Whether haematological traits contribute equally to all ischaemic stroke subtypes is unclear. By analysing summary statistics from genome-wide association studies of haematological traits and MEGASTROKE, Harshfield et al. show that genetically altered levels of several coagulation factors are associated with ischaemic, cardioembolic, and large-artery stroke, but not small-vessel stroke.
Keywords: Mendelian randomization, haematology, clotting cascade, stroke, genetics
Abstract
Thrombosis and platelet activation play a central role in stroke pathogenesis, and antiplatelet and anticoagulant therapies are central to stroke prevention. However, whether haematological traits contribute equally to all ischaemic stroke subtypes is uncertain. Furthermore, identification of associations with new traits may offer novel treatment opportunities. The aim of this research was to ascertain causal relationships between a wide range of haematological traits and ischaemic stroke and its subtypes. We obtained summary statistics from 27 published genome-wide association studies of haematological traits involving over 375 000 individuals, and genetic associations with stroke from the MEGASTROKE Consortium (n = 67 000 stroke cases). Using two-sample Mendelian randomization we analysed the association of genetically elevated levels of 36 blood cell traits (platelets, mature/immature red cells, and myeloid/lymphoid/compound white cells) and 49 haemostasis traits (including clotting cascade factors and markers of platelet function) with risk of developing ischaemic (AIS), cardioembolic (CES), large artery (LAS), and small vessel stroke (SVS). Several factors on the intrinsic clotting pathway were significantly associated (P < 3.85 × 10−4) with CES and LAS, but not with SVS (e.g. reduced factor VIII activity with AIS/CES/LAS; raised factor VIII antigen with AIS/CES; and increased factor XI activity with AIS/CES). On the common pathway, increased gamma (γ′) fibrinogen was significantly associated with AIS/CES. Furthermore, elevated plateletcrit was significantly associated with AIS/CES, eosinophil percentage of white cells with LAS, and thrombin-activatable fibrinolysis inhibitor activation peptide antigen with AIS. We also conducted a follow-up analysis in UK Biobank, which showed that amongst individuals with atrial fibrillation, those with genetically lower levels of factor XI are at reduced risk of AIS compared to those with normal levels of factor XI. These results implicate components of the intrinsic and common pathways of the clotting cascade, as well as several other haematological traits, in the pathogenesis of CES and possibly LAS, but not SVS. The lack of associations with SVS suggests thrombosis may be less important for this stroke subtype. Plateletcrit and factor XI are potentially tractable new targets for secondary prevention of ischaemic stroke, while factor VIII and γ′ fibrinogen require further population-based studies to ascertain their possible aetiological roles.
Introduction
Stroke is the second leading cause of death and disability-adjusted life years worldwide (GBD, 2015 DALYs and HALE Collaborators, 2015; GBD 2015,Mortality and Causes of Death Collaborators, 2015). Stroke represents a syndrome rather than a single disease, and is caused by a number of distinct pathologies. The majority of strokes are caused by cerebral ischaemia resulting in infarction, the most common subtypes of which are large-artery atherosclerotic stroke (LAS), cardioembolic stroke (CES), and lacunar stroke caused by cerebral small vessel disease (SVS) (Markus, 2016).
Blood cell traits, clotting factors, and platelet activation and aggregation pathways are involved in thrombosis and play a central role in the pathogenesis of stroke (Gregg and Goldschmidt-Clermont, 2003). Antithrombotic therapy with both antiplatelet agents and anticoagulants is widely used in the prevention of stroke (Gregg and Goldschmidt-Clermont, 2003), and genetic loci associated with risk of stroke are significantly enriched in targets for antithrombotics (Malik et al., 2018). However, the mechanisms by which disorders associated with these haematological traits and haemostatic pathways result in ischaemic stroke is not fully understood. This information is critical for informing the development of new therapeutic targets for stroke prevention and treatment.
An important question regarding the optimization of secondary prevention treatment is whether thrombosis is an important disease mechanism in the major ischaemic stroke subtypes. Thrombosis and subsequent embolism play a key role in the pathogenesis of both CES and LAS, although trial data demonstrate that anticoagulants are more effective for CES and antiplatelet agents for LAS, suggesting the underlying thrombotic processes differ (Kapil et al., 2017). Whether thrombosis plays an important role in SVS is unknown.
It has been suggested that activation of the coagulation system is a consequence of stroke itself (Brouns and De Deyn, 2009), and therefore determining whether altered coagulation is the cause or consequence of stroke is challenging. One way of overcoming this limitation is the use of Mendelian randomization; this technique is increasingly used to infer causality between exposure and disease through the use of genetic variants associated with the exposure as instrumental variables (Burgess et al., 2018). The use of genetic variants, which are randomly allocated during meiosis, avoids reverse causality and minimizes confounding by environmental factors in a manner analogous to a randomized controlled trial (Burgess et al., 2012).
Recent studies have provided large resources to examine the causal role of haematological traits in ischaemic stroke and its subtypes. The recent MEGASTROKE Consortium provided genome-wide association results from over 67 000 stroke cases and 450 000 controls (Malik et al., 2018). Summary statistics from genome-wide association studies (GWAS) of numerous haematological traits, which in this paper refers to both blood cells (e.g. platelet count) and haemostasis parameters (e.g. factor VIII activity), are also available (Supplementary Table 1). We used two-sample Mendelian randomization, which combines published evidence from different data sources to obtain an estimate of the causal effect (Burgess et al., 2015), to perform a comprehensive analysis of whether perturbations in haematological traits are implicated in stroke risk, and specifically whether certain stroke subtypes cluster with particular haematological traits.
