Abstract
Aim of the study
The diagnosis of hepatocellular carcinoma (HCC) is usually late, due to the lack of early detection of biomarkers for HCC. Metabolomics analysis has emerged as a useful tool for studying human diseases. The objective of the study was to investigate the differences in plasma metabolites between hepatitis C virus (HCV)-induced cirrhosis and HCC.
Material and methods
22 subjects with HCV-related liver cirrhosis and 22 subjects with HCC were enrolled. Clinical, routine laboratory and imaging studies were done. Gas chromatography/mass spectrometry (GC/MS) was used for metabolomics analysis of patients’ plasma samples.
Results
34 known metabolites were detected, of which five metabolites were identified to have the strongest discriminatory power for separation between HCC and cirrhosis groups: octanoic acid (caprylic acid), decanoic (capric acid), oleic acid, oxalic acid and glycine. These are 3 fatty acids (FA), a dicarboxylic acid and a glucogenic amino acid, respectively. No significant correlation was found between the relative intensities of the five metabolites and any of the patient or tumor characteristics (Child-Turcotte-Pugh (CTP) score, Barcelona Clinic Liver Cancer (BCLC) stage, number of focal lesions and size of largest focal lesion). ROC curve analysis was performed and area under the curve (AUC) was calculated, revealing that oleic acid, octanoic (caprylic) acid and glycine had higher positive predictive value than α-fetoprotein.
Conclusions
The study of metabolomics (particularly involving FA) may help define distinct metabolic patterns to distinguish HCV-induced liver cirrhosis from HCC patients. Future research in this field is still needed, particularly concerning HCC treatment strategies which target fatty acid-related metabolic pathways.
Keywords: liver cirrhosis, fatty acids, hepatocellular carcinoma, metabolomics, hepatitis C virus (HCV)
Introduction
Hepatocellular carcinoma (HCC) is increasing in incidence, representing the fifth and ninth most frequently occurring cancer in men and women, respectively [1]. It is considered to be the second leading cause of cancer related mortality in the world, with more than half a million new cases diagnosed worldwide every year. Cirrhosis due to chronic viral hepatitis B or C is now considered the main risk factor for HCC worldwide [2]. Clinically, most patients rarely have symptoms until the later stages of the disease. Thus, the diagnosis is usually late, which is – in turn – responsible for the high morbidity and mortality rates associated with HCC [3]. The only chance of long-term disease-free survival in asymptomatic patients depends on early diagnosis of HCC [4]. Ultrasound imaging and serum α-fetoprotein (AFP) have long been considered to be the classic screening methods for early detection of primary liver cancer. However, around 30% of HCC patients are AFP-negative. Therefore, new screening methods for primary liver cancer are increasingly needed [5, 6].
Metabolomics analysis is a new technology which refers to the scientific study of the small-molecular intermediates and products of metabolism. It is a quantitative measurement of endogenous low molecules, with a relative molecular mass of less than 1000 Daltons, hence identifying the unique chemical patterns produced by specific cellular processes [7, 8]. It is a powerful tool in exploring mechanisms of different diseases, including minimal changes in genes and expression of proteins, which provides ample information on discovery of new biomarkers, disease pathogenesis, diagnosis and personalized treatment [9, 10].
Techniques based on mass spectrometry (MS) such as gas chromatography/mass spectrometry (GC/MS) and liquid chromatography/mass spectrometry (LC/MS) are the most widely used and effective technologies in metabolomics analysis. The former is suitable for analyzing the thermally stable, volatile and gaseous compounds of small molecular mass, while the latter can analyze the more polar compounds with higher relative molecular mass and lower thermal stability [11]. GC/MS is considered to be the gold standard technique in metabolomics [8]. It is a collective system where the volatile and thermally stable compounds are first separated by GC, followed by detection of the eluting compounds by electron-impact mass spectrometers. Human blood is a good source for metabolomics research, as there are large amounts of metabolites in blood. Studies have succeeded in extracting and detecting metabolites from human blood by applying GC/MS [12-14].
The analysis of specific patterns of metabolic alterations associated with HCC can help in providing insight into its etiology and mechanisms. This study aims to compare between the plasma metabolite levels in hepatitis C virus (HCV)-related HCC cases and cirrhotic patients, and to evaluate the capability of candidate metabolites in distinguishing between the two groups.
Material and methods
Study population
Forty-four subjects with HCV-related liver cirrhosis with or without HCC were recruited from the Hepatology Department of the Medical Research Institute Hospital, Alexandria University, Egypt during the period from December 2017 to April 2018. Informed consent was obtained from all participants before the study and it was approved by the local Ethics Committee of the institute in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
A complete history and physical examination were performed for all patients, followed by analysis of the biochemical and radiological profile. The subjects were divided into two groups: 22 patients with liver cirrhosis due to HCV infection and 22 patients with HCC complicating HCV-related cirrhosis. All patients had no prior history of receiving antiviral therapy. HCV infection was identified by positive serum anti-HCV antibodies, which was confirmed by a polymerase chain reaction (PCR) test. Patients had negative serum markers of active infection with hepatitis B virus (HBV), human immunodeficiency virus (HIV) and schistosomiasis. Also, patients with a history of alcohol consumption > 30 g/day, autoimmune diseases, malignancies, diabetes mellitus and non-HCV related liver cirrhosis were excluded from the study.
Clinical and radiological evaluation
Liver cirrhosis was diagnosed based on clinical, laboratory and imaging criteria (coarse echo pattern of the liver on ultrasound), with reporting of the presence/absence of portal hypertension and splenomegaly. Ascites was graded as none, mild/moderate or severe. Child-Turcotte-Pugh (CTP) score and class were used for assessing the severity of liver disease [15].
