Abstract
Sepsis is a multifaceted host response to infection that dramatically affects patient outcomes and the cost of health care. Animal models are necessary to replicate the complexity and heterogeneity of clinical sepsis. However, these models entail a high risk of pain and distress due to tissue trauma, inflammation, endotoxin-mediated hyperalgesia, and other mechanisms. Several recent studies and initiatives address the need to improve the welfare of animals through analgesics and standardize the models used in preclinical sepsis research. Ultimately, the goal is to provide high-fidelity, humane animal models that better replicate the clinical course of sepsis, to provide more effective translation and advance therapeutic discovery. The purpose of this review is to discuss the current understanding of the roles of pain and analgesia in rodent models of sepsis. The current definitions of sepsis along with an overview of pain in human sepsis are described. Finally, welfare concerns associated with animal models of sepsis and the most recent considerations for relief of pain and distress are reviewed.
Abbreviations: CASP, colon ascendens stent peritonitis; CLP, cecal ligation and puncture; SOFA, sequential organ failure assessment
Sepsis is a complex host response to infection that has devastating consequences for many intensive-care patients. In the United States alone, sepsis affects an estimated 1.7 million hospitalized patients.64 The clinical characteristics of sepsis have been identified in 1 of every 3 hospital deaths, and sepsis results in 270,000 deaths annually.64 Septicemia ranks as the most expensive condition treated in hospitals, at an annual cost of approximately $23.7 billion.79 In addition to its effects on mortality and the initial cost of hospitalization, sepsis is associated with high rates of rehospitalization and long-term consequences, including immunosuppression, recurrent infections, acute renal failure, cardiovascular events, cognitive impairment, and chronic pain.71 Considering the significant effect of sepsis on health care, clinically relevant, robust research regarding sepsis is needed.
Animal models, the cornerstone of sepsis research, achieve simultaneous recapitulation of the immunologic, cardiovascular, and metabolic responses of humans to infection. Over the past decade, considerable debate has surrounded both the translational relevance and animal welfare concerns of rodent models of sepsis. The fundamental concerns with translation stem from years of research in models failing to yield defined treatments. Apprehension was then magnified by data suggesting that rodent models could not mimic human inflammation.69 Although the debate continues, the value of rodent models has been reviewed,59 and they remain the most frequently used species in sepsis research.91 With regard to animal welfare in rodent models of sepsis, the use of analgesics has received much attention also. Historically, investigators debated using analgesics in animal models for reasons ranging from perceived species-associated differences in nociception to cost and inconvenience of administration. The immunomodulatory effects of analgesics and their potential to negatively alter inflammation models, despite ethical intentions, were of greatest concern. Recently, these and other debates surrounding animal models—coupled with an improved understanding of the sepsis syndrome—have resulted in targeted initiatives to develop standards for preclinical studies in sepsis.22,29,47,58,61,91
Definition of Sepsis
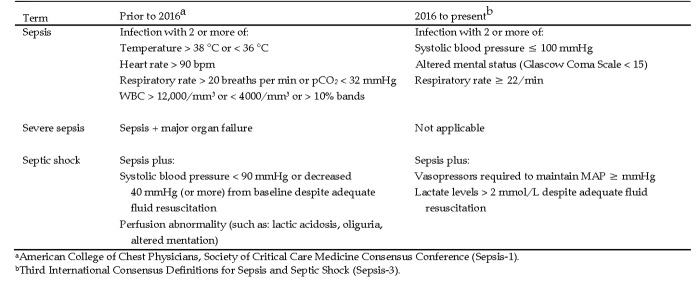
Sepsis has multifaceted pathophysiology that involves numerous systems (for example, immunologic, cardiovascular, neurologic) and many pathways (for example, coagulation, metabolic) to produce an array of heterogeneous clinical scenarios and outcomes. Consequently, no single marker or signature diagnostic test recognizes all clinical cases of sepsis. This complexity underscores the need for an accurate and uniform definition of the syndrome to aid in clinical treatment and epidemiologic reporting. In 2016, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) were published, characterizing sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection.”73 Under this definition, dysfunction is gauged by changes in the 6 organ systems summarized in the Sequential (or Sepsis-related) Organ Failure Assessment (SOFA) Score or an abbreviated version, the quickSOFA (qSOFA), intended for rapid screening (Figure 1). For example, a Glasgow Coma Scale score of 3 to 15 is assigned to reflect a patient's level of consciousness, with higher scores indicating better neurologic function. Septic shock is further defined as profound circulatory, cellular, and metabolic abnormalities associated with a greater risk of mortality than with sepsis alone, with serum lactate levels above a defined threshold and medical intervention required to maintain minimal blood pressure (Figure 1).70,73 These definitions from Sepsis-3 significantly differ from the first consensus reached in 1991 (Sepsis-1) and reaffirmed in 2001 (Sepsis-2). In these earlier statements, sepsis was defined as “the systemic inflammatory response to infection,” which was further refined as severe sepsis when accompanied by organ failure and as septic shock when hypotension persisted despite appropriate fluid resuscitation.8,45 Therefore, prior to 2016, inflammation was the hallmark of sepsis, and clinical diagnosis required the presence or strong suspicion of infection with 2 or more signs of the systemic inflammatory response syndrome.
Figure 1.
Clinical diagnosis of sepsis and septic shock.
The new definitions in Sepsis-3 reflect the current understanding of sepsis pathobiology.73 Reducing the emphasis on proinflammatory responses recognizes the concurrent role of antiinflammatory mediators in the sepsis syndrome and the fact that the systemic inflammatory response syndrome occurs in nonseptic conditions, such as cancer and autoimmune disease. In addition, the new classifications recognize that many other systems contribute to the development of sepsis. This evolution in the understanding of sepsis certainly affects clinical research efforts and may influence welfare in animal models as well. For example, a greater emphasis on organ failure may dictate model choice or endpoint selection, whereas a focus on mental status could influence the use of anesthesia and analgesia.
Pain in Human Sepsis
The prevalence and severity of pain caused by sepsis in human patients are difficult to discern. Pneumonia, the foremost cause of sepsis,56 is often not described as painful; however, conditions such as acute abdomen and joint infection are notably painful. The differences may be inherent to the cause and location of the septic focus or may be due to several factors confounding the detection of pain in sepsis. For example, sepsis often is a secondary complication of other disease processes or medical procedures.56 Therefore, the painful stimulus associated with sepsis may actually be due to comorbidities (for example, diabetic neuropathy, urinary obstruction, traumatic injury) or the result of therapeutic interventions (for example surgery, prolonged immobilization). Furthermore, several pathophysiologic sequelae of sepsis may, in fact, reduce pain perception or undermine pain diagnosis and scoring.2 Dementia and encephalopathy are common in sepsis.66 These conditions impair cognitive awareness of stimuli and may inhibit the ability of a patient to report the presence of pain.4 Likewise, muscle weakness is a prominent feature of sepsis that may limit physical responses used to diagnose pain, and the presence of perivascular edema often interferes with nerve conduction tests.2,84 Frequently used interventions, such as sedation and artificial ventilation,66 may further confuse the recognition of pain in sepsis.
