Abstract
Antibiotics have revolutionized the treatment of bacterial infections. However, it is widely held that there is underinvestment in antibiotics research and development relative to the socially optimal level for a number of reasons. In this article, we discuss whether existing health technology assessment procedures recognize the full economic and societal value of new antibiotics to patients and society when making reimbursement decisions. We present three recommendations for modelling the unique attributes of value that are specific to novel antibiotics. We find, based on a review of the literature, that some of the value elements proposed by our framework have previously been discussed qualitatively by health technology assessment bodies when evaluating antibiotics, but are not yet formally captured via modelling. We present a worked example to show how it may be possible to capture these dimensions of value in a more quantitative manner. We conclude by answering the question of the title as follows: the unique attributes of novel antibiotics should be considered in reimbursement decision making, in a way that captures the full range of benefits these important technologies bring to patients, health care systems, and society.
Keywords: antibiotics, antibiotic resistance, health technology assessment, reimbursement
Antibiotics have changed the way we treat bacterial infections and transformed medicine. Like other biomedical technologies, antibiotics are subject to health technology assessment (HTA) procedures to evaluate the clinical, cost-effectiveness, safety, legal, and ethical implications.1–3 HTA is widely used to support pricing and reimbursement decisions and the development of guidelines about appropriate use.4–7 The unique challenges associated with demonstrating the value of novel antibiotics has been well articulated by Schaffer et al.8 In this article, we offer one approach to capture some of the unique elements of value quantitatively, particularly in systems that employ a cost-effectiveness analysis approach to evaluate new technologies for reimbursement.
It has been argued that the market for antibiotics is subject to market failure, with the result that pharmaceutical companies are underinvesting in antibiotic research relative to the socially optimal level.9–11 This market failure arises from the presence of significant externalities, both positive and negative, which arise from the transmission of infection and the possibility of the emergence and spread of resistant microorganisms. There is gathering global momentum to put in place incentive mechanisms to facilitate the discovery of new antibiotics.
A critical question is, “What is the price worth paying for a novel antibiotic that can treat drug resistant infections?” In particular, we argue that the ability to reduce transmission rates in the general population (transmission value), and the potential to curb resistance through a reduction in selection pressure (diversity value), are important elements to consider in the assessment of the full benefit that antibiotics offer to patients, health care systems, and society. To do would provide an accurate valuation of these technologies based on sound economic theory, and ensure appropriate supply-side incentives.
Framework for Health Technology Assessment of Antibiotics
According to the World Health Organization,12“Health technology assessment (HTA) refers to the systematic evaluation of properties, effects, and/or impacts of health technology. It is a multidisciplinary process to evaluate the social, economic, organisational and ethical issues of a health intervention or health technology.” Yet, conducting HTA for antibiotics and other antimicrobials is challenging because of the externalities associated with antibiotic use.13,14 One aim of this article is to show how to draw on background economic theory to arrive at a practical assessment framework.
A standard ratio used to evaluate new medical technologies in health systems where cost-effectiveness is an important consideration for decision making is the incremental cost effectiveness ratio (ICER). NICE15 defines the ICER as “the ratio of the difference in the mean costs of a technology compared with the next best alternative to the differences in the mean outcomes,” (p85) that is to say the ratio of incremental costs to incremental benefits. In the case of noncommunicable diseases, this ICER can be interpreted as shown in Equation (1).
| (1) |
In this ratio, c is the patient-level incremental cost and v is the incremental benefit to patients receiving treatment, normally measured in units such as quality-adjusted life years (QALYs).
Such an interpretation is entirely appropriate for technologies to treat noncommunicable illness. However, in the case of infectious disease there are costs and benefits from the transmission of disease. How should these additional considerations be included in the ICER? The standard guidelines on how to perform cost-effectiveness analyses are surprisingly quiet on this point. Even the IDSI Reference Case,16 which is intended for use in low and middle-income countries, has little to say, despite the much greater disease burden associated with infectious disease in these countries. Certainly, guidelines on economic cost-benefit analysis tend to err on the side of inclusion: for example, the UK Treasury Green Book17 recommends that “the relevant costs and benefits to government and society of all options should be valued. . . . In this context, relevant costs and benefits are those that can be affected by the decision at hand [our italics].” (p19) Yet HTA, where it uses cost-effectiveness analysis, tends to use some form of cost-utility analysis, which often excludes particular costs and particular benefits.
There has been a significant discussion about the theoretic foundations of cost-utility analysis and the reasons why cost-utility analysis excludes particular considerations that would be included (and indeed monetized) in a more comprehensive cost-benefit analysis (for a recent review, see, e.g., Chapter 2 of Neumann et al3). The narrowest interpretation of cost-utility analysis is that analysis should focus on concerns that fall within the mandate of the Minister of Health: thus productivity impacts or impact on income tax receipts are typically excluded in a cost-utility analysis when the decision-maker perspective is taken. However, on this criterion, there seems to be no justification for excluding the wider costs and benefits of using an antibiotic, beyond the patients treated, as long as these costs fall on the health systems, and the benefits are experienced in the form of health by the patient population of the health system. Therefore, we conclude that even on narrowest interpretation of cost-utility analysis, costs and benefits from changes in the transmission pattern should be included in the analysis. Hence, in this article we propose the modified ICER shown in Equation (2) as being more fully conformant with the health economic theoretical base of HTA. The costs and benefits should be understood as being incremental to the current standard of care.
