Abstract
The use of effective regimens for mitigating pain remain underutilized in research rodents despite the general acceptance of both the ethical imperative and regulatory requirements intended to maximize animal welfare. Factors contributing to this gap between the need for and the actual use of analgesia include lack of sufficient evidence-based data on effective regimens, under-dosing due to labor required to dose analgesics at appropriate intervals, concerns that the use of analgesics may impact study outcomes, and beliefs that rodents recover quickly from invasive procedures and as such do not need analgesics. Fundamentally, any discussion of clinical management of pain in rodents must recognize that nociceptive pathways and pain signaling mechanisms are highly conserved across mammalian species, and that central processing of pain is largely equivalent in rodents and other larger research species such as dogs, cats, or primates. Other obstacles to effective pain management in rodents have been the lack of objective, science-driven data on pain assessment, and the availability of appropriate pharmacological tools for pain mitigation. To address this deficit, we have reviewed and summarized the available publications on pain management in rats, mice and guinea pigs. Different drug classes and specific pharmacokinetic profiles, recommended dosages, and routes of administration are discussed, and updated recommendations are provided. Nonpharmacologic tools for increasing the comfort and wellbeing of research animals are also discussed. The potential adverse effects of analgesics are also reviewed. While gaps still exist in our understanding of clinical pain management in rodents, effective pharmacologic and nonpharmacologic strategies are available that can and should be used to provide analgesia while minimizing adverse effects. The key to effective clinical management of pain is thoughtful planning that incorporates study needs and veterinary guidance, knowledge of the pharmacokinetics and mechanisms of action of drugs being considered, careful attention to individual differences, and establishing an institutional culture that commits to pain management for all species as a central component of animal welfare.
Abbreviations and Acronyms: BW, bodyweight; CFA, Complete Freund adjuvant; ED, Effective dose; FI, Food intake; PONV, postoperative nausea and vomiting; SX, surgical procedure; SR, sustained release
Clinical management of pain in research rodents remains an important ethical and moral issue for IACUC, researchers, and veterinarians today. This is not surprising—pain management in human patients is still poorly characterized and under managed and remains one of the most common reasons that patients seek medical attention.7,22 Some aspects of inconsistency in rodent pain management may be attributable to unknown effective dosages of drugs for different strains of mice and rats, as well as challenges in assessing pain and pain mitigation in these animals. However, our review of the literature revealed that a large proportion of the inconsistent provision of adequate pain relief stems from either explicit or inferred socio-zoologic bias of the research community. For example, several studies have examined the methods of peer-reviewed papers that were published in highly ranked scientific journals and that involved surgery on research animals.26,43,174,199 Repeatedly, these studies demonstrate a significant underuse of peri-operative analgesics in mice and rats26,174,199 in contrast to much better reported use of analgesics in large animal (that is, primate, dog, and pig) surgical studies.43,44 Follow-ups with authors of publications not reporting use of analgesics in mice and rats for painful surgical procedures has not significantly altered these findings, suggesting that it is unlikely to be due to under reporting of analgesic administration.174,199
To begin to address rodent pain consistently in research settings, there must be fundamental recognition that all mammals, at the very least, have near identical nociceptive pathways and pain signaling mechanisms.158 Affective and cognitive processing of pain occurs as much in mice and rats as in primates and dogs, meaning that mice and rats are not somehow ‘less sentient’ species.127,193 Recognizing and admitting this simple concept should give IACUC, researchers, and veterinarians pause before submitting or approving research protocols that do not specify adequate pain relief for mice and rats. An appropriate question to reflect upon in every instance should be, “Would this protocol be approved in a dog or a primate under these same conditions?”. This question would go a long way toward improving consideration for pain management in mice and rats in research settings. An additional challenge for pain management in laboratory animal science has been the lack of objective pain indicators for some species.84,137 Ongoing research has begun to address these gaps, resulting in the development of validated pain assessment tools for mice and rats.
Considerations for types of pain based on underlying mechanisms
Recognition that mice and rats experience pain as much as other mammals is an important consideration when evaluating the types of pain (chronic or acute) to be managed. In both human and veterinary medicine, there is recognition of the importance of properly managing and treating acute pain, for ethical reasons and to prevent the condition from evolving into chronic pain— which is a more difficult condition to treat.97,158 Working estimates are not available for the type, intensity, and duration of pain experienced with different research animal models; however, much of the pain that occurs in induced models is caused by acute peri-procedural pain. This would include most surgical models, models in which animals are instrumented with catheters, implants or other devices, initial injections of irritating substances, such as carrageenan, and many tissue biopsy or invasive sampling methods.
Strict guidelines do not distinguish acute and chronic pain, consistent with recognition that pain occurs along a continuum.28 In human medicine, acute pain is considered to last up to 7 d after an initial event, but this limit can be modified by the severity, extent, and type of injury, and acute pain may last upward of 30 d or longer.114 The pathophysiology of pain initiation and subsequent inflammation has been described previously, with no evidence of any biomarkers that distinguish acute from chronic pain, except that central sensitization is more common in chronic pain.170
Although acute pain may have evolved to provide a protective response to the host, a key distinguishing feature between acute and chronic pain is the lack of any physiologic benefits derived from chronic pain. Chronic pain, particularly when persistent and unrelieved, can severely and negatively impact quality of life29 as a result of the onset of chronic maladaptive stress and hypothalamic-pituitary-adrenal gland axis activation, disruption of sleep, decreased functional and immune system performance, and impairment of social interactions.29 Thus, unless chronic pain is the object of scientific study, this should be between this state and be avoided or minimized by managing pain in its acute stage.
General approaches to clinical pain management in rodents
Multiple pharmacologic agents are available to manage pain in research animals. These agents have different mechanisms and duration of action as well as varying potencies for providing pain relief (see below). This permits the veterinarian, research team, and IACUC to tailor treatments, based on the invasiveness of a given procedure and its potential to cause pain. While the use of standard operating procedures is helpful to ensure consistent pain management in research facilities, it can be counterproductive to take a ‘one-size-fits-all’ approach to pain management in rodents, for example, if only a specific nonsteroidal antiinflammatory drug (NSAID) is used for all painful studies in a facility.
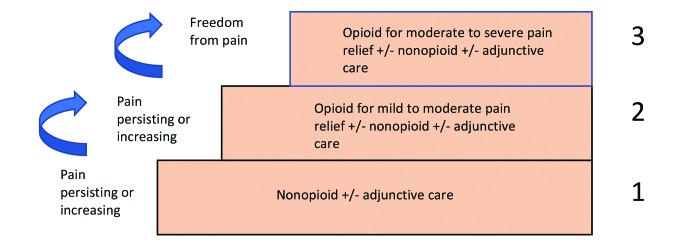
The World Health Organization (WHO) provides a stepped approach to pain management for humans that can be useful to consider when treating research animals (Figure 1).210 Certain steps in this ‘pain ladder’ can be skipped if the level of pain following a procedure is anticipated to be more severe, but it is useful to consider the full range of pharmacologic (and nonpharmacologic) options available for managing pain in research animals. Use of a systematic approach such as this allows treatment to be titrated to the amount of pain expected and observed. This type of approach helps to avoid both under and overuse of pain medications, both of which can be harmful to veterinary patients.
Figure 1.
WHO's Pain Relief Ladder for Patient Management (modified from https://www.who.int/cancer/palliative/painladder/en/). This ladder models an approach for the veterinary clinician, IACUC, and research team to use for pain management in laboratory rodents, based on the anticipated level of invasiveness of procedures being conducted. For example, for a short recovery procedure, such as jugular vein cannulation being conducted by a skilled surgeon, the animal may require peri-operative NSAID treatment in addition to excellent postoperative nursing care. In all cases, regardless of the approved protocol or SOP, each patient should be assessed after the procedure to ensure that pain is being well managed. In the event that an animal appears uncomfortable, an escalation to the next higher level of care in the pain ladder should be considered.
Setting realistic goals for pain management in laboratory rodents
Given the stated difficulties in managing pain adequately in humans, it may not be realistic to assume that all pain can be effectively treated in research animals all the time. This further emphasizes the importance of having a thoughtful plan that is tailored to the procedures being conducted. The plan should include anticipation of pain, early treatment to minimize sensitization, and evaluation of individual animals for a response to therapy. Companion animal pain management guidelines, such as the “PLATTER” approach, can provide a useful approach for systematic management of pain in research animals (see Figure 2).53 Consistent use of this tool by the veterinary team for both clinical cases and research protocols would help to ensure better pain recognition and mitigation in laboratory rodents. Further, this approach could be built into the institutional animal user training program to ensure consistency in analgesia management. Where objective scoring tools do not exist, close observation of animal behavior is necessary and should be conducted noninvasively by an individual familiar with normal preprocedure behavior of the specific animals.
Figure 2.
PLATTER* Approach to Managing Pain in Research Animals.
For new procedures or models with unknown outcomes, it can be useful to conduct detailed individual assessments on a few animals and then generalize these findings to develop a robust scoring system and appropriate pain treatment plan for the larger cohort.58 Because pain and response to treatment can differ between sexes of animals, between animals of different ages, and even between genetically similar animals,115,154 each rodent should be monitored directly after treatment for signs of comfort and wellbeing that indicate a pain-free state. This includes evaluating normal postures, social interactions, grooming, nest-building (in the case of mice), general activity, and food and water intake.
Evidence-based analgesia in rodents
Numerous formularies provide dosing regimens for pain management in rodents. These regimens are primarily based on studies evaluating analgesic efficacy, but also draw from commonly accepted historical practices. Analgesiometry assays used in these studies included variations of the tail flick assay, paw withdrawal, and the hot plate test. While these assays provide some information, they primarily test withdrawal reflexes indicative of nociception, and lack the ability to fully capture the more complex experience and central processing associated with surgical pain.1 More recent studies have attempted to assess pain more comprehensively rather than just nociceptive responses.14 These include assays such as behavioral assessments, grimace scales, vocalizations, and nesting behaviors (for a review, see reference 205).
Most analgesics used for mitigating pain in rodents fall into one of a few classes: opioids (or opioid-like), NSAID or local analgesics. Commonly used agents include buprenorphine, tramadol, meloxicam, carprofen, ketoprofen, ibuprofen, acetaminophen, lidocaine, and bupivacaine. Table 1). provides recommended dosing regimens for mice, rats and guinea pigs for each of these agents based on commonly referenced texts and guidelines.60,83,119 An extensive literature review of pharmacokinetics of these drugs was also conducted. Table 2 presents data on establishment of therapeutic levels of commonly used analgesics. As shown, much of the literature has used “therapeutic levels” that are not based on well-proven studies in rodents, but rather extrapolated from other species, and the current dosing regimens do not appear to be based on achieving those therapeutic levels. The published ranges are also often very large, probably based on a range of analgesic efficacy from a small effect to a much more substantial dampening of pain responses. As such this information is currently of limited value and could benefit from more specific studies performed in rodents, but is included to provide currently available values.
Table 1.
Common currently used analgesic dosing regimens for rodents
| Species | Agent | Dose (mg/kg) | Route | Frequency |
| Mouse | Buprenorphine | 0.05-0.1 | SC | 6-12 h |
| Tramadol | 5-40 | SC, IP | ND | |
| Carprofen | 2-5 | SC | 12-24 h | |
| Meloxicam | 1-5 | SC, PO | 12 h | |
| Ketoprofen | 2-5 | SC | 24 h | |
| Ibuprofen | 30-40 | PO | ND | |
| Acetaminophen | 200 | PO | ND | |
| Rats | Buprenorphine | 0.01-0.1 | SC, IM | 8-12 h |
| Tramadol | 5-20 | SC, IP | ND | |
| Carprofen | 2-5 | SC | 24 h | |
| Meloxicam | 1-2 | SC, PO | 12-24 h | |
| Ketoprofen | 2-5 | SC | 24 h | |
| Ibuprofen | 15 | PO | ND | |
| Acetaminophen | 200 | PO | ND | |
| Guinea pig | Buprenorphine | 0.05 | SC | 6-12 h |
| Carprofen | 2-5 | SC, IM | 12-24 h | |
| Meloxicam | 0.1-0.3 | SC, PO | 24 h | |
| Ibuprofen | 10 | PO | 4 h |
ND = not determined.
Dosages drawn primarily from Flecknell 2016; Hawkins 2012; Kohn and colleagues 2007.
Table 2.
Purported therapeutic plasma levels
An overview of the pharmacokinetic studies for mice, rats, and guinea pigs are summarized in Tables 3 to 5 respectively, and these are discussed in more detail below. The literature was further probed for efficacy studies, and results of these are summarized in a series of tables (Tables 6 to 12), based on species (mouse, rat, or guinea pig) and analgesic drug or class (buprenorphine, nonopioid analgesics, and local anesthetics). The summary of the studies highlighted in these tables suggest, as discussed in more detail below, how the dosing regimens historically used for pain management in rodents may not be adequate,66,105,112,113,130,149,163,211
Table 3.
Pharmacokinetics of analgesics used in mice
| Analgesic | Dose (mg/kg) | Route | Tmax | Cmax (ng/mL) | Duration of action | Reference |
| Buprenorphine | 5 μg/mL | PO-M | 1 h | 7.8 | < 6 h | 94 |
| 15 μg/mL | PO-M | 1 h | 3.0 | 12 h | 94 | |
| 0.03 | SC | 1 h | 0.5 | N/A | 37 | |
| 0.05 | SC | 1 h | 0.5 | N/A | 37 | |
| 0.1 | SC | 8 h | 8.6 | 12 h | 19 | |
| 0.1 | SC | 3 h | 1.3 | N/A | 37 | |
| 0.1 | SC | 1 h | 1.5 | < 6 h | 94 | |
| 0.1 | SC | 2 h | 1.5 | 4 h | 103 | |
| 0.6 | SC | 2 h | 19.1 | 4 h | 112 | |
| 2.0 | SC | 1 h | 20.2 | 12 h | 37 | |
| Buprenorphine SR | 0.1zp | SC | 4 h | 14.5 | 24 h | 112 |
| 0.3zp | SC | 6 h | 0.8 | N/A | 37 | |
| 1.2zp | SC | 0.5 h | 5.0 | 12 h | 37 | |
| 2.2ih | SC | 2 h | 11 | 24 h | 103 | |
| 3.25ag | SC | 6 h | 16.3 | 72 h | 202 | |
| 4.0ih | SC | 24 h | ND | 72-96 h | 80 | |
| Carprofen | 10 | PO-G | 2 h | 20,300 | N/A | 96 |
| 10 | PO-W | 12 h | 17,000 | N/A | 96 | |
| 30 | PO-W | 24 h | 32,000 | N/A | 164 | |
| 5 | SC | 2 h | 525,000 | 12 h | 112 | |
| Meloxicam | 10 | IV | 5 min | 365,000 | 4-6h | 21 |
| 10 | PO | 0.7 h | 18,000 | 4-6h | 21 | |
| 20 | PO-G | 4 h | 16,700 | 24 h | 96 | |
| 1 | SC | 2 h | 4700 | 4 h | 112 | |
| Meloxicam SR | 6zp | SC | 2 h | 7300 | 12-24 h | 112 |
| Tramadol | 25 | IP | 0.08 h | 3010 | 4h | 56 |
| 25 | IV | 0.25 h | 3710 | 2h | 56 | |
| 25 | PO-G | 1 h | 347 | 2 h | 56 | |
| 25 | PO | 1 h | 347 | constant in water | 56 | |
| 25 | SC | 0.25 h | 1870 | 6 h | 56 | |
| EMLA | 18 mg/25g | Top | 0.5 h | 165 | 100 min Toxic at 21.2 mg/kg | 6 |
| 18 mg/25g | Top (open wound) | 0.5 h | 909 | 100 min | 6 | |
| Bupivacaine | 150 µL 0.5% | SC | 1 | 1,000,000 | approximately 4 h Toxic at >0.5% | 73 |
Duration of action = time at which plasma level falls below therapeutic level (see Table 2)
N/A = plasma level did not exceed therapeutic level; ND = not determined
Tmax = time to reach maximum concentration; Cmax = maximum concentration
PO-W = oral in water; PO-M = oral in MediGel; PO-G = oral by gavage; Top = topical; SR= sustained release;
zP = manufactured by Zoopharm, Windsor, CO; ih= inhouse formulation; ag= manufactured by Animalgesics Laboratories, Millersville, MD
Table 5.