Hepatocellular carcinoma cases were diagnosed according to the guidelines of the American Association for the Study of Liver Disease (AASLD) published in 2011, which comprised the presence of a hepatic focal lesion on ultrasound, verified by either a contrast-enhanced triphasic CT-scan study or dynamic contrast-enhanced MRI that showed characteristic criteria for HCC diagnosis (arterial uptake of contrast material followed by washout) [16]. The Barcelona Clinic Liver Cancer (BCLC) system was applied for staging of HCC cases [17].
Biochemical analysis
After an overnight eight-hour fasting period, 10 ml of whole venous blood samples were withdrawn from each subject. One ml was collected in EDTA tubes for complete blood picture and two ml were collected in citrated plasma tubes for prothrombin time and INR determination. Four ml serum samples were prepared for routine clinical chemistry (using an Olympus AU400 clinical chemistry analyzer; Beckman Coulter, Inc.), according to the methods recommended by the International Federation of Clinical Chemistry and Laboratory Medicine [18]. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (total and direct), albumin, alkaline phosphatase, gamma-glutamyl transferase (GGT), blood urea nitrogen (BUN) and creatinine were assessed. In addition, serum AFP level was measured for all patients with cirrhosis and HCC (using the automated IMMULITE 1000 immunoassay analyzer; Siemens Medical Solutions Diagnostics Corporation, Erlangen, Germany). A serum cut-off value equal to or more than 200 ng/ml was considered diagnostic for HCC [19]. Anti-HCV-antibodies were measured by immunoassay technique, and HCV RNA load was quantitatively determined using a real-time PCR system.
The remaining three ml of whole blood were collected in separate EDTA tubes and centrifuged at 2000 rpm for 10 min at 4°C. The plasma was aliquoted into Eppendorf tubes and stored at –80°C for metabolomics measurement.
Metabolomics analysis
Chemicals and reagents: N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) with 1% trimethylchlorosilane (TMCS) of > 99.0% purity, methoxyamine hydrochloride (> 98.0% purity) and pyridine (> 99.8% purity) were commercially obtained from Sigma-Aldrich (St. Louis, MO, USA). High performance liquid chromatography (HPLC) grade methanol was purchased from the Tedia Company (Inc., Fairfield, USA).
Sample preparation: Each 100 μl plasma-sample was thawed at 37°C for 10 min, vortexed and mixed for 15 seconds. 800 μl of methanol, 100 μl of distilled water and 10 μl of “heptadecanoic acid in methanol” (1 mg heptadecanoic acid in 1 ml methanol) were added and mixed. Ten μl per sample were vortex-mixed for one minute, kept on ice for 10 minutes, ultrasonicated at room temperature for 5 minutes, then put again on ice for 10 minutes and centrifuged for 10 min (1200 rpm). The supernatant (200 μl) was transferred into a 5 ml glass centrifugation tube and evaporated to dryness by N2 gas. Next, 50 μl of methoxyamine/pyridine were taken per sample (15 mg/ml = 0.0015 g methoxyamine hydrochloride in 100 μl of pyridine) to the dry tube, vortexed for 30 seconds and left for 16 hours at room temperature with a glass plug. Finally, 50 μl of MSTFA + 1% TMCS derivatization agent were added to the residue, vortex-mixed for 60 seconds and heated in a water bath at 70°C for one hour with a glass plug. The final solution was taken for GC/MS analysis. All the samples were analyzed by GC/MS at random after being preprocessed [12].
GC/MS analysis: Analysis was performed in the research laboratories of City of Scientific Research and Technology Applications, New Borg El-Arab city, Alexandria, Egypt using a Shimadzu GC-2010 gas chromatography instrument coupled with a Shimadzu QP2010 mass spectrometer (Shimadzu Co., Kyoto, Japan). The capillary column used for all analyses was an Agilent DB-5MS with a deactivated fused silica column (inner diameter: 30 m × 0.25 mm, film thickness: 0.25 μm). The column temperature was initially maintained at 80°C for one minute, programmed to 300°C at a rate of 15°C/min, and then held for one min. Ultra high purity helium (99.9%) was used as a carrier gas with a constant flow rate of 1.45 ml/min. The septum purge was turned on with a flow rate of one ml/min all the time. The injector temperature, the interface temperature and the ion source temperature were set at 250°C, 150°C and 230°C, respectively. Ionization was achieved by a 70 eV electron beam. The mass spectrometer was operated under electron impact (EI) in a full-scan mode over the range from m/z 50 to 800 with a 0.5 second scan velocity, and the detector voltage was 0.96 kV [20].
Data processing and statistical analysis
The identification of compounds from the peaks was based on the interpreted tables of m/z values and normalized migration times, by comparing the mass spectrum with Library Spectra v. 2.0 of the National Institutes of Standards and Technology, Gaithersburg, MD (NIST). The identification of metabolites was established by matching masses (m/z) between the peak’s fragmentation pattern and the standard database. Peaks with more than 80% similarity were allocated compound names, while those having less than 80% similarity were listed as unknown metabolites. The chromatograms were subjected to noise reduction before peak area integration. Peaks due to noise, column bleed and MSTFA derivatization procedures were excluded from the data set. Integrated peak areas of multiple derivative peaks which belonged to the same compound were added together and considered as a single compound [21].
SPSS version 20.0 software was used in statistical analysis. The discriminant function analysis test was used to determine which metabolites discriminate between the two groups of the study (HCV cirrhosis versus HCC). The discriminant function analysis model was created by a retrospective stepwise discriminant approach using the data set of the patients. Initial classification functions were applied to determine to which group each case most likely belongs. After alignment and normalization of significant GC data, multivariate statistical analyses were conducted to estimate the sensitivity and specificity of each significant variable in predicting HCC at a defined cut-off value. Clinical, routine laboratory and imaging data of patients were expressed as mean ± standard deviation (SD) or proportions. Comparison between two means was performed using the non-parametric Mann-Whitney U-test for abnormally distributed quantitative variables. Comparison between proportions was determined by the chi square (χ2) test or Fisher’s exact test (FET). Spearman’s correlation coefficient (r) was applied to our results. A p-value equal to or less than 0.05 was considered to be statistically significant. ROC curve analysis was performed using the relative intensity values of the identified plasma metabolites and the area under the curve (AUC) was calculated to determine their individual ability in predicting HCC cases among cirrhotic subjects.
