Abstract
Background:
To date, no biomarker has been able to predict antidepressant response at an early blockade of norepinephrine or serotonin uptake. The transient nocturnal increase in plasma melatonin levels is upregulated by blocking these uptakes. The aim of this study was to test whether fluoxetine increase in urinary 6-sulfatoxymelatonin (aMT6s) is an indicator of serotonin uptake blockade.
Methods:
A total of 20 women (35–45 years of age) recruited from the community had a diagnosis of major depressive disorder confirmed by the Structured Clinical Interview for DSM-IV. Depressive symptoms were evaluated by the Beck Depression Inventory (BDI). Participants were instructed to take 20 mg of fluoxetine every morning. Every 4 weeks, the dose could be increased by 20 mg until symptom remission. The concentration of aMT6s was evaluated in overnight urine samples collected 1 day before and 1 day after the first fluoxetine dose.
Results:
An increase in aMT6s correlated to a decrease in BDI score evaluated on day 45 (ρ = −0.67, p = 0.024) was observed.
Conclusions:
Nocturnal increase in urinary aMT6s after the first day of medication use links the early mechanism of action of fluoxetine to its clinical output 45 days later. Thus, the relationship between urinary aMT6s excretion 1 day before/1 day after is a biomarker for predicting clinical output earlier, reducing illness burden and health care costs.
Keywords: depression, melatonin, mood disorders, pineal gland, SSRI
Introduction
Major depressive disorder (MDD) is a prevalent mental health condition and an important cause of disability around the world.1 Although different classes of medication are available for treatment, a significant number of patients do not show symptom remission.2
Currently, the pathophysiology of MDD is linked to several biological mechanisms. One important mechanism is the depletion of monoaminergic neurotransmitters, such as norepinephrine, serotonin, and dopamine.3 A previous study postulates that spot urine serotonin/dopamine ratio can be used as an objective diagnostic method for adults with MDD.4 Increased availability of these biogenic amines in the synaptic cleft initially reinforces neurotransmission, with an increase in postsynaptic output.5 In the long term, however, it promotes neuroplastic changes. The clinical output is a result of such long-term changes that will only arise if the initial blockade of monoamine uptake occurs.6 Despite years of searching for a biomarker that could predict the later clinical output, no practical method is yet available.
Melatonin, the pineal hormone, an indoleamine derived from serotonin, is synthesized at night under sympathetic control.7 Its production relies on the neurotransmitter norepinephrine and requires the precursor serotonin, both monoamines linked to MDD. Considering that melatonin is metabolized to 6-sulfatoxymelatonin (aMT6s) at first passage in the liver, blocking the neuronal uptake of monoamines would result in an acute increase in neurotransmission in whole body and brain, including the pineal gland.8 In this respect, we have previously shown a significant correlation between increasing aMT6s on the first night of medication with nonselective monoamine reuptake inhibitors (clomipramine and nortriptyline) and clinical antidepressant output.9,10
Melatonin is synthesized by acetylation and methylation of serotonin.7 Although pinealocytes express the enzyme tryptophan hydroxylase (TPH), the relevance of serotonin uptake for melatonin synthesis is not well explored. Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is a first-line antidepressant due to its high efficacy and safety.11 Fluoxetine also exerts its therapeutic effects by reducing central and peripheral levels of cytokines as well as increasing the level of brain-derived neurotrophic factor (BDNF) in the brain.12,13 A previous report indicates that acute fluoxetine treatment enhances serotonin within the pineal gland,8 and this could lead to an increase in melatonin synthesis. As this effect of fluoxetine was thought to be due to a potentiation of the conversion of tryptophan to serotonin, we decided to evaluate whether, also for serotonin uptake blockers, the increase in urinary aMT6s after the first dose could be predictive for improving depressive symptoms.
This study aimed to test whether the urinary concentration of aMT6s is an indicator of initial therapeutic response to fluoxetine, an SSRI widely used as an antidepressant. To this end, we conducted an exploratory prospective longitudinal study of depressed women to assess nocturnal urinary aMT6s as a predictor of antidepressant treatment response.
