Abstract
Pulmonary arterial hypertension is a severe disease for which diagnosis often is delayed. Matrix metalloproteinases have been suggested to play a role in vascular remodeling and pulmonary hypertension development. Our aim was therefore to investigate the potential role of matrix metalloproteinases as biomarkers in diagnosis and differentiation of pulmonary arterial hypertension in relation to various causes of dyspnea and pulmonary hypertension. Using proximity extension assays, 10 matrix metalloproteinases and associated proteins were analyzed in venous plasma from healthy controls (n = 20), as well as patients diagnosed with pulmonary arterial hypertension (n = 48), chronic thromboembolic pulmonary hypertension (n = 20), pulmonary hypertension due to heart failure with preserved (n = 33) or reduced (n = 36) ejection fraction, and heart failure with reduced ejection fraction and heart failure with preserved ejection fraction without pulmonary hypertension (n = 15). Plasma levels of matrix metalloproteinase-2, -7, -9, -12 and TIMP-4 were elevated (p < 0.01) in pulmonary arterial hypertension compared to controls. Plasma levels of matrix metalloproteinase-7 were furthermore lower (p < 0.0081) in pulmonary arterial hypertension than in all the other disease groups, but higher compared to controls (p < 0.0001). Receiver operating characteristic analysis of matrix metalloproteinase-7 resulted in sensitivity of 58.7% and a specificity of 83.3% for detecting pulmonary arterial hypertension among the other disease groups. Plasma matrix metalloproteinase-7 may provide a potential new diagnostic tool to differentiate pulmonary arterial hypertension from other causes of dyspnea, including heart failure with or without pulmonary hypertension and healthy controls. Matrix metalloproteinase-7 may furthermore be involved in the development of pulmonary hypertension and pulmonary arterial hypertension. Future studies investigating the clinical usefulness of matrix metalloproteinase-7 in the differentiation and earlier diagnosis of pulmonary arterial hypertension, as well as its relationship to pulmonary arterial hypertension pathogenesis, are encouraged.
Keywords: pulmonary hypertension, matrix metalloproteinase-7 (MMP-7), biomarkers, matrix metalloproteinases
Pulmonary hypertension (PH) is a pathological condition with impact on morbidity and mortality. It has previously been characterized according to ESC/ERS guidelines by a mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest, as measured by right heart catheterization (RHC),1 but where a new cut-off magnitude at 20 mmHg was suggested at the World Symposium of Pulmonary hypertension 2018.2 PH is sub-classified into pre- or post-capillary PH, depending on whether the pulmonary arterial wedge pressure (PAWP) ≤15 or >15 mmHg, respectively.1 According to the Nice classification,3 pre-capillary PH comprises pulmonary arterial hypertension (PAH, group 1 PH), pulmonary disease and/or hypoxia (group 3 PH), chronic thromboembolic PH (CTEPH, group 4 PH), and PH due to multifactorial and/or unclear factors (group 5 PH). Post-capillary PH consists of PH due to left heart disease (group 2 PH), including PH due to heart failure (HF) with preserved (HFpEF-PH) or reduced (HFrEF-PH) ejection fraction (EF).1,4
PAH, specifically, is a rare disease with poor prognosis, where symptoms such as dyspnea may be diffuse and non-specific.4 Diagnosis of PAH is therefore often hampered by a patient’s and doctor’s delay.5 This is a major clinical challenge as PAH may rapidly progress into more severe stages before treatment is initiated.1 Development of a screening method for patients with unclear dyspnea, that could distinguish various causes of dyspnea and PH from each other, as well as characterize the severity of the disease, would be of great value to reduce the time to diagnosis and potentially enable earlier and more targeted treatment initiation, especially for those suffering from PAH.
The pathogenesis of PAH consists of a vasoconstrictive and a vascular remodeling component. Current treatments of PAH focus mainly on alleviating the vasoconstriction.1 Much less is, however, known about the pathogenesis of the vascular remodeling. Interestingly, remodeling of the extracellular matrix (ECM) plays an important role in the development of vascular remodeling in PAH and occurs early in the disease process.6,7 ECM remodeling affects, for instance, the vascular stiffness of pulmonary arteries as well as favors proliferation of smooth muscle and endothelial cells in distal pulmonary arteries.7,8 The ECM composition is regulated by a balance of proteolytic proteins including matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitors of metalloproteinase (TIMPs).7 MMPs are implicated in vascular remodeling and vascular smooth muscle cell proliferation,9,10 contributing to pathophysiological processes such as ECM turnover, hyperplasia, cell migration, and apoptosis. Thus, MMPs and related proteins are likely to play an important role in the development of vascular remodeling in PAH.11
The aim of the present study was therefore to investigate whether plasma concentrations of MMPs and related proteins are altered in PAH and could be used to differentiate PAH from other causes of PH, such as CTEPH, HF with reduced (HFrEF) or preserved (HFpEF) EF with PH, and HF without PH (HF-NON-PH), and healthy controls. We hypothesized that circulating MMPs and related proteins may be linked to the pathogenesis of PH and influence hemodynamics in the different subtypes of PH, and consequently be of potential use as future blood borne biomarkers to diagnose and differentiate PAH.
