Xpert MTB/RIF (Xpert) and culture are the most reliable methods for tuberculosis diagnosis but are still poorly accessible in many low-resource countries. We aimed to assess the effects of OMNIgene Sputum (OM-S) and ethanol in preserving sputum for Xpert and OM-S for mycobacterial growth indicator tube (MGIT) testing over periods of 15 and 8 days, respectively. Sputum samples were collected from newly diagnosed smear-positive patients.
KEYWORDS: OMNIgene, tuberculosis, Xpert, culture
ABSTRACT
Xpert MTB/RIF (Xpert) and culture are the most reliable methods for tuberculosis diagnosis but are still poorly accessible in many low-resource countries. We aimed to assess the effects of OMNIgene Sputum (OM-S) and ethanol in preserving sputum for Xpert and OM-S for mycobacterial growth indicator tube (MGIT) testing over periods of 15 and 8 days, respectively. Sputum samples were collected from newly diagnosed smear-positive patients. For Xpert, pooled samples were split into 5 aliquots: 3 for Xpert on days 0, 7, and 15 without additive and 2 with either OM-S or ethanol at day 15. For MGIT, 2 aliquots were tested without preservative and 2 with OM-S at 0 and 8 days. Totals of 48 and 47 samples were included in the analysis for Xpert and culture. With Xpert, using day 0 as a reference, untreated samples stored for 7 and 15 days showed concordances of 45/46 (97.8%) and 46/48 (95.8%). For samples preserved with OM-S or ethanol for 15 days compared with untreated samples processed at day 0 or after 15 days, OM-S concordances were 46/48 (95.8%) and 47/48 (97.9%), while those of ethanol were 44/48 (91.7%) and 45/48 (93.8%). With MGIT, concordances between untreated and OM-S-treated samples were 21/41 (51.2%) at day 0 and 21/44 (47.7%) at day 8. In conclusion, Xpert equally detected tuberculosis in OM-S-treated and untreated samples up to 15 days but showed slightly lower detection in ethanol-treated samples. Among OM-S-treated samples, MGIT positivity was significantly lower than in untreated samples at both time points.
INTRODUCTION
Tuberculosis (TB) represents one of the most prevalent infectious diseases in the world, with an estimate of 10 million incidence cases in 2017, the majority from low- or middle-income countries (1). In 2010, the World Health Organization (WHO) endorsed Xpert MTB/RIF (Xpert) (Cepheid, Sunnyvale, CA) for simultaneous detection of TB and resistance to rifampin (2), and the test has been widely adopted for TB diagnosis (1). Nevertheless, Xpert remains unavailable in most primary health care centers where the majority of patients with presumptive TB seek care (3). Culture is the gold standard test to confirm TB, but it is slow and laborious, and due to a requirement for biohazard containment, it is available mainly in high-level laboratories. With Xpert placed at district hospitals and culture at regional hospitals and national reference laboratories in many low-resource countries, sputum samples must be transported from peripheral locations for testing. In some remote settings, high temperatures and long transport make proper sample storage very challenging.
According to the manufacturer’s instructions, specimens to be tested on Xpert should be held at 2 to 8°C for 10 days maximum or be stored at a maximum of 35°C for up to 3 days before processing (4). Even if these limitations hinder access to Xpert, studies on the stability of samples prior to Xpert testing are limited. Fixation of samples with ethanol is a low-cost and effective method of DNA preservation before PCR testing (5); however, data on its application on samples before Xpert testing are not available. Samples for culture should be processed immediately or kept at 2 to 8°C not beyond 3 days.
Long sample storage before culture inoculation is known to increase the contamination rate and affect mycobacterial recovery (6). Cetylpyridinium chlorite is a sample preservative widely used for sample transportation, but this reagent is not compatible with the mycobacterial growth indicator tube (MGIT) technique, commonly used for TB culture (7).
OMNIgene Sputum (OM-S; DNA Genotek Inc., Ottawa, Canada) is another reagent that can be applied to samples prior to testing with both Xpert and MGIT cultures. The reagent stabilizes DNA prior to PCR testing, so samples treated with OM-S may be stored for a maximum of 30 days at a temperature between 4 and 40°C before Xpert testing (8). One study reported good compatibility of OM-S with Xpert in samples transported at room temperature (RT) rather than being subjected to standard procedures, including cold storage (9). However, this study did not systematically compare Xpert performance on OM-S with that of the standard method for the same duration of storage.
