previously shown to result in inefficient DNA repair (Huber et al., 2001). Because homozygous mutation of BRCA1 is lethal, we were only able to study the mice heterozygous for this deletion. For BRCA2, we studied mice that carried a deletion in exon 27, which impairs HR repair by preventing the BRCA2-RAD51 interaction (Donoho et al., 2003). Since this mutation does not result in lethality, both the heterozygote and homozygote mice were available for analysis. We found that BRCA1-mutant heterozygous mice produced fewer oocytes in response to ovarian stimulation compared with wild-type mice (14 ± 7.8 vs. 33.3 ± 0.9; P < 0.05) and had smaller litter sizes after mating (5.6 ± 1.5 vs. 7.6 ± 1.4 pups; P < 0.05). While we did not study the mechanism of reduced litter size in detail and ascribed it to lower ovarian reserve, this reduction could also be due to impaired embryo development, as our recent work with BRCA1 mutant male mice (Stobezki et al., 2019) showed reduced blastocyst formation and implantation rates when BRCA1 mutant males were crossed with wild-type females. The total primordial follicle numbers per ovary were lower in both the newborn (5-day) (2292.5 ± 163.8 vs. 3108 ± 96.1 P < 0.01,) and 4-month-old (408.3 ± 63.4 vs. 702.9 ± 79.5; P < 0.05) BRCA1 mutant heterozygous mice compared with the wild-type mice. These data could suggest that BRCA1 mutations may result in a lower endowment of primordial follicles, and since we did not study older mice, we do not know if follicle loss is accelerated in this model. However, while the extent of DNA damage as assessed by γH2AX expression in primordial follicles was similar at birth, by 4 months of life a higher percentage of primordial follicles became γH2AX-positive in BRCA1 mutant mice compared with wild-type mice (75.7 ± 2.7 vs. 58.8 ± 3.6; P < 0.01). In contrast to BRCA1 mutant mice, there were no differences between the BRCA2 mutant homo- or heterozygote mice and wild-type mice regarding the same variables. Again, the similarities of findings between the studies in women and these transgenic models are striking. As will be seen in the later sections, ovarian aging appears to be predominantly enhanced in women with BRCA1 mutations. As previously explained, this difference between BRCA1 and BRCA2 could be explained by the more complex role of the former in the ATM-mediated DNA DSB repair pathway and the much later decline in the function of BRCA2 with age.
BRCA gene manipulation alters sensitivity to genotoxicity
An independent approach to investigate the importance of DNA DSB repair in human oocytes is to use gene manipulation strategies. To achieve this, we knocked down the key ATM-mediated DNA repair pathway members, such as BRCA1, ATM, MRE11 and Rad51, by small interfering RNAs (siRNA) in mouse GV oocytes and exposed them to genotoxic stress in the form of H2O2. These were compared to similarly exposed mock-injected oocytes (Titus et al., 2013). We found that in the knockdown group, the survival rates were lower, and the percentage of apoptotic oocytes (as assessed by anti-caspase-3 staining) and the γH2AX foci were higher compared to mock-injected oocytes. Interestingly, the overexpression of BRCA1 by microinjection of cDNA in oocytes from old mice resulted in resistance to genotoxic stress and brought the in vitro survival to levels similar to that in young mouse oocytes. These data collectively show that intact ATM-mediated HR repair is acutely critical for oocyte survival and that, potentially, genetic manipulation of old oocytes can restore their ability to repair DNA DSBs to healthy levels.
Role of DNA DSB repair in chemotherapy-induced ovarian aging
We previously showed, in human ovarian tissue organ culture and xenografting models, that exposure to gonadotoxic chemotherapy agents such as doxorubicin results in the induction of DNA DSBs in primordial follicle oocytes and triggers massive apoptotic death (Soleimani et al., 2011). We also observed similar results with cyclophosphamide, a commonly used alkylating agent (Li et al., 2014). We additionally found that exposure to gonadotoxic chemotherapy results in the activation of ATM-mediated DNA DSB repair pathways, potentially rescuing some oocytes from chemotherapy-induced death (Soleimani et al., 2011). In the study by Soleimani et al., we showed that doxorubicin induces DSBs in mouse follicles which results in the activation of ATM-mediated DNA DSB repair mechanisms. However, many follicles with activated ATM pathways remain apoptosis-free (by anti-caspase-3 staining), suggesting that there is reversal of chemo-induced damage. Based on these preliminary findings, we hypothesised that chemotherapy-induced follicle death is determined by the balance between the severity of damage and the ability of the oocytes to repair that damage (Fig. 3). According to this hypothesis, primordial follicle oocytes that cannot repair severe DNA damage are directed to apoptotic death via the ATM-mediated pathway while those that can be repaired via the same pathway survive. In fact, this theory could explain why all primordial follicles are not lost upon exposure to chemotherapy. We further hypothesised that primordial follicles with a better ability to repair DNA DSBs may have a survival advantage (Bedoschi et al., 2019; Soleimani et al., 2011; Govindaraj et al., 2014) and oocytes might first attempt DNA repair then initiate apoptosis if repair fails. Furthermore, since our data suggests that the ability to repair DNA DSBs might decline with age, this may also explain the increased liability to follicle loss in older individuals (Titus et al., 2013). Although a recent study is supportive of our hypotheses (Nguyen et al., 2019), future laboratory and translational studies will be needed for further confirmation.
Figure 3.
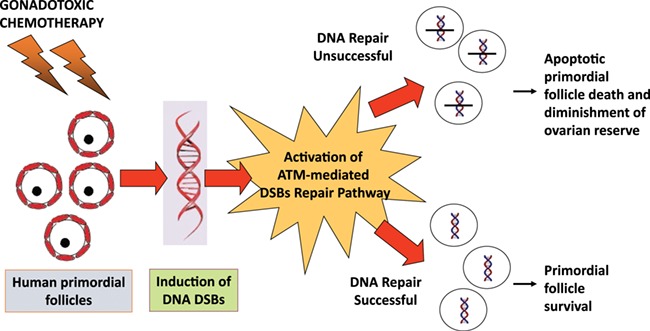
A proposed mechanism of chemotherapy-induced ovarian reserve loss through DNA damage. Primordial follicles have varying abilities to repair DNA double-strand breaks (DSBs) induced by gonadotoxic chemotherapy. When a primordial follicle suffers sufficient DNA damage which cannot be repaired by the ATM-mediated DNA DSB repair pathway, apoptotic pathways will be activated, resulting in follicle death. When on the other hand, DNA DSB repair is successful, the follicle will survive. This mechanism explains why not all follicles suffer the same fate after chemotherapy exposure, in most cases. Recent studies provided strong support for this hypothesis.
