Molecular surveillance of rifampin-resistant Mycobacterium tuberculosis can help to monitor the transmission of the disease. The Xpert MTB/RIF Ultra assay detects mutations in the rifampin resistance-determining region (RRDR) of the rpoB gene by the use of melting temperature (Tm) information from 4 rpoB probes which can fall in one of the 9 different assay-specified Tm windows.
KEYWORDS: Xpert MTB/RIF Ultra, rpoB, tuberculosis
ABSTRACT
Molecular surveillance of rifampin-resistant Mycobacterium tuberculosis can help to monitor the transmission of the disease. The Xpert MTB/RIF Ultra assay detects mutations in the rifampin resistance-determining region (RRDR) of the rpoB gene by the use of melting temperature (Tm) information from 4 rpoB probes which can fall in one of the 9 different assay-specified Tm windows. The large amount of Tm data generated by the assay offers the possibility of an RRDR genotyping approach more accessible than whole-genome sequencing. In this study, we developed an automated algorithm to specifically identify a wide range of mutations in the rpoB RRDR by utilizing the pattern of the Tm of the 4 probes within the 9 windows generated by the Ultra assay. The algorithm builds a RRDR mutation-specific “Tm signature” reference library from a set of known mutations and then identifies the RRDR genotype of an unknown sample by measuring the Tm distances between the test sample and the reference Tm values. Validated using a set of clinical isolates, the algorithm correctly identified RRDR genotypes of 93% samples with a wide range of rpoB single and double mutations. Our analytical approach showed a great potential for fast RRDR mutation identification and may also be used as a stand-alone method for ruling out relapse or transmission between patients. The algorithm can be further modified and optimized for higher accuracy as more Ultra data become available.
INTRODUCTION
Molecular surveillance of rifampin-resistant (RR) tuberculosis (TB) can aid in the detection of disease transmission and help to monitor the effectiveness of public health interventions (1). Although whole-genome sequencing (WGS) has been increasingly used as a surveillance tool (2), the cost and complexity of this approach limit its applicability, especially in the low- and middle-income countries where TB is most prevalent. Fortunately, approximately 95% of rifampin-resistant tuberculosis (RR-TB) cases are caused by a limited number of mutations in a short (81-bp) “core region” of the Mycobacterium tuberculosis rpoB gene (3, 4), also known as the rifampin resistance-determining region (RRDR). This suggests that RR-TB monitoring may be usefully performed using a more focused genotyping approach that limits analysis to the RRDR. As an alternative to WGS, this method would be especially useful if RRDR genotyping could be automatically incorporated into standard diagnostic procedures.
The increasing use of the Xpert MTB/RIF Ultra assay (CE in vitro diagnostic [IVD] medical device not available in the United States) to detect M. tuberculosis and RR may provide an automated means to perform M. tuberculosis RRDR genotyping. The Ultra assay uses 4 overlapping sloppy molecular beacons (SMBs) to probe the M. tuberculosis rpoB RRDR for mutations associated with RR-TB. Rifampin resistance due to a mutation in the RRDR is detected by a shift in the melting temperature (Tm) of at least one of these probes away from the Tm expected to occur in the presence of wild-type (WT), rifampin-susceptible (RS) RRDR sequences (5). Several small-scale studies of clinical M. tuberculosis isolates have suggested that the Tm of individual Ultra assay rpoB SMBs can indeed be used to identify several specific rpoB mutations and to “bin” other rpoB mutations into Tm-defined categories (5, 6). However, the complex effects of 4 different SMBs hybridizing to overlapping regions of the rpoB RRDR amplicon suggested that a single RRDR mutation might destabilize multiple Ultra SMBs. This raised the possibility that individual mutations could produce complex patterns of Tm shifts among the Ultra SMBs, which would enable more-specific differentiation among RRDR mutations and provide a more useful surveillance tool. Further, since different RRDR mutations may be associated with different rifampin MICs (7), a more reliable tool for identifying specific mutations might aid in customizing rifamycin-containing therapy.
We have developed an analytic method that uses the Tm values generated by the Ultra assay to specifically identify most rpoB RRDR mutations. This method combines the Ultra assay Tm values into a “Tm signature” so that the Tm signature of an unknown sample can be compared to the Tm signatures in a reference library of known rpoB RRDR mutants and, thus, the mutation present in the unknown sample be identified. The rare mutations that cannot be specifically identified can be grouped into one of a small number of “mutation groups” that are usually present in the same rpoB RRDR codon. Here, we describe this analytic method and test the overall approach of using Ultra Tm values to identify different RRDR mutations in a wide panel of RR M. tuberculosis isolates.
MATERIALS AND METHODS
Defining the Tm distance (TD) value.
The 4 SMB probes in the Ultra assay can produce many different patterns of Tm values. At its simplest, an RS M. tuberculosis strain produces one Tm value for each probe. For each Ultra test performed on RS M. tuberculosis, the Tm associated with each probe clusters around the mean Tm value produced by that probe in multiple reference tests with rpoB-wild-type M. tuberculosis; thus, any probe that produces a Tm which falls into a larger, assay-defined, probe-specific “wild-type Tm window” is considered to have hybridized to a wild-type target sequence. A sample is defined as RS M. tuberculosis when all the 4 rpoB probes produce Tm values that lie within the 4 wild-type Tm windows. The Ultra assay also defines 5 “mutant Tm” windows. The assay identifies a sample as containing RR M. tuberculosis when at least one of the 4 assay probes produces a Tm value that falls within at least one of the mutant Tm windows. Samples that contain mixtures of RS and RR M. tuberculosis or that contain rpoB core sequences with multiple mutations produce more-complex patterns where one or more probes can produce Tm values in both wild-type and mutant windows at the same time. Mixtures and double mutants can also produce multiple different Tm values within the same Tm window.