Results
Clinical, radiological and routine laboratory evaluation
Males represented 40.9% of patients in the HCC group with a mean age of 60.1 years, versus 63.6% of patients in the cirrhosis group with a mean age of 58.6 years (p > 0.05). There was no statistically significant difference between the two groups regarding CTP score and class (p > 0.05). Triphasic CT evaluation of HCC patients showed that the majority of patients had 2-3 or > 3 focal lesions on presentation (72.8%), the tumor involved both lobes of the liver in 54.5% of patients, with malignant portal vein thrombosis detected in 31.8%, lymph node involvement in only one patient and extrahepatic spread in none. The mean size of the largest focal lesion was 4.43 ±2.01 cm. BCLC staging of HCC patients revealed that more than half of them were at the end stage of the disease (59.1%), while nearly one third of them (31.8%) were at the early stage (Table 1).
Table 1.
Clinical and radiological data of the studied groups
| Variable | HCC (n = 22) n (%) | Cirrhosis (n = 22) n (%) | P-value |
|---|---|---|---|
| Age (years) (X ±SD) | 60.09 ±5.06 | 58.59 ±7.83 | 0.455 |
| Male sex | 9 (40.9) | 14 (63.6) | 0.131 |
| Splenomegaly by US | 12 (54.5) | 20 (90.9) | 0.007* |
| Ascites grade by US | 0.060 | ||
| None | 1 (4.5) | 7 (31.8) | |
| Mild-moderate | 2 (9.1) | 2 (9.1) | |
| Severe | 19 (86.4) | 13 (59.1) | |
| CTP score (X ±SD) | 11.18 ±2.04 | 9.91 ±2.91 | 0.100 |
| CTP class | 0.338 | ||
| Class A | 0 (0.0) | 2 (9.1) | |
| Class B | 9 (40.9) | 9 (40.9) | |
| Class C | 13 (59.1) | 11 (50.0) | |
| PV thrombosis by CT | 7 (31.8) | 0 (0.0) | 0.004* |
| Number of FL by CT | – | – | |
| Single | 6 (27.3) | ||
| Two/three | 8 (36.4) | ||
| More than three | 8 (36.4) | ||
| Size of largest FL (cm) (X ±SD) | 4.43 ±2.01 | – | – |
| Liver lobes involved | – | – | |
| One lobe | 10 (45.5) | ||
| Both lobes | 12 (54.5) | ||
| LN involvement by CT | 1 (4.5) | – | – |
| Extrahepatic spread | 0 (0.0) | – | – |
| BCLC stage of HCC | – | – | |
| Very early stage | 0 (0.0) | ||
| Early stage | 7 (31.8) | ||
| Intermediate stage | 2 (9.1) | ||
| End-stage | 13 (59.1) |
Data are expressed as mean (X) ± standard deviation (SD) or as number (n) and percent (%)
Statistically significant at p < 0.05
HCC – hepatocellular carcinoma, CTP – Child-Turcotte-Pugh, US – ultrasound, CT – triphasic CT-scan, PV – portal vein, FL – focal lesions, LN – lymph nodes, BCLC – Barcelona Clinic Liver Cancer.
The biochemical analysis revealed that there was a statistically significant increase in the levels of AST, ALT, GGT, INR, AFP and white blood cell count (WBC) in the HCC group compared to the cirrhosis group, while there was no statistically significant difference between the two studied groups regarding other parameters (Table 2). Also, there was no statistically significant correlation between serum level of AFP and any of the patient or tumor characteristics (CTP score, BCLC stage, number of focal lesions and size of largest focal lesion (p > 0.05).
Table 2.
Routine laboratory parameters of studied groups
| Parameter | HCC (n = 22) | Cirrhosis (n = 22) | P-value |
|---|---|---|---|
| ALT (U/l) | 89.14 ±86.36 | 27.59 ±12.71 | 0.002* |
| AST (U/l) | 154.14 ±112.44 | 55.59 ±26.66 | 0.001* |
| Albumin (g/dl) | 2.07 ±0.48 | 2.32 ±0.43 | 0.076 |
| Total bilirubin (mg/dl) | 6.17 ±5.29 | 3.80 ±6.44 | 0.189 |
| Direct bilirubin (mg/dl) | 4.02 ±3.98 | 2.32 ±4.63 | 0.199 |
| Alkaline phosphatase (U/l) | 130.95 ±66.30 | 93.95 ±72.56 | 0.085 |
| GGT (U/l) | 80.64 ±50.73 | 49.27 ±47.75 | 0.041* |
| BUN (mg/dl) | 72.41 ±39.15 | 56.91 ±35.50 | 0.176 |
| Creatinine (mg/dl) | 1.20 ±0.38 | 1.33 ±0.54 | 0.357 |
| AFP | 276.21 ±252.92 | 69.93 ±43.82 | 0.002* |
| PT (s) | 19.01 ±3.14 | 17.31 ±4.21 | 0.137 |
| INR | 1.64 ±0.23 | 1.45 ±0.35 | 0.038* |
| Hb (g/dl) | 10.49 ±1.43 | 10.46 ±1.50 | 0.951 |
| RBC (× 106 cells/mm3) | 3.12 ±0.60 | 3.26 ±0.58 | 0.416 |
| WBC (× 103 cells/mm3) | 12.21 ±8.19 | 8.18 ±3.59 | 0.041* |
| Platelets (× 103 cells/mm3) | 117.41 ±46.16 | 118.50 ±62.83 | 0.948 |
Data are expressed as mean ± standard deviation
statistically significant at p < 0.05, n – number
HCC – hepatocellular carcinoma, ALT – alanine transaminase, AST – aspartate transaminase, GGT – gamma-glutamyl transpeptidase, BUN – blood urea nitrogen, AFP – alpha-fetoprotein, PT – prothrombin time, INR – international normalized ratio, Hb – hemoglobin, RBC – red blood cell count, WBC – white blood cell count.