Materials and methods
Data sources
Genetic associations with haematological traits
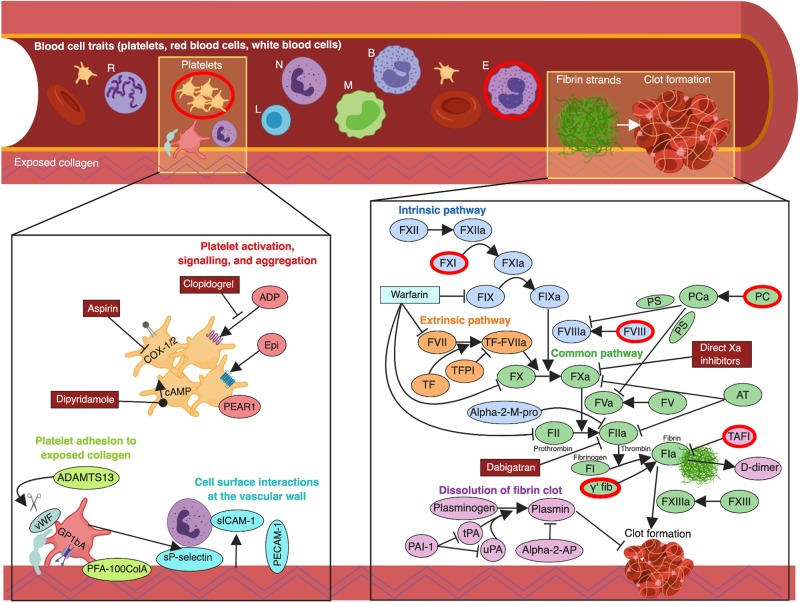
We performed analyses using both blood cell traits (derived from a routine full blood count analysis), and haemostasis traits relating to the coagulation cascade, fibrinolysis, platelet function, and cell surface interactions at the vascular wall. An overview of the relationship between haematological traits and the various biological pathways involved is shown in Fig. 1.
Figure 1.
Overview of haematological pathways and targets for stroke treatment and prevention. arrow = agonize; B = basophils; E = eosinophils; L = lymphocytes; M = monocytes; N = neutrophils; perpendicular symbol = antagonize; R = reticulocytes; scissors = proteolytic cleavage. Traits significantly associated with one or more stroke subtypes are shown with a red border. Created with BioRender (https://biorender.com/).
We obtained genetic association estimates for blood cell traits from a recent publication on the largest GWAS of blood cell traits published to date, which involved 36 blood cell traits measured in 173 480 participants of European ancestry from the UK Biobank and INTERVAL studies (Astle et al., 2016). The traits were classified under six categories: (i) platelets; (ii) mature red cells; (ii) immature red cells; (iv) myeloid white cells; (v) lymphoid white cells; and (vi) compound white cells.
To identify traits relevant to haemostasis we conducted a systematic review of the literature in PubMed (last updated 2 February 2019) searching for GWAS of all applicable traits. Details of the search strategy are provided in the Supplementary material (Appendix 1) and Supplementary Fig. 1. In circumstances where GWAS results for the same trait were available from more than one study, we used summary statistics from the study with the largest sample size. The studies for all traits included in the analysis had made available GWAS summary statistics in the public domain. The number of participants and genetic instruments available from each study are summarized in Supplementary Table 1, and details of the assay methods used by each study are provided in Supplementary Table 2. All methods with available summary statistics were included for traits that were assayed using multiple methods (e.g. factor VIII activity and factor VIII antigen). We used the Reactome database (Fabregat et al., 2018) to classify the haemostasis traits according to the following expertly curated pathways: (i) extrinsic pathway (tissue factor activation); (ii) intrinsic pathway (contact activation); (iii) common pathway; (iv) dissolution of fibrin clot; (v) platelet adhesion to exposed collagen; (vi) platelet activation, signalling, and aggregation; and (vii) cell surface interactions at the vascular wall. For traits that were not easily captured in a single pathway we categorized them based on the pathway that we felt most closely reflected the biology of the trait.
Genetic associations with stroke
We obtained genetic associations with five stroke outcomes [all stroke (AS), all ischaemic strokes (AIS), CES, LAS, and SVS] for two ancestral groupings (European-specific and transancestral meta-analysis) from the MEGASTROKE Consortium, which included data from 67 162 cases and 454 450 control subjects (Malik et al., 2018). Stroke cases were defined based on WHO criteria (i.e. sudden onset neurological changes of presumed vascular origin lasting at least 24 h) with stroke subtypes classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (Adams et al., 1993). The available summary statistics from MEGASTROKE included 60 341 cases with any ischaemic stroke regardless of subtype, of which 9006 were CES, 6688 were LAS, and 11 710 were SVS.
Statistical analyses
Mendelian randomization analysis was used to examine whether haematological traits have a causal effect on the development of ischaemic stroke and/or subtypes of stroke. The key assumptions of Mendelian randomization are that the genetic variant(s) used as instrumental variables (i) are associated with the risk factor; (ii) are not associated with any confounders of the risk factor–outcome association; and (iii) are independently distributed from the outcome conditional on the risk factor and any confounders (Burgess et al., 2018). Genetic variants associated with haematological traits were identified as above and effect estimates were obtained for each instrument and harmonized with the outcome data, ensuring that the effect of each single nucleotide polymorphism (SNP) on the exposure and on the outcome corresponded to the same allele. We performed linkage disequilibrium clumping to ensure that the instruments used for each exposure were independent by selecting only the SNP with the lowest P-value amongst all SNPs with a linkage disequilibrium r2 ≥ 0.001. An estimate of the causal effect was assessed using the inverse-variance weighted (IVW) method. To provide more robust estimates and seek to overcome potential pleiotropy, we performed sensitivity analyses using additional Mendelian randomization models, namely Mendelian randomization-Egger regression, the weighted median estimator, and the simple and weighted mode-based estimators, which facilitated comparison of the causal estimates under different assumptions and using different statistical approaches (Burgess et al., 2018). Analyses were performed separately within the European and transancestral populations from MEGASTROKE. We applied a multiple-testing correction for 130 disease pathway comparisons (13 pathways × two populations × five outcomes), which resulted in a significance threshold of P < 3.85 × 10−4.
To assess the potential for latent pleiotropy, we performed bidirectional Mendelian randomization (also known as reverse Mendelian randomization) for the significantly associated haematological traits (Zheng et al., 2017). Although we were not hypothesizing that ischaemic stroke may actually cause changes in levels of haematological traits, these sensitivity analyses allowed us to assess whether there were any additional factors that may be driving the associations. We also performed a phenome-wide association study (PheWAS) using PhenoScanner (Kamat et al., 2019) to explore whether any of the SNPs significantly associated with both haematological traits and risk of stroke were also associated with other relevant risk factors for stroke and related diseases. This could provide evidence of horizontal pleiotropy, an alternative causal pathway from the SNP to the outcome other than via the haematological trait under investigation, which would violate the assumptions of Mendelian randomization (Burgess et al., 2018).
In view of associations we identified for both factors VIII and XI with CES, we used longitudinal data from 502 616 participants from the UK Biobank to determine whether a strongly significant genetic variant associated with these traits altered the risk of future stroke. We estimated hazard ratios with 95% confidence intervals (CIs) using Cox proportional-hazards regression models adjusted for age and sex, comparing the association with ischaemic stroke risk in 3962 individuals with atrial fibrillation, the most frequent cause of CES (Wolf et al., 1991; Marini et al., 2005; Gretarsdottir et al., 2008; Lubitz et al., 2017; Ntaios et al., 2018), to the association in 498 654 control subjects without atrial fibrillation.