We had previously defined the relatedness of a reference to test Tm signatures as being represented a “distance index” or “D” value (8), which we have renamed here the TD value (Tm distance value) to avoid confusion with Cohen’s d, which measures the effect size of the difference between two means. The TD value for a pair of samples was defined as the Euclidean distance between the samples in Euclidean space. Because the Ultra assay classified Tm values into 9 different Tm windows, we could localize each Tm signature produced by the assay in a 9-dimensional Euclidean space. Tm windows that did not contain a Tm value (for example, a wild-type Tm window for a probe that produced a Tm value in its mutant Tm window due to the presence of a mutation) were classified as having a Tm value of zero. A library of reference Tm values was created as described in Results (Table 1). To calculate the TD value for an unknown test sample, the distance between the 9 data points in the reference Tm signature and the unknown test Tm signature was derived using the following formula:
TABLE 1.
Reference Tm values with standard deviations and Tm windows as defined by the Ultra assaya
| rpoB genotype | Mean or SD |
Value (°C) for Tm windows as defined by the Ultra assay for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rpo1 |
rpo1
Mut |
rpo2 |
rpo2
Mut |
rpo3 |
rpo3
Mut |
rpo4 |
rpo4
MutA |
rpo4
MutB |
||
| Genotypes with one set of reference Tm values | ||||||||||
| WT | Mean | 69.57 | 0 | 73.29 | 0 | 75.96 | 0 | 67.27 | 0 | 0 |
| SD | 0.16 | 0 | 0.16 | 0 | 0.22 | 0 | 0.15 | 0 | 0 | |
| 511CCG | Mean | 0 | 63.52 | 73.22 | 0 | 75.93 | 0 | 67.28 | 0 | 0 |
| SD | 0 | 0.05 | 0.05 | 0 | 0.05 | 0 | 0.05 | 0 | 0 | |
| 513CAGb | Mean | 68.17 | 0 | 75.1 | 0 | 75.93 | 0 | 67.47 | 0 | 0 |
| SD | 0.06 | 0 | 0.17 | 0 | 0.21 | 0 | 0.15 | 0 | 0 | |
| 516GTC | Mean | 70.06 | 0 | 0 | 69.72 | 75.81 | 0 | 67.42 | 0 | 0 |
| SD | 0.12 | 0 | 0 | 0.1 | 0.11 | 0 | 0.18 | 0 | 0 | |
| 516TAC | Mean | 69.71 | 0 | 0 | 68.91 | 75.7 | 0 | 67.39 | 0 | 0 |
| SD | 0.12 | 0 | 0 | 0.17 | 0.14 | 0 | 0.13 | 0 | 0 | |
| 522CAG | Mean | 70.12 | 0 | 0 | 68.83 | 0 | 73.95 | 67.12 | 0 | 0 |
| SD | 0.1 | 0 | 0 | 0.1 | 0 | 0.06 | 0.05 | 0 | 0 | |
| 522TTG | Mean | 69.6 | 0 | 0 | 70.2 | 0 | 73.66 | 67.26 | 0 | 0 |
| SD | 0.07 | 0 | 0 | 0.07 | 0 | 0.09 | 0.05 | 0 | 0 | |
| 526CCC | Mean | 69.47 | 0 | 73.45 | 0 | 0 | 71.7 | 66.08 | 0 | 0 |
| SD | 0.05 | 0 | 0.06 | 0 | 0 | 0 | 0.1 | 0 | 0 | |
| 526TAA | Mean | 69.27 | 0 | 73.17 | 0 | 0 | 71.7 | 67.57 | 0 | 0 |
| SD | 0.06 | 0 | 0.12 | 0 | 0 | 0.1 | 0.06 | 0 | 0 | |
| 526TAC | Mean | 69.44 | 0 | 73.49 | 0 | 0 | 72.83 | 67.29 | 0 | 0 |
| SD | 0.1 | 0 | 0.13 | 0 | 0 | 0.13 | 0.16 | 0 | 0 | |
| 526TCC | Mean | 69.37 | 0 | 73.37 | 0 | 0 | 70.63 | 67.3 | 0 | 0 |
| SD | 0.06 | 0 | 0.15 | 0 | 0 | 0.12 | 0.1 | 0 | 0 | |
| 526TGC | Mean | 69.4 | 0 | 73.43 | 0 | 0 | 71.7 | 68.43 | 0 | 0 |
| SD | 0.1 | 0 | 0.12 | 0 | 0 | 0.1 | 0.06 | 0 | 0 | |
| 531CAG | Mean | 69.27 | 0 | 73.33 | 0 | 0 | 71.23 | 67.1 | 0 | 0 |
| SD | 0.06 | 0 | 0.06 | 0 | 0 | 0.06 | 0 | 0 | 0 | |
| 531TGG | Mean | 69.62 | 0 | 73.78 | 0 | 0 | 73.54 | 0 | 71.06 | 0 |
| SD | 0.08 | 0 | 0.08 | 0 | 0 | 0.09 | 0 | 0.05 | 0 | |
| 531TTG | Mean | 69.5 | 0 | 74.27 | 0 | 0 | 73.44 | 0 | 73.89 | 0 |
| SD | 0.12 | 0 | 0.16 | 0 | 0 | 0.19 | 0 | 0.13 | 0 | |
| 533CCG | Mean | 69.64 | 0 | 73.3 | 0 | 75.98 | 0 | 0 | 0 | 61.68 |
| SD | 0.13 | 0 | 0.12 | 0 | 0.1 | 0 | 0 | 0 | 0.13 | |
| 529AAAc | Mean | 69.53 | 0 | 72.98 | 0 | 0 | 0 | 0 | 0 | 64.08 |
| SD | 0.05 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0.08 | |
| 518AAC del | Mean | 69.53 | 0 | 0 | 65.73 | 75.5 | 0 | 66.93 | 0 | 0 |
