Abstract
Background:
Chronic thromboembolic pulmonary hypertension (CTEPH) may be treated with pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA) and medical therapy (MT). Assessment in a multidisciplinary team of experts (CTEPH team) is currently recommended for treatment decision making. The aim of the present study was to report the effects of such an interdisciplinary concept.
Methods and results:
A total of 160 patients were consulted by the CTEPH team between December 2015 and September 2018. Patient baseline characteristics, CTEPH team decisions and implementation rates of diagnostic and therapeutic procedures were analysed. Change in World Health Organization (WHO) functional class and survival rates were evaluated by treatment strategy. A total of 51 (32%) patients were assessed as operable and 109 (68%) were deemed inoperable. Thirty-one (61% of operable patients) underwent PEA. Patients treated with PEA, BPA(+MT) and MT alone were 50.9 ± 14.7, 62.9 ± 15.1 and 68.9 ± 12.7 years old, respectively. At the follow-up, PEA patients had the highest WHO functional class improvement. Patients treated with BPA(+MT) had significantly better survival than PEA (p = 0.04) and MT patients (p = 0.04; 2-year survival of 92%, 79% and 79%, respectively).
Conclusions:
The CTEPH team ensures that necessary diagnostic procedures are performed. A relatively low proportion of patients was assessed by the CTEPH team as operable and underwent surgery, which in survivors resulted in the best functional improvement. Although patients undergoing BPA(+MT) were older than patients treated with PEA, their survival was better than patients subjected to PEA or MT alone.
The reviews of this paper are available via the supplemental material section.
Keywords: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, multidisciplinary team, pulmonary endarterectomy, survival
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare form of pulmonary hypertension associated with incomplete thrombus resolution, which leads to narrowing and obstruction of pulmonary arteries resulting in high pulmonary vascular resistance (PVR), increased right heart afterload and progression of heart failure if left untreated.1–3
Available treatment methods include surgery [pulmonary endarterectomy (PEA)],4–6 interventional therapy [balloon pulmonary angioplasty (BPA)]7–11 and medical therapy (MT).12 According to European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension, PEA in deep hypothermic circulatory arrest is the treatment of choice for technically operable patients with acceptable risk–benefit ratio.2 Currently riociguat13,14 is recommended for inoperable, persistent or recurrent CTEPH,2 as the only approved drug therapy. However, other pulmonary hypertension specific drugs such as sildenafil,15,16 bosentan,17 macitentan18 and treprostinil19 are also used.12 BPA may be considered in inoperable patients and patients with unfavourable risk–benefit ratio for surgery.2 In line with current guidelines, a multidisciplinary team of experts should assess each patient’s operability and individually choose the most suitable therapeutic strategy.2 A CTEPH team should include physicians experienced in pulmonary hypertension, a cardiologist or pulmonary hypertension physician with expertise in echocardiography, an interventionalist experienced in right heart catheterization (RHC) and a radiologist. At least on-call expertise of a surgeon experienced in PEA should be provided.2
Studies analysing patient subpopulations20,21 qualified by a CTEPH team for various treatment options were published recently. Therefore, our study was aimed not only at analysing the profiles of patients consulted by the CTEPH team and the CTEPH team’s decisions, but also to investigate implementation rates of introduced diagnostic and therapeutic strategies and to examine survival rates and clinical outcomes in different treatment groups.
Patients and methods
Patients consulted by the CTEPH team were referred from a network of Polish pulmonary hypertension centres and had CTEPH diagnosis established according to current guidelines.2 A total of 160 patients had been consulted between December 2015 and September 2018. Each patient was assessed as operable (candidate for PEA) or inoperable, with PEA being considered first-line treatment.2 Patients were regarded as ineligible for surgery owing to high-risk comorbidities or surgically inaccessible localization of thromboembolic lesions. Inoperable patients were offered BPA procedures and MT. In case of patients with very small distal lesions confined to subsegmental pulmonary arteries on imaging, BPA was not performed. Other patients were assigned to combined therapy with MT and BPA. Those who refused BPA received MT alone.
Parameters such as World Health Organization (WHO) functional class, the plasma N-terminal pro-B type natriuretic peptide (NT-proBNP) level concentration and distance covered in the 6 min walk test (6MWT) were retrospectively analysed in all patients consulted by CTEPH team, in subgroups of operable and inoperable patients and in treatment subgroups. In addition, the haemodynamic parameters of pulmonary circulation measured by RHC according to current guidelines22 were examined: right atrial pressure (RAP), mean pulmonary arterial pressure (mPAP), cardiac output (CO), cardiac index, pulmonary capillary wedge pressure (PCWP) and PVR. Frequency of CTEPH development risk factors such as history of acute pulmonary embolism, thrombophilia, cancer history or splenectomy was investigated in both patient subgroups. Moreover, the implementation rate of medical imaging types, upon which CTEPH team decisions were based, was examined. In addition, time periods from diagnostic RHC to CTEPH team evaluation and from CTEPH team evaluation to therapy initialization were measured.
Follow-up was performed at a median of 22 months after CTEPH team evaluation. In surviving patients, WHO functional class was reassessed. Introduced therapeutic methods were analysed. Persistent pulmonary hypertension after PEA was defined as mPAP >25 mmHg by RHC after surgery. Causes of deaths were examined.
