Abstract
Objectives
The aim of this study was to investigate the effects of adding self-lymphatic drainage (SLD) to compression bandaging (CB) therapy rather than manual lymphatic drainage (MLD) in the first phase of complex decongestive therapy (CDT) on arm edema, quality of life, upper extremity function, and anxiety-depression in patients with breast cancer-related lymphedema (BCRL).
Patients and methods
Between January 2015 and January 2017, a total of 24 patients (mean age 58.9±10.3 years; range, 42 to 83 years) with BCRL were randomly assigned to receive CB or CB plus SLD. The edema of the arm was assessed by volume calculation based on the circumference measurements. The Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire (Q-DASH) for upper extremity functions, the Short Form-36 health survey (SF-36) for the quality of life, and the Hospital Anxiety-Depression Scale (HADS) for anxiety and depression were used. The patients were assessed before the treatment, at the end of the treatment, and six months after the treatment.
Results
A significant volume decrease was observed in the affected arm in both groups at the end of the treatment. Statistically significant improvements in the SF-36 and Q-DASH scores were observed in both groups; however, there was no significant change in the HADS-anxiety and depression subscale scores.
Conclusion
Our study results suggest that compression therapy with or without SLD is effective in the treatment of BCRL. However, the addition of SLD to CB in the first phase of CDT rather than MLD seems to provide no additional significant benefit.
Keywords: Breast cancer, compression bandage, self-lymphatic drainage, upper extremity lymphedema
Introduction
Breast cancer is the most common type of cancer among women. Although advances in breast cancer treatment reduce the mortality rates, they are also associated with serious complications.[1] Breast cancer-related lymphedema (BCRL) is one of the most distressing complication of breast cancer treatment.[2] The prevalence is between 8 and 40% as reported in the literature.[3,4]
Lymphedema may develop at any time after the original cancer treatment.[5] Although lymphedema is not a life-threatening disorder, it may precipitate cellulitis, erysipelas, lymphangitis, and occasionally lymphangiosarcoma, if left untreated.[6] It may also cause disfigurement, physical discomfort, and functional impairment. Therefore, anxiety, depression, emotional distress, and impaired quality of life (QoL) are more likely to occur in women with BCRL.[7-9] Effective treatment for BCRL is necessary to prevent these complications.
Currently, there is no curative treatment for lymphedema. The main goals of the treatment are to decrease the excess volume as much as possible, to restore function, and to prevent the development of infection. The gold standard treatment for lymphedema is complex decongestive therapy (CDT).[10,11] It is composed of two phases: an intensive phase which includes patient education, multilayer compression bandages, manual lymphatic drainage (MLD), skin cares, and physical exercises and a maintenance phase which includes self-lymphatic drainage (SLD), compression garments, skin care, and physical exercises.[12] Most studies have shown that CDT is effective in lymphedema treatment.[13,14] However, there are only few studies investigating the effectiveness of each individual component of CDT for the outcomes.[5,12,15-19]
Compression bandaging (CB) is the main component of CDT. Although several studies have demonstrated the effectiveness of CB,[5,20-22] the effectiveness of MLD in CDT still remains an area of controversy. Some authors have suggested that MLD provides no significant benefit when added to compression therapy.[5,16] In addition, MLD is not a cost-effective treatment and its application takes a long period of time (~ 45 to 60 min).[5,16,23] On the other hand, SLD is a simplified version of MLD which can be easily applied by the patient with taking less time (10-15 min) than MLD. The continuing focus on rising health care costs and fiscal restraint has also resulted in a need for cost-effective intervention programs. However, the current literature is limited for the effectiveness of SLD.
In the present study, we aimed to assess the effects of adding SLD to CB therapy rather than MLD in the first phase of CDT on arm edema, QoL, upper extremity function, and anxiety-depression in patients with BCRL.
Patients and Methods
A total of 50 women admitted to the outpatient lymphedema clinic for unilateral BCRL were evaluated between January 2015 and January 2017. Inclusion criteria were as follows: (i) Stage I-II unilateral BCRL, (ii) aged over 18 years, (iii) >3 months after breast cancer treatment, and (iv) willingness to participate in the study. Patients with Stage 3-4 BCRL, those undergoing current radiotherapy (RT) or chemotherapy (CT), patients with an evidence of distant cancer metastasis or cancer recurrence, bilateral breast cancer, congestive heart failure, renal insufficiency, venous or arterial obstruction in the affected arm, infection in the affected arm, and pregnancy were excluded (n=10).
