Is there a causal relationship between delirium and cognitive decline? Casey et al. show that postoperative delirium is associated with an increase in plasma levels of neurofilament light, a neuronal injury biomarker. Levels of neurofilament light correlate with peak delirium severity, suggesting that neuronal injury contributes to delirium pathogenesis.
Keywords: delirium, surgery, neuronal injury, inflammation, cognition
Abstract
While delirium is associated with cognitive decline and dementia, there is limited evidence to support causality for this relationship. Clarification of how delirium may cause cognitive decline, perhaps through evidence of contemporaneous neuronal injury, would enhance plausibility for a causal relationship. Dose-dependence of neuronal injury with delirium severity would further enhance the biological plausibility for this relationship. We tested whether delirium is associated with neuronal injury in 114 surgical patients recruited to a prospective biomarker cohort study. Patients underwent perioperative testing for changes in neurofilament light, a neuronal injury biomarker, as well as a panel of 10 cytokines, with contemporaneous assessment of delirium severity and incidence. A subset of patients underwent preoperative MRI. Initially we confirmed prior reports that neurofilament light levels correlated with markers of neurodegeneration [hippocampal volume (ΔR2 = 0.129, P = 0.015)] and white matter changes including fractional anisotropy of white matter (ΔR2 = 0.417, P < 0.001) with similar effects on mean, axial and radial diffusivity) in our cohort and that surgery was associated with increasing neurofilament light from preoperative levels [mean difference (95% confidence interval, CI) = 0.240 (0.178, 0.301) log10 (pg/ml), P < 0.001], suggesting putative neuronal injury. Next, we tested the relationship with delirium. Neurofilament light rose more sharply in participants with delirium compared to non-sufferers [mean difference (95% CI) = 0.251 (0.136, 0.367) log10 (pg/ml), P < 0.001]. This relationship showed dose-dependence, such that neurofilament light rose proportionately to delirium severity (ΔR2 = 0.199, P < 0.001). Given that inflammation is considered an important driver of postoperative delirium, next we tested whether neurofilament light, as a potential marker of neurotoxicity, may contribute to the pathogenesis of delirium independent of inflammation. From a panel of 10 cytokines, the pro-inflammatory cytokine IL-8 exhibited a strong correlation with delirium severity (ΔR2 = 0.208, P < 0.001). Therefore, we tested whether the change in neurofilament light contributed to delirium severity independent of IL-8. Neurofilament light was independently associated with delirium severity after adjusting for the change in inflammation (ΔR2 = 0.040, P = 0.038). These data suggest delirium is associated with exaggerated increases in neurofilament light and that this putative neurotoxicity may contribute to the pathogenesis of delirium itself, independent of changes in inflammation.
Introduction
Delirium is an acute confusional state, characterized by inattention and cognitive failure, which arises as a physiological consequence of a medical or surgical condition (European Delirium Association and American Delirium Society, 2014). It is associated with increased morbidity and mortality (Witlox et al., 2010; Sanders et al., 2011), high rates of institutionalization (Witlox et al., 2010), and is estimated to cost up to $152 billion per annum in the USA (Leslie et al., 2008). Perhaps most critically, it can herald a decline in cognition (Pandharipande et al., 2013; Inouye et al., 2016) and the onset of dementia (Witlox et al., 2010). One possibility is that delirium results as a failed ‘stress test’ for the brain (Nadelson et al., 2014), revealing underlying neurodegeneration in the presence of an acute physiological stressor. An alternative model is that delirium is associated with neuronal injury and this accelerates any pre-delirium cognitive decline (Halaas et al., 2018); however, data linking delirium to neurotoxicity are scarce. A recent study using CSF levels of the neuronal injury biomarker, neurofilament light (NfL), showed that delirious patients with hip fracture had higher NfL levels than non-delirious controls (Halaas et al., 2018); however, a major limitation of this study is that the NfL levels may represent chronic (Idland et al., 2017; Khalil et al., 2018; Moore et al., 2018), not acute, degeneration. Here we overcome this limitation by studying the change in NfL (from preoperative levels) associated with delirium.