OM-S has the ability to liquefy and decontaminate samples at the same time, offering the possibility to extend their storage until 8 days at temperatures up to 40°C prior to culture inoculation (8). However, studies investigating the effect of OM-S have shown good accuracy but mainly with Lowenstein-Jensen (LJ) culture (10, 11), while those using MGIT have reported contrasting results (12–15).
The objectives of this proof-of-concept study were to determine the effect of OM-S and ethanol when added to samples tested with Xpert after 15 days, to assess OM-S on samples tested with MGIT culture after 8 days, and to investigate the effect of delayed Xpert and MGIT culture testing beyond recommended times for untreated sputum samples.
MATERIALS AND METHODS
Setting.
The study was conducted at Epicentre Mbarara Research Centre, within a regional referral hospital in southwestern Uganda. The biosafety level 3 Epicentre laboratory is quality controlled by the Supranational TB Reference Laboratory of the Tropical Medical Institute of Antwerp (Belgium).
Sample collection.
Xpert and MGIT performances were investigated in phase 1 and phase 2 of the study among newly diagnosed smear-positive (Sm+) adults.
Sm+ patients identified under routine care were referred for informed consent and enrollment at the Epicentre Clinic, where 1 to 3 samples (A, B, and C) were collected within a 1-h interval, to reach total volumes of at least 6 ml for the first phase and 10 ml for the second phase. Samples were pooled to obtain a homogenous bacterial load before being split into aliquots for the different testing strategies. To verify homogeneity, smear microscopy using auramine staining according to WHO/IUATLD AFB microscopy grading (16) was performed on direct, pooled samples and on all the aliquots. Smear-negative (Sm−) pooled samples and insufficient volume samples were excluded from further evaluation. All aliquots were stored at RT between 22 and 26°C in a temperature-controlled laboratory throughout the study investigation period.
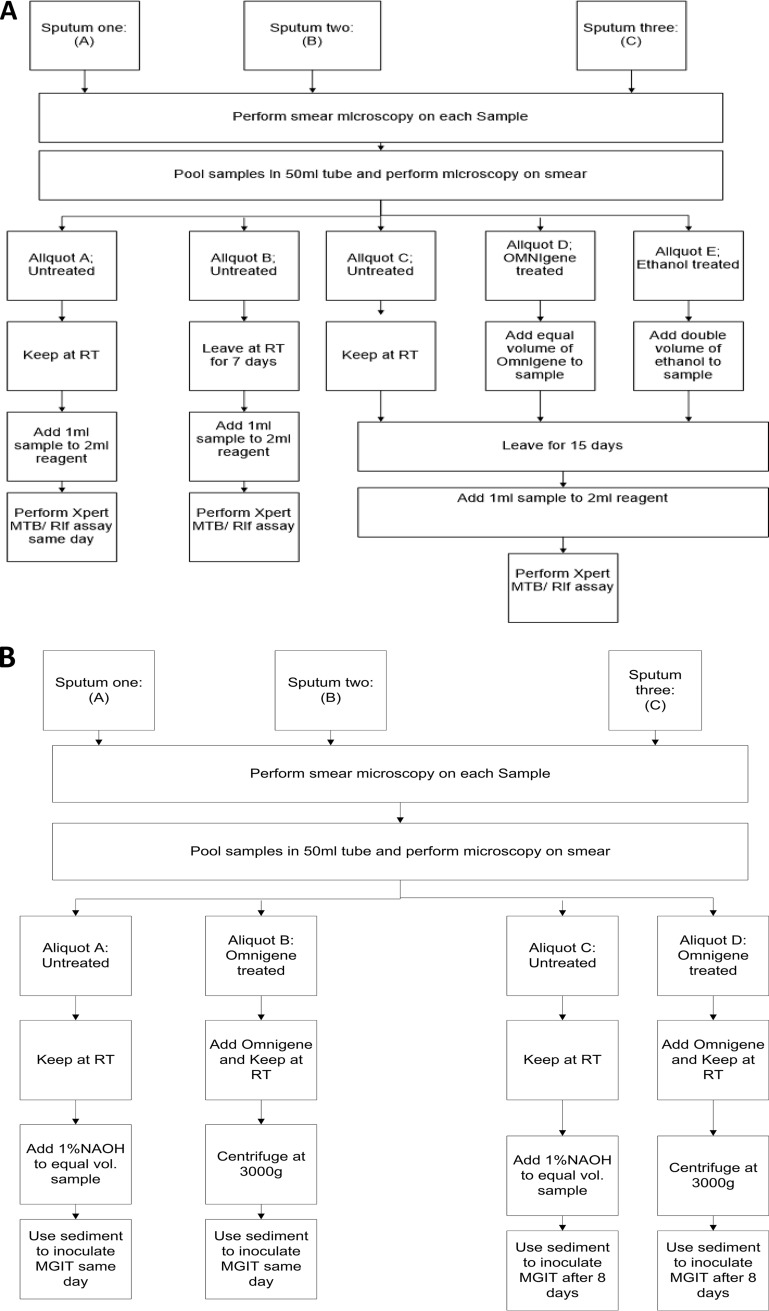
Sample processing and testing. (i) Phase 1: assessment of the effects of OM-S and ethanol on the Xpert test.