Survival analysis was performed for three subgroups of patients: patients treated with PEA, patients treated with BPA (who might have also received MT) and patients treated with MT alone (including patients who received no pulmonary hypertension targeted therapy). Change in WHO functional class was also compared in patients treated with different therapeutic strategies.
The study was conducted according to the Declaration of Helsinki and according to Polish law neither patient’s consent nor approval of internal review board is required for retrospective analysis of available data.
Statistical analysis
For continuous variables, data are presented as mean ± standard deviation (SD). Independent samples Student’s t test and one-way analysis of variance (ANOVA) with post hoc Tukey’s honestly significant difference test were used for comparison of two and multiple groups, respectively. For categorical variables, data are presented as number (%) and Pearson’s chi-squared test was used for comparisons. The Kaplan–Meier method was used for survival analysis, groups were compared by the log-rank test and chi-squared test. p < 0.05 was considered significant. Hazard ratios [95% confidence interval (CI), p value] were calculated using univariable Cox regression. Covariates were selected as potential inputs into the multivariable Cox regression as they fulfilled the inclusion criterium of a univariable p < 0.05. All statistical analyses were performed using STATISTICA 13.1 (STATSOFT, Tulsa, OK, USA).
Results
Diagnostic procedures and CTEPH team decisions
Prior to the evaluation, patients underwent the following diagnostic procedures: RHC, pulmonary angiography, CT angiography, ventilation/perfusion lung scan (VQ scan) and magnetic resonance imaging (MRI); Table 1. Median of time from diagnostic RHC to CTEPH team evaluation was 29 days (11–68 days).
Table 1.
Medical imaging techniques upon which CTEPH team decisions were based.
| Variables | n (%) |
|---|---|
| RHC | 160 (100%) |
| Pulmonary angiography | 160 (100%) |
| CT angiography | 139 (87%) |
| VQ scan | 46 (29%) |
| MRI | 2 (1%) |
CT, computed tomography; MRI, magnetic resonance imaging; RHC, right heart catheterization; VQ scan, ventilation/perfusion lung scan.
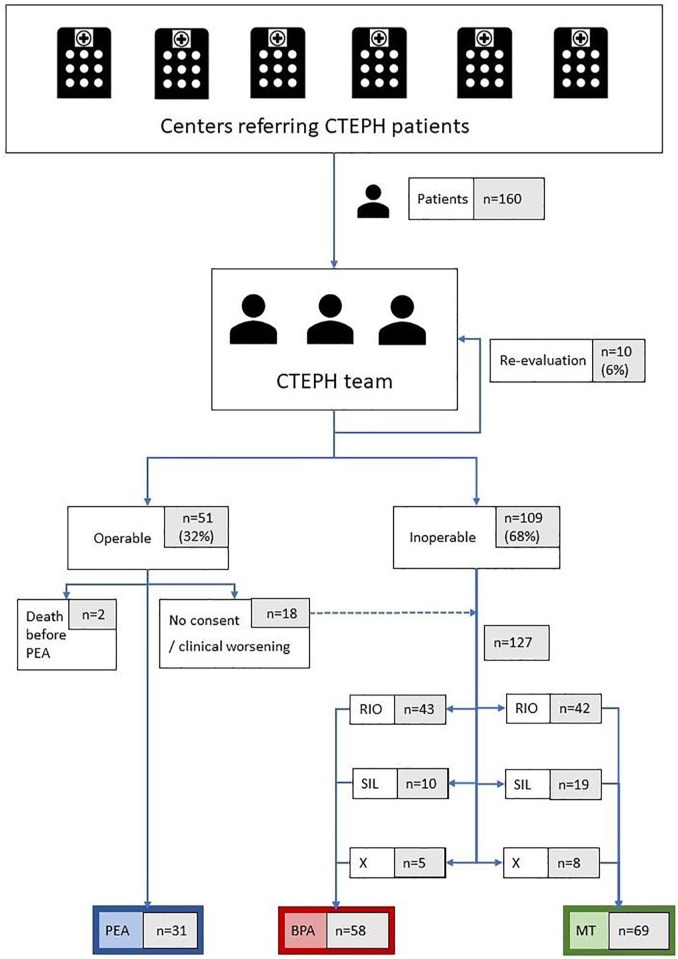
A total of 51 patients (32%) out of 160 were assessed as operable and candidates for PEA whereas 109 (68%) were deemed inoperable: 46 (29%) because of the presence of high-risk comorbidities and 63 (39%) owing to distal localization of thromboembolic material, which is considered as surgically inaccessible6 (Figure 1). In the case of 10 patients (6%), the CTEPH team asked for an additional imaging study to choose the best therapeutic strategy upon re-evaluation.
Figure 1.
Therapy for patients evaluated by the CTEPH team.
BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; MT, medical therapy alone; PEA, pulmonary endarterectomy; RIO, riociguat; SIL, sildenafil; X, no medical therapy.
Baseline characteristics of operable and inoperable patients
Baseline characteristics of patients assessed by the CTEPH team as operable and inoperable were analysed retrospectively. Operable patients were younger (56.7 ± 15.3 versus 66.3 ± 14.6, p < 0.01) and had lower cardiac index (2.2 ± 0.4 l/min/m2 versus 2.5 ± 0.8 l/min/m2, p = 0.02) than those not amenable to surgery. There was no statistically significant difference with respect to other haemodynamic parameters, gender, NT-proBNP level concentration, distance covered in 6MWT, prevalence of thrombophilia, splenectomy, history of acute pulmonary thromboembolism or cancer between these two groups.
