Abstract
Background
Cardiac output is a prognostic marker in patients with pulmonary hypertension. Pulmonary blood flow as a surrogate for cardiac output can be measured non-invasively by inert gas rebreathing. We hypothesized that pulmonary blood flow can predict outcome in patients with pulmonary hypertension.
Methods
From January 2009 to January 2012, we measured pulmonary blood flow by inert gas rebreathing in outpatients with pulmonary hypertension. Patients with pulmonary hypertension confirmed by right heart catheterization and a valid inert gas rebreathing maneuver were followed until January 2016. The investigated outcome was all-cause mortality.
Results
We included 259 patients (mean age 65 ± 13 years, 53% female) with pulmonary hypertension and classified into groups 1 (n = 103), 2 (n = 26), 3 (n = 80), and 4 (n = 50) according to the current pulmonary hypertension classification system. The median time between pulmonary hypertension diagnosis and inert gas rebreathing was 9 (IQR 0; 36) months. During a median follow-up time of 51 (IQR 20; 68) months, 109 patients (42%) died. Parameters significantly associated with survival (in order of decreasing statistical strength) were diffusion capacity of the lung for carbon monoxide (DLCO), 6-minute walk distance (6-MWD), age, NTpro-BNP, WHO functional class, group 3 pulmonary hypertension, and tricuspid annular plane systolic excursion (TAPSE), while baseline hemodynamics and pulmonary blood flow were not. In multivariable Cox regression analysis, DLCO, age, 6-MWD, and TAPSE remained significant and independent predictors of the outcome. DLCO as the strongest parameter also significantly predicted survival in aetiological subgroups except for group 4.
Conclusions
DLCO is a strong and independent predictor for survival in patients with pulmonary hypertension of different aetiologies, while pulmonary blood flow measured by inert gas rebreathing is not.
Keywords: DLCO, inert-gas rebreathing, prognosis, pulmonary blood flow, pulmonary hypertension
Introduction
Pulmonary hypertension (PH) is characterized by increased blood pressure in the pulmonary arteries and can be classified, according to aetiology, into five groups.1 Pulmonary arterial hypertension (PAH), group 1 of the current PH classification system, is a rare disease with a limited prognosis even if it improved since “targeted” medication is available.2 PH due to left heart disease (group 2), lung disease (group 3), or chronic thromboembolic PH (group 4) is much more frequent than PAH and also associated with increased mortality rates.3
Different parameters have been used separately or in combination to predict the prognosis of PH patients.2,4 Clinical parameters like the World Health Organization Functional Class (WHO-FC) can predict survival both at diagnosis and follow-up (on specific treatment).5 Imaging of the heart by echocardiography including the contractile reserve during exercise has been shown to be an independent prognostic marker in patients with severe PH.6 Prognostic value has also been identified for some parameters of cardiac magnetic resonance imaging.7 The 6-minute walking distance (6-MWD) provides prognostic information, and adding peripheral oxygen saturation measurements and heart rate response might improve its prognostic relevance.8–10 Also, several variables determined by cardiopulmonary exercise testing provide prognostic information.11 Levels of natriuretic peptides (BNP, NTpro-BNP) provide prognostic information at the time of diagnosis and during follow-up assessments.12 Haemodynamics assessed by right heart catheter (RHC) provides important prognostic information, both at the time of diagnosis and during follow-up. Right atrial pressure, cardiac output (CO), and mixed venous oxygen saturation are the most robust indicators of right ventricular function and prognosis, whereas mean pulmonary arterial pressure provides little prognostic information.13 Even if RHC is a safe procedure in experienced hands,14 it remains a time-consuming and invasive method precluding its use as a frequent follow-up examination in PH patients. Therefore, other methods to measure CO have been developed. The inert gas rebreathing (IGR) method is a non-invasive technique of CO assessment with good agreement compared to the thermodilution and the Fick method in RHC examinations, which can be easily performed in most patients.15,16
The aim of the present study was to assess the prognostic value of a single CO measurement at rest by the IGR method in a sample of consecutive outpatients with different aetiologies of PH.
Methods
Study design
The present analysis is a single-center study of patients with European descent and known or suspected PH. From January 2009 to January 2012, pulmonary blood flow (PBF) as a surrogate of CO was measured as part of the routine assessment in consecutive outpatients. Patients with PH confirmed by RHC and a valid PBF measurement were followed until January 2016. The investigated outcome was all-cause mortality.
Data were collected prospectively in patients participating in an observational clinical study after written informed consent and retrospectively in the remaining patients. The protocol of the observational study, the data protection strategy, and the retrospective data collection and follow-up of patients were approved by the Ethics Committee of the University of Regensburg and were in accordance with the Declaration of Helsinki.