Pooled samples were split into five equal aliquots: (i) three additive free, with one tested on the collection day, one after 7 days, and one after 15 days, and (ii) two treated with either OM-S or ethanol and tested after 15 days (Fig. 1A).
FIG 1.
(A) Phase 1: assessment of the effects of OM-S and ethanol on the Xpert test. (B) Phase 2: assessment of the effect of OM-S on MGIT culture. RT, room temperature; MGIT, mycobacterial growth indicator tube.
OM-S was added in an equal volume (1:1) and at double the volume (2:1) of ethanol to achieve a 70% final concentration. Then 1 ml of the mixture was combined with 2 ml of sample reagent, mixed, and allowed to settle for 15 min at RT before transference of 2 ml into the Xpert cartridge for testing according to the manufacturer’s protocol (4).
(ii) Phase 2: assessment of the effect of OM-S on MGIT culture.
Pooled samples were split into four equal aliquots: (i) two untreated, with one tested on the collection day and another after 8 days, and (ii) two aliquots added with OM-S and processed on collection day and after 8 days (Fig. 1B).
Aliquots treated with OM-S were added with the reagent in a 1:1 proportion following the manufacturer’s instructions (4), inverted vigorously, and left at RT. On the scheduled day for culture inoculation, the mixture was centrifuged at 3,000 × g for 20 min, the supernatant was discarded, and the sediment was suspended in 1 ml of phosphate buffer before inoculation into an MGIT tube. Untreated aliquots were decontaminated with 1.25% N-acetyl l-cysteine-sodium hydroxide (final concentration) and then centrifuged at 3,000 × g for 20 min. The pellet was resuspended with 1 ml of phosphate buffer and inoculated into MGIT. PANTA (polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin) was used at double concentration according to a modified step of the BD MGIT product insert (7).
Positive cultures were checked for the presence of acid-fast bacilli (AFB) using Ziehl-Neelsen microscopy and tested on blood agar culture to exclude contamination (7). Final identification of Mycobacterium tuberculosis complex (MTC) was performed using the MPT64 (SD Bioline) Rapid diagnostic test. Cultures were classified as negative after 8 weeks of incubation.
Statistical analysis.
A convenient sample size of 50 Sm+ TB patients was proposed for each phase of the study. Laboratory records were doubly entered into the Voozanoo database and analyzed using STATA 12 (TX) software.
Xpert results were categorized as very low/low, medium/high, or negative/not applicable (inconclusive results: either error, invalid result, or no result). Results were presented per stratified aliquot smear results grouped as low (≤1+) and high (>1+) bacillary loads.
To assess the effect of time alone (without preservative) on test performance, the degrees of MTC detection on Xpert were compared between day 0, day 7, and day 15 using the McNemar test for matched data. To assess the effects of both preservatives, the degrees of MTC detection on Xpert were compared between aliquots treated with OM-S and ethanol at day 15 and for each method versus untreated aliquots at day 0 and day 15. Xpert results were considered discordant between aliquots if the difference exceeded one grade of positivity.
