Abstract
Background
In complex older patients, inappropriate medication use and polypharmacy (IMUP) are commonplace and increasing exponentially. Reducing IMUP is a challenge in multiple clinical contexts, including acute admission and family practice, due to several key barriers. In the global effort against this epidemic, educational programs geared toward changing physicians’ prescribing patterns represent an important means of promoting deprescribing.
Methods
This is a nonrandomized, controlled interventional study investigating polypharmacy outcomes and prescribing patterns in patients whose physicians were trained in the Good Palliative-Geriatric Practice (GPGP) method, an algorithm for the reduction of polypharmacy, with patients whose physicians were not. Training involved a one-time, full-day workshop led by a senior geriatrician. Two separate settings were examined. In the inpatient setting, one internal medicine ward was trained and compared with another ward which was not trained. In the family practice setting, 28 physicians were trained and compared with practices of 15 physicians not trained. Patients were above the age of 70, representative of the general geriatric population, and not terminally ill.
Results
In the inpatient arm, the intervention group (n = 100) experienced a decrease in medications prescribed from admission to discharge of 18.5%, compared with a decrease of 1.9% in the control group (n = 100, difference between groups p < 0.0001). In the outpatient arm, the intervention group (n = 100) experienced a decrease in medication number of 6.1% compared with 0.07% in the control group (n = 100, difference between groups p = 0.001) over a 6-month period. Preferential decreases in specific drug classes were observed in both groups, including benzodiazepines, psychotropics, and antihypertensives.
Conclusions
A one-time educational intervention based on GPGP can change prescribing patterns in both outpatient and inpatient settings leading to a moderate reduction in polypharmacy. Future work should focus on longitudinal interventions, and longer-term clinical outcomes such as morbidity, mortality, and quality of life.
Keywords: Deprescribing, polypharmacy, education, family medicine, hospital medicine, palliative, geriatric
Introduction
Over the past century, a dramatic increase in life expectancy coupled with medical science’s success in combating acute illness, has led to the rapid increase in the number of older adults with multiple chronic diseases.1 Advanced healthcare systems are characterized by medical subspecialization and often see little direct collaboration between providers caring for these complex patients.2 This reality proves fertile ground for widespread inappropriate medication use and polypharmacy (IMUP), and adverse drug effects (ADEs). Older people are prescribed a greater number of medications which may be inappropriate,3 fueling the cycle of comorbidity, disability, hospitalization, nursing home placement, and mortality.4,5 When considered on a global scale, the spiraling health, economic, and social consequences of IMUP are tremendous.6 It is clear that frail, older adults are a challenging patient population requiring a medical approach distinct from their younger, healthier counterparts, which must include an emphasis on combating IMUP.1,7 A subpopulation of particular interest has been termed VOCODFLEX:8,9 the Very Old, with Comorbidity, Dementia, Frailty and Limited life Expectancy. In this population the risk–benefit calculus for most medications is tilted strongly toward risk, and the adverse effects of IMUP are prominent. Given the lengthening of life expectancy, the ranks of VOCODFLEX are increasing rapidly, and the dangers of polypharmacy are obvious and widespread, highlighting poly-deprescribing as a key priority in this population.7
According to Topinkova and colleagues10 there is ‘an urgent global need for practical clinical strategies, guidelines, and interventions for reliably improving the quality of pharmacotherapy in older people.’ In this spirit, the International Group for Reducing Inappropriate Medication Use and Polypharmacy (IGRIMUP) was founded in 2013.1,7 The organization’s goal is to combat IMUP via interdisciplinary communication and collaboration, and its ranks currently number nearly 140 leading health professionals from 32 countries. Recently, IGRIMUP proposed 10 comprehensive action recommendations to fight polypharmacy,1 which focus on good geriatric prescribing, as well as educating healthcare professionals about this important task.
Many well-known tools exist to assist the clinician in deprescribing, and these are traditionally classified as explicit (lists or criteria such as Beer’s criteria3 or STOPP/START11) or implicit (judgment-based tools). The Garfinkel Good Palliative-Geriatric Practice (GPGP) algorithm, an implicit method developed by our group, is a comprehensive model for aggressive polydeprescribing. The great advantage of GPGP is its global applicability to any drug in any clinical context. Our group has previously shown it to be effective when implemented by a treating geriatrician in nursing departments.12 Efficacy of the algorithm has been demonstrated in community-dwelling patients,13 including sustained improvement in clinical outcomes as seen in a longer (>3 years) follow-up cohort study.7,14
In additional clinical contexts, including family practice and during acute medical admission, reducing polypharmacy has proven challenging. In a longitudinal study of polydeprescribing in community-dwelling patients, the main barrier to deprescribing was the family doctor’s unwillingness to adopt the consultant geriatrician’s deprescribing recommendations.14 Family physicians often do not feel equipped to stop unnecessary medications, especially those prescribed by other specialists.15,16 Other barriers to deprescribing in this setting include excess time needed to safely deprescribe, pressure due to pay-for-performance, fear of lawsuits, and fear of the patient/family’s reaction.8 In contrast, changes to a patient’s drug regimen implemented during hospitalization may be seen by the family doctor as having greater authority. However, older patients are often discharged from hospital with more medications than on admission.17 These two settings (family practice and internal-medicine admission) are therefore valuable potential targets for deprescribing interventions.
In this light, here, we examine the feasibility of implementing an educational intervention based on GPGP in two clinical settings in Israel: an internal medicine ward of a teaching hospital; and in family practices. The main goal of the study was to evaluate whether the intervention could change participating physicians’ prescribing patterns and reduce the number of medications that older patients were prescribed during the study period.
Both study arms were approved by the ethical (Helsinki) committee of the Wolfson Medical Center, Holon, Israel.