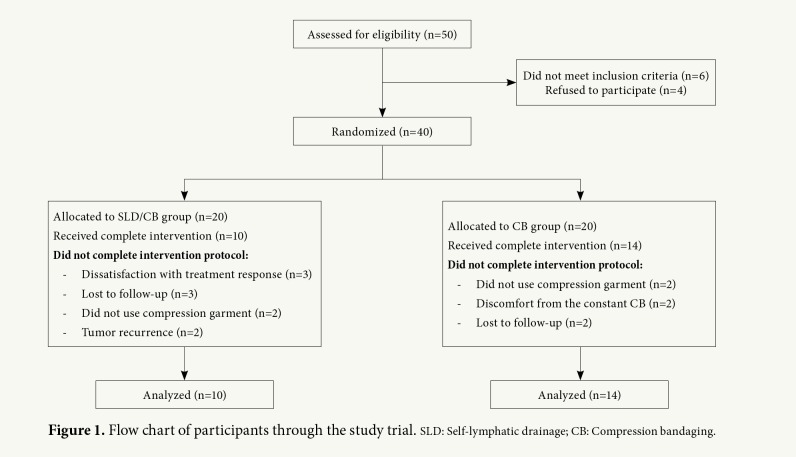
The study included 40 women with unilateral BCRL. Forty patients were randomly assigned following simple randomization procedures (computed random numbers) to receive either CB therapy alone (CB group) or CB plus SLD therapy (CB/SLD group). Fourteen patients in CB group and 10 patients in CB/SLD group completed the study (n=24) (mean age 58.9±10.3 years; range, 42 to 83 years). The flow chart of the study design is shown in Figure 1.
Figure 1. Flow chart of participants through the study trial. SLD: Self-lymphatic drainage; CB: Compression bandaging.
A written informed consent was obtained from each patient. The study protocol was approved by the University of Health Science, Dışkapı Yıldırım Beyazıt Training and Research Hospital Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Demographic and clinical characteristics
Demographic and clinical features of the patients including age, body mass index (BMI), marital status, educational status, and comorbidities were recorded. A medical history related to breast cancer treatment and lymphedema characteristics including pathological diagnosis, cancer stage, type of surgery, the number of lymph nodes removed from axilla, the number of metastatic lymph nodes, CT, RT, hormone therapy, lymphedema stage, lymphedema severity, location of swelling, the time passed since breast cancer surgery, and duration of lymphedema were obtained from each patient.
Circumference measurement and limb volume
The edema of the arm was assessed by circumference measurements and arm volume calculation based on circumference measurements.[24] A retractable, fiber glass tape was used to perform circumference measurements and all measurements were recorded in centimeters (cm). The circumferential measurements of both arms were taken at four points: the metacarpophalangeal (MCP) joints, the wrist, 10-cm distal to the lateral epicondyle, and 10-cm proximal to the lateral epicondyle. Calculation of the limb volume was undertaken using the simplified Frustum Formula (summed truncated cone).[25] These measurements were taken by a single researcher before the treatment, every week during the treatment, at the end of the treatment, and six months after the treatment. Measurements were made at the same time of day. The researcher who performed circumference measurements was blinded to the groups. Lymphedema was defined as a difference of more than 2 cm between the circumferences of the two arms or a difference of more than 10% in volume between the two arms. The staging of lymphedema was performed according the criteria defined by the International Society of Lymphology:[26] Stage 0 (or 1a) is a latent or subclinical condition where swelling is not yet evident despite impaired lymph transport, subtle changes in tissue fluid, and changes in subjective symptoms. Stage 1 represents an early accumulation of fluid relatively high in protein content which subsides with limb elevation. Pitting may occur. Stage 2 indicates that limb elevation alone rarely reduces tissue swelling and pitting is manifest. Late in Stage 2, the limb may or may not pit as excess fat and fibrosis supervenes. Stage 3 encompasses lymphostatic elephantiasis where pitting can be absent and trophic skin changes have developed.[26]
Quality of life
The QoL of the patients levels was assessed using the Short Form-36 (SF-36) health survey. It consists of 36 items in eight domains: physical functioning, role limitations due to physical health, pain, emotional well-being, energy/fatigue, social functioning, role limitations due to emotional problems, and general health. These eight domains can be aggregated into two summary measures: the physical component summary (PCS) and mental component summary (MCS). Each domain is scored according to a standardized scoring protocol. Higher scores indicate better health status. The Turkish validity and reliability studies of the survey were conducted by Kocyigit et al.[27]
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) was used to assess depression and anxiety. It consists of 14 items with two subscales (seven items for anxiety and seven items for depression). Each item is scored 0-3. Each subscale is scored between 0 and 21. Anxiety and depression were defined by a score of ≥8. The Turkish validity and reliability studies of the scale were performed by Aydemir et al.[28]
Upper extremity function
Upper extremity functions of the patients with BCRL were evaluated using the Quick Disabilities of Arm, Shoulder, and Hand (Q-DASH) questionnaire. It has 11 items and each item is scored from 1 to 5. Total score ranges from 0 to 100. Higher scores indicate lower functional level. The reliability and validity studies of the Turkish version were performed by Duger et al.[29] The assessments were performed before the treatment, at the end of the treatment, and six months after the treatment.