Despite these confounding factors, the Centers for Disease Control and Prevention lists “extreme pain or discomfort” as a potential sign of sepsis.14 The most likely source of this pain is inflammation resulting from tissue damage, swelling, vascular leakage, and the release of multiple mediators. Inflammatory pain is a component of many conditions and is worthy of a detailed discussion that may be found elsewhere in this issue. Of specific note, systemic inflammation has been linked to generalized hyperalgesia. Studies in human volunteers revealed that intravenous administration of endotoxin lowered pain thresholds to peripheral pressure, cold, and electrical stimuli18 and to visceral stimuli.7 This effect is believed to be mediated directly or indirectly by proinflammatory cytokines, such as IL1β, that act at peripheral or central sites in the nervous system.18
In addition, neuropathic pain may be a consequence of inflammation or microcirculatory failure in sepsis. An estimated 70% of patients with sepsis develop critical illness polyneuropathy as a result of axonal degeneration in both motor and sensory neurons.84 The immediate clinical manifestation of critical illness polyneuropathy is extreme muscle weakness that becomes apparent during attempts to wean patients off artificial ventilation. However, functional impairment of small nerve fibers occurs early in the course of sepsis,40 and substantial nerve loss is evident in skin biopsies from patients with sepsis.2 As a consequence, patients may experience loss of sensitivity to pain, temperature, and vibration in the distal limbs. Paradoxically, patients may have pain due to the aberrant firing of degenerative nerves. The neuropathic signs may last for months and critical illness polyneuropathy may contribute to the development of chronic pain sequelae.42
Chronic pain is a frequently reported consequence of critical care illnesses, with 31% to 70% of previously septic patients reporting pain 6 mo to a year after discharge from an intensive care unit.5,6 Although the pathophysiology is incompletely understood, sensitization is associated with preexisting pain conditions, prolonged immobilization, and exposure to proinflammatory mediators.5 Severe sepsis and increasing age have been described as significant risk factors for chronic pain after intensive care hospitalization,5 although some studies suggest that chronic pain occurs at the same rate in patients with and without sepsis.5,57
Analgesia in Human Patients with Sepsis
Current recommendations.
Specific recommendations for the type and dosage of analgesics to be used in septic humans are lacking in the literature. Guidelines outlined in the Surviving Sepsis Campaign focus primarily on the management of assisted ventilation.66 The recommendations state that the use of sedation for intermittent or continuous ventilation should be limited, with the rationale that this practice is known to reduce length of stay in ICU and improve outcomes.66 One strategy to achieve this goal is to avoid sedative-only drugs and to use opioids solely.77 Guidelines developed by a task force from the American College of Critical Care Medicine regarding the treatment of pain, agitation, and delirium in intensive-care patients suggest frequent monitoring for pain and the use of pain scoring tools.4 The guidelines recommend opioids as the first choice for nonneuropathic pain, with further consideration to reduce the amount needed by adding NSAID. The addition of gabapentin or carbamazepine to opioids was recommended when neuropathic pain is suspected.4
These recommendations have interesting implications for animal models of sepsis. The recommendations suggest pain is a prominent concern regarding patients with sepsis, warranting routine monitoring. Guidelines frequently dictate the use of some form of analgesia, particularly opioids, in intensive care patients.
Effects of analgesia.
The intricate association of pain and inflammation might suggest that analgesia in patients with sepsis could be a ‘double-edged sword,’ having both positive and negative consequences. Without a doubt, the relief of pain has considerable ethical and physiologic benefit to the host. However, if analgesics alter immune function, in the face of sepsis, this could affect outcomes. Previous definitions of sepsis suggest that poor outcome is associated with overwhelming inflammation, thus implying that reducing inflammation would have positive effects. More recent theories suggest poor outcomes in sepsis are related to immunosuppression, implying that dampening the inflammatory response could have negative effects.
The use of NSAID in patients with sepsis has received much attention and yields variable results. A multicenter study of both acute and chronic use of NSAID in hospitalized patients with emerging bacterial infection found that the risk of sepsis or septic shock was unaffected by NSAID administration. For patients who did develop sepsis, NSAID were associated with a delay from the first sign of infection to the administration of effective antibiotics.44 In another study, the administration of either low-dose aspirin or other NSAID to patients with sepsis was associated with a 10% decrease in mortality. However, the coadministration of aspirin and other NSAID had no benefit, which was attributed to increased risk of bleeding or antiinflammatory effects.74 The lack of dramatic beneficial effects from NSAID may be due to dose timing and the need to identify the current immune status of patients prior to administration. This type of individualized approach to treatment is an ongoing interest in sepsis research that undoubtedly will need to be tested in animal models.
As mentioned earlier, intravenous opioids are recommended frequently for intensive-care patients, including those with sepsis, for pain relief and nonsedated assisted ventilation. The use of these drugs must be carefully considered and monitored in critically ill patients due to their known effects on cardiovascular and respiratory function.4 In addition, the potential immunosuppressive effects of this class of analgesic must be considered. These effects have been reviewed and include inhibition of antibody responses, natural killer cell activity, cytokine expression, chemotaxis, and phagocytic activity.51,67,81 Of note, endogenous morphine levels are elevated in patients with sepsis, and low concentrations of morphine inhibited the in vitro release of the chemotaxin IL8 from neutrophils, suggesting that opioids naturally contribute to the pathophysiology of sepsis.27 With regard to pain relief, the effect of opioid administration in sepsis is difficult to discern due to the aforementioned comorbidities and confounding of pain scoring in critically ill patients. However, one study of 28-d mortality suggested that in-hospital use of opioids has a negative effect on patients with sepsis.90 When adjusted for confounding factors—such as comorbidities, sepsis severity, and infection type—opioid-treated patients had a higher mortality rate (10.35%) than nonopioid-treated patients (2.40%).90 In addition, opioid-treated patients had a higher incidence of positive blood cultures, suggesting that mortality was associated with decreased bacterial clearance rather than hemodynamic effects of the opioids.90 Given that doses, opioid use history, and other factors may have varied between groups in the previous study,90 further study is needed to discern the full effect of the use of opioids in patients with sepsis.
