Abstract
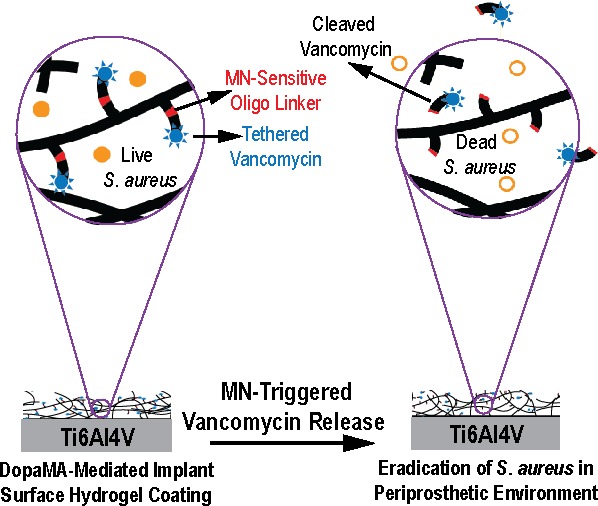
Preventing orthopedic implant-associated bacterial infections remains a critical challenge. Current practices of physically blending high-dose antibiotics with bone cements is known for cytotoxicity while covalently tethering antibiotics to implant surfaces is ineffective in eradicating bacteria from the periprosthetic tissue environment due to the short-range bactericidal actions, which are limited to the implant surface. Here, we covalently functionalize poly(ethylene glycol) dimethacrylate hydrogel coatings with vancomycin via an oligonucleotide linker sensitive to Staphylococcus aureus (S. aureus) micrococcal nuclease (MN) (PEGDMA-Oligo-Vanco). This design enables the timely release of vancomycin in the presence of S. aureus to kill the bacteria both on the implant surface and within the periprosthetic tissue environment. Ti6Al4V intramedullary (IM) pins surface-tethered with dopamine methacrylamide (DopaMA) and uniformly coated with PEGDMA-Oligo-Vanco effectively prevented periprosthetic infections in mouse femoral canals inoculated with bioluminescent S. aureus. Longitudinal bioluminescence monitoring, μCT quantification of femoral bone changes, end point quantification of implant surface bacteria, and histological detection of S. aureus in the periprosthetic tissue environment confirmed rapid and sustained bacterial clearance by the PEGDMA-Oligo-Vanco coating. The observed eradication of bacteria was in stark contrast with the significant bacterial colonization on implants and osteomyelitis development found in the absence of the MN-sensitive bactericidal coating. The effective vancomycin tethering dose presented in this on-demand release strategy was >200 times lower than the typical prophylactic antibiotic contents used in bone cements and may be applied to medical implants and bone/dental cements to prevent periprosthetic infections in high-risk clinical scenarios. This study also supports the timely bactericidal action by MN-triggered release of antibiotics as an effective prophylactic method to bypass the notoriously harder to treat periprosthetic biofilms and osteomyelitis.
Short abstract
Vancomycin covalently attached to implant surface coatings via an oligonucleotide linker sensitive to micrococcal nuclease cleavage eradicates S. aureus from the periprosthetic tissue environment.
Introduction
Periprosthetic infections represent occasional but serious health threats. Bacterial colonization around implants and subsequent biofilm formation are difficult to treat and could lead to implant failure, requiring major revision surgeries associated with high treatment cost, high morbidity, and even mortality.1−3 There are no effective strategies for eradicating established biofilms or bacteria harboring within dense tissues such as the canaliculi of bone due to poor penetration of antibiotics and immune cells into the dense matrices of biofilm and cortical bone.4−7 Thus, prophylactic strategies, particularly in high-risk patient populations and/or following high-risk clinical procedures, that ensure timely elimination of bacteria within the implant microenvironment thereby preventing biofilm formation or bacteria invasion into bone canaliculi would be highly desired.
A current clinical practice for preventing infections in high-risk orthopedic joint replacement surgeries involves physical blending of antibiotics with bone cements. This approach, however, requires high antibiotic loading that could exert local and systemic cytotoxicity. Delivering antibiotics via noncovalent implant surface coatings has been pursued as a safer alternative,8,9 although achieving suitable antibiotic release kinetics to ensure adequate and prompt release remains a challenge.8,10−15 Antibiotics covalently attached to implant surfaces4,16,17 have also been shown to exert bactericidal properties when they are presented via linkers or polymer chains of suitable flexibility/lengths18,19 at a modification site minimally perturbing the bioactivity of the drug.20 A limitation of this covalent surface modification approach, however, is that the antibiotic action is restricted to the immediate surface of the implant. For instance, we recently demonstrated that vancomycin covalently conjugated to the polymer brushes grafted from Ti6Al4V intramedullary (IM) pins was able to significantly reduce the colonization/growth of Staphylococcus aureus (S. aureus) on the metallic implant surface.21 Complete eradication of bacteria within the periprosthetic IM tissue environment, however, was not achieved due to the inability of the covalently tethered vancomycin to diffuse away from the Ti6Al4V surface.
We envision that the covalent attachment of antibiotics to the implant surface via a linker sensitive to unique bacterial enzymatic activities could bridge the gap among the existing approaches. The timely on-demand release of free antibiotics from covalently modified implant surfaces could help combat infections within a broader periprosthetic tissue microenvironment while mitigating cytotoxicity associated with the burst release of high doses of physically entrapped antibiotics or risks for developing bacteria resistance due to inadequate/delayed antibiotic releases.18,22 Various advanced drug delivery systems utilizing external stimuli such as pH, temperature, magnetic field, and ultrasound to trigger drug release were developed.23−27 More recently, endogenous enzymatic activities (especially proteases and nucleases) have been exploited as more biologically relevant and safer alternatives to stimulate on-demand drug release from peptide- and nucleotide-based delivery systems.23 Nonspecific cleavages of the peptide and oligonucleotide in these systems, however, present barriers to the success of this approach. Oligonucleotide sequences with 2′-O-carboxymethyl modifications were recently shown to improve cleavage specificity27 by micrococcal nucleases (MNs) of S. aureus, the Gram-positive bacterium responsible for a third of all orthopedic implant related infections and a major cause of osteomyelitis.3 The therapeutic efficacy of the MN-triggered release of physically entrapped vancomycin in silica nanocapsules was demonstrated in vitro.28,29 However, in vivo efficacy of this approach, especially in the context of on-demand release of covalently tethered antibiotics for combating periprosthetic infections, is unknown.