Study population
The PH outpatient clinic of the University Medical Center Regensburg is a local referral center for patients with known and suspected PH, following mainly patients with precapillary PH (groups 1, 3, and 4 according to the PH classification system) or PH due to left heart disease (group 2) with significant precapillary involvement. Regular outpatient visits are performed every three to six months in stable patients. At every visit, blood tests including measurement of NTpro-BNP, a pulmonary function test including assessment of the diffusion capacity of the lung for carbon monoxide (DLCO), capillary blood gas analysis, and 6-MWT are conducted. In addition, WHO-FC, current medication, and adverse events are recorded in patient’s medical records. An electrocardiogram and an echocardiography are performed at least every second visit, while RHC is performed only if deemed helpful for clinical decision making (e.g. if clinical worsening is not clearly attributable to worsening of PH, or before an escalation of targeted PAH medication). In addition, IGR using the Innocor™ device was routinely performed at every outpatient visit from January 2009 to January 2012.
All patients with PH confirmed by RHC and a valid PBF measurement were eligible for participation. In case of more than one valid PBF measurement, only the first one was used. PH was defined by a mean pulmonary artery pressure of ≥25 mmHg at rest on RHC. Patients were allocated to groups 1 to 4 of the current PH classification system after thorough differential diagnosis according to current guidelines.4 Results of examinations mentioned were extracted from the patient’s medical records at the time of IGR, while the latest RHC measurement before the IGR was used for description.
Right heart catheter
All patients underwent RHC at rest with determination of right atrial pressure, pulmonary arterial pressures, and pulmonary arterial wedge pressure because of suspected PH. CO was determined by thermodilution. Mixed venous oxygen saturation was determined from pulmonary artery blood samples. Pulmonary vascular resistance, cardiac index, and diastolic pressure gradient were calculated. All RHC were performed by the same physician.
Pulmonary function tests, DLCO, and PBF measurements
Spirometry and body plethysmography were used to determine total lung capacity, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and their ratio. DLCO was measured by the single-breath technique without correction for haemoglobin values or lung volumes. PBF was measured by IGR using the Innocor™ device (Innovision ApS, Glamsbjerg, Denmark) as previously described.15 All measurements were carried out by trained personnel in a standardized fashion.
Assessment of outcome
All-cause mortality from the time of PBF measurement was assessed in January 2016 according to medical reports or information from the family physician. If patients did not return for planned follow-up visits, they were contacted by phone. Patients were censored at last contact, lung transplantation, or pulmonary endarterectomy.
Statistical analysis
Descriptive data are presented as mean (±SD) for normally distributed variables and as median and interquartile range for not normally distributed variables. Continuous variables of baseline characteristics were compared by the t-test and categorical variables by the Chi-square test. Correlation was tested by the two-sided Pearson test. Univariate Cox regression analysis was used to analyze the prediction of PBF and other parameters on all-cause mortality. Significant risk factors from the univariable analysis, such as DLCO, 6-MWD, age, NTpro-BNP, WHO-FC, PH group, and echocardiographic tricuspid annular plane systolic excursion (TAPSE), were included as covariates in the multivariate Cox regression. Results are given as hazard ratio estimates with 95% confidence interval and Chi-square values. p-Values of <0.05 were considered significant. Survival curves are shown in Kaplan-Meier plots; logrank test was used to compare estimates of the hazard functions. A Forest plot was used to visualize hazard ratios of survival for DLCO in different aetiological groups and in the total sample. Data were analyzed using the SPSS statistical software package (SPSS 23.0, IBM SPSS Statistics, Armonk, New York, USA).
Results
Patient characteristics
Two hundred fifty-nine consecutive patients (mean age 65 ± 13 years, 53% female) were included from January 2009 to January 2012. Of these, 103 (39.8%), 26 (10.0%), 80 (30.9%), and 50 (19.3%) were allocated to groups 1, 2, 3, and 4 according to the current PH classification system, respectively.1 Patients were predominantly in WHO-FC II or III, and 157 (60.6%) were on targeted PH medication (phosphodiesterase-5-inhibitors, endothelin-receptor-antagonists, prostacyclin analogues, or riociguat). Median time between the diagnostic RHC and PBF measurement was nine (IQR 0; 36) months (Table 1). PH groups were significantly different according to age (oldest in group 2), 6-MWD (shortest in group 3), DLCO (lowest in group 3), PBF (lowest in group 1), mean pulmonary artery pressure (highest in group 1), pulmonary artery wedge pressure (highest in group 2), and pulmonary vascular resistance (highest in group 1). NTpro-BNP, TAPSE, and CO (RHC) were similar between groups (Table 2).