Methods
Inclusion and exclusion criteria
Patients ≥ 18 years included in the Lund Cardio Pulmonary Register (LCPR) cohort, of Region Skåne biobank, between September 2011 and March 2017, diagnosed by RHC according to the existing ESC/ERS guidelines, and differentiated with echocardiography, magnetic resonance imaging, pulmonary scintigraphy, spirometry and/or HRCT, at Skåne University hospital, Lund, Sweden were included. Patients lacking hemodynamic data were excluded.
The study population included patients under investigation for dyspnea, with RHC at baseline diagnosis, divided into PAH (n = 48) and CTEPH (n = 20), as well as, HFpEF-PH (n = 33), HFrEF-PH (n = 36), and HF-NON-PH, (n = 15), as well as 20 healthy volunteers without RHC. HFpEF was defined by EF ≥50% and HFrEF by EF <50%. For subgroup analysis, idiopathic PAH (IPAH) and familial PAH (FPAH) were regarded as one entity (IPAH/FPAH) and the rest of PAH patients as another entity, e.g. connective tissue disease associated PAH (CTD-PAH). All PAH and CTEPH patients were treatment naive. The study was approved by the local ethics committee in Lund (Dnr, 2010/114, 2010/248, 2010/442, 2011/368, and 2015/270). All participants provided informed and written consent.
Plasma sampling and biomarker analyses
Venous plasma samples were collected from the introducer of the patients, placed in the right internal jugular vein, during RHC and stored at −80℃ in the LCPR cohort. Peripheral venous samples were collected from healthy subjects. Plasma samples were analyzed with proximity extension assay (PEA) and quantitative PCR (qPCR). Briefly, antibody pairs with DNA oligonucleotide tails bind the antigen, matching tails hybridize, are elongated with DNA polymerase, and read out by qPCR.12 Plasma biomarker levels were reported in normalized protein expression (NPX) values, where NPX is an arbitrary unit on a log2-scale. Proteins were analyzed using Proseek Multiplex CVD II, CVD III, Oncology II 96-plex immunoassay panels (Olink Proteomics, Uppsala, Sweden).
Included biomarkers
Ten potential biomarkers focused on MMPs and related proteins were analyzed, including MMP-2, -3, -7, -9, -12, TIMP-4 (also known as metalloproteinase inhibitor 4), protein CYR61 (CYR61), thrombospondin-2, WNT1-inducible-signaling pathway protein 1 (WISP-1), and SPARC (also known as osteonectin). NT-proBNP values were for consistency also analyzed with PEA.
Hemodynamics
Hemodynamic data were sampled during RHC performed as part of clinical diagnosis using Swan Ganz catheters (Baxter Health Care Corp, Santa Ana, CA). The parameters registered by RHC included MPAP, PAWP, mean right atrial pressure (MRAP), and mean arterial pressure (MAP). Heart rate (HR) was measured by ECG. Cardiac output (CO) was measured by thermodilution.
Cardiac index (CI), stroke volume (SV), stroke volume index (SVI), left ventricular stroke work index (LVSWI), right ventricular stroke work index (RVSWI), pulmonary vascular resistance (PVR), and transpulmonary gradient (TPG) were calculated by the formulas: CI = CO/body surface area, SV = CO/HR, SVI = SV/body surface area, LVSWI = (MAP − PAWP) × SVI, RVSWI = (MPAP − MRAP) × SVI, PVR = TPG/CO, and TPG = MPAP − PAWP, body surface area = weight0.425 × height0.725 × 0.007184. SvO2 samples were retrieved at the RHC procedure.