| (2) |
In Equation (2), V is the direct benefit of using the new antibiotic for the population of interest, that is, heuristically, if each of the N people benefit to the tune of v QALYS, then V = Nv. It is important to highlight that assessing the direct benefits of antibiotics may be challenging given the nature of the evidence base for these technologies, particularly in view of the difficulty of conducting superiority studies.8,18,19
Vt is the benefit of reduced transmission of the disease to the rest of the population, in terms of QALYs from avoided infections. Vd is the “diversity value”—the benefit at the population level of protecting the existing portfolio of antibiotics, in terms of QALYs flowing from the avoidance of other resistant infections. C is the total purchase and administration cost of using the antibiotic for the population of interest: heuristically, if N people are treated, then C = Nc. S is the total cost savings (e.g., in avoided treatment and reduced bed-days) for the treated population, and St and Sd are the cost savings from avoided transmission and protection of existing antibiotics, respectively. We assume that appropriate economic discount rates are applied to all terms. Assessing the values of these parameters is not straightforward as it will depend on the state of resistance to all the drugs that may be used to treat the target condition.
The framework that we have presented flows from prior discussions in the literature about the economic aspects of the antibiotic resistance.13,14,20,21 We now discuss the recommendations that our framework implies. These recommendations are the consensus view of the authors, based on review of the literature and exposure to policy dialogue in this area, and reflections on the implications of the framework for practice, informed by the empirical study of current practice reported in the following section.
Recommendation 1. Assessment should, as appropriate, include a sensitivity analysis of the impact of resistance to the new antibiotic, both initially and over time. For example, a simple way to model resistance is by means of an exponential decay rate. In this case, sensitivity analysis could take the form of a one-way parametric sensitivity analysis on the parameter capturing the resistance rate. More indepth research may wish to explore more complex mathematical formulations that take into account complexities around genetic selection, pathogen diversity, fitness costs, and transmission.
Using an antibiotic has both positive and negative externalities.20 The negative externality arises because every time the antibiotic is used, it creates selection pressure for resistant bacteria. The current recommendation goes beyond the standard recommendation to use sensitivity analysis in cost-effectiveness analysis as we are recommending that in the case of antibiotics, sensitivity is reported on the resistance parameter (or model) specifically. The rationale for this recommendation is that the extent of this externality is hard to predict and depends on both the mode and volume of use of the antibiotic: in general antibiotics should be used with care to forestall the emergence of resistance. In the case of broad-spectrum antibiotics, the selection pressure associated with use of an antibiotic can affect both the targeted pathogen and other bacteria and this additional cost may need to be included as an extra term in Equation (2). However, we focus on the case of a novel, narrow-spectrum therapy in this article. The positive externalities we consider under Recommendation 3 below.
Recommendation 2. Analysis should take place at the population level.
There is a strong argument for HTA agencies to consider population-level costs and benefits to account for externalities associated with antibiotic use. To implement this change, the parties conducting the assessment must have the appropriate level of scope. In some countries (e.g., Germany), novel inpatient antibiotics are currently assessed by regional or local payers at the hospital level. Yet savings due to transmission and avoided hospitalization may not be captured if the assessment is not done by an assessment body at the appropriate regional or national level. If local or regional payers are reluctant to withhold access to antibiotics on grounds of a mismatch between prices and the benefits that they see locally, central authorities may wish to meet the costs from central funds (as is the case for vaccination, e.g., in the United Kingdom).
Recommendation 3. In addition to the direct costs and benefits associated with treating one patient with an antibiotic, where relevant, the following benefits should also be taken into account:
3.a. Indirect benefits from avoided onward transmission: These are the benefits that accrue from the prevention of transmission from the infected patient to others. Developing a true dynamic disease model that captures these disease dynamics is a significant undertaking, and for some diseases may not even be possible due to inadequate scientific understanding of transmission dynamics. If a reliable and validated dynamic disease is available, it should of course be used. However, often analysts face a choice between fully incorporating these benefits at considerable time and expense, omitting these indirect benefits from the analysis (which means that the overall benefit assessment will be conservative), or incorporating them in a heuristic way that may be open to challenge. Jit and Brisson22 provide a useful guide to the modelling tradeoffs for such decisions.
3.b. Diversity benefits from the protective effects on existing antibiotics currently in use: An important argument for the introduction of a new antibiotic is that it removes the selection pressure from existing antibiotics that are currently in use.21 However, the science of modelling through the impact of a change in treatment on the resistance profile of competing antibiotics, not to mention the health impacts associated with this change in resistance, is still in its early stages. We consider that the best available approach at this point to assessing the diversity benefit, if such benefits are believed to be significant, is to assemble a panel of experts and conduct a formal expert elicitation exercise. Expert elicitation has been increasingly and widely used in HTA in recent years, in questions for which relevant scientific knowledge exists but there is not yet scientific consensus or compelling empirical evidence.23,24
The main questions of this article are whether such recommendations are currently followed, and whether they are feasible within the constraints of HTA practice.