Rodent Models of Sepsis
In a review of top-cited manuscripts on sepsis models from 2003 through 2013, rodent models were used in 94% of the studies, with mouse models predominant at 79% of the total.91 Mice offer several benefits to research, including high fertility, ease of maintenance, the availability of species-specific reagents, and prevalence of genetic modifications.23,48 However, therapeutics deemed efficacious in mouse sepsis models have consistently failed in human clinical trials. This finding suggests that mouse models do not replicate all of the biologic and clinical features of human sepsis, either because of inherent differences between species or the vast differences in supportive care given to humans patients with sepsis as compared with mice.22 In particular, comparisons of the genomic responses between humans and mice suggested that responses to inflammatory stimuli are very different and thus sparked controversy.69 Additional analyses have suggested that mouse models adequately mimic the inflammatory responses of humans.78 Ultimately, mouse models appear to have a prominent place in sepsis research, particularly when applied to unraveling basic science paradigms.59 Large animal models may be more appropriate for final testing of therapeutics,23 and the use of multiple animal models has been encouraged.91
To improve the effectiveness of sepsis models and sepsis research as a whole, there have been several recent recommendations. Experts in the field have suggested strict standardization of specific sepsis models to allow better reproducibility61 and the need to ‘reverse translate,’ that is, confirm that the model replicates the actual mechanism of the human condition, prior to extensive use of the model.22 Recently, the 2017 Wiggers Bernard Conference convened 31 experts in the field of sepsis from 12 countries to develop guidelines for the minimal quality threshold of sepsis models to improve preclinical sepsis research. These guidelines offer recommendations for optimizing study design and host characteristics as well as guidelines to improve clinical relevance, including standards for fluid resuscitation and antibiotic administration.29,47 In addition, the guidelines described recommendations for the use of humane endpoints and, particularly relevant to the current review, the use of analgesics.91
Pain in Rodent Models
Although the treatment of pain may be considered an ethical imperative, pain medications are rarely reported in preclinical sepsis studies.48 In a review of 360 highly cited rodent sepsis studies from 2003 through 2012, 8% described analgesics provided, 87% did not report analgesia, and 5% did not provide analgesia.91 Another report from 2014 indicated that 15% of the analyzed publications reported analgesic use in experimental sepsis.3 Considering the guiding principle that procedures causing pain in humans should be considered painful in animals without direct proof of the contrary,34 it appears that a model that accurately mimics the human condition would have a high probability to cause pain and distress.
The critical illness neuropathy described in humans appears very early in rodent models of sepsis. Within 24 h of cecal ligation and puncture (CLP), a model of sepsis, rats display decreased sciatic nerve excitability due to inactivation of sodium channels.20,55 The electrophysiologic changes occur before notable histologic findings and are associated with higher thresholds for paw withdrawal in response to von Frey filaments.20 Simultaneously, there is an increase in vagal nerve excitability, which is known to modulate the immune system and have antiinflammatory effects in sepsis.40, 42, 85 These findings suggest that pain perception is actually reduced in the early phases after CLP in rats. However, humans with critical illness neuropathy may exhibit acute pain and then develop chronic pain as a result of neuronal degeneration and sensitization.84 This finding has not been studied in animal models of sepsis and should be considered as researchers begin to explore the long-term sequelae of sepsis, requiring chronic animal models.
In addition, rodents are susceptible to endotoxin-induced hyperalgesia. Local injection of endotoxin into the hindpaw of both rats and mice has been used as a model of mechanical and thermal hyperalgesia for decades. The response is mediated through toll-like receptor 4/MyD88 signaling to induce IL1-mediated release of prostaglandins, although prostaglandin-independent mechanisms may also be involved.11,38 The relevance of this mechanism for the induction of pain to all models of sepsis is unknown, because endotoxin release may not be a major component of some models and may not always be clinically relevant in human sepsis.10
Several additional factors can lead to pain and distress in sepsis models. Systemic and local inflammation due to host-pathogen interactions will be a necessary component of all models of sepsis and a major source of painful stimuli. In addition, sepsis models are often considered more clinically relevant when combined with other insults.47 Comorbidities such as aspiration pneumonia, trauma, and burns may be combined with sepsis models, increasing the potential for pain. Likewise, concerns for clinical relevance may support the need for source control and additional painful procedures to remove the focus of infection, as is seen in the clinical setting.47 Lilley and colleagues summarize the routine technical aspects of these models that may induce stress and summarize recommendations for refinement.48 Finally, many of the factors confounding the evaluation of pain in the intensive care setting are present in rodent models also. In particular, the behavioral signs used to evaluate pain are similar to the signs of sepsis, and many pathologic features of sepsis (for example, weakness, encephalopathy) may interfere with other measurements of pain.
Surgical Models of Sepsis
Surgical models of sepsis require a laparotomy to either manipulate the host's natural barrier to abdominal infection or implant infected material. Therefore, these models have tissue injury associated with the surgery that is compounded by extreme local inflammation and the potential for sensitization caused by systemic inflammation. In addition, surgical models may involve a second surgery to remove the focus of infection.47 The effects of pain associated with surgery and with inflammatory models are discussed elsewhere in this journal issue. Therefore, we restrict our discussion to pain and analgesia associated with the specific models of sepsis.
CLP.
CLP has been regarded as the ‘gold standard’ of sepsis models, due to its reproduction of the immunologic and hemodynamic characteristics of human sepsis.60,86 In reports of highly cited literature on sepsis models from 2013 through 2017, CLP was used in 64% of the studies.47 The routine procedure has been reviewed, as have several variations in technique, supportive care, and host factors that allow titration of the effects and influence the outcome of the model.21,46,54 In addition to the surgical trauma and inflammatory response to bacteria, the model creates tissue ischemia and necrosis associated with the cecal ligation.46 Within a few hours, mice demonstrate signs of illness, including hunched posture, piloerection, lethargy, and diarrhea.54 Decreased food consumption, hypothermia, progressive weight loss, and debilitation are prominent signs. Mortality rates vary with the specific model and research goals.91 Animals that recover from the initial insult develop an intraabdominal abscess accompanied by chronic inflammation, splenomegaly, and altered myelopoiesis.19
Colon ascendens stent peritonitis (CASP).