Methods
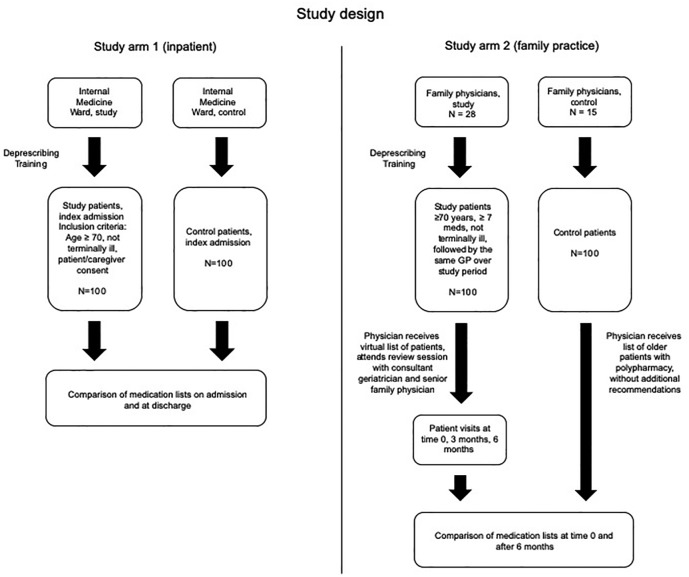
This is a nonrandomized, controlled interventional study investigating the polypharmacy outcomes of patients whose physicians were trained in the GPGP method, with patients whose physicians were not. Two separate settings, acute inpatient and family practice, were examined. Methodological variations were necessary due to the differences in these clinical settings (see Figure 1).
Figure 1.
Study flow diagram.
GP, general practitioner.
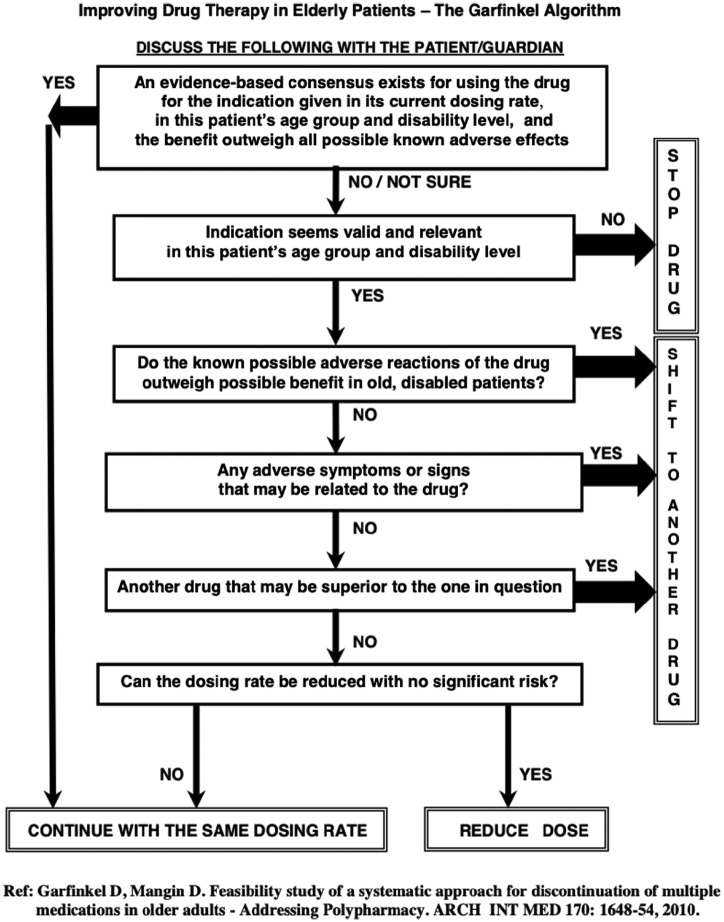
The GPGP method was developed as a comprehensive prescribing paradigm for use in complex geriatric populations.12,13 It involves application of the GPGP algorithm (Appendix 1), which aims to stop all medications, or as many as possible, that are likely to be neither lifesaving nor to improve quality of life based on available evidence in the specific patient population. The algorithm also encourages modifying the drug dose or replacing a potentially harmful drug with a less harmful one. GPGP encourages maximal patient/caregiver involvement to ensure consent and adherence to the intervention. In the original methodology, following a trial period of 3 months, the patient/caregiver can elect to continue with the method or revert to the prior medication list. In this study, however, practitioners used the algorithm mainly as a tool for deprescribing.
Study arm 1 (inpatient)
The intervention group was the physician team of Internal Medicine Ward C at the Wolfson Medical Center in Holon, Israel, who were trained in the GPGP method in December 2016. For the purpose of this study, training in the GPGP method involved a mandatory full-day training session including lectures and a workshop. Content included discussions of up-to-date evidence on geriatric pharmacology, IMUP, and the risks and benefits of deprescribing. A special focus was placed on statins, antihypertensives, aspirin, H2-blockers, proton-pump inhibitors (PPIs), and benzodiazepines (BZDs). Training included clinical case presentations and discussing preliminary results of the ongoing longitudinal study by our group.14 Additional topics included ethical and clinical reasoning with regards to frail and multimorbid older patients, primarily based on the Holmes model.18 This model proposes a framework for the appropriateness of medication prescribing in older patients, based on the interplay of four parameters: life expectancy, goals of care, time to benefit, and treatment targets.
The control group was the comparable team of physicians in Internal Medicine Ward E at the same hospital who did not undergo training and were not aware they were being studied, and whose patients received routine care. Patients were admitted at random from the emergency department to both wards. Patient inclusion criteria were age 70 or above, estimated life expectancy of at least 6 months (absence of terminal illness), and consent of the patient/caregivers. The first 100 patients admitted to each ward who fulfilled the inclusion criteria were included. Data gathered on the patients included demographic characteristics, diagnoses, and number and type of medications upon admission and discharge. In the intervention group, re-evaluation and readjustment of the medication list was performed as needed. All changes were confirmed by a senior physician. The patients/caregivers were educated about all changes as they were made, and an additional explanation about the medication list provided prior to discharge.