Materials and methods
Participants, assessments, and study protocol
Women aged between 35 and 45 years with symptoms of a depressive episode were recruited from the community and primary health care units. The diagnosis of MDD was confirmed with the Structured Clinical Interview for DSM-IV (SCID-IV) performed by a trained psychiatrist. Exclusion criteria were the use of psychotropic drugs, hormones (except oral contraceptives), corticoids, or drugs with action on adrenergic or dopaminergic receptors within 4 weeks prior to the study protocol; suicide risk or severe psychiatric disorder; severe clinical comorbidity; alcohol and drug abuse; pregnancy, irregular menstrual cycles, or menopause; and shift work. Depressive symptoms were evaluated by using the Beck Depression Inventory (BDI) on the first assessment, then again at 1 month, and then every 2 weeks up to 75 days.
Participants were instructed to take 20 mg of fluoxetine every morning, starting on the day after the first psychiatric evaluation. Every 4 weeks, the dose could be increased by 20 mg if there was no clinical response, according to clinical evaluation. In the case of symptom remission at any time, the participant was referred to a primary health care unit for follow-up treatment.
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol was approved by the institutional ethics committee (13-0228 GPPG, Hospital de Clínicas de Porto Alegre), and all participants signed an informed consent form before any study procedure.
Urine collection
Urine samples were collected at the night before and the night after the first fluoxetine dose. To ensure compliance, participants received written guidance and were carefully instructed regarding urine collection. Participants were instructed to collect all their urine during the night, including the first urine in the next morning, and to record the date and hour of voiding. Urine from each micturition was collected in a different vial (maximum volume of 800 mL). All vials were collected from the participants’ homes and delivered to the laboratory, stored at −4°C and processed on the same day.
Urine samples were centrifuged at 2000 rpm for 5 min and the supernatants were collected. Aliquots of each participant were labeled according to the collection time point, whether before or after treatment. The aliquot volume was 1 ml, with a percentage taken from each void proportional to its volume. Samples were stored at −80°C until assayed.
Determination of 6-sulfatoxymelatonin
The concentration of aMT6s in the urine samples was determined by enzyme immunoassay (Melatonin-Sulfate Urine ELISA kit, IBL International GmbH, Hamburg, Germany) using a microtiter plate reader (450 nm, Micronal B-380). The assay had a sensitivity of 1.0 ng/ml. Intra-assay variability [coefficient of variation (CV)] was 5.2–12.2% and inter-assay variability (CV) was 5.1–14.9% for aMT6s levels. Urinary creatinine concentration was measured by the Jaffé method and taken as the reference for the aMT6s concentration.14
Values are expressed as nanograms of aMT6s per milligram of creatinine. The variation in aMT6s was defined by the difference between urinary aMT6s/creatinine levels from the night after the first dose of fluoxetine, and the night before treatment.
Statistical analysis
The average values of BDI scores and aMT6s levels showed a nonparametric distribution and were, therefore, expressed as the median and interquartile range (IQR). Spearman’s rho correlation coefficients were used to analyze the relationship between delta aMT6s levels and improvement in BDI scores (i.e. the difference from baseline) between pretreatment evaluation and evaluation after 30 and 45 days of treatment. PASW Statistics, version 18.0, was used for all statistical analyses (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results
Baseline characteristics and cohort MDD history
The initial study sample consisted of 20 women (mean age of 40.19 ± 3.76) with a confirmed diagnosis of a major depressive episode with no current antidepressant treatment; 18 of the women (85.71%) had an education level greater than 11 years, and 11 (52.4%) were currently working.