Survey of Current Practice in the Assessment of Antibiotics
We conducted a review of HTA assessments of antibiotics across the European Union in order to understand how HTA agencies currently assess antibiotics, comparing against the framework outlined in the previous section. Only agencies that published their recommendations in English, German, Spanish, French, and/or Dutch were included. We were able to include in our analysis five nations and eight different agencies (HAS from France, IQWiG and DIMDI from Germany, ZI from Netherlands, AETS from Spain, SMC from Scotland, NICE from England, and AWMSG from Wales).
To establish which reports to evaluate, we examined the list of publications available at the website of each selected agency from 2000 through April 2016, and selected those related to antibiotics that contained complete HTA reports (defined as those ones that included at least a comparative clinical effectiveness, efficacy, safety, and economic assessment of the drug). After the online search, each agency was directly contacted to request additional antibiotic HTA reports. For comparison purposes, the selected antibiotic was required to have gone through the full HTA process in at least two of the selected HTA agencies.
From each selected antibiotic HTA report, we reviewed and looked for mention of the unique characteristics of antimicrobials taken into consideration by each individual agency, particularly relating to the development spread of resistance. In total, these agencies produced 35 antibiotic HTA reports, of which 17 were determined to fulfil our inclusion criteria (4 from HAS, 1 from IQWiG, 2 from ZI, 4 from SMC, 3 from NICE, and 3 from AWMSG).
Based on the aforementioned criteria, the following antibiotics were selected:
Aztreonam lysine (Cayston) 75 mg powder and solvent for nebulizer solution by Gilead Sciences
Ceftaroline fosamil (Zinforo) 600 mg powder for concentration for solution for infusion by AztraZeneca
Colistimethate sodium (Colobreathe) 1,662,500 IU hard capsules, inhalation powder by Forest Laboratories
Fidaxomicin (Dificlir) 200 mg film-coated tablets by Astellas Pharma
Tigecycline (Tygacil) 50 mg vial of powder for intravenous infusion by Wyeth
The results of the review were as follows:
When evaluating ceftaroline fosamil, both SMC and AWMSG made brief comments concerning the development of resistance. HAS specifically had concerns regarding a secondary indication for community-acquired pneumonia (CAP) due to the high risk of developing resistance, the broad spectrum nature, and the availability of narrower-spectrum antibiotics.
HAS was able to evaluate resistance by comparing the percentage of drug-resistant isolates of colistimethate sodium against tobramycin after 0 and 24 weeks of use, but NICE did not mention this point.
Tigecycline was evaluated by SMC and HAS. The latter briefly discussed the necessity of new drugs with new mechanisms of action and that tigecycline will likely provide additional treatment options for managing infectious diseases (potentially indicating awareness of the importance of diversity in protecting against the spread of resistance).
When reviewing fidaxomicin, AWMSG acknowledged the benefit of a new class of antibiotic with a novel mechanism of action, and showed concern for the development of future resistance against this new product. The Dutch ZI and French HAS only made brief comments related to the possibility of developing resistance; in addition, the latter makes reference to the introduction of fidaxomicin as an additional tool in helping reducing the spread of resistant bacteria. NICE (through a NICE-advice report) and SMC did not address any issues relating to transmission or diversity value in their respective reports.
To further provide a qualitative sense of the way in which the components of our framework surface, we focus on the case of fidaxomicin where we found explicit recognition of transmission and diversity value in reports from the AWMSG and HAS:
CHMP [Committee for Medicinal Products for Human Use] also noted that fidaxomicin belongs to a novel antibiotic class, which it considered important from an antibiotic resistance perspective, as it limits the risks for cross-resistance.25
Under satisfactory conditions of use, this proprietary medicinal product may have an impact in terms of reducing the ecological risk linked to the spread of resistant bacteria. [Fidaxomicin] is therefore likely to provide a partial response to a public health need.26
We conclude from this review that there is awareness of the distinctive nature and dimension of value of antibiotics within HTA agencies, and these considerations do surface in discussions about assessment, and may be taken into account qualitatively. However, standard HTA methods do not include the additional sources of value of antibiotics in a systematic way, although the background health economic theory that guides HTA suggests that they should. This challenge is recognized, for example, by the European Commission27 call to “develop new or improved methodological HTA approaches and foster methodological consensus-building.”
Methods
Worked Example: CRAB Monotherapy Treatment
In order to demonstrate how analysis might be conducted in line with the recommendations above, we present a worked example. The model is based on a hypothetical antibiotic described by Spellberg and Rex28 (henceforth, SR), who conducted a cost-effectiveness analysis associated with the introduction of the new antibiotic in the United States. The purpose of this is not to conduct an actual analysis that would support reimbursement decisions about this antibiotic (since it does not, in fact, exist) but to sketch how an antibiotic might be assessed using the ideas of our framework.
The hypothetical SR monotherapy targets carbapenem-resistant Acinetobacter baumannii (CRAB), which is a resilient microorganism, with the ability to survive in the environment for long periods of time by acquiring resistance genes, rendering the infections they cause unable to be treated by certain antibiotics. The carbapenem class of antibiotics are last line drugs that are often used to treat multidrug-resistant infections within hospitals, particularly intensive care units (ICUs). Therefore, CRAB is considered an important infection-causing organism within the ICU setting. For simplicity, we assume a 100% therapy uptake rate.