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences). Continuous variables were assessed using the Kolmogorov–Smirnov test of normality and presented as mean ± standard deviation (SD) or using a median minimum–maximum. The number of medications was compared between the two groups during the same period following the educational program given to the study group. Comparison was performed using the Student’s t test for independent samples for independent variables, or the Mann–Whitney U test, as needed. The heterogeneity in patient’s characteristics and other potential confounders were taken into consideration and included in the mode (odds ratios with 95% confidence intervals). All tests were two sided and considered significant when p < 0.05.
Results: study arm 1
In arm 1 of the study (inpatient), n = 100 for each of the study and intervention groups. Table 1 presents patient characteristics (age, sex) as well as prevalence of comorbidities, which were comparable between groups. Important exceptions were a higher rate of falls in the control group, and of recurrent infections and chronic pulmonary disease in the intervention group (the latter two almost reaching statistical significance.)
Table 1.
Arm 1: demographics and comorbidity.
| Intervention n = 100 |
Control n = 100 |
p value | |
|---|---|---|---|
| Age (mean + SD) | 82.5 + 6.4 | 82.0 + 7.6 | 0.880 |
| Female sex | 56 | 55 | 0.887 |
| Baseline medications (mean + SD) | 9.27 ± 2.0 | 8.81 ± 1.9 | 0.102 |
| Smoker | 26 | 21 | 0.404 |
| Diabetes mellitus | 64 | 62 | 0.770 |
| Hyperlipidemia | 81 | 89 | 0.113 |
| Hypertension | 98 | 96 | 0.407 |
| Cardiovascular disease | 72 | 81 | 0.133 |
| Congestive heart failure | 54 | 53 | 0.887 |
| Stroke | 34 | 25 | 0.163 |
| Chronic pulmonary disease | 32 | 20 | 0.053 |
| History of cancer* | 25 | 18 | 0.228 |
| Falls** | 8 | 20 | 0.014 |
| Hypothyroidism | 26 | 25 | 0.871 |
| Recurrent infections** | 8 | 2 | 0.052 |
Diagnosis of cancer: cure or remission.
More than one per year.
SD, standard deviation.
Table 2 presents for both study and control groups: a cross-section of the number of medications in each medication class at baseline, the number of deprescribing events, the number of new prescribing events, and the cross-section of the number of medications at study completion. Due to the heterogeneity in the baseline cross-sectional medication profile for the study and control groups, we considered the percent change for a given drug class to be a more useful parameter than the absolute number of prescribing and deprescribing events. Statistically significant percent changes are marked with an asterisk.
Table 2.
Arm 1: prescribing by drug class in each group.
| Medication | Study group |
Control group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| On admission (n) | Stop (n) | Add (n) | On discharge (n) | Rate of change | On admission (n) | Stop (n) | Add (n) | On discharge (n) | Rate of change | |
| CCBs* | 51 | 15 | 1 | 37 | −27.5% | 37 | 7 | 8 | 38 | +2.7% |
| ARBs* | 42 | 11 | 3 | 33 | −26.2% | 34 | 6 | 6 | 30 | +11.8% |
| ACEI | 27 | 9 | 0 | 19 | −29.6% | 29 | 6 | 2 | 29 | 0.0% |
| Alpha blockers | 13 | 6 | 1 | 12 | −7.7% | 16 | 4 | 6 | 15 | −6.3% |
| Beta blockers | 70 | 3 | 2 | 65 | −7.1% | 74 | 5 | 3 | 76 | +2.7% |
| Clonidine* | 14 | 0 | 6 | 20 | +42.9% | 9 | 3 | 0 | 6 | −33.3% |
| Spironolactone | 6 | 3 | 0 | 3 | −50.0% | 13 | 7 | 2 | 8 | −38.5% |
| Thiazides* | 11 | 6 | 0 | 5 | −54.5% | 17 | 12 | 0 | 5 | −70.6% |
| Furosemide* | 52 | 6 | 7 | 53 | +1.9% | 40 | 4 | 16 | 52 | +30.0% |
| Antiarrhythmics | 15 | 4 | 4 | 15 | 0.0% | 17 | 2 | 4 | 19 | +11.8% |
| Statins* | 67 | 19 | 0 | 48 | −28.4% | 61 | 13 | 3 | 51 | −16.4% |
| Insulin | 24 | 2 | 4 | 26 | +8.3% | 10 | 1 | 6 | 15 | +50.0% |
| Metformin* | 26 | 6 | 0 | 20 | −23.1% | 29 | 9 | 0 | 20 | −31.0% |
| Other antidiabetics* | 24 | 12 | 1 | 13 | −45.8% | 31 | 12 | 5 | 24 | −22.6% |
| Anticoagulants | 30 | 1 | 9 | 38 | +26.6% | 35 | 0 | 7 | 42 | +20.0% |
| P2Y12 inhibitor | 29 | 4 | 0 | 25 | −13.8% | 23 | 6 | 4 | 21 | −8.7% |
| Aspirin* | 50 | 19 | 5 | 36 | −28.0% | 50 | 12 | 5 | 43 | −14.0% |
| BZD* | 35 | 26 | 0 | 9 | −74.3% | 45 | 12 | 8 | 41 | −8.9% |
| Thyroid hormone | 23 | 1 | 0 | 22 | −4.3% | 27 | 1 | 0 | 26 | −3.7% |
| Steroids | 11 | 4 | 1 | 8 | −27.3% | 6 | 1 | 2 | 7 | +16.7% |
| PPI | 71 | 1 | 2 | 72 | +1.4% | 72 | 0 | 7 | 75 | +4.2% |
| Antipsychotics* | 13 | 6 | 0 | 7 | −46.2% | 6 | 2 | 3 | 7 | +16.7% |
| Antidepressants/SSRI* | 34 /21 | 12 | 0 | 22 /11 | −35.3%/−47.6% | 33 /21 | 7 | 2 | 28 /20 | −15.1%/−4.8% |
| Anti-Parkinson drugs | 12 | 3 | 0 | 9 | −25.0% | 7 | 1 | 0 | 6 | −14.3% |
| Bronchodilator inhalers* | 13 | 2 | 13 | 24 | +84.6% | 8 | 1 | 13 | 20 | +250.0% |
| Other medications* | 63 | 33 | 2 | 32 | −49.2% | 56 | 13 | 23 | 66 | +17.9% |
| Total* | 826 | 214 | 61 | 673 | −18.5% | 785 | 147 | 135 | 770 | −1.9% |
Difference between groups’ rate of change reaching statistical significance (p < 0.05).