| SD | 0.21 | 0 | 0 | 0.38 | 0.26 | 0 | 0.06 | 0 | 0 | |
| 510CTG_516TAC | Mean | 0 | 65.73 | 0 | 69.07 | 75.77 | 0 | 67.47 | 0 | 0 |
| SD | 0 | 0.06 | 0 | 0.12 | 0.12 | 0 | 0.06 | 0 | 0 | |
| 511CCG_516GGC | Mean | 0 | 65.9 | 71.87 | 0 | 75.83 | 0 | 67.47 | 0 | 0 |
| SD | 0 | 0.1 | 0.15 | 0 | 0.12 | 0 | 0.06 | 0 | 0 | |
| 512CGC_526AAC | Mean | 0 | 63.7 | 73.67 | 0 | 0 | 72.53 | 67.53 | 0 | 0 |
| SD | 0 | 0.1 | 0.12 | 0 | 0 | 0.15 | 0.06 | 0 | 0 | |
| 512GGC_531TGG | Mean | 0 | 66.75 | 74.05 | 0 | 0 | 73.65 | 0 | 71.1 | 0 |
| SD | 0 | 0.06 | 0.06 | 0 | 0 | 0.13 | 0 | 0.08 | 0 | |
| 513CTA_523GAG | Mean | 0 | 0 | 71.55 | 0 | 0 | 72.28 | 67.08 | 0 | 0 |
| SD | 0 | 0 | 0.06 | 0 | 0 | 0.05 | 0.13 | 0 | 0 | |
| 513CTA_526TAA | Mean | 0 | 0 | 73.23 | 0 | 0 | 71.8 | 67.7 | 0 | 0 |
| SD | 0 | 0 | 0.15 | 0 | 0 | 0.1 | 0.1 | 0 | 0 | |
| 515ATT_516TAC | Mean | 69.87 | 0 | 0 | 68.74 | 75.81 | 0 | 67.2 | 0 | 0 |
| SD | 0.17 | 0 | 0 | 0.19 | 0.12 | 0 | 0.1 | 0 | 0 | |
| 515ATT_526AAC | Mean | 69.6 | 0 | 0 | 70.03 | 0 | 72.5 | 67.17 | 0 | 0 |
| SD | 0.1 | 0 | 0 | 0.06 | 0 | 0 | 0.12 | 0 | 0 | |
| 516GGC_533CCG | Mean | 70.85 | 0 | 71.77 | 0 | 75.78 | 0 | 0 | 0 | 61.62 |
| SD | 0.1 | 0 | 0.05 | 0 | 0.08 | 0 | 0 | 0 | 0.13 | |
| 516TAC_531TTG | Mean | 69.8 | 0 | 0 | 68.5 | 0 | 73.23 | 0 | 73.8 | 0 |
| SD | 0.1 | 0 | 0 | 0.1 | 0 | 0.06 | 0 | 0.1 | 0 | |
| 522TTG_531GCG | Mean | 69.57 | 0 | 0 | 70.3 | 0 | 72.87 | 67.7 | 0 | 0 |
| SD | 0.31 | 0 | 0 | 0.1 | 0 | 0.12 | 0.1 | 0 | 0 | |
| 530ATG_531TTC | Mean | 69.5 | 0 | 73.63 | 0 | 0 | 69.53 | 0 | 0 | 64 |
| SD | 0.17 | 0 | 0.21 | 0 | 0 | 0.12 | 0 | 0 | 0.1 | |
| 512ACC_515ATT_526AAC | Mean | 0 | 61.62 | 0 | 69.9 | 0 | 72.53 | 67.48 | 0 | 0 |
| SD | 0 | 0.04 | 0 | 0.06 | 0 | 0.08 | 0.1 | 0 | 0 | |
| Genotypes with two sets of reference Tm values | ||||||||||
| 526AGC-1 | Mean | 69.4 | 0 | 73.5 | 0 | 0 | 71.5 | 68.9 | 0 | 0 |
| SD | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 526AGC-2c | Mean | 69.53 | 0 | 73.61 | 0 | 0 | 71.59 | 0 | 0 | 0 |
| SD | 0.11 | 0 | 0.18 | 0 | 0 | 0.13 | 0 | 0 | 0 | |
| 526CGC-1 | Mean | 69.51 | 0 | 73.46 | 0 | 0 | 74.25 | 0 | 69.36 | 0 |
| SD | 0.03 | 0 | 0.05 | 0 | 0 | 0.05 | 0 | 0.07 | 0 | |
| 526CGC-2c | Mean | 69.55 | 0 | 73.49 | 0 | 0 | 0 | 0 | 69.39 | 0 |
| SD | 0.08 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0.09 | 0 | |
| 509AGG_526CGC-1 | Mean | 0 | 66.1 | 73.47 | 0 | 0 | 74.27 | 0 | 69.43 | 0 |
| SD | 0 | 0.15 | 0.05 | 0 | 0 | 0.05 | 0 | 0.1 | 0 | |
| 509AGG_526CGC-2c | Mean | 0 | 66.09 | 73.55 | 0 | 0 | 0 | 0 | 69.3 | 0 |
| SD | 0 | 0.14 | 0.14 | 0 | 0 | 0 | 0 | 0.21 | 0 | |
| Genotype with three sets of reference Tm values | ||||||||||
| 518GAC_533CCG-1c | Mean | 69.42 | 0 | 0 | 0 | 75.77 | 0 | 0 | 0 | 61.45 |
| SD | 0.13 | 0 | 0 | 0 | 0.12 | 0 | 0 | 0 | 0.14 | |
| 518GAC_533CCG-2 | Mean | 69.6 | 0 | 71.5 | 0 | 75.9 | 0 | 0 | 0 | 61.7 |
| SD | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 518GAC_533CCG-3 | Mean | 69.2 | 0 | 0 | 71.1 | 75.6 | 0 | 0 | 0 | 61.3 |
| SD | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Grouped genotypes | ||||||||||
| 526AAC or 526CTC | Mean | 69.38 | 0 | 73.29 | 0 | 0 | 72.41 | 67.47 | 0 | 0 |
| SD | 0.13 | 0 | 0.1 | 0 | 0 | 0.06 | 0.09 | 0 | 0 | |
| 526GAC or 531TTC | Mean | 69.42 | 0 | 73.56 | 0 | 0 | 72.19 | 68.15 | 0 | 0 |
| SD | 0.17 | 0 | 0.23 | 0 | 0 | 0.21 | 0.15 | 0 | 0 | |
| 516GAC del or 516GTC_518GAC | Mean | 69.65 | 0 | 0 | 66.75 | 75.75 | 0 | 67.14 | 0 | 0 |
| SD | 0.09 | 0 | 0 | 0.13 | 0.09 | 0 | 0.11 | 0 | 0 | |
n = 9 Tm windows. SD information was unavailable for 3 references because the cases are too rare to provide meaningful SD data.