Of the 20 women, 7 (35%) were in their first episode of MDD. For the 14 women (65%) who reported a previous episode, time living with MDD ranged from 4 months to 12 years, with a median of 2 years. Of the total 20, 2 women did not recall the time of diagnosis; 7 (35%) reported previous effective treatment with SSRI (fluoxetine, n = 4; sertraline, n = 2; citalopram, n = 1), 2 (10%) with psychotherapy, 2 (10%) with more than one approach including use of medication and psychotherapy, and 11 (55%) reported no previous treatments. All participants that reported use SSRI had a previous adequate response to the medication.
Cohort results
The participants had a median BDI score of 28.5 (IQR = 24.25–36.5) in the first evaluation. After 45 days of treatment with an initial 20-mg dose of fluoxetine, the average score was significantly lower (median = 20, IQR = 14–25).
A significant correlation was found between the before/after difference in urinary aMT6s excretion and the decrease in BDI score on day 45 (ρ = −0.67, p = 0.024). That is, the higher the delta aMT6s level, the greater the decrease in depressive symptoms as assessed by the BDI (Figure 1). A total of 11 participants were included in this correlation analysis because 9 had already achieved symptom remission before the evaluation on day 45. No correlation was found for day 30 (ρ = −0.23, p = 0.374). Two participants decided to interrupt the study protocol before the second evaluation.
Figure 1.
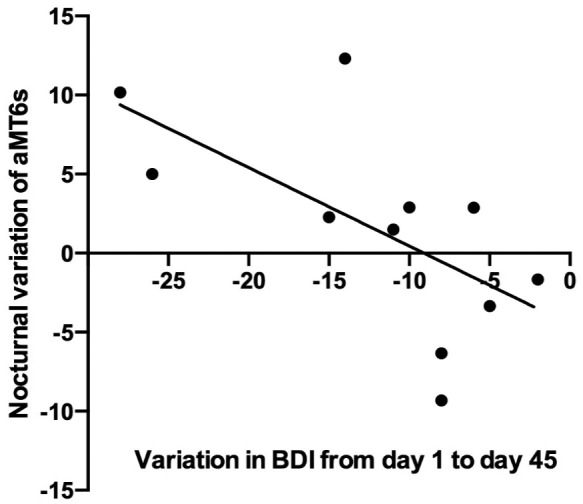
Correlations of nocturnal variation in aMT6s after the first dose of fluoxetine with reduction in BDI scores after 45 days of treatment (n = 11).
aMT6s, 6-sulfatoxymelatonin; BDI, Beck depression inventory.
Discussion
We aim to establish a reliable and evidence-based biomarker for predicting the clinical output of MDD treated with fluoxetine already on the first day of medication.
Circulating melatonin indicates nocturnal darkness. Lights on at night immediately blocks pineal melatonin synthesis.15 As melatonin suffers the first-pass liver effect, the excretion of the metabolite, aMT6s, reflects pineal sympathetic input. We have explored this temporal phenomenon to evaluate the sympathetic input in the pineal gland,9,10 as well as the level of serotonin in pinealocytes (present study). The present exploratory study shows that aMT6s predicts fluoxetine clinical output.
