Abstract
Background
Low back pain (LBP) continues to be a severe global healthy problem, and a lot of patients would undergo conservative or surgical treatments. However, the improving capacity of spinal load sharing during activities of daily living (ADLs) after interventions is largely unknown. The objective of this study was to quantitatively predict the improvement of spinal musculoskeletal loadings during level walking and stair climbing after two simulated interventions.
Material and Methods
Twenty-six healthy adults and seven lumbar disc herniation patients performed level walking and stair climbing in sequence. The spinal movement was recorded using a motion capture system. The experimental data were applied to drive a musculoskeletal model to calculate all the lumbar joint resultant forces and muscle activities of seventeen main trunk muscle groups. Rehabilitation and reconstruction were selected as the representative of conservative and surgical treatment, respectively. The spinal load sharing after rehabilitation and reconstruction was predicted by replacing the patients' spine rhythm with healthy subjects' spine rhythm and altering the center of rotation at the L5S1 level, respectively.
Results
During both level walking and stair climbing, the joint resultant forces of the lower lumbar intervertebral discs were predicted to reduce after the two simulated inventions. In addition, the maximum muscle activities of the most trunk muscle groups decreased after simulated rehabilitation and conversely increased after simulated reconstruction.
Conclusion
The predictions revealed the different compensatory responses on the spinal load sharing after two simulated interventions, severing as guidance for making preoperative planning and rehabilitation planning.
1. Introduction
According to the report in the literature, low back pain (LBP) continues to be one of the most serious global health problems [1] and causes tremendous direct and indirect economic costs [2–4]. One of the explanations for LBP is disc prolapse inducing nerve root compression. In most cases, the herniation could recover naturally [5], but there are still 5% to 10% of patients with disc herniation who would undergo surgery [6].
Spinal reconstruction has emerged as an effective method to restore the mechanical stability and prevent further pathological development. However, the center of rotation (COR) [7–9], which is a measure of spinal motion quality, will be altered by spinal reconstruction. It has been reported that the alternation of lumbar COR could cause considerable changes in muscle forces using a musculoskeletal model [10]. In addition, the finite element analysis has also shown that the facet forces, ligament loads, and disc stresses are strongly correlated with the location of COR [11]. In an in vitro study, it was found that the higher position of COR correlated with the lower facet force [12]. However, most relevant studies only focused on the single spinal functional unit (FSU) such as L5S1 [13, 14]. Additionally, the musculoskeletal model, the finite element model, and cadaveric lumbar spine were usually driven by some fixed load conditions or default constant spine rhythm. To the authors' knowledge, none of the previous studies have reported the effect of different COR locations on the load sharing of the five FSUs' joint resultant forces and multiple trunk muscle groups' activities during level walking and stair climbing.
According to Panjabi's theory [15], the stabilizing system of spine included two subsystems: (1) the spinal column and (2) the spinal muscles. Spinal reconstruction could directly change the motion characteristics of the spinal column. The strength or activation pattern of spinal muscles could be improved by effective rehabilitation. It has been found that rehabilitation for LBP patients would lead to greater improvement in the flexion relaxation response of back muscles [16] and change the muscle onsets of the lumbar erector spinae [17]. In addition, muscle activation to improve trunk stability by rehabilitation was highly related with rehabilitation strategies [17–21]. Since the origin and insertion of every muscle fascicle were around the spinal column, the resultant muscle activity was strongly associated with the spine rhythm [22]. The spine rhythm was denoted by each lumbar segmental motion contribution to the total lumbar motion. Both reconstruction and rehabilitation could improve the spinal load sharing. However, it is not clear which treatment is better to improve the spinal load sharing and to what extent the two kinds of treatment could improve the spinal load sharing.
Therefore, the objective of this study was to quantitatively predict the improvement of joint resultant forces of five lumbar intervertebral discs and muscle activities of seventeen main trunk muscle groups during level walking and stair climbing after simulated rehabilitation and simulated reconstruction for LBP caused by lumbar disc herniation (LDH). In this study, the recovery to healthy people's spine rhythm was considered as the ideal result of rehabilitation. Therefore, rehabilitation was simulated by replacing the patients' spine rhythm by healthy people's spine rhythm. The spinal reconstruction was simulated by changing the position of COR in the musculoskeletal model. Thus, we have the following two hypotheses:
The spine rhythm in healthy people would be better for spinal load sharing than that in LDH patients
The redistribution of lumbar joint resultant forces and main muscle groups' activities after simulated reconstruction was different from that after simulated rehabilitation
2. Methods
2.1. Subject
Twenty-six healthy male adults (age: mean 23.6 years (SD 1.92 years), height: mean 169.9 cm (SD 5.9 cm), weight: 63.5 kg (SD 8.4 kg)) and seven male LDH patients (age: mean 28.7 years (SD 4.5 years), height: mean 170.1 cm (SD 3.4 cm), weight: mean 67.4 kg (SD 5.3 kg)) were recruited for this study. The healthy controls were included if they were reported no visible motor dysfunction, no back pain, no surgery in recent one year, and no intense exercise 24 hours before trial. The patient groups were included if: (1) they had the ability to conduct basic activities of daily living such as level walking; (2) they were suffering lumbar disc herniation; (3) the herniation occurred in the lower lumbar region; and (4) the symptom had reached the criteria for surgery. In this study, the disc herniation was found to happen at the L4L5 level in three-seventh cases, at the L5S1 level in another three-seventh cases, and at both L4L5 and L5S1 levels in one-seventh cases. This study was approved by the department of orthopedics of Shenzhen Second People's Hospital in China. All the participants were given informed consent before trial.