Table 1.
Clinical parameters of the 259 analyzed patients.
| Patients (n) | 259 |
| Age at PBF measurement (years) | 65 ± 13 |
| Female sex (n; %) | 138 (53%) |
| WHO-FC 1/2/3/4 (%) | 4/40/49/7 |
| 6-minute walk distance (m) | 344 ± 113 |
| NTpro-BNP (pg/ml) | 1620 ± 1940 |
| TAPSE (mm) | 18.3 ± 4.9 |
| DLCO (% predicted) | 49 ± 22 |
| Mean PA pressure at PH diagnosis (mmHg) | 42 ± 11 |
| Cardiac output at PH diagnosis (l/min) | 4.9 ± 1.5 |
| Innocor-PBF (l/min) | 4.3 ± 1.3 |
| Time between PH diagnosis and PBF measurement (months) | 9 [0; 36] |
| PAH-Medication w/o CCB (n) | 157 |
| Phosphodiesterase-5-inhibitor (n) | 129 |
| Endothelin-receptor-antagonist (n) | 69 |
| Prostacyclin (n) | 13 |
| Riociguat (n) | 1 |
| PH-monotherapy (n) | 108 |
| PH-dual/triple combination therapy (n) | 43/6 |
| CCB (n) | 47 |
| Anticoagulation | 193 (76%) |
Note: Results are provided as mean ± standard deviation for normally distributed and as median (interquartile range) for non-normally distributed variables.
CCB: calcium channel blocker; DLCO: diffusion capacity of the lung for carbon monoxide; PA: pulmonary artery; PBF: pulmonary blood flow; PH: pulmonary hypertension; TAPSE: tricuspid annular plane systolic excursion; WHO-FC: World Health Organization functional class.
Table 2.
Clinical and hemodynamic parameters of the 259 analyzed patients differentiated by aetiology.
| All patients | Group 1 | Group 2 | Group 3 | Group 4 | pa | |
|---|---|---|---|---|---|---|
| Number | 259 | 103 | 26 | 80 | 50 | – |
| Age (years) | 65 ± 13 | 60 ± 13 | 70 ± 13 | 68 ± 10 | 67 ± 12 | <0.001 |
| 6-minute-walk distance (m) | 344 ± 113 | 375 ± 112 | 325 ± 113 | 302 ± 86 | 332 ± 128 | 0.002 |
| Ntpro-BNP (pg/ml) | 1620 ± 1940 | 1447 ± 1825 | 1661 ± 1236 | 1631 ± 2026 | 1948 ± 2292 | 0.555 |
| TAPSE (mm) | 18.3 ± 4.9 | 18.4 ± 4.6 | 17.4 ± 4.9 | 18.3 ± 4.9 | 18.3 ± 5.5 | 0.859 |
| DLCO (% pred) | 49 ± 22 | 57 ± 19 | 54 ± 19 | 33 ± 18 | 56 ± 18 | <0.001 |
| Mean PAP Dx (mmHg) | 42 ± 11 | 46 ± 12 | 39 ± 10 | 37 ± 9 | 42 ± 9 | <0.001 |
| PAWP Dx (mmHg) | 10 ± 6 | 9 ± 4 | 20 ± 5 | 9 ± 5 | 9 ± 4 | <0.001 |
| PVR Dx (WU) | 7.3 ± 4.3 | 8.8 ± 5.0 | 4.1 ± 2.5 | 6.2 ± 3.2 | 7.8 ± 3.6 | <0.001 |
| CO Dx (l/min) | 4.9 ± 1.5 | 4.8 ± 1.6 | 5.2 ± 1.7 | 5.0 ± 1.4 | 4.9. ± 1.5 | 0.608 |
| Innocor-PBF (l/min) | 4.3 ± 1.3 | 4.1 ± 1.3 | 4.6. ± 1.4 | 4.2 ± 1.3 | 4.4 ± 1.2 | 0.372 |
| Innocor-PBF (l/min/m2) | 2.3 ± 0.6 | 2.2 ± 0.6 | 2.6 ± 0.8 | 2.3 ± 0.6 | 2.4 ± 0.6 | 0.033 |
Note: Results are provided as mean ± standard deviation.
CO: cardiac output; DLCO: diffusion capacity of the lung for carbon monoxide; Dx: diagnosis; PAP: pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; PBF: pulmonary blood flow; PVR: pulmonary vascular resistance; TAPSE: tricuspid annular plane systolic excursion; WU: Wood unit.
Difference between the groups (t-test for continuous variables).