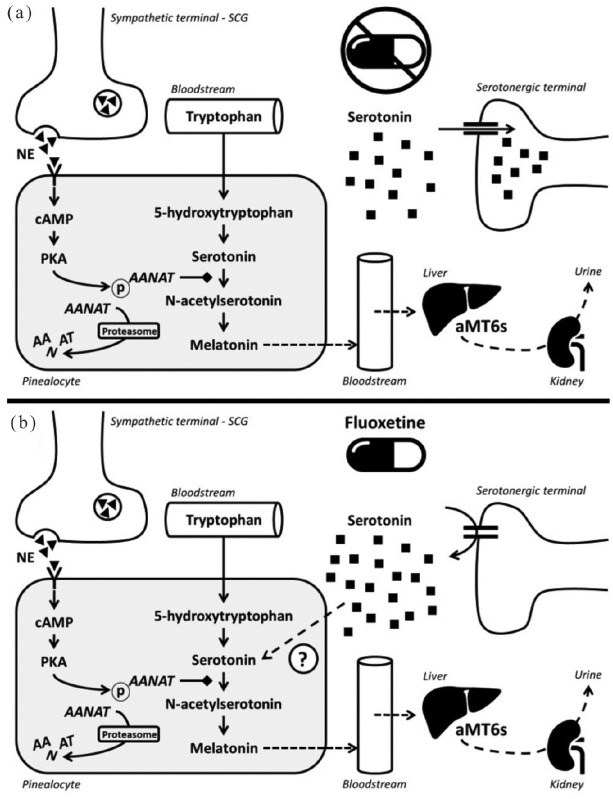
Melatonin synthesis occurs in the pineal gland mainly at night (in the absence of light), from the essential amino acid tryptophan. Four enzymes sequentially participate in melatonin biosynthesis. TPH converts tryptophan to l-5-hydroxytryptophan, which is then converted by the aromatic l-amino acid decarboxylase (AADC) to serotonin [5-hydroxytryptamine (5-HT)], with vitamin B6 as a cofactor. Serotonin is acetylated through the action of arylalkylamine N-acetyltransferase (AANAT), forming N-acetylserotonin, which is converted to melatonin by N-acetylserotonin O-methyltransferase (ASMT)16 (Figure 2).
Figure 2.
Schematic representation of melatonin production cascade and aMT6s excretion (a) and a theoretic model of the action of fluoxetine (b). Fluoxetine increases serotonin in the synaptic cleft, resulting in an increase in the pinealocyte cytoplasmic content. Increased melatonin synthesis will result in enhanced aMT6s excretion. Thus, the relationship between the urinary concentration of aMT6s before and after a single dose of fluoxetine could be taken as a biomarker for inhibiting serotonin uptake and for a positive clinical output after 45 days of treatment.
AANAT, arylalkylamine N-acetyltransferase; aMT6s, 6-sulfatoxymelatonin; NE, norepinephrine; SCG, superior cervical ganglion.
Fluoxetine has increased the production of melatonin in some studies,17,18 but produced no effects in others.19,20 Such evidence indicates that there might be significant individual differences in the pineal response to fluoxetine. It is important to clarify that aMT6s is well correlated with plasma melatonin.21 By analyzing the nocturnal variation in aMT6s after a single dose of medication (24-h period), it is possible to assume that this measurement depends on the integrity of the above-described monoaminergic cascade (Figure 2). Following the initial increase in neurotransmitter turnover, the clinical output is expected to occur as the neuroplastic process takes place. Based on our data, we can assume that an acute responsiveness of this system to the action of fluoxetine (meaning an increase in aMT6s following serotonin blockade) might indicate higher chances of symptom improvement.
This study aims to investigate whether urinary nocturnal aMT6s could be a predictor of a positive therapeutic outcome of MDD. The internal validity of the present work was reinforced by selecting a homogenous sample of women of high educational level. However, the chosen sample could limit the generalization of our findings to other clinical populations. Furthermore, we did not collect information on anthropometrics and inflammatory profile. Hence, it is necessary to replicate this protocol in a larger and heterogeneous sample considering other confounding variables. This way, future studies could analyze the accuracy of this marker to include it in clinical guidelines.
The present study establishes a proof of concept that aMT6s, the major melatonin metabolite, is a biomarker for antidepressant treatment with monoaminergic blockers. The melatonin production pathway represents a common intersection point of the biogenic amines that reinforce neurotransmission and postsynaptic output in the initial phase of treatment. In the case of clomipramine and nortriptyline, norepinephrine blockade is reflected in the increased pineal signaling for the conversion of serotonin to N-acetylserotonin,9,10 thereby increasing melatonin production. In contrast, a novel antidepressant, agomelatine is a potent agonist of melatonin (MT), MT1, and MT2 receptor types, and an antagonist of the serotonin (5HT), 5-HT2C receptor.22 With the present study, we postulate that increased serotonin in the synaptic cleft becomes a substrate to produce melatonin within the pinealocyte.