In the CASP model, a midline laparotomy is used to isolate the ascending colon and secure a plastic stent distal to the ileocecal junction to allow fecal flow into the abdomen.80 The severity of the model can be modulated by changing the diameter of the stent. A generalized peritonitis results with bacteremia, distant organ seeding, and organ dysfunction.49 When compared with a severe model of CLP (18-gauge, double puncture, and 1.5-cm cecal ligation), CASP resulted in greater inflammatory cytokine concentrations and bacterial loads in various organs, peritoneal lavage fluid, and blood.49 Similar to those with CLP, mice with CASP display decreased mobility, piloerection, and decreased food consumption within a few hours of surgery; those with severe peritonitis may die within 48 h.80 An advantage of CASP is that it does not result in a focus of necrotic tissue or chronic abscess that may confound the interpretation of the inflammatory response; however, the CASP model requires more surgical skill than the CLP model and therefore has not been used as extensively as CLP.76
Implantation of infected material.
Implantation models have been used infrequently and involve the insertion of infected material, often a fibrin clot, into the abdominal cavity. The model in rats achieves a sepsis syndrome similar to that seen with the other surgical models.1,83 The implantation model differs from the other surgical models in that, rather than the induction of polymicrobial infection, a single organism is used, which has been described as a drawback for modeling abdominal sepsis. However, a defined and titratable inoculum might be an advantage in some cases.
Current recommendations.
The current recommendation for preclinical models from the 2017 Wiggers Bernard Conference is that “analgesics recommended for surgical sepsis should be consistent with ethical considerations.”91 The guidelines recognize pain as a source of experimental variability and advocate standard use and reporting of analgesics in surgical models of sepsis.
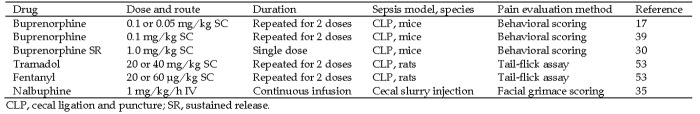
Although NSAID are tested in animal models as treatments for sepsis, their routine use for pain relief in animal models would be problematic due to their obvious effects on inflammation. However, some opioids and locally instilled lidocaine have been used in sepsis models.76,91 Buprenorphine, the most commonly used analgesic in rodent models, has been examined in sepsis induced by CLP in 3 strains of mice.17,33,39 However, the actual efficacy of the drug in those studies could not be discerned according to the behavioral parameters selected. This difficulty may have been a function of the stage of illness due to sepsis and confounding of pain detection, as is seen in humans. In comparison, when compared with buprenorphine HCl, sustained-release buprenorphine was associated with decreased pain behaviors at 12 and 24 h after surgery in a murine CLP model,30 suggesting that continuous dosing may be more beneficial. Tramadol and fentanyl both have demonstrated efficient minimization of pain detected by tail flick assay within 24 h of CLP in rats.53 In fact, tramadol and fentanyl demonstrated efficacy at doses found to be subeffective in sham-operated animals.53 In addition, local lidocaine or bupivacaine has been used at the incision site in surgical models of sepsis either alone or in conjunction with systemic analgesics.9,43,89 Studies evaluating analgesic efficacy in sepsis have used variable drugs, dosages, and durations of administration (Figure 2). Generally, 2 d of routine analgesia followed by as needed administration are required for rodent laparotomy at this institution. However, further research is needed to determine how long analgesics should be administered to address the entire septic episode.
Figure 2.
Assessment of analgesia efficacy in rodent sepsis studies.
Nonsurgical Models
Compared with surgical options, nonsurgical models raise fewer concerns with animal welfare but can still potentiate painful side effects. Nonsurgical models typically generate systemic inflammatory responses through intraperitoneal or intravenous injections of bacteria or fecal slurries. Overall, few studies specifically examine the level of pain or the use of analgesics in these models.
Endotoxemia models.
In a review of rodent sepsis models, endotoxin administration was used in 23% of the studies, second only to CLP.47 Historically, endotoxin has been used to provide a low-cost, reproducible method to study systemic inflammation and a shock-like state, with some similarity to sepsis. However, bolus LPS essentially represents an intoxication model rather than true sepsis. Endotoxemia leads to high levels of plasma inflammatory cytokines that peak earlier and resolve faster than in other sepsis models.63,68 In addition, distinct differences exist in the sensitivity to endotoxin between species, with humans being much more sensitive.46,62 As such, recent expert recommendations state endotoxin injection, although useful for studies of acute inflammation, should not be considered as a model of sepsis in preclinical studies.47 Instead, sepsis models should replicate host–pathogen interactions using live bacteria.47
Live bacteria models.
Live bacteria models vary widely in terms of the route of infection, frequency of administration, bacteria strain, and size of inoculum. These factors combine with animal characteristics to determine model severity. Recent recommendations for sepsis models suggest that the bacteria inoculum should be representative of the virulence factors and patterns of antibiotic resistance found in human sepsis. Therefore, the implication is to prefer clinical isolates over laboratory strains.47 Escherichia coli, Staphylococcus, and Pseudomonas are commonly chosen pathogens prevalent in human sepsis cases. If strain and size of inoculum are standardized, the model may be more reproducible.48 The disadvantages of this model are the use of a single strain of bacteria may not be representative of abdominal sepsis, and a large bolus of bacteria may mimic the effects of a single LPS dose due to the rapid lysis of bacteria by complement.15 In addition, intravenous boluses may not lead to colonization or bacteria replication.10
Cecal slurry injection models.
Cecal slurry injection models consist of intraperitoneal injection of a standardized quantity of cecal contents from a donor to instigate bacterial peritonitis.52 The progression of disease and mortality rates may be titrated by varying the dose of cecal slurry. Within a few hours of injection, adult mice display mild piloerection and decreased movement, progressing to respiratory depression, labored breathing, and decreased responsiveness within 12 h.72 In comparison to an endotoxin model, the cecal slurry injection had a similar acute course with recovery after 72 h, although cytokine concentrations were not as high as seen with endotoxin. In contrast, the CLP model has a more protracted course with animals continuing to decline beyond 72 h.68 Comparisons of leukocyte gene expression demonstrated pronounced differences between low-mortality versions of the CLP and cecal slurry models.26 The major advantage of the cecal slurry model is the avoidance of tissue trauma and ischemia produced by surgical models of polymicrobial peritonitis. In addition, the cecal slurry model can be used in situations where surgery is experimentally prohibited or technically challenging, as is the case in 5- to 7-d-old mice used in models of neonatal sepsis.24,26,88 One drawback of the model is the need to prepare a fresh slurry prior to each experiment. This disadvantage may be avoided by storing the slurry frozen in 15% glycerol–PBS, yielding a uniform and viable inoculum.75
Extraabdominal models of sepsis.