Pharmacokinetics of buprenorphine in guinea pigs
| Dose (mg/kg) | Route | Tmax | Cmax (ng/mL) | Duration of action | Reference |
| 0.2 | IV | 1.5 m | 46.7 | 6 h | 179 |
| 0.2 | PO | 1.2 h | 2.4 | 3-6 h | 179 |
| 0.05 | SC | 1 h | 2.3 | < 6 h | 189 |
| 0.15 SRzp | SC | 1 h | 2-2.3 | 6 h | 216 |
| 0.3 SRzp | SC | 26 h | 1.34 | 24-48 h | 189 |
| 0.3 SRzp | SC | 1 h | 6.9-11.5 | 48 h | 216 |
| 0.48 SRag | SC | 48 h | 1.2 | 72-96 h | 163 |
| 0.6 SRzp | SC | 1 h | 64-71 | 72 h | 216 |
Duration of action = time at which plasma level falls below therapeutic level (see Table 2).
SR = sustained-release formulation
Tmax = time to reach maximum concentration
Cmax = maximum concentration
zP = manufactured by Zoopharm, Windsor, CO; ag= manufactured by Animalgesics Laboratories, Millersville, MD
Table 6.
Mouse efficacy studies of buprenorphine
| Dose (mg/kg) | Route | Test | Duration of action | Comments | Reference |
| 0.5-2 | IP | HP, TF | 105-135 min | 3 | |
| 0.5-6.8 | IP | TF | ED70 at 0.5-2 mg/kg | Effective dose decreased with doses > 4.5 mg/kg | 118 |
| 2.4 | IP | SX | No effect | Dosed on day 1 and 7 postoperative | 87 |
| 1 | PO-N | SX | Reduced blood corticosterone | 200 | |
| 0.75 | PO-F | HP, Lap | Up to 4 h | Suggest one SC dose followed by medicated feed for up to 20 h | 155 |
| 4.2 | PO-F | HP, Lap | Up to 4 h | Suggest one SC dose followed by medicated feed for up to 20 h | 155 |
| 0.5-5.0 | SC | HP, TF, WT | ED50 1.5 mg/kg | 206 | |
| 0.001-0.1 | SC | Lap | Up to 90 min at 0.05-0.1 mg/kg | 140 | |
| 0.01, 0.05 | SC | Lap | Partially effective at high dose | 211 | |
| 0.05 | SC | Lap | 5 h | 148 | |
| 0.05 | SC | SX | Minimal effect | Dosed twice d for 2 d. Decrease BW, increase arterial pressure, decrease HR | 173 |
| 0.1 | SC | CLP | No effect | 90 | |
| 0.1 | SC | HP, Lap | 4 h | Dosed q8h for 24 h | 103 |
| 0.1 | SC | Lap | No effect | Dosed q12h for 3d | 113 |
| 0.1 | SC | Lap | No effect | 124 | |
| 0.1 | SC | Lap, VF | 2-8 h | Dosed q12h for 48 h. Suggest multimodal with carprofen | 164 |
| 0.1 | SC | SX | Partial efficacy to 12h | Dosed q12h for 3 d | 203 |
| 0.25-5 | SC | TF | ED50- 0.25 mg/kg | 171 | |
| ED30 1-5 mg/kg | |||||
| ED50- 10 mg/kg | |||||
| ED80- 50 mg/kg | |||||
| 0.3 | SC | HP | No effect | 25 | |
| 0.5 | SC | SX | No effect | Dosed q8h for 48 h | 99 |
| 0.6 | SC | SX | Low level pain up to 24 h | 57 | |
| 1.0 | SC | HP | 12 h | 25 | |
| 1.5 | SC | HP, TF | 4 h | 88 | |
| 2 | SC | Lap | 6 h | Dosed once, or q6h for 18 h. Increase in blood pressure at 6 h | 70 |
| 2 | SC | HP, TF | 3-5 h | 66 | |
| 0.6 SR | SC | Lap | up to 24 h | 113 | |
| 1.0 SR | SC | CLP | 24 h | Improved clinical score | 90 |
| 1.5 SR | SC | HP, TF | up to 48 h | 88 | |
| 2.2 SR | SC | HP, Lap | 24-48 h | 103 | |
Effects are based on a single dose of analgesic unless otherwise described in the comments.
PO-n = oral in Nutella; PO-F = oral in feed
CLP = cecal ligation and puncture; SX = surgical model; Lap = Laparotomy
HP = hot plate assay
TF = tail flick assay
VF = von Frey test
WT = writhing test
zP = manufactured by Zoopharm, Windsor, CO; ih= inhouse formulation
Table 12.
Guinea pig efficacy studies of buprenorphine, NSAIDs and local analgesics
| Analgesic | Dose (mg/kg) | Route | Test | Duration of action | Comments | Reference |
| Buprenorphine | ||||||
| 0.05 | SC | RS | 12-24 h | Dosed q12h for 72. | 189 | |
| 1-5 | SC | Pin prick | ED50 3.0 mg/kg; ED75 4-5 mg/kg at 30 min post administration | 35 | ||
| 0.3 SRzp | SC | RS | 6 h | 189 | ||
| 0.48 SRag | SC | Lap, VF | Up to 96 h | No change in behavior compared with analgesia only group | 163 | |
| 0.6 mmol | IM | PW | 4 h | 213 | ||
| Carprofen | 1 | SC | Lap, VF | Ineffective | Pain indices 2-8 h postoperative that resolved by 24 h | 49 |
| 4 | SC | Lap, VF | Partially effective | Dosed daily for 3 d. Pain indices 8 h postoperative that resolved by 24 h | 163 | |
| Meloxicam | 0.2 | SC | Lap | Dosed daily for 2 d. Received local bupivacaine and/or lidocaine. No effect. | 52 |
Lap = laparotomy; PW = paw withdrawal assay; RS = Randall-Selitto test; VF = von Frey test;
Table 4.
Pharmacokinetics of analgesics used in rats
| Analgesic | Dose (mg/kg) | Route | Tmax | Cmax (ng/mL) | Duration of action | Reference |
| Buprenorphine | 0.05 | SC | 0.5 h | 1.5 | 2 h | 72 |
| 0.1 | SC | 4 h | 2.7 | 8-24 h | 64 | |
| 0.4 | PO-N | 2 h | 1.25 | 14 h | 72 | |
| 0.9 SRzp | SC | 4 h | 2.8 | 24-48 h | 64 | |
| 1.2 SRzp | SC | 4 h | 2.8 | 24 h | 64 | |
| 1.2 SRzp | SC | 24 h | 1.01 | 24 h | 160 | |
| Ketoprofen | 2.5 | IV | <5 min | 10,000 | 48 h | 181 |
| 10 | IV | <5 min | 100,000 | 24 h | 181 | |
| 3.2 | PO | 0.5 h | 2730 | 24 h | 143 | |
| 10 | PO | 0.5 h | 11,700 | 90-360 min | 4 | |
| 0.5 | SC | ND | 0.73 | N/A | 195 | |
| 1.0 | SC | ND | 1.79 | N/A | 195 | |
| 5.0 | SC | ND | 8.43 | Measured at 2 h | 195 | |
| Meloxicam | 1 | IV | < 0.25 h | 5000 | 24 h | 21 |
| 0.3 | PO | 4.5-6.5 h | 2300-3200 | ND | 21 | |
| Tramadol | 20 | IP | 10 min | 3187 | 300 min | 186 |
| 20 | IV | < 10 min | 23,314 | 300 min | 186 | |
| Bupivacaine | 2% 300 µL | SC | 2 h | 7000 | Waned by 10 h | 74 |
Duration of action = time at which plasma level falls below therapeutic level (see Table 2).
SR = sustained-release
N/A = indicates plasma level did not exceed therapeutic level
ND = not determined
Tmax = time to reach maximum concentration
Cmax = maximum concentration
PO-n = oral in Nutella
zP = manufactured by Zoopharm, Windsor, CO
Table 7.
Mouse efficacy studies of nonopioid analgesics
| Agent | Dose (mg/kg) | Route | Test | Duration of action | Comments | Reference |
| Acetaminophen | 50 | IP | Lap | 1 h | 149 | |
| 320 | PO | SX | No effect on activity | 87 | ||
| 160,320 | PO | CFA | Up to 90 min | 156 | ||
| 100-450 | SC | Lap | No effect | 140 | ||
| Carprofen | 30 | PO-W | Lap, VF | In effective | Medicated water provided for 72 h | 164 |
| 5-25 | SC | Lap | 90 min at 20-25 mg/kg | Suggest 29 mg/kg | 140 | |
| 5 | SC | Lap | Burrowing latency similar to anesthesia alone | 105 | ||
| 5 | SC | Lap | Activity and burrowing no different than anesthesia alone | 104 | ||
| 5 | SC | Lap | Nest complexity improved slightly at high dose | 106 | ||
| 50 | ||||||
| Flunixin | 2.5 | SC | Lap | No effect | 70 | |
| Gabapentin | 1 | IP | VF | 3 h | Returned to baseline by 24 h | 159 |
| 3 | ||||||
| 3-100 | IP | DN, FT, HP, TF | FT ED50 9.3 mg/kg | 153 | ||
| HP ED50 16.5 mg/kg | ||||||
| TF ED50 17.6 mg/kg | ||||||
| 50 | IP | CCI, VF | ED50 7 mg/kg | 45 | ||
| Ibuprofen | 200 | TF | No effect | 217 | ||
| 40, 80 | PO | CFA | 150 min | 156 | ||
| 40 | PO-W | SX | No effect on activity | 87 | ||
| 2.5-20 | SC | CFA, VF | ED50 10 mg/kg | 38 | ||
| 200 | SC | TF | In effective at 45 min | 121 | ||
| Ketoprofen | FT, WT | FT- ED50 100 mg/kg | 68 | |||
| WT- ED80 10 mg/kg | ||||||
| 30 | IP | WT | ED50 30 mg/kg | 151 | ||
| 1-20 | SC | Lap | 90 min at 20 mg/kg | Suggest 65 mg/kg | 140 | |
| Meloxicam | 1 | IP | HP, FT, WT | FT ED50 3 mg/kg, 10 mg/kg HP ED50 3 mg/kg,10 mg/kg | 180 | |
| 3 | WT- ED80 10 mg/kg | |||||
| 10 | ||||||
| 2 | SC | Lap | Dosed once daily for 3 d. Reduced activity for 24 h postoperative | 203 | ||
| 2 | SC | SX | Partially effective | Dosed with 2 mg/kg preoperative, then 1 mg/kg daily for 2 d. Improved BW, increase arterial pressures and HR | 173 | |
| 5 | SC | Lap | No effect | 149 | ||
| 5 | SC | SX | No effect | Dosed once daily for 2 d | 99 | |
| 5 | SC | Lap | 1 h | 148 | ||
| 5 | SC | Lap | Corticosterone normalized at 20 mg/kg; All effective based on ethogram | 212 | ||
| 10 | ||||||
| 20 | ||||||
| 20 | SC | SX | 1 h | Reduced MGS and behaviors | 130 | |
| Tramadol | FT, TF, WT | FT ED50 2.8 mg/kg | 150 | |||
| TF ED25- 2.4 mg/kg | ||||||
| WT- ED50 1.86 mg/kg | ||||||
| 20 | Lap | No effect | 123 | |||
| 20 | SC | SX | Minimal effect | Dosed daily for 2 d. Decrease BW, increase arterial pressure, decrease HR | 173 | |
| 3-100 | IP | DN, FT, HP, TF | FT ED50 3.5 mg/kg | 153 | ||
| HP ED50 12.5 mg/kg | ||||||
| TF ED50 9.7 mg/kg | ||||||
| 10-100 | IP | CFA | ED50 25 mg/kg | 152 | ||
| 10-80 | IP | HP | 30-60 min | ED50 70 mg/kg | 145 | |
| 10 | IP | TF | Increased latency at 20 and 40 mg/kg | 55 | ||
| 20 | ||||||
| 40 | ||||||
| 50 | IP | HP, TF | 30-60 min | ED50 50 mg/kg; Trace minerals increased effectiveness | 5 | |
| 40,80 | PO | CFA | 45-90 min | 156 | ||
| SC | HP | ED50 14.8 mg/kg | 175 | |||
| ED80 71.9 mg/kg | ||||||
| 3.2 | SC | WT | ED50 3.2 mg/kg | 59 | ||
Effects are based on a single dose of analgesic unless otherwise described in the comments.
PO-W- oral in water; ED = effective dose; SX- surgical model; Lap- laparotomy
CLP = cecal ligation and puncture; CCI = chronic constriction injury
CFA = Complete Freund’s adjuvant; DN = diabetic neuropathy; FT = Formalin test; HP = hot plate assay; NP = neuropathic pain; PW = paw withdrawal test; TF = tail-flick assay; VF = von Frey test; WT = writhing test
Table 9.