First we validate prior findings that (i) plasma NfL is associated with markers of neurodegeneration such as white matter injury (Moore et al., 2018) or grey matter atrophy (Idland et al., 2017; Khalil et al., 2018); and (ii) that surgery itself is associated with a rise in NfL (Evered et al., 2018), suggesting it may provoke neuronal injury. Next, we tested our primary hypothesis, that postoperative delirium, identified at least once on postoperative Days 1–4, is associated with a greater rise in NfL on postoperative Day 1 than in participants who did not incur delirium. Causality for any relationship would be supported by dose-dependence; hence, we tested whether NfL levels correlate with peak delirium severity. Given that inflammation is considered the primary precipitant for postoperative delirium (van Gool et al., 2010; Sanders, 2011; Cunningham and Maclullich, 2013), we tested whether neuronal injury (as evidenced by an increase in NfL) contributes to the pathogenesis of delirium independent of inflammation.
Materials and methods
The data are derived from two ongoing prospective perioperative cohort studies registered with ClinicalTrials.gov (NCT03124303, NCT02926417) and approved by the University of Wisconsin-Madison Institutional Review Board (2015-0374, 2015-0960). In brief, 114 adult patients were recruited who were scheduled for major elective non-intracranial, non-cardiac surgery (defined as requiring at least a 2-day hospital stay; see Supplementary Fig. 1 for STROBE diagram and Supplementary Table 1 for inclusion and exclusion criteria).
Participants had blood draws preoperatively and on each of the first (up to) four postoperative Days the patient was in hospital, resulting in 487 blood samples. Preoperatively and twice daily, postoperatively participants underwent delirium assessments with the Confusion Assessment Method (CAM)/3D-CAM, or the CAM-ICU if the patient was intubated. Delirium severity was assessed with the validated Delirium Rating Scale-98 (DRS). No patient was intubated for more than 96 h postoperatively allowing collection of at least one DRS measure per participant without intubation.
Plasma samples were collected in EDTA-containing tubes preoperatively, and in the morning (06.00–10.00) of each postoperative day, stored at −80°C, and sent for cytokine multiplex assay (Eve technology, Canada). The cytokines are listed in Table 1. In addition, samples were sent to the University of Gothenburg for analysis of NfL, using a single-molecule array method, as previously described in detail (Gisslen et al., 2016). The measurements were performed by board-certified laboratory technicians in one round of experiments using one batch of reagents. Intra-assay coefficients of variation were 5.1% for a quality control sample with an NfL concentration of 10.9 pg/ml and 9.6% for a quality control sample with a concentration of 150 pg/ml. The lower limit of quantification was 6.7 pg/ml.
Table 1.
Associations between each analyte and peak DRS scores and postoperative change in NfL
| Association with peak DRS | Association with rise in NfL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | ΔR2 | t-value | DF | P-value | Bonferroni | ΔR2 | t-value | DF | P-value | Bonferroni |
| IL-1B | 0.060 | 2.508 | 89 | 0.014 | 0.153 | 0.061 | 2.942 | 85 | 0.004 | 0.042 |
| IL-1ra | 0.024 | 0.888 | 89 | 0.377 | 1.000 | 0.057 | 1.876 | 85 | 0.064 | 0.640 |
| IL-2 | 0.014 | 0.925 | 89 | 0.357 | 1.000 | 0.060 | 2.379 | 85 | 0.020 | 0.196 |
| IL-4 | 0.014 | 1.051 | 89 | 0.296 | 1.000 | 0.022 | 1.277 | 85 | 0.205 | 1.000 |
| IL-6 | 0.008 | 0.723 | 89 | 0.471 | 1.000 | 0.027 | 1.642 | 85 | 0.104 | 1.000 |
| IL-8 | 0.208 | 4.422 | 89 | <0.001 | <0.001 | 0.222 | 5.427 | 85 | <0.001 | <0.001 |
| IL-10 | 0.126 | 2.071 | 89 | 0.041 | 0.454 | 0.163 | 3.501 | 85 | 0.001 | 0.007 |
| IL-12p70 | 0.002 | 0.276 | 89 | 0.783 | 1.000 | 0.034 | 0.986 | 85 | 0.327 | 1.000 |
| MCP-1 | 0.069 | 2.529 | 89 | 0.013 | 0.145 | 0.063 | 2.264 | 85 | 0.026 | 0.261 |
| TNFa | <0.001 | 0.172 | 89 | 0.864 | 1.000 | 0.037 | 1.543 | 85 | 0.127 | 1.000 |
| NfL | 0.199 | 3.408 | 85 | 0.001 | 0.011 | - | - | - | - | - |
Peak DRS or rise in NfL were regressed on each analyte, individually, while statistically controlling for age and sex. Effect sizes were quantified as ΔR2, i.e. the proportion of the total variance in the outcome explained by this predictor.