2.2. Experimental Protocol
In this study, the spinal and pelvic movements were captured by placing the optical markers on the bony landmarks. The bony landmarks included the spinous processes of the third and seventh thoracic vertebra (T3 and T7) and of the first, third, and fifth lumbar vertebra (L1, L3, and L5) and left and right posterior superior iliac spine (LPSIS and RPSIS) and the iliac crest (IC) [23, 24]. Before trials, one surgeon helped to find these landmarks and place the optical markers. Then, individuals were instructed to walk at self-selected, roughly constant speed with a moderate range of arm swing. Subsequently, participants were guided to stand on the ground in front of the staircase and then climb the staircase at a self-selected pace and place only one foot on each staircase. Before data collection, the participants had to practice the two activities until they felt they could perform them naturally.
Before trial, the participant maintained a neutral upright standing position for at least five seconds to collect the baseline data. Then, the participants performed level walking and stair climbing in sequence. Each activity was repeated three times. During the two activities, the markers were captured by Optotrak Certus motion analysis system (Northern Digital Inc., Ontario, Canada) at the sample rate of 100 Hz.
2.3. Musculoskeletal Model
In the AnyBody Managed model repository (AMMR, version 1.6) of an AnyBody Modeling system, a generic FacetJointModel was selected since it could predict the muscle forces and intradiscal forces in a redundant system. The details of the model were described and validated in the literature [25–27]. In brief, the model consisted of one pelvic segment, five lumbar vertebrae, and one lumped thoracic segment. The connection between the adjacent segment was a spherical joint which was a simplified model of the intervertebral disc. The location of each joint referred to the work by Pearcy and Bogduk [28]. In this model, there were more than one hundred muscle fascicles around pelvis and spine. These muscles were divided into several main muscle groups based on its function, namely, rectus abdominis (RA), left and right erector spinae (ES), left and right lumbar multifidus (LM), left and right thoracic multifidus (TM), left and right oblique externus (OE), left and right oblique internus (OI), left and right psoas major (PM), left and right quadratus lumborum (QL), and left and right semispinalis (SS). All the muscles fascicles were solved as a force component using the minimum-maximum optimization algorithm and could only exert tensile force [25, 26, 29].
2.4. The Spine Rhythm and Simulation
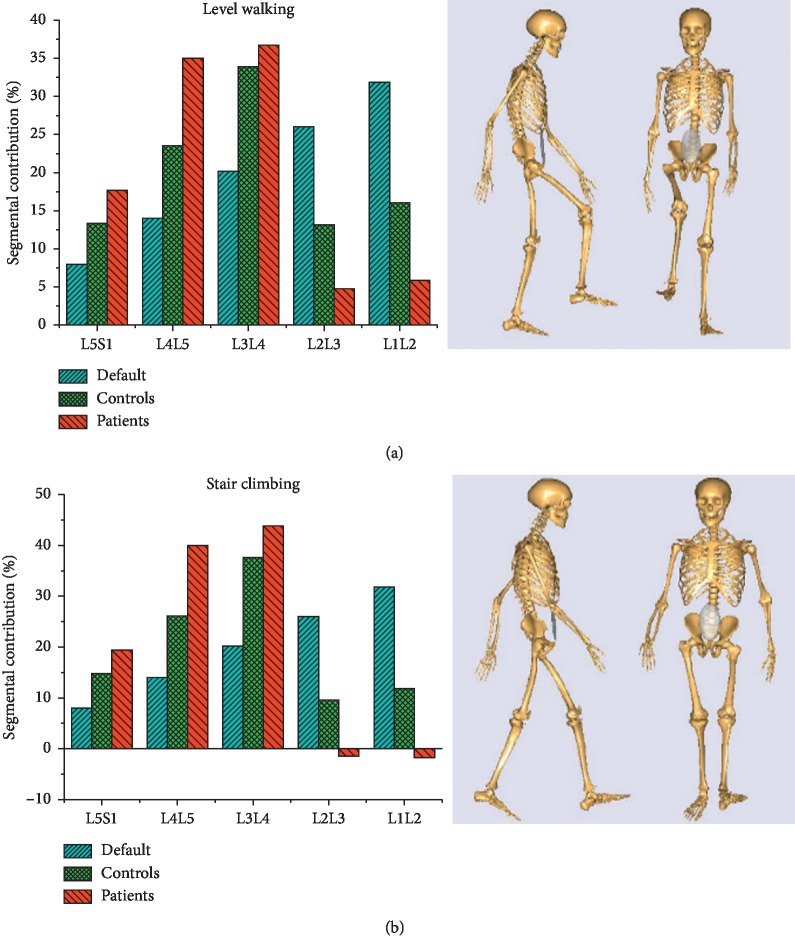
In the spine model, the motion of every segment was driven by the spine rhythm which represented the contribution of every segment to the total spinal motion. In the AnyBody model system, the default spine rhythm was constant without consideration of the individual difference. In this study, every subject's spine model was driven by the individual spine rhythm which was determined by captured marker coordinates [23]. The features of default, control group, and patient group's spine rhythms during level walking and stair climbing were represented by the average across the gait cycle and are shown in Figure 1.