Introduced therapeutic procedures
A total of 51 patients (32%) were assessed by CTEPH team as operable (Figure 1). Subsequently, 31 (61% of operable patients) underwent PEA, 2 (4%) died before the intervention and 18 patients (35%) became candidates for other treatment options as they refused surgery (10 patients) or became ineligible for surgery owing to clinical worsening (8 patients). Out of 31 PEA patients, 6 were diagnosed with persistent CTEPH and required further treatment: 3 of them received sildenafil, 1 received riociguat and 2 were treated with riociguat and BPA. A total of 109 patients (68%) were deemed inoperable by the CTEPH team. Out of 127 patients who did not undergo PEA, as being primary or secondary inoperable or lacking consent (Figure 1), 58 (46%) were treated with BPA and 69 (54%) were offered MT alone with sildenafil or riociguat. Average number of BPA sessions per patient was 4.4 ± 2.3. Among BPA patients 53 (91%) additionally received MT. Median times from CTEPH team evaluation to PEA, to first BPA procedure and to riociguat therapy initiation were 43 (19–154) days, 150 (55–292) days and 30 (7–118) days, respectively.
Characteristics of patients treated with PEA, BPA (with or without MT) and with MT alone
PEA, BPA and MT patients were 50.9 ± 14.7, 62.9 ± 15.1 and 68.9 ± 12.7 years old, respectively (p < 0.01). Post hoc analysis showed that the PEA cohort was significantly younger than the BPA cohort (p < 0.001) and MT cohort (p < 0.001). The BPA cohort was also younger than the MT cohort (p = 0.04); see Table 2. Patients subjected to PEA had suffered more often from thrombophilia than those subjected to BPA and treated with MT alone (8 (26%) versus 6 (10%) versus 4 (6%), p = 0.01). There was no significant difference with respect to gender, NT-proBNP level concentration, distance covered in 6MWT, splenectomy, history of acute pulmonary thromboembolism or cancer between the treatment groups (Table 2). Patients in all treatment groups showed similar distribution of presented WHO functional class with slightly lower percentage in class IV of BPA than PEA patients and higher than MT patients (Table 2). No significant difference was observed with respect to haemodynamic parameters (Table 3).
Table 2.
Baseline characteristics of the 160 CTEPH patients treated with PEA, BPA (with or without MT) and with MT alone (riociguat/sildenafil).
| Variable | All patients (n = 160) | PEA (n = 31) | BPA (n = 58) | MT (n = 69) | p value |
|---|---|---|---|---|---|
| Age, years | 63.2 ± 15.4 | 50.9 ± 14.7 | 62.9 ± 15.1 | 68.9 ± 12.7 | <0.01 |
| Gender, female | 95 (59%) | 17 (55%) | 33 (57%) | 45 (65%) | 0.41 |
| History of acute pulmonary embolism | 118 (74%) | 26 (84%) | 43 (74%) | 49 (71%) | 0.29 |
| Thrombophilia | 18 (11%) | 8 (26%) | 6 (10%) | 4 (6%) | 0.01 |
| Cancer history | 17 (11%) | 1 (3%) | 6 (10%) | 10 (14%) | 0.27 |
| Splenectomy | 10 (6%) | 1 (3%) | 6 (10%) | 2 (3%) | 0.28 |
| NT-proBNP, pg/ml | 2639 ± 3926 | 1894 ± 2475 | 3005 ± 4650 | 2666 ± 3772 | 0.47 |
| 6MWT, m | 310 ± 128 | 328 ± 131 | 342 ± 142 | 280 ± 354 | 0.09 |
| WHO functional class I/II/III/IV | 0/31(19%) /110(69%) /19(12%) | 0/5(16%) /20(65%) /6(19%) | 0/11(19%) /39(67%) /8(14%) | 0/15(21%) /51(72%) /5(7%) | - |
BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; MT, medical therapy (riociguat/sildenafil) alone; NT-proBNP, N-terminal pro-brain natriuretic peptide; PEA, pulmonary endarterectomy; WHO, World Health Organization; 6MWT, 6-minute walk test.
Data are presented as mean ± SD or count (percentage).
Table 3.
Haemodynamics of the 160 CTEPH patients treated with PEA, BPA (with or without MT) and with MT alone (riociguat/sildenafil).
| Haemodynamics | All patients (n = 160) | PEA (n = 31) | BPA (n = 58) | MT (n = 69) | p value |
|---|---|---|---|---|---|
| RAP, mmHg | 9.8 ± 5.4 | 10.6 ± 6.7 | 8.7 ± 4.9 | 9.0 ± 5.2 | 0.29 |
| mPAP, mmHg | 46.7 ± 11.6 | 48.1 ± 9.5 | 48.5 ± 10.7 | 44.6 ± 12.9 | 0.14 |
| PCWP, mmHg | 10.7 ± 3.7 | 10.5 ± 3.4 | 10.8 ± 4.4 | 10.7 ± 3.1 | 0.92 |
| CO, l/min | 4.4 ± 1.3 | 4.4 ± 1.3 | 4.3 ± 1.3 | 4.5 ± 1.4 | 0.81 |
| Cardiac index, l/min/m2 | 2.4 ± 0.7 | 2.2 ± 0.5 | 2.3 ± 0.7 | 2.5 ± 0.8 | 0.16 |
| PVR, dyn·s/cm5 | 734 ± 357 | 762 ± 328 | 763 ± 344 | 696 ± 376 | 0.53 |
BPA, balloon pulmonary angioplasty; CO, cardiac output; CTEPH, chronic thromboembolic pulmonary hypertension; mPAP, mean pulmonary artery pressure; MT, medical therapy (riociguat/sildenafil) alone; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCPW, pulmonary capillary wedge pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; RAP, right atrial pressure.