Rat efficacy studies of buprenorphine
| Dose (mg/kg) | Route | Test | Duration of action | Comments | Reference |
| 0.01 | IM | Lap, TF | No effect | Dosed q12h for 72 h | 41 |
| 0.1 | IM | Lap, TF | Dosed q12h for 72 h. BW and food intake similar to saline treatment; TF increased latency | 41 | |
| 0.02-0.2 | IP | TF | 24 h at 0.2 mg/kg | Hyperalgesia at 0.01 mg/kg | 207 |
| 8 µg/kg | IV | TF | 4 h | 161 | |
| 0.4 | PO | Lap | 270-390 min | Observations limited to 390 min postoperative | 176 |
| 0.5-10 | PO-G | TF | 2 h at 5-10 mg/kg | 138 | |
| 0.5 | PO-G | HP | 3-5 h | 129 | |
| 0.1-0.4 | PO-J | Lap | Increased BW all treatment groups | 62 | |
| 0.5 | PO-J | HP | 1 h | 129 | |
| 0.5 | PO-J | Lap | Dosed q12h for 36 h. Not effective based on BW | 98 | |
| 0.5- 2.0 | PO-N | HP | 60-120 at 1 mg/kg | 92 | |
| 0.4 | PO-N | SX | No change in corticosterone; no change in activity 5h post op; BW loss less than control | 72 | |
| 0.3-3.0 | SC | HP, TF | ED50 0.4 mg/kg | 206 | |
| 0.03 | SC | Lap | Dosed q12h for 72 h. Decrease BW | 20 | |
| 0.03 | SC | PW | 24 h | Reduce RGS | 133 |
| 0.05 | SC | SX | Dosed q8-12h for 120 h. Improved gait | 27 | |
| 0.05 | SC | HP, SX, VF | No effect | Dosed q12h for 72 h | 34 |
| 0.05 | SC | HP | 1 h | 107 | |
| 0.05 | SC | SX | No effect | Dosed preoperative and 18 h postoperative supplemented with 0.25 mg/kg POJ | 117 |
| 0.05 | SC | HP | 3-5 h | 129 | |
| 0.05 | SC | 2 h | 138 | ||
| 0.05 | SC | HP | Dosed q12h for 60 h. PW latency increased; Minimal effect | 141 | |
| 0.05 | SC | Lap | 270-390 min postoperative | Observations limited to 390 min postoperative | 176 |
| 0.05 | SC | SX, VF, HP | Up to 96 h | Dosed q12h for 72 h. Reduced mechanical and thermal sensitivity. | 184 |
| 0.05 | SC | Lap | Lower ethogram score | 160 | |
| 0.1 | SC | FT | 6 h | 1 | |
| 0.1 | SC | HP | 30-240 min | 92 | |
| 0.1 | SC | Lap | Dosed q12h for 72 h. Lower ethogram score | 160 | |
| 0.25-0.1 | SC | VF | Increase threshold | 196 | |
| 0.2 | SC | PW, SX | PW no effect at 24h; no effect on vertical rises | 64 | |
| 0.5 | SC | SX | Increase corticosterone levels | 72 | |
| 0.5 | SC | SX | No effect | Supplemented with 0.25 mg/kg POJ | 117 |
| 0.5 | SC | HP, TF | 6-8 h | 66 | |
| 0.3 SRzp | SC | HP, SX, VF | No effect | 34 | |
| 0.65 SRzp | SC | HP | 4-48 h | 107 | |
| 1.2 SRzp | SC | HP, SX, VF | HP increase latency at 24h; VF no significant difference to baseline | 34 | |
| 1.2 SRzp | SC | SX, PW | Up to 48 h | Increase vertical rises compared with buprenorphine | 64 |
| 1.2 SRzp | SC | HP | 24-72h | 107 | |
| 1.2 SRzp | SC | SX, VF, HP | Up to 96 h | Reduced mechanical and thermal sensitivity. | 184 |
| 1.2 SRzp | SC | Lap | Dosed q12h for 72 h. Lower ethogram score | 160 | |
| 4.5 SRzp | SC | HP, SX, VF | HP increased latency at 24 h at 4.5 mg/kg; VF no effect; Sedative effect with 4.5 mg/kg | 34 |
Effects are based on a single dose of analgesic unless otherwise described in the comments.
PO-n = oral in Nutella; PO-J = oral in gelatin; PO-G = oral by gavage
SX = surgical model; Lap = Laparotomy
HP = hot plate assay; PW = paw withdrawal test; TF = tail-flick assay; VF = von Frey test
zP = manufactured by Zoopharm, Windsor, CO
Table 10.
Rat efficacy studies of nonopioid analgesics
| Agent | Dose (mg/kg) | Route | Test | Duration of action | Comments | Ref |
| Acetaminophen | 100, 300 | PO | VF | No effect | Dosed daily for 2 d | 196 |
| 20-1000 | PO | HP, TNT, VF | 30-120 min at 100 and 1000 mg/kg | VF ED50 32.8 mg/kg | 188 | |
| 4.48 mg/mL | PO-W | SX | No effect | 27 | ||
| Carprofen | 2 | PO-G | PW, VF | 6-9 h | 201 | |
| 5 | PO-G | SX, VF, HP | Up to 48 h | Medicated feed provided 2 d preoperative and 2 d postoperative. Reduced mechanical pain, but not thermal. | 184 | |
| 5 | SC | Lap | Dosed preoperative and 4 and 24 h postoperative. Increased activity | 23 | ||
| 5 | SC | Lap | 270-390 min | Observation limited to 390 min postoperative | 176 | |
| 5, 10 | SC | CFA | No effect | 166 | ||
| Gabapentin | 25-200 | IP | FT | Effective at 100 and 200 mg/kg | 157 | |
| 30-300 | IP | CCI TF, VF | TF increase at 300 mg/kg; VF ED50 34 mg/kg; cold allodynia ED50 103 mg/kg | 95 | ||
| 5-20 | IP | HP, VF | Increase thresholds 10-20 mg/kg | 81 | ||
| 300 | PO | CFA | No effect | 139 | ||
| 30-300 | PO-G | RS | 1-4 h at 300 mg/kg | 85 | ||
| 02-4 h at 100 mg/kg | ||||||
| 3 h at 30 mg/kg | ||||||
| 10-100 | SC | VF | Nominal effect at 100 mg/kg | 167 | ||
| 90 | SC | TF | 30-90 min | 146 | ||
| Ibuprofen | 0.3-30 | PO | CFA | No return to baseline gait | 139 | |
| 20 | PO | SX | Dosed q8-12h for 120 h. Improved gait | 27 | ||
| 31, 100 | SC | CFA | WT bearing within 30-90 min; | Rearing increase at 100 mg/kg; Burrowing increased | 178 | |
| Ketoprofen | HP, PW | 6 h at 30-100 mg/kg | 68 | |||
| 3 | IM | Lap, TF | Dosed q12h for 72 h. No effect | 41 | ||
| 3,5 | IM | Lap | Dosed preoperative and 9-12 h postoperative. Reduced BW and FI; single and double dose have similar effect | 40 | ||
| 1,3.2,10 | PO | HP | 30-60 min | ED90 3.2 and 10 mg/kg | 4 | |
| 0.5-10 | SC | PW, VF | Guarding reduced 2-24 h at 5 and 10 mg/kg; no effect on PW or VF | 195 | ||
| 40 | SC | Lap | Reduced RGS similar to morphine | 111 | ||
| Meloxicam | 1 | SC | Lap | Dosed daily for 3 d. Lower ethogram score; no difference from 2 mg/kg dose | 160 | |
| 2 | SC | Lap | Dosed daily for 3 d. Lower ethogram score | 160 | ||
| 2, then 1 | SC | Lap | Dosed daily for 3 d. Improved BW, FI | 20 | ||
| 4.0 SRzp | SC | SX, VF, HP | Up to 48 h | Reduced mechanical pain, but not thermal. | 184 | |
| Naproxen | 50-100 | IP | CFA | Weight bearing increased at 30 min; increase burrowing | 178 | |
| 50-150 | IP | CFA | Effective at 50 mg/kg; higher dose no benefit | 177 | ||
| Tramadol | 0.625-40 | IP | HP, VF | ED50 10 mg/kg; ED80 40 mg/kg | 144 | |
| 10 | IP | HP | Dosed q12h for 60 h. In effective | 141 | ||
| 10-30 | IP | HP | ED40 30 mg/kg | 65 | ||
| 10-40 | IP | VF | 15-30 min at 20 mg/kg; 15-120 min at 40 mg/kg | 116 | ||
| 11 | IP | TF | 75 min | 218 | ||
| 12.5 | IP | Lap | Dosed preoperative and 4 and 24 h postoperative. No effect on activity, wheel running, BW | 23 | ||
| 1-25 | IP | TF | Increase latency at 15 and 25 mg/kg; motor function impaired > 15 mg/kg | 135 | ||
| 4-50 | IP | HP, TF | Increase latency at 12.5-50 mg/kg; heavy sedation > 25 mg/kg | 24 | ||
| 5-20 | IP | CFA | 60-90 min | Increase latency at 10 and 20 mg/kg | 214 | |
| 5-40 | IP | TF | 30-120 min | ED50 20 mg/kg; ED80 40 mg/kg | 100 | |
| 3-30 | PO | HP, TNT, VF | 30-120 min at 10 and 30 mg/kg | VF ED50 4.8 mg/kg | 188 | |
| 4-50 | PO-J | HP, TF | No effect | 24 | ||
| 0.45 | SC | TF | 30-90 min | 162 | ||
| 20 | SC | FT | Reduced pain scores | 67 | ||
| 4-50 | SC | HP, TF | Increased latency at 25-50 mg/kg; heavy sedation | 24 | ||
PO-W = oral in water; PO-G = oral by gavage; PO-J = oral in gelatin; SX = surgical model; Lap = Laparotomy; TNT = tibial nerve translocation; CCI = chronic constriction injury
CLP = cecal ligation and puncture; CFA = Complete Freund’s adjuvant; DN = diabetic neuropathy; FT = Formalin test; HP = hot plate assay; RS = Randall-Selitto test; TF = tail-flick assay; VF = von Frey test; zP = manufactured by Zoopharm, Windsor, CO
Buprenorphine
Buprenorphine, one of the most commonly used analgesics in rodents, is typically dosed subcutaneously twice a day, yet pharmacokinetic data demonstrate that mice and rats rarely achieve a plasma level greater than the purported therapeutic level beyond 4 to 6 h. Oral formulations provided continuously in feed or gels, and sustained-release formulations enhance the duration of action of buprenorphine. When buprenorphine is provided in MediGel or Nutella, the duration of effect can be up to 12 to 14 h 72,94However, the mouse studies found considerable variation in the amount ingested.94 Sustained-release formulations of buprenorphine regularly achieve a plasma level greater than therapeutic levels for more than 12 h, and often up to 24 h 37,103,112,202However, manufacturer guidelines (Zoopharm, Windsor, CO) suggest that dosing once every 72 h is sufficient. These findings suggest that the commonly used twice-daily dosing schedule of buprenorphine does not achieve an adequate duration of analgesia. Efficacy studies in rodents support these findings as they infrequently achieve clinical analgesia beyond 8 h, unless sustained-release formulations are used.
Nonsteroidal antiinflammatory drugs (NSAID)
The pharmacokinetics of nonopioid analgesics demonstrate similar pharmacokinetic and efficacy trends as buprenorphine. The commonly used dosages of NSAID in rodents fail to routinely provide plasma levels greater than therapeutic levels. Carprofen given at 5 mg/kg SC to mice has a duration of effect for 12 h;112 however, the common dosing interval can be up to once daily. Efficacy studies demonstrate a minimal effect beyond the first 6 h postoperatively.176 While carprofen administered in the drinking water achieved sustainable therapeutic levels up to 35 h, the study did not evaluate the efficacy of this route of administration in a postoperative model.96 Meloxicam at 1 mg/kg SC in mice has a duration of effect of 4 h,112 and when given orally at 10 mg/kg has a duration of action of 4 to 6 h.21 However, when given at a higher oral dose of 20 mg/kg or in a sustained release formulation meloxicam has a duration of effect lasting up to 24 h.96,112 Mice provided meloxicam in the drinking water refused to consume it.96 Efficacy studies of meloxicam support pharmacokinetic studies in that 5 mg/kg appears to have no effect on postoperative analgesia in mice and clinically higher doses up to 20 mg/kg may be required for analgesia in mice.130,212Other NSAID have demonstrated similar findings of shorter duration of action in mice, which may be overcome with higher doses, such as ketoprofen at 10 to 20 mg/kg.140 Doses of 1 or 4 mg/kg SC appear to be similarly ineffective in guinea pigs.49,163 However, in rats, a 2 mg/kg SC dose reduced behavioral signs of pain in a laparotomy model.160
Local anesthetics
Local anesthetics have a short duration of action; 30 min with lidocaine and up to 60 min with bupivacaine. There are formulations that prolong the analgesic efficacy of local anesthetics, and these formulations can increase the duration of action to 24 to 48 h.
Recommendations
Dosing regimens for these analgesics should be carefully reconsidered in light of recent pharmacokinetic and efficacy studies . The frequency of dosing should be based on these pharmacokinetic studies as well as cage-side clinical assessments of pain, although clinical assessments should consider the ability of rodents to mask signs of pain. Table 13 provides our updated recommendations that address the inadequate dosing intervals that are widely used (and currently considered acceptable practice by many IACUC).61 Given the inconsistent findings associated with the efficacy studies on NSAID, the dosing regimens recommended in Table 13 are based on current studies using more recent techniques to identify pain, such as facial grimace score, and pharmacokinetic studies. Although several studies have evaluated voluntary ingestion of medical gels or feedstuff, routine use requires caution as rodents will reduce feed and water intake during the postoperative period and voluntary ingestion can be variable, resulting in inadequate dosing.
Table 13.
Updated analgesic dosing recommendations
| Species | Agent | Dose (mg/kg) | Route | Frequency |
| Mouse | Buprenorphine | 0.1-0.5 | SC | 4-6 h |
| Buprenorphine SRzp | 0.6 | SC | 48 h | |
| Tramadol | 80 | SC | 24 h | |
| Carprofen | 5 | SC | 12 h | |
| 20 | SC | 24 h | ||
| Meloxicam | 5-10 | SC | 8-12 h | |
| Ketoprofen | 20 | SC | 24 h | |
| Rats | Buprenorphine | 0.05-0.0.1 | SC | 6-8 h |
| 0.5-0.6 | PO | 24 h | ||
| Buprenorphine SRzp | 1.2 | SC | 48 h | |
| Tramadol | 20-40 | PO | 24 h | |
| 5 | SC | 24 h | ||
| Carprofen | 5 | SC | 24 h | |
| Meloxicam | 1 | SC | 12-24 h | |
| Ketoprofen | 5 | SC | 24 h | |
| Guinea pig | Buprenorphine | 0.05 | SC | 6 h |
| Buprenorphine SRzp,ag | 0.3-0.48 | SC | 48 h | |
| Carprofen | 4 | SC | 12-24 h | |
| Meloxicam | 0.2 | SC | 12-24 h |
Modified from Flecknell 2018.61
SR = sustained release; # - provided in food treat, should be observed ingesting
zP = manufactured by Zoopharm, Windsor, CO; ag= manufactured by Animalgesics Laboratories, Millersville, MD.
Note: caution should be taken with higher doses of NSAIDs. Multimodal analgesia recommended to allow effective use of lower doses.
Multimodal analgesia
Another aspect of analgesic therapy that may overcome the current dosing challenges is multimodal analgesia. Multimodal analgesia combines multiple analgesics with different mechanisms of action into the treatment regimen, which often results in an increased efficacy while using lower dosages of the individual agents. Multimodal analgesia is commonly used in human and veterinary medicine for pain management.12,13,17,42,50,126 Evidence that multimodal analgesia is effective in rodents is summarized in Table 14. In a tail-flick assay, the effects of ibuprofen were enhanced with opioids.217 The effective dose of gabapentin and tramadol were both reduced when given in combination in a diabetic neuropathy model evaluating analgesia using the tail-flick assay, hot plate, and formalin test.153 Similarly, the analgesic effect of tramadol was improved when ketoprofen was given concurrently using the writhing test, tail-flick assay, and formalin test.150,152 Opioids also enhance the effects of tramadol.59,175 In a murine laparotomy model, mice were treated with either buprenorphine alone or in combination with carprofen, administered in the drinking water.164 The combination of buprenorphine and carprofen provided the best analgesia, compared with buprenorphine alone, and carprofen alone failed to provide any analgesia. A similar study was performed in a guinea pig ovariohysterectomy model.163 Guinea pigs were treated at induction with an extended-release formulation of buprenorphine, carprofen or multimodal treatment. The frequency of behaviors indicative of pain was reduced in the multimodal treatment group compared with buprenorphine or carprofen alone.