Other studies have also supported that oocytes have the capacity for repairing DNA damage induced by chemotherapy and aging through ATM-mediated HR pathway and that such repair mechanisms can be altered by targeting Bax and Rad51 (Kujio et al., 2010). Those studies found an inverse relationship between Bax and Rad51 expression. While the percentage of mature oocytes staining positive for Rad51 decreased with age, the Bax levels increased with age, and in a Bax-knockout model, oocytes exhibited improved DNA repair. These again suggest that there is a close relationship between chemotherapy-induced aging and ATM-mediated DNA DSB repair mechanisms.
Role of DNA DSB repair and BRCA gene function in oocyte quality
Age-dependent maternal aneuploidy is one of the key manifestations of ovarian aging (Anaya et al., 2013; Vaskivuo et al., 2001). Although the exact mechanism of the age-related increase in oocyte aneuploidy is not known, oocytes are exposed to numerous factors during the extended years that they remain in the prophase of first meiotic division which may impair oocyte quality (Jones, 2008). As DNA repair efficiency declines with age, DSBs accumulate and an increasing fraction of oocytes are eliminated to prevent genetic transition (Titus et al., 2013; Oktay et al., 2015; Rinaldi et al., 2017). It has been shown that failure to repair DSBs or misrepair may result in deletions, translocations and chromosome loss in oocytes (Di Giacomo et al., 2005; Cloutier et al., 2015). In mice, it has been shown that the declining function of BRCA1 may result in impaired meiotic spindle assembly and reduced chiasmata (Xiong et al., 2008). In addition, BRCA1 and the members of the ATM-mediated DNA DSB repair family are involved in the modulation of cohesins which regulate sister chromatid cohesion and migrate to DSB repair sites (Watrin and Peters, 2006). It has been demonstrated that in mouse oocyte chromosomes, the cohesin complex declines gradually as the mice age (Lister et al., 2010). In fact, evidence has been presented to show that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes (Chiang et al., 2010). Interestingly, a subunit of the Cohesin complex, SMC1, is regulated by the ATM-mediated HR pathway (Chiang et al. 2010), suggesting the possibility that the age-related decline in ATM-mediated DNA repair function may also negatively affect the function of cohesins. Furthermore, it has been demonstrated that old mouse oocytes may have shorter telomeres than those of young oocytes (Yamada-Fukunaga et al., 2013). BRCA1 and BRCA2 are also involved in telomere maintenance during aging process (McPherson et al., 2006; Cabuy et al., 2009; Rosen 2013) and BRCA1 mutations result in shortened telomeres (Rosen, 2013).
Another mechanism by which declining ATM-mediated HR may affect oocyte quality is through reduced chiasmata maintenance and frequency. At prophase I, when primordial follicle oocytes are arrested, HR occurs between sister chromatids. The same DNA repair mechanisms that naturally occur during HR are used by oocytes to mend DSBs (Shinohara et al., 2000). While this process increases genetic diversity, it is possible that it also plays a role in stabilising the metaphase plate by creating physical bonds between the chromatids. In fact, the crossovers associated with the HR events can have stabilising or destabilising effect on the genome. In meiosis, crossovers are highly regulated, such that at least one crossover occurs between each pair of homologous chromosomes, to ensure proper chromosome segregation, but excess numbers of crossovers are suppressed (Champion and Hawley, 2002). In mice, it has long been observed that the frequency of chiasmata decreases with age (Henderson and Edwards, 1968) and, in some strains of mice, age-related increases in aneuploidy rates in the offspring are associated with decreases in the frequency of recombination between chromosome homologs (Jones, 2008).
To sum up, the existing data suggest a regulatory role for ATM-mediated DSB repair/HR mechanisms in oocyte meiotic function and chromatid cohesion. However, further laboratory and translational research will be required to determine whether a decline in the function of the ATM-mediated DNA DSB repair pathway may play a role in age-induced aneuploidy.
Clinical Assessment
Ultimate confirmation of the role of BRCA function and DNA repair in ovarian aging and reproduction should come for clinical studies. Within the past decade, there have been numerous studies that have assessed various aspects of ovarian function in women with BRCA mutations. These include those that have studied: (i) the oocyte yield after ovarian stimulation, (ii) age at natural menopausal, (iii) serum anti-Mullerian hormone (AMH) levels, (iv) primordial follicle density and DNA damage in human ovarian tissue and (v) fertility outcomes. It is however important to understand the limitations of studying healthy women with BRCA mutations as they may only represent the tip of the iceberg (Fig. 4). Those with most severe BRCA dysfunction would either develop breast, ovarian or other cancers or undergo risk reducing salpingo-oophorectomy and lose their reproductive capacity at earlier ages. This would result in the elimination of individuals with the most severely impaired DNA repair mechanisms from the potential pool of subjects for research. In fact, initial observations on the impact of BRCA mutations on response to ovarian stimulation (Oktay et al., 2010) and serum AMH levels (Titus et al., 2013) were made on a small number of affected women undergoing fertility preservation procedures. These severely affected women would otherwise have received chemotherapy and their ovarian function could not have been properly assessed. Hence, studies that are studying unaffected women with BRCA mutations likely represent those with relatively lower impairment in DNA repair capacity and hence the magnitude of difference in ovarian function outcomes may also be smaller than in affected women. Given this caveat, we reviewed the available clinical data.
Figure 4.
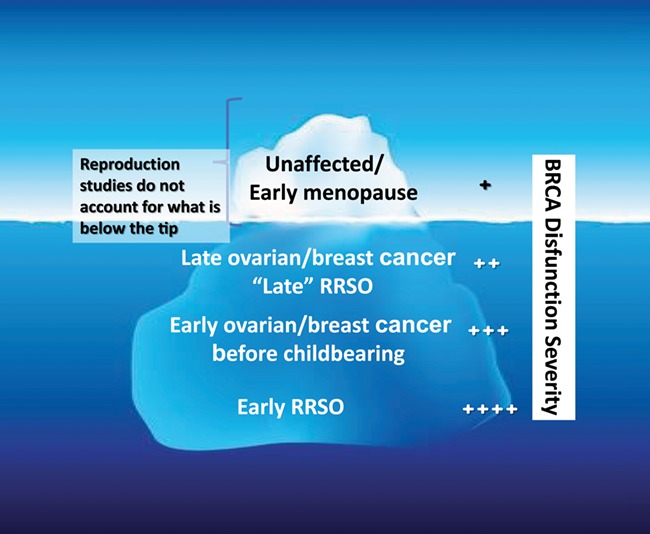
Explanation of the limitations in detecting BRCA mutation-related decline in ovarian reserve and fertility. RRSO: risk-reducing salpingo-oophorectomy.