In order to estimate the benefits associated with this hypothetical monotherapy being adopted in Europe, we adapted the methods used in SR and applied them to the European incidence statistics of CRAB infections. Data from the ECDC (European Centre for Disease Prevention and Control) point prevalence survey29 was used to estimate the incidence of CRAB infections in Europe, by extracting the incidence of healthcare associated infections and applying A. baumannii infection and Carbapenem resistance rates, as presented in Equation (3) and Table 1.
Table 1.
European CRAB Incidence Estimates
| Point Estimate | Source | |
|---|---|---|
| Annual health care–associated infection incidence in Europe | 4,000,000 | ECDC |
| % of Acinetobacter baumannii infections | 2.7% | ECDC |
| Carbapenem resistance rate | 40% | ECDC |
| Annual incidence of CRAB infections in Europe | 43,200 | Estimated |
CRAB, carbapenem-resistant Acinetobacter baumannii; ECDC, European Centre for Disease Prevention and Control.
| (3) |
Our Equation (2) contains terms relating to benefits and savings from both direct treatment and avoided transmission. To assess these benefits, we start with the existing annual incidence of CRAB infections in Europe. We then consider a scenario where the new monotherapy has been in use for some time and therefore annual incidence been reduced by x% due to avoided transmission. In this new steady state, (1−x)% of the current incidence will contribute to direct treatment benefits and x% will contribute to the transmission benefits.
Insights into the value of x can be obtained from dynamic disease modelling. A dynamic disease model for A. baumannii is presented in Doan et al.30 Within the model, 98% of A. baumannii transmission was estimated as environmental, driven by bacterial shedding from individuals both colonized and infected with A. baumannii, as opposed to direct transmission between patients within the ICU. CRAB monotherapy targets the bacterial shedding by removing CRAB from infected patients and therefore reducing the source of environmental bacteria. The bacterial shedding rates estimated by the article give us a basis for estimating the reduced transmission offered by the monotherapy. According to the bacterial shedding rates within this model, infected individuals account for 43% of all bacterial shedding into the environment. Assuming a 100% therapy uptake rate targeting infected individuals, we use a 40% reduction in transmission of overall CRAB infections, after 1 year of CRAB monotherapy being introduced as the primary treatment option for suspected CRAB infections.
In order to estimate the costs, savings, and benefits for the fraction of the population receiving curative treatment, we used the methods of SR directly. The costs for resistant infections were extracted from SR (converting dollars into euros) while the price of a course of the new monotherapy was set at €25,000 (€16,000 more than estimated by SR), in order to provide a “worst case scenario” of the new monotherapy. The life years gained and the quality of those life years for each treated patient were extracted from SR. Key parameters are shown in Table 2, with the lowest and highest estimates for sensitivity analysis purposes where appropriate, as well as the resulting computed European incidence rates. Costs, savings, and benefits are calculated using Equations (4), (5), and (6):
Table 2.
Parameters Used to Estimate Direct Costs and Savings
| Point Estimate | Low | High | Source | |
|---|---|---|---|---|
| Cost of treating resistant case | €14,913 | €1,000 | €25,685 | Adapted from SR |
| Cost of novel therapy | €25,000 | €8,900 | €40,000 | Adapted from SR |
| Reduced transmission rate | 40% | Estimated | ||
| Reduced mortality rate | 10% | SR | ||
| Cost reduction per effective therapy | 50% | SR | ||
| Life-years gained | 8 | 6 | 10 | SR |
| Utility value of quality of life gained | 0.6 | 0.4 | 0.8 | SR |
SR, Spellberg and Rex.28
| (4) |
| (5) |
| (6) |
In order to estimate the savings and benefits accruing from avoided transmission, that is the benefit enjoyed by the fraction the population that does not experience illness as a result of the use of the new monotherapy by other people, we model as shown in Equations (7) and (8). The cost of treating a resistance case in Equation (7) is the cost of treatment with the old technology because, in the counterfactual world in which the new technology does not exist, these patients would be treated with the old technology.
| (7) |
| (8) |
The original CRAB mortality rate was applied since these individuals avoided a CRAB infection altogether. In addition, both the life years gained and quality improvement were increased, with the reasoning of improved life quality following the prevention of an infection, as opposed to recovery following treatment (Table 3).
Table 3.
Parameters Used to Estimate Transmission Costs and Savings
| Point Estimate | Low | High | Source | |
|---|---|---|---|---|
| CRAB incidence | 43,200 | ECDC | ||
| Reduced transmission rate | 40% | Estimated | ||
| Cost of treating resistant case | €14,913 | €1,000 | €25,685 | Adapted from SR |
| CRAB mortality rate | 20% | SR, ICU estimates | ||
| Life-years gained from avoided infection | 12 | SR, ICU estimates | ||
| Utility value of quality of life gained from avoided infection | 0.8 | SR, ICU estimates |
CRAB, carbapenem-resistant Acinetobacter baumannii; ECDC, European Centre for Disease Prevention and Control; ICU, intensive care unit; SR, Spellberg and Rex.28
Since polymyxins are currently used to treat carbapenem-resistant infections, the SR monotherapy would substitute for polymyxins in the treatment of CRAB, and hence the new therapy would reduce the selection pressure on organisms to develop polymyxin resistance. Thus, the new therapy would improve the treatment success rate of infections that are often treated with polymyxins. The diversity savings and benefits in the context of this example relate to the effects of the reduction in polymyxin use and the subsequent reduction in resistance.