Silent mutation.
References with only 3 positive Tm values.
In this context, the 9 ΔTm values correspond to the differences of rpo1, rpo2, rpo3, rpo4, rpo1 Mut, rpo2 Mut, rpo3 Mut, rpo4 MutA or rpo4 MutB Tm values of a reference (known) Tm signature with the corresponding Tm values of an unknown test Tm signature. A series of TD values are generated by calculating the TD value of the sample against each reference in the library, and the smallest TD value, i.e., the TD-1 value corresponding to the difference between a specific reference Tm signature and a test Tm signature, identifies the most likely mutation present in the test sample. The next closest match is identified by the TD-2 value. The calculation of TD values and matching TD-1 and TD-2 values was done using an R script which is available upon request.
Samples that have more than 4 positive Tm values are identified as “mixtures.” If the Tm signature includes Tm values in all 4 wild-type windows plus a Tm value in one mutant Tm window, the mutant in the mixture would also be identified. Any other sample with more than 4 positive Tm values would be identified simply as a heteroresistant mixture because the specific mutations in the mixture could not be identified in these cases. This process was done using the R script.
Testing of DNA, BCG, and clinical isolates with the Ultra assay.
The mutant DNA samples used to generate the reference library of Tm signatures and to perform the initial validation study were obtained from the TDR-TB Strain Bank, International Tuberculosis Research Center (Masan, South Korea), and Foundation of Innovative New Diagnostics (FIND). A Mycobacterium bovis BCG strain (ATCC catalog no. 35734) was used to generate the representative reference Tm signature for the WT Tm pattern. The M. tuberculosis cultures used in the blind validation study were obtained from FIND. For the DNA samples, the Ultra cartridge was preloaded with the DNA of interest and was then placed into a GeneXpert instrument (Cepheid, USA). A modified assay protocol was used that allows direct testing of DNA instead of sputum or cells (5). BCG cells were processed in a biosafety cabinet and tested using Ultra cartridges following the standard procedure. Each genotype was tested at least 3 times, including multiple tests performed on the same sputum sample and/or on multiple sputa. For algorithm evaluation, clinical isolates provided by FIND were processed in a biosafety level 3 (BSL3) facility and tested on Ultra cartridges using version 2 of the Ultra protocol following the standard procedure specified by Cepheid.
Tm reference samples.
Clinical DNA samples (n = 81) containing 40 different rpoB core region mutants as well as BCG were used to build the reference. The samples that had been tested using version 1 of the Ultra protocol were reanalyzed using the version 2 protocol. Tm values were recorded, and windows with no Tm value were given a value of zero so that every sample had 9 Tm values, and means of each of the 9 Tm values were calculated for each reference in the R script.
Human subject approval.
This study did not use any human subjects.
RESULTS
Deriving reference Tm signatures specific to rifampin resistance mutations.