Figure 1.
Three types of mean spine rhythm and schematic during (a) level walking and (b) stair climbing.
2.5. The Selection of the Optimized COR and Secondary Simulation
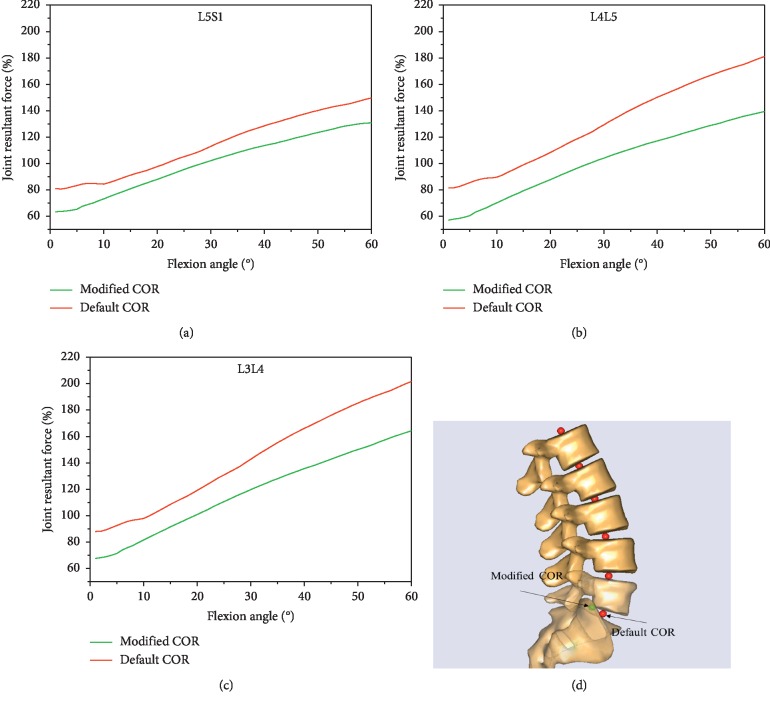
In this study, the position of COR was set offset from default COR for L5S1 (Figure 2). The offset was from 10 mm anterior to 10 mm posterior with an interval of 1 mm and 10 mm inferior to 10 mm superior with an interval of 1 mm. Then, the musculoskeletal model was driven to perform trunk flexion using the default lumbar spine rhythm under each offset COR. During simulation, the intradiscal forces were recorded. Finally, one position of the COR at L5S1 was selected because the joint resultant forces at L3L4, L4L5, and L5S1 levels were significantly decreased (Figure 2). Afterward, the musculoskeletal model was modified by resetting the default COR with new COR and then driven by every patient's spine rhythm during level walking and stair climbing.
Figure 2.
The position of the modified center of rotation and predicted the joint resultant forces at L3L4, L4L5, and L5S1 for default and modified center of rotation. The green line indicates the modified center of rotation. The red line indicates the default center of rotation.
2.6. Data Analysis
For the two ADLs, the gait cycle was defined as the time interval between subsequent heel strikes of the same leg. Then, the data of muscle activities and intradiscal forces were intercepted by the time window of the analyzed cycle. The intercepted data were time-normalized to 0–100% with 101 points. Moreover, the intradiscal forces were normalized to the body weight of every subject. The improvement at each time point was determined by the value of the variable before intervention subtracting from the value of the variable after the intervention. Data analysis was performed using custom-made programs implemented in MATLAB (the MathWorks, Inc.).