The basis for calculation of this benefit is the total European ICU population. We calculate the number of ICU infections resistant to carbapenems (i.e., the number of ICU infections that are likely to be treated with polymyxins) and multiply that population by the estimated average cost savings and QALYs gained from reducing the selection pressure on polymyxins. The equations we use are listed as (9) and (10):
| (9) |
| (10) |
In the absence of a polymyxin prescription rate, the carbapenem prescription and resistance rates are used as a proxy, as polymyxins are likely to be used to treat carbapenem-resistance ICU infections. To estimate the number of European ICU stays, we used figures from a reference concerning the number of ICU beds across a number of European countries31 and applied ICU occupancy rates from another source.32 In the case of a real therapy we would recommend performing a formal expert elicitation to assess the extent of mortality and cost reductions resulting from reduced selection pressure, but as the technology to be evaluated in this case is hypothetical, we asked a clinical expert to provide us with a reasonable range of numbers. Our parameter estimates are shown in Table 4. Note that they are not based on an assumption that the SR monotherapy will eliminate polymyxin-resistance, but that it will reduce the selection pressure on polymyxins such that treatment costs reduce by 5% to 8% and mortality reduces by 2.5% to 3.5%.
Table 4.
Parameters Used to Estimate Diversity Benefits
| Point Estimate | Low | High | Source | |
|---|---|---|---|---|
| Estimated no. of ICU stays | 1,910,975 | Estimated | ||
| Carbapenem prescription rate | 2.5% | 1% | 5.5% | ECDC |
| Carbapenem resistance rate | 40% | ECDC | ||
| Cost of treating resistant case | €14,913 | €1,000 | €25,685 | Adjusted from SR |
| Estimated reduction in costs of treating ICU HAIs | 7% | 5% | 8% | Expert judgement |
| Estimated reduction in mortality of ICUs HAIs | 3% | 2.5% | 3.5% | Expert judgement |
ECDC, European Centre for Disease Prevention and Control; HAI, health-associated infections; ICU, intensive care unit; SR, Spellberg and Rex.28
Author TB is employed by Hoffman-La Roche and his participation in this project is as in-kind contribution to the project by his employer. Other than that, the funding source had no role in the study.
Results
We used the above reasoning to assess direct, transmission and diversity cost, savings, and benefits. Table 5 summarizes these calculated estimates for the parameter ranges given in Tables 2, 3, and 4. High cost and low benefits/savings estimates are pessimistic; low cost and high benefits/savings estimates are optimistic.
Table 5.
Costs, Savings, and Benefits
| Point Estimate | Optimistic | Pessimistic | |
|---|---|---|---|
| Direct cost (C, mEUR) | 648 | 231 | 1,037 |
| Direct savings (S, mEUR) | 193 | 333 | 13 |
| Direct benefits (V) | 12,442 | 20,736 | 6,221 |
| Transmission savings (St, mEUR) | 258 | 444 | 17 |
| Transmission benefits (Vt) | 33,178 | 33,178 | 33,178 |
| Diversity savings (Sd, mEUR) | 20 | 86 | 0 |
| Diversity benefits (Vd) | 2,752 | 11,772 | 459 |
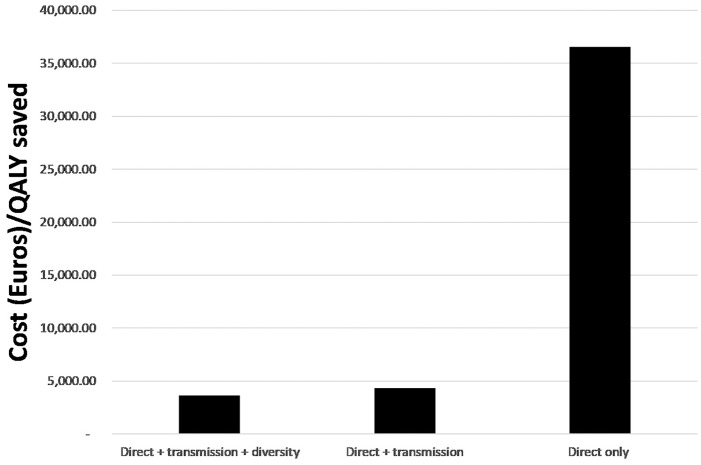
We used these numbers to calculate a cost per QALY saved for the SR monotherapy, which reflects the multiple sources of value as per Recommendation 3. The point estimate is €3661 per QALY. Figure 1 shows ICERs calculated using only the “Direct” only components, “Direct + transmission” components, and “Direct + transmission + diversity” components, highlighting the important of considering the transmission benefits in a comprehensive analysis.
Figure 1.
Incremental cost-effectiveness ratios (ICERs) from considering only the “Direct” only components, “Direct + transmission” components, and “Direct + transmission + diversity” components. QALY, quality-adjusted life year.