Study design
All patients were given information about lymphedema, skin care, and physical exercises. Compression bandaging was applied to all patients. Short stretch bandages were used to achieve continuous pressure during work as well as during rest periods. The bandage was wrapped in proximal direction with pressure gradually decreasing. The bandage was kept on for 23 h and replaced at the next day. The clinician applied all the compression bandages for five days per week.
In CB/SLD group, SLD was instructed by the clinician and performed by subjects for 10 to 15 min each day before CB. The patients were given a leaflet describing the SLD sequence. It was applied sequentially to neck, non-affected axilla, anterior chest wall, affected inguinal region, lateral trunk, affected shoulder, affected upper arm, affected forearm, affected hand and fingers. The patients were instructed to use a relaxed hand to gently stretch the skin in the direction away from the swollen area, repeating the movements 10 times in various positions. At every visit, the patients were asked to indicate whether they performed SLD regularly. Their technique was monitored weekly during the study and each participant kept a diary recording the areas covered and time taken each day for SLD.
The treatment phase was performed, until no changes were observed in the limb circumference measurements obtained every week. The median duration of treatment phase was five (range 4 to 8) weeks in CB group and six (range 4-20) weeks in CB/SLD group (Table 1). In the treatment phase, the patients were evaluated every week. After the treatment period, all patients were prescribed custom fitted compression garments that deliver 20 to 30 mmHg (Class 2) of pressure. They were asked to wear the garments during the day. Self-lymphatic drainage was not applied in the maintenance phase in either group. In this phase, the patients were evaluated every three months and limb circumference was used to measure the change.
Table 1. Demographic and disease related characteristics of the patients with breast cancer-related lymphedema.
| CB Group (n=14) | CB/SLD Group (n=10) | p | |||||||
| n | % Mean±SD | Median | Min-Max | n | % Mean±SD | Median | Min-Max | ||
| Age (year) | 61.64±11.69 | 5 | 0-13 | 55.20±7.15 | 8 | 0-11 | 0.131 0.382 0.080 1.000 | ||
| BMI (kg/m2) | 32.73±5.80 | 30.88±3.62 | |||||||
| Education (year) Marital status | |||||||||
| Married | 9 | 64,3 | 7 | 70 | |||||
| Single | 5 | 36,7 | 3 | 30 | |||||
| Arm dominance | 0,163 | ||||||||
| Right | 14 | 100 | 8 | 80 | |||||
| Left | 0 | 0 | 2 | 20 | |||||
| Comorbidity | 0,665 | ||||||||
| Yes | 11 | 78,6 | 7 | 70 | |||||
| No | 3 | 21,4 | 3 | 30 | |||||
| Pathological diagnosis | 1,000 | ||||||||
| İnfiltrative ductal carcinoma | 12 | 85,7 | 9 | 90 | |||||
| İnfiltrative lobular carcinoma | 2 | 14,3 | 1 | 10 | |||||
| Cancer stage | * | ||||||||
| Stage 1 | 3 | 21,4 | 2 | 20 | |||||
| Stage 2 | 4 | 28,6 | 4 | 40 | |||||
| Stage 3 | 7 | 50 | 4 | 40 | |||||
| Type of surgery | 1,000 | ||||||||
| BCS+ALND | 1 | 7,1 | 1 | 10 | |||||
| MRM+ALND | 13 | 92,9 | 9 | 90 | |||||
| Lymph nodes removed from axilla | 23 | 11-52 | 17,5 | 10-22 | 0,071 | ||||
| Metastatic lymph nodes | 1,50 | 0-33 | 2,50 | 0-6 | 0,663 | ||||
| Chemotherapy | 0,615 | ||||||||
| Yes | 11 | 78,6 | 9 | 90 | |||||
| No | 3 | 21,4 | 1 | 10 | |||||
| Radiotherapy | 0,388 | ||||||||
| Yes | 8 | 57,1 | 8 | 80 | |||||
| No | 6 | 42,9 | 2 | 20 | |||||
| Hormone therapy | 0,678 | ||||||||
| Yes | 8 | 57,1 | 7 | 70 | |||||
| No | 6 | 42,9 | 3 | 30 | |||||
| Affected arm | 0,240 | ||||||||