Statistics
Data analysis was performed using GraphPad Prism version 8.0.2 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com. Data were presented as median (interquartile range) when applicable. Data distribution was analyzed using frequency distribution. Kruskal–Wallis test with uncorrected Dunn’s multiple comparison test was performed to compare biomarkers’ levels in controls and for all included subgroups. To limit false positive results, the Kruskal–Wallis tests were analyzed with a Benjamini and Hochberg false discovery rate (FDR) test (q = 0.05).13 Uncorrected Dunn’s multiple comparison test was done on the significant Kruskal–Wallis tests and were in turn analyzed with FDR (q = 0.05). After FDR analysis, the threshold for statistical significance was set to <0.021. The FDR analysis was made on Kruskal–Wallis and subsequent multiple comparison tests. Presented p-values are raw and denoted as significant if <0.021. For other statistical tests, a p-value <0.05 was considered statistically significant. Mann–Whitney’s test was used for subgroup analysis of PAH. Correlations using Spearman’s coefficient were performed on MMP-7 in relation to MRAP, MPAP, CI, SVI, RVSWI, PVR, as well as to NT-proBNP and SvO2. Spearman correlation was additionally performed on the MMP-7 levels between all the groups. To evaluate MMP-7 as a predictor of PAH among the other disease groups, receiver operating characteristic (ROC) analysis was performed on MMP-7 comparing PAH versus other PH groups and HF-NON-PH. Youden’s index was used to determine cut-offs for sensitivity and specificity. Mann–Whitney’s test was used to investigate potential gender difference in MMP-7 levels between males and females and was performed on all the separate patient groups as well as all groups together.
Results
Population characteristics
Characteristics of the patient groups and controls are presented in Table 1. In the PAH group, 21 patients (43.8%) were diagnosed with IPAH, two patients (4.2%) with FPAH, 21 patients (43.7%) with systemic sclerosis associated PAH (SSc-PAH), and four patients (8.3%) with other CTD-PAH. In the PAH subgroup analysis, IPAH/FPAH were regarded as one entity with 23 patients (47.9%), and CTD-PAH as the other with 25 patients (52.1%).
Table 1.
Population characteristics and hemodynamics.
| Control (n = 20) | PAH (n = 48) | CTEPH (n = 20) | HFpEF-PH (n = 33) | HFrEF-PH (n = 36) | HF-NON-PH (n = 15) | |
|---|---|---|---|---|---|---|
| Females (%) | 50 | 83 | 65 | 64 | 19 | 53 |
| Age (years) | 41 (26.8–50.5) | 71.5 (64–76) | 75 (70.8–77.8) | 75 (68.5–83) | 54 (47.3–59.5) | 60 (46–76) |
| BSA (m2) | 1.9 (1.8–2) | 1.7 (1.6–2) | 1.8 (1.8–2) | 1.9 (1.7–2.1) | 2 (1.9–2.1) | 2 (1.7–2.1) |
| MAP (mmHg) | 96 (89.4–104) | 98.5 (94–110.3) | 98 (91.5–104.5) | 79.5 (75.3–88.8) | 89 (80–96) | |
| MPAP (mmHg) | 43 (37–54.8) | 42 (35–54.3) | 34 (28.5–46) | 34.5 (29–40.8) | 20 (17–22) | |
| PAWP (mmHg) | 8 (6–11) | 9.5 (7–13) | 18 (16–22.5) | 25 (19–28)a | 15 (9–18) | |
| MRAP (mmHg) | 7 (4–11) | 5.5 (3.3–8) | 10 (6.5–14) | 14.5 (9–17) | 6 (2–16) | |
| HR (beats/min) | 77.5 (70–94.3) | 75 (69.5–88) | 70 (61.5–82.5) | 71 (68.3–86) | 72 (60–84) | |
| CO (l/min) | 3.8 (3.0–5.1) | 4 (3.5–4.7) | 4.5 (3.7–5.7) | 3.2 (2.8–4.0) | 3.3 (3.0–4.4) | |
| CI (l/min/m2) | 2.2 (1.8–2.8) | 2.3 (1.9–2.5) | 2.4 (2.1–2.8) | 1.6 (1.4–1.9) | 1.9 (1.6–2.2) | |
| SV (ml/beat) | 51.2 (40.8–56.3) | 56.3 (45.8–65.1) | 61.7 (48.8–83.7) | 45.1 (36.0–54.5) | 54.8 (44.8–58.8) | |
| SVI (ml/beat/m2) | 28.7 (22.6–35) | 30.5 (26.3–32.5) | 33.8 (28.1–42.3) | 22.5 (18.2–27.2) | 29 (25.2–31.9) | |
| PVR (WU) | 9.5 (6.2–11.8) | 9.3 (5.9–10.8) | 3.6 (2.4–4.9) | 3.0 (2.3–3.7)a | 1.5 (1.0–2.0) | |
| LWSWI (mmHg × ml/m2) | 2488 (2045–3213) | 2508 (2330–3187) | 2664 (2189–3308) | 1152 (957–1636)a | 2168 (1650–2716) | |
| RVSWI (mmHg × ml/m2 | 990.5 (807.2–1246) | 1111 (844.5–1298) | 831.5 (670.7–1140) | 439.6 (305.8–649.3) | 382.4 (195.5–494.5) | |
| NT-proBNP (AU) | 3.1 (2.1–3.8) | 2.6 (1.0–4.2) | 2.9 (2.4–3.3) | 4.9 (4.1–5.4) | 3.2 (1.3–4.4) | |
| SvO2 (%) | 59.3 (51.1–66.2) | 62.5 (54.9–67.9) | 64.1 (57.8–66.8) | 50.3 (46.5–55.2) | 61.2 (58.5–69.2) | |
| Creatinine (µmol/l) | 90 (70.8–113.5) | 88 (73–122.5) | 99 (79–117) | 121 (90–145) | 93 (80.5–123) | |
Variables are presented as median (interquartile range).