Metabolomics analysis
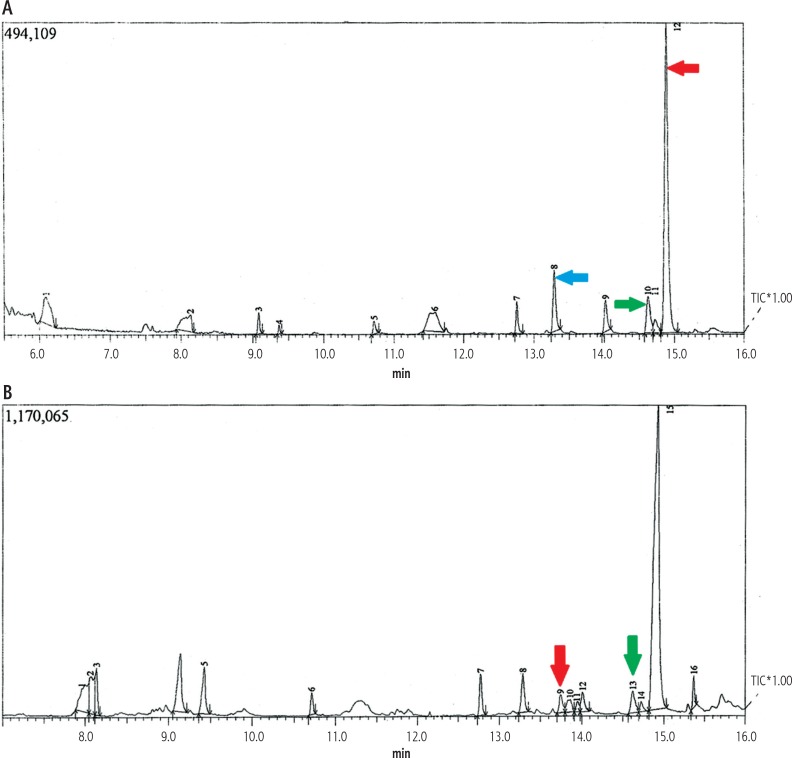
Examples of GC-MS total ion chromatograms (TIC) of plasma samples derived from the studied groups are shown in Fig. 1. Around 61 signals were detected in the samples using mass spectral deconvolution software for peak detection. However, many of them were not consistently found in other samples or presented too low abundance or too poor spectral quality to be accurately assigned to specific metabolites. A total of 34 peaks could be auto-identified by the NIST library through comparing the fragmentation patterns composed of all fragment ions, as shown in Table 3. The remaining peaks which could not be identified were not reported. These metabolites are suggested to be involved in energy metabolism, lipid metabolism, protein metabolism, and amino acid metabolism.
Fig. 1.
Example of GC-MS total ion chromatogram (TIC) of plasma sample of HCC versus cirrhosis patient. A) HCC patient chromatogram demonstrating high peaks of decanoic acid (blue arrow), oleic acid (green arrow), and glycine (red arrow); B) Cirrhosis patient chromatogram demonstrating low peaks of glycine (red arrow) and oleic acid (green arrow), while decanoic acid was undetectable
Table 3.
Discriminant analysis of different plasma metabolites in the studied groups
| No. | Identified metabolites | Retention time (min) | m/z ratio | HCC n = 22 | Cirrhosis n = 22 | Wilks’ lambda | P-value |
|---|---|---|---|---|---|---|---|
| 1 | Dihydroxyacetophenone | 5.793 | 281.00 | 5.132 ±3.905 | 5.475 ±1.021 | 0.971 | 0.078 |
| 2 | Trisiloxane | 6.136 | 73.00 | 8.220 ±4.657 | 7.545 ±4.232 | 0.949 | 0.084 |
| 3 | Benzenedicarboxaldehyde | 6.958 | 133.00 | 2.733 ±1.942 | 1.043 ±0.986 | 0.983 | 0.400 |
| 4 | Carbamic acid | 7.304 | 149.00 | 7.645 ±7.578 | 2.817 ±1.910 | 0.917 | 0.058 |
| 5 | 3-ethyl 2-methylhexane | 7.465 | 84.00 | 5.857 ±1.443 | 4.675 ±0.007 | 0.940 | 0.069 |
| 6 | Silane | 7.585 | 207.00 | 2.860 ±0.000 | 1.193 ±0.543 | 0.995 | 0.635 |
| 7 | Pyridinecarbonitrile | 7.811 | 221.00 | 5.66 ±0.000 | 14.120 ±0.000 | 0.994 | 0.605 |
| 8 | Caprylic acid | 8.106 | 57.00 | 7.334 ±2.623 | 2.711 ±1.802 | 0.985 | 0.023* |
| 9 | Oxomalonic acid | 8.087 | 57.00 | 3.770 ±0.000 | 2.736 ±2.006 | 0.988 | 0.480 |
| 10 | Oxalic acid | 8.110 | 57.00 | 3.223 ±1.001 | 2.112 ±0.701 | 0.934 | 0.001* |
| 11 | Neohexane | 9.050 | 57.00 | 3.950 ±0.000 | 1.090 ±0.000 | 0.952 | 0.155 |
| 12 | Enanthic acid | 9.078 | 57.00 | 2.945 ±0.306 | 3.983 ±2.448 | 1.000 | 0.985 |
| 13 | Caproic acid | 9.084 | 57.00 | 2.945 ±0.306 | 3.983 ±2.448 | 1.000 | 0.985 |
| 14 | Butane | 9.315 | 57.00 | 2.731 ±1.228 | 4.770 ±0.000 | 0.976 | 0.319 |
| 15 | Iodododecane | 9.386 | 57.00 | 8.570 ±0.000 | 2.060 ±0.000 | 0.949 | 0.141 |
| 16 | Valeric acid | 9.629 | 191.00 | 3.3120 ±2.000 | 3.146 ±2.153 | 0.997 | 0.704 |
| 17 | Glutaric acid | 9.697 | 191.00 | 3.312 ±2.000 | 3.146 ±2.153 | 0.997 | 0.704 |
| 18 | Methoxy benzoic acid | 10.617 | 135.00 | 3.801 ±2.025 | 2.564 ±1.593 | 0.961 | 0.199 |
| 19 | Ethanol | 12.668 | 179.00 | 5.025 ±2.147 | 3.634 ±2.023 | 0.973 | 0.290 |
| 20 | Hypoxanthine | 13.115 | 55.00 | 1.800 ±0.000 | 0.650 ±0.000 | 0.982 | 0.389 |
| 21 | Arachidic acid | 13.133 | 73.00 | 3.129 ±2.491 | 3.424 ±5.271 | 0.991 | 0.535 |