BRCA mutations and ovarian response to stimulation
We identified five studies comparing oocyte yield after ovarian stimulation between BRCA carriers and controls (Oktay et al., 2010; Shapira et al., 2015; Derks-Smeets et al., 2017; Lambertini et al., 2018; Turan et al., 2018). Oktay et al. studied women with breast cancer who underwent fertility preservation before treatment and found that BRCA1 mutation carriers produced lower number of oocytes compared with controls (7.4 [95% CI, 3.1 to 17.7] in BRCA1 mutation carriers vs. 12.4 [95% CI, 10.8 to 14.2] in BRCA mutation negative and untested low risk controls; P = 0.025) (Oktay et al., 2010). However, the number of women with BRCA2 mutations was small and therefore the conclusions regarding the latter were not robust. In another study where fertility preservation cycle outcomes were compared between women with breast cancer and other malignancy types, it was found that BRCA mutation carriers produced lower numbers of oocytes after ovarian stimulation (Turan et al., 2018) even though the study was limited by the retrospective design. Lambertini et al. reported a trend for a lower number of oocytes (6.5 vs. 9; P = 0.145) and cryopreserved oocytes (3.5 vs. 6; P = 0.121) in BRCA-mutated breast cancer patients compared to controls in a retrospectively designed study. A low response rate occurred more frequently in the BRCA mutation group (40 vs. 11%; P = 0.147) and they required higher dose of gonadotropins (2775 vs. 2025 IU; P = 0.085) and longer stimulation (11.5 vs. 9 days; P = 0.110) (Lambertini et al., 2018). The authors indicated that although these differences were clinically significant, they could only show a statistical trend due to inadequate study power. In the study by Shapira et al., however, BRCA mutation carriers showed normal ovarian response to ovarian stimulation (Shapira et al., 2015). However, that study had several limitations, including the retrospective design, relatively young age of the cohort, inclusion of both affected and unaffected women, and inclusion of those who underwent in vitro fertilisation for preimplantation genetic diagnosis. In addition, varying ovarian stimulation protocols were included in that study. In another study, ovarian stimulation outcomes were compared between the unaffected BRCA mutation carriers aged 31.4 ± 3.7 years and controls who had mean age of 32.1 ± 4.1 years (Derks-Smeets et al., 2017). Although ovarian response rate was similar between the groups, the number of mature oocytes was found to be reduced in BRCA1 but not BRCA2 mutation carriers. In summary, although there are some limitations, the preponderance of evidence suggests that response to ovarian stimulation is reduced in women with BRCA mutations. However, randomised controlled studies comparing uniform groups are required to achieve more conclusive results.
BRCA mutations and menopausal age
We found five studies which assessed the influence of BRCA mutation status on age at natural menopause (Rzepka Gorska et al., 2006; Collins et al., 2013; Finch et al., 2013; Lin et al., 2013; van Tilborg et al., 2016a), and three of those found that age at natural menopause was lower in women with BRCA carriers compared with controls (Rzepka Gorska et al., 2006; Finch et al., 2013; Lin et al., 2013). Lin et al. compared unaffected BRCA1/2 mutated women in the Study of Women’s Health Across the Nation (SWAN) group and found that the median age at natural menopause in BRCA1/2 carriers was significantly earlier than in the control group (50 vs. 53 years, P value <0.001) (Lin et al., 2013). In another study by Finch et al., while the mean age at natural menopause was 48.8 and 49.2 years for unaffected BRCA1 BRCA2 mutation carriers, it was 50.3 years for controls (Finch et al., 2013). Different from these two studies, Rzepka Gorska et al. compared menopause age between carriers of the BRCA1 mutation and patients without mutation of BRCA. All women had a breast cancer diagnosis and it was found that the mean age at menopause was 45.3 years in carriers of the BRCA1 mutation while it was 48.2 in patients without the mutation (Rzepka Gorska et al., 2006). Two studies found no difference for age at natural menopause between the groups (Collins et al., 2013; van Tilborg et al., 2016a). In the study by Van Tilborg, the authors indicated that they could not support or refute that women with BRCA mutations have earlier age at natural menopause due to various types of selection bias including more women being censored at age >40 years if using oral contraceptive pills and a higher incidence of surgical menopause among carriers due to risk reducing bilateral salpingo-oophorectomy (28.5 vs. 7.5% in controls). Interestingly, when the authors separately analysed the earliest born cohort, which was less likely to be subjected to the aforementioned censoring biases (such as early prophylactic risk reducing bilateral salpingo-oophorectomy) due to lack of availability of BRCA mutation testing at that time, they did detect earlier age at natural menopause among women later found to be BRCA mutation carriers (van Tilborg et al., 2016a). In the case of the study by Collins et al., the outcome was the percentage of women reaching natural menopause during the study period. Although 445 women with BRCA1 and 361 women with BRCA2 mutations were initially included in the study, only a small fraction of these women reached natural menopause (11 and %13, respectively) due to risk reducing bilateral salpingo-oophorectomy (Collins et al., 2013). Moreover, higher number of carriers (40% for BRCA1 carriers and 29% for BRCA2 carriers) than controls (7% for both) were censored at cancer diagnosis, highlighting the difficulty in monitoring age at natural menopause with BRCA mutation carriers. Interestingly, a later study on the same cohort by the same group of investigators found that AMH levels were lower in women with BRCA mutations (Phillips et al., 2016). In summary, despite the fact that risk reducing salpingo-oophorectomy limited the methodology in some studies, women with BRCA mutations appear to experience menopause at earlier age.
BRCA mutations and AMH levels
There is a direct relationship between the number of early-stage follicles and serum AMH levels, and it is one of the best predictors known today to evaluate ovarian reserve (van Rooij et al., 2002). We identified 10 studies which investigated the association between BRCA mutations and AMH levels. Although there were some contradictory studies, the majority found lower AMH levels in women with mutations (Titus et al., 2013; Michaelson-Cohen et al., 2014; Wang et al., 2014; Giordano et al., 2016; Philips et al., 2016; van Tilborg et al., 2016b; Johnson et al., 2017; Ben-Aharon et al., 2018; Lambertini et al., 2018; Gunnala et al., 2019). These studies showed heterogeneity and differences in design (Table I). First, of the 10 publications, affected women with BRCA mutations were studied in only three (Titus et al., 2013; Gunnala et al., 2018; Lambertini et al., 2018). Second, studies compared various combinations of untested healthy women and women who tested negative for BRCA mutations in control groups to affected and/or unaffected women with or without BRCA mutation-type specification as the study groups. This heterogeneity makes it difficult to compare these studies. Third, in numerous studies, no adjustment was made for important confounding factors such as smoking, oral contraceptive use and body mass index. Fourth, AMH levels are not normally distributed and can vary by as much as three orders of magnitude. Negative studies, other than the one by Von Tilborg et al., did not adjust data for non-normal distribution. And finally, some studies included relatively younger women with mutations (van Tilborg et al., 2016b; Johnson et al., 2017) and one did not have sufficient power identifying a trend for lower AMH in affected women with BRCA mutations (Lambertini et al., 2018). Studying younger women will reduce the likelihood of detecting differences in ovarian reserve as the decline in the function of intact BRCA allele may not be significant until after age 35 years (Oktay et al., 2014; Giordano et al., 2016).