Measured values below the range of detection were imputed as 0.001 for cytokines (n = 78/4870 cytokine assays) and 6.7 pg/ml for NfL (n = 3/487 assays) based on the lower limit of quantification. Data were then log-transformed to correct the strong rightward-skew and examined for multivariate outliers using the robust Mahalanobis distance (Garrett, 1989). Nine samples were excluded from analysis because of extreme deviation of Mahalanobis distance values from the expected chi-squared distribution. Six patients were excluded (one experienced alcohol withdrawal delirium, and others had cancelled or aborted surgery). After outlier exclusion, 460 samples remained from 108 participants, 93 of whom had both preoperative and postoperative Day 1 blood samples necessary to analyse the perioperative change in NfL.
Imaging
Of the patients recruited to IPOD-B3, 52 patients consented to preoperative imaging. Six scans were discarded because of motion artefact, resulting in preoperative scans from 46 patients (nine delirious). MRI data were collected on a 3.0 T whole-body scanner (General Electric Medical Systems) with an 8-channel head coil. White matter injury was quantified with diffusion tensor imaging (DTI, scan parameters in the Supplementary material). The DTI data were corrected for distortions induced by eddy currents and head movements (Andersson and Sotiropoulos, 2016), skull-stripped to remove background noise and non-tissue components and voxel-wise diffusion tensor model was fit using FSL (Smith et al., 2004; Jenkinson et al., 2012), yielding scalar maps for fractional anisotropy, axial diffusivity, mean diffusivity and radial diffusivity. It is worth noting that these metrics may not uniformally change with age in all brain regions (Kumar et al., 2013), though changes are often associated with neurodegenerative disease (Alexander et al., 2007). All scalar maps were transformed to a population-matched template space (Schwarz et al., 2017), which is optimal for non-linear registration (Avants et al., 2009). Voxel-wise skeletonized white matter tract maps were obtained for fractional anisotropy, axial diffusivity, mean diffusivity and radial diffusivity with tract-based spatial statistics (TBSS) (Smith et al., 2006). Additional details may be found in the Supplementary material.
Grey matter atrophy was characterized based on high resolution T1-weighted scans, and T2-fluid attenuated inversion recovery (FLAIR) (Supplementary material). Cortical thickness (mm) measures were obtained using FreeSurfer v6.0 (https://surfer.nmr.mgh.harvard.edu/). All scans were processed using T1+T2-FLAIR multimodal recon-all processing stream, which includes motion correction, skull-stripping, registration, segmentation, smoothing, and parcellation mapping. Slices were manually inspected for segmentation errors and artefacts. Hippocampal volumes were defined based on the automated labelling procedure outlined by Fischl and extracted using the asegstats2table tool (Fischl et al., 2002). Left and right hippocampal volumes were averaged per participant.
Statistical analysis
The sample size was determined based on a prior study that showed a rise in postoperative NfL levels (Evered et al., 2018). Assuming that delirium harboured the burden of the NfL increase from preoperative levels, 90 patients would provide 80% power (alpha 5%) to show a difference in NfL levels of 15 pg/ml (standard deviation 24 pg/ml) assuming a delirium incidence of 33%. The significance level for all tests was set at P < 0.05 with Bonferroni correction for multiple comparisons where appropriate. Linear regression was conducted in R with age and sex as covariates. In the case of hippocampal volume, estimated intracranial volume was used in place of sex. Mean differences are reported as mean difference [95% confidence interval (CI)]. For DTI data, voxel-wise statistical approach [randomise tool with threshold-free cluster enhancement (Smith and Nichols, 2009)] was adopted to study the association with NfL (log10) on a group level. Positive and negative contrasts were examined with age and sex as covariates for 10 000 permutations with familywise error (FWE) rate controlled for multiple comparisons.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Patient demographics
The delirium incidence was 39/108 (36.1%) and the mean peak DRS was 21.2 for delirious subjects and 6.8 for non-delirious subjects. No significant differences were identified in delirious versus non-delirious participants in age (69.5 versus 72.0 years old, t = 1.654, P = 0.104), or sex (proportion female: 46.2% versus 39.1%, χ2 = 0.506, P = 0.477). However, delirious participants underwent more vascular surgery (64.1% versus 37.7%, χ2 = 6.98, P = 0.008), and generally underwent higher risk surgery (National Surgical Quality Improvement Program Risk of Death; 5% versus 2%, P = 0.010; see Supplementary Table 2 for demographic information).