| 22 | Palmitic acid | 13.221 | 73.00 | 3.129 ±2.491 | 3.424 ±5.271 | 0.991 | 0.535 |
| 23 | Pentadecylic acid | 13.234 | 73.00 | 3.129 ±2.491 | 3.424 ±5.271 | 0.991 | 0.535 |
| 24 | Heptadecanoic acid | 13.893 | 57.00 | 1.920 ±1.392 | 1.983 ±1.352 | 0.988 | 0.484 |
| 25 | Propionic acid | 14.547 | 57.00 | 1.382 ±1.268 | 0.616 ±0.220 | 0.929 | 0.081 |
| 26 | Capric acid | 14.601 | 73.00 | 0.713 ±0.210 | 0.343 ±0.208 | 0.923 | 0.009* |
| 27 | Oleic acid | 14.621 | 55.00 | 4.743 ±1.442 | 1.602 ±0.333 | 0.929 | 0.041* |
| 28 | Stearic acid | 14.747 | 60.00 | 4.460 ±0.057 | 2.440 ±2.258 | 0.997 | 0.721 |
| 29 | Glycine | 14.774 | 223.00 | 55.245 ±1.734 | 50.611 ±2.341 | 0.939 | 0.015* |
| 30 | Methionine | 15.153 | 223.00 | 1.071 ±0.663 | 0.947 ±0.658 | 0.995 | 0.639 |
| 31 | L-Leucine | 15.193 | 223.00 | 1.071 ±0.663 | 0.947 ±0.658 | 0.995 | 0.639 |
| 32 | Butylhydroquinone | 15.273 | 295.00 | 1.737 ±0.313 | 1.525 ±0.335 | 0.994 | 0.102 |
| 33 | Acrylic acid | 16.164 | 311.00 | 14.200 ±0.000 | 6.070 ±6.772 | 0.996 | 0.674 |
| 34 | Isophthalic acid | 16.904 | 267.00 | 57.710 ±0.000 | 50.360 ±19.638 | 0.976 | 0.318 |
Relative intensities of plasma metabolites in the two study groups (HCC vs. cirrhosis) are given as their peak areas, and expressed as mean ± standard deviation
Statistically significant at p ≤ 0.05, n – number
HCC – hepatocellular carcinoma.
Five metabolites were finally selected among all variables with the strongest discriminatory power for separation between the HCC group and the liver cirrhosis group. The five peaks were identified as octanoic acid (caprylic acid), decanoic (capric acid), oleic acid, oxalic acid and glycine, which were all significantly higher in the HCC patients compared to the HCV-cirrhotic group.
When statistically comparing the mean values of the relative intensities of the five plasma metabolites between the two studied groups, they were found to be significantly higher among the HCC group compared to the cirrhosis group (Table 4). However, no statistically significant correlation was found between the relative intensities of the five metabolites and any of the patient or tumor characteristics (CTP score, BCLC stage, number of focal lesions and size of largest focal lesion), as shown in Table 5.
Table 4.
Comparison between the two studied groups according to the relative intensities of the five identified plasma metabolites
| HCC group (n = 22) | Cirrhosis group (n = 22) | Test of sig. | P-value | |
|---|---|---|---|---|
| Octanoic (caprylic) acid | ||||
| Range | 3.5-11.2 | 0.9-6.4 | U = 9.0* | < 0.001* |
| Mean ±SD | 7.3 ±2.6 | 2.7 ±1.8 | ||
| Oxalic acid | ||||
| Range | 1.6-4.7 | 1-3.1 | t = 2.774* | 0.011* |
| Mean ±SD | 3.2 ±1.1 | 2.1 ±0.7 | ||
| Decanoic (capric) acid | ||||
| Range | 0.3-1.1 | 0-0.7 | U = 22.0* | 0.003* |
| Mean ±SD | 0.7 ±0.2 | 0.3 ±0.2 | ||
| Oleic acid | ||||
| Range | 2.6-6.9 | 1-2.1 | t = 7.053* | < 0.001* |
| Mean ±SD | 4.7 ±1.4 | 1.6 ±0.3 | ||
| Glycine | ||||
| Range | 52.1-57.3 | 45.8-53.6 | t = 5.369* | < 0.001* |
| Mean ±SD | 55.2 ±1.7 | 50.6 ±2.3 | ||
U – Mann-Whitney test, t – Student’s t-test, p – p value for comparison between the two studied groups
statistically significant at p < 0.05
Table 5.