Outcome
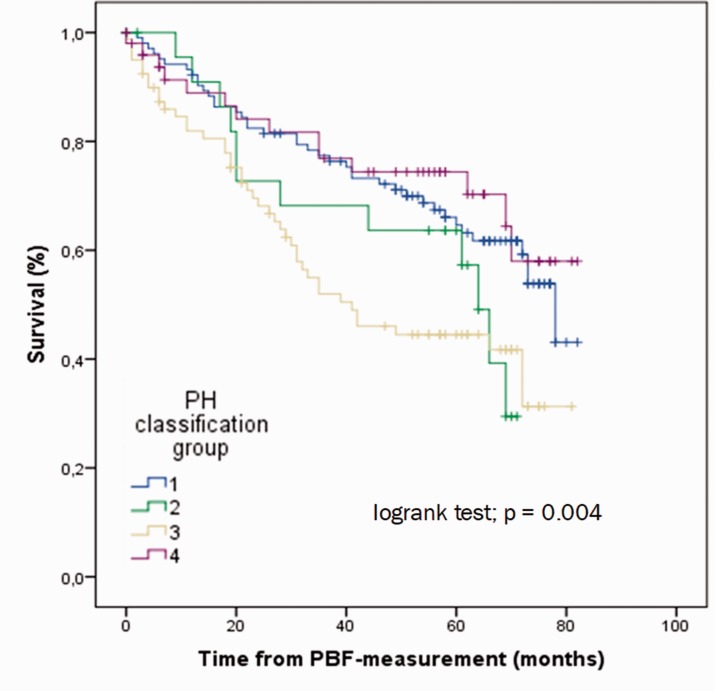
During a median follow-up time of 51 (IQR 20; 86) months, 109 patients (42%) died. Patients with PH groups 2 and 3 had the worst prognosis (Fig. 1). The deceased patients were significantly older, had a shorter 6-MWD, higher NTpro-BNP, lower TAPSE, and worse DLCO (Table 3). Parameters significantly associated with survival (in order of decreasing statistical strength) were DLCO (χ2 = 46.9, p < 0.001), 6-MWD, age, NTpro-BNP, WHO-FC, group 3 PH, and TAPSE (Table 4). PBF (measured by IGR) and CO (measured by RHC) were significantly correlated (r = 0.475, p < 0.001). PBF as well as CO, pulmonary arterial mean pressure, and right atrial pressure were no predictors of survival in the total sample, neither in continuous nor in dichotomous analyses (every p > 0.05).
Fig. 1.
Survival according to pulmonary hypertension aetiological groups.
PH: pulmonary hypertension; PBF: pulmonary blood flow.
Table 3.
Clinical and hemodynamic parameters of the 259 analyzed subjects differentiated by survival status.
| Survived | Died | pa | |
|---|---|---|---|
| Number | 150 | 109 | – |
| Age (years) | 62 ± 14 | 69 ± 10 | <0.001 |
| 6-minute walk distance | 373 ± 115 | 297 ± 92 | <0.001 |
| NT pro-BNP (pg/ml) | 1250 ± 1792 | 2121 ± 2027 | 0.001 |
| TAPSE (mm) | 19.0 ± 4.9 | 17.2 ± 4.7 | 0.008 |
| DLCO (% predicted) | 55 ± 21 | 41 ± 20 | <0.001 |
| Mean PAP at Dx (mmHg) | 42 ± 11 | 42 ± 11 | 0.946 |
| PAWP Dx (mmHg) | 10 ± 6 | 10 ± 5 | 0.565 |
| PVR Dx (WU) | 7.2 ± 4.3 | 7.5 ± 4.3 | 0.620 |
| CO Dx (l/min) | 5.0 ± 1.6 | 4.7 ± 1.4 | 0.098 |
| Innocor-PBF (l/min) | 4.4 ± 1.4 | 4.1 ± 1.3 | 0.108 |
| Innocor-PBF (l/min/m2) | 2.3 ± 0.6 | 2.3 ± 0.6 | 0.556 |
| Time between Dx of PH and PBF measurement | 8 [0; 42] | 9 [0; 29] | 0.020 |
Note: Results are provided as mean ± standard deviation.
CO: cardiac output; DLCO: diffusion capacity of the lung for carbon monoxide; Dx: diagnosis; PAP: pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; PBF: pulmonary blood flow; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; TAPSE: tricuspid annular plane systolic excursion; WU: Wood unit.
Difference between the groups (t-test for continuous variables).
Table 4.