Neurofilament light as a marker of neurodegeneration
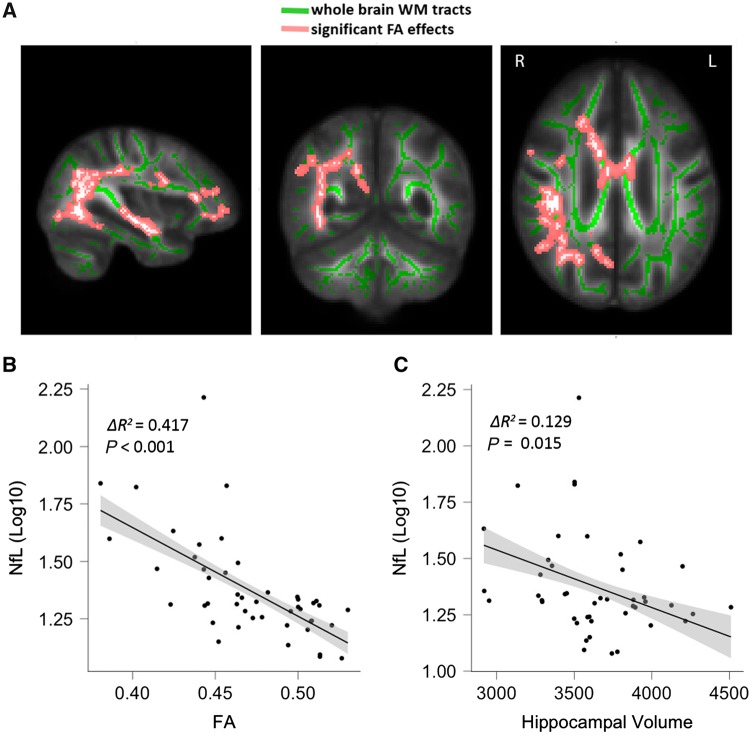
Initially, we confirmed that preoperative NfL was associated with preoperative markers of neurodegeneration in our cohort. Of the 102 subjects with baseline plasma samples, 46 had usable imaging data acquired. The median number of days between the blood draw and MRI was 2 (interquartile range 0–7 days). NfL was associated with altered white matter integrity in all four DTI measures. While reduced fractional anisotropy was associated with elevated NfL levels (voxel-wise statistics: voxels 9263, t = 4.66, FWE P = 0.045; mean statistics: ΔR2 = 0.417, P < 0.001), a proportional relationship was found between each of the diffusivity measures, i.e. axial diffusivity (voxel-wise statistics: voxels 1532, t = 4.50, FWE P = 0.027; mean statistics: ΔR2 = 0.333, P < 0.001), mean diffusivity (voxel-wise statistics: voxels 2968, t = 5.19, FWE P = 0.032; mean statistics: ΔR2 = 0.395, P < 0.001), radial diffusivity (voxel-wise statistics: voxels 10776, t = 7.03, FWE P = 0.032; mean statistics: ΔR2 = 0.488, P < 0.001) and NfL (Fig. 1A and B, Supplementary Figs 2–4 and Supplementary Table 3). Common to all measures, white matter neurodegeneration extended on the right-sided internal capsule, superior and posterior corona radiata, external capsule and superior longitudinal fasciculus. Similarly, reduced hippocampal volume correlated with increased NfL (ΔR2 = 0.129, t = 2.53, P = 0.015, Fig. 1C).
Figure 1.