Table I.
Studies investigating the association between BRCA mutation and AMH levels.
| Study (year) | Design | Population | Findings | Limitations |
|---|---|---|---|---|
| Titus et al., (2013) | Prospective | Affected women BRCA1+ (n = 13, mean age: 34.8 ± 4.8 years) BRCA2+ (n = 9) BRCA1 + 2 (n = 2) BRCA− (n = 60, mean age: 36.3 ± 3.5 years) | Lower serum AMH with BRCA1 (P = 0.01) but not BRCA2 (P = 0.127) mutations compared to BRCA− controls | Small sample size. |
| Wang et al. (2014) | Cross-sectional | Unaffected women BRCA1+ (n = 62, mean age: 35.5 ± 5.2 years) BRCA2 + (n = 27, mean age: 35.6 ± 6.2 years) BRCA—(n = 54, mean age: 39.3 ± 3.7 years) | After adjusting for age and BMI, AMH was lower in BRCA1 (P = 0.026) but not BRCA2 carriers (P = 0.470) vs. BRCA− controls | No adjustment for potential confounders such as oral contraceptive use and smoking. |
| Phillips et al. (2016) | Cross-sectional | Unaffected women BRCA1+ (n = 172, mean age: 34.2 ± 5.7 years) BRCA1− (n = 216, mean age: 35.8 ± 5.8 years) BRCA2+ (n = 147 mean age: 34.4 ± 5.6 years) BRCA2− (n = 158 mean age: 35.8 ± 5.6 years) | After adjusting for smoking, age and body mass index, AMH was lower in BRCA1 (P = 0.02) but not in BRCA2 carriers (P = 0.94) compared to BRCA− controls. | The results may not be generalisable to those with severe mutations since unaffected women were included. |
| Johnson et al. (2017) | Prospective cohort | Unaffected women BRCA1 + (n = 55 mean age: 31.4 ± 5.5 years) BRCA2 + (n = 50 mean age: 30.9 ± 6.2 years) BRCA− (n = 26 mean age: 34.3 ± 6.7 years) Low risk controls (n = 64 mean age: 30.9 ± 5.6 years) | Significantly lower AMH among BRCA2 mutation carriers compared with low-risk control women | Relatively younger study groups. BMI was available for only 41% of the subjects. Affected women and women with RRSO were excluded. |
| Lambertini et al. (2018) | Secondary analysis of database from two previous studies | Affected women BRCA + (n = 29) BRCA− (n = 72) Median age 31 years [interquartile range 28–33 years] | Reduced reproductive potential in BRCA+ cohort. Median AMH 1.8 μg/l and 2.6 μg/l in the BRCA+ and BRCA− cohorts, respectively (P = 0.109). Trend for fewer oocytes being retrieved (P = 0.145) and cryopreserved (P = 0.121) in BRCA+ cohort 0.121). | Retrospective design, small sample size. |
| Michaelson-Cohen et al. (2014) | Retrospective | Unaffected women BRCA 1/2 + (n = 41 mean age: 33.2 ± 3.9 years) Healthy controls? (n = 324) | No difference in AMH | No adjustment for potential confounders Women with polycystic ovary syndrome were not excluded, BRCA1 and 2 mutation carriers were analysed collectively. AMH was not log-adjusted. |
| Giordano et al. (2016) | Prospective | Unaffected women BRCA1+ (n = 68, 66% aged under 35 years) BRCA− (n = 56, 41% aged under 35 years) | With adjustment for BMI, duration of birth control, smoking, gravidity, parity and age >35, BRCA1 was strongly associated with a low AMH (P = 0.037) | Small sample size. |
| Van Tilborg et al. (2016a) | Cross-sectional | Unaffected women BRCA 1/2+ (n = 124, median age: 29 [20–45] years) Non carriers (n = 131, median age: 31 [18–44] yrs) | Linear regression analysis adjusted for age, current smoking and current hormonal contraceptive use and did not detect lower AMH levels in BRCA mutation carriers | Relatively young mean age. BRCA1 and 2 mutation carriers analysed collectively. |
| Ben-Aharon et al. (2018) | Prospective | Unaffected women BRCA+ (n = 33, median age: 35 years) BRCA− (n = 15, median age: 34 years) | Lower serum AMH level in BRCA mutation carriers compared to negative controls | Small sample size. No adjustment for potential confounders. |
| Gunnala et al. (2019) | Retrospective cohort | Affected and unaffected women BRCA+ (n = 57, mean age: 32.4 ± 3.6 years) BRCA1+ (n = 31, mean age: 32.0 ± 3.5 years) BRCA2+ (n = 18, mean age: 33.4 ± 3.5 years) BRCA− (n = 738, mean age: 35.5 ± 4.3 years) | With adjustment for age and BMI, no difference was found in AMH levels. | Small sample size No adjustment for potential confounders Lack of log transformation of AMH Assume the controls are in fact BRCA-negative even though they were not tested. Selection bias (including patients that underwent fertility preservation and those with severe mutations that underwent RRSO were not taken into account) |
Abbreviations: AMH, anti-Mullerian hormone; BMI, body mass index; RRSO, risk reducing salpingo-oophorectomy
We analysed the impact of mutations in BRCA1 versus BRCA2 on AMH level compared to those who tested negative and found that AMH levels were lower in young women with germline BRCA1 mutations but not in women with BRCA2 mutations (Titus et al., 2013). However, our study was not sufficiently powered for subgroup analysis. In 2014, Wang et al. found lower AMH levels in unaffected BRCA1 carriers compared with noncarriers. Similar to Titus et al. and Wang et al., Giordano et al. showed that women who are BRCA1 mutation positive had significant decline in AMH levels especially after age 35 compared to women without BRCA mutations after adjusting for confounders such as BMI, duration of birth control, smoking, gravidity, parity and age > 35 (Giordano et al., 2016). The findings of two other studies were consistent with the aforementioned three studies (Philips et al., 2016; Ben-Aharon et al., 2018). While the studies where subgroup analysis was feasible showed lower AMH levels in women with BRCA1 mutations, one study found significantly lower AMH levels in BRCA2 but not BRCA1 mutation carriers compared with low-risk control (Johnson et al., 2017). In summary, although some studies pose weaknesses, the majority of studies support that women with BRCA mutations, particularly of the BRCA1 gene, have lower serum AMH levels.