Mendelian randomization analyses were performed in R version 3.4.4 (R Core Team, 2018) using the ‘TwoSampleMR’ package version 0.4.21 (Hemani et al., 2018), and Cox proportional-hazards regression estimates were obtained using Stata version 15.1 (StataCorp, 2018). Two-sided P-values and 95% CIs are presented.
Data availability
The GWAS summary statistics used to perform the analyses described in this study were obtained from publicly available published data. All data generated or analysed during this study are included in this published article and its Supplementary material.
Results
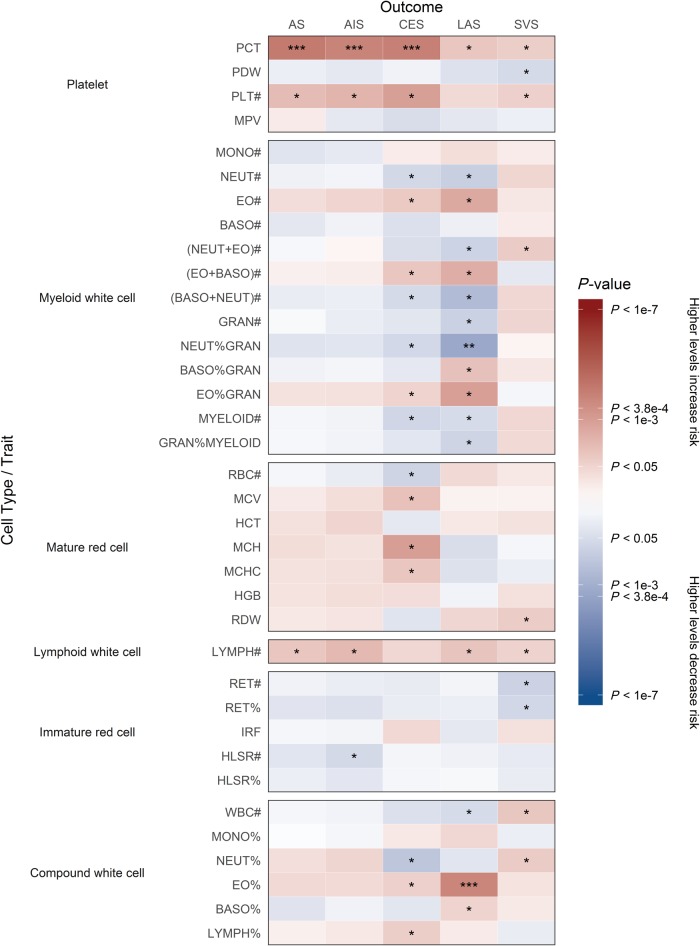
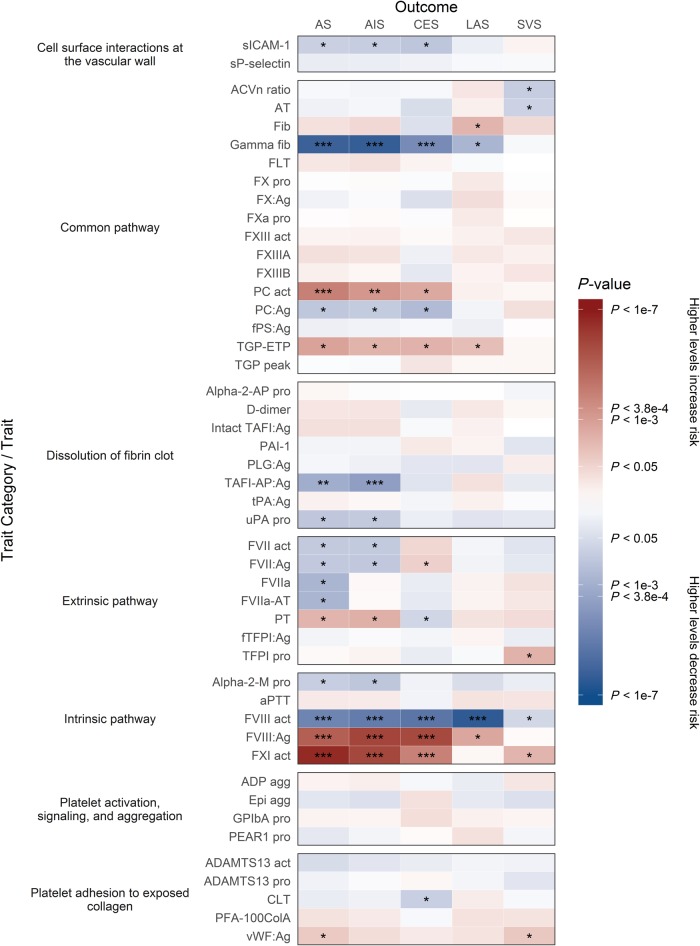
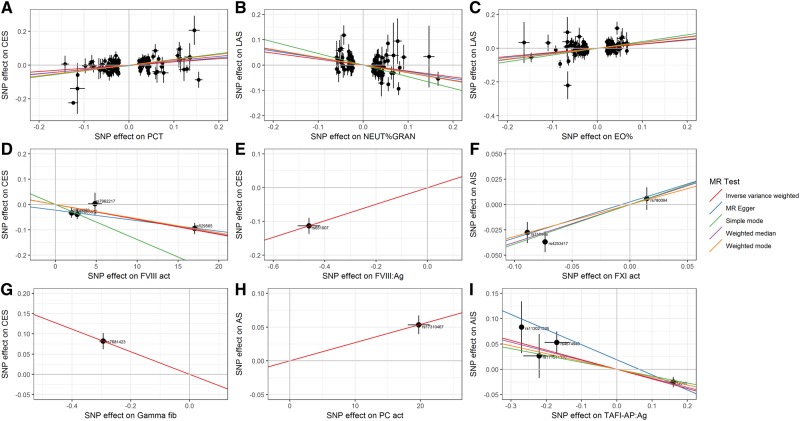
From our systematic literature review of haematological traits we obtained published GWAS summary statistics for 36 blood cell traits and 49 haemostasis traits from 27 publications involving over 375 000 individuals (Supplementary Table 1) (Barbalic et al., 2010; Johnson et al., 2010; Smith et al., 2010, 2011; Athanasiadis et al., 2011; Lovely et al., 2011; Paré et al., 2011; Edelstein et al., 2012; Huang et al., 2012, 2014; Oudot-Mellakh et al., 2012; de la Morena-Barrio et al., 2013; Williams et al., 2013; Ma et al., 2014; Rocanin-Arjo et al., 2014; de Vries et al., 2015, 2016; Tang et al., 2015; Astle et al., 2016; van Loon et al., 2016; Dennis et al., 2017; Pankow et al., 2017; Sennblad et al., 2017; Kanai et al., 2018; Olson et al., 2018; Stanne et al., 2018; Sun et al., 2018). Figures 2 and 3 summarize the direction and magnitude of the association estimates for each haematological trait with each stroke subtype, with stronger associations indicated by darker colours (red in the positive direction and blue in the negative direction) and asterisks to indicate the level of statistical significance. Figure 4 shows scatterplots of the associations of each genetic variant plotted against their association with stroke subtypes for all traits that had significant (P < 3.85 × 10−4) or marginally significant (P < 1 × 10−3) associations: plateletcrit (PCT), neutrophil percentage of granulocytes (NEUT%GRAN), eosinophil percentage of white cells (EO%), factor VIII activity, factor VIII antigen, factor XI activity, gamma (γ′) fibrinogen, protein C (PC) activity, and thrombin-activatable fibrinolysis inhibitor activation peptide antigen (TAFI-AP:Ag). Figure 1 highlights significant associations from the Mendelian randomization analysis, provides a representation of the core components of each of the haematological pathways, and indicates existing drug targets for stroke prevention and treatment.