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; BZD, benzodiazepine; CCB, calcium-channel blocker; PPI, proton-pump inhibitor; SSRI, selective serotonin reuptake inhibitor.
In the study arm, 826 medications were present at baseline (average 9.27 ± 2.0 per patient), 214 medications were deprescribed (25.9% rate of deprescription), 61 medications were newly prescribed (0.61 per patient), leading to 673 medications at discharge (7.64 ± 2.0), and a rate of change of −18.5%. In the control arm, 785 medications were present at baseline (8.81 ± 1.9), 147 deprescribed (18.7% rate of deprescription), 135 medications were newly prescribed (1.35 per patient), and, leading to 770 medications at discharge (8.65 ± 2.1), and a rate of change of −1.9%. The difference in medications at baseline was not statistically significant (p = 0.102) although the difference at discharge was (p < 0.0001). Comparing the rate of medication deprescription and new prescriptions between groups, the study group saw a relative increase in deprescribing of 38.5%, and a relative decrease in new prescribing by −54.8%.
With regards to statistically significant differences in specific medication groups (see p values in Table 2), for cardiovascular medications, the percent change for statins was −28.4% versus −16.4% between study and control, with no new statin prescriptions in the study group. Both groups had an increase in furosemide, although the percent change was higher in the control group (+30% control versus +1.9% intervention.) As for antihypertensives, calcium-channel blockers (CCBs) and angiotensin-receptor blockers (ARBs) were stopped at a significantly greater rate in the intervention group versus control group, while thiazides were stopped at a greater rate in the control group. The percent change for clonidine was +42.9% in the intervention group versus −33.3% in the control group. When grouped together as a class, blood-pressure-reducing medications, excluding diuretics, were deprescribed significantly more in the study group (p = 0.015, not presented in the table.)
Metformin was stopped slightly more in the control group (−31.0% versus −23.1%) while other oral antidiabetics were stopped more in the intervention group (−45.8% versus −22.6%). The percent change for insulin was higher in the control group (+50% versus +8.3%) but this was not statistically significant.
For psychoactive medications, the percent change for BZDs was −74.3% versus −8.9% in the study and control groups, respectively; for antipsychotics, percent change was −46.2% versus +16.7%, and for antidepressants, −35.3% versus −15.1% in the study and control groups, respectively. The study group did not offer new prescriptions for any of these classes on discharge.
The group entitled ‘other medications’ was very heterogeneous and composed of rare medications/drugs taken by only a few patients that did not fit any of the other categories presented in Table 2. They included bisphosphonates, hypnotics, antiemetics, laxatives, analgesics, vitamins and minerals, and others. The rate of change for this group of medications was −49.2% versus +17.9% in the study versus control group.
Methods: study arm 2 (family practice)
The intervention group included family physicians in the Central District of Israel who were recruited to undergo the GPGP training program. The program was similar to that described in arm 1, and participation was mandatory. Following training, each participating physician received a computerized list of their patients 70 years or older prescribed at least seven chronic medications over the preceding 6 months, who had a life expectancy of at least 6 months (absence of terminal illness). Patients not followed by the same family doctor over the study period were excluded. The physicians were asked to perform a virtual medication review based on the GPGP algorithm. The study group then participated in a group session to discuss the proposed changes. This session was moderated by a senior geriatrician from our team (DG) and a senior family physician administrator who was the direct supervisor of the participating physicians. Following this, the participants were asked to invite the patients on their lists to a comprehensive baseline visit (time 0), at which time initial medication changes could be made. Extended patient visits for this purpose were facilitated. The patients were invited for additional follow ups at 3 months and 6 months, and changes in medications (number and class) were recorded. The control group was a comparable group of family physicians from the Southern District of Israel who did not undergo the training program. They received a random list of patients similar to those in the study group and were informed that these patients were receiving seven medications or more, but were not requested to manage the patients differently.
Data collected included demographic characteristics and number and class of medications actually dispensed to the patient in the 6 months before and after time 0. Excluded were over-the-counter medications, as well as eyedrops, and topical skin preparations. The sample size of 100 patients was selected based on expected medium effect of the intervention (0.45 SD), with significance level of 5% (two tailed) and power of 80%. An additional 20% were recruited to allow for dropouts. Randomization was performed from a list of eligible patients, using the ‘Research Randomizer’ computer software. The primary outcome was the total number of medications before, and 6 months after, the intervention. The secondary outcomes were percent change by class of drugs, as well as a difference in the rate of hospitalization (intragroup and intergroup) 6 months after the intervention. Additionally, participating physicians were requested to fill out feedback questionnaires regarding their experience using the GPGP algorithm (Appendix 2).
Statistical comparisons of the number of drugs in the study group, before and after the intervention, were performed using a paired Student’s t test. Statistical comparisons between the study and control groups were performed by using the two-tailed t test. Statistical analysis of categorical variables between the study and control groups were performed by using the Chi-square test. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS package for Windows (version 2.0).
Results: study arm 2
In arm 2 of the study (family practice), 28 family doctors participated in the intervention program and their patients represent the study group (n = 100) versus a control group of 15 physicians with n = 100 control patients. There was no significant difference between groups in age (80.5 years, range 70–96 years), sex (51% female), nor baseline number of drugs, 10.97 ± 2.7 versus 10.5 ± 2.2, in control and study groups, respectively (Table 3; p = 0.149.) Comorbidities were not examined in this arm of the study. At 6 months after the intervention, in the control group, 11.21 ± 2.91 drugs were taken on average per patient, trending an increase from baseline (p = 0.062). In the intervention group, 10.04 ± 2.16 were taken on average per patient. The magnitude of this reduction, although small, was significant (p = 0.005). The difference between groups postintervention was also significant (p = 0.001).