The abdominal cavity is the site of infection in 60% of preclinical sepsis models.47 However, pneumonia is the most common source of sepsis in humans. In addition to pneumonia and intraabdominal infections, urinary tract and soft tissue infections are the top 4 causes of sepsis.56,65 According to these data, experts in the field suggest strong consideration be given to the use of multiple clinically relevant models, including those with an extraabdominal focus of infection. These models would closely mimic the clinical scenario and promote a better understanding of the systemic pathophysiology involved in sepsis.47 Overall, the extraabdominal models tend to be less technically involved than the majority of intraabdominal models. Pneumonia models may require brief anesthesia for intranasal or intratracheal instillation of bacteria. Alternatively, aerosol inhalation may be used.16,41 S. pneumoniae, K. pneumoniae, and P. aeruginosa are frequently used to mimic the human clinical condition.47 In each case, model progression depends on the bacterial strain and dose.82 In a mouse model of K. pneumoniae, mice displayed decreased respiratory rate, labored breathing, and decreased activity within 6 to 24 h of inoculation.28 Urosepsis models generally involve injecting bacteria into the bladder to create an ascending urinary tract infection.12,37 Soft-tissue infection models may be created by simple subcutaneous injection of bacteria such as S. pyogenes. Mice may exhibit systemic signs and high mortality rates within 2 or 3 d, consistent with other sepsis models.13 Depending on the research goals, extraabdominal models may be used to study local pathology as compared with sepsis; therefore, the welfare concerns may vary with these models.48
Current recommendations.
The pain category for nonsurgical models of sepsis is not always be clear, and few data exist on the use of analgesics in nonsurgical models. Considerations for analgesia in surgical animal models and human intensive care patients often focus on the invasive procedures or comorbidities experienced rather than the septic state. For nonsurgical models, the initial lack of an invasive procedure to create sepsis may reduce the perceived need for analgesia. In a study comparing the CLP model with the cecal slurry model, buprenorphine administration was reported only for the surgical model.26 Likewise, a study using the cecal slurry model reported less than 5% of mice received analgesia for pain immediately after the injection. However, continuous infusion of nalbuphine reduced facial grimace scores at 24 h in a cecal slurry model in rats.35 Although clinical scoring was unaffected, the facial grimace scoring suggested that this nonsurgical model did cause pain in the absence of an invasive procedure. This finding suggests that additional studies defining the role of pain and analgesia in nonsurgical models of sepsis are warranted. In the meantime, consistent with guidelines for intensive care patients, recommendations for preclinical sepsis models are to consider analgesics for nonsurgical sepsis and to apply rigorous monitoring routines aimed at determining the need for analgesics.91
Effects of Analgesics on Models of Sepsis
Overall, analgesics are recommended for models of sepsis, but the effects of their use must be considered. The immunosuppressive effects of some opioids are well documented in rodents. Morphine is used frequently in human medicine. However, when delivered by slow-release subcutaneous pellet to mice, morphine is associated with overgrowth and subsequent translocation of gram-positive bacteria into the blood.31,50 This side effect has been used as a model of sepsis and has often been suggested as a rationale for omitting analgesics from sepsis models. Currently, there is evidence that less-immunosuppressive opioids can be used in some surgical models. In murine CLP models, overall survival and select immune cell counts and cytokine levels from the peritoneal cavity and blood were largely unaffected by buprenorphine administration in 3 strains of female mice.17,33,39 Likewise, sustained-release buprenorphine caused no differences in survival or blood levels of MCP1 and IL6 when compared with buprenorphine HCl in a CLP model using male mice.30 However, buprenorphine did yield dose-dependent adverse effects on overall mortality and blood neutrophil counts at 12 h after surgery in male C57BL/6 mice,17 and variation in survival curves was noted when the stage of estrous cycle was factored with analgesic administration in BALB/c mice.39 Fentanyl had little effect on survival and multiple inflammatory markers after CLP in rats. In the same study, tramadol likewise showed little effect on inflammatory markers;53 however, high doses of tramadol increased mortality in the rat CLP model and had similar effects in a mouse laparotomy model.87 Both fentanyl and tramadol were associated with differences in blood pressure, and caution was suggested for sepsis studies focused on cardiovascular effects.53 Although continuous lidocaine and bupivacaine infusions have demonstrated antiinflammatory effects that promoted survival in a mouse CLP model,25 the use of low, local doses of lidocaine and appropriate controls likely will avoid a significant effect on research outcomes. These studies demonstrate that judicious use of opioids will not greatly impact some studies of inflammation in sepsis but are not an exhaustive test of all systems. With the expanded definition of sepsis to include the importance of multiple systems and pathways, additional studies of the effects of opioids in surgical models may be necessary. Ultimately, the choice of analgesic is determined by research goals.
Clinical Scoring in Models of Sepsis
Scoring systems allow standardized evaluation and reporting of pain and distress in animal models. They can be used to inform the need for analgesia or humane euthanasia. In sepsis models, they have the added advantage of mimicking the methods in which sepsis is diagnosed, monitored and treated. The most recent definition of sepsis defines clinical criteria based on the SOFA Score and the Glasgow Coma Score for diagnosis and monitoring the progression of sepsis in human patients.73 For preclinical studies, specific recommendations from the 2017 Wiggers Bernard Conference now suggest “development and validation of standardized criteria to monitor the well-being of septic animals” and “development and validation of standardized criteria for euthanasia of septic animals.”91 In some rodent studies, scoring systems used to evaluate pain had variable success and may have been confounded when the assessment variables were similar to the signs of sepsis.17,30,33,36 However, very similar criteria were readily adapted to evaluate wellbeing and predict endpoints. The Murine Sepsis Score consists of 7 readily observable, clinical variables related to activity, appearance, and respiration and predicted death in a model of fecal slurry peritonitis with 100% specificity.72 Another scoring tool, the Mouse Clinical Assessment Score for Sepsis, evaluates an extraabdominal model of pneumonia-associated sepsis. Using 8 humane endpoints with 4 stages of severity to assess disease progression, the Mouse Clinical Assessment Score for Sepsis correlated well with cytokine levels and other mediators.32 These scoring systems appear readily adaptable to other models of sepsis, although modification may be necessary to account for the effects of study- and model-specific factors, including the anesthesia and surgery needed to create some models of sepsis.72 The use of scoring systems likely will have immediate benefits for the welfare of animals in sepsis studies. Additional benefits of scoring sepsis severity are the ability to gauge the relative severity of different sepsis models and to compare results between laboratories using similar models.