3. Results
3.1. The Effect of Two Simulated Interventions on the Joint Resultant Forces during Level Walking and Stair Climbing
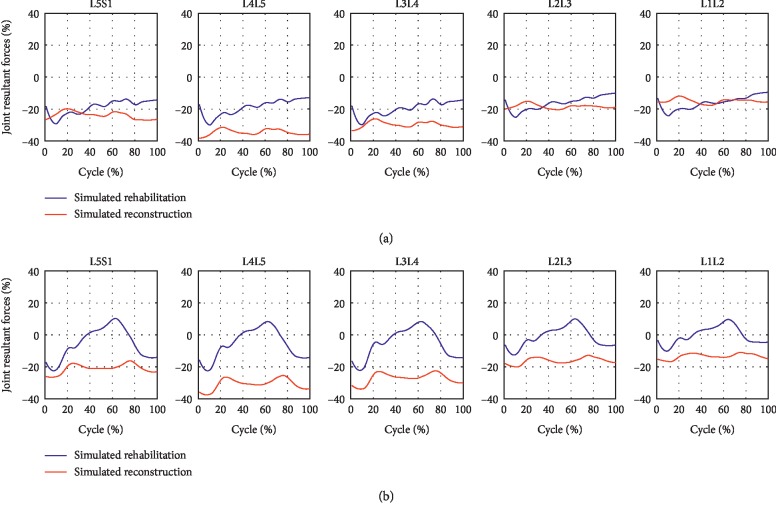
Figure 3 illustrates the effect of two interventions on joint resultant forces during level walking and stair climbing. During level walking, the two interventions both decreased the joint resultant forces acting on all the five lumbar intervertebral discs. However, there were more decreases after simulated reconstruction than simulated rehabilitation. During stair climbing, all the five lumbar motion segment units showed larger reductions in joint resultant forces throughout the gait cycle after simulated reconstruction, while the improvement after simulated rehabilitation varied across the gait cycle. However, the joint resultant forces acting at the L5S1, L4L5, and L3L4 levels were decreased in average after simulated rehabilitation.
Figure 3.
The improvement of joint resultant forces during (a) level walking and (b) stair climbing after the two interventions.
3.2. The Effect of Two Simulated Interventions on the Muscle Activities during Level Walking and Stair Climbing