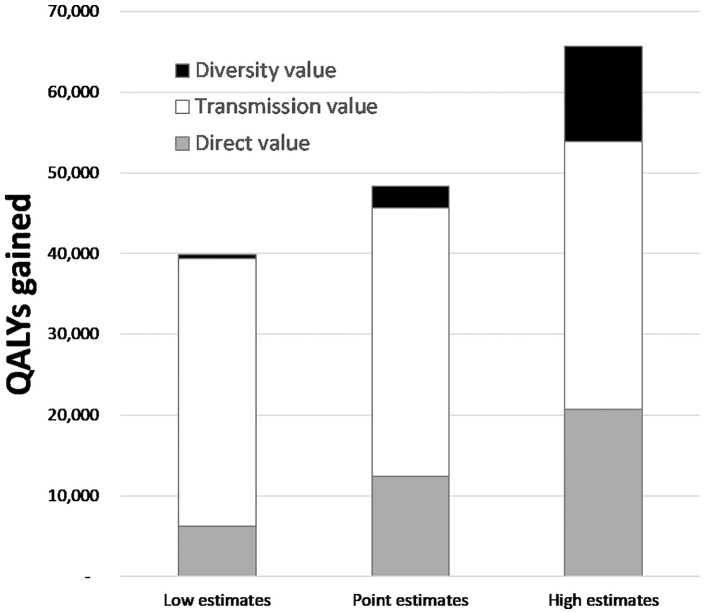
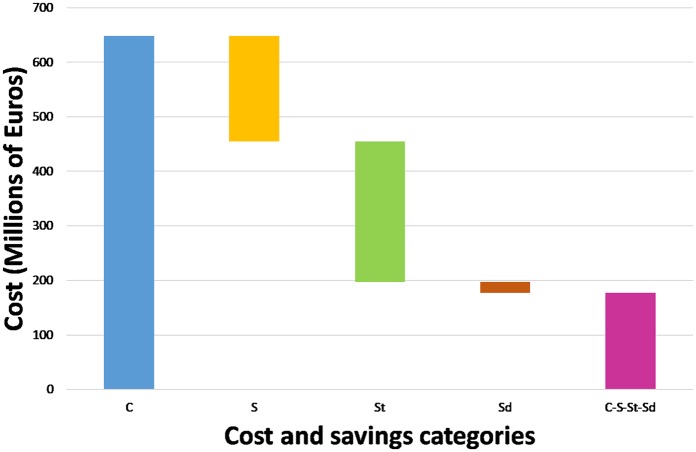
Following Recommendation 2, our estimates are calculated at the population level, and Figures 2 and 3 give insight into the scale and composition of these numbers by showing the breakdown of the benefits and how the different sorts of savings (partially) compensate for the treatment costs. (In Figure 2, the direct component of the value is represented by the gray area, the transmission component by the white area, and the diversity component by the black area of the bar.)
Figure 2.
Breakdown of total benefit by type of value. QALY, quality-adjusted life year.
Figure 3.
How savings from avoided illness might mitigate treatment cost.
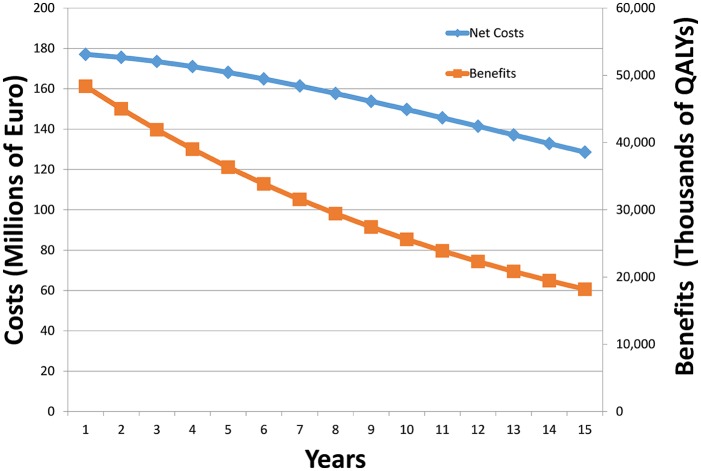
Recommendation 1 is to perform sensitivity analysis to account for resistance. As resistance rates are hard to predict due to fundamental scientific uncertainty, as well as uncertainty about background conditions in the health system, we stress that such sensitivity analysis should not be seen as a forecast, but rather as a “what-if” tool that can be used to sensitize decision makers to possible future experience with this technology. Note that if only direct costs and benefits are considered, increasing resistance will reduce the population treated, but will not necessarily change the cost-effectiveness calculation (as population size appears in the numerator and denominator of the cost-effectiveness ratio and so cancels out). However, if our three categories of costs and benefits are considered, as not all these are directly proportional to population treated, resistance may affect the cost-effectiveness ratio in ways which are hard to predict. A simple way to illustrate this is to reduce each of three categories of costs and benefits by a fixed factor over time (reflecting the decline of the size of the population enjoying the benefit or incurring the cost). To make this point clear, if we apply an annual decay of 5%, 8%, and 3% for direct, transmission, and diversity benefit, respectively, we get the following trajectory for costs and benefits over 15 years as shown in Figure 4. This shows that, given these numbers, the gap between the costs and benefits increases as the years progress (in fact the cost-effectiveness worsens from €3,661 to €7,067 per QALY by year 15).
Figure 4.
Sensitivity analysis to show possible effects of antibiotic resistance over time. QALY, quality-adjusted life year.