Table 14.
Published multimodal analgesic efficacy studies
| Species | Multimodal analgesics | Dose (mg/kg) | Route | Model | Comments | Reference |
| Mouse | Buprenorphine Carprofen | 0.1 | SC | Lap | Buprenorphine dosed q12h, carprofen medicated water provided for 72 h. Improved analgesia for 2-8 h postoperative | 164 |
| 30 | PO-W | |||||
| Gabapentin | 3-100 | IP | TF, HP, FT | Reduced ED50 for each analgesic | 153 | |
| Tramadol | 3-100 | IP | ||||
| Tramadol | 10-100 | IP | TF, HP, FT | ED50 reduced with Keto | 152 | |
| Ketoprofen | 30-250 | IP | ||||
| Buprenorphine | 0.05 | SC | Lap | Buprenorphine dosed once preoperative. Melox was given 24 h postoperative | 148 | |
| Meloxicam | 5 | SC | ||||
| Meloxicam | 5 | SC | Lap | No effect | 149 | |
| Acetaminophen | 50 | IP | ||||
| Ibuprofen | 200 | IP | TF | Opioids enhanced latency | 217 | |
| Tramadol | SC | WT, HP | Opioids reduced ED50 | 59,175 | ||
| Rat | Buprenorphine | 0.03 | SC | PW | Similar effect to buprenorphine alone | 133 |
| Meloxicam | 2 | SC | ||||
| Buprenorphine | 0.05 | SC | SX | Buprenorphine dosed q8-12h, meloxicam daily. No effect; 8 h dosing resulted in pica | 183 | |
| Meloxicam | 2 | SC | ||||
| Acetaminophen | 20-1000 | PO | HP, VF | ED50 reduced of each | 188 | |
| Tramadol | 3-30 | PO | ||||
| Carprofen | 5 | SC | Lap | Dosed preoperative and 4 and 24 h postoperative. Increased activity with tramadol | 23 | |
| Tramadol | 12.5 | IP | ||||
| Gabapentin | 5-20 | IP | HP, VF | Potentiates opioids | 81,146,162,167, | |
| Tramadol | 10 | SC | HP | Tramadol dosed q12h for 60 h, gabapentin dosed daily. Minimal effect | 141 | |
| Gabapentin | 80 | SC | ||||
| Tramadol | 10 | SC | SX | Tramadol dosed q8-12h and gabapentin dosed daily for 120 h, No effect | 27 | |
| Gabapentin | 80 | SC | ||||
| Levobupivacaine | 0.3% 50 µL | SC | SX | Enhanced with ibuprofen and epinephrine | 122 | |
| Ibuprofen | 2 mg/mL 50 µL | SC | ||||
| Lidocaine | 22.6 mmol/kg | SC | VF | Increased threshold | 31 | |
| Naloxone | 43.2 mmol/kg | |||||
| Guinea pig | Meloxicam | 0.2 | SC | Lap | No effect | 52 |
| Bupivacaine | 1 | SC | ||||
| Lidocaine | 1 | SC | ||||
| Buprenorphine SRag | 0.48 | SC | Lap | Improved analgesia compared with carprofen alone | 163 | |
| Carprofen | 4 | SC | ||||
PO-W = Oral by water
ag= manufactured by Animalgesics Laboratories, Millersville, MD
Experiments assessing analgesic efficacy are challenging and complicated by species, strain, model, and environment. Nonetheless, studies evaluating alternative dosing regimens and multimodal therapies would further expand our knowledge base and provide better options for pain control. These studies must include proper control groups, including a “no treatment” group when not ethically precluded. However, sufficient data are available at this time to warrant the use of shorter dosing intervals for some of these drugs, and/or use of multimodal regimens. Many of the studies evaluating rodent pain have found that the most significant signs of pain occur within the first 12 to 24 h postoperatively. Multimodal therapies could be extremely beneficial during this critical postoperative time, including the administration of local anesthetic at the site of the incision, which could greatly reduce postoperative pain.10,18
Routes of administration
Administration of analgesic drugs to rodents must consider their small body size, stress associated with handling, the half-life of drugs, bioavailability, and factors that impact compliance with administration, such as difficulty in method of administration, time needed to administer the drug, and frequency of dosing required to achieve effective levels.
Parenteral administration
Parenteral routes remain the most common route of administration for analgesics. Based on retrospective reviews of analgesic administration reported in the literature, buprenorphine and carprofen are the most commonly used analgesics in rats and mice, and are most frequently administered subcutaneously.91,199 Intraperitoneal and intramuscular injections have been reported but less commonly. Parenteral routes also offer more reliable and consistent rates of absorption and bioavailability, compared with oral administration.204 While intraperitoneal injections might provide slightly faster absorption, subcutaneous injections are relatively easy for personnel to administer, can be performed with minimal and short-lasting restraint, and have less potential for adverse effects such as injection into an organ, and/or peritonitis. An often unrecognized characteristic of intraperitoneally administered substances is that absorption occurs largely through mesenteric vessels and are at least partially subject to first-pass hepatic metabolism.136
Buprenorphine, carprofen, and meloxicam, 3 commonly administered analgesics in rats and mice, are all available in injectable formulations but require dilution to be administered at appropriate dosages in mice. Carprofen and meloxicam were shown to be stable under a variety of environmental conditions (light compared with dark, and room temperature compared with 4 °C) for up to 7 d when diluted in reverse osmosis water.96 Although this study evaluated oral administration, it provides evidence of the stability of these drugs, even after dilution.
Sustained-release formulations are increasingly available, and based on personal and listserv communications appear to be gaining widespread acceptance in the US. As early as 1994 investigators were exploring use of liposomal preparations to extend the duration of action of local anesthetics such as bupivacaine,75 and systemic opioids such as morphine.78 The first commercially available formulation of a systemically absorbed analgesic for use in rodents was Buprenorphine-SR-LAB (Zoopharm, Windsor, CO) and its use for analgesia in rats was first published in 2011.64 Since that time, 14 other publications in rodents have included mice, rats, guinea pigs, and prairie dogs. Sustained-release meloxicam is also commercially available; however literature showing its efficacy and sustained plasma levels beyond 24 h in rodents are still lacking.112,184 These sustained-release formulations, based on use of biodegradable polymers, offer many advantages including decreased handling (and thus stress) to the animal, decreased personnel time, and more consistent and sustained plasma and tissue drug levels, which decrease the potential for breakthrough pain that can occur if standard formulations are dosed too infrequently.63 However, their use needs to be carefully considered and drawbacks weighed against their benefits. For example, current formulations require use of very small volumes for mice. This makes accurate dosing very challenging and over-dosing is a possibility. Also, absorption is variable and initial plasma concentrations can be quite high. Animals should be watched carefully during the first 4 to 8 h for signs of adverse opioid-induced effects, such as sedation, respiratory depression, and/or pica; however, other than pica in rats, other opioid-induced effects have not been appreciably seen in the authors’ collective experiences. Lastly, the delay until an analgesic response is achieved must be factored into the pain management plan.
Regional anesthesia
Delivery of local anesthetics as a means of providing incisional or regional anesthesia and analgesia is a well-established and effective procedure. The relatively short duration of action and inability to redose in rodents has limited its utility to primarily 3 applications: (1) as part of a multimodal pain management plan, (2) as the sole pain management in minimally invasive procedures, such as small skin incisions for a subcutaneous implant, and (3) to provide some minimal analgesia when no systemic analgesia can be administered for scientific reasons. See Tables 8 and 11 for a summary of published efficacy studies in mice and rats respectively.
Table 8.
Mouse efficacy studies of local anesthetics
| Agent | Dose | Route | Test | Duration of action | Comments | Reference |
| Bupivacaine | 0.5% | Immer | TBX | No effect | Immersion for 30 s | 48 |
| 0.25% to 0.5% 50 µL | SC | HP, TF | 5-15 min at 0.25 mg/kg | Epinephrine at 1:200000 increased duration to 60 min | 190 | |
| 30-45 min at 0.5 mg/kg | ||||||
| 0.5% 150 µL | SC | Electric | 1-2 h | 73 | ||
| 10% in polymer | SC | HP, SNB | Up to 30 h | 187 | ||
| 333 mg/kg in polymer | SC | HP, SNB | Up to 48 h | 192 | ||
| 0.015% to 0.5% 150 µL | SC | Electric | 15 min low dose; 60 min high dose | 77 | ||
| 0.12% 100 µL | SC | TF | 30-45 min | 191 | ||
| 0.75% 20 µL | SC | TBX, TF | < 5 min | In effective for TBX | 108 | |
| 1.1% 40 µL | SC | TF | 45 min | Epinephrine increased duration to 80 min | 75 | |
| 5 mg/kg | SC | Lap | Up to 60 min | Reduced mouse grimace scale | 130 | |
| EMLA | Top | Tail vein injection | No effect | 47 | ||
| Top | TBX | No effect | 48 | |||
| Lidocaine | 2-4mM | Immer | TF | 5 min | 120 | |
| 0.5% 40 µL | SC | TF | 5-30 min | Epinephrine at 1:200000 increased duration up to 100 min | 76 | |
| 1% | ||||||
| 2% | ||||||
| 2% 20 µL | SC | TBX, TF | < 5 min | In effective for TBX | 108 | |
Effects are based on a single dose of analgesic unless otherwise described in the comments.
Immer = immersion; TBX = tail biopsy; SNB = sciatic nerve block; Electric = electrical stimulus
Table 11.
Rat efficacy studies of local analgesics
| Agent | Dose | Route | Test | Duration of action | Comments | Ref |
| Bupivacaine | PN | SNB | 7 h | Liposomal formulation increased duration to 21 h | 54 | |
| 1-6 mg/kg liposomal formula | SC | VF | 2 h | 110 | ||
| 2 mg/kg | SC | VF | — | 110 | ||
| 2% 300 µL | SC | VF | 25 min | 74 | ||
| 2% liposomal Equation 300 µL | SC | VF | 200 min | 74 | ||
| 5-15 mg/mL | SC | HP | 120-200 min | Latency increased in dose dependent manner | 93 | |
| Levobupivacaine | 0.3% 50 µL | SC | SX | 3-24 h | 122 | |
| Lidocaine | 2% 400 µL | HP, CCI | Reduced scratching behavior | 15 | ||
| 1.5-13.8 mmol/kg | SC | VF | 15-30 min 13.8mmol | ED50 5.4 mmol/kg; ED75 8.0 mmol/kg | 32 | |
| 2% 600 µL | SC | VF | ED50 0.13% | 33 | ||
| 4.4-62.2 mmol/kg | SC | VF | 15-30 min at 62.2 mmol/kg | ED50 13.3 mmol/kg; ED80 36.7 mmol/kg | 31 | |
| 2% gel | Top | TF | 20 min | 9 | ||
| Pramoxine | 12-120 mmol/kg | SC | VF | 15-30 min at 120 mmol | ED50 42.1 mmol/kg; ED75 63.9 mmol/kg | 32 |
| Procaine | 2% 600 µL | SC | VF | ED50 0.44% | 33 | |
| Ropivacaine | 2 mg/mL 300 µL | ID | Lap, VF | Up to 24 h | Less disturbed circadian rhythm, HR, BP | 30 |
PN = perineural; SNB = sciatic nerve block; CCI = chronic constriction injury
ID = intradermal; Top = topical; HR = heart rate; BP = blood pressure; HP = hot plate assay; TF = tail-flick assay; VF = von Frey test
Oral administration
Bioavailability must be considered for any drug administered orally. Voluntary consumption will be variable between animals and both food and water consumption are often decreased after a surgical procedure.8,87,197 If the drug is administered in a “treat” to encourage consumption, animals may need to be singly housed to ensure equal access and consumption. This could add another level of stress and an additional research variable. Absorption in the intestinal tract can be highly variable and affected by the amount of digesta in the tract, gastrointestinal motility, and other factors. The analgesics themselves may even impact GI motility.125,165 Oral opioids are commonly used in humans but their primary use is for chronic pain, and there is a paucity of information on oral opioids in rodents. First pass metabolism is an impeding factor as opioids are degraded and lose a significant percentage of their bioavailability.
Oral gavage ensures exact dosing and delivery to all animals in the cohort. However, this method can be time consuming and the handling, restraint, and procedure itself may be stressful to the animals. Administration of analgesics in the drinking water is an attractive option and has been tested in a variety of paradigms in both mice and rats, but this method has numerous drawbacks to widespread use. Palatability and neophobia must be evaluated in each instance, as decreased water consumption will significantly impact the analgesic dosing.16,194 Further, decreased consumption may compound an already negative hydration state due to the surgery and associated blood/fluid loss. The solubility of oral solutions is another consideration. Ibuprofen and acetaminophen in pediatric suspensions tend to settle out of solution and both are relatively insoluble in water.63 A study evaluating rats given acetaminophen in drinking water found no difference in paw pressure latency compared with control rats and treated rats consumed less.39 This same study also compared buprenorphine in drinking water to intramuscular injection. An increased latency response was measured in high dose buprenorphine (2.9 mg/kg/day equivalent to 0.02mg/ mL water) in drinking water comparable to that seen with IM buprenorphine, and neophobia was not seen. However, one group measured a decreased response to hot plate sensitivity in rats provided acetaminophen elixir at a concentration of 4.48 mg/mL in drinking water.147 While consumption of acetaminophen treated water was greater than 50% less than tap water on Day 1, the neophobic response decreased substantially by Day 2. In addition, rats drank significantly more acetaminophen the day after surgery compared with no-surgery controls. In a study by Ingrao and colleagues male C57BL/6 mice consumed carprofen willingly when diluted in their drinking water but not meloxicam.96 Buprenorphine added to drinking water at 0.009 mg/mL (calculated to deliver approximately 10 times published subcutaneous doses) did not negatively affect volume of water consumed in female C57BL/6 mice, and resulted in therapeutic blood levels at many of the time points evaluated.182 This differs from the results obtained from a study in rats in which a measurable neophobic response was seen.101 Despite those encouraging results, interindividual differences in water consumption were seen as well as sporadic consumption during the daytime (light phase), resulting in variability in serum concentrations.
Delivery of analgesia by consumption in diet or a food treat has met with some success and offers the advantage of less stress on the animals since they do not need to be handled and restrained for dosing. Buprenorphine has been administered to rats in gelatin,62,134 in hazelnut chocolate spread to rats and mice,2,71,109 and in commercially available gels such as Medi-Gel (Clear H2O, Portland, ME) in mice.94 Indeed, in some studies, oral consumption provided longer lasting blood levels of drug than subcutaneous injection,109 for which the duration of action in mice is not long enough to provide continuous analgesia when dosed only every 8 to 12 h. However, consistent themes in all of these studies were variability in consumption, both in quantity and time of day that led the authors to conclude that these methods may be unreliable for provision of consistent and continuous analgesia. Further, in almost all of these studies animals were singly housed. NSAID have also been provided in “treat” forms, including carprofen-containing tablets (Rodent MDs, Bio-Serv, Frenchtown, NJ), and carprofen containing sucralose gel (MediGel CPF, Clear H2O, Portland, ME).