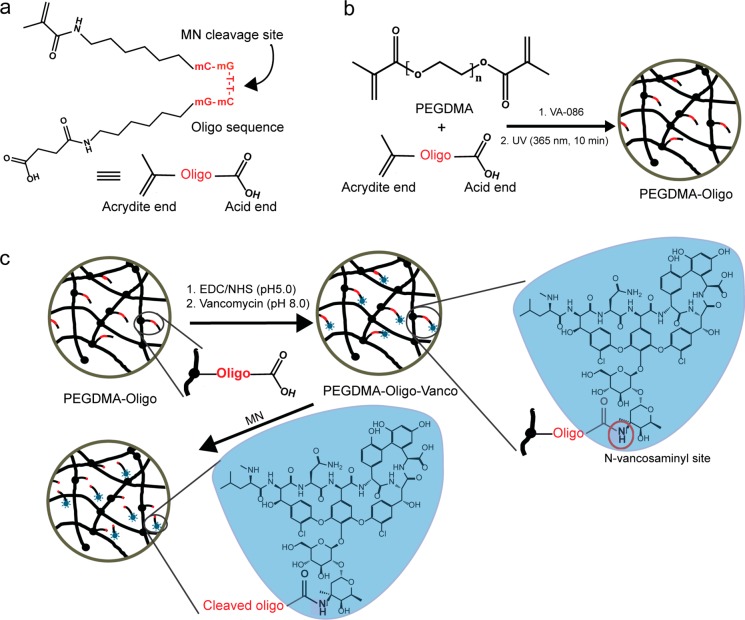
Here, we covalently functionalize PEGDMA hydrogel with vancomycin via a 2′-O-carboxymethyl modified oligonucleotide linker sensitive to S. aureus MN and evaluate its antibacterial activity in vitro and antiperiprosthetic infection properties in vivo upon application to metallic implants as a surface coating using a rodent femoral canal infection model. The oligonucleotide linker (abbreviated as oligo, Figure 1a) consists of a carboxylic acid and an acrydite on either end of the mC–mG–T–T–mC–mG sequence, which was previously shown to exhibit enhanced stability to mouse and human serum but sensitivity to S. aureus MN cleavages.28−30 The acrydite end is used to covalently conjugate the probe to the PEGDMA matrix during radical polymerization (Figure 1b) while the carboxylic acid end of the oligo linker is used to form an amide linkage with the N-vancosaminyl group of vancomycin via EDC/NHS chemistry (Figure 1c). Vancomycin, a glycopeptide antibiotic acting at the Gram-positive bacterial cell walls to block peptidoglycan synthesis, is considered the most effective in treating infections caused by Staphylococcus including Methicillin-resistant S. aureus.31 It inhibits the transpeptidation and transglycosylation steps of bacterial cell wall biosynthesis through the binding of the l-Lys–d-Ala–d-Ala termini of the nascent peptidoglycan precursor through H-bonds. Chemical modification at the N-vancosaminyl site is known to present minimal perturbation to this binding.32,33 The flexible C5 spacers between the nucleotide sequence and the bifunctional end groups are designed to relieve steric hindrance during amidation with vancomycin via the N-vancosaminyl site and to ensure adequate rotational freedom of the oligo upon covalent attachment to the hydrogel matrix. In the presence of metabolically active S. aureus, the bacterial MN is expected to cleave the oligo at the unmodified T–T position, releasing the vancomycin with a small overhung fragment at the N-vancosaminyl site (Figure 1c). We recently showed that the minimal effective concentration of vancomycin (against S. aureus) was not significantly altered upon modification with an N-vancosaminyl oligo(ethylene glycol) overhang,21 which is of comparable length of the amidated oligo fragment overhang upon MN cleavage.
Figure 1.
Depiction of the PEGDMA-Oligo-Vanco hydrogel network and MN-triggered vancomycin release. (a) Oligonucleotide (Oligo) sequence modified with bifunctional end groups. (b) PEGDMA-Oligo hydrogel formation. (c) PEGDMA-Oligo-Vanco hydrogel formation and MN-triggered vancomycin release.
Results and Discussion
Synthesis of PEGDMA-Oligo and PEGDMA-Oligo-Vanco Hydrogels
We first validated the cleavage of the oligo probe (synthesized and purified by Integrated DNA Technologies) by MN (source: S. aureus strain ATCC 27735; Worthington Biochemical Corporation) prior to its conjugation with vancomycin or PEGDMA hydrogel. Gel permeation chromatography (GPC) and UV detection at 260 nm revealed two fragments upon treatment of the oligo by MN (0.1 U/μL) in PBS (pH 7.4, 10 mM) containing a physiological concentration of calcium ions (Figure 2a, blue trace). In the absence of calcium ions, MN showed very little cleavage of oligo (Figure 2a, red trace).
Figure 2.