| Dominant | 6 | 42,9 | 7 | 70 | |||||
| Non-dominant | 8 | 78,6 | 3 | 30 | |||||
| Location of swelling | * | ||||||||
| FA | 1 | 7,1 | 1 | 10 | |||||
| UA | 6 | 42,9 | 2 | 20 | |||||
| Whole arm | 1 | 7,1 | 1 | 10 | |||||
| Wrist+FA+UA | 5 | 35,7 | 3 | 30 | |||||
| FA+UA | 1 | 7,1 | 3 | 30 | |||||
| LE stage | 1,000 | ||||||||
| Stage 1 | 3 | 21,4 | 2 | 20 | |||||
| Stage 2 | 11 | 78,6 | 8 | 80 | |||||
| LE severity | * | ||||||||
| Mild | 3 | 21,4 | 2 | 20 | |||||
| Moderate | 10 | 71,4 | 6 | 60 | |||||
| Severe | 1 | 7,1 | 2 | 20 | |||||
| Time from surgery (month) | 21 | 3-83 | 21,5 | 3-252 | 0,780 | ||||
| Duration of LE (month) | 2 | 1-12 | 1 | 1-36 | 0,791 | ||||
| Duration of treatment phase (week) | 5 | 4-8 | 6 | 4-20 | 0,255 | ||||
| CB: Compression bandaging; SLD: Self-lymphatic drainage; SD: Standard deviation; Min: Minimum; Max: Maximum; BMI: Body mass index; BCS: Breast conserving surgery; ALND: Axiller lymph node dissection; MRM: Modified radical mastectomy; FA: Forearm; UA: Upper arm; LE: Lymphedema; * p-values for location of swelling , cancer stage and LE severity couldn’t be calculated since the counts are too small. | |||||||||
Statistical analysis
Statistical analysis was performed using the SPSS version 11.5 software (SPSS Inc., Chicago, IL, USA) in the manner of per-protocol approach. Normality of continuous variables were evaluated using the Shapiro-Wilks test. The Levene test was used to evaluate the homogeneity of variances for continuous variables. Normally distributed variables were expressed in mean ± standard deviation (SD), while other continuous and discrete variables, and categorical variables were expressed in median (min-max) and number and percentage, respectively. The independent sample t-test and Mann Whitney U test were used for inter-group comparisons of continuous and discrete variables. The Pearson chi-square and Fisher's exact tests were used to compare the categorical variables. Repeated measures analysis of variance (ANOVA) and Friedman test were used for intra-group comparisons of the repeated measures. As independent intra- and inter-group comparisons increased type-1 error of the tests, Bonferroni correction was applied. A p value of less than 0.05 was considered statistically significant.
Results
A total of 24 patients completed the study. All patients previously underwent unilateral breast cancer surgery including an axillary node dissection. Most of the patients developed lymphedema after the first year of surgery. There was no significant difference between the groups in demographic and clinical characteristics (p>0.05). Demographic and clinical characteristics of the patients are shown in Table 1.
Edema of arm
There was a significant volume decrease in both groups at the end of the treatment from baseline (p=0.006 and p=0.002, respectively). Volume decrease of the affected arm was similar in both groups and no significant difference was observed between two groups (p=0.939). Six months after the intensive phase, the affected arm volumes in both groups remained at the same level which were achieved at the end of the treatment (p=0.909). A significant reduction in the percentage volume difference between two sides were found at the end of the treatment from baseline in both groups (p=0.002 and p=0.004, respectively). There was no significant difference in the percentage volume difference at the end of the treatment and six month after the treatment between the two groups (p=0.818 and p=0.967, respectively). The affected arm volumes and volume differences of the groups are shown in Table 2.