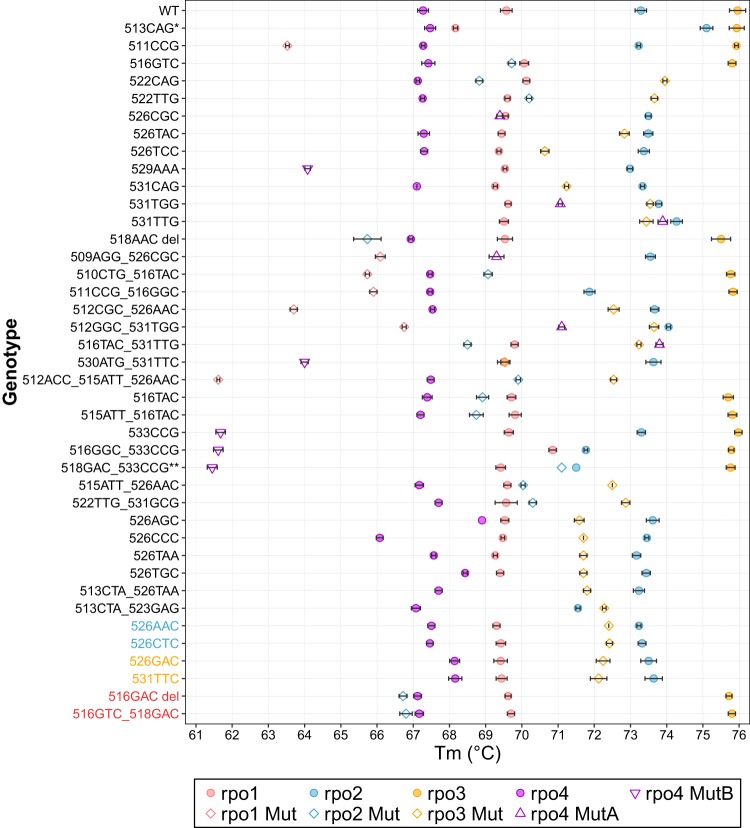
Each Ultra assay generates a series of Tm values. Each of the generated Tm values that falls inside a Tm window can be localized into one of 9 Tm windows and used for subsequent analysis; those that fall outside a Tm window are excluded. The Tm values that are localized into Tm windows can be combined into a Tm signature. We investigated whether Tm signatures specific to individual RRDR mutations could be identified. In order to use Tm signatures generated by the Ultra assay for this purpose, we first needed to generate a reference library of Tm signatures that were specific for each mutation or each small “mutation group” of related mutations. We tested 82 samples comprising 41 different rpoB genotypes (1 WT genotype and 40 mutant genotypes) and recorded the mean Tm value present in each window for each mutation (Table 1). Most samples generated 4 positive Tm values; however, some mutations were found to generate only 3 Tm values because the 4th Tm value fell in a range that did not correspond to the defined Tm windows of the assay. A Tm value of zero was assigned to any window that did not contain a Tm value generated by the assay. An initial analysis performed using mean values for all test replicates showed that combining the Tm values in the 5 mutant and 4 wild-type windows enabled us to distinguish almost all mutations (Fig. 1, codons in black) except for 3 “mutation groups” consisting of two mutations each (Fig. 1, codons in color).
FIG 1.
Mean Ultra Tm value ± 1 SD determined for 41 rpoB genotypes. Mutations from 511CCG through 512ACC_515ATT_526AAC showed unique mutant Tm window patterns. Mutations from 516TAC through 513CTA_523GAG had undistinguishable mutant Tm window patterns, but they showed different WT Tm window patterns. Colored labels represent 3 mutant groups, each containing 2 mutations that have similar Tm patterns. The single asterisk (*) indicates that 513CAG is a silent mutation (Q513Q) and all 4 Tm values fall within WT windows. The double asterisks (**) indicate that the rpo2 Tm value of 518GAC_533CCG could fall within the WT window or the Mut window or neither.
A repeat analysis of individual (not mean) test results showed that slight variations in Tm values between some replicates (Fig. 1) would result in some errors in RRDR mutation identification unless a larger library of all possible Tm signature patterns corresponding to individual mutations was developed. For example, certain mutations such as 526CGC generated 3 Tm values in some replicates but 4 Tm values in others because the fourth Tm was very close to the edge of a defined Tm window and could be detected only if it fell inside the Tm window and was not detected if it fell outside the window. In such cases, we created 2 sets of Tm reference patterns: one that contained 4 positive Tm values and an alternate set that contained only 3 Tm values. In rare cases, we found samples that, upon repeat testing, produced Tm values that were very close to those within two or more Tm windows (for example, if 2 adjacent windows were in close proximity with a gap of <0.2 degree, a Tm at the very edge of one window would be able to cross to the other window due to minor Tm variations within standard deviation [SD] limits, during repeat runs). In these cases, 3 sets of alternative reference Tm signatures were made: one with the probe Tm value in one window, one with the probe Tm in an adjacent window, and one with the probe Tm in a temperature range that was between these two windows and which was not detected by the assay (Tm of zero for that window). However, this was observed only in case of the double mutant 518GAC_533CCG, for which we designated three Tm signature variants in the reference library (Table 1). In total, we determined that 43 different Tm signatures would best identify the 41 different rpoB RRDR genotypes in our test set. Of these, 31 genotypes could be identified by a single Tm signature each, one double mutant was identified by any of 3 possible signatures, and 3 mutations were identified by either of 2 possible signatures. The remaining 6 mutations produced Tm signatures that were not significantly different (P value > 0.19) but were found to match in pairs. This enabled us to divide these 6 mutations into 3 groups of two different mutations in each group, with each group being identified by its group-specific Tm signature (Table 1).
Internal validation of Tm signatures.