BSA: body surface area; CI: cardiac index; CO: cardiac output; CTEPH: chronic thromboembolic pulmonary hypertension; HF-NON-PH: HF without PH; HFpEF-PH: PH due to heart failure (HF) with preserved ejection fraction (EF); HFrEF-PH: PH due to HF with reduced EF; HR: heart rate; LVSWI: left ventricular stroke work index; MAP: mean arterial pressure; MPAP: mean pulmonary artery pressure; MRAP: mean right atrial pressure; PAH: pulmonary arterial hypertension; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; RVSWI: right ventricular stroke work index; SV: stroke volume.
Indicates n = 35 due to one patient being unable to go through all tests.
MMP-7
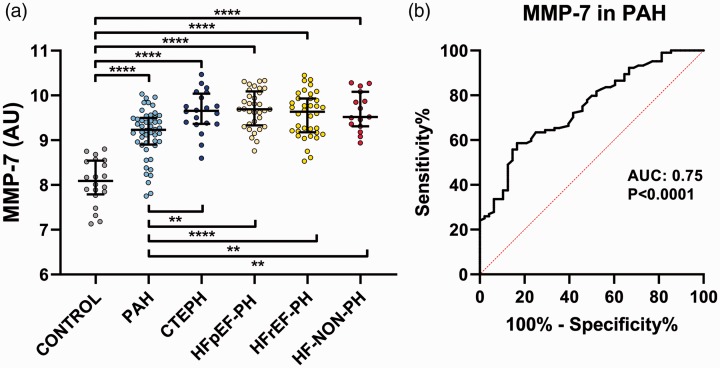
For PAH, plasma MMP-7 levels were higher than the controls (p < 0.0001) but lower compared to the other disease groups (p < 0.0081). For PAH, plasma MMP-7 levels were lower compared to the other disease groups (p < 0.0081), and higher compared to controls (p < 0.0001) (Fig. 1a). ROC analysis for plasma MMP-7 in PAH versus other PH groups and HF-NON-PH resulted in an area under the curve of 0.75 (95% CI 0.67–0.83) (p < 0.0001), as well as a sensitivity of 58.7% and a specificity of 83.3% (Fig. 1b). Subgroup analysis of plasma MMP-7 in IPAH and CTD-PAH showed no difference (p = 0.31). Spearman correlations of MMP-7 levels between all groups showed a negative correlation rs=−0.57 (p = 0.029) between control and HF-NON-PH. Analysis for gender difference in MMP-7 with Mann–Whitney’s test was non-significant.
Fig. 1.
(a) Plasma levels and difference of MMP-7 in PH subgroups. MMP-7 levels in PAH were higher than in controls (p < 0.0001) but lower than in the other disease groups (p < 0.0081). (b) ROC curve of MMP-7 levels in PAH versus other groups. AUC: area under the curve; CTEPH: chronic thromboembolic PH; HFpEF-PH: PH due to HF with preserved EF; HFrEF-PH: PH due to HF with reduced EF; HF-NON-PH: HF without PH; MMP-7: matrix metalloproteinase-7, PAH: pulmonary arterial hypertension; ** indicates p value <0.01; ****indicates p-value < 0.0001.
Other MMPs and related proteins
The plasma levels of MMP-12 (p < 0.007), TIMP-4 (p < 0.005), thrombospondin-2 (p < 0.0003), and WISP-1 (p < 0.0017) were significantly lower in controls compared to all the disease groups. For PAH, all plasma biomarkers were significantly elevated compared to controls (p < 0.0099), except for MMP-3 and SPARC. Plasma MMP-3 levels were furthermore lower (p < 0.0118) in PAH than in the other PH groups (Table 2, supplementary table 1, supplementary fig. 1).
Table 2.