Table 3.
Arm 2: primary outcome: number of medications prescribed before and 6 months after the intervention.
| Study group (n = 100) | Control group (n = 100) | p value | |
|---|---|---|---|
| Drugs at baseline (mean ± SD) | 10.5 ± 2.2 | 10.97 ± 2.7 | 0.149 |
| Drugs at 6 months (mean ± SD) | 10.04 ± 2.16 | 11.21 ± 2.9 | 0.001 |
| p value | 0.005 | 0.062 |
SD, standard deviation.
Table 4 presents the change in number of patients taking a specific drug class 6 months after the intervention, in the control and study groups. Statistically significant percent changes were seen in the study group for statins (−8.0%), thiazides (−27.3%), CCBs (−10.6%), BZDs and Z drugs (−10.6%), and vitamins/minerals (−10.4%). The overall percent change for the control and intervention groups was −0.07% versus −6.1%, respectively. It is to be noted that some individuals were taking more than one drug in a specific group, explaining the percent change decrease for the control group in this tabulation despite the overall increase in drugs prescribed.
Table 4.
Arm 2: number of patients prescribed specific drug groups.
| Drug group | Study group |
Control group |
||||
|---|---|---|---|---|---|---|
| Baseline | After 6 months | Rate of change | Baseline | After 6 months | Rate of change | |
| Statins | 87 | 80 | −8.0%* | 81 | 80 | −1.20% |
| Beta blockers | 67 | 66 | −1.50% | 71 | 69 | −2.80% |
| Alpha blockers | 40 | 37 | −7.50% | 33 | 33 | 0.00% |
| ACEIs/ARBs | 78 | 73 | −6.40% | 82 | 79 | −3.60% |
| Thiazides | 33 | 24 | −27.3%* | 39 | 39 | 0.00% |
| CCBs | 66 | 59 | −10.6%* | 57 | 58 | 1.80% |
| Nitrates | 18 | 19 | 5.60% | 11 | 12 | 9.10% |
| Antiaggregants | 84 | 82 | −2.40% | 75 | 74 | −1.30% |
| Anticoagulants | 15 | 17 | 13.30% | 18 | 18 | 0.00% |
| BZD/Z drugs | 66 | 59 | −10.6%* | 77 | 74 | −3.80% |
| PPI | 59 | 58 | −1.70% | 60 | 58 | −3.30% |
| NSAIDs | 0 | 1 | – | 1 | 1 | 0.00% |
| Laxatives | 17 | 17 | 0.00% | 23 | 25 | 8.70% |
| TCAs | 6 | 5 | −16.60% | 2 | 1 | −50.00% |
| SSRIs/SNRIs | 18 | 16 | −11.10% | 37 | 37 | 0.00% |
| Vitamins/minerals | 77 | 69 | −10.4%* | 87 | 87 | 0.00% |
| Antidiabetics | 72 | 72 | 0.00% | 79 | 82 | 3.80% |
| Total | 803 | 754 | −6.1%* | 833 | 827 | −0.07% |
Statistically significant reduction in the study group, p < 0.05.
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; BZD, benzodiazepine; CCB, calcium-channel blocker; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
There was no significant difference in the change of the number of hospitalizations between the study and the control groups (p = 0.693, data not presented in table form). There was no change in the number of hospitalizations within each group 1 year before and after the intervention (p = 0.744) and (p = 0.816) in the intervention study group and in the control group, respectively.
In terms of feedback, no family doctors contacted the geriatrician or pharmacist consultants over the course of the study. The poststudy questionnaire was returned by only 8/28 participants; 5/8 respondents indicated lack of time in using the algorithm, while 3/8 noted difficulty in using it; 5/8 cited the patient being in ‘stable condition’ as a reason for not changing medications, and 2/8 experienced the patient rejecting the physician’s suggestion to deprescribe.
Discussion
As early as the last decade of the 20th century, polypharmacy began to emerge as a serious clinical problem in the geriatric population, being observed in community dwellers,19 those admitted to nursing facilities,20 as well as in the acutely hospitalized.21,22 Several works have demonstrated a positive correlation between medication use (number, type, and inappropriateness) and hospitalizations among older patients, particularly those consuming seven medications or more.23,24 As the factors fueling IMUP multiply, its clinical, social, and economic adverse effects become multifarious, and increase exponentially.6 With the goal of combating the global IMUP pandemic, IGRIMUP published a list of 10 action recommendations, some of them emphasizing the importance of educating healthcare professionals about polypharmacy and deprescribing. In the ethos of these action recommendations, we propose the GPGP algorithm as an effective and versatile tool to assist the clinician in optimizing geriatric prescribing. GPGP is the subject of the only ongoing longitudinal study in the field of deprescribing, which has thus far shown that rational polydeprescribing is associated with improved clinical outcomes, an effect that persists over years of follow up.14 However, we recognize that obstacles exist in the widespread implementation of the GPGP method among nongeriatricians. It can be time consuming, requires specialized multidisciplinary knowledge, and most of all, demands a paradigm shift from the prevalent guideline-based practice. We considered that formal training in GPGP may be necessary to render it more accessible. In this light, the present study evaluates the efficacy of an education intervention based on GPGP, the results suggesting that such a method is effective in changing prescribing patterns in both the community and in-hospital settings.