BRCA mutations and histopathologic examination of ovarian tissues
The ultimate proof of reduced ovarian reserve in women with BRCA mutations should come from the direct assessment of the primordial follicle reserve. There have been multiple studies that have looked at the primordial follicle density in women with BRCA mutations. Pavone et al. examined ovaries of women who underwent risk reducing salpingo-oophorectomy because of BRCA (+) status or a strong family history of breast or ovarian cancer (mean age: 37.3 years) and compared them to those which were removed for benign indications (mean age: 36.5 years) (Pavone et al., 2014). They found that women with BRCA mutations had significantly decreased follicle counts compared to controls. We investigated primordial follicle density and DNA DSB accumulation in ovarian sections of unaffected BRCA mutation carriers (mean age: 36.5 ± 4.7 years) and compared them to the ovarian tissue from age-matched organ donor cadavers (Lin et al., 2017). We found that women with BRCA mutations had significantly lower primordial follicle density and increased DNA damage in primordial follicles compared to controls. We also found that the differences further diverged after age 30, indicating the acceleration of DNA damage and follicle death in women with BRCA mutations (Fig. 5). Lambertini et al. investigated the follicle density from women with breast cancer who underwent ovarian cryopreservation and found that women in the BRCA-positive cohort tended to have lower number of oocytes per fragment (0.08 vs. 0.14; P = 0.193) and per square millimetre (0.33 vs. 0.78; P = 0.153) than those in the BRCA-negative cohort (Lambertini et al., 2018). These differences did not reach statistical significance due to insufficient study power, according to the authors. These findings were further confirmed by Ben-Aharon et al., who showed that women with BRCA mutations have fewer primordial, primary, secondary and antral follicles in their ovaries compared to noncarriers (Ben-Aharon et al., 2018). Moreover, they showed that the levels of protein kinase B and AMH mRNAs, markers of cell survival and ovarian reserve respectively, were significantly lower in ovaries of BRCA carriers. In addition, in the same study, FGF23, which is known to increase with age, was higher in BRCA carriers than in the control group, suggesting that women with BRCA mutations may have overall accelerated aging. While none of these studies could practically assess total ovarian follicle counts and are subject to inherent variability of sampling ovarian cortex, these results collectively attest that women with BRCA mutations have lower ovarian reserve and possibly an overall accelerated aging status. In summary, there is evidence that primordial follicle numbers are reduced in women with BRCA mutations. While there is some evidence that follicle loss is accelerated in these women with age, we cannot rule out the possibility that women with BRCA mutations have started life with lower primordial follicle endowments.
Figure 5.
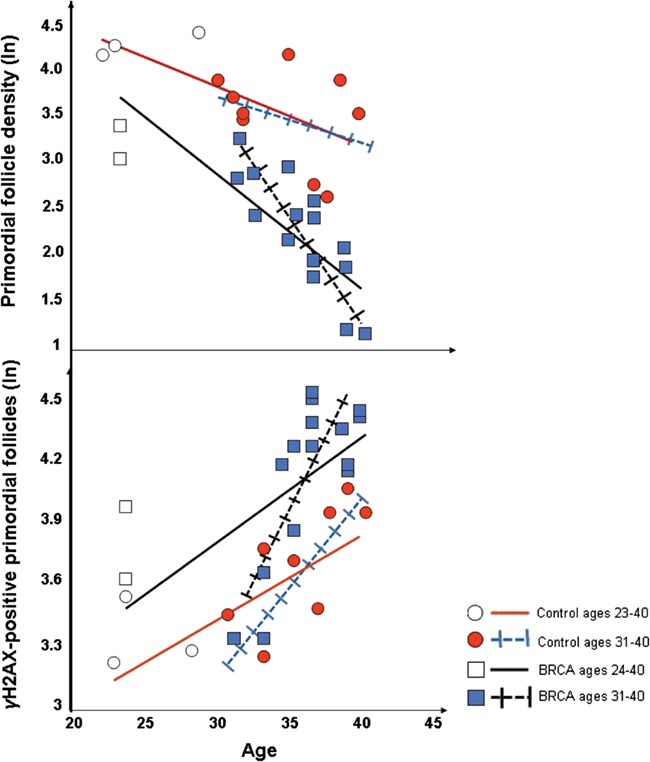
Impact of BRCA mutations on ovarian aging. In women with BRCA mutations, (A) primordial follicle density is lower and declines faster with age compared to controls, and (B) a higher fraction of primordial follicles accumulate DNA DSBs with age (as indicated by γH2AX expression). Both processes are accelerated after age 30.
BRCA mutations and fertility
We identified six studies investigating the association of BRCA mutations with fertility (Moslehi et al., 2010; Pal et al., 2010; Smith et al., 2012; Finch et al., 2013; Friedman et al., 2016; Giordano et al., 2016). Although four did not detect any differences between BRCA mutation carriers and controls (Pal et al., 2010; Moslehi et al., 2010; Friedman et al., 2016; Finch et al., 2013), one study detected lower gravidity and parity in women with BRCA1 mutations (Giordano et al., 2016) and interestingly, one study found increased fertility in women with BRCA mutations (Smith et al., 2012). However, these studies had numerous limitations. They were questionnaire-based retrospective analyses which may be subject to recall bias. Furthermore, the studies were not uniform in studying affected versus unaffected women and the findings were not adjusted for confounding factors such as oral contraceptive use and smoking as well as age. Only one study found significantly lower gravidity and parity in women with BRCA1 mutations compared to those without (43 vs. 68%; P = 0.007 for pregnancy and 40 vs. 60%; P = 0.04 for term birth in BRCA1 mutation positive versus negative respectively) (Giordano et al., 2016). Interestingly, while 66% of women with BRCA mutation were under 35 years, it was 41% in BRCA negative group in this study, a major limitation in studying fecundity in a BRCA mutation-positive population is again the exclusion of most severely affected cases by previous risk reducing salpingo-oophorectomy, cancer or premature ovarian failure from the study cohort. This leaves a less severely impacted group which may not have clinically significant differences from controls. Moreover, as was discussed before, in women with BRCA mutations there is still an intact allele which suffices for the maintenance of DNA repair. The function of the intact allele seems to begin to decline after age 30–35, resulting in clinical consequences probably after age 37–40 (Finch et al., 2013; Titus et al., 2013; Oktay et al., 2014; Oktay et al., 2015). This means that clinically significant differences may not be detectable in younger women. In fact, this point was raised in the discussion of the report by Finch et al. (2013) and in the accompanying commentary (Santoro, 2013).