Conclusion
Relevant international guidelines for the pharmacological management of depression suggest shifting the therapeutic approach if patients do not reach symptom remission after 4–8 weeks.2,23 Here, we show a clear correlation between the pharmacological effect, that is, increase in nocturnal urinary excretion of aMT6s in the first night of medication, with the therapeutic output, that is, reduction in BDI scores after 45 days. It is noteworthy that urinary aMT6s excretion is a significant predictor of clinical output for clomipramine and nortriptyline, two nonselective monoamine reuptake inhibitors. Thus, refinement of this prognostic test has the potential to enhance treatment planning in depressed individuals, minimizing suffering, improving quality of life, and reducing health care costs.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Data for 6-Sulfatoxymelatonin predicts treatment response to fluoxetine in major depressive disorder by Juliana Jury Freitas, Nicóli Bertuol Xavier, André Comiran Tonon, Alicia Carissimi, Leandro Timm Pizutti, Carlos Augusto Vieira Ilgenfritz, Regina Pekelmann Markus and Maria Paz Hidalgo in Therapeutic Advances in Psychopharmacology
Acknowledgments
Authors Regina Pekelmann Markus and Maria Paz Hidalgo contributed equally to this study. The authors are grateful to Camila Morelatto de Souza, Felipe Gutierrez, Ana Paula Francisco, Flávia Amorim, Paula Chiamenti, and Daiane Machado for their participation in the clinical evaluation of participants.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: The following grants supported this study: São Paulo Research Foundation (FAPESP), #2013/13691-1 to RPM; National Council for Science Technology and Innovation (CNPq) #470301/2013-9 to MPH, Rio Grande do Sul State Foundation (FAPERGS) # PQg FAPERGS 04/2012 to MPH, FAPERGS/MS/CNPq/SESRS (PPSUS-2017), and Research Support Fund at Hospital de Clínicas de Porto Alegre (FIPE-HCPA) #13-0228 to MPH. The following received fellowships: undergraduates CAVI from BIC-UFRGS; JFI and ACT from PIBIC/CNPq - HCPA; AC from Higher Education Personnel Improvement Coordination (CAPES); 305331/2012-4 PQ/CNPq to MPH; senior fellowship to RPM from CNPq.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: André Comiran Tonon  https://orcid.org/0000-0003-4818-3144
https://orcid.org/0000-0003-4818-3144
Contributor Information
Juliana Jury Freitas, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
Nicóli Bertuol Xavier, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
André Comiran Tonon, Laboratório de Cronobiologia e Sono do HCPA/UFRGS, Ramiro Barcelos, 2350, Centro de Pesquisa Clínica, sala 21617, Porto Alegre, Rio Grande do Sul, CEP 90035-003, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
Alicia Carissimi, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
Leandro Timm Pizutti, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
Carlos Augusto Vieira Ilgenfritz, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil.
Regina Pekelmann Markus, Laboratório de Cronofarmacologia, Departamento de Fisiologia, Instituto de Biociência, Universidade de São Paulo, São Paulo, Brazil; Departamento de Psiquiatria e Medicina Legal da Faculdade de Medicina, UFRGS, Porto Alegre, Brazil.
Maria Paz Hidalgo, Laboratório de Cronobiologia e Sono do Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; Programa de Pós-Graduação em Psiquiatria e Ciências do Comportamento, UFRGS, Porto Alegre, Brazil.