Table 2. Arm volumes and volume differences of the groups.
| CB group (n=14) | CB/SLD group (n=10) | ||
| Mean±SD | Mean±SD | p* | |
| Volume of the affected arm (mL) | |||
| Pretreatment | 507.0±103.3 | 522.9±61.0 | 0.669 |
| At the end of the treatment | 469.9±108.0 | 472.9±62.3 | 0.939 |
| Six months after the treatment | 479.0±103.1 | 483.4±68.6 | 0.909 |
| p** | 0.006 | 0.002 | |
| Volume difference between two sides | |||
| Pretreatment | 24.7±15.8 | 25.1±11.3 | 0.712 |
| At the end of the treatment | 14.4±8.1 | 15.4±9.8 | 0.818 |
| Six months after the treatment | 17.8±10.5 | 16.7±11.1 | 0.967 |
| p** | 0.002 | 0.004 | |
| CB: Compression bandage; SLD: Self lymphatic drainage; SD: Standard deviation; * Inter-group analysis, ** Intra-group analysis. | |||
Upper extremity function
Intra-group analysis showed that the Q-DASH scores at the end of the treatment and six months after the treatment were significantly different from the baseline in both groups (p<0.001 and p<0.001, respectively). There was no statistically significant difference in the Q-DASH scores between the groups (p=0.868, p=0.932, and p=0.831, respectively). The Q-DASH scores and score changes of the groups are shown in Table 3.
Table 3. Q-DASH, SF-36 and HADS scores of the groups.
| CB group (n=14) | CB/SLD group (n=10) | ||
| Mean±SD | Mean±SD | p* | |
| Q-DASH | |||
| Pretreatment | 54.4±20.2 | 55.7±16.2 | 0.868 |
| At the end of the treatment | 35.7±13.1 | 34.8±17.5 | 0.932 |
| Six months after the treatment | 27.7±9.5 | 28.6±9.7 | 0.831 |
| p** | <0.001 | <0.001 | |
| SF-36 PCS | |||
| Pretreatment | 39.6±7.4 | 33.7±11.0 | 0.127 |
| At the end of the treatment | 48.1±12.4 | 41.5±9.4 | 0.174 |
| Six months after the treatment | 57.9±15.2 | 63.9±17.6 | 0.380 |
| p** | 0.004 | <0.001 | |
| SF-36 MCS | |||
| Pretreatment | 52.2±11.8 | 55.8±10.9 | 0.934 |
| At the end of the treatment | 63.3±13.0 | 56.3±9.2 | 0.159 |
| Six months after the treatment | 68.1±11.3 | 70.6±14.9 | 0.653 |
| p** | 0.003 | 0.003 | |
| HADS-A | |||
| Pretreatment | 6.1±4.7 | 4.9±3.4 | 0.486 |
| At the end of the treatment | 4.6±2.9 | 4.2±3.7 | 0.746 |
| Six months after the treatment | 4.7±2.1 | 3.4±2.4 | 0.741 |
| p** | 0.082 | 0.333 | |
| HADS-D | |||
| Pretreatment | 4.1±3.5 | 4.9±4.6 | 0.625 |
| At the end of the treatment | 3.1±2.4 | 4.2±4.8 | 0.463 |
| Six months after the treatment | 1.9±1.8 | 2.0±1.5 | 0.841 |
| p** | 0.087 | 0.053 | |
| Q-DASH: Quick Disabilities of Arm, Shoulder and Hand; SF-36: Short form-36; HADS: Hospital Anxiety and Depression Scale; CB: Compression bandage; SLD: Self lymphatic drainage; SD: Standard deviation; PCS: Physical Component Summary; MCS: Mental Component Summary; HADS-A: Hospital Anxiety and Depression Scale-Anxiety; HADS-D: Hospital Anxiety and Depression Scale- Depression; * Inter-group analysis, ** Intra-group analysis. | |||
Quality of life
Intra-group analysis showed that SF-36 physical and mental subscale scores significantly increased at the end of the treatment and six months after the treatment in both groups (p=0.004 and p<0.001, respectively). We found no statistically difference between the groups in the follow-up SF-36 physical and mental subscale scores (for SF-36 physical p=0.127, p=0.174, and p=0.380, respectively and for SF-36 mental p=0.934, p=0.159, and p=0.653, respectively). The SF-36 scores and score changes of the groups are shown in Table 3.