Although they are imperative for research advances, animal models of sepsis present substantial welfare concerns. The inherent nature of an inflammatory disease process and the technical aspects of the models (surgery, peritonitis, comorbidities, and so forth) present many potential sources of pain and distress. Currently, experts in the field recommend refinement of these models by the use of analgesics and scoring systems. Studies suggest judicious and limited use of less immunosuppressive opioids may not dramatically affect the outcomes of similarly conducted CLP studies, and the authors of the current review recommend buprenorphine for CLP models of sepsis. However, more research is needed to assess the true efficacy of pain management for both surgical and nonsurgical models of sepsis, and the final selection of analgesics must consider the goals of individual scientific studies. In addition, careful monitoring and clear humane end points are imperative to minimize animal pain and distress in preclinical sepsis studies and should be determined on a case-by-case basis. Overall, refinements likely will enhance animal welfare in animal models of sepsis and allow further scientific advancements in sepsis research.
Acknowledgments
The authors’ work is supported in part by National Institutes of Health grant GM112799.
References
- 1.Ahrenholz DH, Simmons RL. 1980. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery 88:41–47. [PubMed] [Google Scholar]
- 2.Axer H, Grimm A, Pausch C, Teschner U, Zinke J, Eisenach S, Beck S, Guntinas-Lichius O, Brunkhorst FM, Witte OW. 2016. The impairment of small nerve fibers in severe sepsis and septic shock. Crit Care 20:1–9. 10.1186/s13054-016-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bara M, Joffe AR. 2014. The ethical dimension in published animal research in critical care: the public face of science. Crit Care 18:1–7. 10.1186/cc13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care Medicine 2013. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41:263–306. 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 5.Battle CE, Lovett S, Hutchings H. 2013. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care 17:1–8. 10.1186/cc12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumbach P, Gotz T, Gunther A, Weiss T, Meissner W. 2016. Prevalence and characteristics of chronic intensive care–related pain: the role of severe sepsis and septic shock. Crit Care Med 44:1129–1137. 10.1097/CCM.0000000000001635. [DOI] [PubMed] [Google Scholar]
- 7.Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit JS, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S. 2012. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain 153:794–799. 10.1016/j.pain.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee, American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655. 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 9.Brown I, Bellevue O, Shawo A, Woldesemayat H, Lyo V, Rayikanti B, Lee M, Uzosike ED, Kasravi S, Harris HW. 2015. Low-dose cyclophosphamide improves survival in a murine treatment model of sepsis. Shock 43:92–98. 10.1097/SHK.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4:854–865. 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 11.Calil IL, Zarpelon AC, Guerrero AT, Alves-Filho JC, Ferreira SH, Cunha FQ, Cunha TM, Verri WA., Jr 2014. Lipopolysaccharide induces inflammatory hyperalgesia triggering a TLR4/MyD88-dependent cytokine cascade in the mice paw. PLoS One 9:1–8. 10.1371/journal.pone.0090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey AJ, Tan CK, Ipe DS, Sullivan MJ, Cripps AW, Schembri MA, Ulett GC. 2016. Urinary tract infection of mice to model human disease: Practicalities, implications and limitations. Crit Rev Microbiol 42:780–799. [DOI] [PubMed] [Google Scholar]
- 13.Castiglia V, Piersigilli A, Ebner F, Janos M, Goldmann O, Dambock U, Kroger A, Weiss S, Knapp S, Jamieson AM, Kirschning C, Kalinke U, Strobl B, Muller M, Stoiber D, Lienenklaus S, Kovarik P. 2016. Type I interferon signaling prevents IL1β-driven lethal systemic hyperinflammation during invasive bacterial infection of soft tissue. Cell Host Microbe 19:375–387. 10.1016/j.chom.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control. [Internet]. 2016. Making health care safer: think sepsis. Time matters. [Cited 27 March 2019]. Available at: https://www.cdc.gov/vitalsigns/sepsis/index.html [Google Scholar]
- 15.Chen P, Stanojcic M, Jeschke MG. 2014. Differences between murine and human sepsis. Surg Clin North Am 94:1135–1149. 10.1016/j.suc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM, 2nd, Karl IE, Hotchkiss RS, Buchman TG. 2003. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med 31:1630–1637. 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 17.Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365. [PMC free article] [PubMed] [Google Scholar]
- 18.de Goeij M, van Eijk LT, Vanelderen P, Wilder-Smith OH, Vissers KC, van der Hoeven JG, Kox M, Scheffer GJ, Pickkers P. 2013. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One 8:1–9. 10.1371/journal.pone.0084159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. 2007. MyD88-dependent expansion of an immature GR1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204:1463–1474. 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diniz LRL, Portella VG, da Silva Alves KS, Araujo P, de Albuquerque RLC, Jr, Cavalcante de Albuquerque AA, Coelho-de-Souza AN, Leal-Cardoso JH. 2018. Electrophysiologic alterations in the excitability of the sciatic and vagus nerves during early stages of sepsis. J Pain Res 11:783–790. 10.2147/JPR.S144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 67:6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efron PA, Mohr AM, Moore FA, Moldawer LL. 2015. The future of murine sepsis and trauma research models. J Leukoc Biol 98:945–952. 10.1189/jlb.5MR0315-127R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink MP. 2014. Animal models of sepsis. Virulence 5:143–153. 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka K, Kalish F, Zhao H, Lu S, Wong S, Wong RJ, Stevenson DK. 2017. Induction of heme oxygenase 1 attenuates the severity of sepsis in a nonsurgical preterm mouse model. Shock 47:242–250. 10.1097/SHK.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 25.Gallos G, Jones DR, Nasr SH, Emala CW, Lee HT. 2004. Local anesthetics reduce mortality and protect against renal and hepatic dysfunction in murine septic peritonitis. Anesthesiology 101:902–911. 10.1097/00000542-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Szpila BE, Larson S, Joseph A, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. 2014. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol 192:3156–3165. 10.4049/jimmunol.1301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glattard E, Welters ID, Lavaux T, Muller AH, Laux A, Zhang D, Schmidt AR, Delalande F, Laventie BJ, Dirrig-Grosch S, Colin DA, Van Dorsselaer A, Aunis D, Metz-Boutigue MH, Schneider F, Goumon Y. 2010. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS One 5:1–14. 10.1371/journal.pone.0008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonçalves MC, Horewicz VV, Luckemeyer DD, Prudente AS, Assreuy J. 2017. Experimental sepsis severity score associated to mortality and bacterial spreading is related to bacterial load and inflammatory profile of different tissues. Inflammation 40:1553–1565. 10.1007/s10753-017-0596-3. [DOI] [PubMed] [Google Scholar]