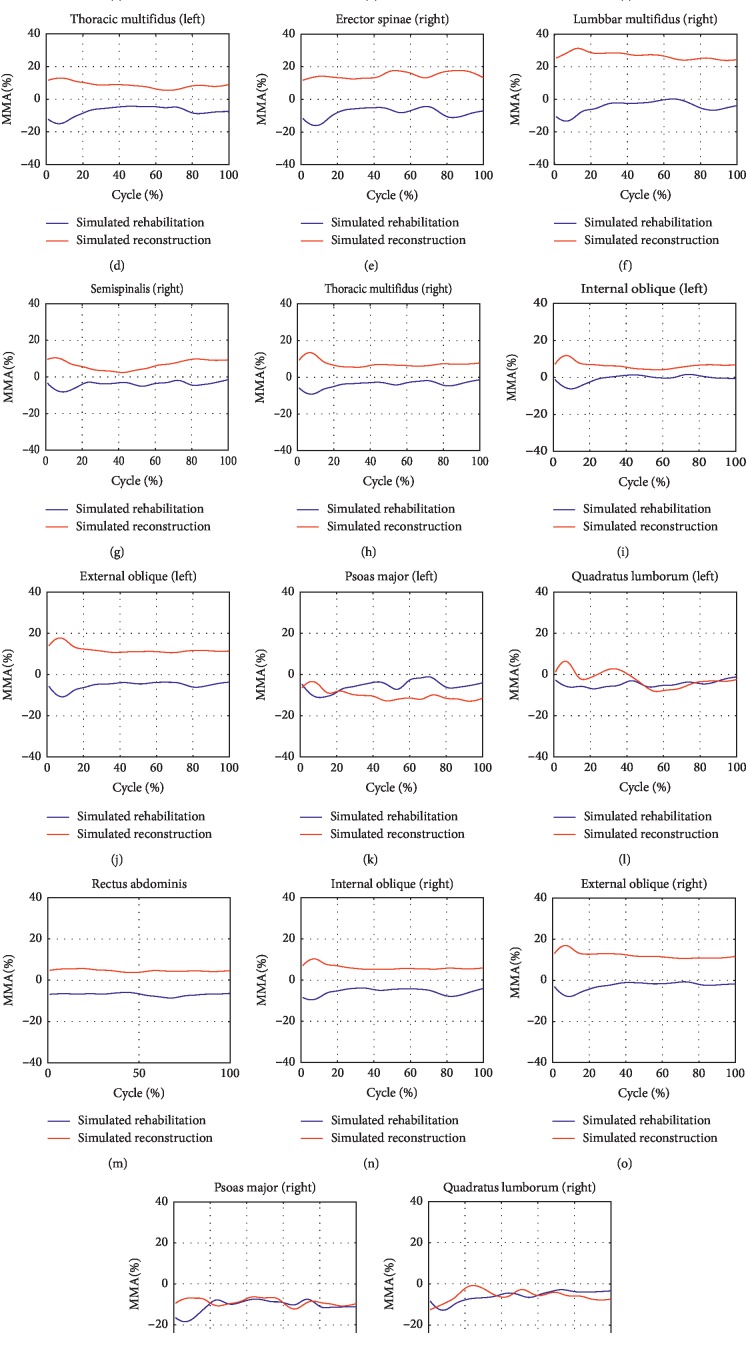
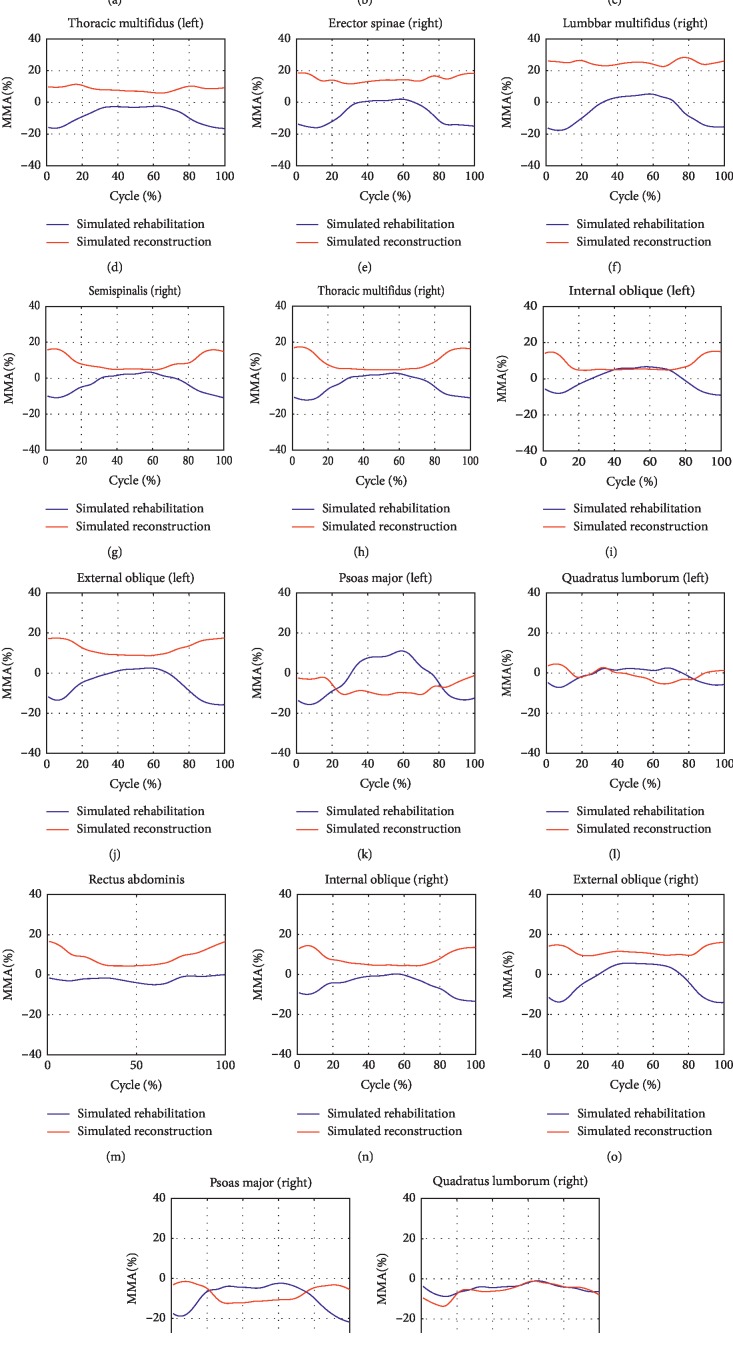
The maximum muscle activities (MMAs) of all the seventeen muscle groups were found to possess reductions during level walking after simulated rehabilitation (Figure 4). In contrast, larger MMAs after simulated reconstruction were found in RA and two sides of ES, LM, SS, TM, IO, and EO. During stair climbing, the MMAs of all the back muscle groups and five of the nine front muscle groups were improved after simulated rehabilitation (Figure 5). In contrary to simulated rehabilitation, there were increases in the MMAs of all the back muscle groups after simulated reconstruction. In addition, seven of the nine front muscle groups showed more MMAs after simulated reconstruction.
Figure 4.
The improvement of maximum muscle activities of the seventeen main trunk muscle groups during level walking after the two interventions.
Figure 5.
The improvement of maximum muscle activities of the seventeen main trunk muscle groups during stair climbing after the two interventions.