Another consideration for self-administration in water or food is the time of consumption. Mice and rats consume most of their feed and water during the dark cycle.198 If surgery occurs in the morning of any given day, and the animals do not consume significant quantities of the medication until that night, they will lack pain management during the most crucial initial 12-h postoperative period. Therefore, beginning drug administration prior to the painful procedure (for example surgery) is recommended to overcome both the neophobic response and circadian rhythm impact on consumption to ensure that sufficient blood levels are attained preemptively.
Transdermal administration: Transdermal patches are effective for delivery of analgesia in humans and larger animals, but their practical application to rats and mice is so far limited. Two studies have evaluated the Buprederm patch (Samyang Pharmaceutical Center, Daejeong, Korea) in mice.168,215 Analgesia, as measured by tail-flick latency, was most pronounced 3 to 6 h after application and an effect was measurable for 24 h.215
Timing of administration
The concept of preemptive analgesia is now well established in the pain management of human patients. A PubMed search conducted in December 2018 with keywords “pre-emptive” and “analgesia” produced 412 results. Many of these related to dental, spinal, and other orthopedic procedures. The clinical justification for preemptive analgesia is based on preventing central sensitization of nerve fibers by noxious stimuli occurring peripherally. This excitation results in a lowered pain threshold and hyperalgesia.11,209 Indeed, a number of studies in humans have demonstrated that preemptive use of local anesthetics decreased the amount of analgesia required postoperatively and decreased hyperalgesia associated with some injuries.46,51,142 Preemptive analgesia should provide similar benefits in animals by enhancing ability to ameliorate pain resulting in faster recovery periods. Preoperative administration of buprenorphine 30 min prior to surgery in rats resulted in less reduction in food intake than those given buprenorphine after surgery.86 Preoperative administration of pethidine to rats undergoing ovariohysterectomy surgery prevented postoperative hyperalgesia.128
Analgesia should be administered preoperatively whenever short surgical periods are anticipated and an inhalant such as isoflurane or sevoflurane is the sole anesthetic used. The time to onset of action of the analgesic must be considered in planning time of administration. Even drugs given subcutaneously or intraperitoneally are expected to take 15 to 30 min to achieve therapeutic levels. Orally administered drugs will take even longer due to time needed for intestinal absorption and first-pass metabolism in the liver. The increased use of sustained-release formulations offers many advantages, as previously discussed; however, these agents generally take longer to reach effective plasma levels than their standard formulations. An animal that is anesthetized with isoflurane for a 30-min surgical procedure and does not receive a dose of SR-buprenorphine until after the surgery is completed will likely experience unrelieved pain for 30 to 60 min during the postoperative recovery period.
If the surgical period is sufficiently long that an analgesic can be administered under anesthesia and reach effective tissue levels before anesthetic recovery, then this provides another reasonable option. The advantage is that the animal will not be subject to an additional handling (and thus stress) event prior to anesthesia. Another advantage of preoperative or perioperative analgesia is the anesthetic-sparing effect that many of these drugs provide.89,169 Thus, incorporating administration of analgesics into the anesthetic management plan is another method of providing balanced anesthesia that reduces some of the adverse effects of individual anesthetic agents.
Adjunct (nonpharmacologic) considerations
The goal of pain management is to keep patients as comfortable as possible. Nonpharmacologic interventions that may reduce pain should be considered during postoperative recovery in mice and rats . Animals subjected to procedures resulting in more chronic discomfort or pain are also good candidates for adjunct care. Training researchers in gentle handling techniques and methods to evaluate animals in a nonintrusive manner will minimize incidental stress. Taking time to habituate animals (particularly rats) to handling in advance of the invasive period can further reduce handling stress at both the time of surgery and during postoperative recovery. Skilled surgeons will minimize the degree to which the surgical procedure itself contributes unnecessarily to pain caused by excess tissue trauma, secondary infections, tissue swelling, or other inflammatory responses.119 Selection of appropriate materials such as synthetic suture material and implanted materials that evoke less tissue response and effective sterilization of surgical materials are also important considerations.
General principles of supportive nursing care apply to all species, including laboratory rodents. Providing additional external heat will help with anesthetic recovery and prevent discomfort associated with hypothermia; soft dry bedding in a solid bottom cage similarly will provide a more comfortable recovery period. Bedding material that does not stick to the animal's eyes, nose or mouth, such as a paper chip or shredded paper nesting material, should be used.60 Housing animals in a quiet area that is not heavily trafficked will minimize another potential source of stress. Food and water should be easily accessible to the animals without having to stand up on their hind limbs and stretch to reach it, particularly for orthopedic, invasive abdominal or spinal cord surgeries. Food pellets can be placed on the cage floor and soaked with water to encourage consumption. For animals needing even more supportive care, a variety of high-calorie supplements are available as well as gels as a ready source of hydration. Animals that have had a procedure impacting their mouth, jaw, and/or surrounding tissues may benefit from a soft diet until healed. Administration of fluids either subcutaneously or intraperitoneally may also be beneficial, both in anesthetic recovery and also in preventing dehydration during a period of inappetence. Recommended volumes are 1 to 2 mL for mice and 5 to 10 mL for rats depending on body weight.60 Larger volumes should be divided into two doses and administered at 2 separate sites.
Side effects of analgesia use in rodents
Analgesic drugs should be administered with care because of inherent side effects that result from their structure, chemical characteristics, and mechanism of action, and because of the potential for overdose effects when administered at high or extra-label doses. Even correct doses of analgesics can have unintended side effects if animals are not managed appropriately after a procedure. Figure 3 shows an example of renal tubular injury induced by flunixin meglumine. In addition to renal effects, other unintended side effects of some classes of NSAIDs include gastric and duodenal ulcers, and even intestinal perforations.208 The higher dosages of NSAID currently being recommended narrow the therapeutic window and caution should be taken when dosing beyond 3 consecutive days. In addition, hydration status should be assured to minimize risk of acute kidney injury. Opioid use is similarly confounded by side effects, and intoxication is generally associated with cardiorespiratory depression, sedation, constipation, and cognitive impairment.82 Different classes of opioids will have different mechanisms of action, and different side effects. For example, buprenorphine has been associated with pica and obstruction in rodents when used at high doses.36 A common side effect of postoperative opioid administration in humans and other animal species that is rarely considered in mice and rats is postoperative nausea and vomiting (PONV).172,185 Although most rodents cannot vomit, they may experience nausea after opioid administration. If rodents experience some version of PONV after opioid administration, then this could be associated with acute postprocedural weight loss. Dogs develop PONV more frequently after morphine administration than buprenorphine.172
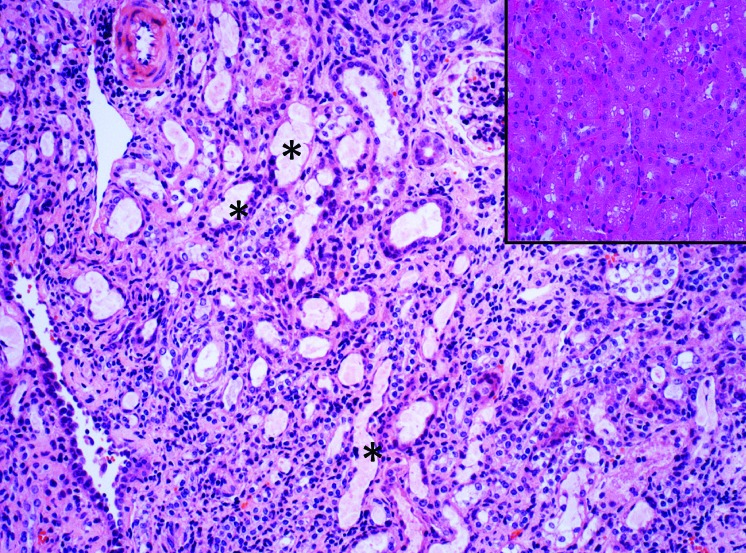
Figure 3.
Photomicrograph of a mouse kidney demonstrating acute renal tubular dilatation and necrosis. This mouse was one of several animals that was treated with 2.5 mg/kg flunixin meglumine (Banamine) SC, a potent NSAID, following a 20-min surgical procedure conducted under ketamine/xylazine anesthesia. Fluids were not given after surgery, anesthesia was not reversed, and the cage of recumbent recovering mice was placed under a heat lamp. Animals were euthanized less than 24h after surgery following poor recovery. Renal injury was attributed to acute renal ischemia secondary to NSAID use that was compounded by mild to moderate clinical dehydration. Inset: Normal appearance of renal cortical tubules from an untreated mouse. (x100 H and E)
Pain on injection may occur with some analgesic drugs and in particular NSAID. The intramuscular route is best avoided for injection in small rodents because swelling, necrosis and subsequent sloughing has been associated with administering acidic agents into their small muscle mass.204 Some sustained-release formulations of buprenorphine have been associated with skin irritation and necrosis.64
Adverse effects of analgesics can be reduced with a number of strategies. Combining analgesic drugs with different mechanisms of action to reduce the overall dose required for any single agent, by using topical and local anesthetics, and incorporating other adjunctive forms of care for animals (see adjunct considerations above) all reduce the deficits associated with analgesia administration. Research teams should work together with their clinical veterinarian to select the safest and most appropriate analgesia plan for their studies.
Conclusions
Like other mammals, laboratory rodents are sentient species and require the same considerations for peri-procedural treatment care that will minimize pain and suffering. The key to effective clinical management of pain is advance planning and anticipation of outcomes. A large number of pharmacologic and nonpharmacologic agents can be used alone or in combination to provide effective care while minimizing potential adverse effects. While the list of pharmacological agents considered most effective in rodents has not changed much in the last decade, we have presented an evidence-based approach to formulate our current recommendations, including consideration of dosing intervals, use of sustained-release formulations, and multimodal approaches to pain management. Based on the available evidence and dosing practices, rodents are often provided inadequate analgesia. However, a significant constraint is that more frequent dosing may require more frequent handling, restraint, and thus increased stress. Analgesics are potent agents with known side effects, and treatment plans should always be developed in conjunction with clinical veterinary input. More research is needed on the duration of effect of analgesia for many drugs and on better dose titrations for achieving and sustaining optimal analgesic blood drug levels. While valid scientific reasons may require withholding analgesic drugs to mice and rats after painful procedures, the vast majority of cases have no prohibitions to analgesic use. If potential experimental effects or interactions with specific analgesic agents are unknown or suspected, investigators and veterinarians should be encouraged to work collaboratively to design and conduct pilot studies before concluding that analgesia will not be provided. A commitment to appropriately managing pain in all research animals represents a commitment to compassionate care and a goal that all those working with animals in research should be striving toward.