Anxiety-depression
We found that the HADS-anxiety and depression subscale scores were not significantly different at the end of the treatment and six months after the treatment in both groups (for HADS-anxiety p=0.082 and p=0.333, respectively and for HADS-depression p=0.087 and p=0.053, respectively). In addition, there was no significant difference in the HADS-anxiety scores and depression scores between the groups (for HADS-anxiety p=0.486, p=0.746, and p=0.741, respectively and for HADS-depression p=0.625, p=0.463, and p=0.841, respectively). The HADS scores of the groups are shown in Table 3.
Discussion
Breast cancer-related lymphedema is a common and debilitating complication of breast cancer treatment.[30] There is no curative treatment for this condition, and the main goals of the treatment is to reduce swelling, to increase the joint mobility, to decrease discomfort, and to improve upper extremity functions and QoL. As a treatment option, CDT is the most popular and widespread approach. Its efficacy has been proved in previous studies.[31-33] However, most of these studies have addressed into the combined effects of this comprehensive treatment program. It is necessary to assess the effectiveness of each individual component of CDT and to identify optimal treatment program. However, CDT is an expensive intervention and takes an extended period of time. Particularly MLD, which is applied before the compression treatment, is time-consuming and troublesome which limits the use of MLD. On the other hand, SLD, a simplified version of MLD, can be learned and applied by the patient easily. There is no study comparing the effects of CB alone with CB plus SLD in the literature. Therefore, in the present study, we investigated the efficacy of SLD combined with CB in the intensive phase of CDT in patients with BRCL.
Compression is largely described as the key component of lymphedema treatment in the updated best practice guidelines of the International Lymphedema Framework.[34] Compressive therapies are very important for CDT both in the first intensive phase and in the second maintenance phase. Recent studies have shown that compression therapy has the most effective impact on reducing edema volume of affected arm.[5,20,35] In our study, compression bandages resulted in a significant decrease on affected arm volume at the end of the treatment in intensive phase. In the maintenance phase, in which compression garments were prescribed, reduction in arm volume was preserved at six months after the treatment.
Furthermore, SLD is a simplified version of MLD and recommended during the maintenance phase of CDT. It can be learned and applied by the patient or the caregiver. It is easy to perform and takes only 10-15 min, compared to MLD. There are only a few studies to investigate the effects of SLD for upper extremity lymphedema treatment.[12,15,16,36] In a previous study, 13 patients who developed BCRL were treated with SLD and 15 patients with MLD for two weeks and the treatment was followed by CB in both groups. The percent reduction in arm volume was 33.8% in the MLD group and 22% in the SLD group. The authors found no significant difference between MLD and SLD.[36] Williams et al.[12] conducted a randomized-controlled study evaluating the efficacy of MLD and SLD in 31 patients with BCRL. Group A patients received daily three-week MLD therapy followed by a six-week off period and then received a three-week SLD therapy. Group B patients received a three-week SLD therapy followed by a six-week off period and then received a three-week MLD. Group A patients had a significant decrease in the arm volume and upper arm skin thickness. Signs of impaired sensation such as pain, weight, sleep disturbance decreased and QoL were significantly improved. There was no statistically significant difference between two groups in assessing trunk edema and QoL. In our study, the efficacy of adding SLD to compression bandage rather than MLD during the intensive phase of CDT was investigated, and no additional benefit was observed. However, clinical experience suggests that SLD is useful in patients where MLD is unable to be performed, and it is useful in the long-term, as it provides self-care after CDT. Therefore, SLD should be instructed to patients with BCRL and their caregivers. Learning should be reinforced with printed materials. Education should be continued periodically and patients should be encouraged to practice.
In our study, we found that upper extremity functions were significantly improved in both groups, although there was no significant difference between the groups. In other words, upper extremity functions did not change when SLD was added to the treatment. Bruggada et al.[37] conducted a randomized-controlled study evaluating the efficacy of CDT and a home-based program involving SLD, skin care, and remedial exercises. They reported that CDT in combination with home-based program was effective in improving upper extremity function. However, there is no study investigating the efficacy of SLD therapy alone on upper extremity function in the literature.
The QoL of patients after breast cancer treatment is adversely affected. Aesthetic deformation after lymphedema development, decreased functional skills caused by swelling, and psychological stress may affect patients' QoL negatively. In a previous study investigating the effects of CDT on the QoL in patients with BCRL, SF-36 scores were significantly improved at the end of treatment and at six months compared to pre-treatment values.[38] Similarly, we observed that physical and mental functioning significantly improved at the end of the treatment and at six months compared to pre-treatment values. However, the addition of SLD therapy to compression treatment did not change the results. There is no study investigating the effects of adding SLD therapy to compression therapy on QoL in patients with BCRL in the literature.