4. Discussion
The goal of this study was to predict the effect of two simulated interventions for LDH on the intradiscal forces acting on the five lumbar intervertebral discs and maximum muscle activities of seventeen main muscle groups in the spinal region during two common ADLs.
The findings showed that there were reductions in the joint resultant forces at L5S1, L4L5, and L3L4 levels and the MMAs of the majority of the seventeen muscle groups during the two ADLs after simulated rehabilitation, supporting the first hypothesis. More decreases were found in joint resultant forces after simulated reconstruction than simulated rehabilitation during the two ADLs. In addition, the majority of the seventeen muscle groups demonstrated smaller MMAs after simulated rehabilitation but larger MMAs after simulated reconstruction. These findings supported the second hypothesis.
In the first hypothesis, the reductions of joint resultant forces were expected since decompression was one of the main therapeutic purposes for LDH. Apart from decompression, the muscle activities were also decreased after simulated rehabilitation, which was also expected since LBP could induce increases in lumbar muscle activities during functional tasks [30, 31]. The improvement in joint resultant forces and trunk muscle activities may be explained within the context of proper spine rhythm. To perform specific ADL, the central nervous system (CNS) would allocate motion for every FSU. The spinal column and musculature, which were two primary stabilizing systems in Panjabi's model of spinal stability, would respond for the motion allocation. In Arjmand et al.'s studies [22, 32], subjective alteration of spine rhythm in healthy people induced the changes in the spinal loads and muscle activities, which was in consistent with Panjabi's spinal stabilizing theory. In patients with LDH, the CNS adopted different spine rhythm strategies (Figure 1) due to subjective fear or habitual protective behavior. Likely, the spinal column and musculature exhibited adaptive response for this spine rhythm. However, this adaptation imposed extra burdens on lumbar discs and trunk muscles because the healthy spine rhythm might be an optimal strategy for diminishing compression force [33]. Moreover, this study found that healthy spine rhythm could also lowered the burden of musculature. So, the final purpose of rehabilitation might be the recovery of the healthy spine rhythm.
In the second hypothesis, the joint resultant forces were decreased after the alteration of COR. Additionally, the effect of decompression was better after simulated reconstruction than simulated rehabilitation. The simulated rehabilitation changed the motion distribution on FSU, but the structure and motion quality of every FSU were not altered. However, the structure of FSU, which was deemed as the base of the spine, was restored after simulated reconstruction. The restoration of the spinal base might contribute to better decompression. Noteworthy was that the obvious decompression in the lower lumbar region occurred under both default spine rhythm and patient' spine rhythm even though the two rhythms are quite different (Figure 1). It could be concluded that the alternation of COR played a greater role in decompression than the alteration of spine rhythm. Different with that after simulated rehabilitation, the decreases in joint resultant forces were accompanied by the increases in MMAs of the most main trunk muscle groups, especially in back muscle groups. Han et al. [10] have also reported that the muscle forces and activation patterns could be strongly affected by the location of COR, inducing considerable higher muscle forces. In this study, the COR moved posteriorly. The lever arms of the back muscle fascicles became shorter. So, to stabilize the spinal system, it is an adaption to increase muscle forces drastically.
In closing, understanding the load sharing in the spinal region and grading the load conditions would be beneficial for the selection of treatment in clinical examination. Our findings show that both interventions could reduce the joint resultant forces. However, in consideration of huge tissue injuries and possible extra burden on muscles caused by COR offset after reconstruction, rehabilitation should be a prior intervention. Reconstruction would be advised when the larger decompression was essential. In the future study, we will assess every patient's spinal load sharing pattern and find the correlation between spinal load sharing pattern and the therapy effect, which would guide the clinical treatment in specific.
5. Limitations
In this study, there were several limitations. Firstly, reduction in joint resultant forces was selected as the inclusive criteria for optimal COR, which might be a little arbitrary. The optimization of COR should take intradiscal forces, facet forces, muscle forces, and ligament forces into consideration. However, it was difficult to allocate weight for every variable. So, the selection for optimal COR was just a simplified method. Secondly, the prediction for reconstruction omitted the tissue injuries caused by surgery, which might affect the amplitude of some muscle forces. Thirdly, the interaction between spine rhythm and COR was not taken into consideration.
6. Conclusions
This study shows that both simulated rehabilitation and simulated reconstruction would affect the load sharing in the spine stabilizing system. Spinal loading decrease for lumbar intervertebral discs in the pathological region was predicted after both interventions. However, simulated rehabilitation reduced the muscle activities, while simulated reconstruction increased muscle activities. Considering the rapid decompression and better effects after simulated reconstruction and improvement of muscle activities after simulated rehabilitation, the combination of reconstruction and rehabilitation might be a better treatment choice for severe LDH patients. Besides, the prediction of loading characteristics during ADLs after two interventions might provide a crucial insight into the preoperative planning and rehabilitation planning.