References
- 1.Abbott FV, Bonder M. 1997. Options for management of acute pain in the rat. Vet Rec 140:553–557. 10.1136/vr.140.21.553. [DOI] [PubMed] [Google Scholar]
- 2.Abelson KSP, Jacobsen KR, Sundbom R, Kalliokoski O, Hau J. 2012. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab Anim 46:349–351. 10.1258/la.2012.012028. [DOI] [PubMed] [Google Scholar]
- 3.Ageel AM. 1986. Effects of desipramine and chlorimipramine on buprenorphine analgesia in mice. Jpn J Pharmacol 41:139–145. 10.1254/jjp.41.139. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Carrasco JC, Rodríguez-Silverio J, Jiménez-Andrade JM, Carrasco-Portugal M del C, Flores-Murrieta FJ. 2014. Relationship between blood levels and the antihyperalgesic effect of ketoprofen in the rat: ketoprofen pk/pd modeling in rats. Drug Dev Res 75:189–194. [DOI] [PubMed] [Google Scholar]
- 5.Alexa T, Marza A, Voloseniuc T, Tamba B. 2015. Enhanced analgesic effects of tramadol and common trace element coadministration in mice. J Neurosci Res 93:1534–1541. 10.1002/jnr.23609. [DOI] [PubMed] [Google Scholar]
- 6.Al-Musawi A, Matar K, Kombian SB, Andersson L. 2012. A pharmacokinetic study of a topical anesthetic (EMLA) in mouse soft tissue laceration. Dent Traumatol 28:483–487. 10.1111/j.1600-9657.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 7.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. 2003. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97:534–540. 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 8.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:1–10. 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atabaki R, Hassanpour-Ezatti M. 2014. Improvement of lidocaine local anesthetic action using lallemantia royleana seed mucilage as an excipient. Iran J Pharm Res 13:1431–1436. [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey M, Corcoran T, Schug S, Toner A. 2018. Perioperative lidocaine infusions for the prevention of chronic postsurgical pain: a systematic review and meta-analysis of efficacy and safety. Pain 159:1696–1704. https://doi:10.1097/j.pain.0000000000001273 [DOI] [PubMed] [Google Scholar]
- 11.Bailey PM, Child CS. 1987. Endocrine response to surgery. p 100–116. In: Kaufman L, editor. Anaesthesia review 4. London (United Kingdom): Churchill Livingstone. [Google Scholar]
- 12.Barazanchi AWH, MacFater WS, Rahiri JL, Tutone S, Hill AG, Joshi GP, PROSPECT collaboration 2018. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth 121:787–803. 10.1016/j.bja.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Barker JC, Dibartola K, Wee C, Andonian N, Abdel-rasoul M, Lowery D, Janis JE. 2018. Preoperative multimodal analgesia decreases postanesthesia care unit narcotic use and pain scores in outpatient breast surgery. Plast Reconstr Surg 142:443e–450e. 10.1097/PRS.0000000000004804. [DOI] [PubMed] [Google Scholar]
- 14.Barrot M. 2012. Tests and models of nociception and pain in rodents. Neuroscience 211:39–50. 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Batista LM, Batista IM, Almeida JP, Carvalho CH, de Castro-Costa SB, de Castro-Costa CM. 2009. Preemptive analgesic effect of lidocaine in a chronic neuropathic pain model. Arq Neuropsiquiatr 67:1088–1092. 10.1590/S0004-282X2009000600024. [DOI] [PubMed] [Google Scholar]
- 16.Bauer DJ, Christenson TJ, Clark KR, Powell SK, Swain RA. 2003. Acetaminophen as a postsurgical analgesic in rats: a practical solution to neophobia. Contemp Top Lab Anim Sci 42:20–25. [PubMed] [Google Scholar]
- 17.Berry SH. 2015. Analgesia in the perioperative period. Vet Clin North Am Small Anim Pract 45:1013–1027. 10.1016/j.cvsm.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Bicket MC, Cohen SP. 2018. Lidocaine infusions and preventative analgesia: can the answer to our prayers be hiding right under our noses? Pain 159:1677–1678. [DOI] [PubMed] [Google Scholar]
- 19.Blankenship-Paris TL, Dutton JW, Goulding DR, McGee CA, Kissling GE, Myers PH. 2016. Evaluation of buprenorphine hydrochloride Pluronic gel formulation in male C57BL/6NCrl mice. Lab Anim (NY) 45:370–379. 10.1038/laban.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourque SL, Adams MA, Nakatsu K, Winterborn A. 2010. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49:617–622. [PMC free article] [PubMed] [Google Scholar]
- 21.Busch U, Schmid J, Heinzel G, Schmaus H, Baierl J, Huber C, Roth W. 1998. Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos 26:576–584. [PubMed] [Google Scholar]
- 22.Buvanendran A, Fiala J, Patel KA, Golden AD, Moric M, Kroin JS. 2015. The incidence and severity of postoperative pain following inpatient surgery. Pain Med 16:2277–2283. 10.1111/pme.12751. [DOI] [PubMed] [Google Scholar]
- 23.Cannon CZ, Kissling GE, Goulding DR, King-Herbert AP, Blankenship-Paris T. 2011. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim (NY) 40:85–93. 10.1038/laban0311-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon CZ, Kissling GE, Hoenerhoff MJ, King-Herbert AP, Blankenship-Paris T. 2010. Evaluation of dosages and routes of administration of tramadol analgesia in rats using hot-plate and tail-flick tests. Lab Anim (NY) 39:342–351. 10.1038/laban1110-342. [DOI] [PubMed] [Google Scholar]
- 25.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 26.Carbone L, Austin J. 2016. Pain and laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 11:1–24. 10.1371/journal.pone.0155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caro AC, Tucker JJ, Yannascoli SM, Dunkman AA, Thomas SJ, Soslowsky LJ. 2014. Efficacy of various analgesics on shoulder function and rotator cuff tendon-to-bone healing in a rat (Rattus norvegicus) model. J Am Assoc Lab Anim Sci 53:185–192. [PMC free article] [PubMed] [Google Scholar]
- 28.Carr DB, Goudas LC. 1999. Acute pain. Lancet 353:2051–2058. 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 29.Chapman CR, Gavrin J. 1999. Suffering: the contributions of persistent pain. Lancet 353:2233–2237. 10.1016/S0140-6736(99)01308-2. [DOI] [PubMed] [Google Scholar]
- 30.Charlet A, Rodeau JL, Poisbeau P. 2011. Radiotelemetric and symptomatic evaluation of pain in the rat after laparotomy: long-term benefits of perioperative ropivacaine care. J Pain 12:246–256. 10.1016/j.jpain.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Chen YW, Shieh JP, Liu KS, Wang JJ, Hung CH. 2017. Naloxone prolongs cutaneous nociceptive block by lidocaine in rats. Fundam Clin Pharmacol 31:636–642. [DOI] [PubMed] [Google Scholar]
- 32.Chou AK, Chiu CC, Chen YW, Wang JJ, Hung CH. 2018. Skin nociceptive block with pramoxine delivery by subcutaneous injection in rats. Pharmacol Rep 70:1180–1184. 10.1016/j.pharep.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Chu CC, Wu SZ, Su WL, Shieh JP, Kao CH, Ho ST, Wang JJ. 2008. Subcutaneous injection of inhaled anesthetics produces cutaneous analgesia. Can J Anaesth 55:290–294. 10.1007/BF03017206. [DOI] [PubMed] [Google Scholar]
- 34.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 35.Cichewicz DL, Welch SP, Smith FL. 2005. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal Δ9-tetrahydrocannabinol. Eur J Pharmacol 525:74–82. 10.1016/j.ejphar.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 36.Clark JA, Jr, Myers PH, Goelz MF, Thigpen JE, Forsythe DB. 1997. Pica behavior associated with buprenorphine administration in the rat. Lab Anim Sci 47:300–303. [PubMed] [Google Scholar]
- 37.Clark TS, Clark DD, Hoyt RF., Jr 2014. Pharmacokinetic comparison of sustained- release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 38.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. 2012. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884. 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper DM, Delong D, Gilleti CS. 1997. Analgesic efficacy of acetaminophen and buprenorphine administered in the drinking water of rats. Contemp Top Lab Anim Sci 36:58–62. [PubMed] [Google Scholar]
- 40.Cooper DM, Hoffman W, Tomlinson K, Lee HY. 2008. Refinement of the dosage and dosing schedule of ketoprofen for postoperative analgesia in Sprague–Dawley rats. Lab Anim (NY) 37:271–275. 10.1038/laban0608-271. [DOI] [PubMed] [Google Scholar]
- 41.Cooper DM, Hoffman W, Wheat N, Lee HY. 2005. Duration of effects on clinical parameters and referred hyperalgesia in rats after abdominal surgery and multiple doses of analgesic. Comp Med 55:344–353. [PubMed] [Google Scholar]
- 42.Corletto F. 2007. Multimodal and balanced analgesia. Vet Res Commun 31 S1:59–63. 10.1007/s11259-007-0085-5. [DOI] [PubMed] [Google Scholar]
- 43.Coulter CA, Flecknell PA, Richardson CA. 2009. Reported analgesic administration to rabbits, pigs, sheep, dogs and non-human primates undergoing experimental surgical procedures. Lab Anim 43:232–238. 10.1258/la.2008.008021. [DOI] [PubMed] [Google Scholar]
- 44.Coulter CA, Flecknell PA, Leach MC, Richardson CA. 2011. Reported analgesic administration to rabbits undergoing experimental surgical procedures. BMC Vet Res 7:12 10.1186/1746-6148-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe MS, Wilson CD, Leishman E, Prather PL, Bradshaw HB, Banks ML, Kinsey SG. 2017. The monoacylglycerol lipase inhibitor KML29 with gabapentin synergistically produces analgesia in mice: KML29 and gabapentin synergistically reduce pain. Br J Pharmacol 174:4523–4539. 10.1111/bph.14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahl JB, Brennum J, Arendt-Nielsen L, Jensen TS, Kehlet H. 1993. The effect of pre- versus postinjury infiltration with lidocaine on thermal and mechanical hyperalgesia after heat injury to the skin. Pain 53:43–51. 10.1016/0304-3959(93)90054-S. [DOI] [PubMed] [Google Scholar]
- 47.David JM, Duarte Vogel S, Longo K, Sanchez D, Lawson G. 2014. The use of eutectic mixture of lidocaine and prilocaine in mice (Mus musculus) for tail vein injections. Vet Anaesth Analg 41:654–659. 10.1111/vaa.12177. [DOI] [PubMed] [Google Scholar]
- 48.Dudley ES, Johnson RA, French DC, Boivin GP. 2016. Effects of topical anesthetics on behavior, plasma corticosterone, and blood glucose levels after tail biopsy of C57BL/6NHSD mice (Mus musculus). J Am Assoc Lab Anim Sci 55:443–450. [PMC free article] [PubMed] [Google Scholar]
- 49.Dunbar ML, David EM, Aline MR, Lofgren JL. 2016. Validation of a behavioral ethogram for assessing postoperative pain in guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 55:29–34. [PMC free article] [PubMed] [Google Scholar]
- 50.Dunkman WJ, Manning MW. 2018. Enhanced recovery after surgery and multimodal strategies for analgesia. Surg Clin North Am 98:1171–1184. 10.1016/j.suc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Ejlersen E, Andersen HB, Eliasen K, Mogensen T. 1992. A comparison between preincisional and postincisional lidocaine infiltration and postoperative pain. Anesth Analg 74:495–498. [DOI] [PubMed] [Google Scholar]
- 52.Ellen Y, Flecknell P, Leach M. 2016. Evaluation of using behavioural changes to assess postoperative pain in the guinea pig (Cavia Porcellus). PLoS One 11:1–20. 10.1371/journal.pone.0161941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epstein M, Rodan I, Griffenhagen G, Kadrlik J, Petty M, Robertson S, Simpson W. 2015. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc 51:67–84. 10.5326/JAAHA-MS-7331. [DOI] [PubMed] [Google Scholar]
- 54.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. 2009. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci USA 106:7125–7130. 10.1073/pnas.0900598106. Erratum: Proc Natl Acad Sci USA 2011. 108: 4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erhan E, Onal A, Kocabas S, Parlar A, Yegul I, Kosay S. 2005. Ondansetron does not block tramadol-induced analgesia in mice. Methods Find Exp Clin Pharmacol 27:629–632. 10.1358/mf.2005.27.9.939337. [DOI] [PubMed] [Google Scholar]
- 56.Evangelista Vaz R, Draganov DI, Rapp C, Avenel F, Steiner G, Arras M, Bergadano A. 2018. Preliminary pharmacokinetics of tramadol hydrochloride after administration via different routes in male and female B6 mice. Vet Anaesth Analg 45:111–122. 10.1016/j.vaa.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Faller KME, McAndrew DJ, Schneider JE, Lygate CA. 2015. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the Mouse Grimace Scale. Exp Physiol 100:164–172. 10.1113/expphysiol.2014.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fenwick N, Duffus SEG, Griffin G. 2014. Pain management for animals used in science: views of scientists and veterinarians in Canada. Animals (Basel) 4:494–514. 10.3390/ani4030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Dueñas V, Poveda R, Sánchez S, Ciruela F. 2012. Synergistic interaction between fentanyl and a tramadol:paracetamol combination on the inhibition of nociception in mice. J Pharmacol Sci 118:299–302. 10.1254/jphs.11161SC. [DOI] [PubMed] [Google Scholar]
- 60.Flecknell PA. 2016. Laboratory animal anaesthesia. London (United Kingdom): Academic Press. [Google Scholar]
- 61.Flecknell P. 2018. Rodent analgesia: assessment and therapeutics. Vet J 232:70–77. 10.1016/j.tvjl.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Flecknell PA, Roughan JV. 1999. Use of oral buprenorphine (‘Buprenorphine jello’) for postoperative analgesia in rats—a clinical trial. Lab Anim 33:169–174. 10.1258/002367799780578381. [DOI] [PubMed] [Google Scholar]
- 63.Foley PL. 2014. Current options for providing sustained analgesia to laboratory animals. Lab Anim (NY) 43:364–371. 10.1038/laban.590. [DOI] [PubMed] [Google Scholar]
- 64.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 65.Fowler M, Clifford JL, Garza TH, Slater TM, Arizpe HM, Novak J, Petz LN, Loyd DR. 2014. A rat model of full thickness thermal injury characterized by thermal hyperalgesia, mechanical allodynia, pronociceptive peptide release and tramadol analgesia. Burns 40:759–771. 10.1016/j.burns.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13. [PubMed] [Google Scholar]
- 67.Gerçek A, Eti Z, Göğüş FY, Sav A. 2004. The analgesic and anti-inflammatory effects of subcutaneous bupivacaine, morphine and tramadol in rats. Agri 16:53–58. [PubMed] [Google Scholar]
- 68.Girard P, Verniers D, Coppé M-C, Pansart Y, Gillardin J-M. 2008. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur J Pharmacol 584:263–271. 10.1016/j.ejphar.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Giraudel JM, Diquelou A, Laroute V, Lees P, Toutain PL. 2005. Pharmacokinetic/pharmacodynamic modelling of NSAIDs in a model of reversible inflammation in the cat. Br J Pharmacol 146:642–653. 10.1038/sj.bjp.0706372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goecke JC, Awad H, Lawson JC, Boivin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44. [PubMed] [Google Scholar]
- 71.Goldkuhl R, Hau J, Abelson KSP. 2010. Effects of voluntarily-ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behaviour in permanently catheterised rats. In Vivo 24:131–135. [PubMed] [Google Scholar]
- 72.Goldkuhl R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2010. Plasma concentrations of corticosterone and buprenorphine in rats subjected to jugular vein catheterization. Lab Anim 44:337–343. 10.1258/la.2010.009115. [DOI] [PubMed] [Google Scholar]
- 73.Grant GJ, Barenholz Y, Piskoun B, Bansinath M, Turndorf H, Bolotin EM. 2001. DRV liposomal bupivacaine: preparation, characterization, and in vivo evaluation in mice. Pharm Res 18:336–343. 10.1023/A:1011059131348. [DOI] [PubMed] [Google Scholar]
- 74.Grant GJ, Lax J, Susser L, Zakowski M, Weissman E. 1997. Wound infiltration with liposomal bupivacaine prolongs analgesia in rats. Acta Anaesthesiol Scand 41:204–207. 10.1111/j.1399-6576.1997.tb04666.x. [DOI] [PubMed] [Google Scholar]
- 75.Grant GJ, Vermeulen K, Langerman L, Zakowski M, Turndorf H. 1994. Prolonged analgesia with liposomal bupivacaine in a mouse model. Reg Anesth 19:264–269. [PubMed] [Google Scholar]
- 76.Grant GJ, Zakowski MI, Vermeulen K, Langerman L, Ramanathan S, Turndorf H. 1993. Assessing local anesthetic effect using the mouse tail flick test. J Pharmacol Toxicol Methods 29:223–226. 10.1016/1056-8719(93)90029-E. [DOI] [PubMed] [Google Scholar]
- 77.Grant GJ, Piskoun B, Lin A, Bansinath M. 2000. An in vivo method for the quantitative evaluation of local anesthetics. J Pharmacol Toxicol Methods 43:69–72. 10.1016/S1056-8719(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 78.Grant GJ, Vermeulen K, Zakowski M, Stenner M, Turndorf H, Langerman L. 1994. Prolonged analgesia and decreased toxicity with liposomal morphine in a mouse model. Anesth Analg 79:706–709. 10.1213/00000539-199410000-00015. [DOI] [PubMed] [Google Scholar]
- 79.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343. 10.1038/laban.152. [DOI] [PubMed] [Google Scholar]
- 80.Guarnieri M, Tyler BM, DeTolla L, Zhao M, Kobrin B. 2014. Subcutaneous implants for long-acting drug therapy in laboratory animals may generate unintended drug reservoirs. J Pharm Bioallied Sci 6:38–42. 10.4103/0975-7406.124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. 2014. Gabapentin enhances antinociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci 17:753–759. [PMC free article] [PubMed] [Google Scholar]
- 82.Haroutounian S. 2018. [Postoperative opioids, endocrine changes,and immunosuppression.] Schmerz 32:374–380. 10.1007/s00482-018-0319-1. [Article in German]. [DOI] [PubMed] [Google Scholar]
- 83.Hawkins M, Pascoe P. 2012. Anesthesia, analgesia, and sedation of small mammals. p 429–451. Chapter 31 In: Quesenberry KE, Carpenter JW, editors. Ferrets, rabbits and rodents clinical medicine and surgery. St Louis (MO): Saunders; 10.1016/B978-1-4160-6621-7.00031-2 [DOI] [Google Scholar]
- 84.Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395. 10.1258/002367702320389044. [DOI] [PubMed] [Google Scholar]
- 85.Hayashida K, DeGoes S, Curry R, Eisenach JC. 2007. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology 106:557–562. 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 86.Hayes JH, Flecknell PA. 1999. A comparison of pre- and post-surgical administration of bupivacaine or buprenorphine following laparotomy in the rat. Lab Anim 33:16–23. 10.1258/002367799780578534. [DOI] [PubMed] [Google Scholar]
- 87.Hayes KE, Raucci JA, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23. [PubMed] [Google Scholar]
- 88.Healy JR, Tonkin JL, Kamarec SR, Saludes MA, Ibrahim SY, Matsumoto RR, Wimsatt JH. 2014. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. Am J Vet Res 75:619–625. 10.2460/ajvr.75.7.619. [DOI] [PubMed] [Google Scholar]
- 89.Hedenqvist P, Roughan JV, Flecknell PA. 2000. Effects of repeated anaesthesia with ketamine/medetomidine and of pre-anaesthetic administration of buprenorphine in rats. Lab Anim 34:207–211. 10.1258/002367700780457536. [DOI] [PubMed] [Google Scholar]
- 90.Herndon NL, Bandyopadhyay S, Hod EA, Prestia KA. 2016. Sustained-release buprenorphine improves postsurgical clinical condition but does not alter survival or cytokine levels in a murine model of polymicrobial sepsis. Comp Med 66:455–462. [PMC free article] [PubMed] [Google Scholar]
- 91.Herrmann K, Flecknell P. 2019. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX 36:65–80. 10.14573/altex.1804011. [DOI] [PubMed] [Google Scholar]
- 92.Hestehave S, Munro G, Pedersen TB, Abelson KSP. 2017. Antinociceptive effects of voluntarily ingested buprenorphine in the hot-plate test in laboratory rats. Lab Anim 51:264–272. 10.1177/0023677216668553. [DOI] [PubMed] [Google Scholar]
- 93.Hoare T, Young S, Lawlor MW, Kohane DS. 2012. Thermoresponsive nanogels for prolonged duration local anesthesia. Acta Biomater 8:3596–3605. 10.1016/j.actbio.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hovard A, Teilmann A, Hau J, Abelson K. 2015. The applicability of a gel delivery system for self-administration of buprenorphine to laboratory mice. Lab Anim 49:40–45. 10.1177/0023677214551108. [DOI] [PubMed] [Google Scholar]
- 95.Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, Fontana DJ. 1997. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol 324:153–160. 10.1016/S0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- 96.Ingrao JC, Johnson R, Tor E, Gu Y, Litman M, Turner PV. 2013. Aqueous stability and oral pharmacokinetics of meloxicam and carprofen in male C57BL/6 mice. J Am Assoc Lab Anim Sci 52:553–559. [PMC free article] [PubMed] [Google Scholar]
- 97.Interagency Pain Research Coordinating Committee. [Internet]. 2015. National pain strategy. [Cited 01 April 2019]. Available at https://iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf.