Review of the literature reveals that diagnosis and treatment of breast cancer are associated with psychological problems such as anxiety, depression, anger, and uncertainty about future. Morgan et al.[39] reported that patients with BCRL experienced greater functional disorder, poor psychological balance, more anxiety, and depression than the general population. In contrast to previous studies, we found that the HADS anxiety and depression subscale scores were all within normal range in both groups. In both groups, the HADS scores decreased at the end of the treatment, compared to baseline, although this decrease was not statistically significant. The discrepancy in the results can be attributed to small sample size in our study and the use of different assessment methods.
Nonetheless, there are several limitations to this study. First, the number of patients was relatively small which makes drawing any conclusion about the effectiveness of SLD difficult. Second, we measured circumference at three predefined points on the arm and calculated changes in girth at these points. The segment length determined by the circumference measurements has not been standardized with variations of 3 cm, 4 cm and 10 cm reported in the literature. However, using the smallest segment length reported in the literature would give the most accurate geometric volume measurement.[40] Therefore, using fewer point for volume calculation as in our study is another limitation for accurate volume measurement.
In conclusion, our study results suggest that compression therapy provides a significant edema reduction in the intensive phase of CDT. It is also an effective treatment modality in preserving the existing volume in the maintenance phase. However, SLD rather than MLD in the intensive phase may not provide an additional support to the treatment. We believe that further large-scale studies are needed to shed light into the effects of SLD in the intensive phase of CDT in patients with BCRL.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Ung OA, Dylke ES, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast. 2016;28:29–36. doi: 10.1016/j.breast.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment--a nationwide study of prevalence and associated factors. Breast. 2010;19:506–515. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Lopez Penha TR, Slangen JJ, Heuts EM, Voogd AC, Von Meyenfeldt MF. Prevalence of lymphoedema more than five years after breast cancer treatment. Eur J Surg Oncol. 2011;37:1059–1063. doi: 10.1016/j.ejso.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: a randomized controlled trial. Breast Cancer Res Treat. 2004;86:95–106. doi: 10.1023/B:BREA.0000032978.67677.9f. [DOI] [PubMed] [Google Scholar]
- 6.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral del Moral O, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:5396–5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177:184–187. doi: 10.1016/s0002-9610(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 8.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83:2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90:76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]
- 10.The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology Executive Committee. Lymphology. 1995;28:113–117. [PubMed] [Google Scholar]
- 11.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 12.Williams AF, Vadgama A, Franks PJ, Mortimer PS. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur J Cancer Care (Engl) 2002;11:254–261. doi: 10.1046/j.1365-2354.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- 13.Szuba A, Cooke JP, Yousuf S, Rockson SG. Decongestive lymphatic therapy for patients with cancer-related or primary lymphedema. Am J Med. 2000;109:296–300. doi: 10.1016/s0002-9343(00)00503-9. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Todo Y, Kaneuchi M, Handa Y, Watanabe K, Yamamoto R. Study of edema reduction patterns during the treatment phase of complex decongestive physiotherapy for extremity lymphedema. Lymphology. 2008;41:80–86. [PubMed] [Google Scholar]
- 15.Hornsby R. The use of compression to treat lymphoedema. Prof Nurse. 1995;11:127–128. [PubMed] [Google Scholar]
- 16.Andersen L, Højris I, Erlandsen M, Andersen J. Treatment of breast-cancer-related lymphedema with or without manual lymphatic drainage--a randomized study. Acta Oncol. 2000;39:399–405. doi: 10.1080/028418600750013186. [DOI] [PubMed] [Google Scholar]