Acknowledgments
The authors thank Shenzhen Second People's Hospital, Tsinghua University, National Research Center for Rehabilitation Technical Aids in China, Shenzhen University, and Sun Yat-sen University. The authors also thank all the participants. This research was funded by the Innovation Commission of Science and Technology of Shenzhen Municipality (grant nos. KJYY20170405161248988 and KQTD20170331100838136), Guangdong Medical Science and Technology Research Fund Project (grant no. A2019070), Xinjiang Uygur Autonomous Region Science and Technology Support Project Plan (grant no. 2016E02065), and the National Natural Science Foundation of China (grant no. 61533004).
Data Availability
The data used to support the findings of this study were supplied by Dr. Wenyu Zhou under license and so cannot be made freely available. Requests for access to these data should be made to the corresponding author Dr. Wenyu Zhou.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Katikireddi S. V. Global, regional, and national incidence and prevalence, and years lived with disability for 328 diseases and injuries in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma V. Y., Chan L., Carruthers K. J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Archives of Physical Medicine and Rehabilitation. 2014;95(5):986–995. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambeek L. C., van Tulder M. W., Swinkels I. C. S., Koppes L. L. J., Anema J. R., van Mechelen W. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine. 2011;36(13):1050–1058. doi: 10.1097/brs.0b013e3181e70488. [DOI] [PubMed] [Google Scholar]
- 4.Dagenais S., Caro J., Haldeman S. Psy28 a systematic review of low back pain cost of illness studies in the United States and internationally. Value in Health. 2008;11(3):A160–A161. doi: 10.1016/s1098-3015(10)70509-x. [DOI] [PubMed] [Google Scholar]
- 5.WEBER H. Spine update the natural history of disc herniation and the influence of intervention. Spine. 1994;19(19):2234–2238. doi: 10.1097/00007632-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Deyo R. A., Loeser J. D., Bigos S. J. Herniated lumbar intervertebral disk. Annals of Internal Medicine. 1990;112(8):598–603. doi: 10.7326/0003-4819-112-8-598. [DOI] [PubMed] [Google Scholar]
- 7.Schilling C., Pfeiffer M., Grupp T. M., Blömer W., Rohlmann A. The effect of design parameters of interspinous implants on kinematics and load bearing: an in vitro study. European Spine Journal. 2014;23(4):762–771. doi: 10.1007/s00586-014-3237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahng T.-A., Kim Y. E., Moon K. Y. Comparison of the biomechanical effect of pedicle-based dynamic stabilization: a study using finite element analysis. The Spine Journal. 2013;13(1):85–94. doi: 10.1016/j.spinee.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Baillargeon E., Anderst W. J. Sensitivity, reliability and accuracy of the instant center of rotation calculation in the cervical spine during in vivo dynamic flexion-extension. Journal of Biomechanics. 2013;46(4):670–676. doi: 10.1016/j.jbiomech.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han K. S., Kim K., Park W. M., Lim D. S., Kim Y. H. Effect of centers of rotation on spinal loads and muscle forces in total disk replacement of lumbar spine. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine. 2013;227(H5):543–550. doi: 10.1177/0954411912474742. [DOI] [PubMed] [Google Scholar]
- 11.Alapan Y., Sezer S., Demir C., Kaner T., İnceoğlu S. Load sharing in lumbar spinal segment as a function of location of center of rotation. Journal of Neurosurgery: Spine. 2014;20(5):542–549. doi: 10.3171/2014.1.spine13426. [DOI] [PubMed] [Google Scholar]
- 12.Rousseau M.-A., Bradford D. S., Hadi T. M., Pedersen K. L., Lotz J. C. The instant axis of rotation influences facet forces at L5/S1 during flexion/extension and lateral bending. European Spine Journal. 2006;15(3):299–307. doi: 10.1007/s00586-005-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marras W. S., Ferguson S. A., Burr D., Davis K. G., Gupta P. Functional impairment as a predictor of spine loading. Spine. 2005;30(7):729–737. doi: 10.1097/01.brs.0000157459.36970.62. [DOI] [PubMed] [Google Scholar]
- 14.Marras W. S., Davis K. G., Ferguson S. A., Lucas B. R., Gupta P. Spine loading characteristics of patients with low back pain compared with asymptomatic individuals. Spine. 2001;26(23):2566–2574. doi: 10.1097/00007632-200112010-00009. [DOI] [PubMed] [Google Scholar]
- 15.Panjabi M. M. Clinical spinal instability and low back pain. Journal of Electromyography and Kinesiology. 2003;13(4):371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 16.Marshall P. W., Murphy B. A. Muscle activation changes after exercise rehabilitation for chronic low back pain. Archives Of Physical Medicine And Rehabilitation. 2008;89(7):1305–1313. doi: 10.1016/j.apmr.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 17.Brooks C., Kennedy S., Marshall P. W. M. Specific trunk and general exercise elicit similar changes in anticipatory postural adjustments in patients with chronic low back pain. Spine. 2012;37(25):E1543–E1550. doi: 10.1097/brs.0b013e31826feac0. [DOI] [PubMed] [Google Scholar]