Hazard ratios for mortality in 259 subjects in univariable and multivariable Cox regression analyses.
| Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|
| HR (95% CI) | χ2 | p | HR (95% CI) | p | |
| DLCO (% predicted) | 0.966 (0.956; 0.976) | 46.9 | <0.001 | 0.975 (0.959; 0.991) | 0.002 |
| 6-minute walk distance | 0.994 (0.992; 0.006) | 33.0 | <0.001 | 0.996 (0.992; 0.999) | 0.014 |
| Age (years) | 1.050 (1.030; 1.070) | 26.5 | <0.001 | 1.043 (1.009; 1.078) | 0.012 |
| NTpro-BNP (pg/ml) | 1.000 (1.000; 1.000) | 20.0 | <0.001 | 1.000 (1.000; 1.000) | 0.410 |
| WHO-FC I/II vs. III/IV | 0.498 (0.334; 0.742) | 12.2 | 0.001 | 1.574 (0.807; 3.072) | 0.183 |
| PH group 3 vs. 1/2/4 | 1.899 (1.290; 2.794) | 10.9 | 0.001 | 1.262 (0.673; 2.364) | 0.468 |
| TAPSE (mm) | 0.937 (0.895; 0.981) | 7.7 | 0.005 | 0.920 (0.859; 0.986) | 0.018 |
| PAH-medication y/n | 0.762 (0.511; 1.137) | 1.8 | 0.183 | ||
| Innocor-PBF (l/min) | 0.910 (0.786; 1.055) | 1.6 | 0.212 | ||
| Innocor-PBF (l/min/m2) | 0.956 (0.710; 1.286) | 0.1 | 0.765 | ||
Note: Shown are the hazard ratios by univariable and multivariable Cox regression analyses and 95% confidence intervals (95% CI). The variables used in the multivariable regression model were the significant variables from the univariable regression model: DLCO, 6-minute walk distance, age, NTpro-BNP, WHO-FC I/II vs. III/IV, PH group 3 vs. 1/2/4, and TAPSE.
DLCO: diffusion capacity of the lung for carbon monoxide; HR: hazard ratio; PAH: pulmonary arterial hypertension; PBF: pulmonary blood flow; PH: pulmonary hypertension; TAPSE: tricuspid annular plane systolic excursion; WHO-FC: World Health Organization functional class.
In a multivariable Cox regression model including the significant predictors of mortality, DLCO, 6-MWD, age, and TAPSE remained significant and independent predictors for the outcome (Table 4).
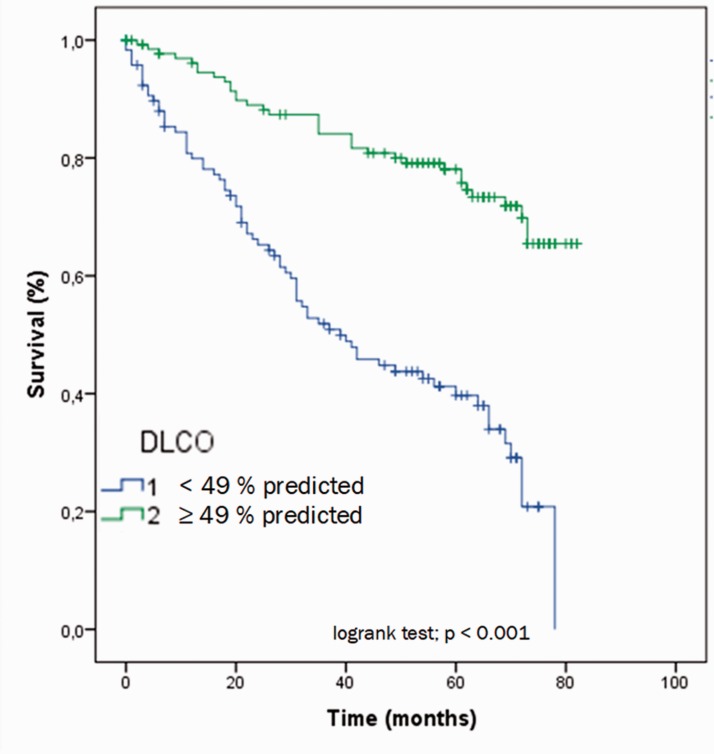
If DLCO was adjusted for possible restrictive or obstructive lung disease (as ratio of DLCO and FVC or FEV1, respectively), it was still a significant predictor of survival (HR 0.179 (95% CI 0.051; 0.629), χ2 = 56.7, p = 0.007 for FEV1 and HR 0.235 (95% CI 0.0.68; 0.805), χ2 = 54.4, p = 0.021 for FVC, respectively). Dichotomization of DLCO by its median (49% of predicted) led to a clear differentiation between mortality rates right from the beginning of follow-up (HR 0.267 (95% CI 0.177; 0.404), p < 0.001; Fig. 2). After three years of follow-up, 87% of patients with DLCO above the median were alive vs. only 52% of those below the median.