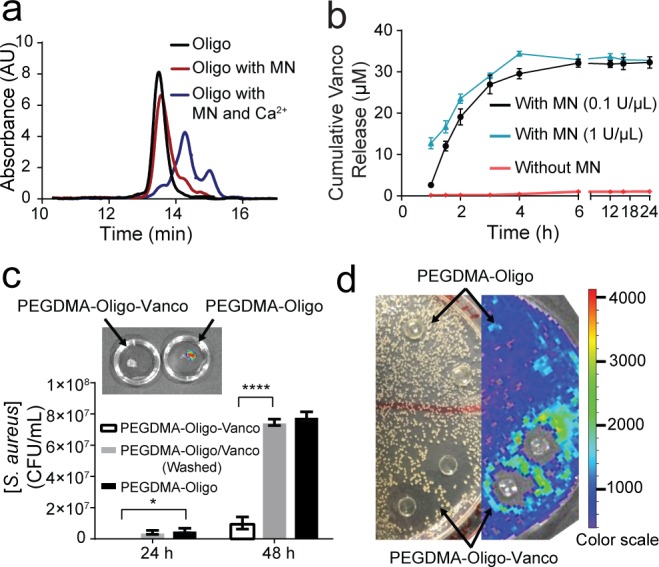
MN-triggered oligo cleavage and the antibacterial activities of PEGDMA-Oligo-Vanco hydrogel in vitro. (a) GPC traces of intact oligo (black) and oligo upon treatment with MN with (blue) and without (red) Ca2+. (b) Cumulative vancomycin (Vanco) release from PEGDMA-Oligo-Vanco hydrogel incubated with (black and blue) and without (red) MN. Differences at all given time points were significant (p ≤ 0.0001). (c) Total bacterial counts after 24 and 48 h of Xen-29 S. aureus culture in LB media containing PEGDMA-Oligo-Vanco, washed PEGDMA-Oligo/Vanco, or PEGDMA-Oligo hydrogels (n = 3; inset, corresponding IVIS image of the PEGDMA-Oligo-Vanco and PEGDMA-Oligo hydrogels retrieved after 48 h in S. aureus culture). (d) Photograph (left) and IVIS image (right) of an LB agar plate of Xen-29 S. aureus culture 24 h after placement of PEGDMA-Oligo-Vanco and PEGDMA-Oligo hydrogel discs (n = 2 shown) over the agar plate. Error bars represent standard deviations. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 (two-way ANOVA).
Upon confirmation of the sensitivity of the oligo to MN cleavage, it was covalently incorporated with PEGDMA during photopolymerization. A leaching experiment showed that 90 ± 5.0% oligo was effectively tethered to a 15 w/v% PEGDMA hydrogel, achieving 1.1 μg oligo/mg PEGDMA incorporation content, as revealed by the detection of untethered oligo (absorption at 260 nm) leached into deionized (DI) water upon extensive equilibration of the PEGDMA-Oligo hydrogel in PBS (48 h).
Covalent attachment of vancomycin to PEGDMA-Oligo hydrogel was carried out using EDC/NHS chemistry resulting in the PEGDMA-Oligo-Vanco hydrogel. To examine the release of covalently tethered vancomycin from the hydrogel, PEGDMA-Oligo-Vanco hydrogel discs (50 μL, 8 mm in diameter, n = 3) were incubated in PBS (pH 7.4, 10 mM) containing a physiological concentration of calcium ions with or without the presence of MN (0.1 and 1.0 U/μL). The concentration of released vancomycin was monitored by UV spectroscopy at 280 nm. As shown in Figure 2b, the release of vancomycin was only detected in the groups spiked with MN, supporting cleavage of the oligo linker and release of vancomycin by MN activity. The initial release (first 4 h) of vancomycin was slightly higher with the higher MN concentration, although the total vancomycin released over 24 h was the same. Assuming complete release of vancomycin after 24 h, the amount of vancomycin loaded on the 15 w/v% PEGDMA hydrogel matrix was 90 μg/mm3 hydrogel (or 24 mg Vanco/40 g PEGDMA; note that the minimum inhibitory concentration of vancomycin was ∼2 μg/mL21). This vancomycin covalent loading content was significantly lower than the noncovalent prophylactic antibiotics incorporation content in commercial bone cement (e.g., 0.5–1.0 g gentamycin/40 g bone cement, 1.0 g tobramycin/40 g bone cement).34−37
In Vitro Antibacterial Activity of PEGDMA-Oligo-Vanco Hydrogel
The therapeutic efficacy of PEGDMA-Oligo-Vanco hydrogel was first evaluated by in vitro bacterial cultures. PEGDMA-Oligo-Vanco and PEGDMA-Oligo hydrogel discs (50 μL, 8 mm in diameter, n = 3) were incubated in Luria–Broth (LB) containing 130 CFU of S. aureus at 37 °C. To account for potential contribution from residue unconjugated vancomycin, another control group with the same content of vancomycin physically entrapped (without EDC/NHS) at the time of PEGDMA-Oligo hydrogel formation was also prepared (PEGDMA-Oligo/Vanco). All hydrogels were washed for 72 h in DI water before being placed in Xen-29 S. aureus suspension culture. The Xen29 S. aureus, emitting bioluminescence when metabolically active due to the expression of luciferase,38 enables convenient visualization in vitro and longitudinal monitoring in vivo by an in vivo imaging system (IVIS-100, PerkinElmer). As shown in Figure 2c, the PEGDMA-Oligo-Vanco hydrogel was able to significantly inhibit the bacterial growth by 8-fold after 48 h. Of note, IVIS imaging showed no signs of bacterial attachment and colonization on the surface of the PEGDMA-Oligo-Vanco hydrogel upon its retrieval from the suspension culture after 48 h whereas significant colonization of S. aureus was detected on the PEGDMA-Oligo control hydrogel (Figure 2c, inset). The cleavage of the oligo linker by live S. aureus and the ability of released vancomycin to diffuse out of the hydrogel to exert antibiotic activities were further validated by the clear zone development around the PEGDMA-Oligo-Vanco hydrogels placed on an agar plate of the S. aureus culture. No clear zone was developed around the PEGDMA-Oligo control. These in vitro outcomes suggest that, when applied to the surface of implants, the PEGDMA-Oligo-Vanco hydrogel coating may also inhibit S. aureus surface attachment and colonization and suppress or even eradicate bacterial growth in its vicinity.