Data are presented as mean ± SD.
Follow-up
Median follow-up time after CTEPH team for all the patients was 22 (16–30) months.
In the PEA group, 7 (23%) of 31 patients died during the follow-up period; 4 patients (13%) died in the hospital directly after the surgery (on days 1, 4, 11 and 21). Causes of death included reperfusion oedema, perioperative bleeding complication, persistent pulmonary hypertension, infection, stroke as well as unknown cause. In the BPA (+MT) subgroup, 5 patients (9%) died during the follow-up period as a result of pneumonia, renal failure, malignancy, death on day 1 following BPA procedure and a sudden death that could not be explained. In the MT subgroup, 14 patients (20%) died during the follow-up period. The main cause of death was progressive right heart failure and other causes included biventricular heart failure, infection, respiratory insufficiency, pulmonary haemorrhage, malignancy and sudden death that could not be explained.
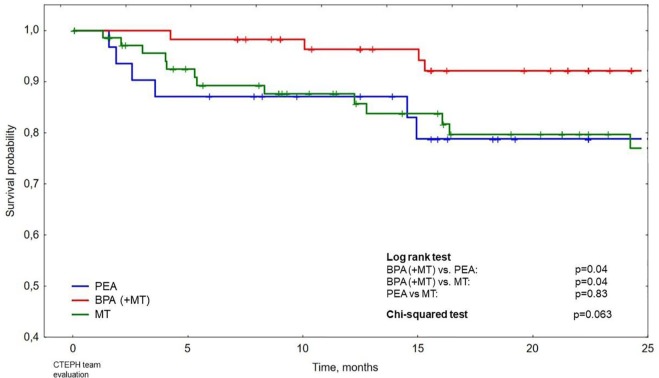
Survival after CTEPH team evaluation in the subgroups treated with PEA, BPA and MT alone is presented with Kaplan–Meier curves in Figure 2. The difference between survival of patients treated with BPA and those treated with PEA as well as the difference between survival of patients treated with BPA and those treated with MT alone are statistically significant (in both cases p = 0.04 in log-rank test). The 2-year survival for patients treated with BPA, PEA and MT alone was 92% (95% CI 85–99%), 79% (95% CI 64–94%) and 79% (95% CI 69–90%), respectively. Other patient characteristics that proved to be statistically significant correlates of mortality such as age, WHO functional class and RAP are presented in Table 4.
Figure 2.
Survival after chronic thromboembolic pulmonary hypertension (CTEPH) team evaluation in patients treated with pulmonary endarterectomy (PEA), balloon pulmonary angioplasty [(BPA) with or without medical therapy (MT)] and with MT alone (riociguat/sildenafil).
Table 4.
Correlates of mortality for all patients.
|
Univariable regression
|
Multivariable regression
|
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 (1.00–1.06) | 0.046 | 1.03 (1.0–1.07) | 0.041 |
| PEA versus BPA | 3.08 (0.98–9.72) | 0.055 | ||
| MT versus BPA | 2.78 (1.00–7.71) | 0.050 | ||
| BPA | 0.34 (0.13–0.90) | 0.031 | 0.35 (0.13–0.94) | 0.037 |
| WHO FC IV versus II–III | 3.10 (1.30–7.42) | 0.011 | 2.50 (1.00–6.27) | 0.050 |
| RAP | 1.09 (1.04–1.16) | 0.001 | 1.13 (1.05–1.21) | 0.001 |
| PVR | 1.10 (1.01–1.20) | 0.029 | ||
Cox univariable and multivariable regression.
BPA, balloon pulmonary angioplasty; CI, confidence interval; CTEPH, chronic thromboembolic pulmonary hypertension; WHO FC, World Health Organization functional class; HR, hazard ratio; PVR, pulmonary vascular resistance; RAP, right atrial pressure.
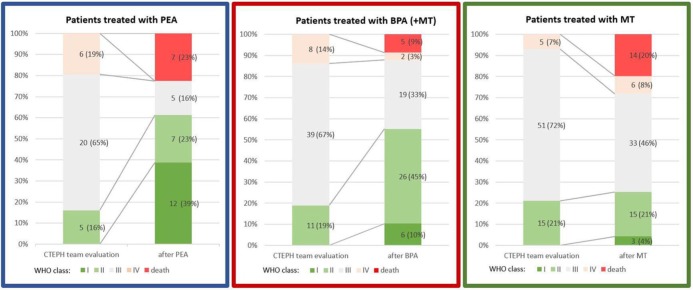
Change in WHO functional class following the treatment in these patient subgroups is presented in Figure 3. Greatest improvement was noted at the follow-up in surviving patients who underwent PEA, 3.0 ± 0.6 versus 1.7 ± 0.8; in surviving patients subjected to BPA it was 2.9 ± 0.6 versus 2.3 ± 0.7, whereas in surviving patients treated with MT alone it was insignificant, 2.9 ± 0.5 versus 2.7 ± 0.7.