Protein levels and comparison in all groups.
| BIOMARKER | Control (n = 20) | PAH (n = 48) | CTEPH (n = 20) | HFpEF-PH (n = 33) | HFrEF-PH (n = 36) | HF-NON-PH (n = 15) |
|---|---|---|---|---|---|---|
| MMP-2 | 2.96 (2.85–3.04) | 3.34 (2.85–3.62)† | 3.26 (2.81–3.48) | 3.47 (3.24–3.82)† | 3.78 (3.52–4.22)†‡§ | 3.32 (2.88–3.87)†¥ |
| MMP-3 | 6.47 (5.96–7.17) | 6.20 (5.62–6.88) | 6.92 (6.30–7.65)‡ | 7.02 (6.34–7.75)‡ | 7.19 (6.51–7.74)‡ | 6.69 (6.09–7.38) |
| MMP-7 | 8.09 (7.79–8.54) | 9.23 (8.90–9.50)† | 9.66 (9.37–10.0)†‡ | 9.69 (9.33–10.1)†‡ | 9.64 (9.18–9.93)†‡ | 9.52 (9.31–10.1)†‡ |
| MMP-9 | 3.36 (2.91–3.78) | 4.0 (3.32–4.70)† | 4.72 (4.05–5.16)† | 3.47 (3.05–4.16)§ | 3.92 (3.39–4.49)† | 3.51 (3.31–4.24)§ |
| MMP-12 | 6.25 (5.74–6.66) | 7.44 (6.88–7.99)† | 7.35 (6.94–7.80)† | 7.49 (7.16–8.28)† | 6.94 (6.32–7.58)†‡# | 7.15 (6.78–7.78)† |
| TIMP4 | 3.93 (3.71–4.05) | 4.31 (3.99–4.87)† | 4.96 (4.54–5.42)†‡ | 4.76 (4.34–5.37)†‡ | 4.39 (4.02–4.59)†§# | 4.49 (4.21–4.82)† |
| CYR61 | 4.46 (4.09–4.78) | 5.02 (4.63–5.28)† | 4.87 (4.51–5.36)† | 4.79 (4.48–5.11) | 4.77 (4.56–5.39)† | 4.52 (4.27–4.79)‡¥ |
| SPARC | 5.73 (5.64–5.85) | 5.75 (5.62–5.85) | 6.05 (5.86–6.13)†‡ | 5.81 (5.67–5.97)§ | 5.87 (5.69–5.99)‡ | 5.86 (5.69–5.97) |
| Thrombo- spondin-2 | 5.20 (5.10–5.28) | 5.58 (5.32–5.74)† | 5.67 (5.46–5.87)† | 5.57 (5.39–5.76)† | 5.73 (5.57–5.83)†‡ | 5.55 (5.34–5.72)† |
| WISP-1 | 3.65 (3.46–3.91) | 4.29 (4.05–4.76)† | 4.43 (3.95–4.82)† | 4.78 (4.47–5.23)†‡ | 4.74 (4.29–5.16)†‡ | 4.29 (4.11–4.75)† |
Variables are presented in arbitrary units (AU) as median (interquartile range).
CTEPH: chronic thromboembolic pulmonary hypertension; CYR61: protein CYR61; HF-NON-PH: HF without PH; HFpEF-PH: PH due to heart failure (HF) with preserved ejection fraction (EF); HFrEF-PH: PH due to HF with reduced EF; MMP: matrix metalloproteinase; PAH: pulmonary arterial hypertension; TIMP-4; tissue inhibitor of metalloproteinases 4; WISP-1: WNT1-inducible-signaling pathway protein 1.
Statistics: †significantly different compared to controls; ‡significantly different compared to PAH; §significantly different compared to CTEPH; #significantly different compared to HFpEF-PH; ¥significantly different compared to HFrEF-PH. Significant after FDR analysis as p-value below the FDR threshold p < 0.021.
Discussion
Given the low median survival, from diagnosis, of untreated CTD and IPAH patients of 1–2.8 years and their non-specific symptoms, earlier diagnosis is crucial to improve outcome.5,14 In the present study, we demonstrate that plasma MMP-7 levels differ in PAH compared to the other groups of PH such as CTEPH as well as HFrEF-PH and HFpEF-PH, and HF-NON-PH as well as from healthy individuals.
Remodeling of ECM and the resulting increase in vascular stiffness in PAH is an early process that precedes abnormal pulmonary artery pressure and elevated PVR. An expansion of ECM in the pulmonary vascular wall furthermore leads to fibrosis.7 As MMPs are involved in the ECM remodeling process including vascular remodeling and stiffness,8,15 we investigated whether MMP plasma levels may be used to differentiate PAH from other causes of PH, including CTEPH and HFrEF and HFpEF with PH, as well as HF-NON-PH. In accordance with our hypothesis, several of the MMPs and associated proteins were found to be deranged in PAH with increased plasma levels. Circulating plasma levels of MMP-2, -7, -9, -12, and the MMP inhibitor TIMP-4, were increased in PAH as compared to healthy controls, indicating a potential involvement in PAH pathophysiology. More specifically, plasma MMP-7 enabled differentiation of PAH patients from controls, CTEPH, as well as HF with or without PH. Plasma MMP-7 could consequently have the potential as a future tool for PAH diagnosis. However, plasma MMP-7’s intermediate level between controls and the other disease conditions may hamper its use as a clear discriminator between the various states of disease.