- 17.LiL , YuanL , ChenX , WangQ , TianJ , YangK , etal Current treatments for breast cancer-related lymphoedema: a systematic review. Asian Pac J Cancer Prev. 2016;17:4875–4883. doi: 10.22034/APJCP.2016.17.11.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson K, Albertsson M, Ingvar C, Ekdahl C. Effects of compression bandaging with or without manual lymph drainage treatment in patients with postoperative arm lymphedema. Lymphology. 1999;32:103–110. [PubMed] [Google Scholar]
- 19.Didem K, Ufuk YS, Serdar S, Zümre A. The comparison of two different physiotherapy methods in treatment of lymphedema after breast surgery. Breast Cancer Res Treat. 2005;93:49–54. doi: 10.1007/s10549-005-3781-2. [DOI] [PubMed] [Google Scholar]
- 20.Partsch H. Assessing the effectiveness of multilayer inelastic bandaging. Journal of Lymphoedema. 2007;2:55–61. [Google Scholar]
- 21.Whitaker J, Williams A, Pope D, Elwell R, Thomas M, Charles H, et al. Clinical audit of a lymphoedema bandaging system: a foam roll and cohesive short stretch bandages. J Wound Care. 2015;24:83–84. doi: 10.12968/jowc.2015.24.3.83. [DOI] [PubMed] [Google Scholar]
- 22.Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18:639–646. doi: 10.1093/annonc/mdl182. [DOI] [PubMed] [Google Scholar]
- 23.Selfe J, Karki A, Simonen R, Malkia E. Efficacy of physical therapy methods and exercise after a breast cancer option: a systematic review. Crit Rev Phys Rehabil Med. 2001;13:159–190. [Google Scholar]
- 24.Megens AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82:1639–1644. doi: 10.1053/apmr.2001.26822. [DOI] [PubMed] [Google Scholar]
- 25.Meijer RS, Rietman JS, Geertzen JH, Bosmans JC, Dijkstra PU. Validity and intra- and interobserver reliability of an indirect volume measurements in patients with upper extremity lymphedema. Lymphology. 2004;37:127–133. [PubMed] [Google Scholar]
- 26.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. treatment of peripheral lymphedema. 2013 consensus document of the international society of lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 27.Koçyiğit H, Aydemir Ö, Ölmez N, Memiş A. SF-36’nın Türkçe için geçerliliği ve güvenilirliği. İlaç ve Tedavi Dergisi. 1999;12:102–106. [Google Scholar]
- 28.Aydemir Ö, Güvenir T, Küey L, Kültür S. Hastane Anksiyete ve Depresyon Ölçeği Türkçe formunun geçerlilik ve güvenilirliği. Türk Psikiyatri Dergisi. 1997;8141:280–287. [Google Scholar]
- 29.Düger T, Yakut E, Öksüz Ç, Yörükan S, Bilgütay BS, Ayhan Ç, et al. Kol, omuz ve el sorunları (Disabilities of Arm, Shoulder and Hand- DASH) anketi Türkçe uyarlamasının güvenirliği ve geçerliği. Fizyoter Rehabil. 2006;17:99–107. [Google Scholar]
- 30.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 31.Rockson SG, Miller LT, Senie R, Brennan MJ, Casley-Smith JR, Földi E, et al. American Cancer Society Lymphedema Workshop. Workgroup III: Diagnosis and management of lymphedema. Cancer. 1998;83:2882–2885. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2882::aid-cncr45>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PMR. 2012;4:580–601. doi: 10.1016/j.pmrj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Karadibak D, Yavuzsen T, Saydam S. Prospective trial of intensive decongestive physiotherapy for upper extremity lymphedema. J Surg Oncol. 2008;97:572–577. doi: 10.1002/jso.21035. [DOI] [PubMed] [Google Scholar]
- 34.Lymphoedema Framework. Best Practice for the management of lymphoedema. 2. London: Medical Education Partnership; 2012. [Google Scholar]
- 35.Zuther JE, Norton S. Lymphedema Management: The Comprehensive Guide for Practitioners. 3. Stuttgart: Thieme-Verlag; 2013. [Google Scholar]
- 36.Sitzia J, Sobrido L, Harlow W. Manual lymphatic drainage compared with simple lymphatic drainage in the treatment of post-mastectomy lymphoedema: a pilot randomized trial. Physiotherapy. 2002;88:99–107. [Google Scholar]
- 37.Buragadda S, Alhusaini AA, Melam GR, Arora N. Effect of complete decongestive therapy and a home program for patients with post mastectomy lymphedema. J Phys Ther Sci. 2015;27:2743–2748. doi: 10.1589/jpts.27.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SJ, Park YD. Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl) 2008;17:463–468. doi: 10.1111/j.1365-2354.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 39.Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J. 2005;2:47–62. doi: 10.1111/j.1742-4801.2005.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sander AP, Hajer NM, Hemenway K, Miller AC. Upper- extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82:1201–1212. [PubMed] [Google Scholar]