Figure 3.
Change in World Health Organization (WHO) functional class in patients treated with pulmonary endarterectomy (PEA), balloon pulmonary angioplasty [(BPA) with or without medical therapy (MT)] and MT alone (riociguat/sildenafil).
Discussion
Diagnostic procedures required by CTEPH team
Prior to CTEPH team evaluation, all patients underwent RHC and pulmonary angiography, 139 (87%) received CT angiography and 46 (29%) VQ scan. A Korean single-centre retrospective analysis21 found that in a subgroup of patients managed by a CTEPH team, implementation rates of RHC (97.6% versus 10.8%, p < 0.001) and pulmonary angiography (97.6% versus 18.9%, p < 0.001) were higher than in patients treated prior to the introduction of the CTEPH team. This is in line with our experience of a CTEPH team ensuring that necessary diagnostic procedures are performed prior to treatment initialization, in particular guaranteeing proper differentiation of CTEPH from other types of pulmonary hypertension.
Low rate of PEA
A total of 51 (32%) patients were assessed as eligible for PEA whereas 109 (68%) were deemed inoperable. This is a low rate of PEA qualification as compared with the international prospective registry in which 63% CTEPH patients were considered operable and 57% (between 12% and 61% in various countries) underwent surgery.23 However in the pre-BPA era in our centre, 59% of CTEPH patients underwent PEA,24 which is consistent with the international registry operability rates.23 A decreasing proportion of patients undergoing surgery was also previously noted in a French study with an operability rate of 30% (146 out of 484 patients) in the BPA era.20 Interestingly, Korean cohorts showed opposite evolution with 32.4% and 59.5% of CTEPH patients undergoing PEA prior to and after the introduction of a CTEPH team, respectively.21
A significant number of patients (29%) were deemed inoperable owing to high-risk comorbidities that included chronic obstructive pulmonary disease, renal failure, morbid obesity, cachexia, concomitant left ventricular failure, coronary disease, untreated obstructive sleep apnoea, advanced cancer, frailty, antiphospholipid syndrome with thrombocytopenia, cerebral vascular disease, diabetes mellitus, cardiac arrythmias, carotid artery stenosis and liver cirrhosis. In our study as surgically inaccessible were regarded lesions confined to segmental and subsegmental arteries seen on imaging or a disease with an increase in PVR to a large extent caused by a distal vasculopathy. However, the final decision was subjective and depended on the experience of the surgical team. A relatively high proportion of patients (39%) were ineligible for surgery owing to a surgically inaccessible localization of thromboembolic disease. This observation may be explained by the fact that BPA interventions were started in Poland in 2013 and the riociguat approval and reimbursement program being introduced in 2015. Thus, patients who had been previously regarded as ineligible for PEA and for whom new treatment options emerged, might have been now referred to the CTEPH team contributing to a higher number of inoperable patients among the consulted cohort.
Among patients qualified for PEA, 18 (35%) did not undergo surgery. Some of them experienced clinical worsening prior to the procedure. Others were not willing to consider surgery which may be associated with reluctance to accept the risk of surgery or patients’ increasing awareness of other emerging treatment options.
Importantly, eight patients who had not undergone surgery owing to clinical worsening were subsequently treated with MT alone and, thus, they could have negatively influenced the outcome of patients in MT group.
Differences between treatment subgroups
Significant age differences were noted between operable and inoperable patients (56.7 ± 15.3 versus 66.3 ± 14.6, p < 0.01) and between different treatment groups (PEA, BPA and MT patients were 50.9 ± 14.7, 62.9 ± 15.1 and 68.9 ± 12.7 years old, respectively, p < 0.01). Even though age alone should not be considered as a contraindication for PEA,25 older patients more often suffer from severe comorbidities, which may be associated with unacceptable surgical risk–benefit ratio. Indeed, also in our study a substantial age difference was noted between patients deemed inoperable owing to high-risk comorbidities and those who qualified for surgery (73.0 ± 13.1 versus 56.7 ± 15.3, p < 0.01). Age differences between various treatment groups noted in our cohort are consistent with observations from a French report20 analysing patients subjected to PEA and BPA (60 ± 14 versus 64 ± 14 years, p < 0.01) and the international registry of 697 CTEPH patients in the pre-BPA era who did or did not receive surgery (60 versus 67 years).26
In our analysis, patients assessed as operable by the CTEPH team had lower cardiac index than inoperable patients. In the international CTEPH pre-BPA era registry, cardiac index was lower in patients who received surgery than those who did not.26 Similarly, in Amsallem’s study, cardiac index in PEA patients was lower than in BPA patients.20 However, a comparison between our patient subgroups ultimately subjected to three different therapeutic strategies showed no significant differences with respect to cardiac index or any other haemodynamic parameters (Table 3). In addition, patients subjected to PEA had more often suffered from thrombophilia than those subjected to BPA and treated with MT alone (8 (26%) versus 6 (10%) versus 4 (6%), p = 0.01) (Table 2), which is in line with a previous study (thrombophilia prevalence of 17.6% in PEA patients versus 10.7% in BPA patients, p = 0.04).20 In addition, higher rates of splenectomy were previously described for patients undergoing BPA than PEA (9.6% versus 1.4%, p < 0.01)20 Our data suggest a similar trend but without statistical significance (splenectomy in 1 (3%), 6 (10%) and 2 (3%) of patients who were treated with PEA, BPA and MT, respectively, p = 0.28).