In the context of this example, ignoring transmission and diversity effects in the analysis will have a substantial impact on both accept/reject decisions and pricing decisions, and specifically would lead to rejecting or underpricing a welfare-improving technology. Although the analysis is for a notional rather than a real technology, this observation is fully consistent with the qualitative policy discourse in this area, which stresses how ignoring the wider effects of antibiotics has led to chronic underinvestment in this critically important area of technology.
Discussion
This article looks for a middle course between theory-based directives from health economics that are challenging to implement and pragmatic rule-based approaches to evaluating antibiotics that ignore the role of AMR entirely. It is important to realize that all such assessments of antibiotics are conditional on an assumed treatment scenario: more conservative use for example may increase V and Vt in the long run, but may compromise Vd. This underscores that there has to be close coordination between the agency making the reimbursement decision and the agencies responsible for the development of treatment guidelines and for monitoring compliance.
Although the recommendations we proposed in the Framework section of the article are not consistent with current HTA practice, HTA agencies will have to include such considerations if the full value of new antibiotic therapies are to be recognized in decision making. Moreover, taking these considerations into account is logically implied by the background health economic theory, which is supposed to guide and give normative authority to HTA.
It is true that advocates of many other therapeutic areas often present arguments as to why these are also considered unique and, therefore, should be assessed differently by HTA authorities (e.g., orphan drugs, targeted oncology medicines, and agents targeting neurodegenerative diseases). However, as argued in the previous literature, there are sound health economic grounds for considering an expanded concept of value such as the one proposed in this article, and implementing these methodologies is not an insurmountable feat. Accordingly our answer to the question of the title of this article is that HTA agencies should evaluate the unique attributes of novel antibiotics, in a way that takes into consideration the full economic and societal value of these important technologies to patients, health care systems, and society. Otherwise, the value of these essential medicines could be substantially underrecognized, leading to continued market failure, underinvestment, and inadequate innovation to address the problem of rising antimicrobial resistance.
Supplemental Material
Supplemental material, mdmpnp.rjf_online_supp for How Should the Value Attributes of Novel Antibiotics Be Considered in Reimbursement Decision Making? by Alec Morton, Abigail Colson, Axel Leporowski, Anna Trett, Taimur Bhatti and Ramanan Laxminarayan in MDM Policy & Practice
Acknowledgments
The study has received support from the Innovative Medicines Initiative Joint Undertaking under Grant Agreement No. 115618 (Driving re-investment in R&D and responsible antibiotic use, DRIVE-AB; www.drive-ab.eu), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. However, this work does not necessarily represent the view of all DRIVE-AB partners and all opinions and remaining errors are the authors. The authors would like to thank David Findlay, Sumanth Gandra, Timo Goeschl, Ka Lum, Christine Luxemburger, Itamar Megiddo, John Rex, Ursula Theuretzbacher, and Adrian Towse for helpful discussions and comments. We thank the editor and three anonymous reviewers for thoughtful comments which have improved the article.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This project is funded by the Innovative Medicines Initiative, which is a joint initiative of the European Commission and EFPIA, the European Pharmaceutical Industry Association. TB is an employee of Hoffman-La Roche, a pharmaceutical company that invests in the research and development of antibiotics. AM has received a speakers’ fee from the Office of Health Economics for participation in a workshop on health technology assessment for new antibiotics, sponsored by pharmaceutical companies. AT was previously employed by Astellas Pharma, which produces the antibiotic brand Dificlir, which is discussed in Section 3.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study has received support from the Innovative Medicines Initiative Joint Undertaking under Grant Agreement No. 115618 (Driving re-investment in R&D and responsible antibiotic use, DRIVE-AB; www.drive-ab.eu), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution.
Ethical Approval: The article is conceptual in nature and does not require ethical clearance.
ORCID iD: Alec Morton  https://orcid.org/0000-0003-3803-8517
https://orcid.org/0000-0003-3803-8517
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at http://journals.sagepub.com/home/mpp.
Contributor Information
Alec Morton, University of Strathclyde, Glasgow, UK.
Abigail Colson, University of Strathclyde, Glasgow, UK.
Axel Leporowski, University of Heidelberg, Heidelberg, Germany.
Anna Trett, University of Strathclyde, Glasgow, UK; Center for Disease Dynamics, Economics & Policy, New Delhi, India.
Taimur Bhatti, F. Hoffmann-La Roche Ltd., Pharmaceuticals Division, Basel, Switzerland.
Ramanan Laxminarayan, University of Strathclyde, Glasgow, UK; Center for Disease Dynamics, Economics & Policy, New Delhi, India; Princeton Environmental Institute, Princeton University, Princeton, New Jersey.