Relation between relative intensities of the five plasma metabolites and different patient and tumor characteristics
| Octanoic (caprylic) acid | Decanoic (capric) acid | Oleic acid | Oxalic acid | Glycine | |
|---|---|---|---|---|---|
| CTP-score (n = 44) |
r = –0.131 p = 0.542 |
r = 0.171 p = 0.426 |
r = 0.244 p = 0.251 |
r = 0.246 p = 0.256 |
r = 0.182 p = 0.395 |
| BCLC stage* (n = 20) |
U = 9.0 p = 0.833 |
U = 8.0 p = 0.667 |
t = 1.351 p = 0.214 |
t = 0.389 p = 0.707 |
t = 0.572 p = 0.583 |
| Number of FL (n = 22) |
H = 0.932 p = 0.628 |
H = 1.682 p = 0.431 |
F = 0.332 p = 0.727 |
F = 1.223 p = 0.344 |
F = 1.448 p = 0.291 |
| Size of largest FL (n = 22) |
r = 0.124 p = 0.717 |
r = 0.417 p = 0.202 |
r = 0.096 p = 0.778 |
r = 0.272 p = 0.418 |
r = 0.453 p = 0.161 |
CTP – Child-Turcotte-Pugh, BCLC – Barcelona Clinic Liver Cancer
(*Intermediate stage patients were excluded from analysis due to small sample size, n = 2)
FL – focal lesion, n = number of patients, r – Pearson coefficient, U – Mann-Whitney test, t – Student’s t-test, F – ANOVA test, H – Kruskal Wallis test, p – level of significance between the different categories (statistically significant at p ≤ 0.05)
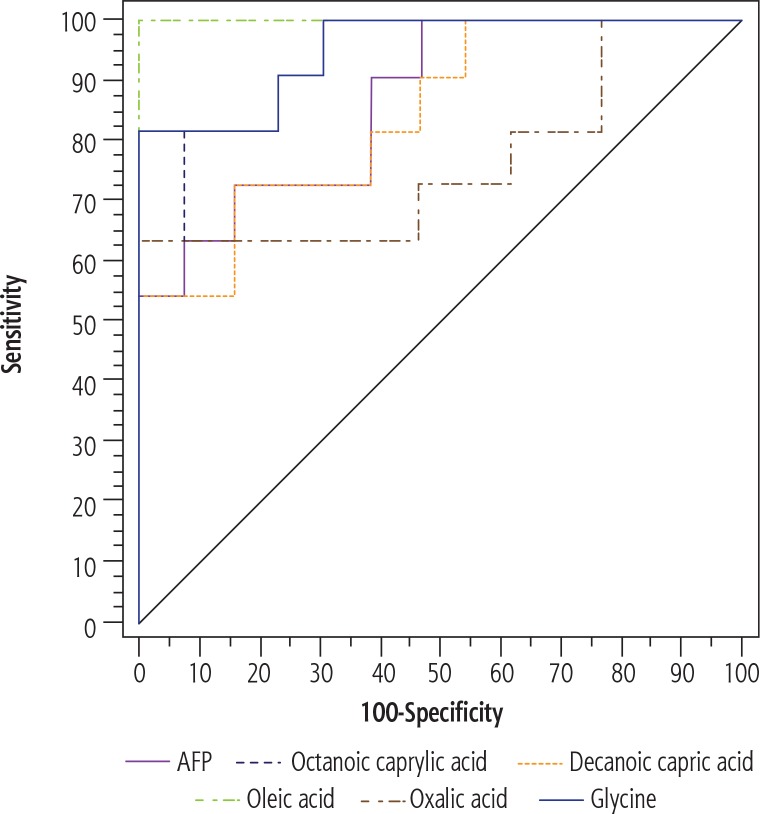
ROC curve analysis was performed using the relative intensity values of the identified plasma metabolites in comparison to serum concentration of AFP. The area under the curve (AUC) was calculated to determine their individual ability in predicting HCC cases among cirrhotic subjects, revealing that oleic acid, octanoic (caprylic) acid and glycine had higher positive predictive value than AFP (Table 6, Fig. 2).
Table 6.
Sensitivity and specificity of AFP versus plasma metabolites in predicting HCC cases among cirrhotic patients
| AUC | P | 95% CI | Cut-off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| AFP | 0.867* | 0.002* | 0.725-1.009 | > 95 | 72.73 | 84.62 | 80.0 | 78.6 |
| Octanoic (caprylic) acid | 0.937* | < 0.001* | 0.847-1.028 | > 5.003 | 81.82 | 92.31 | 90.0 | 85.7 |
| Oxalic acid | 0.762* | 0.030* | 0.552-0.973 | > 3.142 | 63.64 | 100.0 | 100.0 | 76.5 |
| Decanoic (capric) acid | 0.846* | 0.004* | 0.691-1.001 | > 0.512 | 72.73 | 84.62 | 80.0 | 78.6 |
| Oleic acid | 1.000* | < 0.001* | 1.000-1.000 | > 2.087 | 100.0 | 100.0 | 100.0 | 100.0 |
| Glycine | 0.951* | < 0.001* | 0.871-1.031 | > 53.57 | 81.82 | 100.0 | 100.0 | 86.7 |
AUC – area under curve, p – value: probability value, CI – confidence interval, NPV – negative predictive value, PPV – positive predictive value, AFP – alpha-fetoprotein
statistically significant at p ≤ 0.05
Fig. 2.