Figure 2.
Mendelian randomization results showing causal estimates for association of blood cell traits with stroke and its subtypes. Mendelian randomization results are grouped by Reactome pathways according to blood cell traits: (i) platelets; (ii) mature red cells; (iii) immature red cells; (iv) myeloid white cells; (v) lymphoid white cells; and (vi) compound white cells. Refer to Supplementary Table 1 for a description of each trait. Colours show magnitude and direction of P-value of association for estimate of causal effect using inverse-variance weighted Mendelian randomization approach. Asterisks indicate the significance of the P-value for the most significant association (either European or transancestral population): *P < 0.05; **P < 1 × 10−3; ***P < 3.85 × 10−4.
Figure 3.
Mendelian randomization results showing causal estimates for association of haemostasis traits with stroke and its subtypes. Mendelian randomization results are grouped by Reactome pathways according to haemostasis traits: (i) extrinsic pathway of fibrin clot formation (tissue factor activation); (ii) intrinsic pathway of fibrin clot formation (contact activation); (iii) common pathway of fibrin clot formation; (iv) dissolution of fibrin clot; (v) platelet adhesion to exposed collagen; (vi) platelet activation, signalling, and aggregation; and (vii) cell surface interactions at the vascular wall. Refer to Supplementary Table 1 for a description of each trait. Colours show magnitude and direction of P-value of association for estimate of causal effect using inverse-variance weighted Mendelian randomization approach. Asterisks also indicate the significance of the P-value for the most significant association (either European or transancestral population): *P < 0.05; **P < 1 × 10−3; ***P < 3.85 × 10−4.
Figure 4.
Genetic associations with haematological traits and stroke subtypes with significant causal estimates. The associations of each genetic variant associated with haematological traits with significant (P < 3.85 × 10−4) or marginally significant (P < 1 × 10−3) causal estimates are plotted against their association with selected stroke subtypes. Circles represent the associated change in levels of the trait and corresponding increased risk of stroke for each variant. The horizontal and vertical lines through each circle represent the corresponding 95% CIs for the genetic associations. Associations were oriented to the effect allele of each trait. Coloured lines show the slope (causal estimate) of the trait on stroke obtained using a variety of different Mendelian randomization (MR) approaches. Trait/outcome/population: (A) PCT/CES/European; (B) NEUT%GRAN/LAS/transancestral; (C) EO%/LAS/transancestral; (D) factor VIII activity/CES/European; (E) factor VIII antigen/CES/European; (F) factor XI activity/AIS/European; (G) γ′ fibrinogen/CES/transancestral; (H) protein C activity/CES/transancestral population; and (I) TAFI-AP:Ag/AIS/transancestral. See Supplementary Table 1 for a description of each trait.
Blood cell traits
Amongst the 36 blood cell traits, we observed the strongest associations for PCT (the volume fraction of blood occupied by platelets) and EO%. We found that genetically elevated levels of PCT were associated with significantly increased risk of AS, AIS, and CES in both European-only and transancestral populations (Figs 2, 4A and Supplementary Table 3), and increased EO% levels were significantly associated with LAS in both populations (Figs 2 and 4C). Decreased NEUT%GRAN levels were also marginally significantly associated with LAS in both populations (Figs 2 and 4B).
Haemostasis traits
We examined three interconnected pathways of the clotting cascade. On the intrinsic (contact activation) pathway, factors VIII and XI were significantly associated with risk of stroke (Figs 3, 4D–F and Supplementary Table 4). Genetically elevated levels of factor VIII antigen and factor XI activity, and lower levels of factor VIII activity, were strongly associated with increased risk of AS, AIS, and CES, predominantly in both European-only and transancestral populations, and also with LAS in Europeans for factor VIII activity. Nearly all of the traits on the extrinsic (tissue factor activation) pathway had suggestive associations but were not statistically significant (Fig. 3).
On the common pathway, genetically lowered levels of γ′ fibrinogen (which contains the alternately spliced isoform of the fibrinogen gamma chain) were significantly associated with increased risk of AS, AIS, and CES in both populations (Figs 3 and 4G). Additionally, elevated protein C activity levels were significantly associated with increased risk of AS in both populations and marginally associated with AIS in a transancestral population, and there was suggestive evidence that it may also be associated with CES in both populations (Figs 3 and 4H). Elevated levels of total fibrinogen also exhibited suggestive evidence of association with LAS, though in the opposite direction to γ′ fibrinogen.
The remaining pathways that we examined were related to dissolution of fibrin clots (fibrinolysis); platelet adhesion to exposed collagen; platelet activation, signalling, and aggregation; and cell surface interactions at the vascular wall (Fig. 3). Genetically lowered levels of TAFI-AP:Ag, which is involved in the dissolution of fibrin clots, were significantly associated with increased risk of AIS in a transancestral population (Figs 3 and 4I).