- 29.Hellman J, Bahrami S, Boros M, Chaudry IH, Fritsch G, Gozdzik W, Inoue S, Radermacher P, Singer M, Osuchowski MF, Huber-Lang M. 2019. Part III: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for fluid resuscitation and antimicrobial therapy endpoints. Shock 51:33–43. [DOI] [PubMed] [Google Scholar]
- 30.Herndon NL, Bandyopadhyay S, Hod EA, Prestia KA. 2016. Sustained-release buprenorphine improves postsurgical clinical condition but does not alter survival or cytokine levels in a murine model of polymicrobial sepsis. Comp Med 66:455–462. [PMC free article] [PubMed] [Google Scholar]
- 31.Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. 1997. Morphine induces sepsis in mice. J Infect Dis 176:183–188. 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- 32.Huet O, Ramsey D, Miljavec S, Jenney A, Aubron C, Aprico A, Stefanovic N, Balkau B, Head GA, de Haan JB, Chin-Dusting JP. 2013. Ensuring animal welfare while meeting scientific aims using a murine pneumonia model of septic shock. Shock 39:488–494. 10.1097/SHK.0b013e3182939831. [DOI] [PubMed] [Google Scholar]
- 33.Hugunin KM, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260. 10.1097/SHK.0b013e3181cdc412. [DOI] [PubMed] [Google Scholar]
- 34.Interagency Research Animal Committee. 1985. US government principles for utilization and care of vertebrate animals used in testing, research, and training.Fed Regist 50:20864–20865. [PubMed] [Google Scholar]
- 35.Jeger V, Arrigo M, Hildenbrand FF, Muller D, Jirkof P, Hauffe T, Seifert B, Arras M, Spahn DR, Bettex D, Rudiger A. 2017. Improving animal welfare using continuous nalbuphine infusion in a long-term rat model of sepsis. Intensive Care Med Exp 5:1–13. 10.1186/s40635-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeger V, Hauffe T, Nicholls-Vuille F, Bettex D, Rudiger A. 2016. Analgesia in clinically relevant rodent models of sepsis. Lab Anim 50:418–426. 10.1177/0023677216675009. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JR, Magistro G, Clabots C, Porter S, Manges A, Thuras P, Schubert S. 2018. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 (‘clonal group A’) cystitis isolate in murine models of urinary tract infection and sepsis. Microb Pathog 120:128–131. 10.1016/j.micpath.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 38.Kanaan SA, Safieh-Garabedian B, Haddad JJ, Atweh SF, Abdelnoor AM, Jabbur SJ, Saade NE. 1997. Effects of various analgesic and antiinflammatory drugs on endotoxin-induced hyperalgesia in rats and mice. Pharmacology 54:285–297. 10.1159/000139498. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy LH, Hwang H, Wolfe AM, Hauptman J, Nemzek-Hamlin JA. 2014. Effects of buprenorphine and estrous cycle in a murine model of cecal ligation and puncture. Comp Med 64:270–282. [PMC free article] [PubMed] [Google Scholar]
- 40.Khan J, Harrison TB, Rich MM, Moss M. 2006. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 67:1421–1425. 10.1212/01.wnl.0000239826.63523.8e. [DOI] [PubMed] [Google Scholar]
- 41.Knapp S, Schultz MJ, van der Poll T. 2005. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock 24 Suppl 1:12–18. 10.1097/01.shk.0000191385.41689.f3. [DOI] [PubMed] [Google Scholar]
- 42.Latronico N, Shehu I, Seghelini E. 2005. Neuromuscular sequelae of critical illness. Curr Opin Crit Care 11:381–390. 10.1097/01.ccx.0000168530.30702.3e. [DOI] [PubMed] [Google Scholar]
- 43.Laufenberg LJ, Kazi AA, Lang CH. 2014. Salutary effect of aurintricarboxylic acid on endotoxin- and sepsis-induced changes in muscle protein synthesis and inflammation. Shock 41:420–428. 10.1097/SHK.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Legras A, Giraudeau B, Jonville-Bera AP, Camus C, Francois B, Runge I, Kouatchet A, Veinstein A, Tayoro J, Villers D, Autret-Leca E. 2009. A multicentre case-control study of nonsteroidal antiinflammatory drugs as a risk factor for severe sepsis and septic shock. Crit Care 13:R43 10.1186/cc7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256. 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 46.Lewis AJ, Seymour CW, Rosengart MR. 2016. Current murine models of sepsis. Surg Infect (Larchmt) 17:385–393. 10.1089/sur.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Libert C, Ayala A, Bauer M, Cavaillon JM, Deutschman C, Frostell C, Knapp S, Kozlov AV, Wang P, Osuchowski MF, Remick DG. 2019. Part II: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for types of infections and organ dysfunction endpoints. Shock 51:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lilley E, Armstrong R, Clark N, Gray P, Hawkins P, Mason K, López-Salesansky N, Stark A-K, Jackson SK, Thiemermann C, Nandi M. 2015. Refinement of animal models of sepsis and septic shock. Shock 43:304–316. 10.1097/SHK.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 49.Maier S, Traeger T, Entleutner M, Westerholt A, Kleist B, Huser N, Holzmann B, Stier A, Pfeffer K, Heidecke CD. 2004. Cecal ligation and puncture versus colon ascendens stent peritonitis: 2 distinct animal models for polymicrobial sepsis. Shock 21:505–511. 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 50.Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S. 2015. Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL17A neutralization. Sci Rep 5:1–17. 10.1038/srep10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina PE. 2006. Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med 259:138–154. 10.1111/j.1365-2796.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 52.Mourelatos MG, Enzer N, Ferguson JL, Rypins EB, Burhop KE, Law WR. 1996. The effects of diaspirin cross-linked hemoglobin in sepsis. Shock 5:141–148. 10.1097/00024382-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Nardi GM, Bet AC, Sordi R, Fernandes D, Assreuy J. 2013. Opioid analgesics in experimental sepsis: effects on physiological, biochemical, and haemodynamic parameters. Fundam Clin Pharmacol 27:347–353. 10.1111/j.1472-8206.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 54.Nemzek JA, Hugunin KM, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal wellbeing. Comp Med 58:120–128. [PMC free article] [PubMed] [Google Scholar]
- 55.Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. 2009. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest 119:1150–1158. 10.1172/JCI36570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novosad SA, Sapiano MR, Grigg C, Lake J, Robyn M, Dumyati G, Felsen C, Blog D, Dufort E, Zansky S, Wiedeman K, Avery L, Dantes RB, Jernigan JA, Magill SS, Fiore A, Epstein L. 2016. Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. MMWR Morb Mortal Wkly Rep 65:864–869. 10.15585/mmwr.mm6533e1. [DOI] [PubMed] [Google Scholar]