Combination of treatment modalities
The CTEPH team decides whether patients should be qualified for surgery, but as presented in Figure 1, real-life therapeutic management is far more complex and often involves polytherapy and further decisions concerning multiple BPA sessions and pharmacological treatment. PEA was regarded as a treatment of choice. Inoperable patients were offered BPA procedures and pharmacotherapy, that is, mainly riociguat (53%) or sildenafil (18%). BPA patients underwent multiple BPA sessions, as a recent study showed that extensive revascularization by BPA improves clinical outcome.27 Taking into consideration how diverse treatment combinations were used (Figure 1) there were many possible ways of splitting patients into subgroups for the comparison of treatment outcomes. We decided to perform follow-up on three groups of patients as presented in Figure 1.
Comparison with the pre-CTEPH team era
Prior to the introduction of the BPA program and the CTEPH team, an analysis of introduced therapeutic strategies and their long-term outcomes in 112 consecutive patients with CTEPH referred to our centre between 1998 and 2008 had been performed.24 Treatment strategies had been limited to PEA and off-label targeted MT with sildenafil, bosentan, iloprost or treprostinil, as no interventional treatment procedures had been performed at the time.
A total of 59% of patients had undergone PEA and 41% remained on MT alone, which in comparison with our current cohort shows a decline in the number of patients receiving surgery.
In the previous study patients’ inoperability was caused by distal localization of lesions in 26%, by comorbidities in 9%, by patient’s refusal in 5% and by death prior to the surgery in 1% of all the referred patients. In our current cohort, patients were considered primary or secondary inoperable owing to the distal localization of lesions, high-risk comorbidities, refusal, clinical exacerbation and death prior to the surgery in 29%, 39%, 6%, 5% and 1% of cases, respectively. The higher number of patients currently ineligible for surgery owing to high-risk comorbidities may be partially explained by the older age of the current CTEPH population (63.2 ± 15.4 versus 53.9 ± 14.7). The introduction of a new interventional procedure may also negatively affect implementation rates of surgery. In addition, an interventional cardiologist’s participation in patient evaluation by the CTEPH team could be potentially associated with a lower rate of surgery qualification.
Survival analysis
Survival significantly better than in the other groups was noted for the patients in the BPA subgroup (Figure 2). This can be explained by high in-hospital mortality in patients who underwent PEA: 4 patients (13%). Other centres reported perioperative mortality in the range 3.4–12.5%,20,21,26,28 and the international CTEPH registry showed in-hospital mortality rates of 3.4%, 4.5% and 8.8% in centres performing more than 50, 11–50 and 1–10 PEAs per year, respectively.26 The University of California San Diego Medical Center, the most experienced institution in PEA, reported in-hospital mortality for the last 500 operations as low as 2.2%.4
This high perioperative mortality rate is confounding especially when compared with our previous report analysing the results of PEA performed by the same surgical team and revealing a drop in perioperative mortality between the periods 1998–2004 and 2004–2008 (from 9.7% to 6.3%).24 Results observed in current cohort may be attributed to the small sample size, that is, only 31 patients receiving surgery. Among previously identified PEA mortality risk factors the most important is preoperative PVR4,6,29,30 with PVR higher than 1000 dynes/s/cm–5 associated with 3–4 times higher mortality.4 Other previously described independent risk factors for in-hospital mortality include distance covered in 6MWT,29 cardiac index29 and post-operative PVR.28 However, in our cohort these parameters were not significantly worse in surgical than in nonsurgical patients. PVR of 762 ± 328 WU in patients subjected to surgery was not significantly worse than those reported previously in patients undergoing PEA.4,6,20,24,28 However, 6MWT distance of 328 ± 131 m was slightly lower than values normally described in operated patients such as 371 ± 124 m20 or 353 ± 134 m24 or contrasting 350 m in hospital survivors versus 290 m in hospital nonsurvivors.28 This might suggest that lower functional capacity in our patients might have contributed to high perioperative mortality.
WHO functional class improvement
Improvement in WHO functional class at the follow-up in surviving patients who underwent PEA was much higher than for other treatment strategies (Figure 3), which is consistent with studies reporting CTEPH patients’ substantial clinical improvement following surgery.24,28,31
Role of BPA and MT in CTEPH
Importantly, our analysis showed also better survival of patients treated with BPA and MT than with MT alone (p = 0.04). The 2-year survival in these groups was 92% versus 79%. Both groups have significantly lower mortality rates than our 1998–2008 medically treated CTEPH cohort in which 2-year survival was 68%,24 which shows the progress made in 20 years in treatment of nonoperable CTEPH patients enabled by the development of pulmonary hypertension-specific pharmacotherapy and interventional methods. Excellent BPA outcomes were previously reported in a Japanese multicentre registry with BPA survival rates of 96.8% and 94.5% after 2 and 3 years, respectively, which is comparable with the survival of PEA patients at experienced centres.32 This indicates that BPA has become an important treatment option for carefully selected patients. Recently, riociguat has acquired a recommendation in the case of inoperable CTEPH.2,13,14 The ongoing randomized prospective RACE study (ClinicalTrials.gov identifier: NCT02634203) comparing BPA with riociguat treatment in nonoperable CTEPH patients may enable a direct comparison between these two treatments. However, in our opinion they should not be seen as alternatives, but rather as complementary methods. Even though further research is needed to objectively assess the outcomes of this polytherapy, it could prove to be valuable in particular for inoperable patients.