In study arm 1, we examined the effect of the intervention in the inpatient setting. The patients were a heterogeneous group of patients 70 years and older, representative of the general inpatient geriatric population. We chose an age cut-off of 70 instead of the traditional 65, due to the general robustness of patients below the age of 70. We believe this would have resulted in a population with less polypharmacy and generally less reflective of the geriatric patient populations such of VOCODFLEX for which GPGP was designed, and for whom we posit the greatest benefit to be reaped through polydeprescribing. At the end of the study, the intervention resulted in a significant net decrease in the number of medications prescribed by 18.5%, or 1.6 medications less per patient at discharge, versus no significant change in the control group. In relative terms, a percent change rise in deprescription of 38.5%, and decrease in new prescriptions of 54.8% on leaving hospital was seen between the groups. Additionally, specific drug classes were deprescribed with an increased preponderance: statins, antihypertensives, oral antihyperglycemics, aspirin, BZDs, antipsychotics, antidepressants, and ‘other’ medications. This suggests that several key messages presented to the participating physicians during the education intervention were implemented and changed prescribing practices.
We were interested whether the study participants developed deprescribing judgment (implicit skills) or were simply adhering to a set of learned criteria (explicit skills). The drug classes preferentially deprescribed in the study group suggests the acquisition of implicit skills. In their favor, the control group deprescribed more thiazides, drugs ‘classically’ attributed to side effects in older patients (falls, hyponatremia); accordingly, the control group indeed had more patients with recurrent falls. The intervention group deprescribed antihypertensives of other classes such as angiotensin-converting-enzyme inhibitor (ACEI)/ARB, which are not classic targets for deprescribing and are not prominent on lists of ‘drugs to stop,’3,11 as well as the grouping of all antihypertensives. This could reflect a newfound sensitivity to moderate blood pressure goals in frail older patients.25 In a similar vein, nonmetformin oral hypoglycemics were stopped more, possibly reflecting a sensitivity to lenient glycosylation (A1C) goals and the adverse effects in older patients of medication-induced hypoglycemia.26,27 Of great importance, the study group stopped psychoactive medications (BZDs, antipsychotics, antidepressants) to a far greater extent than the control group; additionally, no patients left hospital with a new prescription for one of these drug classes. This suggests that the physicians recognized the prominent role these medications play in geriatric IMUP-related morbidity, including their contribution to the specific hospitalization, or their potential to lead to future ADEs. Miscellaneous ‘other drugs’ were also stopped at a much greater rate in the intervention group, possibly reflecting a newfound willingness to stop drugs taken for unclear indications, as well as recognition that the absolute number of drugs taken correlates with ADEs.28
In some areas of prescribing, the intervention was less successful. Certain drugs appeared problematic to deprescribe, including PPIs and beta blockers, the former being notorious for overprescription.29 Possible reasons for this could be that these drugs appear benign, when in fact the study physicians may have been unfamiliar with their side-effect profiles. Another reason could be a fear of gastrointestinal bleeding events with withdrawal of the PPI. Future interventions could involve a focus on deprescribing these medications, and in the case of PPIs, possibly using a deprescribing protocol.29 Another issue is idiosyncratic or biased prescribing patterns, as can be seen in the case of clonidine in the intervention group, which was prescribed far more. This could be explained by a greater proportion of patients with refractory hypertension, although it seems more likely to be due to participating physicians’ preference for the drug, who were unaware of its side-effect profile in older patients.
An effect similar to that observed in arm 1 but of lesser magnitude was seen in study arm 2. We examined the effect of the educational intervention on family physicians and their patients, which was a heterogeneous group reflective of the general outpatient geriatric population aged 70 years and older. The intervention resulted in a significant net decrease in the number of medications prescribed by 6.1%, 6 months after the intervention (0.45 drugs less per patient), versus no significant change in the control group. Additionally, specific drug classes were deprescribed with an increased preponderance: statins, CCBs, thiazides, BZDs, and vitamins and minerals.
The results in the outpatient arm were less impressive than in the inpatient arm. The effect size was small, and we felt that the participating physicians were not enthusiastic about deprescribing. They did not use the resources at their disposal (such as contacting the senior geriatrician and pharmacist available for assistance), and few gave the requested feedback. Among those who did provide feedback, none indicated that they were afraid to deprescribe, although other obstacles were highlighted. Some patients refused the suggested changes; patient education about polypharmacy is also critical to the deprescribing endeavor.30 One physician wrote a comment that summarizes part of the problem: ‘Doctors are obliged to comply with the family practice’s guidelines, which do not differentiate between patients based on age or frailty. In addition, family doctors are subject to complaints from patients and their families and also from medical systems; even family doctors who believe in the importance of reducing polypharmacy do not feel they are supported by the system.’ These comments are in line with others who found that even physicians aware of the harm of IMUP and who are willing to make alterations find it difficult to change well-established patterns of care, especially in clinical environments not conducive to change.31 These perspectives underscore the multitude of obstacles to deprescribing in the family practice setting, highlighting the centrality of change on the healthcare policy level, which could provide a more permissive environment for deprescribing.
Despite these obstacles, even on a preliminary level, it was demonstrated in the outpatient setting as well that the intervention changed prescribing patterns to favor deprescribing. In contrast, in the control group, a trend toward an increased number of medications over time was apparent. We also observed a general decrease in a specific number of classes, including those emphasized during the training sessions, both those ‘classically’ targeted for deprescribing such as thiazides, and those that are not (CCBs, statins.) This may reflect a shift toward geriatric-friendly prescribing habits. It is noteworthy that the timing of the study coincided with new guidelines for addressing hypertension and hyperlipidemia in older patients,32–34 possibly supporting a perception that it is safe to deprescribe these drug classes in certain patients. Although a gap exists between guidelines and daily practice,31 Wang and coworkers concluded that altering clinical targets may motivate physicians to reduce or minimize prescribed medication.35 Another success was the deprescribing of more BZDs and Z drugs, a class well-known for their difficulty to deprescribe in the setting of chronic use.36
We have previously shown that patients who undergo polydeprescribing do not experience a worsening in clinical outcomes such as morbidity and mortality.14 This finding was also reflected in the outpatient arm of the present study, which did not observe an intragroup or intergroup change in the number of hospitalizations over a 6-month follow up. That being said, rate of hospitalization was a secondary outcome, the study not having been powered to detect a statistical difference between groups.