MMP-7 is one of the smallest MMPs acting on a wide range of substrates including proteoglycans; fibronectin; casein; and gelatin types I, II, IV, and V.16 MMP-7 cleaves pro TNF-α, syndecan-1, and E-cadherin into soluble forms.16 MMP-7 may furthermore have a pro-apoptotic effect through the release of FAS-ligand and an anti-apoptotic effect via heparin-binding epidermal growth factor (HB-EGF).16
Interestingly, it has been reported that plasma MMP-7 levels increase with older age.17 We found that plasma MMP-7 levels were increased in PAH compared to controls, but not as much as in CTEPH, HFrEF and HFpEF with PH, or in HF-NON-PH. Given that PAH patients tended to be the oldest among the PH groups, the relative lower plasma MMP-7 in PAH compared to the other disease groups was disproportional. Thus, the age-related increase of MMP-7 may be small and only add little to the MMP-7 increase. Although age difference may contribute to the increased levels of MMP-7 in PAH, it cannot satisfactorily explain the entire increase as CTEPH patients are of the same age and yet have higher MMP-7 levels. In addition, as we found no difference between IPAH/FPAH and CTD-PAH, the difference in MMP-7 levels seems to be common for both PAH groups.
The MMP-7 sensitivity of 58.7% and specificity of 83.3% for PAH in our study suggest that MMP-7 could potentially be used as an indicator for PAH in a dyspnea population and potentially lead to earlier referral for further diagnostic procedures. In addition, it could be a valuable addition as a potential future tool for differentiating the PAH subgroup from other PH subgroups including HFpEF, where the latter group may be misinterpreted as “atypical” PAH patients, due to an excessive vasoconstriction with elevated PVR. It is furthermore of interest to speculate whether MMP-7 also may be of importance in HFrEF-PH, where induction of an elevated vascular resistance may impose a contraindication for heart transplantation. Thus, interestingly, MMP-7 may potentially be used in combination with other PAH biomarkers to enable future “deep phenotyping,” addressed in the current state-of-the art article in diagnosis of PH, as PAH patients may have a unique profile or characteristic pattern of proteomics.18 This indicates a need for further investigation of MMP-7 and other potential PAH biomarkers in lager studies.
MMP-7 produced by cancer cells has furthermore been found to reactivate the inactive form of VEGF by degrading connective tissue growth factor (CTGF), suggesting a regulative role of MMP-7 on VEGF activity.19 MMP-7 actions have also been indicated to degrade sVEGFR-1/sFlt-1 allowing reactivation of bound VEGF165.20 VEGF165 activity is also reactivated by MMP-7 acting on CTGF.21 MMP-7 may consequently play a protective role in PAH, as increased VEGF-A has a protective effect against PH in hypoxia and monocrotaline animal models.22,23 In a previous study in our group, sFlt-1 was suggested to have a potential role in PAH biology, as sFlt-1 levels decreased after initiation of PAH treatment.24 However, in the present study, no significant correlations were found between MMP-7 and hemodynamics, suggesting that MMP-7 may not directly drive severity of the PAH disease.
Additionally, MMP-7 could have a regulatory effect protecting from PAH development through degrading s-Flt-1 and CTGF, thereby releasing VEGF-A. This is of great importance as it may explain why the MMP-7 levels were less increased in PAH patients compared with the other disease groups. With less degradation of sFlt-1 and CTGF there would be lower levels of VEGF-A and less protection from PAH development.
Of interest, MMP-7 may contribute to the development of PAH via pathways involved in other pathological conditions. For instance, MMP-7 is linked to progression of several malignancies including oral squamous cell carcinoma, prostate cancer, pancreatic cancer, breast cancer, and non-small cell lung cancer.25 In addition, MMP-7 is also thought to promote fibrosis by cleaving of E-cadherin and other factors such as HB-EGF which promotes lung fibroblast proliferation.26 MMP-7 action on E-cadherin enables β-catenin signaling which is associated with increased vascular smooth muscle cell proliferation and is increased in PAH pulmonary arterial smooth muscle cells.27,28 In systemic sclerosis, in which fibrosis is an important pathophysiological mechanism,29 plasma/circulating MMP-7 levels are increased, including in SSc-PAH patients.30 Thus, as dysregulated MMP-7 has been proposed to contribute to the pathology of tumor progression and lung fibrosis through E-cadherin shedding and related β-catenin signaling,28,31 MMP-7 could potentially contribute to PAH pathology via the E-cadherin/β-catenin and HB-EGF signaling pathways.