Fig. 2.
Survival according to diffusion capacity of the lung for carbon monoxide above vs. below median (49% of the predicted).
DLCO: diffusion capacity of the lung for carbon monoxide.
Prognostic factors in different PH groups
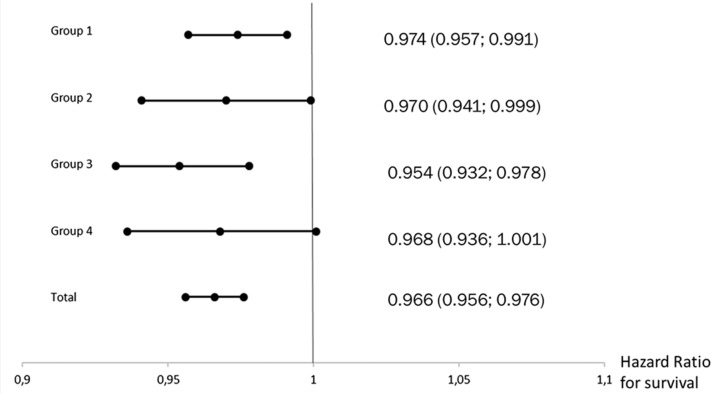
DLCO as the strongest parameter also significantly predicted survival in subgroups of patients according to the PH classification system except for group 4 (HR 0.968 (95% CI 0.936; 1.001), p = 0.059) (Fig. 3). PBF was a significant predictor of survival only in patients of PH group 3 (HR 0.753 (95% CI 0.598; 0.949), p = 0.016). CO showed a significant predictive value only in patients of group 4 (HR 0.477 (95% CI 0.274; 0.832), p = 0.009), mean pulmonary arterial pressure in groups 2 and 3 (HR 1.067 (95% CI 1.003; 1.135), p = 0.040 and HR 1.032 (95% CI 1.002; 1.064), p = 0.039, respectively), and right atrial pressure only in group 4 (HR 1.090 (HR 1.005; 1.182), p = 0.036).
Fig. 3.
Forest plot showing hazard ratios of survival for diffusion capacity of the lung for carbon monoxide in different aetiological groups and in the total sample.
Discussion
The present analysis yielded the novel finding that DLCO was strongly associated with survival in patients with PH across different aetiologies during a mean follow-up of 44 months, while PBF measured by IGR was not. This outcome was independent from other risk factors significantly associated with survival like 6-MWD, age, NTpro-BNP, WHO-FC (I/II vs. III/IV), PH aetiology (group 3 vs. 1/2/4), and TAPSE. Patients with a DLCO below the median (49% of predicted value) had a HR of 3.74 (95% CI 2.45; 5.65) (p < 0.001) for all-cause mortality.
Prognostic value of hemodynamic parameters
The invasively measured hemodynamic parameters right atrial pressure and CO have shown to be robust indicators of right ventricular function and prognosis in patients with idiopathic PAH at both diagnosis and follow-up.13 Both parameters had no significant prognostic value in the present analysis, neither in the entire group nor in the largest subgroup of patients with PAH. This may be explained by the fact that we assessed survival from the time of PBF measurement and not from the diagnostic RHC. Therefore, subsequent targeted therapy could have influenced this otherwise valid association at least in patients with PAH.
Use of non-invasive IGR is well established and validated for assessing CO in the absence of a significant intrapulmonary shunt.15 Farina et al. could show that CO can be reliably measured by IGR in patients with PH.17 However, we were not able to confirm our hypothesis that a single IGR measurement in outpatients with PH of different aetiologies is prognostically relevant. In patients with PH due to left heart and lung diseases and chronic thromboembolic PH (CTEPH), this could be an expected finding as an association between CO and prognosis in these PH groups is not concisely shown in the literature. However, in patients with PAH, this finding is unexpected and may be due to technical reasons of our measurement. The Innocor™ device measures arterial oxygen saturation by pulsoxymetry to correct the measured PBF for possible intrapulmonary shunts, i.e. the CO is calculated from the PBF plus estimated shunt flow. However, Farina et al. have shown that the algorithm used within the Innocor™ device is inaccurate in patients with PAH and hypoxemia.17 Therefore, we decided to only measure the PBF as surrogate for CO. However, due to the fact that PBF is measured by rebreathing, only the blood flow in ventilated areas (ventilation/perfusion matched) is captured which leads to a systemic underestimation of CO.18 As inhomogeneous ventilation/perfusion may vary considerably even among PAH patients (and maybe due to pulmonary comorbidities), this could also have influenced the missing association between PBF and survival in our patients.