- 98.Jablonski P, Howden B. 2002. Oral buprenorphine and aspirin analgesia in rats undergoing liver transplantation. Lab Anim 36:134–143. 10.1258/0023677021912415. [DOI] [PubMed] [Google Scholar]
- 99.Jacobsen KR, Fauerby N, Raida Z, Kalliokoski O, Hau J, Johansen FF, Abelson KS. 2013. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med 63:105–113. [PMC free article] [PubMed] [Google Scholar]
- 100.Jang HS, Jang IS, Lee MG. 2010. The effects of tramadol on electroencephalographic spectral parameters and analgesia in rats. Korean J Physiol Pharmacol 14:191–198. 10.4196/kjpp.2010.14.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jessen L, Christensen S, Bjerrum OJ. 2007. The antinociceptive efficacy of buprenorphine administered through the drinking water of rats. Lab Anim 41:185–196. 10.1258/002367707780378131. [DOI] [PubMed] [Google Scholar]
- 102.Jeunesse EC, Bargues IA, Toutain CE, Lacroix MZ, Letellier IM, Giraudel JM, Toutain PL. 2011. Paw inflammation model in dogs for preclinical pharmacokinetic/pharmacodynamic investigations of nonsteroidal antiinflammatory drugs. J Pharmacol Exp Ther 338:548–558. 10.1124/jpet.110.178350. [DOI] [PubMed] [Google Scholar]
- 103.Jirkof P, Tourvieille A, Cinelli P, Arras M. 2015. Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Lab Anim 49:177–187. 10.1177/0023677214562849. [DOI] [PubMed] [Google Scholar]
- 104.Jirkof P, Cesarovic N, Rettich A, Fleischmann T, Arras M. 2012. Individual housing of female mice: influence on postsurgical behaviour and recovery. Lab Anim 46:325–334. 10.1258/la.2012.012027. [DOI] [PubMed] [Google Scholar]
- 105.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. 2010. Burrowing behavior as an indicator of postlaparotomy pain in mice. Front Behav Neurosci 4:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, Arras M. 2013. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 47:153–161. 10.1177/0023677213475603. [DOI] [PubMed] [Google Scholar]
- 107.Johnson RA. 2016. Voluntary running-wheel activity, arterial blood gases, and thermal antinociception in rats after 3 buprenorphine formulations. J Am Assoc Lab Anim Sci 55:306–311. [PMC free article] [PubMed] [Google Scholar]
- 108.Jones CP, Carver S, Kendall LV. 2012. Evaluation of common anesthetic and analgesic techniques for tail biopsy in mice. J Am Assoc Lab Anim Sci 51:808–814. [PMC free article] [PubMed] [Google Scholar]
- 109.Kalliokoski O, Jacobsen KR, Hau J, Abelson KSP. 2011. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J 187:251–254. 10.1016/j.tvjl.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Kang SC, Jampachaisri K, Seymour TL, Felt SA, Pacharinsak C. 2017. Use of liposomal bupivacaine for postoperative analgesia in an incisional pain model in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 56:63–68. [PMC free article] [PubMed] [Google Scholar]
- 111.Kawano T, Takahashi T, Iwata H, Morikawa A, Imori S, Waki S, Tamura T, Yamazaki F, Eguchi S, Kumagai N, Yokoyama M. 2014. Effects of ketoprofen for prevention of postoperative cognitive dysfunction in aged rats. J Anesth 28:932–936. 10.1007/s00540-014-1821-y. [DOI] [PubMed] [Google Scholar]
- 112.Kendall LV, Hansen RJ, Dorsey K, Kang S, Lunghofer PJ, Gustafson DL. 2014. Pharmacokinetics of sustained-release analgesics in mice. J Am Assoc Lab Anim Sci 53:478–484. [PMC free article] [PubMed] [Google Scholar]
- 113.Kendall LV, Wegenast DJ, Smith BJ, Dorsey KM, Kang S, Lee NY, Hess AM. 2016. Efficacy of sustained-release buprenorphine in an experimental laparotomy model in female mice. J Am Assoc Lab Anim Sci 55:66–73. [PMC free article] [PubMed] [Google Scholar]
- 114.Kent ML, Tighe PJ, Belfer I, Brennan TJ, Bruehl S, Brummett CM, Buckenmaier CC, Buvanendran A, Cohen RI, Desjardins P, Edwards D, Fillingim R, Gewandter J, Gordon DB, Hurley RW, Kehlet H, Loeser JD, Mackey S, McLean SA, Polomano R, Rahman S, Raja S, Rowbotham M, Suresh S, Schachtel B, Schreiber K, Schumacher M, Stacey B, Stanos S, Todd K, Turk DC, Weisman SJ, Wu C, Carr DB, Dworkin RH, Terman G. 2017. The ACTTION-APS-AAPM pain taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. J Pain 18:479–489. 10.1016/j.jpain.2017.02.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kest B, Wilson SG, Mogil JS. 1999. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther 289:1370–1375. [PubMed] [Google Scholar]
- 116.Kimura M, Obata H, Saito S. 2012. Antihypersensitivity effects of tramadol hydrochloride in a rat model of postoperative pain. Anesth Analg 115:443–449. 10.1213/ANE.0b013e31825683c3. [DOI] [PubMed] [Google Scholar]
- 117.Kirsch JH, Klaus JA, Blizzard KK, Hurn PD, Murphy SJ. 2002. Pain evaluation and response to buprenorphine in rats subjected to sham middle cerebral artery occlusion. Contemp Top Lab Anim Sci 41:9–14. [PubMed] [Google Scholar]
- 118.Kögel B, Christoph T, Straßburger W, Friderichs E. 2005. Interaction of μ-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur J Pain 9:599–611. 10.1016/j.ejpain.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK. 2007. Guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci 46:97–108. [PubMed] [Google Scholar]
- 120.Kolesnikov YA, Chereshnev I, Pasternak GW. 2000. Analgesic synergy between topical lidocaine and topical opioids. J Pharmacol Exp Ther 295:546–551. [PubMed] [Google Scholar]
- 121.Kolesnikov YA, Wilson RS, Pasternak GW. 2003. The synergistic analgesic interactions between hydrocodone and ibuprofen. Anesth Analg 97:1721–1723. 10.1213/01.ANE.0000087801.20395.97. [DOI] [PubMed] [Google Scholar]
- 122.Korat PS, Kapupara PP. 2017. Local infiltration of the surgical wound with levobupivacaine, ibuprofen, and epinephrine in postoperative pain: an experimental study. Biomed Pharmacother 96:104–111. 10.1016/j.biopha.2017.09.131. [DOI] [PubMed] [Google Scholar]
- 123.Koutroli E, Alexakos P, Kakazanis Z, Symeon I, Balafas E, Voyiatzaki C, Kostomitsopoulos N. 2014. Effects of using the analgesic tramadol in mice undergoing embryo transfer surgery. Lab Anim (NY) 43:167–172. 10.1038/laban.518. [DOI] [PubMed] [Google Scholar]
- 124.Krueger KL, Fujiwara Y. 2008. The use of buprenorphine as an analgesic after rodent embryo transfer. Lab Anim (NY) 37:87–90. 10.1038/laban0208-87. [DOI] [PubMed] [Google Scholar]
- 125.Kurz A, Sessler DI. 2003. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 63:649–671. 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 126.Lamont LA. 2008. Multimodal pain management in veterinary medicine: the physiologic basis of pharmacologic therapies. Vet Clin North Am Small Anim Pract 38:1173–1186. 10.1016/j.cvsm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 127.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 128.Lascelles BD, Waterman AE, Cripps PJ, Livingston A, Henderson G. 1995. Central sensitization as a result of surgical pain: investigation of the pre-emptive value of pethidine for ovariohysterectomy in the rat. Pain 62:201–212. 10.1016/0304-3959(94)00266-H. [DOI] [PubMed] [Google Scholar]
- 129.Leach MC, Forrester AR, Flecknell PA. 2010. Influence of preferred foodstuffs on the antinociceptive effects of orally administered buprenorphine in laboratory rats. Lab Anim 44:54–58. 10.1258/la.2009.009029. [DOI] [PubMed] [Google Scholar]
- 130.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. 2012. The assessment of post-vasectomy pain in mice using behaviour and the mouse grimace scale. PLoS One 7:1–9. 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lees P, Giraudel J, Landoni MF, Toutain PL. 2004. PK-PD integration and PK-PD modelling of nonsteroidal antiinflammatory drugs: principles and applications in veterinary pharmacology. J Vet Pharmacol Ther 27:491–502. 10.1111/j.1365-2885.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 132.Lees P, Landoni MF, Giraudel J, Toutain PL. 2004. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther 27:479–490. 10.1111/j.1365-2885.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 133.Leung V, Zhang E, Pang DS. 2016. Real-time application of the rat grimace scale as a welfare refinement in laboratory rats. Sci Rep 6:1–12. 10.1038/srep31667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liles JH, Flecknell P, Roughan J, Cruz-Madorran I. 1998. Influence of oral buprenorphine, oral naltrexone or morphine on the effects of laparotomy in the rat. Lab Anim 32:149–161. 10.1258/002367798780600025. [DOI] [PubMed] [Google Scholar]
- 135.Loram LC, Mitchell D, Skosana M, Fick LG. 2007. Tramadol is more effective than morphine and amitriptyline against ischaemic pain but not thermal pain in rats. Pharmacol Res 56:80–85. 10.1016/j.phrs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Lukas G, Brindle SD, Greengard P. 1971. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178:562–564. [PubMed] [Google Scholar]
- 137.Magalhães-Sant'Ana M, Sandøe P, Olsson I. 2009. Painful dilemmas: the ethics of animal-based pain research. Anim Welf 18:49–63. [Google Scholar]
- 138.Martin LBE, Thompson AC, Martin T, Kristal MB. 2001. Analgesic efficacy of orally administered buprenorphine in rats. Comp Med 51:43–48. [PubMed] [Google Scholar]
- 139.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. 2007. Inflammation-induced reduction of spontaneous activity by adjuvant: a novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther 320:194–201. 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 140.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 141.McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, Yeomans DC, Felt SA. 2011. Analgesic effects of tramadol, tramadol–gabapentin, and buprenorphine in an incisional model of pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 50:192–197. [PMC free article] [PubMed] [Google Scholar]
- 142.McQuay HJ, Carroll D, Moore RA. 1988. Postoperative orthopaedic pain — the effect of opiate premedication and local anaesthetic blocks. Pain 33:291–295. 10.1016/0304-3959(88)90287-4. [DOI] [PubMed] [Google Scholar]
- 143.Medina-López R, Vara-Gama N, Soria-Arteche O, Moreno-Rocha L, López-Muñoz F. 2018. Pharmacokinetics and pharmacodynamics of (s)-ketoprofen co-administered with caffeine: a preclinical study in arthritic rats. Pharmaceutics 10:20 10.3390/pharmaceutics10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Merlos M, Portillo-Salido E, Brenchat A, Aubel B, Buxens J, Fisas A, Codony X, Romero L, Zamanillo D, Vela JM. 2018. Administration of a co-crystal of tramadol and celecoxib in a 1:1 molecular ratio produces synergistic antinociceptive effects in a postoperative pain model in rats. Eur J Pharmacol 833:370–378. 10.1016/j.ejphar.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 145.Meymandi M-S, Keyhanfar F. 2012. Pregabalin antinociception and its interaction with tramadol in actue model of pain. Pharmacol Rep 64:576–585. 10.1016/S1734-1140(12)70853-8. [DOI] [PubMed] [Google Scholar]
- 146.Meymandi M-S, Sepehri G, Mobasher M. 2006. Gabapentin enhances the analgesic response to morphine in acute model of pain in male rats. Pharmacol Biochem Behav 85:185–189. 10.1016/j.pbb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 147.Mickley GA, Hoxha Z, Biada JM, Kenmuir CL, Bacik SE. 2006. Acetaminophen self-administered in the drinking water increases the pain threshold of rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 45:48–54. [PubMed] [Google Scholar]
- 148.Miller AL, Kitson GL, Skalkoyannis B, Flecknell PA, Leach MC. 2016. Using the mouse grimace scale and behaviour to assess pain in CBA mice following vasectomy. Appl Anim Behav Sci 181:160–165. 10.1016/j.applanim.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller AL, Wright-Williams SL, Flecknell PA, Roughan JV. 2012. A comparison of abdominal and scrotal approach methods of vasectomy and the influence of analgesic treatment in laboratory mice. Lab Anim 46:304–310. 10.1258/la.2012.012078. [DOI] [PubMed] [Google Scholar]
- 150.Miranda HF, Puig MM, Romero MA, Prieto JC. 2009. Effects of tramadol and dexketoprofen on analgesia and gastrointestinal transit in mice. Fundam Clin Pharmacol 23:81–88. 10.1111/j.1472-8206.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 151.Miranda HF, Prieto JC, Puig MM, Pinardi G. 2008. Isobolographic analysis of multimodal analgesia in an animal model of visceral acute pain. Pharmacol Biochem Behav 88:481–486. 10.1016/j.pbb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 152.Miranda HF, Romero MA, Puig MM. 2012. Antinociceptive and anti-exudative synergism between dexketoprofen and tramadol in a model of inflammatory pain in mice: Synergy in analgesia. Fundam Clin Pharmacol 26:373–382. 10.1111/j.1472-8206.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 153.Miranda HF, Sierralta F, Aranda N, Poblete P, Noriega V, Prieto JC. 2018. Synergism between gabapentin-tramadol in experimental diabetic neuropathic pain. Fundam Clin Pharmacol 32:581–588. 10.1111/fcp.12400. [DOI] [PubMed] [Google Scholar]
- 154.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. 1999. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80:67–82. 10.1016/S0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 155.Molina-Cimadevila MJ, Segura S, Merino C, Ruiz-Reig N, Andrés B, de Madaria E. 2014. Oral self-administration of buprenorphine in the diet for analgesia in mice. Lab Anim 48:216–224. 10.1177/0023677214532454. [DOI] [PubMed] [Google Scholar]