References
- 1. Banta D, Kristensen FB, Jonsson E. A history of health technology assessment at the European level. Int J Technol Assess Health Care. 2009;25(Suppl. 1):68–73. [DOI] [PubMed] [Google Scholar]
- 2. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 3. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. 2nd ed. Oxford: Oxford University Press; 2017. [Google Scholar]
- 4. Sorenson C, Drummond M, Kanavos P. Ensuring Value for Money in Health Care: The Role of Health Technology Assessment in the European Union. Observatory Studies Series, No. 11. Copenhagen: World Health Organization Regional Office for Europe; 2008. [Google Scholar]
- 5. Glassman A, Chalkidou K. Priority-Setting in Health: Building Institutions for Smarter Public Spending. Washington: Center for Global Development; 2012. [Google Scholar]
- 6. Chalkidou K, Glassman A, Marten R, et al. Priority-setting for achieving universal health coverage. Bull World Health Organ. 2016;94(6):462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glassman A, Giedion U, Smith PC. What’s In, What’s Out: Designing Benefits for Universal Health Coverage. Washington: Brookings Institution Press; 2017. [Google Scholar]
- 8. Schaffer SK, West P, Towse A, et al. Assessing the Value of New Antibiotics: Additional Elements of Value for Health Technology Assessment Decisions. London: Office of Health Economics; 2017. [Google Scholar]
- 9. Morel CM, Mossialos E. Stoking the antibiotic pipeline. BMJ. 2010;340:c2115. [DOI] [PubMed] [Google Scholar]
- 10. Sertkaya A, Eyraud JT, Birkenbach A, et al. Analytical framework for examining the value of antibacterial products. Available from: https://scholarship.law.bu.edu/faculty_scholarship/5/.
- 11. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 12. World Health Organization. Health technology assessment. Available from: http://www.who.int/medical_devices/assessment/en/.
- 13. Coast J, Smith RD, Millar MR. Superbugs: should antimicrobial resistance be included as a cost in economic evaluation? Health Econ. 1996;5(3):217–26. [DOI] [PubMed] [Google Scholar]
- 14. Coast J, Smith R, Karcher AM, Wilton P, Millar M. Superbugs II: how should economic evaluation be conducted for interventions which aim to contain antimicrobial resistance? Health Econ. 2002;11(7):637–47. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]
- 16. Claxton K, Revill P, Sculpher M, Wilkinson T, Cairns J, Briggs A. The Gates Reference Case for Economic Evaluation. Seattle: The Bill and Melinda Gates Foundation; 2014. [Google Scholar]
- 17. HM Treasury. The Green Book: Appraisal and Evaluation in Central Government. London: TSO; 2003. [Google Scholar]
- 18. Boucher HW, Ambrose PG, Chambers HF, et al. White paper: developing antimicrobial drugs for resistant pathogens, narrow-spectrum indications, and unmet needs. J Infect Dis. 2017;216(2):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rex JH, Talbot GH, Goldberger MJ, et al. Progress in the fight against multidrug-resistant bacteria 2005–2016: modern non-inferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis. 2017;65(1):141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laxminarayan R, Brown GM. Economics of antibiotic resistance: a theory of optimal use. J Environ Econ Manage. 2001;42(2):183–206. [Google Scholar]
- 21. Laxminarayan R, Weitzman ML. On the implications of endogenous resistance to medications. J Health Econ. 2002;21(4):709–18. [DOI] [PubMed] [Google Scholar]
- 22. Jit M, Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: a primer. Pharmacoeconomics. 2011;29(5):371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grigore B, Peters J, Hyde C, Stein K. Methods to elicit probability distributions from experts: a systematic review of reported practice in health technology assessment. Pharmacoeconomics. 2013;31(11):991–1003. [DOI] [PubMed] [Google Scholar]
- 24. Soares M, Bojke L. Expert elicitation to inform health technology assessment. In: Dias L, Morton A, Quigley J, eds. Elicitation—The Science and Art of Structuring Judgement. New York: Springer; 2017. p 479–94. [Google Scholar]
- 25. All Wales Medicines Strategy Group. Fidaxomicin (Dificlir®) 200 mg Film-Coated Tablets (AWMSG Secretariat Assessment Report Advice No. 3712). Pernath: All Wales Therapeutic and Toxicology Center; 2012. [Google Scholar]
- 26. HAS Commission de la Transparence. Le projet d’avis adopté par la Commission de la Transparence le 18 juillet 2012 a fait l’objet d’une audition le 17 octobre 2012: DIFICLIR 200 mg, comprimé pelliculé - Boite de 20 comprimés (CIP: 2012;222:376-7), Boite de 100 comprimés (CIP: 2012;582: 403-6). Available from: https://www.has-sante.fr/upload/docs/application/pdf/2012-11/dificlir_17102012_avis_ct_12235.pdf.
- 27. European Commission. A European one health action plan against antimicrobial resistance (AMR). Available from: https://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf.
- 28. Spellberg B, Rex JH. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov. 2013;12(12):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eurostat. Main tables: annual national accounts. Available from: http://ec.europa.eu/eurostat/web/national-accounts/data/main-tables.
- 30. Doan TN, Kong DC, Marshall C, Kirkpatrick CM, McBryde ES. Modeling the impact of interventions against Acinetobacter baumannii transmission in intensive care units. Virulence. 2016;7(2):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murthy S, Wunsch H. Clinical review: international comparisons in critical care—lessons learned. Crit Care. 2012;16(2):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tierney LT, Conroy KM. Optimal occupancy in the ICU: a literature review. Aust Crit Care. 2014;27(2):77–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, mdmpnp.rjf_online_supp for How Should the Value Attributes of Novel Antibiotics Be Considered in Reimbursement Decision Making? by Alec Morton, Abigail Colson, Axel Leporowski, Anna Trett, Taimur Bhatti and Ramanan Laxminarayan in MDM Policy & Practice