In the present analysis, PBF predicted mortality only in patients with underlying lung disease. This cannot be explained by the mechanism discussed above (underestimation of CO by PBF) and may just reflect the severity of the underlying lung disease. Further studies evaluating the impact of serial PBF measurements on the prognosis of PH are warranted.
DLCO as prognostic parameter in PH
In the present analysis, among other parameters significantly associated with survival (6-MWD, age, NTpro-BNP, WHO functional class, group 3 PH, and TAPSE), DLCO was the most powerful one. Some studies reported on DLCO as a prognostic marker for patients with PH before. In idiopathic PAH, DLCO is usually normal or only mildly impaired,19 but patients with a low DLCO (<45% of predicted value) have a particularly high mortality.20 Szturmowicz et al. found an increased mortality in idiopathic PAH patients with an DLCO <55% predicted.21 Kang et al. described a low DLCO as poor prognostic factor in patients with PAH associated with connective tissue disease.22 Hoeper et al. found a significantly worse survival rate in patients with PH due to heart failure with preserved ejection fraction and DLCO <45% predicted.23 Similar findings have been reported in patients with chronic lung disease and PH.24–26 Suda et al. described a decreased DLCO to be associated with poor outcome in 91 medically treated CTEPH patients.27 In the present analysis, the DLCO was at no specified time during treatment a significant predictor for survival in the entire sample of PH patients and in the different aetiological groups except for CTEPH. This may be explained by the small sample size of this subgroup, as the HR is similar compared to the other PH groups (HR 0.968; CI 95% (0.936; 1.001), p = 0.059).
DLCO is influenced by many factors like the alveolar capillary volume, the permeability of the alveolar membrane, the lung perfusion, the haemoglobin concentration, smoking habits, and potential intrapulmonary or intracardiac shunts. Regarding this broad spectrum of factors influencing DLCO, it is conceivable that its strong prognostic capability is not only reflecting one single pathomechanism. An impaired DLCO may therefore reflect a number of disorders like parenchymal lung diseases, obstructive or restrictive ventilatory abnormalities, heart failure, anemia, smoking, and others, which are known to have an impact on prognosis and might exist as underlying cause or comorbidities in PH patients. Like DLCO, also the 6-MWD has a number of possible influencing factors and does not only reflect cardiac function. Interestingly, this parameter was the second most powerful prognostic parameter in our present analysis. 6-MWD has proven its prognostic value despite of – or just because of – the quantity of influencing factors (e.g. age, cardiac function, ventilatory, orthopedic, frailty, and others) before.28
The strengths of the present study are the large sample size and the thorough phenotyping of a sample of outpatients with PH of different aetiologies, which reflects the spectrum of PH patients currently seen at referral centers and allowed adjusted analysis for many known prognostic factors in PH. In addition, the long follow-up has to be mentioned as an advantage. The following limitations warrant discussion: First, PBF was not measured simultaneously with CO by RHC, but nine months later in median. Although PBF and CO correlated well, there might be a relevant change of CO during this time interval due to disease progression or targeted medication. However, neither invasively measured hemodynamics at diagnosis nor PBF during follow-up were associated with survival in the present study. Second, the data derive from a single center, which may have created bias. However, this ensured a homogeneous exploration of patients in a well-experienced PH center, and all RHCs were done by the same physician, which minimizes interobserver variability.
In summary, our findings show that DLCO is strongly and independently associated with survival in a large sample of patients with PH of different aetiologies. Therefore, DLCO should be considered in the risk assessment of patients with PH and evaluated regarding its potential as therapeutic target.
Acknowledgements
We thank all participating patients of the PH outpatient department of the University Medical Center Regensburg. We thank the nurses for their expert work in performing IGR: Christiane Cordes, Vera Oelve, and Margit Rothaug. This study was part of Nicoletta Mergenthaler’s doctoral thesis.
Authors’ contribution
TJL was responsible for the conception, hypotheses delineation, and design of the study. All authors were involved in the statistical data analysis. TJL and SS were writing the article and revised it prior to submission. All authors read and approved the final version of the article.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Ethical approval
The protocol of the observational study, the data protection strategy, and the retrospective data collection and follow-up of patients were approved by the Ethics Committee of the University of Regensburg and were in accordance with the Declaration of Helsinki.
Funding
SS received lecture fees from Actelion Pharmaceuticals Ltd. TJL received personal fees from Actelion, MSD, Pfizer, and OMT orphan.