Hydrogel Coating of Ti6Al4V IM Pins
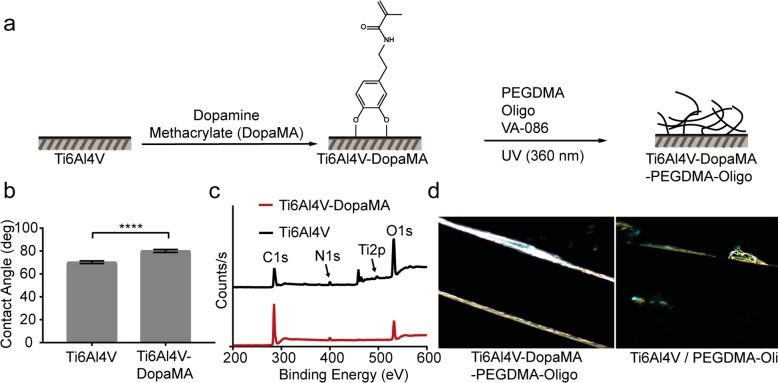
To evaluate the ability of PEGDMA-Oligo-Vanco hydrogel coating to prevent or mitigate periprosthetic infections in vivo, we applied the coating to the surface of Ti6Al4V IM pins emulating the metallic hardware used in orthopedic surgeries. To enhance the adhesion and stability of the hydrogel coating to the metallic substrate, the Ti6Al4V surface was first treated with dopamine methacrylate (DopaMA, Figure 3a). The catechol group from DopaMA is known for high affinity for surface oxides of Ti6Al4V39 whereas the methacrylate is designed to covalently polymerize with the PEGDMA matrix during hydrogel coating application. Dip-coating the metallic substrate with DopaMA is expected to promote a more uniform and stable surface coating of the functionalized PEGDMA. The choice of the methacrylate surface group also makes it possible to covalently bond with poly(methyl methacrylate) (PMMA) bone cement if desired. Successful surface modification of Ti6Al4V plates (1 cm × cm) with DopaMA was confirmed by water contact angle measurements and X-ray photoelectron spectroscopy (XPS) analyses. A statistically significant increase in water contact angle (Figure 3b) was observed upon DopaMA surface coating, consistent with the increased surface hydrophobicity due to the bonding of the more hydrophilic catechol unit with surface oxides, exposing the hydrophobic methacrylate toward the air. Decreases in XPS signal intensities for Ti 2p and O 1s and an increase in intensity for C 1s were also observed upon DopaMA treatment, consistent with the surface coverage by the organic molecules (Figure 3c). Hydrogel precursors for PEGDMA-Oligo were then photopolymerized onto the DopaMA-coated Ti6Al4V surface. As shown by dark-field optical microscopy (Figure 3d), the DopaMA intermediate improved the uniformity of the PEGDMA-Oligo coating on the Ti6Al4V pins (0.5 mm in diameter; to be inserted into mouse femoral canal in subsequent in vivo studies). Vancomycin was then covalently attached to the hydrogel coating by EDC/NHS chemistry as described earlier. The antibacterial capability of PEGDMA-Oligo-Vanco-coated Ti6Al4V pins was validated by in vitro bacterial culture on LB agar plates (Figure S1).
Figure 3.
Surface modification and characterization of Ti6Al4V plates. (a) Schematic of sequential DopaMA and PEGDMA-Oligo hydrogel coatings on Ti6Al4V substrates. (b) Water contact angle (n = 6) of Ti6Al4V and Ti6Al4V-DopaMA. Error bars represent standard deviations, **** p ≤ 0.0001. (c) XPS scans on the Ti6Al4V surfaces before and after DopaMA immobilization. (d) Dark field optical micrographs of PEGDMA-Oligo coating on Ti6Al4V-DopaMA vs Ti6Al4V IM pins (1 mm in diameter). Magnification: 50×.
In Vivo Antibacterial Activity of Ti6Al4V Pins Coated with PEGDMA-Oligo-Vanco Hydrogel
A rodent femoral canal infection model40,41 was used to investigate the antibacterial activity of the hydrogel-coated Ti6Al4V IM pins in vivo. A low dose (40 CFU) of bioluminescent Xen29 S. aureus was inoculated in the reamed femoral canal of skeletally mature CL57BL/6 mice (6–10 weeks old, males) before an unmodified Ti6Al4V pin, or a Ti6Al4V pin coated with PEGDMA-Oligo-Vanco or PEGDMA-Oligo hydrogel, was inserted (Figure S2). Our previous study showed that this low bacteria inoculation dose was sufficient in establishing infection in the mouse femoral canal.21 The degree of infection was evaluated with longitudinal IVIS monitoring of the inoculated bacteria, quantification of total bacterial counts on retrieved pins, longitudinal microcomputed tomography (μCT) quantification of femoral bone changes, and histological staining of explanted femurs at the 21 day end point.
IVIS detected bioluminescence in the femurs receiving the IM pins coated with PEGDMA-Oligo control hydrogel at 2 days postoperation which was sustained over the course of 21 days (Figure 4a, bottom panel; Figure 4b, red bars). The longitudinal detection of bioluminescence in the no-vancomycin control coating group further validated the establishment of infection with the inoculation of 40 CFU Xen29 S. aureus. By stark contrast, no obvious bioluminescence was visualized from the femurs inserted with the IM pins coated with PEGDMA-Oligo-Vanco at any time point during the 21 day follow-up (Figure 4a, bottom panel), and the quantification of bioluminescent signals confirmed significant reduction in intensity by >95% at 2 days postoperation compared to the control groups (pins with PEGDMA-Oligo coating, Figure 4b, black bars; or unmodified pins, Figure S3), and complete disappearance after day 7 (Figure 4b, red bars). To confirm the elimination of bacterial burden by the PEGDMA-Oligo-Vanco coating in vivo, the IM pins were harvested on day 21 and thoroughly vortexed (5 min in LB media) before the suspensions were cultured on LB agar plates for bacterial counts. Consistent with the IVIS imaging data, no bacteria were recovered from the retrieved IM pins with PEGDMA-Oligo-Vanco coating, supporting complete eradication of periprosthetically bound bacteria while >500 CFU S. aureus were recovered from the retrieved IM pins with the PEGDMA-Oligo control coating (Figure 4c).
Figure 4.