Conclusions
In conclusion, available basic science, translational and clinical data indicate that intact BRCA function and related ATM-mediated DNA DSB repair might have an important role in the maintenance of ovarian reserve. Based on these combined data, we generated a hypothesis to explain ovarian aging by the age-related impairment of BRCA-related DNA DSB repair efficiency (Fig. 6). However, it is likely that many other factors and mechanisms are involved in oocyte aging besides diminished DNA repair. These possibly include endocrine, paracrine, genetic and metabolic factors. While clinical studies have not always provided uniform results due to numerous limitations, the preponderance of evidence indicates that women with BRCA mutations have reduced ovarian reserve. Evidence is also accumulating that women with pathogenic BRCA mutations may also have accelerated somatic aging. Nevertheless, future large prospective studies are needed to better understand the clinical significance of BRCA and similar mutations on fertility and aging in general. Moreover, an increasing number of novel mutations are being detected in other members of the ATM-mediated DNA DSB repair pathway in women with breast and ovarian cancers. These discoveries may create an opportunity to clinically study the role of other genes in the ATM-mediated DNA DSB repair pathway in ovarian aging. Furthermore, additional laboratory studies are needed to prove and better delineate the mechanisms by which DNA DSB repair deficiency may cause aneuploidy in oocytes (Fig. 6). If these mechanisms are clearly understood, we may be able to develop targeted treatments to reverse the age-related decline in DNA DSB repair function and slow down ovarian aging.
Figure 6.
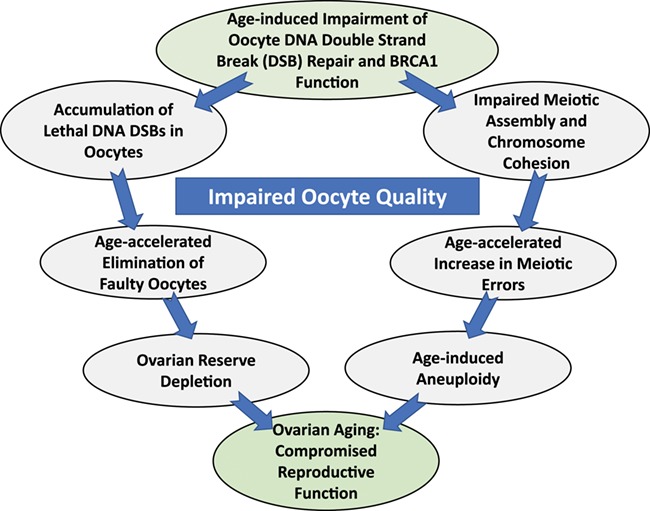
DNA repair theory of oocyte aging. The DNA DSB repair function declines with age, and this decline is accelerated after age 37. As a result, on the one hand an increasing fraction of primordial follicles accumulate severe DNA double-strand DNA breaks, which results in accelerated apoptotic loss of follicles and ovarian reserve diminishment. In parallel, since ATM-mediated DNA DSB repair and BRCA function are important in meiotic function and chromosome cohesion, aneuploidy risk increases with age, again in an accelerated fashion. If proven, this theory may explain age-related decline in oocyte reserve and quality and its acceleration after about age 37.
Authors’ roles
Conception of the idea: Kutluk Oktay; manuscript writing: both authors; final approval of manuscript: both authors.
Funding
National Institutes of Health (NICHD RO1HD053112).
Conflict of interest
None declared.
References
- Almeida CP, Ferreira MCF, Silveira CO, Campos JR, Borges IT, Baeta PG, Silva FHS, Reis FM, Del Puerto HL. Clinical correlation of apoptosis in human granulosa cells-a review. Cell Biol Int 2018;42:1276–1281. [DOI] [PubMed] [Google Scholar]
- Anaya Y, Tran ND. Delayed childbearing: impacts on fecundity and treatment. Med J Obstet Gynecol 2013;1:1009. [Google Scholar]
- Ashwood-Smith MJ. Edwards RG. DNA repair by oocytes. Mol Hum Reprod 1996;2:46–51. [DOI] [PubMed] [Google Scholar]
- Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med 2019;61:68–75. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I, Levi M, Margel D, Yerushalmi R, Rizel S, Perry S, Sharon E, Hasky N, Abir R, Fisch B et al. Premature ovarian aging in BRCA carriers: a prototype of systemic precocious aging? Oncotarget 2018;9:15931–15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 2014;343:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabuy E, Newton C, Slijepcevic P. BRCA1 knock-down causes telomere dysfunction in mammary epithelial cells. Cytogenet Genome Res 2009;122:336–342. [DOI] [PubMed] [Google Scholar]
- Cannan WJ, Pederson DS. Mechanisms and consequences of double-strand DNA break formation in chromatin. J Cell Physiol 2016;231:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Difilippantonio S, Difilippantonio MJ et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 2003;114:371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ et al. Genomic instability in mice lacking histone H2AX. Science 2002;296:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion MD, Hawley RS. Playing for half the deck: the molecular biology of meiosis. Nat Cell Biol 2002;4:s50–s56. [DOI] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010;20:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod 2007;77:312–319. [DOI] [PubMed] [Google Scholar]
- Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer Metastasis Rev 2009;28:225–232. [DOI] [PubMed] [Google Scholar]
- Cloutier JM, Mahadevaiah SK, ElInati E, Nussenzweig A, Toth A, Turner JM. Histone H2AFX links meiotic chromosome asynapsis to prophase I oocyte loss in mammals. PLoS Genet 2015;11:e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IS, Bar C, Paz-Elizur T, Ainbinder E, Leopold K, de Wind N, Geacintov N, Livneh Z. DNA lesion identity drives choice of damage tolerance pathway in murine cell chromosomes. Nucleic Acids Res 2015;43:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, Birch KE, Hopper JL, Phillips KA. Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their noncarrier relatives? Results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol 2013;31:3920–3925. [DOI] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks-Smeets IAP, van Tilborg TC, van A, Smits L, Torrance HL, Meijer-Hoogeveen M et al. BRCA1 mutation carriers have a lower number of mature oocytes after ovarian stimulation for IVF/PGD. J Assist Reprod Genet 2017;34:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A 2005;102:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelmann M, Spehalski E, Stoneham T, Buis J, Wu Y, Sekiguchi JM, Ferguson DO. Multiple functions of MRN in end-joining pathways during isotype class switching. Nat Struct Mol Biol 2009;16:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho G, Brenneman MA, Cui TX, Donoviel D, Vogel H, Goodwin EH, Chen DJ, Hasty P. Deletion of Brca 2 exon 27 causes hypersensitivity to DNA crosslinks, chromosomal instability, and reduced life span in mice. Genes Chromosomes Cancer 2003;36:317–331. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992;7:1342–1346. [DOI] [PubMed] [Google Scholar]
- Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, Neuhausen SL, Kim-Sing C, Tung N, Karlan B et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril 2013;99:1724–1728. [DOI] [PubMed] [Google Scholar]
- Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P, Neuhausen SL, Isaacs C, Weber B, Foulkes WD, Moller P et al. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res 2006;8:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell 2001;7:249–262. [DOI] [PubMed] [Google Scholar]