As mentioned above, the pattern of intermediate MMP-7 levels in PAH between controls and the other disease groups is intriguing and could suggest a complex interplay of MMP-7 and the different disease groups, and that MMP-7 may be involved in the vascular remodeling induced by various causes of PH. Consequently, although the MMP-7 levels in PAH were different from the other groups, the intermediate values could make MMP-7 challenging to use as a biomarker in specifically identifying PAH. Thus, additional larger studies are needed to validate our results and the precise role of MMP-7.
In the present study, we also found increased levels of MMP-2, MMP-9, as well as TIMP-4 in PAH. Increased levels of MMP-2 and -9 have previously also been observed in a monocrotaline rat model of PH.32 Hiremath et al.33 found increased levels of MMP-9 and normal levels of MMP-2 in IPAH. Tiede et al.34 reported increased plasma levels of MMP-2 in CTD-PAH and lower levels of TIMP4 in IPAH. This difference may be attributed to our PAH population including both SSc-PAH and IPAH. MMP-2 and TIMP-4 have earlier been seen increased in PH compared with controls,35 a result which is in line with this study. The present study, however, indicated that an increase of MMP-2 and TIMP-4 is also found in HF-NON-PH.
The present study also included, in addition to PH groups and controls, a more commonly occurring group suffering from dyspnea, namely HF patients, also those without PH.1,36 The present study thereby shows that MMP-7 can identify PAH in a wider population suffering from dyspnea as well as healthy subjects. Biomarker levels were measured in PAH specific treatment naive patients with no interference of PAH drugs. The difference in MMP levels in the disease groups relative controls could, however, have been influenced by that the controls in this study are somewhat younger than the disease groups. The PAH population in the present study are somewhat older than in some other registries. A median age of 71.5 for PAH diagnosis is however consistent with the Swedish PAH register37 as well as the COMPERA registry’s median age of idiopathic PAH diagnosis of 71 years.38
Whether the circulating plasma levels of the biomarkers truly reflect remodeling of the pulmonary vasculature, the heart and/or reflect levels locally in the tissues was not investigated. The present study, however, indicates that MMP-7 may reflect PAH pathology, but its precise role is not yet clear.
Despite that the patient populations consist of all available patients in the LCPR register, they are somewhat small. Thus, the results could in addition be interpreted as less conclusive. The aim of the present study was, however, to investigate whether MMPs could differentiate PAH, in an effort to discover new biomarkers for PAH. Consequently, although the possibility of no differences due to the small patient groups, MMP-7 is interesting as a potential future biomarker. Larger studies are needed to investigate if the present results are useful in the clinic.
In conclusion, the present study indicates that plasma MMP-7 may be used as a future biomarker to identify PH and differentiate patients with PAH from healthy controls, and potentially also from those with CTEPH, HFrEF and HFpEF with PH, as well as HF-NON-PH. Such a discrimination may enable earlier PAH diagnosis and treatment initiation. Future larger studies are urged for to evaluate the clinical usefulness of MMP-7 as a biomarker for PAH and PH as well as its precise role in PAH pathogenesis.
Supplemental Material
Supplemental material, PUL895414 Supplemental Material for Matrix metalloproteinase 7 in diagnosis and differentiation of pulmonary arterial hypertension by Mattias Arvidsson, Abdulla Ahmed, Habib Bouzina and Göran Rådegran in Pulmonary Circulation
Acknowledgements
We acknowledge the support of the staff at the Hemodynamic Lab, The Section for Heart Failure and Valvular Disease, Skåne University Hospital, Lund, Sweden; and The Department of Clinical Sciences, Lund, Cardiology, Lund University, Lund, Sweden. Special thanks to Anneli Ahlqvist for support in assembling plasma samples and LCPR registration. In addition, we acknowledge the biobank services and retrieval of blood samples from LCPR performed at Labmedicine Skåne, University and Regional Laboratories, Region Skåne, Sweden.
Contributorship
MA, AA, HB, and GR made a substantial contribution to the concept or design of the work, as well as acquisition, analysis or interpretation of data, MA drafted the manuscript. MA, AA, HB, and GR revised it critically for important intellectual content and approved the final version to be published.
Conflict of interest
Arvidsson and Ahmed report no conflicts of interest. Bouzina reports personal lecture fees from Actelion Pharmaceuticals Sweden AB. Bouzina has received unrestricted research grants from the Swedish Society of Pulmonary Hypertension on behalf of GlaxoSmithKline. Rådegran reports personal lecture fees from Actelion Pharmaceuticals, Bayer Health Care, GlaxoSmithKline and Nordic Infucare outside the submitted work. Rådegran has received unrestricted research grants from Actelion Pharmaceuticals and GlaxoSmithKline. Rådegran is, and has been primary-, or co-, investigator in clinical PAH trials for GlaxoSmithKline, Actelion Pharmaceuticals, Pfizer, Bayer and United Therapeutics, and in clinical heart transplantation immunosuppression trials for Novartis.