Associations of NfL with imaging markers of neurodegeneration. (A) T-map showing the anatomical location of changes in fractional anisotropy (FA) that correlate with NfL. (B) Raw data for correlation of fractional anisotropy and NfL. Fractional anisotropy values were averaged over all significant voxels. (C) Association of hippocampal volume and NfL. WM = white matter.
Neurofilament light rises after surgery
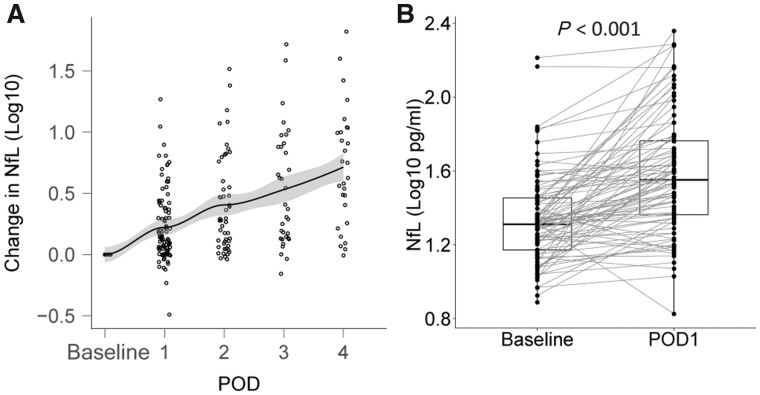
Across all patients, NfL levels rose postoperatively and tended to gradually increase with time (Fig. 2A). This continued postoperative rise may be due to on-going neuronal injury, slow clearance of NfL or perhaps selection bias (as patients with more minor operations were discharged earlier than those with more severe operations). For the latter reason, we concentrated on the postoperative Day 1 data for the primary analyses (93 patients with paired preoperative–postoperative Day 1 samples). On postoperative Day 1, NfL was higher than preoperative levels [mean difference (95% CI) = 0.240 (0.178, 0.301) log10(pg/ml), P < 0.001 Fig. 2B]. As an exploratory analysis we performed regression for perioperative risk factors for a rise in NfL on postoperative Day 1, finding associations with intraoperative measures of surgical severity, operative time and blood loss (Supplementary Table 4).
Figure 2.
The postoperative change in NfL. (A) Time course of NfL change from baseline levels over first 4 days after surgery. (B) Change in NFL from baseline to postoperative Day 1 (POD1).
Primary outcome: neurofilament light is associated with delirium
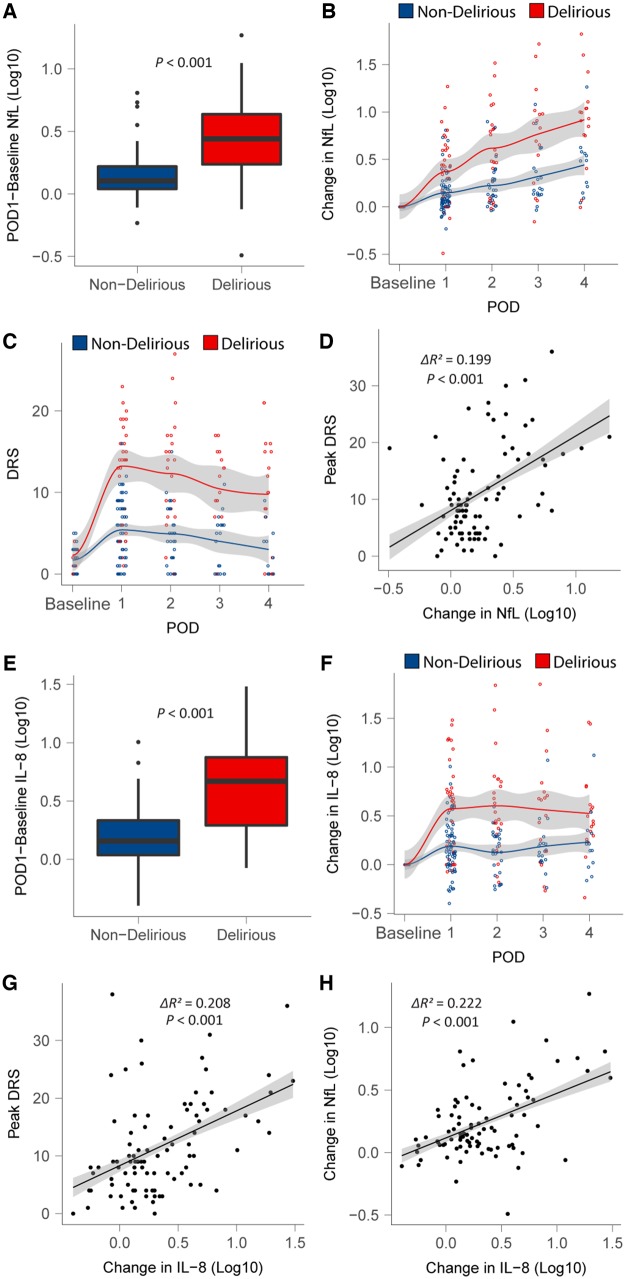
Next, we tested our primary hypothesis that postoperative Day 1 NfL would be higher in participants who ever incurred delirium than non-delirious controls. While there were no significant differences in NfL levels between the groups preoperatively (data not shown, P > 0.05), postoperative Day 1 NfL levels rose in both groups; however, the rise was more profound in the delirious group (t = 4.32, P < 0.001, Fig. 3A and B, see Supplementary Fig. 5 for individual subject changes). Similar rises were evident in subjects who were delirious on Day 1 or who became delirious later (Supplementary Fig. 6). Next, we assessed the presence of dose dependence in this group difference by correlating NfL with delirium severity. Delirium severity typically peaked on postoperative Day 1 (Fig. 3C) and NfL correlated with delirium severity (peak DRS, ΔR2 = 0.199, t = 4.65, P < 0.001, Fig. 3D). Sensitivity analyses excluding subjects from IPOD-B2 or controlling for postoperative sedation did not affect the results (data not shown).
Figure 3.