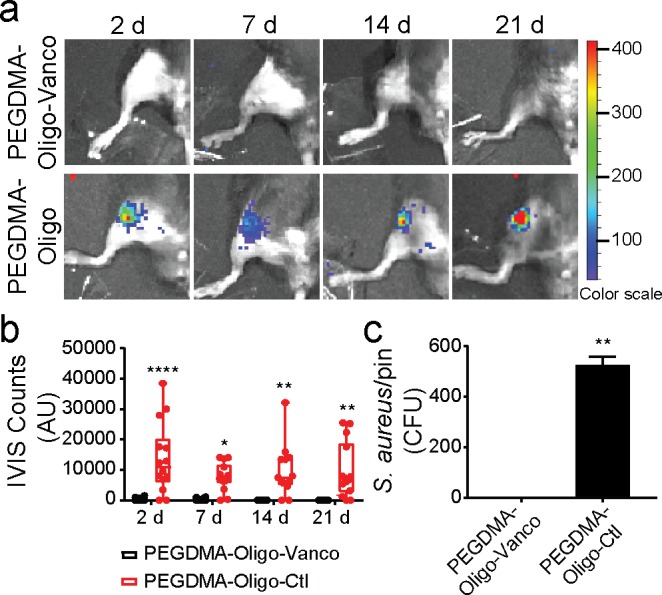
Complete eradication of S. aureus inoculated in the mouse femoral canal by PEGDMA-Oligo-Vanco coating. (a) IVIS images of mouse femurs injected with 40 CFU Xen-29 S. aureus and inserted with IM pins with PEGDMA-Oligo-Vanco or PEGDMA-Oligo coatings at 2, 7, 14, and 21 days. (b) Quantification of longitudinal bioluminescence signals of mouse femurs injected with 40 CFU Xen-29 S. aureus and inserted with the different hydrogel-coated pins at 2, 7, 14, and 21 days (n = 14). (c) S. aureus recovery from 21 day explanted pins (n = 11). Error bars represent standard deviations. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 (two-way ANOVA for part b; Student’s t-test for part c).
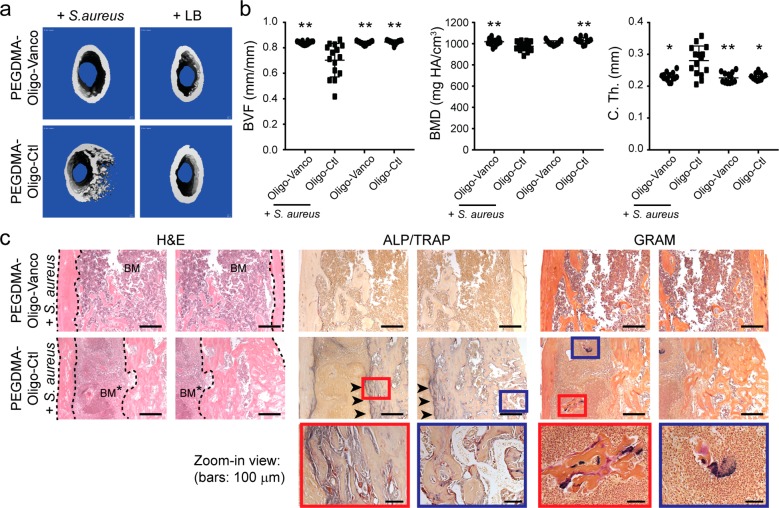
μCT imaging was carried out postoperation to confirm the proper positioning of inserted pins in all groups (Figure S4), and at the 3 week end point to determine the degree of infection within the femoral region of interest (ROI). Established local infections (osteomyelitis) could deteriorate the bone quality over time, causing decreases in bone volume fraction (BVF)42 and bone mineral density (BMD),43 and resulting in cortical thickening44 in the affected area. Accordingly, these properties were quantified by end point μCT in both infected and uninfected femurs treated with IM pins with or without hydrogel coatings. 3D μCT axial slice images at the end point revealed osteolysis and an increase in cortical thickness (C. Th.), most clearly observed in the distal region of the infected femurs treated with unmodified pins (Figure S5a) or pins coated with PEGDMA-Oligo control hydrogel (Figure 5a). These changes were confirmed by the significant decreases in BVF and BMD accompanying the increase in C. Th. in the PEGDMA-Oligo + S. aureus group (Figure 5b) and the unmodified pin + S. aureus group (Figure S5b) compared to the uninfected groups or the infected group treated with pins coated with PEGDMA-Oligo-Vanco. The PEGDMA-Oligo-Vanco + S. aureus group showed no significant differences in BVF, BMD, or C. Th. when compared to uninfected control groups, suggesting that the coating effectively prevented the changes in bone architecture often seen in osteomyelitis.
Figure 5.
Prevention of the development of osteomyelitis in mouse femoral canal inoculated with S. aureus by PEGDMA-Oligo-Vanco coating. (a) 3D μCT axial images of the distal femoral region 21 days after the insertion of Ti6Al4V IM pins (pins excluded during contouring) with different hydrogel coatings, with or without the inoculation of 40-CFU Xen-29 S. aureus. (b) Quantification of femoral BVF, BMD, and C. Th. of infected and uninfected femurs 21 days after the insertion of Ti6Al4V IM pins with PEGDMA-Oligo-Vanco or PEGDMA-Oligo coatings. n = 11–14. Error bars represent standard deviations. * p ≤ 0.05, ** p ≤ 0.01 as compared to the PEGDMA-Oligo control coating + S. aureus group (one-way ANOVA). (c) H&E, ALP (blue)/TRAP (red), and Gram staining (bacteria stain blue) of explanted femurs in the infected group with PEGDMA-Oligo-Vanco coating or PEGDMA-Oligo control coating at 21 days postoperation. Dashed lines outline the cortical bone; BM = bone marrow; BM* = infected bone marrow; arrowheads indicate regions of enhanced ALP/TRAP activities; higher magnification views of the regions within the blue and red boxes are shown in the bottom row. Scale bars = 500 μm (top and middle rows) or 100 μm (bottom row).