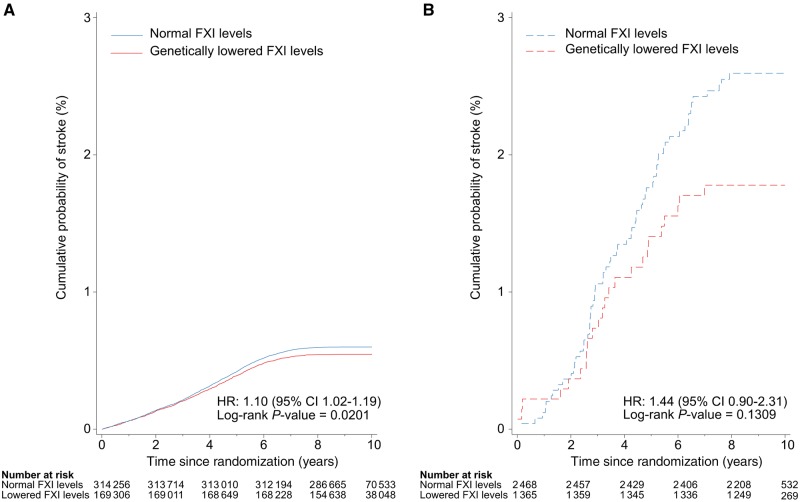
We also examined whether genetic variants significantly associated with factors VIII and XI were associated with risk of future stroke in atrial fibrillation cases and controls within the UK Biobank. rs710446 in the KNG1 locus was selected as it was the SNP most strongly associated with factor XI activity and was also significantly associated with factor VIII activity. The hazard ratios for the association of rs710446 with ischaemic stroke were 1.44 (95% CI 0.90–2.31) in individuals with atrial fibrillation and 1.10 (95% CI 1.02–1.19) in controls without atrial fibrillation (Fig. 5). However, the absolute magnitude of the reduction in cumulative probability of stroke was much greater in individuals with atrial fibrillation, implying that treatments designed to reduce the risk of stroke by targeting factor VIII or factor XI levels are much more likely to be successful in patients with atrial fibrillation.
Figure 5.
Role of genetic variants associated with haematological traits in mediating association of atrial fibrillation with risk of ischaemic stroke. Cumulative probability of ischaemic stroke in individuals with normal and genetically lowered levels of factor XI (FXI) activity (based on rs710446 alleles in KNG1 locus) in UK Biobank participants (A) without atrial fibrillation and (B) with atrial fibrillation.
Bidirectional Mendelian randomization and phenome-wide scan
In the reverse Mendelian randomization analyses, we found that AS and AIS were significantly associated with PCT, EO%, factor VIII activity, factor VIII antigen, factor XI activity, γ′ fibrinogen, and protein C activity. Many of these associations were found in both the European and transancestral populations and were confirmed using multiple Mendelian randomization methods (Supplementary Table 5). Our PheWAS found that the SNPs significantly associated with haematological traits and risk of stroke subtypes were also significantly associated with other relevant risk factors for stroke and related diseases, such as body mass index, systolic blood pressure, total cholesterol, LDL cholesterol, triglycerides, smoking status, coronary artery disease, and Alzheimer’s disease (Supplementary Tables 6 and 7).
Discussion
This study provides a comprehensive description of the relationship between haematological traits and the risk of stroke and its subtypes. It highlights that a number of haematological traits are causal risk factors for stroke, but that these associations differ according to stroke subtype. The findings demonstrate that inter-individual genetic variation affecting several blood cell traits (PCT and EO%), components of the intrinsic (factors VIII and XI) and common (γ′ fibrinogen and PC activity) pathways of the clotting cascade, and a fibrinolysis inhibitor (TAFI-AP:Ag), significantly influence ischaemic stroke risk, particularly CES and (to a lesser extent) LAS, but not SVS.
Our findings have a number of clinical implications. First, they suggest that thrombosis, particularly due to abnormalities in the intrinsic and common pathways of the clotting cascade, plays an important role in the pathogenesis of both CES and LAS, but we found no associations with SVS. Embolism from cardiac pathology causes CES, while embolism from large-artery stenosis is believed to be the major pathogenic mechanism underlying LAS (Markus, 2016). Therefore, it is not surprising that genetic variants that increase thrombosis and thus increase the likelihood of formation of potential emboli are risk factors for both conditions. The role of thrombosis in SVS is much less certain. Case-control studies have suggested that platelet activation and coagulation may be less important for this subtype (Lavallée et al., 2013), while increasing the intensity of antiplatelet therapy has not been shown to result in any further reduction of recurrent stroke in patients with SVS (SPS3 Investigators et al., 2012). Furthermore, a previous candidate gene study showed the ABO locus, found to be associated with increased coagulation, was associated with CES and LAS but not SVS (Williams et al., 2013). Taken together, these data raise the possibility that SVS is not driven by perturbations of platelets or the clotting cascade, implying that therapies designed to acutely dissolve thrombus (thrombolysis) or prevent thrombosis in the longer term (antithrombotic therapy) may not be effective in SVS. A further consideration is that small vessel pathology can cause both ischaemic SVS and deep intracerebral haemorrhage (Markus, 2016), meaning that optimizing the type and intensity of antithrombotic therapy is particularly important in this patient group. These hypotheses now need examining in randomized trials in well-phenotyped cohorts of patients with SVS.
Second, our analysis has identified new potential targets for pharmacological therapy and risk reduction of acute ischaemic stroke, particularly CES. Existing antiplatelet medication used to treat and prevent ischaemic stroke—including aspirin, clopidogrel, and dipyridamole—affect different signalling pathways involved in platelet activation and aggregation, thereby leading to reduced platelet function (Fig. 1). Our results suggest that reduction of PCT should also be considered. PCT is normally tightly controlled by the hormone thrombopoietin (TPO). TPO mimetics are drugs used for the treatment of immune thrombocytopenia, characterized by accelerated platelet clearance from the circulation and low platelet count and PCT (Kuter, 2009; Tang et al., 2017). Therefore, a possible avenue for pharmacological research in acute ischaemic stroke would be the converse, i.e. antagonism of TPO or its haematopoietic stem cell receptor, MPL, in order to reduce PCT levels.