Relationship of NFL and IL-8 to delirium. (A and E) Difference in NfL/IL-8 from baseline to postoperative Day 1 (POD1) by delirium outcome. (B and F) Time course of NfL/IL-8 change from baseline levels over first 4 days after surgery by delirium outcome. (C) DRS scores over time divided by delirium status. (D and G) Correlation between change in NfL/IL-8 from baseline to POD1 and peak DRS scores. (H) Correlation between the change in values from baseline to POD1 for NFL and IL-8 following surgery.
Relationship to inflammation
To identify any relationship to inflammation we undertook two correlational approaches: correlations of inflammatory cytokines with (i) delirium severity; and (ii) with NfL adjusting for multiple comparisons using the Bonferroni correction (Table 1). After Bonferroni correction, our results showed strong correlations between postoperative Day 1 rises in both IL-8 and NfL and delirium severity (Table 1, P < 0.001, Bonferroni adjusted P = 0.011, respectively). IL-1β and IL-10 correlated with NfL but not delirium severity. Similar to NfL, IL-8 rose to higher levels in the delirious group (Fig. 3E–G) and were driven by similar perioperative factors (Supplementary Table 5). IL-8 and NfL were also strongly correlated (Fig. 3H and Table 1). Given this strong correlation we tested whether preoperative IL-8 may also be a marker of neurodegeneration (as it shared significant signal with NfL); however, preoperative IL-8 was not associated with hippocampal volume (Supplementary Fig. 7) or DTI metrics (P > 0.05, data not shown) in our dataset, confirming that IL-8 is not a marker of neurodegeneration.
Given the strong correlation between delirium severity and IL-8 and the known role of inflammation in postoperative delirium, we undertook linear regression to identify if NfL may contribute to delirium pathogenesis independent of inflammation. Trail Making Test B (Lindroth et al., 2019) and operative time were associated with delirium severity in a regression model of clinical predictors (Supplementary Table 6). To understand the mechanism through which surgery precipitates delirium, while controlling confounders, we included age, sex, NfL, IL-8 and Trail Making Test B in a regression model designed to identify whether NfL contributes to delirium severity independently of IL-8. Intraoperative factors have also been associated with delirium (Rudolph and Marcantonio, 2011), consistent with IL-8 and NfL being mediators of the surgery-induced delirium, operative time is colinear with both IL-8 and NfL (Supplementary Tables 4 and 5). Hence as operative time is the clinical predictor, and IL-8 and NfL are the putative mechanisms, with the added issue of statistical collinearity of these predictors, operative time was not included in the model. We identified that change in NfL is associated with delirium severity, independent of changes in inflammatory burden (ΔR2 = 0.040, t = 2.11, P = 0.038; see Supplementary Table 7 for full regression results). This suggests that neuronal injury may exert a small, independent effect in the pathogenesis of delirium.