Histological staining of explanted femurs was used to corroborate the morphological changes observed by μCT and to visualize any colonization or penetration of bacteria along the endosteal surface or within the canaliculi of the cortical bone. Hematoxylin and eosin (H&E) staining revealed normal cortical bone structure and bone marrow morphology in the PEGDMA-Oligo-Vanco + S. aureus group while pronounced cortical thickening was found in the PEGDMA-Oligo control + S. aureus (Figure 5c) and unmodified Ti6Al4V + S. aureus (Figure S6) groups, consistent with μCT findings. A highly cellularized bone marrow canal was also detected in the infected groups treated with unmodified pins (Figure S6, bottom row, *) or pins with the PEGDMA-Oligo control coating (Figure 5c, middle row, *), consistent with elevated cellular responses to an active local infection. No alkaline phosphatase (ALP, blue, osteoblasts) or tartrate-resistant acid phosphatase (TRAP, red, osteoclasts) activities, indicative of active bone remodeling, were found in the PEGDMA-Oligo-Vanco + S. aureus group (Figure 5c and Figure S6, top rows) or the uninfected groups (Figure S6, middle 2 rows). By contrast, infected femurs treated with unmodified pins (Figure S6, bottom row) or pins with the PEGDMA-Oligo control coating (Figure 5c, middle and bottom rows) exhibited coupled osteoblastic and osteoclastic activities within the cortical bone, consistent with the observed cortical thickening and enhanced remodeling within the cortices. Gram staining revealed colonized bacteria (blue) within the bone marrow canal of the PEGDMA-Oligo control + S. aureus (Figure 5c, middle and bottom rows) and unmodified pins + S. aureus groups (Figure S6, bottom row, inset) but not in the PEGDMA-Oligo-Vanco + S. aureus group (Figure 5c and Figure S6, top rows) or the control groups without S. aureus inoculation (Figure S6, 2 middle rows). No bacteria were detected in the cortical bone canaliculi by optical microscopy.
These μCT and histological findings strongly corroborated the bioluminescence data and end point bacterial counts on the retrieved pins to demonstrate the effectiveness of PEGDMA-Oligo-Vanco-coated Ti6Al4V pins in eradicating S. aureus within the marrow canal in a timely manner to prevent their colonization on the implant surface or invasion into the cortical bone. These outcomes represent a significant improvement over the short-range suppression of bacterial colonization on Ti6Al4V IM pins achieved by vancomycin covalently conjugated to either surface oxides40 or to implant surface-grafted polymer brushes.21 The on-demand release of vancomycin from the hydrogel coating enabled the bactericidal properties of the freed vancomycin to be exerted in the broader periprosthetic tissue environment in a timely manner, clearing the bacteria before they had a chance to colonize on implant surfaces or invade the bone.
Scavenger organs harvested at 21 days postoperation revealed no difference among any of the groups treated with hydrogel-coated pins (both infected and uninfected) versus the healthy controls (Figure S7), supporting the safety of the coatings including the subsequently released vancomycin within the time frame examined. Such a safety profile is not surprising given that the polymethacrylate chemistry is employed in commercial bone cements, and the covalent vancomycin loading dose of 0.6 μg/mg PEGDMA applied to the Ti6Al4V IM pin was 200–400-fold lower than the common prophylactic antibiotics loading doses in commercial bone cement.34−37
Taken together, these in vivo results support outstanding bactericidal properties of the PEGDMA-Oligo-Vanco coating applied to Ti6Al4V pins to protect against S. aureus infections, eradicating the bacteria from both the implant surface and its periprosthetic bony tissue environment. The low dose of 40 CFU of S. aureus inoculated was sufficient to establish infection in the untreated groups and emulates a realistic clinical setting where, following standard debridement, gross infections prior to implantation are unlikely; it supports the validity of this model for examining the efficacy of prophylactic bactericidal coatings. By contrast, the literature shows that, in the absence of an MN-sensitive linker, antibiotics covalently attached to metallic implants can only reduce the bacterial colonization/biofilm formation on the implant surface16,21,40 but are unable to release free-diffusing vancomycin to the broader periprosthetic tissue space to prevent the invasion, proliferation, and colonization to tissue and ultimately the development of chronic infections. A limitation of the current rodent study is the limited postoperation monitoring duration. To assess the long-term protection of this coating strategy against periprosthetic infections and its impact on implant osteointegration, a large animal study employing clinically used metallic implants, with/without porous surface topography, for up to 6 months would be desired. In addition, examining whether this coating strategy may be extended to bone cements and dental resins will also help determine the scope of its potential clinical translation.
Conclusions
In summary, the PEGDMA-Oligo-Vanco hydrogel coating applied to the surface of Ti6Al4V effectively prevented S. aureus colonization on the surface of the implants and eradicated local infection both in vitro and in vivo. The vancomycin was cleaved and released from the hydrogel coating in the intramedullary space of mouse femurs inoculated with S. aureus, resulting in timely eradication of S. aureus from the implant surface and within the marrow cavity and preventing invasion of bacteria into cortical bone and the subsequent development of osteomyelitis. Conceptually, our findings support that early and timely bactericidal action by MN-triggered release of antibiotics is an effective prophylactic method to bypass the notoriously harder to treat periprosthetic biofilms and osteomyelitis.
From a translational perspective, the PEG-based polymethacrylate gelling mechanism was compatible with the solidification of bone cement and dental resin and, thus, may be applied as prophylactic standard care to prevent implant-associated biofilm formation and osteomyelitis. The DopaMA intermediate coating applied to the metallic implant surfaces ensures a stable and uniform hydrogel coating on the metallic implant surface. Finally, the low effective antibiotic tethering dose (e.g., more than 2 orders of magnitude reduction compared to prophylactic antibiotics physically blended with bone cement) and the MN-sensitive on-demand release mechanism improve both the efficacy (timely release) and safety of local antibiotics delivery.
Acknowledgments
The authors thank April Mason-Savas for histology support. XPS was performed at the Center for Nanoscale Systems (CNS) at Harvard University supported by the National Science Foundation award 1541959. This work was supported in part by a National Institute of Health grant (R01AR068418).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.9b00870.