Interestingly, a recent study showed that even though in patients treated before 2008 PEA enabled significantly better survival than medication, in a group of CTEPH patients who initiated treatment in 2009–2016, no difference in survival between PEA, BPA and medication was observed.33 With recent cases of patients treated with both PEA and BPA,34,35 it has been suggested that in the future some patients may benefit most from various combinations of surgical therapy, percutaneous therapy and MT.5
Study limitations
Our study is limited by its retrospective and nonrandomized nature. Our observations about patient survival and changes in WHO functional class are subject to strong selection bias as qualification for surgery is not a randomized process. In fact, high-risk comorbidities were the very reason for patients to be deemed inoperable. As a result, non-PEA groups have included a disproportionately high number of high-risk patients.
In addition, the possible contribution of the recent introduction of riociguat and the BPA program to the higher prevalence of the distal form of CTEPH is another important limitation.
The fact that many patients were subjected to more than one treatment strategy makes comparison between separate treatment options difficult and specific treatment combination groups are too small for a comparison of treatment outcomes. Lack of RHC data at the follow-up prevents more comprehensive comparison of treatment outcomes.
Current analysis might be biased by a difficult to explain high death rate in the PEA subgroup.
A CTEPH team constitutes a current standard according to the ESC guidelines.2 Its implementation has probably improved and coordinated the complex CTEPH treatment. Comparison between patients evaluated by a CTEPH team and those not subjected to this strategy has been limited by the amount of data gathered in our previous study.
Conclusion
A CTEPH team ensures that procedures necessary for the diagnosis and choice of a suitable therapeutic strategy are performed. A relatively low proportion of patients were assessed by the CTEPH team as operable and underwent surgery as compared with previous reports. Those who survived PEA had better functional improvement than patients treated with other methods. Even though patients undergoing BPA with MT were older than patients treated with PEA and had potentially more high-risk comorbidities, their survival was better than the survival of patients subjected to PEA or MT alone.
Supplemental Material
Supplemental material, Author_Response_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_3_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Acknowledgments
We would like to express our gratitude to Mrs. Małgorzata Suder (certified nurse) for her excellent assistance to CTEPH team activities.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funds from the statutory activity of the Centre of Postgraduate Medical Education in Warsaw, Poland (grant number 501-1-54-25-18) and Medical University of Białystok, Poland (grant number N/ST/2B/17/002/1153).
Conflict of interest statement: SD, PB, EM, MK and AT report grants and personal fees from Actelion, MSD, Bayer and AOP Orphan. LC reports personal fees and conference participation fees from Actelion, MSD, Bayer, and AOP Orphan. The remaining authors have no conflicts of interest to declare.
ORCID iD: Szymon Darocha  https://orcid.org/0000-0001-8298-9243
https://orcid.org/0000-0001-8298-9243
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Anna Siennicka, 1st Chair and Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Szymon Darocha, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, Fryderyk Chopin Hospital in European Health Centre Otwock, Borowa 14/18, Otwock, Mazowieckie, 05-400, Poland.
Marta Banaszkiewicz, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, European Health Centre Otwock, Centre of Postgraduate Medical Education, Poland.
Piotr Kędzierski, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, European Health Centre Otwock, Centre of Postgraduate Medical Education, Poland.
Anna Dobosiewicz, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, European Health Centre Otwock, Centre of Postgraduate Medical Education, Poland.
Piotr Błaszczak, Department of Cardiology, Cardinal Wyszynski’ Hospital, Lublin, Poland.
Małgorzata Peregud-Pogorzelska, Department of Cardiology, Pomeranian Medical University, Szczecin, Poland.
Jarosław Damian Kasprzak, 1st Department of Cardiology, Biegański Hospital, Medical University of Łódź, Łódź, Poland.
Michał Tomaszewski, Department of Cardiology, Medical University of Lublin, Lublin, Poland.
Ewa Mroczek, Department of Cardiology, Regional Specialist Hospital, Research and Development Center, Wrocław, Poland.
Bożena Zięba, University Clinical Centre, Medical University of Gdańsk, Gdańsk, Poland.
Danuta Karasek, 2nd Department of Cardiology, Faculty of Health Sciences, Collegium Medicum, Nicolaus, Copernicus University, Poland.
Katarzyna Ptaszyńska-Kopczyńska, Department of Cardiology, Medical University of Białystok, Białystok, Poland.
Katarzyna Mizia-Stec, First Department of Cardiology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Poland.
Tatiana Mularek-Kubzdela, Department of Cardiology, Poznan University of Medical Sciences, Poznan, Poland.
Anna Doboszyńska, Pulmonary Department, Pulmonary Hospital, University of Warmia and Mazury, Olsztyn, Poland.
Ewa Lewicka, Department of Cardiology and Electrotherapy, Medical University of Gdansk, Gdansk, Poland.
Marcin Ruchała, Department of Cardiology, Cardinal Wyszynski’ Hospital, Lublin, Poland.
Maciej Lewandowski, Department of Cardiology, Pomeranian Medical University, Szczecin, Poland.
Sylwia Łukasik, Department of Cardiology, Medical University of Lublin, Lublin, Poland.