The intrinsic pathway of the clotting cascade is stimulated in vivo by methods including contact with negatively-charged polyphosphates released from activated platelets (Long et al., 2016). It culminates in the coupling together of factor IX with its cofactor factor VIII to activate factor X. The only currently available anticoagulant used in stroke that acts on the intrinsic pathway is warfarin, which diminishes hepatic synthesis of factor IX as well as prothrombin (factor II) and factor X in the common pathway and factor VII in the extrinsic pathway. Disadvantages of warfarin include the inconvenience of regular blood tests for monitoring and annualized risk of major bleeding of ˜3% (Hylek et al., 2014). This makes our finding that higher factor XI activity is significantly associated with an increased risk of AIS, and particularly CES, especially pertinent, as agents that reduce factor XI synthesis in the liver (Zhang et al., 2010) and inhibit the action of factor XI’s activated form (factor XIa) (Lin et al., 2006; Tucker et al., 2009; Donkor et al., 2017; Woodruff et al., 2017) have already been developed. Phase I and II clinical trials of antisense oligonucleotides in several hundred individuals have shown that they reduce factor XI levels, have a major bleeding risk of only 0–1% (Liu et al., 2011), and are superior in reducing radiologically detected asymptomatic deep vein thrombosis (Büller et al., 2015). Our study extends the finding of a recent publication on the association of factor XI levels with AIS and CES in nearly four times as many stroke cases and 14 times as many control subjects (Gill et al., 2018). Taken together with the early phase clinical trial data, there is now strong support for the need for randomized clinical trials of the use of factor XI inhibitors versus standard-of-care in ischaemic stroke, such as the international secondary stroke prevention trial using factor XIa inhibitor BMS-986177 (United States National Institutes of Health, 2019).
Our results indicate that factor VIII may also be a druggable target to consider for the prevention of both CES and LAS. To validate our findings, we demonstrated that the genetic variant most strongly associated with factor VIII activity and also associated with factor XI activity predicted risk of future stroke in patients with atrial fibrillation in the UK Biobank. Notably, the effect estimate was stronger in individuals with atrial fibrillation compared to those without atrial fibrillation, albeit the association in atrial fibrillation cases was not statistically significant, which is likely due to insufficient power. This suggests that drugs designed to inhibit factor XI activity (Bane and Gailani, 2014) or increase factor VIII activity might be effective at reducing the risk of ischaemic stroke in patients with atrial fibrillation, although studies with larger numbers of atrial fibrillation cases and randomized controlled trials would be needed to validate this finding. Additionally, the opposing direction of effect of factor VIII on stroke risk when measured by different assays means that further clarification of this finding in other population-based studies is required.
Through enzymatic cleavage of factor X to its activated form, factor Xa is the start of the common pathway that results in the generation of a fibrin clot. Existing anticoagulants used for secondary stroke prevention that affect this pathway include direct factor Xa and thrombin (factor IIa) inhibitors apixaban, rivaroxaban, and dabigatran (Fig. 1). However, our finding that increased levels of γ′ fibrinogen reduce the risk of AIS and CES would suggest that other distinct molecular pathways could potentially modify ischaemic stroke risk. γ′ fibrinogen results from the alternate splicing of one of the three genes (FGG), that is translated into fibrinogen and normally comprises 7% of circulating fibrinogen (Lovely et al., 2011), and has distinct biochemical properties (Gersh et al., 2009). After an acute ischaemic stroke, increased γ′ fibrinogen levels have been associated with higher scores for short-term disability (van den Herik et al., 2011); however, the raised γ′ fibrinogen may reflect an acute phase response to stroke and it is difficult to ascribe causation (Farrell, 2012). Indeed, given that γ′ fibrinogen demonstrates defective platelet aggregation compared with fibrinogen (Farrell et al., 1992), there is a biologically plausible mechanism by which γ′ fibrinogen is protective against ischaemic stroke that warrants further examination in prospective longitudinal studies.
One of the strengths of our analysis is that we show that several haematological traits are still strongly significant despite correcting for multiple statistical tests across all traits and outcomes. We only corrected for the number of disease–pathway comparisons analysed rather than the total number of unique traits, as this would have been too conservative due to correlations between many highly related traits. Some traits, such as factors X and XIII, were only measured in small studies (e.g. ˜2000 individuals or less), so they were penalized after the correction for multiple testing was applied and limited our power to detect associations with stroke. Likewise, there were approximately six times more cases available for all stroke than for subtypes of ischaemic stroke, so our power to detect statistically significant associations was also more limited for the ischaemic stroke subtypes (CES, LAS, and SVS). Additionally, many of the traits exhibited suggestive evidence (P < 0.05) that genetically altered levels of these traits could lead to increased risk of one or more stroke subtypes, though they did not reach the threshold for statistical significance after correction for multiple testing and therefore could be spurious findings. Further examination of more detailed associations between haematological traits and stroke subtypes than was accomplished in this analysis will only be feasible when larger samples sizes of well-phenotyped stroke cases become available.
Our study also has a few limitations. Our systematic review of GWAS of haematological studies was limited to studies listed in PubMed published in English, so there may be other studies that we missed. Additionally, two-sample Mendelian randomization should ideally be applied using non-overlapping datasets for obtaining genetic associations with the exposure and the outcome to reduce weak instrument bias (Burgess et al., 2018). However, overlap between participants was unavoidable for some traits because the largest available published GWAS of those traits were measured in studies that were also included as part of the MEGASTROKE Consortium, such as the CHARGE Consortium, which comprised 10.7% of the total European stroke cases, and the BioBank Japan Project, which comprised 24.2% of the total non-European stroke cases (Malik et al., 2018). Summary statistics from CHARGE and BioBank Japan were used to obtain genetic associations with the exposure for seven of the 85 traits. Moreover, there was significant heterogeneity amongst the different types of haemostasis traits that may limit their direct comparability. Several traits such as factor VIII antigen and protein C activity were only associated with a single independent SNP, so the causal estimates for these traits are less robust than for other traits that were associated with a large number of independent SNPs. Furthermore, the small number of participants included in some of the studies (e.g. 352 for antithrombin and 116 for PFA-100ColA) means that their genetic associations are less reliable than for the traits that were measured in tens or hundreds of thousands of individuals (e.g. 164 339 for PCT), which in turn limits the reliability of the causal inferences for these traits. Finally, the significant associations detected in the reverse Mendelian randomization analyses and PheWAS suggest that there is a possibility of horizontal pleiotropy if the outcomes were affected through other traits or pathways to those under investigation, so these findings should be interpreted with caution. Nevertheless, despite these limitations, we have applied a rigorous analysis approach to ensure our reported findings are as robust as possible.