Methods and supplementary figures showing LB agar plate containing PEGDMA-Oligo-Vanco and PEGDMA-Oligo-coated Ti6Al4V pins after 24 h of S. aureus culture, in vivo study design, longitudinal bioluminescence intensities of mouse femoral canals inserted with IM pins with or without PEGDMA-Oligo-Vanco coating, postoperation μCT axial images, 3D μCT axial images of the distal femoral region, histology staining of explanted femurs, and heart, lung, liver, spleen, pancreas, kidney, and rib retrieved from the mice receiving various IM pin treatments in uninfected vs infected femoral canals for 21 days (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang T.; Wang C.; Zhou S.; Xu J.; Jiang W.; Tan L.; Fu J. Nanovalves-Based Bacteria-Triggered, Self-Defensive Antibacterial Coating: Using Combination Therapy, Dual Stimuli-Responsiveness, and Multiple Release Modes for Treatment of Implant-Associated Infections. Chem. Mater. 2017, 29 (19), 8325–8337. 10.1021/acs.chemmater.7b02678. [DOI] [Google Scholar]
- Zhao L.; Chu P. K.; Zhang Y.; Wu Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res., Part B 2009, 91 (1), 470–480. 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- Campoccia D.; Montanaro L.; Arciola C. R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27 (11), 2331–2339. 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Pichavant L.; Carrie H.; Nguyen M. N.; Plawinski L.; Durrieu M. C.; Heroguez V. Vancomycin Functionalized Nanoparticles for Bactericidal Biomaterial Surfaces. Biomacromolecules 2016, 17 (4), 1339–1346. 10.1021/acs.biomac.5b01727. [DOI] [PubMed] [Google Scholar]
- Cloutier M.; Mantovani D.; Rosei F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33 (11), 637–652. 10.1016/j.tibtech.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Wu Z.; Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- de Mesy Bentley K. L.; Trombetta R.; Nishitani K.; Bello-Irizarry S. N.; Ninomiya M.; Zhang L.; Chung H. L.; McGrath J. L.; Daiss J. L.; Awad H. A.; Kates S. L.; Schwarz E. M. Evidence of Staphylococcus aureus deformation, proliferation and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J. Bone Miner. Res. 2017, 32 (5), 985–990. 10.1002/jbmr.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Chen Q.; Zhang Y.; Diba M.; Kolwijck E.; Shao J.; Jansen J. A.; Yang F.; Boccaccini A. R.; Leeuwenburgh S. C. Electrophoretic Deposition of Chitosan Coatings Modified with Gelatin Nanospheres To Tune the Release of Antibiotics. ACS Appl. Mater. Interfaces 2016, 8 (22), 13785–13792. 10.1021/acsami.6b03454. [DOI] [PubMed] [Google Scholar]
- Bakhshandeh S.; GorginKaraji Z.; Lietaert K.; Fluit A. C.; Boel C. H. E.; Vogely H. C.; Vermonden T.; Hennink W. E.; Weinans H.; Zadpoor A. A.; Amin Yavari S. Simultaneous Delivery of Multiple Antibacterial Agents from Additively Manufactured Porous Biomaterials to Fully Eradicate Planktonic and Adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9 (31), 25691–25699. 10.1021/acsami.7b04950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich A.; Kuss M. A.; Duan B.; Kielian T. 3D Bioprinted Scaffolds Containing Viable Macrophages and Antibiotics Promote Clearance of Staphylococcus aureus Craniotomy-Associated Biofilm Infection. ACS Appl. Mater. Interfaces 2019, 11 (13), 12298–12307. 10.1021/acsami.9b00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuk I.; Jariwala F.; Attygalle A. B.; Wu Y.; Libera M. R.; Sukhishvili S. A. Self-Defensive Layer-by-Layer Films with Bacteria-Triggered Antibiotic Release. ACS Nano 2014, 8 (8), 7733–7745. 10.1021/nn500674g. [DOI] [PubMed] [Google Scholar]
- Anderson E. M.; Noble M. L.; Garty S.; Ma H.; Bryers J. D.; Shen T. T.; Ratner B. D. Sustained release of antibiotic from poly(2-hydroxyethyl methacrylate) to prevent blinding infections after cataract surgery. Biomaterials 2009, 30 (29), 5675–5681. 10.1016/j.biomaterials.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. S.; William Costerton J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358 (9276), 135–138. 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Wang B.; Liu H.; Sun L.; Jin Y.; Ding X.; Li L.; Ji J.; Chen H. Construction of High Drug Loading and Enzymatic Degradable Multilayer Films for Self-Defense Drug Release and Long Term Biofilm Inhibition. Biomacromolecules 2018, 19 (1), 85–93. 10.1021/acs.biomac.7b01268. [DOI] [PubMed] [Google Scholar]
- Taubes G. The bacteria fight back. Science 2008, 321, 356–361. 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- Chen C. P.; Jing R. Y.; Wickstrom E. Covalent Attachment of Daptomycin to Ti6Al4V Alloy Surfaces by a Thioether Linkage to Inhibit Colonization by Staphylococcus aureus. ACS Omega 2017, 2 (4), 1645–1652. 10.1021/acsomega.6b00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie B.; Long T.; Ao H.; Zhou J.; Tang T.; Yue B. Covalent Immobilization of Enoxacin onto Titanium Implant Surfaces for Inhibiting Multiple Bacterial Species Infection and In Vivo Methicillin-Resistant Staphylococcus aureus Infection Prophylaxis. Antimicrob. Agents Chemother. 2017, 61 (1), e01766-16 10.1128/AAC.01766-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose B.; Antoci V.; Zeiger A. R.; Wickstrom E.; Hickok N. J. Vancomycin Covalently Bonded to Titanium Beads Kills Staphylococcus aureus. Chem. Biol. 2005, 12 (9), 1041–1048. 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Lawson M. C.; Shoemaker R.; Hoth K. B.; Bowman C. N.; Anseth K. S. Polymerizable vancomycin derivatives for bactericidal biomaterial surface modification: structure-function evaluation. Biomacromolecules 2009, 10 (8), 2221–2234. 10.1021/bm900410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406 (6797), 775–781. 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Braun B. M.; Skelly J. D.; Ayers D. C.; Song J. Significant Suppression of Staphylococcus Aureus Colonization on Intramedullary Ti6Al4V Implants Surface-Grafted with Vancomycin-Bearing Polymer Brushes. ACS Appl. Mater. Interfaces 2019, 11 (32), 28641–28647. 10.1021/acsami.9b07648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits E.; Kucharikova S.; Van Dijck P.; Erdtmann M.; Krona A.; Lovenklev M.; Frohlich M.; Dovgan B.; Impellizzeri F.; Braem A.; Vleugels J.; Robijns S. C.; Steenackers H. P.; Vanderleyden J.; De Brucker K.; Thevissen K.; Cammue B. P.; Fauvart M.; Verstraeten N.; Michiels J. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. 2016, 34 (12), 2191–2198. 10.1002/jor.23238. [DOI] [PubMed] [Google Scholar]