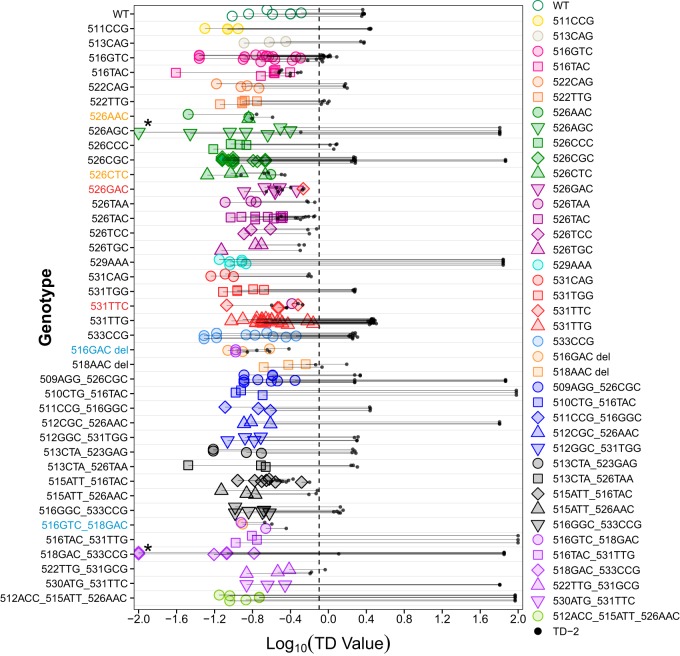
We next assessed how well the Tm signature reference library could be used to identify rpoB RRDR mutations using our TD value calculation method to determine the relatedness of a test sample to all the Tm signatures in the reference library. In this initial validation study, as a challenge set, we retested the samples used to generate the reference Tm signature library. This was done due to the limited availability of additional RR mutant samples. The output generated by these multiple comparisons consisted of the closest (TD-1) and the next-closest (TD-2) matches of the test sample to the mutations in the Tm signature library along with the TD values for each match. The genotype of a sample was identified using TD-1 and TD-2 values following a method illustrated in Fig. 2 and following processing through the use of the R script described in Materials and Methods. In this method, a perfect match results in a TD value of zero, and the cutoff value for TD-1 and TD-2 for calling a match is set to 0.8 because the maximum TD-1 value observed was 0.69 (log10 0.69 = −0.16 in Fig. 3). We first used a reference library without grouping the 6 mutations described earlier to test whether grouping was actually necessary. The majority of the 41 genotypes tested were correctly identified by examination of their TD-1 values alone (Fig. 3), which was expected since these tests were performed on the same samples as were used to generate the Tm signature references. Only 6 genotypes (526AAC, 526CTC, 526GAC, 531TTC, 516GAC deletion, and double mutant 516GTC 518GAC) in the challenge set were not matched to the correct references by TD-1 values alone in some replicates (colored labels in Fig. 3). These were the same 6 mutations belonging to the 3 sets that were grouped together in the final Tm reference library (Grouped genotypes in Table 1). The samples were tested against the final Tm reference library (which now included these 6 mutations grouped in 3 different sets of 2 mutations each), and all genotypes were correctly identified with TD-1 values between 0 and 0.69, of which 21 replicates from 7 genotypes had TD-2 – TD-1 = <0.25 (see Table S1 in the supplemental material).
FIG 2.
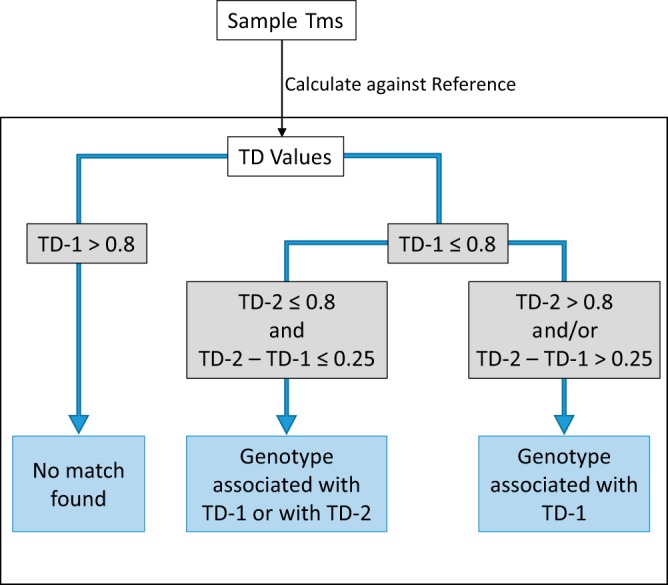
Algorithm for identifying mutants based on TD values.
FIG 3.
TD-1 and TD-2 values determined for each sample in logarithmic transformation. Each genotype was tested on multiple samples or for multiple times on one sample to generate at least 3 replicates. Horizontal lines indicate the distance between TD-1 (colored data points) and TD-2 (black dots) of individual replicates. Colors and shapes of each data point represent the reference mutant corresponding to TD-1 values. The vertical dashed line indicates the cutoff for calling a mutant. *, 3 samples of 2 genotypes had TD-1 values of 0; therefore, 0.01 was added to their TD-1 values before logarithmic transformation was performed.
Confirming the TD algorithm on clinical isolates.
To validate the performance of our TD distance calculation approach to RRDR mutation identification, a new and previously untested set of 33 clinical isolates with various RRDR mutations was tested in a blind manner. Each RRDR mutation was also confirmed by Sanger sequencing. All isolates were tested using the Ultra assay. The Tm values generated by each assay were then analyzed by running the R script. Of the 33 isolates tested, 3 (2 514TTT and 1 531ATG) did not generate a match (i.e., all TD-1 values were >0.8) because they were not in the Tm reference library. Of the remaining 30 isolates, 19 were correctly identified by their TD-1 values alone (range, 0.1 to 0.56) and 8 were correctly identified by either their TD-1 value or their TD-2 value (ranges, 0.22 to 0.53 and 0.35 to 0.66, respectively), with one of these two closest matches representing the correct RRDR mutation. Finally, 3 samples were misidentified (Table 2), one of which (Isolate-15 in Table 2) had a mutation (515ATA_526AAC) that did not exist in our Tm reference library. This isolate was, therefore, incorrectly matched to an existing reference (515ATT_526AAC) with a TD-1 value of 0.15. Note that even in the absence of this particular double mutation in our reference library, a similar double mutation in the same two codons was identified as a possible correct match. The other 2 misidentified samples (Isolate-3 and Isolate-21 in Table 2) had mutations that existed in the Tm reference library; however, their correct references generated TD values that were higher than their TD-2 values for the mismatched mutations. However, the codons containing these mutations were identified correctly in each case also, despite the errors in identifying specific mutations within that codon. Thus, excluding samples whose genotypes did not exist in our reference library, the TD algorithm identified 27 of 29 mutations (93%), including 19/29 (65.5%) where a single mutation was correctly identified and 8/29 (27.6%) where a mutation group was correctly identified.
TABLE 2.