Łukasz Chrzanowski, 1st Department of Cardiology, Biegański Hospital, Medical University of Łódź, Łódź, Poland.
Dariusz Zieliński, Department of Cardiac Surgery, Medicover Hospital, Warsaw, Poland.
Adam Torbicki, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, European Health Centre Otwock, Centre of Postgraduate Medical Education, Poland.
Marcin Kurzyna, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, European Health Centre Otwock, Centre of Postgraduate Medical Education, Poland.
References
- 1. Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endor. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 3. Fedullo P, Kerr KM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011; 183: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 4. Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. [DOI] [PubMed] [Google Scholar]
- 5. Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 7. Darocha S, Pietura R, Pietrasik A, et al. Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ J 2017; 81: 552–557. [DOI] [PubMed] [Google Scholar]
- 8. Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 9. Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. [DOI] [PubMed] [Google Scholar]
- 10. Kurzyna M, Darocha S, Pietura R, et al. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Polish Hear J 2017; 75: 645–654. [DOI] [PubMed] [Google Scholar]
- 11. Araszkiewicz A, Jankiewicz S, Łanocha M, et al. Optical coherence tomography improves the results of balloon pulmonary angioplasty in inoperable chronic thrombo-embolic pulmonary hypertension. Adv Interv Cardiol 2017; 2: 180–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pepke-Zaba J, Ghofrani H, Hoeper MM. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 14. Simonneau G, D’Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 15. Darocha S, Banaszkiewicz M, Pietrasik A, et al. Sequential treatment with sildenafil and riociguat in patients with persistent or inoperable chronic thromboembolic pulmonary hypertension improves functional class and pulmonary hemodynamics. Int J Cardiol 2018; 269: 283–288. [DOI] [PubMed] [Google Scholar]
- 16. Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. [DOI] [PubMed] [Google Scholar]
- 17. Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 18. Ghofrani H-A, Simonneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017; 5: 785–794. [DOI] [PubMed] [Google Scholar]
- 19. Sadushi-Kolici R, Jansa P, Kopec G, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med 2019; 7: 239–248. [DOI] [PubMed] [Google Scholar]
- 20. Amsallem M, Guihaire J, Arthur Ataam J, et al. Impact of the initiation of balloon pulmonary angioplasty program on referral of patients with chronic thromboembolic pulmonary hypertension to surgery. J Hear Lung Transplant 2018; 37: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 21. Oh DK, Song J-M, Park D-W, et al. The effect of a multidisciplinary team on the implementation rates of major diagnostic and therapeutic procedures of chronic thromboembolic pulmonary hypertension. Heart Lung. Epub ahead of print 14 August 2018. DOI: 10.1016/j.hrtlng.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 22. Kurzyna M, Araszkiewicz A, Błaszczak P, et al. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Polish Hear J 2015; 73: 63–68. [DOI] [PubMed] [Google Scholar]
- 23. Pepke-zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH) results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 24. Wieteska M, Biederman A, Kurzyna M, et al. Outcome of medically versus surgically treated patients with chronic thromboembolic pulmonary hypertension. Clin Appl Thromb 2014; 22: 92–99. [DOI] [PubMed] [Google Scholar]
- 25. Newnham M, Hernández-Sánchez J, Dunning J, et al. Age should not be a barrier for pulmonary endarterectomy in carefully. Eur Respir J 2017; 50: 1701804. [DOI] [PubMed] [Google Scholar]
- 26. Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 27. Shinkura Y, Nakayama K, Yanaka K. Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018; 13: 2060–2068. [DOI] [PubMed] [Google Scholar]
- 28. Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 29. Condliffe R, Kiely DG, Gibbs JSR, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J 2008; 33: 332–338. [DOI] [PubMed] [Google Scholar]
- 30. Hartz RS, Byrne JG, Levitsky S, et al. Predictors of mortality in pulmonary thromboendarterectomy. Ann Thorac Surg 1996; 62: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 31. Condliffe R, Kiely DG, Gibbs JSR, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 32. Ogawa A, Satoh T, Fukuda T, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Qual Outcomes 2017; 10: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Miwa H, Tanabe N, Jujo T, et al. Long-term outcome of chronic thromboembolic pulmonary hypertension at a single Japanese pulmonary endarterectomy center. Circ J 2018; 82: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 34. Araszkiewicz A, Darocha S, Pietrasik A, et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2018; 278: 232–237. [DOI] [PubMed] [Google Scholar]
- 35. Wiedenroth CB, Liebetrau C, Breithecker A, et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Hear Lung Transplant. Epub ahead of print 30 October 2015. DOI: 10.1016/j.healun.2015.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team by Anna Siennicka, Szymon Darocha, Marta Banaszkiewicz, Piotr Kędzierski, Anna Dobosiewicz, Piotr Błaszczak, Małgorzata Peregud-Pogorzelska, Jarosław Damian Kasprzak, Michał Tomaszewski, Ewa Mroczek, Bożena Zięba, Danuta Karasek, Katarzyna Ptaszyńska-Kopczyńska, Katarzyna Mizia-Stec, Tatiana Mularek-Kubzdela, Anna Doboszyńska, Ewa Lewicka, Marcin Ruchała, Maciej Lewandowski, Sylwia Łukasik, Łukasz Chrzanowski, Dariusz Zieliński, Adam Torbicki and Marcin Kurzyna in Therapeutic Advances in Respiratory Disease