- Tang F.; Li L.; Chen D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24 (12), 1504–1534. 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- Li Z.; Yuan D.; Jin G.; Tan B. H.; He C. Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliveries. ACS Appl. Mater. Interfaces 2016, 8 (3), 1842–1853. 10.1021/acsami.5b09822. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Horev B.; Hwang G.; Klein M. I.; Koo H.; Benoit D. S. Characterization and optimization of pH-responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 2016, 4 (18), 3075–3085. 10.1039/C5TB02054A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Mesiano A. J.; Venkatasubramanian S.; Gross S. H.; Harris J. M.; Russell A. J. Activity and stability of enzymes incorporated into acrylic polymers. J. Am. Chem. Soc. 1995, 117 (17), 4843–4850. 10.1021/ja00122a014. [DOI] [Google Scholar]
- Yan M.; Ge J.; Liu Z.; Ouyang P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 2006, 128 (34), 11008–11009. 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- Hernandez F. J.; Huang L.; Olson M. E.; Powers K. M.; Hernandez L. I.; Meyerholz D. K.; Thedens D. R.; Behlke M. A.; Horswill A. R.; McNamara J. O. 2nd Noninvasive imaging of Staphylococcus aureus infections with a nuclease-activated probe. Nat. Med. 2014, 20 (3), 301–306. 10.1038/nm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F. J.; Hernandez L. I.; Kavruk M.; Arica Y. M.; Bayramoglu G.; Borsa B. A.; Oktem H. A.; Schafer T.; Ozalp V. C. NanoKeepers: stimuli responsive nanocapsules for programmed specific targeting and drug delivery. Chem. Commun. (Cambridge, U. K.) 2014, 50 (67), 9489–9492. 10.1039/C4CC04248D. [DOI] [PubMed] [Google Scholar]
- Borsa B. A.; Tuna B. G.; Hernandez F. J.; Hernandez L. I.; Bayramoglu G.; Arica M. Y.; Ozalp V. C. Staphylococcus aureus detection in blood samples by silica nanoparticle-oligonucleotides conjugates. Biosens. Bioelectron. 2016, 86, 27–32. 10.1016/j.bios.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Grundmann H.; Aires-de-Sousa M.; Boyce J.; Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368 (9538), 874–885. 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- Kahne D.; Leimkuhler C.; Lu W.; Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 2005, 105 (2), 425–448. 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- Lawson M. C.; Shoemaker R.; Hoth K. B.; Bowman C. N.; Anseth K. S. Polymerizable Vancomycin Derivatives for Bactericidal Biomaterial Surface Modification: Structure-Function Evaluation. Biomacromolecules 2009, 10 (8), 2221–2234. 10.1021/bm900410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistolfi A.; Massazza G.; Verné E.; Massè A.; Deledda D.; Ferraris S.; Miola M.; Galetto F.; Crova M. Antibiotic-Loaded Cement in Orthopedic Surgery: A Review. ISRN Orthop 2011, 2011, 1. 10.5402/2011/290851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neut D.; de Groot E. P.; Kowalski R. S.; van Horn J. R.; van der Mei H. C.; Busscher H. J. Gentamicin-loaded bone cement with clindamycin or fusidic acid added: biofilm formation and antibiotic release. J. Biomed. Mater. Res., Part A 2005, 73 (2), 165–170. 10.1002/jbm.a.30253. [DOI] [PubMed] [Google Scholar]
- Meyer J.; Piller G.; Spiegel C. A.; Hetzel S.; Squire M. Vacuum-mixing significantly changes antibiotic elution characteristics of commercially available antibiotic-impregnated bone cements. J. Bone Joint Surg. Am. 2011, 93 (22), 2049–2056. 10.2106/JBJS.J.01777. [DOI] [PubMed] [Google Scholar]
- Jiranek W. A.; Hanssen A. D.; Greenwald A. S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Joint Surg. Am. 2006, 88 (11), 2487–2500. 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- Francis K. P.; Joh D.; Bellinger-Kawahara C.; Hawkinson M. J.; Purchio T. F.; Contag P. R. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun. 2000, 68 (6), 3594–3600. 10.1128/IAI.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Q.; Hsueh N.; Chai C. L. L. The Chemistry of Bioinspired Catechol(amine)-Based Coatings. ACS Biomater. Sci. Eng. 2019, 5 (6), 2708–2724. 10.1021/acsbiomaterials.9b00281. [DOI] [PubMed] [Google Scholar]
- Antoci V. Jr.; Adams C. S.; Hickok N. J.; Shapiro I. M.; Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin. Orthop. Relat. Res. 2007, 461, 88–95. 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- Adams C. S.; Antoci V. Jr.; Harrison G.; Patal P.; Freeman T. A.; Shapiro I. M.; Parvizi J.; Hickok N. J.; Radin S.; Ducheyne P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27 (6), 701–709. 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- Stadelmann V. A.; Potapova I.; Camenisch K.; Nehrbass D.; Richards R. G.; Moriarty T. F. In Vivo MicroCT Monitoring of Osteomyelitis in a Rat Model. BioMed Res. Int. 2015, 2015, 587857. 10.1155/2015/587857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niska J. A.; Meganck J. A.; Pribaz J. R.; Shahbazian J. H.; Lim E.; Zhang N.; Rice B. W.; Akin A.; Ramos R. I.; Bernthal N. M.; Francis K. P.; Miller L. S. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and muCT imaging in an orthopaedic implant infection in mice. PLoS One 2012, 7 (10), e47397 10.1371/journal.pone.0047397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odekerken J. C.; Walenkamp G. H.; Brans B. T.; Welting T. J.; Arts J. J. The longitudinal assessment of osteomyelitis development by molecular imaging in a rabbit model. BioMed Res. Int. 2014, 2014, 424652. 10.1155/2014/424652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.