ROC curves of AFP and plasma metabolites demonstrating their different abilities to predict HCC cases among cirrhotic patients
Discussion
Hepatocellular carcinoma is often advanced and incurable at presentation, which is partially attributed to the absence of appropriate biomarkers for early diagnosis [22]. The technology of metabolomics has emerged as a useful analytical tool for human disease study, because of its high sensitivity and capability to simultaneously measure many metabolites [23].
The objective of the present study was to investigate the unique differences in plasma metabolites between HCV-cirrhotic and HCC patients. Based on non-targeted metabolomic analysis, the present work detected 61 metabolites, of which 34 metabolites were known compounds. Five metabolites were successfully identified with the strongest discriminatory power for distinguishing between the HCC group and the liver cirrhosis group, namely octanoic acid (caprylic acid), decanoic (capric acid), oleic acid, oxalic acid and glycine. The first two of the identified metabolites are medium-chain saturated FA, oleic acid is a monounsaturated FA, oxalic acid is a saturated dicarboxylic acid, while glycine is a simple glucogenic amino acid. ROC curve analysis demonstrated that oleic acid, octanoic (caprylic) acid and glycine had higher ability than AFP in predicting HCC cases among HCV-cirrhotic patients.
Hepatocellular carcinoma, as well as other malignant tumors, is known to generate a catabolic state in the body. The liver, being the principal metabolic hub of fats, carbohydrates and proteins, undergoes major metabolic alterations under these circumstances. HCC malignant cells require building blocks to provide material for cellular membranes, signaling molecules and energy as they proliferate and spread. Glycolysis, of course, acts as the primary source of energy [24]. In addition, gluconeogenesis from lipids and proteins also plays a key role, which consequently increases the turnover of glucogenic amino acids (e.g. glycine, alanine) and free FA [25, 26]. This might be one explanation for the findings of our metabolomics analysis. In fact, a metabolomics study by Di Poto et al. identified glycine as having better performance than AFP, and oxalic acid as clearly distinguishing HCC cases from HCV-cirrhotic controls [27]. Another metabolomics study by Muir et al. demonstrated elevated oleic, adrenic, and osbond acids in the plasma of patients with nonalcoholic steatohepatitis-associated hepatocellular carcinoma [28]. Furthermore, a third study by Qiu et al. used chromatography–mass spectrometry to prove that linoleic acid, oleic acid, arachidonic acid and palmitic acid were potential fatty acid biomarkers of HCC patients [29].
Cancer-induced dysregulation of FA metabolism has been receiving particular attention in recent years. It was suggested that cancer cells fulfill their requirement for energy and building materials either by upregulating de novo FA synthesis, or by altering FA oxidation [30]. At this point, studies seem to disagree on how FA regulation is involved in tumorigenesis. While some studies linked the downregulation of FA oxidation with HCC [31], others associated increased catabolism of certain saturated lipids with high AFP levels in the serum of HCC patients, concluding that lipidomics analysis may provide new biomarkers for HCC [32-34]. Li et al. also demonstrated that aberrant lipid metabolism was an evident feature of HCC, and that the severity of the condition correlated with higher tissue concentrations of saturated triglycerides (TG) and lower concentrations of polyunsaturated TG [35]. Lin et al. revealed similar outcomes and concluded that their findings offer the biomedical potential to use the altered lipid metabolism as a diagnostic marker for cancer cells, which – in turn – opens the opportunity for treating aggressive HCC by targeting altered lipid metabolism pathways [36].
Nevertheless, our results showed no correlation between patient/tumor characteristics (CTP score, BCLC stage, number of focal lesions and size of largest focal lesion) and the relative intensities of the identified plasma metabolites. In comparison, Muir et al. found a great discrepancy among the different identified fatty acids and their relation to HCC tumor size/burden in mice and humans. While some of them showed a positive correlation (e.g. oleic and adrenic acids), others showed negative (e.g. margaric and linoleic acids) or no correlation with tumor size/burden [28].
In Egypt, cases of hepatocellular carcinoma are mostly secondary to HCV-induced liver cirrhosis [37]. HCV infection seems to have a synergistic effect on lipid turnover, namely by encouraging lipogenesis and steatosis to provide a lipid-rich environment for viral replication [38]. This is achieved by augmenting the expression and activation of specific transcription factors that activate the synthesis of FA, triglycerides and cholesterol, causing their accumulation in the liver [39]. Added to the previously described HCC-related alteration of lipid metabolism, these findings emphasize the role of dysregulated FA particularly in HCV-induced HCC carcinogenesis, and indicates that interfering with lipogenesis may represent a potential therapeutic strategy for these cases.
Numerous therapeutic agents are already targeting key and/or lipogenic enzymes and pathways in lipid metabolism and have shown good efficacy against several cancers. However, this progress in therapeutic agents for HCC seems to be lagging behind. This might be explained by the great genetic and biochemical variability among HCC patients, which makes it difficult to classify them based on lipid metabolism. A second explanation is the complexity of FA metabolism itself, which involves an active balance between synthesis and catabolism [40].
Conclusions
From this perspective, we conclude that the study of metabolomics may help define distinct metabolic patterns, particularly in FA metabolism, which may distinguish HCV-induced liver cirrhosis from HCC patients, hence aiding in early diagnosis of this fatal condition. Future research in this field is still needed, particularly concerning HCC treatment strategies which target fatty acid-related metabolic pathways.
Acknowledgements
The authors would like to acknowledge Prof. Dr. Moustafa Mohamed Saleh Abbassy (Institute of Graduate Studies and Research, Alexandria University, Egypt) for his valuable and effective help regarding GC-MS technique.
Disclosure
The authors report no conflict of interest.