- 18.Xiao J., Sun J., Gao J., Wang H., Yang X. The activity of surface electromyographic signal of selected muscles during classic rehabilitation exercise. Rehabilitation Research and Practice. 2016;2016:9. doi: 10.1155/2016/4796875.4796875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B. C. Y., McGill S. M. Effect of long-term isometric training on core/torso stiffness. Journal of Strength and Conditioning Research. 2015;29(6):1515–1526. doi: 10.1519/jsc.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 20.Hubley-Kozey C. L., Vezina M. J. Muscle activation during exercises to improve trunk stability in men with low back pain. Archives Of Physical Medicine And Rehabilitation. 2002;83(8):1100–1108. doi: 10.1053/apmr.2002.33063. [DOI] [PubMed] [Google Scholar]
- 21.Vezina M. J., Hubley-Kozey C. L. Muscle activation in therapeutic exercises to improve trunk stability. Archives of Physical Medicine and Rehabilitation. 2000;81(10):1370–1379. doi: 10.1053/apmr.2000.16349. [DOI] [PubMed] [Google Scholar]
- 22.Arshad R., Zander T., Dreischarf M., Schmidt H. Influence of lumbar spine rhythms and intra-abdominal pressure on spinal loads and trunk muscle forces during upper body inclination. Medical Engineering & Physics. 2016;38(4):333–338. doi: 10.1016/j.medengphy.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Kuai S., Guan X., Zhou W., et al. Continuous lumbar spine rhythms during level walking, stair climbing and trunk flexion in people with and without lumbar disc herniation. Gait & Posture. 2018;63:296–301. doi: 10.1016/j.gaitpost.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Shengzheng K., Weiqiang L., Run J., Wenyu Z. The effect of lumbar disc herniation on spine loading characteristics during trunk flexion and two types of picking up activities. Journal of Healthcare Engineering. 2017;2017:10. doi: 10.1155/2017/6294503.6294503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophy M., Senan N. A. F., Lotz J. C., O’Reilly O. M. A Musculoskeletal model for the lumbar spine. Biomechanics and Modeling in Mechanobiology. 2012;11(1-2):19–34. doi: 10.1007/s10237-011-0290-6. [DOI] [PubMed] [Google Scholar]
- 26.de Zee M., Hansen L., Wong C., Rasmussen J., Simonsen E. B. A generic detailed rigid-body lumbar spine model. Journal of Biomechanics. 2007;40(6):1219–1227. doi: 10.1016/j.jbiomech.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 27.de Zee M., Mikkelsen P., Wong C., Simonsen E. B., Rasmussen J. The influence of the center of rotation position on facet joint loading and end plate loading investigated with musculoskeletal simulation. Proceedings of the Xxiind Congress of the International Society of Biomechanics; July 2009; Cape Town, South Africa. [Google Scholar]
- 28.Pearcy M. J., Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine. 1988;13(9):1033–1041. doi: 10.1097/00007632-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Han K.-S., Zander T., Taylor W. R., Rohlmann A. An enhanced and validated generic thoraco-lumbar spine model for prediction of muscle forces. Medical Engineering & Physics. 2012;34(6):709–716. doi: 10.1016/j.medengphy.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 30.D’hooge R., Hodges P., Tsao H., Hall L., Macdonald D., Danneels L. Altered trunk muscle coordination during rapid trunk flexion in people in remission of recurrent low back pain. Journal of Electromyography and Kinesiology. 2013;23(1):173–181. doi: 10.1016/j.jelekin.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Dubois J.-D., Piché M., Cantin V., Descarreaux M. Effect of experimental low back pain on neuromuscular control of the trunk in healthy volunteers and patients with chronic low back pain. Journal of Electromyography and Kinesiology. 2011;21(5):774–781. doi: 10.1016/j.jelekin.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Arjmand N., Shirazi-Adl A. Biomechanics of changes in lumbar posture in static lifting. Spine. 2005;30(23):2637–2648. doi: 10.1097/01.brs.0000187907.02910.4f. [DOI] [PubMed] [Google Scholar]
- 33.Tafazzol A., Arjmand N., Shirazi-Adl A., Parnianpour M. Lumbopelvic rhythm during forward and backward sagittal trunk rotations: combined in vivo measurement with inertial tracking device and biomechanical modeling. Clinical Biomechanics. 2014;29(1):7–13. doi: 10.1016/j.clinbiomech.2013.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study were supplied by Dr. Wenyu Zhou under license and so cannot be made freely available. Requests for access to these data should be made to the corresponding author Dr. Wenyu Zhou.