The MGIT positivity rate was stratified by smear categories: negative and low (≤1+) and high (>1+) bacillary loads. To assess the effect of time alone, untreated samples were compared at day 0 and day 8. To assess the effect of OM-S on MGIT, OM-S-treated aliquots at day 8 were compared to untreated aliquots at day 0 and day 8 along with OM-S-treated aliquots at day 0. To investigate the effect of OM-S on mycobacterial viability, treated and untreated aliquots were compared at day 0.
Finally, mean time to culture positivity and standard deviation (SD) were calculated among untreated and OM-S-treated aliquots at day 0 and day 8 and compared using a paired t test.
Ethical approval.
Approvals were received from the Mbarara University Research Ethics Committee, the Uganda National Council for Science and Technology, and the ITM Ethical Review Board.
RESULTS
Phase 1: assessment of the effects of OM-S and ethanol on the Xpert test.
Between May 2016 and October 2017, the study enrolled 52 patients in phase 1. Of these, 2 submitted insufficient sample volume and were excluded. Fifteen patients (30%) provided 6 ml of sample, which did not require additional sample collection, 32 (64%) provided 2 samples, and 3 (6.2%) provided 3 samples, for a total of 88 samples. After pooling of samples, 2/50 (4%) aliquots were Sm− and excluded from further analysis. Of the 48 remaining samples, 10 (20.8%) were scanty positive, 14 (29.2%) had a grade of 1+, 10 (20.8%) had a grade of 2+, and 14 (29.2%) had a grade of 3+. All aliquots obtained from the same sample showed either the same grade of positivity or 1 grade level of difference, except for 5 samples (identifiers [ID] 107, 115, 140, 144, and 145) (Table 1).
TABLE 1.
Individual Xpert test resultsa
| Lab ID | Sm pooled sample | UN day 0 |
UN day 7 |
UN day 15 |
OM day 15 |
ETH day 15 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sm | MTB Xpert | RMP Xpert | Sm | MTB xpert | RMP Xpert | Sm | MTB xpert | RMP xpert | Sm | MTB Xpert | RMP xpert | Sm | MTB xpert | RMP Xpert | ||
| 101 | 3+ | 3+ | High | S | 2+ | High | S | 2+ | Medium | S | 2+ | Medium | S | 2+ | High | S |
| 103 | 1+ | 1+ | Low | S | 1+ | Low | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Low | S |
| 104 | 1+ | 2+ | Medium | S | 2+ | Medium | S | 1+ | High | S | 1+ | Medium | S | 1+ | Medium | S |
| 105 | 1+ | 3 AFB | Low | S | 5 AFB | Low | S | 1 AFB | Low | S | 2 AFB | Low | S | 2 AFB | Low | S |
| 106 | 1+ | 1+ | Low | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S |
| 107 | 1+ | 1+ | High | S | 2+ | High | S | 3+ | High | S | 2+ | High | S | 1+ | High | S |
| 108 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 109 | 3 AFB | 1+ | Low | S | 1+ | Error | NA | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S |
| 110 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 111 | 1+ | 7 AFB | Low | S | 1+ | Low | S | 1+ | Low | S | 1+ | Low | S | 1+ | Low | S |
| 112 | 2+ | 1+ | High | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | High | S | 1+ | Medium | S |
| 113 | 1+ | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S |
| 114 | 2+ | 2+ | Medium | S | 1+ | Medium | S | 2+ | Medium | S | 2+ | Medium | S | 1+ | Low | S |
| 115 | 1 AFB | 2 AFB | Low | S | 1+ | Low | S | 1 AFB | Low | S | Negative | Low | S | Negative | Negative | NA |
| 117 | 1+ | 10 AFB | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 10 AFB | Medium | S | 11 AFB | Medium | S |
| 118 | 3+ | 2+ | Medium | S | 2+ | High | S | 2+ | High | S | 2+ | High | S | 2+ | Medium | S |
| 120 | 1 AFB | Negative | High | S | Negative | Low | R | Negative | Low | R | Negative | Low | R | 1 AFB | Negative | NA |