References
- 1.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;(47 Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See comment in PubMed Commons below. Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779. doi: 10.1586/17474124.2015.1028363. [DOI] [PubMed] [Google Scholar]
- 3.Moustafa T, Fickert P, Magnes C, et al. Alterations in lipid metabolism mediates inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012;142:140–151. doi: 10.1053/j.gastro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.Jia HL, Xing XJ, Ye QH, et al. Application of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008;30:440–443. [PubMed] [Google Scholar]
- 6.Tao Qin, Hu Liu, Qi Song, et al. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2247–2253. doi: 10.1158/1055-9965.EPI-10-0302. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Zhu Y, Cong Q, et al. Metabolomics research progress on liver diseases. Can J Gastroenterol Hepatol. 2017;2017:8467192. doi: 10.1155/2017/8467192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue R, Dong L, Wu H, et al. Gas chromatography/mass spectrometry screening of serum metabolomic biomarkers in hepatitis B virus infected cirrhosis patients. Clin Chem Lab Med. 2009;47:305–310. doi: 10.1515/CCLM.2009.083. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 10.Taylor J, King RD, Altmann T, et al. Application of metabolomics to plant genotype discrimination using statistics and machine learning. Bioinformatics. 2002;18:S241–248. doi: 10.1093/bioinformatics/18.suppl_2.s241. [DOI] [PubMed] [Google Scholar]
- 11.Krone N, Hughes BA, Lavery GG, et al. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J Steroid Biochem Mol Biol. 2010;121:496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu K, Sheng GP, Sheng JF, et al. A metabolomic investigation on the biochemical perturbation in liver failure patients caused by hepatitis B virus. J Proteome Res. 2007;6:2413–2419. doi: 10.1021/pr060591d. [DOI] [PubMed] [Google Scholar]
- 13.O’Hagan S, Dunn WB, Knowles JD, et al. Closed-loop, multi-objective optimization of two-dimensional gas chromatography/ mass spectrometry for serum metabolomics. Anal Chem. 2007;79:464–476. doi: 10.1021/ac061443+. [DOI] [PubMed] [Google Scholar]
- 14.Xue RY, Lin ZX, Deng CH, et al. A serum metabolomic investigation on hepatocellular carcinoma patients by chemical derivatization followed by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3061–3068. doi: 10.1002/rcm.3708. [DOI] [PubMed] [Google Scholar]
- 15.Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: The model for end-stage liver disease – should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 16.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 17.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 18.Pan BS, Zhang WZ, Chen MS, et al. A study for harmonization and reference range of serum ALT measurement in Shanghai. Lab Med. 2005;20:417–420. [Google Scholar]
- 19.Chan SL, Mo F, Johnson PJ, et al. Performance of serum α-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 2014;16:366–372. doi: 10.1111/hpb.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Wang Y, Yun Y, et al. A potential tool for diagnosis of male infertility: Plasma metabolomics based on GC-MS. Talanta. 2016;147:82–89. doi: 10.1016/j.talanta.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Wang W, Lv S, et al. Metabonomics study of liver cancer based on ultra performance liquid chromatography coupled to mass spectrometry with HILIC and RPLC separations. Anal Chim Acta. 2009;650:3–9. doi: 10.1016/j.aca.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Ressom HW, Xiao JF, Tuli L, et al. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitian AI, Nelson DR, Liu C, et al. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/ MS-MS. Liver Int. 2014;34:1428–1444. doi: 10.1111/liv.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterhouse C, Jeanpretre N, Keilson J. Gluconeogenesis from alanine in patients with progressive malignant disease. Cancer Res. 1968;39:1968–1972. [PubMed] [Google Scholar]
- 25.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 26.Bi XZ, Lin QS, Foo TW, et al. Proteomic analysis of colorectal cancer reveals alterations in metabolic pathways: mechanism of tumor genesis. Mol Cell Proteomics. 2006;5:1119–1130. doi: 10.1074/mcp.M500432-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Di Poto C, He S, Varghese RS, et al. Identification of race-associated metabolite biomarkers for hepatocellular carcinoma in patients with liver cirrhosis and hepatitis C virus infection. PLoS One. 2018;13:e0192748. doi: 10.1371/journal.pone.0192748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir K, Hazim A, He Y, et al. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer Res. 2013;73:4722–4731. doi: 10.1158/0008-5472.CAN-12-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu JF, Zhang KL, Zhang XJ, et al. Abnormalities in plasma phospholipid fatty acid profiles of patients with hepatocellular carcinoma. Lipids. 2015;50:977–985. doi: 10.1007/s11745-015-4060-6. [DOI] [PubMed] [Google Scholar]
- 30.Currie E, Schulze A, Zechner R, et al. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjornson E, Mukhopadhyay B, Asplund A, et al. Stratification of hepatocellular carcinoma patients based on acetate utilization. Cell Rep. 2015;13:2014–2026. doi: 10.1016/j.celrep.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 32.Beyoglu D, Imbeaud S, Maurhofer O, et al. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58:229–238. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito S, Ojima H, Ichikawa H, et al. Molecular background of alpha-fetoprotein in liver cancer cells as revealed by global RNA expression analysis. Cancer Sci. 2008;99:2402–2409. doi: 10.1111/j.1349-7006.2008.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson AD, Maurhofer O, Beyoglu D, et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71:6590–6600. doi: 10.1158/0008-5472.CAN-11-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Guan M, Lin Y, et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by liver lipidomics. Int J Mol Sci. 2017;18:E2550. doi: 10.3390/ijms18122550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Ding Y, Wang Y, et al. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology. 2017;66:432–448. doi: 10.1002/hep.29033. [DOI] [PubMed] [Google Scholar]
- 37.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 38.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waris G, Felmlee DJ, Negro F, et al. Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol. 2007;81:8122–8130. doi: 10.1128/JVI.00125-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Safaei A, Arefi Oskouie A, Mohebbi SR, et al. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 2016;9:158–173. [PMC free article] [PubMed] [Google Scholar]