In conclusion, we provide the first comprehensive analysis of the role of genetically elevated blood cell and haemostasis traits on risk of stroke. Of the 85 different traits that we analysed, PCT, EO%, factor VIII, factor XI, γ′ fibrinogen, protein C activity, and TAFI-AP:Ag have been identified as novel risk factors for ischaemic stroke, and we provide further support for the role of raised factor XI in its aetiology. Haematological traits also differentially associate with various stroke subtypes, suggesting the role of thrombosis may differ for different stroke subtypes. Methods for reducing PCT and factor XI levels should now be further examined in experimental and clinical research settings as potential new targets for secondary stroke prevention. We also demonstrate that this approach may identify potential drug targets for novel antithrombotic drugs.
Supplementary Material
Acknowledgements
The authors would like to thank William Astle (Medical Research Council Biostatistics Unit, Department of Public Health & Primary Care, University of Cambridge), for his comments on the design and implementation of the analyses. He was not compensated for his work on this study.
Funding
This study was supported by a British Heart Foundation Programme grant (RG/16/4/32218) and the European Union’s Horizon 2020 research and innovation programme under grant agreement No 667375 (CoSTREAM). It also received infrastructural support from the UK National Institute for Health Research (Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust)a. This research was conducted using the UK Biobank resource (application 36509). The MEGASTROKE project received funding from sources specified at http://megastroke.org/acknowledgements.html (a list of members and affiliations is provided in Supplementary material, Appendix 2). M.C.S. is supported by a UK Medical Research Council Clinical Research Training Fellowship (MR/R002363/1). H.S.M. and W.H.O. are supported by the National Institute for Health Researcha (Senior Investigator Awards). W.H.O. also receives research support from the European Commission, the UK Medical Research Council, and NHS Blood and Transplanta. aThe views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Competing interests
The authors report no competing interests.
Glossary
Abbreviations
- AIS =
all ischaemic stroke
- AS =
all stroke
- CES =
cardioembolic stroke
- EO% =
eosinophil percentage of white cells
- GWAS =
genome-wide association study
- LAS =
large artery stroke
- PCT =
plateletcrit
- SNP =
single nucleotide polymorphism
- SVS =
small vessel stroke
- TAFI-AP:Ag =
thrombin-activatable fibrinolysis inhibitor activation peptide antigen
References
- Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016; 167: 1415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis G, Buil A, Souto JC, Borrell M, López S, Martinez-Perez A, et al. A genome-wide association study of the protein C anticoagulant pathway. PLoS One 2011; 6: e29168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bane CE, Gailani D. Factor XI as a target for antithrombotic therapy. Drug Discov Today 2014; 19: 1454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet 2010; 19: 1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 2009; 111: 483–95. [DOI] [PubMed] [Google Scholar]
- Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015; 372: 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ 2012; 345: e7325. [DOI] [PubMed] [Google Scholar]
- Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genomics Hum Genet 2018; 19: 303–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium EPICI. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 2015; 30: 543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J, Medina-Rivera A, Truong V, Antounians L, Zwingerman N, Carrasco G, et al. Leveraging cell type specific regulatory regions to detect SNPs associated with tissue factor pathway inhibitor plasma levels. Genet Epidemiol 2017; 41: 455–66. [DOI] [PubMed] [Google Scholar]
- de la Morena-Barrio ME, Buil A, Antón AI, Martínez-Martínez I, Miñano A, Gutiérrez-Gallego R, et al. Identification of antithrombin-modulating genes. Role of LARGE, a gene encoding a bifunctional glycosyltransferase, in the secretion of proteins? PLoS One 2013; 8: e64998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PS, Boender J, Sonneveld MAH, Rivadeneira F, Ikram MA, Rottensteiner H, et al. Genetic variants in the ADAMTS13 and SUPT3H genes are associated with ADAMTS13 activity. Blood 2015; 125: 3949–55. [DOI] [PubMed] [Google Scholar]
- de Vries PS, Chasman DI, Sabater-Lleal M, Chen M-H, Huffman JE, Steri M, et al. A meta-analysis of 120 246 individuals identifies 18 new loci for fibrinogen concentration. Hum Mol Genet 2016; 25: 358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor DA, Bhakta V, Eltringham-Smith LJ, Stafford AR, Weitz JI, Sheffield WP. Selection and characterization of a DNA aptamer inhibiting coagulation factor XIa. Sci Rep 2017; 7: 2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein LC, Luna EJ, Gibson IB, Bray M, Jin Y, Kondkar A, et al. Human genome-wide association and mouse knockout approaches identify platelet supervillin as an inhibitor of thrombus formation under shear stress. Circulation 2012; 125: 2762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, et al. The reactome pathway knowledgebase. Nucleic Acids Res 2018; 46: D649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DH. γ′ Fibrinogen as a novel marker of thrombotic disease. Clin Chem Lab Med 2012; 50: 1903–9. [DOI] [PubMed] [Google Scholar]
- Farrell DH, Thiagarajan P, Chung DW, Davie EW. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci USA 1992; 89: 10729–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1603–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh KC, Nagaswami C, Weisel JW, Lord ST. The presence of γ′ chain impairs fibrin polymerization. Thromb Res 2009; 124: 356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D, Georgakis MK, Laffan M, Sabater-Lleal M, Malik R, Tzoulaki I, et al. Genetically determined FXI (factor XI) levels and risk of stroke. Stroke 2018; 49: 2761–3. [DOI] [PubMed] [Google Scholar]
- Gregg D, Goldschmidt-Clermont PJ. Platelets and cardiovascular disease. Circulation 2003; 108: e88–90. [DOI] [PubMed] [Google Scholar]
- Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol 2008; 64: 402–9. [DOI] [PubMed] [Google Scholar]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018; 7: e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Huffman JE, Yamakuchi M, Trompet S, Asselbergs FW, Sabater-Lleal M, et al. Genome-wide association study for circulating tissue plasminogen activator levels and functional follow-up implicates endothelial STXBP5 and STX2. Arterioscler Thromb Vasc Biol 2014; 34: 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sabater-Lleal M, Asselbergs FW, Tregouet D, Shin S-Y, Ding J, et al. Genome-wide association study for circulating levels of PAI-1 provides novel insights into its regulation. Blood 2012; 120: 4873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol 2014; 63: 2141–7. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Yanek LR, Chen MH, Faraday N, Larson MG, Tofler G, et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet 2010; 42: 608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations [Internet]. Bioinformatics. 2019. Available from: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Akiyama M, Takahashi A, Matoba N, Momozawa Y, Ikeda M, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet 2018; 50: 390–400. [DOI] [PubMed] [Google Scholar]