| 121 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | Medium | S | 3+ | High | S | 3+ | Medium | S |
| 122 | 7 AFB | 8 AFB | Medium | S | 7 AFB | Medium | S | Negative | Medium | S | 15 AFB | Medium | S | 12 AFB | Medium | S |
| 123 | 15 AFB | 1+ | High | S | 2+ | High | S | 2+ | High | S | 2+ | High | S | 2+ | Medium | S |
| 124 | 1+ | 13 AFB | Medium | S | 3 AFB | Medium | S | 2 AFB | Medium | S | 1+ | Medium | S | 2 AFB | Medium | S |
| 125 | 2+ | 15 AFB | Medium | S | 1+ | High | S | 1+ | High | S | 12 AFB | Medium | S | 1+ | Medium | S |
| 126 | 1+ | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Low | S |
| 127 | 2+ | 1+ | Medium | S | 2+ | High | S | 2+ | High | S | 2+ | Medium | S | 2+ | Medium | S |
| 128 | 3+ | 2+ | Medium | S | 3+ | Medium | S | 3+ | Medium | S | 2+ | Medium | S | 2+ | Medium | S |
| 130 | 2+ | 2+ | Medium | S | 1+ | Medium | S | 2+ | Medium | S | 2+ | Medium | S | 2+ | Medium | S |
| 131 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 132 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 133 | 3+ | 3+ | High | S | 3+ | Medium | S | 2+ | Medium | S | 3+ | High | S | 3+ | Medium | S |
| 134 | 2 AFB | 3 AFB | Low | S | 1 AFB | very Low | S | 2 AFB | Very Low | S | 3 AFB | Very Low | S | 1 AFB | very Low | S |
| 135 | 2+ | 3+ | High | S | 2+ | High | S | 2+ | Medium | S | 2+ | Medium | S | 2+ | Medium | S |
| 136 | 6 AFB | 8 AFB | Medium | S | 3 AFB | Medium | S | 5 AFB | Medium | S | 5 AFB | Low | S | 2 AFB | Medium | S |
| 137 | 1+ | 2+ | Medium | S | 1+ | Low | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S |
| 138 | 2+ | 3+ | Medium | S | 2+ | Medium | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 139 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | Medium | S | 3+ | High | S | 3+ | Medium | S |
| 140 | 2+ | 3+ | Medium | S | 1+ | Medium | S | 3+ | High | S | 2+ | Medium | S | 3+ | Medium | S |
| 142 | 2+ | 1+ | Medium | S | 2+ | High | S | 2+ | High | S | 2+ | High | S | 2+ | Very low | S |
| 143 | 3 AFB | 1 AFB | Low | S | 6 AFB | Medium | S | 5 AFB | Medium | S | 2 AFB | Medium | S | 2 AFB | Low | S |
| 144 | 2+ | 2+ | High | S | 1+ | Error | NA | 3+ | High | S | 3+ | High | S | 3+ | Low | S |
| 145 | 3+ | 2+ | Medium | S | 1+ | Medium | S | 2+ | High | S | 3+ | Medium | S | 3+ | Medium | S |
| 147 | 1+ | 1+ | High | S | 1+ | Medium | S | 2+ | High | S | 1+ | High | S | 1+ | Medium | S |
| 149 | 3+ | 2+ | Medium | S | 2+ | High | S | 1+ | High | S | 2+ | High | S | 2+ | High | S |
| 150 | 1+ | 1+ | High | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S | 1+ | Medium | S |
| 151 | 3+ | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S | 3+ | High | S |
| 152 | 3+ | 2+ | Medium | S | 3+ | High | S | 3+ | Low | S | 3+ | High | S | 3+ | High | S |
| 153 | 2 AFB | 2 AFB | Very low | S | 2 AFB | Low | S | Negative | Medium | S | Negative | Medium | S | Negative | High | S |
| 154 | 3 AFB | 2 AFB | Medium | S | 1+ | Medium | S | 5 AFB | Low | S | 1+ | Low | S | 1+ | Medium | S |
| 155 | 1+ | 4 AFB | Medium | S | 7 AFB | Medium | S | 5 AFB | Low | S | 1 AFB | Medium | S | 8 AFB | Medium | S |
RPM, rifampin; Sm, smear microscopy; MTC, Mycobacterium tuberculosis complex; AFB, acid-fast bacilli; UN, untreated sample; ETH, ethanol-treated sample; S, susceptible; R, resistant; NA, not applicable. A numeral followed by “AFB” indicates the number of bacilli in a scanty positive sample.
Xpert detected MTC in all aliquots except 4; it produced 2 invalid results for 1+ untreated aliquots tested at day 7 (ID 109 and 144) and 2 negative results for aliquots treated with ethanol: 1 Sm− and 1 scanty positive aliquot (ID 115 and 120) (Table 2).