While in both study arms a statistically significant increase in net deprescribing and in specific drug classes was seen in the intervention group, several differences were apparent between the two settings. The effect size was greater in the inpatient setting, reflecting that it may be easier for nongeriatricians to deprescribe when a patient is admitted with an acute medical problem. Conceptually, when the patient is admitted with a decompensation, the physician may view that outpatient care has failed and that a change in prescribing is merited. When the physician is sensitized or primed to the role of polypharmacy in acute presentations in the old patient via an education intervention, fertile ground is created for deprescribing. In contrast, in the family practice setting, it may be more difficult to convince the clinician to deprescribe when the patient is felt to be ‘stable’ (as noted by several family doctor participants in the feedback questionnaires.) Ostini and colleagues comment that the concept of ‘prescribing inertia’ provides a framework for understanding why prescribing might continue when it should not.15
Some readers may evaluate the results of this study to be somewhat disappointing, given that it only demonstrated a small-moderate decrease in the number of medications prescribed. This study was designed by geriatricians, and for the consultant geriatrician, one of the core aims may be to deprescribe as many medications as possible that are judged as inappropriate or unnecessary. To place these results in perspective, we must emphasize the paradigmatic differences between the roles of the geriatrician, the family physician, and the internist. When the patient is admitted with an acute medical problem to the general medical ward, it is frequently necessary to commence new treatments, for example anticoagulation for new-onset atrial fibrillation, platelet antiaggregants for a new vascular event, diuretics for new or worsening heart failure, and so forth. New prescribing of these medications should not be viewed as a ‘failure’; they are a clinical necessity.
Additionally, in terms of good geriatric care, we wonder if the educational intervention biased the study group to stopping medications even when there might be an indication to initiate beneficial medications. Many prescribing tools including STOPP/START highlight the addition of absent beneficial medications.11 Some examples include initiating antidepressant therapy in depressed older patients (while the study group stopped antidepressants, very few were initiated), as well as identifying patients at risk for osteoporotic fracture and initiating preventative pharmacotherapy. While the study demonstrated an acquired ability to deprescribe inappropriate or unnecessary medications, it was not designed to evaluate other components of good prescribing. Other examples not evaluated in this study that are delineated in the GPGP algorithm include dose correction and the replacement of a potentially harmful drug with a more appropriate drug.
In the outpatient setting, it could be helpful, conceptually, to reframe the problem of polypharmacy in terms of good preventive care in older patients. Just as atherosclerosis can be depicted as a multifactorial problem that merits addressing its multiple synergistic causes (diabetes, cholesterol, smoking, exercise, and so forth), so too, polypharmacy could be conceptualized as a multifactorial problem justifying the mitigation of its various parts (maximal reduction in the total number of medications.) As has been discussed above, evidence describing the plethora of short-term, as well as long-term complications due to polypharmacy should be emphasized.
It is important to note that not all the patients in the present study were VOCODFLEX, and therefore, in some of the younger and more robust study patients, adherence to evidence-based guidelines for the general population may in fact have been appropriate. This renders the results of our study more generalizable to the general geriatric population. In future studies, it will be important to define measures of frailty, function, and cognition in order to differentiate between more robust patients who may benefit from certain drug interventions, and VOCODFLEX who would not.
Limitations
Both arms of the study have several limitations, including small sample sizes of inherently heterogeneous patients, and in the inpatient arm, a single medical ward with a small group of treating physicians. Participation bias on the part of the physicians was an important factor. While we were able to demonstrate the direct effect of an educational intervention on physicians’ prescribing patterns, we judged the scale of this study to be too small to analyze long-term clinical outcomes such as morbidity, mortality, and quality of life. Hopefully this will be the subject of future work. In the inpatient arm, we did not have access to data on future hospitalization, which would have provided an important data point. Another limitation in the inpatient arm was that we were unable to know if the treating physician in the community continued the changes in prescribing proposed by the inpatient team or reverted to the previous medication list. This point, which touches on the critical issue of continuity of care, should be the focus of future work. In terms of durability of the effect, we wonder if a multisession intervention would be more effective. The follow-up time was short, and it would be interesting to study the retention of deprescribing principles over time.
Conclusion
Educational interventions geared to changing inappropriate prescribing in older patients are a key tool in the global fight against IMUP. This study demonstrates that a one-time educational intervention based on GPGP can be effective in changing prescribing patterns in family practitioners and internists, resulting in a decrease in the number of medications prescribed. The GPGP algorithm provides a useful basis for teaching good geriatric prescribing. Internists and family practitioners do not fulfill the same role as geriatricians and therefore, cannot be expected to function in an identical fashion. Nevertheless, deprescribing must take place over all care settings including acute inpatient and family practice.37 Future work should emphasize methods which aim to remove the multitude of deprescribing barriers in order to aid practitioners in this critical task.
Appendices
Appendix 1.
The Garfinkel Good Palliative-Geriatric Practice algorithm.13
Appendix 2
Feedback questionnaire for study arm 2.
Dear Dr _________________________
This feedback questionnaire is with regards to your participation in the Quality of Prescribing and Polypharmacy Project, via participation in the prescribing workshop and the use of the GPGP algorithm.
From the list below, please select the most appropriate reasons which explain lack of intervention to reduce polypharmacy in the following patients in your care, adjacent to their names listed below:
(1) I used the algorithm and according to it, there was no need for any change in prescribing.
(2) The algorithm was difficult to use, and therefore I didn’t use it.
(3) I didn’t have enough time to devote the thought necessary to employ the algorithm.
(4) I felt uncomfortable about making a treatment change on my own and I didn’t have time to consult with another physician.
(5) The patient’s medical status was stable, and therefore I didn’t feel it was necessary to make any medication changes.
(6) There isn’t enough of an evidence base for the use of the algorithm, therefore I preferred not to work with it.
(7) I consulted with a specialist and we decided that it wouldn’t be right to change the patient’s medications at this time.
(8) Making changes would be appropriate at this time but I didn’t believe the patient would be capable of complying with the recommendations.
(9) I recommended deprescribing medications but the patient did not agree with the changes.