- 57.Orwelius L, Lobo C, Teixeira Pinto A, Carneiro A, Costa-Pereira A, Granja C. 2013. Sepsis patients do not differ in health-related quality of life compared with other ICU patients. Acta Anaesthesiol Scand 57:1201–1205. 10.1111/aas.12164. [DOI] [PubMed] [Google Scholar]
- 58.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, Chaudry IH, Coopersmith CM, Deutschman C, Drechsler S, Efron P, Frostell C, Fritsch G, Gozdzik W, Hellman J, Huber-Lang M, Inoue S, Knapp S, Kozlov AV, Libert C, Marshall JC, Moldawer LL, Radermacher P, Redl H, Remick DG, Singer M, Thiemermann C, Wang P, Wiersinga WJ, Xiao X, Zingarelli B. 2018. Minimum quality threshold in preclinical sepsis studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Intensive Care Med Exp 6:1–6. 10.1186/s40635-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, Cuzzocrea S, Jiang Y, Junger WG, Hirasawa H, Hotchkiss RS, Li XA, Radermacher P, Redl H, Salomao R, Soebandrio A, Thiemermann C, Vincent JL, Ward P, Yao YM, Yu HP, Zingarelli B, Chaudry IH. 2014. Abandon the mouse research ship? Not just yet! Shock 41:463–475. 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker SJ, Watkins PE. 2001. Experimental models of gram-negative sepsis. Br J Surg 88:22–30. 10.1046/j.1365-2168.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- 61.Remick DG, Ayala A, Chaudry IH, Coopersmith CM, Deutschman C, Hellman J, Moldawer L, Osuchowski MF. 2019. Premise for standardized sepsis models. Shock 51:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remick DG, Newcomb DE, Bolgos GL, Call DR. 2000. Comparison of the mortality and inflammatory response of 2 models of sepsis: lipopolysaccharide versus cecal ligation and puncture. Shock 13:110–116. 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 63.Remick DG, Ward PA. 2005. Evaluation of endotoxin models for the study of sepsis. Shock 24 Suppl 1:7–11. 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 64.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M. 2017. Incidence and trends of sepsis in US hospitals using clinical versus claims data, 2009–2014. JAMA 318:1241–1249. 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee C, Gohil S, Klompas M. 2014. Regulatory mandates for sepsis care—reasons for caution. N Engl J Med 370:1673–1676. 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock, 2016. Intensive Care Med 43:304–377. 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 67.Sacerdote P. 2006. Opioids and the immune system. Palliat Med 20 Suppl 1:s9–s15. [PubMed] [Google Scholar]
- 68.Seemann S, Zohles F, Lupp A. 2017. Comprehensive comparison of 3 different animal models for systemic inflammation. J Biomed Sci 24:1–17. 10.1186/s12929-017-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110:3507–3512. 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. 2016. Developing a new definition and assessing new clinical criteria for septic shock: for the 3rd international consensus definitions for sepsis and septic shock. JAMA 315:775–787. 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shankar-Hari M, Rubenfeld GD. 2016. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Dis Rep 18:1–9. 10.1007/s11908-016-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, Mele T. 2014. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes 7:1–11. 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The 3rd international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sossdorf M, Otto GP, Boettel J, Winning J, Lösche W. 2013. Benefit of low-dose aspirin and nonsteroidal antiinflammatory drugs in septic patients. Crit Care 17:1–2. 10.1186/cc11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. 2014. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One 9:1–15. 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. 2017. Murine models of sepsis and trauma: can we bridge the gap? ILAR J 58:90–105. 10.1093/ilar/ilx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strøm T, Martinussen T, Toft P. 2010. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomized trial. Lancet 375:475–480. 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 78.Takao K, Miyakawa T. 2015. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natil Acad Sci U S A 112:1167–1172. 10.1073/pnas.1401965111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torio CM, Moore BJ. [Internet]. 2016. National inpatient hospital costs: the most expensive conditions by payer, 2013. [Cited 27 March 2019]. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. [PubMed]
- 80.Traeger T, Koerner P, Kessler W, Cziupka K, Diedrich S, Busemann A, Heidecke CD, Maier S. 2010. Colon ascendens stent peritonitis (CASP)—a standardized model for polymicrobial abdominal sepsis. J Vis Exp (46). 10.3791/2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vallejo R, de Leon-Casasola O, Benyamin R. 2004. Opioid therapy and immunosuppression: a review. Am J Ther 11:354–365. 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 82.van der Poll T. 2012. Preclinical sepsis models. Surg Infect (Larchmt) 13:287–292. 10.1089/sur.2012.105. [DOI] [PubMed] [Google Scholar]
- 83.Verweij WR, Namavar F, Schouten WF, Kostense PJ, Pellenkoft M, De Graaff J, MacClaren DM. 1993. In vitro activity of peritoneal cells from rats after intraabdominal infection with Bacteroides fragilis and Escherichia coli. J Med Microbiol 38:13–18. 10.1099/00222615-38-1-13. [DOI] [PubMed] [Google Scholar]
- 84.Visser LH. 2006. Critical illness polyneuropathy and myopathy: clinical features, risk factors, and prognosis. Eur J Neurol 13:1203–1212. 10.1111/j.1468-1331.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 85.Wang DW, Yin YM, Yao YM. 2016. Vagal modulation of the inflammatory response in sepsis. Int Rev Immunol 35:415–433. 10.3109/08830185.2015.1127369. [DOI] [PubMed] [Google Scholar]
- 86.Wichterman KA, Baue AE, Chaudry IH. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res 29:189–201. 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 87.Wolfe AM, Kennedy LH, Na JJ, Nemzek-Hamlin JA. 2015. Efficacy of tramadol as a sole analgesic for postoperative pain in male and female mice. J Am Assoc Lab Anim Sci 54:411–419. [PMC free article] [PubMed] [Google Scholar]
- 88.Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer LL. 2007. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28:675–683. [DOI] [PubMed] [Google Scholar]
- 89.Xu G, Feng Y, Li D, Zhou Q, Chao W, Zou L. 2018. Importance of the complement alternative pathway in serum chemotactic activity during sepsis. Shock 50:435–441. 10.1097/SHK.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang R, Meng J, Lian Q, Chen X, Bauman B, Chu H, Segura B, Roy S. 2018. Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: a retrospective cohort study using electronic health records. PLoS One 13:1–8. 10.1371/journal.pone.0190362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zingarelli B, Coopersmith CM, Drechsler S, Efron P, Marshall JC, Moldawer L, Wiersinga WJ, Xiao X, Osuchowski MF, Thiemermann C. 2019. Part I: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for study design and humane modeling endpoints. Shock 51:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]