Guarantor
SS is the guarantor of this work and, as such, had full access to all data of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ORCID iD
Stefan Stadler https://orcid.org/0000-0002-8783-618X
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1): ). pii: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50(2): ). pii: 1700740. [DOI] [PubMed] [Google Scholar]
- 3.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Chung L, Zamanian RT, et al. Functional class improvement and 3-year survival outcomes in patients with pulmonary arterial hypertension in the REVEAL Registry. Chest 2013; 144: 160–168. [DOI] [PubMed] [Google Scholar]
- 6.Grünig E, Tiede H, Enyimayew EO, et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013; 128: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 7.Swift AJ, Rajaram S, Marshall H, et al. Black blood MRI has diagnostic and prognostic value in the assessment of patients with pulmonary hypertension. Eur Radiol 2012; 22: 695–702. [DOI] [PubMed] [Google Scholar]
- 8.Fritz JS, Blair C, Oudiz RJ, et al. Baseline and follow-up 6-min walk distance and brain natriuretic peptide predict 2-year mortality in pulmonary arterial hypertension. Chest 2013; 143: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provencher S, Chemla D, Hervé P, et al. Heart rate responses during the 6-minute walk test in pulmonary arterial hypertension. Eur Respir J 2006; 27: 114–120. [DOI] [PubMed] [Google Scholar]
- 10.Paciocco G, Martinez FJ, Bossone E, et al. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J 2001; 17: 647–652. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg FC, Arzt M, Lange T, et al. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail 2013; 15: 771–775. [DOI] [PubMed] [Google Scholar]
- 12.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J 2008; 32: 503–512. [DOI] [PubMed] [Google Scholar]
- 13.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 14.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006; 48: 2546–2552. [DOI] [PubMed] [Google Scholar]
- 15.Peyton PJ, Thompson B. Agreement of an inert gas rebreathing device with thermodilution and the direct oxygen Fick method in measurement of pulmonary blood flow. J Clin Monit Comput 2004; 18: 373–378. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Maier R, Tongers J, et al. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 1999; 160: 535–541. [DOI] [PubMed] [Google Scholar]
- 17.Farina S, Teruzzi G, Cattadori G, et al. Noninvasive cardiac output measurement by inert gas rebreathing in suspected pulmonary hypertension. Am J Cardiol 2014; 113: 546–551. [DOI] [PubMed] [Google Scholar]
- 18.Gama de Abreu M, Winkler T, Pahlitzsch T, et al. Performance of the partial CO2 rebreathing technique under different hemodynamic and ventilation/perfusion matching conditions. Crit Care Med 2003; 31: 543–551. [DOI] [PubMed] [Google Scholar]
- 19.Steenhuis LH, Groen HJ, Koëter GH, et al. Diffusion capacity and haemodynamics in primary and chronic thromboembolic pulmonary hypertension. Eur Respir J 2000; 16: 276–281. [DOI] [PubMed] [Google Scholar]
- 20.Chandra S, Shah SJ, Thenappan T, et al. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J Heart Lung Transplant 2010; 29: 181–187. [DOI] [PubMed] [Google Scholar]
- 21.Szturmowicz M, Kacprzak A, Franczuk M, et al. Low DLCO in idiopathic pulmonary arterial hypertension – clinical correlates and prognostic significance. Pneumonol Alergol Pol 2016; 84: 87–94. [DOI] [PubMed] [Google Scholar]
- 22.Kang KY, Jeon CH, Choi SJ, et al. Survival and prognostic factors in patients with connective tissue disease-associated pulmonary hypertension diagnosed by echocardiography: results from a Korean nationwide registry. Int J Rheum Dis 2017; 20: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Meyer K, Rademacher J, et al. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail 2016; 4: 441–449. [DOI] [PubMed] [Google Scholar]
- 24.Chaouat A, Bugnet A-S, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. [DOI] [PubMed] [Google Scholar]
- 25.Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010; 35: 105–111. [DOI] [PubMed] [Google Scholar]
- 26.Rose L, Prins KW, Archer SL, et al. Survival in pulmonary hypertension due to chronic lung disease: influence of low diffusion capacity of the lungs for carbon monoxide. J Heart Lung Transplant 2019; 38: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suda R, Tanabe N, Ishida K, et al. Prognostic and pathophysiological marker for patients with chronic thromboembolic pulmonary hypertension: usefulness of diffusing capacity for carbon monoxide at diagnosis. Respirology 2017; 22: 179–186. [DOI] [PubMed] [Google Scholar]
- 28.Zelniker TA, Huscher D, Vonk-Noordegraaf A, et al. The 6MWT as a prognostic tool in pulmonary arterial hypertension: results from the COMPERA registry. Clin Res Cardiol 2018; 107: 460–470. [DOI] [PubMed] [Google Scholar]