- Kapil N, Datta YH, Alakbarova N, Bershad E, Selim M, Liebeskind DS, et al. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin Appl Thromb 2017; 23: 301–18. [DOI] [PubMed] [Google Scholar]
- Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med 2009; 60: 193–206. [DOI] [PubMed] [Google Scholar]
- Lavallée PC, Labreuche J, Faille D, Huisse M-G, Nicaise-Roland P, Dehoux M, et al. Circulating markers of endothelial dysfunction and platelet activation in patients with severe symptomatic cerebral small vessel disease. Cerebrovasc Dis 2013; 36: 131–8. [DOI] [PubMed] [Google Scholar]
- Lin J, Deng H, Jin L, Pandey P, Quinn J, Cantin S, et al. Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem 2006; 49: 7781–91. [DOI] [PubMed] [Google Scholar]
- Liu Q, Bethune C, Dessouki E, Grundy J, Monia BP, Bhanot S. ISIS-FXIRx, a novel and specific antisense inhibitor of factor XI, caused significant reduction in FXI antigen and activity and increased aPTT without causing bleeding in healthy volunteers. Blood 2011; 118: 209. [Google Scholar]
- Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 2016; 14: 427–37. [DOI] [PubMed] [Google Scholar]
- Lovely RS, Yang Q, Massaro JM, Wang J, D’Agostino RB, O’Donnell CJ, et al. Assessment of genetic determinants of the association of γ′ fibrinogen in relation to cardiovascular disease. Arterioscler Thromb Vasc Biol 2011; 31: 2345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz SA, Parsons OE, Anderson CD, Benjamin EJ, Malik R, Weng L-C, et al. Atrial fibrillation genetic risk and ischemic stroke mechanisms. Stroke 2017; 48: 1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ozel AB, Ramdas S, McGee B, Khoriaty R, Siemieniak D, et al. Genetic variants in PLG, LPA, and SIGLEC 14 as well as smoking contribute to plasma plasminogen levels. Blood 2014; 124: 3155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018; 50: 524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Stroke 2005; 36: 1115–9. [DOI] [PubMed] [Google Scholar]
- Markus H. Stroke: causes and clinical features. Medicine 2016; 44: 515–20. [Google Scholar]
- Ntaios G, Sagris D, Gioulekas F, Galanis P, Pardali C, Vemmou A, et al. 20-year trends of characteristics and outcomes of stroke patients with atrial fibrillation. Int J Stroke 2018; 13: 707–16. [DOI] [PubMed] [Google Scholar]
- Olson NC, Raffield LM, Lange LA, Lange EM, Longstreth WT, Chauhan G, et al. Associations of activated coagulation factor VII and factor VIIa-antithrombin levels with genome-wide polymorphisms and cardiovascular disease risk. J Thromb Haemost 2018; 16: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudot-Mellakh T, Cohen W, Germain M, Saut N, Kallel C, Zelenika D, et al. Genome wide association study for plasma levels of natural anticoagulant inhibitors and protein C anticoagulant pathway: the MARTHA project. Br J Haematol 2012; 157: 230–9. [DOI] [PubMed] [Google Scholar]
- Pankow JS, Tang W, Pankratz N, Guan W, Weng L-C, Cushman M, et al. Identification of genetic variants linking protein C and lipoprotein metabolism: the ARIC study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol 2017; 37: 589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré G, Ridker PM, Rose L, Barbalic M, Dupuis J, Dehghan A, et al. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet 2011; 7: e1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocanin-Arjo A, Cohen W, Carcaillon L, Frére C, Saut N, Letenneur L, et al. A meta-analysis of genome-wide association studies identifies ORM1 as a novel gene controlling thrombin generation potential. Blood 2014; 123: 777–85. [DOI] [PubMed] [Google Scholar]
- Sennblad B, Basu S, Mazur J, Suchon P, Martinez-Perez A, van Hylckama Vlieg A, et al. Genome-wide association study with additional genetic and post-transcriptional analyses reveals novel regulators of plasma factor XI levels. Hum Mol Genet 2017; 26: 637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NL, Chen M-HH, Dehghan A, Strachan DP, Basu S, Soranzo N, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation 2010; 121: 1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NL, Huffman JE, Strachan DP, Huang J, Dehghan A, Trompet S, et al. Genetic predictors of fibrin D-dimer levels in healthy adults. Circulation 2011; 123: 1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators SPS3, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012; 367: 817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanne T, Olsson M, Lorentzen E, Pedersen A, Gummesson A, Gils A, et al. A genome-wide study of common and rare genetic variants associated with circulating thrombin activatable fibrinolysis inhibitor. Thromb Haemost 2018; 118: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature 2018; 558: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Cushman M, Green D, Rich SS, Lange LA, Yang Q, et al. Gene-centric approach identifies new and known loci for FVIII activity and VWF antigen levels in European Americans and African Americans. Am J Hematol 2015; 90: 534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-T, He P, Li Y-Z, Chen H-Z, Chang X-L, Xie Q-D, et al. Diagnostic value of platelet indices and bone marrow megakaryocytic parameters in immune thrombocytopenic purpura. Blood Coagul Fibrinolysis 2017; 28: 83–90. [DOI] [PubMed] [Google Scholar]
- Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJT, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood 2009; 113: 936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States National Institutes of Health. Oral factor XIa inhibitor for the prevention of new ischemic stroke in patients receiving aspirin and clopidogrel following acute ischemic stroke or transient ischemic attack (TIA) [Internet]. 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03766581.
- van den Herik E, Cheung E, de Lau L, den Hertog H, Leebeek F, Dippel D, et al. γ’/total fibrinogen ratio is associated with short-term outcome in ischaemic stroke. Thromb Haemost 2011; 105: 430–4. [DOI] [PubMed] [Google Scholar]
- van Loon J, Dehghan A, Weihong T, Trompet S, McArdle WL, Asselbergs FFW, et al. Genome-wide association studies identify genetic loci for low von Willebrand factor levels. Eur J Hum Genet 2016; 24: 1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams FMK, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the ABO locus: the EuroCLOT study. Ann Neurol 2013; 73: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–8. [DOI] [PubMed] [Google Scholar]
- Woodruff RS, Ivanov I, Verhamme IM, Sun M-F, Gailani D, Sullenger BA. Generation and characterization of aptamers targeting factor XIa. Thromb Res 2017; 156: 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Löwenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood 2010; 116: 4684–92. [DOI] [PubMed] [Google Scholar]
- Zheng J, Baird D, Borges M-C, Bowden J, Hemani G, Haycock P, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep 2017; 4: 330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GWAS summary statistics used to perform the analyses described in this study were obtained from publicly available published data. All data generated or analysed during this study are included in this published article and its Supplementary material.