Discussion
Our data provide preliminary evidence that postoperative delirium is associated with neuronal injury. NfL, IL-8 and delirium severity were all correlated with each other, suggesting ‘dose-dependence’ and increasing the plausibility for causal relationships between these variables. Despite these strong correlations, both change in NfL and change in IL-8 independently contributed to the pathogenesis of delirium that appeared to be driven by surgical severity in this cohort. While we recommend our results are confirmed in future studies, our data increase plausibility that delirium may accelerate cognitive decline (Fong et al., 2009; Pandharipande et al., 2013; Inouye et al., 2016) through neuronal injury.
Our study does have some limitations. First, it is a relatively small study that should be verified in future biomarker cohorts. Second, while we have conducted focused hypothesis-driven tests, with rigorous adjustment for multiple comparisons where appropriate (Bonferroni) and adjustment for an important precipitant for postoperative delirium, inflammation, we are not able to exhaustively adjust for all possible confounders in our analyses (e.g. blood electrolyte levels). This is a limitation of all observational research but nonetheless emphasizes that our data are merely suggestive of, and do not show, a causal relationship. While our study fulfils many of the Bradford-Hill criteria for causality including a biological gradient with delirium severity, temporality and biological plausibility, reproducibility of our findings will be critical. Nonetheless, to truly establish causality, experimental studies will be required. Third, the correlation of IL-8 and NfL raises the possibility that systemic inflammation may play a role in the neuronal injury associated with NfL rise, as suggested by both animal (Skelly et al., 2019) and clinical (Davis et al., 2014) studies. IL-8 has been associated with delirium previously (Hall et al., 2018) and may act as marker of innate immune activation leading to invasion of the brain with myeloid cells (Degos et al., 2013). Though the collinearity of IL-8 and NfL likely also reflects a common driver, surgical severity, which may lead to delirium through independent inflammatory and neuronal injury effects. Whether NfL rises in a cohort without such inflammatory injury should be tested in a large cohort of delirium with mixed aetiology. One prediction could be that delirium associated with other aetiologies, such as medications, may be less neurotoxic and therefore less likely to lead to cognitive decline. It is also of interest that NfL continued to rise postoperatively in our cohort, while delirium severity (and IL-8) appeared to plateau. To understand this phenomenon, a greater understanding of NfL biology would help, notably whether the incremental rise in NfL is due to on-going neuronal injury or slow clearance from plasma. Fourth, we were unable to show that preoperative NfL was associated with delirium, despite association with MRI markers of neurodegeneration. Given that imaging markers of neurodegeneration have been associated with delirium in prior studies (Cavallari et al., 2016; Shioiri et al., 2016), it is unclear whether we were underpowered to detect the effect or if NfL does not predict subsequent delirium. Therefore, NfL may serve as a mechanistic marker but may not have clinical utility as a predictive variable. However, we stress this was a secondary outcome of our study and we are embarking on a larger study to test any predictive association.
Despite these important limitations, our study has many strengths including rigorous delirium assessments (without missing data for delirium), contemporaneous collection of biomarkers (covering both preoperative and postoperative phases of care) and robust statistical approaches. Furthermore, we verified that NfL could detect features of preoperative neurodegeneration in our relatively homogenous population of elderly patients scheduled for major surgery. It is also important to note that we tested a large battery of inflammatory markers in parallel, and confirmed that IL-8 was not a marker of neurodegeneration. This study provides important proof of concept data suggesting that delirium is associated with neuronal injury. To contextualize our findings, the absolute rise in NfL in delirious subjects is typically greater than rises associated with concussion (Shahim et al., 2018) and in some cases were similar to subjects with stroke (Tiedt et al., 2018). We recommend that a large observational cohort or registry of studies that covers a mixed aetiology of delirium is conducted to (i) confirm these observations; (ii) identify the relative role of neuronal injury in inflammation-driven and other aetiology driven delirium; and (iii) to link to long-term cognitive outcomes.