References
- 1. Wang J, Wu X, Lai W, et al. Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ Open 2017; 7: e017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mojtabai R. Nonremission and time to remission among remitters in major depressive disorder: revisiting STAR*D. Depress Anxiety 2017; 34: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 3. Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 2010; 9: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wijaya CS, Lee JJZ, Husain SF, et al. Differentiating medicated patients suffering from major depressive disorder from healthy controls by spot urine measurement of monoamines and steroid hormones. Int J Environ Res Public Health 2018; 15: pii: E865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wainwright SR, Galea LAM. The neural plasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast 2013; 2013: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nierenberg AA, Farabaugh AH, Alpert JE, et al. Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry 2000; 157: 1423–1428. [DOI] [PubMed] [Google Scholar]
- 7. Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev 2003; 55: 325–395. [DOI] [PubMed] [Google Scholar]
- 8. Tsuiki K, Yamamoto YL, Diksic M. Effect of acute fluoxetine treatment on the brain serotonin synthesis as measured by the alpha-methyl-L-tryptophan autoradiographic method. J Neurochem 1995; 65: 250–256. [DOI] [PubMed] [Google Scholar]
- 9. Hidalgo MPL, Caumo W, Dantas G, et al. 6-Sulfatoxymelatonin as a predictor of clinical outcome in depressive patients. Hum Psychopharmacol 2011; 26: 252–257. [DOI] [PubMed] [Google Scholar]
- 10. Markus RP, Franco DG, Carvalho LA, et al. Acute increase in urinary 6-sulfatoximelatonin after clomipramine, as a predictive measure for emotional improvement. J Psychopharmacol (Oxford) 2010; 24: 855–860. [DOI] [PubMed] [Google Scholar]
- 11. Tran BX, Ha GH, Vu GT, et al. Indices of change, expectations, and popularity of biological treatments for major depressive disorder between 1988 and 2017: a scientometric analysis. Int J Environ Res Public Health 2019; 16: pii: E2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu Y, Ho CS, Liu X, et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One 2017; 12: e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu Y, Ho CS, McIntyre RS, et al. Effects of vortioxetine and fluoxetine on the level of brain derived neurotrophic factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res Bull 2018; 142: 1–7. [DOI] [PubMed] [Google Scholar]
- 14. Klante G, Brinschwitz T, Secci K, et al. Creatinine is an appropriate reference for urinary sulphatoxymelatonin of laboratory animals and humans. J Pineal Res 1997; 23: 191–197. [DOI] [PubMed] [Google Scholar]
- 15. Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science 1980; 210: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 16. Klein DC, Berg GR, Weller J. Melatonin synthesis: adenosine 3’,5’-monophosphate and norepinephrine stimulate N-acetyltransferase. Science 1970; 168: 979–980. [DOI] [PubMed] [Google Scholar]
- 17. Míguez JM, Simonneaux V, Pévet P. Evidence for a regulatory role of melatonin on serotonin release and uptake in the pineal gland. J Neuroendocrinol 1995; 7: 949–956. [DOI] [PubMed] [Google Scholar]
- 18. Reierson GW, Mastronardi CA, Licinio J, et al. Chronic fluoxetine treatment increases daytime melatonin synthesis in the rodent. Clin Pharmacol 2009; 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNulty JA, Colin V. Sympathetic denervation and chronic serotonin uptake blockade by fluoxetine do not affect pineal gland 5-hydroxyindole acetic acid: evidence that oxidative deamination of pineal serotonin is a property of the pinealocyte. J Neural Transm Gen Sect 1992; 89: 93–101. [DOI] [PubMed] [Google Scholar]
- 20. Monteleone P, Orazzo C, Natale M, et al. Lack of effect of short-term fluoxetine administration on nighttime plasma melatonin levels in healthy subjects. Biol Psychiatry 1994; 35: 139–142. [DOI] [PubMed] [Google Scholar]
- 21. Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med 2008; 4: 66–69. [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Y, Ho CS, McIntyre RS, et al. Agomelatine-induced modulation of brain-derived neurotrophic factor (BDNF) in the rat hippocampus. Life Sci 2018; 210: 177–184. [DOI] [PubMed] [Google Scholar]
- 23. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. AJP 2006; 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Data for 6-Sulfatoxymelatonin predicts treatment response to fluoxetine in major depressive disorder by Juliana Jury Freitas, Nicóli Bertuol Xavier, André Comiran Tonon, Alicia Carissimi, Leandro Timm Pizutti, Carlos Augusto Vieira Ilgenfritz, Regina Pekelmann Markus and Maria Paz Hidalgo in Therapeutic Advances in Psychopharmacology