- 156.Montilla-García Á, Tejada MÁ, Perazzoli G, Entrena JM, Portillo-Salido E, Fernández-Segura E, Cañizares FJ, Cobos EJ. 2017. Grip strength in mice with joint inflammation: a rheumatology function test sensitive to pain and analgesia. Neuropharmacology 125:231–242. 10.1016/j.neuropharm.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 157.Munro G, Dyhr H, Grunnet M. 2010. Selective potentiation of gabapentin-mediated antinociception in the rat formalin test by the nicotinic acetylcholine receptor agonist ABT-594. Neuropharmacology 59:208–217. 10.1016/j.neuropharm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 158.National Research Council. 2009. Recognition and alleviation of pain in laboratory animals, Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 159.Nishiyori M, Ueda H. 2008. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain 4:1–6. 10.1186/1744-8069-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nunamaker EA, Goldman JL, Adams CR, Fortman JD. 2018. Evaluation of analgesic efficacy of meloxicam and 2 formulations of buprenorphine after laparotomy in female Sprague–Dawley rats. J Am Assoc Lab Anim Sci 57:498–507. 10.30802/AALAS-JAALAS-17-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ohtani M, Kotaki H, Sawada Y, Iga T. 1995. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 272:505–510. [PubMed] [Google Scholar]
- 162.Okulicz-Kozaryn I, Leppert W, Mikolajczak P, Kaminska E. 2013. Analgesic effects of tramadol in combination with adjuvant drugs: an experimental study in rats. Pharmacol 91:7–11. 10.1159/000343632. [DOI] [PubMed] [Google Scholar]
- 163.Oliver VL, Athavale S, Simon KE, Kendall LV, Nemzek JA, Lofgren JL. 2017. Evaluation of pain assessment techniques and analgesia efficacy in a female guinea pig (Cavia porcellus) model of surgical pain. J Am Assoc Lab Anim Sci 56:425–435. [PMC free article] [PubMed] [Google Scholar]
- 164.Oliver VL, Thurston SE, Lofgren JL. 2018. Using cageside measures to evaluate analgesic efficacy in mice (Mus musculus) after surgery. J Am Assoc Lab Anim Sci 57:186–201. [PMC free article] [PubMed] [Google Scholar]
- 165.Pairet M, Ruckebusch Y. 1989. On the relevance of nonsteroidal antiinflammatory drugs in the prevention of paralytic ileus in rodents. J Pharm Pharmacol 41:757–761. 10.1111/j.2042-7158.1989.tb06360.x. [DOI] [PubMed] [Google Scholar]
- 166.Pais-Vieira M, Lima D, Galhardo V. 2009. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett 463:98–102. 10.1016/j.neulet.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 167.Papathanasiou T, Juul RV, Gabel-Jensen C, Kreilgaard M, Heegaard AM, Lund TM. 2017. Quantification of the pharmacodynamic interaction of morphine and gabapentin using a response surface approach. AAPS J 19:1804–1813. 10.1208/s12248-017-0140-2. [DOI] [PubMed] [Google Scholar]
- 168.Park I, Kim D, Song J, In CH, Jeong SW, Lee SH, Min B, Lee D, Kim SO. 2008. BupredermTM, a new transdermal delivery system of buprenorphine: pharmacokinetic, efficacy and skin irritancy studies. Pharm Res 25:1052–1062. 10.1007/s11095-007-9470-6. [DOI] [PubMed] [Google Scholar]
- 169.Penderis J, Franklin RJ. 2005. Effects of pre- versus post-anaesthetic buprenorphine on propofol-anaesthetized rats. Vet Anaesth Analg 32:256–260. 10.1111/j.1467-2995.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 170.Peterson NC, Nunamaker EA, Turner PV. 2017. To treat or not to treat: the effects of pain on experimental parameters. Comp Med 67:469–482. [PMC free article] [PubMed] [Google Scholar]
- 171.Pick CG, Peter Y, Schreiber S, Weizman R. 1997. Pharmacological characterization of buprenorphine, a mixed agonist–antagonist with κ3 analgesia. Brain Res 744:41–46. 10.1016/S0006-8993(96)01069-4. [DOI] [PubMed] [Google Scholar]
- 172.Ramsey D, Fleck T, Berg T, Nederveld S, DeLong D, Tena J-K, Aleo M, McCall R. 2014. Cerenia prevents perioperative nausea and vomiting and improves recovery in dogs undergoing routine surgery. Int J Appl Res Vet Med 12:228–237. [Google Scholar]
- 173.Rätsep MT, Barrette VF, Winterborn A, Adams MA, Croy BA. 2013. Hemodynamic and behavioral differences after administration of meloxicam, buprenorphine, or tramadol as analgesics for telemeter implantation in mice. J Am Assoc Lab Anim Sci 52:560–566. [PMC free article] [PubMed] [Google Scholar]
- 174.Richardson CA, Flecknell PA. 2005. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127. [DOI] [PubMed] [Google Scholar]
- 175.Romero A, Miranda HF, Puig MM. 2010. Antinociceptive effects of morphine, fentanyl, tramadol and their combination, in morphine-tolerant mice. Pharmacol Biochem Behav 97:363–369. 10.1016/j.pbb.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 176.Roughan JV, Flecknell PA. 2004. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol 15:461–472. 10.1097/00008877-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 177.Rutten K, Robens A, Read SJ, Christoph T. 2014. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. Eur J Pain 18:213–222. 10.1002/j.1532-2149.2013.00359.x. [DOI] [PubMed] [Google Scholar]
- 178.Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. 2014. Burrowing as a nonreflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation: Burrowing as a nonreflex behavioural readout. Eur J Pain 18:204–212. 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 179.Sadar MJ, Knych HK, Drazenovich TL, Paul-Murphy JR. 2018. Pharmacokinetics of buprenorphine after intravenous and oral transmucosal administration in guinea pigs (Cavia porcellus). Am J Vet Res 79:260–266. 10.2460/ajvr.79.3.260. [DOI] [PubMed] [Google Scholar]
- 180.Santos ARS, Vedana EMA, De Freitas GAG. 1998. Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47:302–307. 10.1007/s000110050333. [DOI] [PubMed] [Google Scholar]
- 181.Satterwhite JH, Boudinot FD. 1992. Pharmacokinetics of ketoprofen in rats: effect of age and dose. Biopharm Drug Dispos 13:197–212. 10.1002/bdd.2510130306. [DOI] [PubMed] [Google Scholar]
- 182.Sauer M, Fleischmann T, Lipiski M, Arras M, Jirkof P. 2016. Buprenorphine via drinking water and combined oral-injection protocols for pain relief in mice. Appl Anim Behav Sci 185:103–112. 10.1016/j.applanim.2016.09.009. [DOI] [Google Scholar]
- 183.Schaap MWH, Uilenreef JJ, Mitsogiannis MD, van ’t Klooster JG, Arndt SS, Hellebrekers LJ. 2012. Optimizing the dosing interval of buprenorphine in a multimodal postoperative analgesic strategy in the rat: minimizing side-effects without affecting weight gain and food intake. Lab Anim 46:287–292. 10.1258/la.2012.012058. [DOI] [PubMed] [Google Scholar]
- 184.Seymour TL, Adams SC, Felt SA, Jampachaisri K, Yeomans DC, Pacharinsak C. 2016. Postoperative analgesia due to sustained- release buprenorphine, sustained-release meloxicam, and carprofen gel in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 55:300–305. [PMC free article] [PubMed] [Google Scholar]
- 185.Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. 2016. Postoperative nausea and vomiting: a simple yet complex problem. Anesth Essays Res 10:388–396. 10.4103/0259-1162.179310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sheikholeslami B, Gholami M, Lavasani H, Rouini M. 2016. Evaluation of the route dependency of the pharmacokinetics and neuro-pharmacokinetics of tramadol and its main metabolites in rats. Eur J Pharm Sci 92:55–63. 10.1016/j.ejps.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 187.Shikanov A, Domb AJ, Weiniger CF. 2007. Long acting local anesthetic–polymer formulation to prolong the effect of analgesia. J Control Release 117:97–103. 10.1016/j.jconrel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 188.Shinozaki T, Yamada T, Nonaka T, Yamamoto T. 2015. Acetaminophen and non-steroidal anti-inflammatory drugs interact with morphine and tramadol analgesia for the treatment of neuropathic pain in rats. J Anesth 29:386–395. 10.1007/s00540-014-1953-0. [DOI] [PubMed] [Google Scholar]
- 189.Smith BJ, Wegenast DJ, Hansen RJ, Hess AM, Kendall LV. 2016. Pharmacokinetics and paw withdrawal pressure in female guinea pigs (Cavia porcellus) treated with sustained-release buprenorphine and buprenorphine hydrochloride. J Am Assoc Lab Anim Sci 55:789–793. [PMC free article] [PubMed] [Google Scholar]
- 190.Smith FL. 1997. Regional cutaneous differences in the duration of bupivacaine local anesthesia in mice. Life Sci 60:1613–1621. 10.1016/S0024-3205(97)00128-8. [DOI] [PubMed] [Google Scholar]
- 191.Smith FL, Davis RW, Carter R. 2001. Influence of voltage-sensitive Ca++channel drugs on bupivacaine infiltration anesthesia in mice. Anesthesiology 95:1189–1197. [DOI] [PubMed] [Google Scholar]
- 192.Sokolsky-Papkov M, Golovanevski L, Domb AJ, Weiniger CF. 2009. Prolonged local anesthetic action through slow release from poly (lactic acid co castor oil). Pharm Res 26:32–39. 10.1007/s11095-008-9699-8. [DOI] [PubMed] [Google Scholar]
- 193.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. 2011. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Speth RC, Smith MS, Brogan RS. 2001. Regarding the inadvisability of administering postoperative analgesics in the drinking water of rats (Rattus norvegicus). Contemp Top Lab Anim Sci 40:15–17. [PubMed] [Google Scholar]
- 195.Spofford CM, Ashmawi H, Subieta A, Buevich F, Moses A, Baker M, Brennan TJ. 2009. Ketoprofen produces modality-specific inhibition of pain behaviors in rats after plantar incision. Anesth Analg 109:1992–1999. 10.1213/ANE.0b013e3181bbd9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.St A Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34. [PubMed] [Google Scholar]
- 197.Stasiak KL, Maul D, French E, Hellyer PW, Vandewoude S. 2003. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci 42:13–20. [PubMed] [Google Scholar]
- 198.Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586. 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- 200.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Postoperative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342. [PubMed] [Google Scholar]
- 201.Teixeira FM, Castro LL, Ferreira RT, Pires PA, Vanderlinde FA, Medeiros MA. 2012. High-frequency electroacupuncture versus carprofen in an incisional pain model in rats. Braz J Med Biol Res 45:1209–1214. 10.1590/S0100-879X2012007500133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Traul KA, Romero JB, Brayton C, DeTolla L, Forbes-McBean N, Halquist MS, Karnes HT, Sarabia-Estrada R, Tomlinson MJ, Tyler BM, Ye X, Zadnik P, Guarnieri M. 2015. Safety studies of post-surgical buprenorphine therapy for mice. Lab Anim 49:100–110. 10.1177/0023677214554216. [DOI] [PubMed] [Google Scholar]
- 203.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191. [PMC free article] [PubMed] [Google Scholar]
- 204.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 205.Turner P, Pang D, Lofgren J, 2019. A review of pain assessment methods in laboratory rodents. Comp Med 69:EPub ahead of print. [DOI] [PMC free article] [PubMed]
- 206.Tyers MB. 1980. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol 69:503–512. 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wala EP, Holtman JR., Jr 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651:89–95. 10.1016/j.ejphar.2010.10.083. [DOI] [PubMed] [Google Scholar]
- 208.Wallace JL, McKnight W, Reuter BK, Vergnolle N. 2000. Nsaid-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119:706–714. 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 209.Woolf CJ, Wall PD. 1986. Morphine-sensitive and morphine-insensitive actions of C-fibre input on the rat spinal cord. Neurosci Lett 64:221–225. 10.1016/0304-3940(86)90104-7. [DOI] [PubMed] [Google Scholar]
- 210.World Health Organization. [Internet]. 2019. WHO's pain relief ladder for patient management. [Cited 01 April 2019]. Available at https://www.who.int/cancer/palliative/painladder/en/.
- 211.Wright-Williams S, Flecknell PA, Roughan JV. 2013. Comparative effects of vasectomy surgery and buprenorphine treatment on faecal corticosterone concentrations and behaviour assessed by manual and automated analysis methods in C57 and C3H mice. PLoS One 8:1–13. 10.1371/journal.pone.0075948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wright-Williams SL, Courade J, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mouse. Pain 130:108–118. 10.1016/j.pain.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 213.Wu SZ, Liu KS, Chu KS, Cheng KI, Kuei CH, Wang JJ, Tzeng JI. 2006. The depot of buprenorphine decanoate produced a dose-related long-lasting antinociceptive effect in guinea pigs. Acta Anaesthesiol Taiwan 44:161–168. [PubMed] [Google Scholar]
- 214.Xie H, Dong ZQ, Ma F, Bauer WR, Wang X, Wu GC. 2008. Involvement of serotonin 2A receptors in the analgesic effect of tramadol in mono-arthritic rats. Brain Res 1210:76–83. 10.1016/j.brainres.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 215.Yun M, Jeong S, Pai C, Arndt S. 2010. Pharmacokinetic-pharmacodynamic modeling of the analgesic effect of buprederm(TM) in mice. Health (N Y) 2:824–831. [Google Scholar]
- 216.Zanetti AS, Putta SK, Casebolt DB, Louie SG. 2017. Pharmacokinetics and adverse effects of 3 sustained-release buprenorphine dosages in healthy guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 56:768–778. [PMC free article] [PubMed] [Google Scholar]
- 217.Zelcer S, Kolesnikov Y, Kovalyshyn I, Pasternak DA, Pasternak GW. 2005. Selective potentiation of opioid analgesia by nonsteroidal antiinflammatory drugs. Brain Res 1040:151–156. 10.1016/j.brainres.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 218.Zhang Y, Du L, Pan H, Li L, Su X. 2011. Enhanced analgesic effects of propacetamol and tramadol combination in rats and mice. Biol Pharm Bull 34:349–353. 10.1248/bpb.34.349. [DOI] [PubMed] [Google Scholar]