Conclusions
Herein, we show that delirium is associated with increases in NfL, a putative neuronal injury biomarker. The change is dose-dependent supporting (but not proving) a causal relationship. We propose that these findings be confirmed in a larger prospective cohort of mixed aetiology delirium that also investigates associations with long-term changes in cognition.
Funding
R.D.S. is supported by NIH K23 AG055700 and R01 AG063849-01. H.L. is supported by NHBLI 5T32HL091816-07.
Competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no competing interests that may be relevant to the submitted work. H.Z. has served at scientific advisory boards for Roche Diagnostics, Wave, Samumed and CogRx, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg (all unrelated to the submitted work). K.B. has served as a consultant or at advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper.
Supplementary Material
Glossary
Abbreviations
- DRS =
Delirium Rating Scale-98
- NfL =
neurofilament light
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4: 316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight j 2009; 2: 1–35. [Google Scholar]
- Cavallari M, Dai W, Guttmann CR, Meier DS, Ngo LH, Hshieh TT, et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 2013; 28: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DH, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry 2014; 23: 403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 2013; 118: 527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Delirium Association, American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014; 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN studies. JAMA Neurol 2018; 75: 542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009; 72: 1570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett RG. The chi-square plot: a tool for multivariate outlier recognition. J Geochem Explor 1989; 32: 319–41. [Google Scholar]
- Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas NB, Blennow K, Idland AV, Wyller TB, Raeder J, Frihagen F, et al. Neurofilament light in serum and cerebrospinal fluid of hip fracture patients with delirium. Dement Geriatr Cogn Disord 2018; 46: 346–57. [DOI] [PubMed] [Google Scholar]
- Hall RJ, Watne LO, Cunningham E, Zetterberg H, Shenkin SD, Wyller TB, et al. CSF biomarkers in delirium: a systematic review. Int J Geriatr Psychiatry 2018; 33: 1479–500. [DOI] [PubMed] [Google Scholar]
- Idland AV, Sala-Llonch R, Borza T, Watne LO, Wyller TB, Braekhus A, et al. CSF neurofilament light levels predict hippocampal atrophy in cognitively healthy older adults. Neurobiol Aging 2017; 49: 138–44. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer's Dement 2016; 12: 766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Khalil M, Teunissen CE, Otto M,, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14: 577–89. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM. Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Res 2013; 1512: 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth H, Bratzke L, Twadell S, Rowley P, Kildow J, Danner M, et al. Predicting postoperative delirium severity in older adults: the role of surgical risk and executive function. Int J Geriatr Psychiatry 2019; 34: 1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EE, Hohman TJ, Badami FS, Pechman KR, Osborn KE, Acosta LMY, et al. Neurofilament relates to white matter microstructure in older adults. Neurobiol Aging 2018; 70: 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth 2014; 112: 440–51. [DOI] [PubMed] [Google Scholar]
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses 2011; 77: 140–3. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ 2011; 343: d4331. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ, et al. The Mayo Clinic adult life span template: better quantification across the life span. Alzheimer's Dement 2017; 13: P93–4. [Google Scholar]
- Shahim P, Tegner Y, Marklund N, Blennow K, Zetterberg H. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 2018; 90: e1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioiri A, Kurumaji A, Takeuchi T, Nemoto K, Arai H, Nishikawa T. A Decrease in the volume of gray matter as a risk factor for postoperative delirium revealed by an atlas-based method. Am J Geriatr Psychiatry 2016; 24: 528–36. [DOI] [PubMed] [Google Scholar]
- Skelly DT, Griffin EW, Murray CL, Harney S, O'Boyle C, Hennessy E, et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry 2019; 24: 1533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M,, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–19. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011; 112: 1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology 2018; 91: e1338–47. [DOI] [PubMed] [Google Scholar]
- van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010; 375: 773–5. [DOI] [PubMed] [Google Scholar]
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010; 304: 443–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.