- Giordano S, Garrett-Mayer E, Mittal N, Smith K, Shulman L, Passaglia C, Gradishar W, Pavone ME. Association of BRCA1 mutations with impaired ovarian reserve: connection between infertility and breast/ovarian cancer risk. J Adolesc Young Adult Oncol 2016;5:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraj V, Keralapura Basavaraju R, Rao AJ. Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod Biomed Online 2015;30:303–310. [DOI] [PubMed] [Google Scholar]
- Govindaraj V, Krishnagiri H, Chakraborty P, Vasudevan M, Rao AJ. Age-related changes in gene expression patterns of immature and aged rat primordial follicles. Syst Biol Reprod Med 2017;63:37–48. [DOI] [PubMed] [Google Scholar]
- Grøndahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod 2010;25:957–968. [DOI] [PubMed] [Google Scholar]
- Gunnala V, Fields J, Irani M, D'Angelo D, Xu K, Schattman G, Rosenwaks Z. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril 2019;111:363–371. [DOI] [PubMed] [Google Scholar]
- He DJ, Wang L, Zhang ZB, Guo K, Li JZ, He XC, Cui QH, Zheng P. Maternal gene Ooep may participate in homologous recombination-mediated DNA double-strand break repair in mouse oocytes. Zool Res 2018;39:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature 1968;218:22–28. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Yang TW, Sarkisian CJ, Master SR, Deng CX, Chodosh LA. Impaired DNA damage response in cells expressing an exon 11-deleted murine Brca 1 variant that localizes to nuclear foci. Mol Cell Biol 2001;21:4005–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustedt N, Durocher D. The control of DNA repair by the cell cycle. Nat Cell Biol 2016;19:1–9. [DOI] [PubMed] [Google Scholar]
- Hutt K, Kerr JB, Scott CL, Findlay JK, Strasser A. How to best preserve oocytes in female cancer patients exposed to DNA damage inducing therapeutics. Cell Death Differ 2013;20:967–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki A, Schoenmakers S, Baarends WM. DNA double strand breakrepair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics 2010;5:255–266. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Sammel MD, Domchek S, Schanne A, Prewitt M, Gracia C. Antimüllerian hormone levels are lower in BRCA2 mutation carriers. Fertil Steril 2017;107:1256, e6–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RD, Jasin M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem Soc Trans 2001;29:196–201. [DOI] [PubMed] [Google Scholar]
- Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update 2008;14:143–158. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell 2012;48:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, Kurita T. Transient inhibition of p 53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ 2019;26:502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, Perez GI. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad 51. PLoS One 2010;5:e 9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche BJ, Orazio NI, Weitzman MD. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett 2010;584:3682–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA Jr, Desir J, Delbaere A, t'Kint de Roodenbeke MD, de Azambuja E, Ignatiadis M et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol 2018;29:237–243. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre 11-Rad 50-Nbs 1 complex. Science 2005;308:551–554. [DOI] [PubMed] [Google Scholar]
- Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod 2014;29:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab 2017;102:3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, Cedars M, Rosen M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer 2013;119:1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips J, Kaina B. DNA double-strand breaks trigger apoptosis in p 53-deficient fibroblasts. Carcinogenesis 2001;22:579–585. [DOI] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo 2. Curr Biol 2010;20:1511–1521. [DOI] [PubMed] [Google Scholar]
- Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer 2018;89:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes NF. Toh GW. DNA repair: the importance of phosphorylating histone H2AX. Curr Biol 2005;15:R99–R102. [DOI] [PubMed] [Google Scholar]
- Luo Y, Hartford SA, Zeng R, Southard TL, Shima N, Schimenti JC. Hypersensitivity of primordial germ cells to compromised replication associated DNA repair involves ATM-p 53-p 21 signaling. PLoS Genet 2014;10:e1004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V et al. Cloning and expression of the rat p 8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem 1997;272:32360–32369. [DOI] [PubMed] [Google Scholar]
- Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R. Carroll J. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun 2015;6:8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, Shehabeldin A, Porras A, Karaskova J, Vukovic B et al. A role for Brca 1 in chromosome end maintenance. Hum Mol Genet 2006;15:831–838. [DOI] [PubMed] [Google Scholar]
- Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer 2014;24:233–237. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Singh R, Lessner L, Friedman JM. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol 2010;22:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QN, Zerafa N, Liew SH, Findlay JK, Hickey M, Hutt KJ. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod 2019Apr 6. pii: gaz 020. doi: 10.1093/molehr/gaz020. [DOI] [PubMed] [Google Scholar]
- Nishihara K, Huang R, Zhao J, Shahane SA, Witt KL, Smith-Roe SL, Tice RR, Takeda S, Xia M. Identification of genotoxic compounds using isogenic DNA repair deficient DT40 cell lines on a quantitative high throughput screening platform. Mutagenesis 2016;31:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010;28:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Turan V, Titus S, Stobezki R, Mutations LLBRCA. DNA repair deficiency. and Ovarian Aging Biol Reprod 2015;93:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T, Keefe D, Sun P, Narod SA. Fertility in women with BRCA mutations: a case-control study. Fertil Steril 2010;93:1805–1808. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000;10:886–895. [DOI] [PubMed] [Google Scholar]
- Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, Schink JC, Woodruff TK. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci 2014;21:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE. Schlessinger D, Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol Reprod 2015;92:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Casellas R, Reina-San-Martin B et al. AID is required to initiate Nbs 1/γ-H2AX focus formation and mutations at sites of class switching. Nature 2001;414:660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Cote J. Cellular machineries for chromosomal DNA repair. Genes Dev 2004;18:602–616. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M. Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod 2016;31:1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Rao HBDP, Yun Y, Sandhu S, Fong JH, Sapre M, Nguyen M, Tham A, Van BW, Chng TYH et al. Impeding DNA break repair enables oocyte quality control. Mol Cell 2018;72:211, e3–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N. And Matzuk, MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004;305:1157–1159. [DOI] [PubMed] [Google Scholar]