Ethical approval
The study was approved by the local ethics committee in Lund (Dnr, 2010/114, 2010/248, 2010/442, 2011/368 and, 2015/270).
Funding
The present work was supported by unrestricted research grants from the Swedish Society of Pulmonary Hypertension, ALF and Actelion Pharmaceuticals AB. The foundations had no role in the literature review selection, analysis and interpretation of the data, or publication of the manuscript.
ORCID iDs
Mattias Arvidsson https://orcid.org/0000-0001-5315-7819 Habib Bouzina https://orcid.org/0000-0002-9956-7060
References
- 1.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 4.Grignola JC. Hemodynamic assessment of pulmonary hypertension. World J Cardiol 2011; 3: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev 2012; 21: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 2016; 126: 3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thenappan T, Chan SY, Weir EK. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2018; 315: H1322–H1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Jin M, Yang F, et al. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm 2013; 2013: 928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res 2009; 81: 178–186. [DOI] [PubMed] [Google Scholar]
- 11.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J 2012; 40: 766–782. [DOI] [PubMed] [Google Scholar]
- 12.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLOS One 2014; 9: e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995; 57: 289–300. [Google Scholar]
- 14.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 15.Ponticos M, Smith BD. Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis. J Biomed Res 2014; 28: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 2017; 147: 1–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnema DD, Webb CS, Pennington WR, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail 2007; 13: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J 2019; 53: 1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito TK, Ishii G, Chiba H, et al. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene 2007; 26: 7194–7203. [DOI] [PubMed] [Google Scholar]
- 20.Ito TK, Ishii G, Saito S, et al. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009; 113: 2363–2369. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto G, Inoki I, Fujii Y, et al. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 2002; 277: 36288–36295. [DOI] [PubMed] [Google Scholar]
- 22.Partovian C, Adnot S, Raffestin B, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 2000; 23: 762–771. [DOI] [PubMed] [Google Scholar]
- 23.Campbell Andrew IM, Zhao Y, Sandhu R, et al. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 2001; 104: 2242–2248. [DOI] [PubMed] [Google Scholar]
- 24.Bouzina H, Nielsen S, Scheele, et al. Plasma sFlt-1 fluctuations in response to pulmonary arterial hypertension treatment. Cadiovasc Pharm Open Access 2017; 6: 207.
- 25.Han J-C, Li X-D, Du J, et al. Elevated matrix metalloproteinase-7 expression promotes metastasis in human lung carcinoma. World J Surg Oncol 2015; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig VJ, Zhang L, Hagood JS, et al. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2015; 53: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi J, Orcholski M, Yuan K, et al. PDGF-dependent beta-catenin activation is associated with abnormal pulmonary artery smooth muscle cell proliferation in pulmonary arterial hypertension. FEBS Lett 2016; 590: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rims CR, McGuire JK. Matrilysin (MMP-7) catalytic activity regulates β-catenin localization and signaling activation in lung epithelial cells. Exp Lung Res 2014; 40: 126–136. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Wei J, Tourtellotte WG, et al. Fibrosis in systemic sclerosis: common and unique pathobiology. Fibrogenesis Tissue Repair 2012; 5: S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moinzadeh P, Krieg T, Hellmich M, et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Exp Dermatol 2011; 20: 770–773. [DOI] [PubMed] [Google Scholar]
- 31.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003; 162: 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XM, Shi K, Li JJ, et al. Effects of angiotensin II intervention on MMP-2, MMP-9, TIMP-1, and collagen expression in rats with pulmonary hypertension. Genet Mol Res 2015; 14: 1707–1717. [DOI] [PubMed] [Google Scholar]
- 33.Hiremath J, Thanikachalam S, Parikh K, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010; 29: 137–149. [DOI] [PubMed] [Google Scholar]
- 34.Tiede SL, Wassenberg M, Christ K, et al. Biomarkers of tissue remodeling predict survival in patients with pulmonary hypertension. Int J Cardiol 2016; 223: 821–826. [DOI] [PubMed] [Google Scholar]
- 35.Schumann C, Lepper PM, Frank H, et al. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 2010; 15: 523–532. [DOI] [PubMed] [Google Scholar]
- 36.Coats AJS, Pieske B, Linde C, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 37.Rådegran G, Kjellström B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J 2016; 50: 243–250. [DOI] [PubMed] [Google Scholar]
- 38.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL895414 Supplemental Material for Matrix metalloproteinase 7 in diagnosis and differentiation of pulmonary arterial hypertension by Mattias Arvidsson, Abdulla Ahmed, Habib Bouzina and Göran Rådegran in Pulmonary Circulation