Clinical isolates tested using TD value-based algorithm
| Sample | rpoB genotype | Result | Correct call? | Note |
|---|---|---|---|---|
| Isolate-1 | 526AAC | 526AAC or 526CTC or 526TAC | Yes | |
| Isolate-2 | 516GTC | 516GTC | Yes | |
| Isolate-3 | 526GAC | 526TAA or 526AAC or 526CTC | No | TD-1 = 0.39, TD-2 = 0.56, TD value with correct reference = 0.58 |
| Isolate-4 | 531TTG | 531TTG | Yes | |
| Isolate-5 | 526TAC | 526AAC or 526CTC or 526TAC | Yes | |
| Isolate-6 | 531TTG | 531TTG | Yes | |
| Isolate-7 | 531TGG | 531TGG | Yes | |
| Isolate-8 | 516GTC | 516GTC or 516TAC | Yes | |
| Isolate-9 | 516GTC | 516GTC | Yes | |
| Isolate-10 | 531TTG | 531TTG | Yes | |
| Isolate-11 | 511CCG | 511CCG (mixture) | Yes | |
| Isolate-12 | 526GAC | 526GAC or 531TTC | Yes | |
| Isolate-13 | 531TTG | 531TTG | Yes | |
| Isolate-14 | 526TAC | 526AAC or 526CTC or 526TAC | Yes | |
| Isolate-15 | 515ATA_526AAC | 515ATT_526AAC | No | |
| Isolate-16 | 516GTC | 516GTC or 516TAC | Yes | |
| Isolate-17 | 526CTC | 526AAC or 526CTC | Yes | |
| Isolate-18 | 514TTT | No match | TD-1 = 2.58 | |
| Isolate-19 | 514TTT | No match | TD-1 = 2.64 | |
| Isolate-20 | 516GTC | 516GTC | Yes | |
| Isolate-21 | 516GTC | 516TAC or 515ATT_516TAC | No | TD-1 = 0.53, TD-2 = 0.66, TD value with correct reference = 0.71 |
| Isolate-22 | 531TTG | 531TTG | Yes | |
| Isolate-23 | 531TTG | 531TTG | Yes | |
| Isolate-24 | 526TAC | 526AAC or 526CTC or 526TAC | Yes | |
| Isolate-25 | 531TGG | 531TGG | Yes | |
| Isolate-26 | 516GTC | 516GTC or 516TAC | Yes | |
| Isolate-27 | 531TTG | 531TTG | Yes | |
| Isolate-28 | 511CCG | 511CCG | Yes | |
| Isolate-29 | 526AAC | 526AAC or 526CTC | Yes | |
| Isolate-30 | 531ATG | No match | TD-1 = 3.04 | |
| Isolate-31 | 526TAC | 526AAC or 526CTC or 526TAC | Yes | |
| Isolate-32 | 531TTG | 531TTG | Yes | |
| Isolate-33 | 531TTG | 531TTG | Yes |
DISCUSSION
We have developed a method of identifying mutations in the M. tuberculosis rpoB core region. Our TD value-based method identified a wide range of mutations with high resolution, using an approach that is easy to automate. Our approach looks at the sequence-specific patterns generated by all the possible Tm values from four SMB probes covering the entire RRDR and not individual Tm values or Tm differences between WT and mutant sequences. Thus, it is not limited to identifying mutations associated only with RR, and silent mutations in the target region of the Ultra assay were able to be similarly identified. For example, the Ultra assay was designed to call the silent mutation 513CAG (Q513Q) as “RIF resistance not detected” (5) and the Tm values of all 4 probes tested against this silent mutation fell into WT Tm windows. However, our method distinguished this mutation from others with a true WT sequence due to specific patterns generated by WT and Q513Q silent mutation, which were recognized robustly using our algorithm. Our approach also showed improved mutation detection compared to alternative approaches that are based purely on whether a Tm value is present or absent in a Tm window or on Tm values present only in the mutant windows. For example, rpoB mutants 526AGC, 526CCC, 526TAA, and 526TGC generated the same Tm window patterns (rpo1 WT, rpo2 WT, rpo3 Mut, and rpo4 WT) and had almost identical mutant Tm values in the same mutant Tm window (rpo3). In spite of that, our method was able to specifically identify each mutation because our approach uses both the presence of a Tm and the actual Tm values within each window to identify a mutation (Fig. 1). Results showing differing Tm values in the rpo4 WT window for these mutations even in cases in which they fell within the same window were enough for the algorithm to distinguish these mutation variants in the same codon (Table 1). Most of the M. tuberculosis isolates with so-called “disputed mutations,” which can be difficult to identify as RR using phenotypic methods due to their borderline RR MICs (511CCG, 516TAC, 533CCG, and 526AGC) (9), were also specifically identified. Two other such mutations, 526AAC and 526CTC (9, 10), were identified as a group due to their identical Tm signatures. Differentiating these disputed mutations from the others may help to explain at least some of the occasional discordances between Ultra and phenotypic results, where samples that were identified as RR by Ultra assay were identified as RS in phenotypic tests (11).
The other advantage of our method is that it can correctly identify the RRDR mutations in samples that generate only 3 positive Tm values if at least one of the 3 Tm values falls into a mutant Tm window. The latest version of the Ultra assay (Xpert MTB/RIF Ultra V2) detects samples with 3 Tm values as RR but does so only when either the rpo3 or rpo4 Tm values fall within the “Mut” or “MutA” Tm window, respectively, and when the rpo1 and rpo2 Tm values fall into wild-type windows (internal Rutgers/Cepheid study data: this information has not yet been published). The rare RRDR mutations that result in 3 Tm values but which do not meet these criteria, such as 529AAA, would be detected as “RIF Indeterminate” by the Ultra assay, but these mutations would still be identified as RR by our method. In our Tm reference library, there are 4 RRDR genotypes with only 3 positive Tm values, and each has a unique Tm signature (Table 1). Our method can also identify RRDR mutant genotypes in many heteroresistant samples, i.e., test samples that contain DNA from both WT and RRDR mutant M. tuberculosis strains. Heteroresistance is identified by the presence of >4 Tm values in the 9 Tm windows. The RRDR mutant genotype can be identified in these mixed samples if the Ultra assay produces a Tm value in each WT window plus an additional Tm value in a mutant window. All other signatures produced by heteroresistant samples are identified simply as a mixture.