(10) I am not the treating physician for this patient.
| Patient’s name | ID | Main reason for not deprescribing | Additional reasons for not deprescribing |
|---|---|---|---|
We would be pleased to receive any additional comments:
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Aaron Jason Bilek  https://orcid.org/0000-0001-6720-1841
https://orcid.org/0000-0001-6720-1841
Doron Garfinkel  https://orcid.org/0000-0002-3171-9881
https://orcid.org/0000-0002-3171-9881
Contributor Information
Aaron Jason Bilek, Geriatrics Department, Tel Aviv Sourasky Medical Center, Weizmann Street 6, Tel Aviv 64239, Israel.
Yuval Levy, Deputy Director General Hospital, Sheba Medical Center, Tel Hashomer, Israel.
Haneen Kab, Pharmacy Department at Hebrew University, Jerusalem, Israel.
Pavel Andreev, Department of Medicine C, Wolfson Medical Center, Holon, Israel.
Doron Garfinkel, Medical Center, Ramat Gan, Israel Homecare Hospice, Israel Cancer Association, Givatayim, Israel IGRIMUP (International Group for Reducing Inappropriate Medication Use and Polypharmacy), Bat Yam, Israel.
References
- 1. Mangin D, Bahat G, Golomb BA, et al. International group for reducing inappropriate medication use & polypharmacy (IGRIMUP): position statement and 10 recommendations for action. Drugs Aging 2018; 35: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294: 716–724. [DOI] [PubMed] [Google Scholar]
- 3. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019; 67: 674–694. [DOI] [PubMed] [Google Scholar]
- 4. Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmac 2007; 5: 345–351. [DOI] [PubMed] [Google Scholar]
- 5. Gnjidic D, Hilmer SN, Blyth FM, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther 2012; 91: 521–528. [DOI] [PubMed] [Google Scholar]
- 6. Garfinkel D. Geriatric Boom Catastrophe - A major medical, economic and social nightmare of the 21st century. Proceedings of the 16th Congress of the International Association of Gerontology, 18–23 August 1997; Adelaide, Australia. [Google Scholar]
- 7. Garfinkel D. Poly-de-prescribing vs. polypharmacy – the weapon to fight an iatrogenic epidemic. An overview. Europ J Geriatr Gerontol 2019; 1(1): 1–10. [Google Scholar]
- 8. Garfinkel D. and IGRIMUP. Overview of current and future research and clinical directions for drug discontinuation: psychological, traditional and professional obstacles to deprescribing. Eur J Hosp Pharm 2017; 24: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garfinkel D, Ilhan B, Bahat G. Routine deprescribing of chronic medications to combat polypharmacy. Ther Adv Drug Saf 2015; 6: 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topinková E, Baeyens JP, Michel JP, et al. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging 2012; 29: 477–494. [DOI] [PubMed] [Google Scholar]
- 11. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2014; 44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J 2007; 9: 430–434. [PubMed] [Google Scholar]
- 13. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 14. Garfinkel D. Poly-de-prescribing to treat polypharmacy: efficacy and safety. Ther Adv Drug Saf 2018; 9: 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostini R, Hegney D, Jackson C, et al. Knowing how to stop: ceasing prescribing when the medicine is no longer required. J Manag Care Pharm 2012; 18: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anthierens S, Tansens A, Petrovic M, et al. Qualitative insights into general practitioners views on polypharmacy. BMC Fam Pract 2010; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thillainadesan J, Gnjidic D, Green S, et al. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging 2018; 35: 303–319. [DOI] [PubMed] [Google Scholar]
- 18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166: 605–609. [DOI] [PubMed] [Google Scholar]
- 19. Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003; 348: 1556–1564. [DOI] [PubMed] [Google Scholar]
- 20. Cooper J. Adverse drug reaction-related hospitalizations of nursing facility patients: a 4-year study. South Med J 1999; 92: 485–490. [DOI] [PubMed] [Google Scholar]
- 21. Azad N, Tierney M, Victor G, et al. Adverse drug events in the elderly population admitted to a tertiary care hospital. J Healthc Manag 2002; 47: 295–305. [PubMed] [Google Scholar]
- 22. Spinewine A, Swine C, Dhillon S, et al. Appropriateness of use of medicines in elderly inpatients: qualitative study. BMJ 2005; 331: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flaherty JH, Perry HM, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci 2000; 55: M554–M559. [DOI] [PubMed] [Google Scholar]
- 24. Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging 2005; 22: 69–82. [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 26. Farrell B, Black C, Thompson W, et al. Deprescribing antihyperglycemic agents in older persons: evidence-based clinical practice guideline. Can Fam Physician 2017; 63: 832–843. [PMC free article] [PubMed] [Google Scholar]
- 27. Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51(Suppl. 5): S265–S280. [DOI] [PubMed] [Google Scholar]
- 28. Viktil KK, Blix HS, Moger TA, et al. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol 2007; 63: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician 2017; 63: 354–364. [PMC free article] [PubMed] [Google Scholar]
- 30. Reeve E, To J, Hendrix I, et al. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 2013; 30: 793–807. [DOI] [PubMed] [Google Scholar]
- 31. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003; 362: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 32. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 33. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357. [DOI] [PubMed] [Google Scholar]
- 34. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014; 129(Suppl. 2): S1–S45. [DOI] [PubMed] [Google Scholar]
- 35. Wang K, Camargo M, Veluswamy R. Evidence-based strategies to reduce polypharmacy: a review. OA Elderly Medicine 2013; 1: 5. [Google Scholar]
- 36. Pottie K, Thompson W, Davies S, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician 2018; 64: 339–351. [PMC free article] [PubMed] [Google Scholar]
- 37. Garfinkel D, Ilin N, Waller A, et al. Inappropriate medication use and polypharmacy in end-stage cancer patients: isn’t it the family doctor’s role to de-prescribe much earlier? Int J Clin Pract 2018; 72: e13061. [DOI] [PubMed] [Google Scholar]