- Rao R, Pointdujour-Lim R, Ganguly A, Shields CL. Multifocal choroidal melanoma in a patient with germ line BRCA-associated proteın 1 Mutatıon. Retin Cases Brief Rep Winter 2018;12:1–4. [DOI] [PubMed] [Google Scholar]
- Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 2017;67:1026, e2–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue A, Lafrance M, Gauthier MC, McDonald D, Hendzel M, West SC, Jasin M, Masson JY. Interplay between human DNA repair proteins at a unique double-strand break in vivo. EMBO J 2006;25:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 2002;17:3065–3071. [DOI] [PubMed] [Google Scholar]
- Roos WP. Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med 2006;12:440–450. [DOI] [PubMed] [Google Scholar]
- Roos WP. Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett 2013;332:237–248. [DOI] [PubMed] [Google Scholar]
- Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet 2013;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepka-Gorska I, Tarnowski B, Chudecka-Głaz A, Gorski B, Zielinska D, Tołoczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat 2006;100:59–63. [DOI] [PubMed] [Google Scholar]
- Saha J, Davis AJ. Unsolved mystery: the role of BRCA1 in DNA end-joining. J Radiat Res 2016;57:i18–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro BRCA mutations and fertility: do not push the envelope! Fertil Steril 2013;99:1560. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125) IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002;158:486–492. [DOI] [PubMed] [Google Scholar]
- Shapira M, Raanani H, Feldman B, Srebnik N, Dereck-Haim S, Manela D et al. BRCA mutation carriers show normal ovarian response in in vitro fertilization cycles. Fertil Steril 2015;104:1162–1167. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB et al. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol 2008;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Gasior S, Bishop D, Shinohara A. Tid 1/Rdh 54 promotes colocalization of rad 51 and dmc 1 during meiotic recombination. Proc Natl Acad Sci USA 2000;97:10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. Proc Biol Sci 2012;279:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online 2007;14:700–708. [DOI] [PubMed] [Google Scholar]
- Stobezki R, Titus S, Oktay K. Declining BRCA-mediated DNA repair in sperm aging and its prevention by sphingosine-1-phosphate. Reprod Sci 2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p 63 protects the female germ line during meiotic arrest. Nature 2006;444:624–628. [DOI] [PubMed] [Google Scholar]
- Sun XL, Jiang H, Han DX, Fu Y, Liu JB, Gao Y, Hu SM, Yuan B, Zhang JB. The activated DNA double-strand break repair pathway in cumulus cells from aging patients may be used as a convincing predictor of poor outcomes after in vitro fertilization-embryo transfer treatment. PLoS One 2018;13:e0204524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol 2006;7:739–750. [DOI] [PubMed] [Google Scholar]
- Suwaki N, Klare K, Tarsounas M. RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol 2011;22:898–905. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood 2006;107:4223–4233. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Schild D. Recombinational DNA repair and human disease. Mutat Res 2002;509:49–78. [DOI] [PubMed] [Google Scholar]
- van Tilborg TC, Broekmans FJ, Pijpe A, Schrijver LH, Mooij TM, Oosterwijk JC et al. Do BRCA1/2 mutation carriers have an earlier onset of natural menopause? Menopause 2016a;23:903–910. [DOI] [PubMed] [Google Scholar]
- van Tilborg TC, Derks-Smeets IA, Bos AM, Oosterwijk JC, van Golde RJ, Die-Smulders CE et al. Serum AMH levels in healthy women from BRCA1/2 mutated families: are they reduced? Hum Reprod 2016b;31:2651–2659. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda T, Fleming AB, Nickoloff JA, Osley MA. Chromatin remodelling at a DNA double strand break site in Saccharomyces cerevisiae. Nature 2005;438:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci 2018;25:26–32. [DOI] [PubMed] [Google Scholar]
- Uhrhammer N, Bay JO, Bignon YJ. Seventh international workshop on ataxia-telangiectasia. Cancer Res 1998;58:3480–3485. [PubMed] [Google Scholar]
- Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenback F, Heikinheimo M, Tapanainen JS. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab 2001;86:3421–3429. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Beyond cancer genomics: after the end of the beginning. Curr Opin Genet Dev 2012;22:1–2. [DOI] [PubMed] [Google Scholar]
- Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA 2003;100:12871–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril 2014;102:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrin E, Peters J. Cohesin and DNA damage repair. Exp Cell Res 2006;312:2687–2693. [DOI] [PubMed] [Google Scholar]
- White RR, Vijg J. Do DNA double-strand breaks drive aging? Mol Cell 2016;63:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lu LY, Yu X. The role of BRCA1 in DNA damage response. Protein Cell 2010;1:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong B, Li S, Ai JS, Yin S, Ouyang YC, Sun SC, Chen DY, Sun QY. BRCA1 is required for meiotic spindle assembly and spindle assembly checkpoint activation in mouse oocytes. Biol Reprod 2008;79:718–726. [DOI] [PubMed] [Google Scholar]
- Xu QH, Wang FC, Xiang YL, Zhang XX, Zhao ZA, Gao Z, Liu WB, Lu XK, Liu YS, Yu XJ et al. Maternal BCAS2 protects genomic integrity in mouse early embryonic development. Development 2015;142:3943–3953. [DOI] [PubMed] [Google Scholar]
- Yamada-Fukunaga T, Yamada M, Hamatani T, Chikazawa N, Ogawa S, Akutsu H, Miura T, Miyado K, Tarı’n JJ, Kuji N et al. Age-associated telomere shortening in mouse oocytes. Reprod Biol Endocrinol 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 2004;95:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki-Dizaji M, Akrami SM, Abolhassani H, Rezaei N, Aghamohammadi A. Ataxia telangiectasia syndrome: moonlighting ATM. Expert Rev Clin Immunol 2017;13:1155–1172. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, Wu X, Keefe DL, Liu L. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet 2015;32:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Yan LY, Zhang XX, Lu XK, Wang TR, Yan J, Liu XQ, Qiao J, Li L. Identification of a human subcortical maternal complex. Mol Hum Reprod 2015;21:320–329. [DOI] [PubMed] [Google Scholar]