A current limitation of our approach is that it is entirely based on known information. A mutation that has not yet been included in our Tm signature library would either be called “No Match” or, if it has a Tm signature close enough to one of the Tm signatures in the library, would be misidentified as the RDRR mutant with the closest-matching signature. In our study performed with clinical isolates, mutations of 3 isolates could not be identified and a double mutation was misidentified because their genotypes were not present in our library. Assays that produce Tm values that represent larger-than-expected variations from reference Tm values may also result in RRDR misidentifications. Two RRDR mutations in the clinical isolates appear to have been misidentified for this reason (see Fig. S1 in the supplemental material). In these two cases, the TD values of the correct mutation were slightly greater than the TD-2 values generated by our approach (0.55 versus 0.53 and 0.73 versus 0.67, respectively). However, the mutant codon was still correctly identified in these two samples, which underscores the utility of our approach. Furthermore, an expansion of our Tm signature library resulting from the addition of more M. tuberculosis samples and including clinically relevant “deletion” mutants is likely to produce more-precise mean values that could improve mutation identification. It is also possible that once enough data have been collected, it would be possible to modify our R identification tool to include the distribution of reference Tm values in addition to the mean values and to calculate the possibilities corresponding to each genotype call, which could further improve the specificity of our mutation identification.
Our study demonstrated the feasibility of using the wide range of Tm profiles generated in the Ultra assay and over 100 independent GeneXpert modules to specifically identify clinically prevalent RR mutations and opened the possibility of using Ultra data as an epidemiological tool. Given the performance of this approach applied to a large number of GeneXpert modules, this method should be widely applicable to others using a shared Tm signature reference library. As more Tm data are collected and added to the Tm signature reference library, the enrichment of the data will further evolve and optimize the algorithm for higher accuracy and improve its applicability. In clinical practice, the use of TD values may also be valuable as a stand-alone method to distinguish two clinical samples that clearly have different genotypes from samples that may have the same genotype, and this information could rule out transmission or relapse.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health (NIH) grant AI111397, a grant from the Foundation for Innovative New Diagnostics. Research support was also provided by Cepheid.
A.M.S., D.K., K.T., D.L., D.P., R.K., and S.C. are employed by Cepheid Inc., which sells the Xpert MTB/RIF Ultra product. D.A. and S.C. earn royalties from the sale of Xpert MTB/RIF Ultra.
Footnotes
Supplemental material is available online only.
For a commentary on this article, see 10.1128/JCM.01504-19.
REFERENCES
- 1.Conceicao EC, Guimaraes AED, Lopes ML, Furlaneto IP, Rodrigues YC, da Conceicao ML, Barros WA, Cardoso NC, Sharma A, Lima L, Gomes HM, Duarte RS, Frota C, Rutaihwa LK, Gagneux S, Suffys PN, Lima K. 2018. Analysis of potential household transmission events of tuberculosis in the city of Belem, Brazil. Tuberculosis (Edinb) 113:125–129. doi: 10.1016/j.tube.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Kohl TA, Diel R, Harmsen D, Rothganger J, Walter KM, Merker M, Weniger T, Niemann S. 2014. Whole-genome-based Mycobacterium tuberculosis surveillance: a standardized, portable, and expandable approach. J Clin Microbiol 52:2479–2486. doi: 10.1128/JCM.00567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musser JM. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev 8:496–514. doi: 10.1128/CMR.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nusrath Unissa A, Hanna LE. 2017. Molecular mechanisms of action, resistance, detection to the first-line anti tuberculosis drugs: rifampicin and pyrazinamide in the post whole genome sequencing era. Tuberculosis (Edinb) 105:96–107. doi: 10.1016/j.tube.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, Glass J, Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Alland D. 2017. The New Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 8:e00812-17. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng KCS, van Deun A, Meehan CJ, Torrea G, Driesen M, Gabriels S, Rigouts L, Andre E, de Jong BC. 2018. Xpert Ultra can unambiguously identify specific rifampin resistance-conferring mutations. J Clin Microbiol 56:e00686-18. doi: 10.1128/JCM.00686-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. 2014. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 52:2157–2162. doi: 10.1128/JCM.00691-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravorty S, Aladegbami B, Burday M, Levi M, Marras SAE, Shah D, El-Hajj HH, Kramer FR, Alland D. 2010. Rapid universal identification of bacterial pathogens from clinical cultures by using a novel sloppy molecular beacon melting temperature signature technique. J Clin Microbiol 48:258–267. doi: 10.1128/JCM.01725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, Rigouts L, Rusch-Gerdes S, Wright A. 2009. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol 47:3501–3506. doi: 10.1128/JCM.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Deun A, Aung KJM, Bola V, Lebeke R, Hossain MA, de Rijk WB, Rigouts L, Gumusboga A, Torrea G, de Jong BC. 2013. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 51:2633–2640. doi: 10.1128/JCM.00553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, Ghebrekristos Y, Anyango I, Murithi W, Dietze R, Lyrio Peres R, Skrahina A, Auchynka V, Chopra KK, Hanif M, Liu X, Yuan X, Boehme CC, Ellner JJ, Denkinger CM, Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, et al. 2018. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.