TABLE 2.
Correlation between Xpert and smear grade for all samplesa
| Xpert result | No. (%) with indicated smear grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤1+ |
>1+ |
|||||||||
| UND0 | UND7 | UND15 | OMD15 | ETHD15 | UND0 | UND7 | UND15 | OMD15 | ETHD15 | |
| Very low/low | 9 (34.6) | 8 (28.6) | 7 (29.2) | 9 (34.6) | 8 (28.6) | 0 | 0 | 1 (4.2) | 0 | 2 (9.1) |
| Medium/high | 17 (65) | 18 (64.3) | 17 (70.8) | 17 (65.4) | 18 (64.3) | 22 (100) | 20 (100) | 23 (95.8) | 24 (100) | 20 (90.9) |
| Negative/invalid | 0 | 2 (7.1) | 0 | 0 | 2 (7.1) | 0 | 0 | 0 | 0 | 0 |
| Total | 26 | 28 | 24 | 26 | 28 | 22 | 20 | 24 | 24 | 22 |
UND0, UND7, and UND15, untreated aliquot tested at days 0, 7, and 15, respectively; OMD15, aliquot treated with OM-S tested at day 15; ETH15, aliquot treated with ethanol tested at day 15.
Xpert performance for untreated specimens.
When we compared untreated aliquots obtained from the same sample and tested at days 0 and 15, we found that Xpert detected MTC in all (P value = 1). Except with two samples (ID 120 and 153), the Xpert grades varied within one degree of positivity (Table 3). Aliquot 120 was high at day 0 but low at all other time points. In contrast, aliquot 153 was very low at day 0 and medium at day 15.
TABLE 3.
Comparison of Xpert results in untreated samples at days 0, 7, and 15
| Result at day 0 | No. of samples with indicated result |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UND7 |
UND15 |
||||||||||||
| Negative | Very low | Low | Med | High | NA | Negative | Very low | Low | Med | High | NA | ||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Very low | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1a | 0 | 0 | 1 |
| Low | 0 | 1 | 4 | 2 | 0 | 1 | 0 | 1 | 3 | 4 | 0 | 0 | 8 |
| Med | 0 | 0 | 1 | 15 | 6 | 0 | 0 | 0 | 3 | 10 | 9 | 0 | 22 |
| High | 0 | 0 | 1b | 4 | 11 | 1 | 0 | 0 | 1b | 7 | 9 | 0 | 17 |
| Invalid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 1 | 7 | 21 | 17 | 2 | 0 | 1 | 7 | 22 | 18 | 0 | 48 |
ID 153.
ID 120.
Using day 0 as a reference and excluding invalid results, 45/46 (97.8%) aliquots had results concordant with those of day 7, while 46/48 (95.8%) aliquots had results concordant with those of day 15.
Effects of OM-S and ethanol specimen treatment on Xpert performance.
The results from the comparison between aliquots tested with OM-S or ethanol and versus untreated aliquots at day 0 and day 15 are shown in Table 4.
TABLE 4.
Comparison of Xpert results for OM-S- and ethanol-treated aliquots at different days
| Treatment | Result | No. of samples with indicated result |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UND0 |
UND15 |
|||||||||||
| Negative | Very low | Low | Med | High | Negative | Very low | Low | Med | High | |||
| OMD15 | Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Very low | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Low | 0 | 0 | 3 | 2 | 1a | 0 | 0 | 5 | 1 | 0 | 6 | |
| Med | 0 | 1b | 4 | 15 | 3 | 0 | 0 | 1 | 17 | 5 | 23 | |
| High | 0 | 0 | 0 | 5 | 13 | 0 | 0 | 1c | 4 | 13 | 18 | |
| Invalid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 1 | 8 | 22 | 17 | 0 | 1 | 7 | 22 | 18 | 48 | |
| ETH15 | Negative | 0 | 0 | 1 | 0 | 1a | 0 | 0 | 2 | 0 | 0 | 2 |
| Very low | 0 | 0 | 1 | 1d | 0 | 0 | 1 | 0 | 0 | 1d | 2 | |
| Low | 0 | 0 | 4 | 2 | 1e | 0 | 0 | 2 | 4 | 1c | 7 | |
| Med | 0 | 0 | 2 | 16 | 8 | 0 | 0 | 2 | 16 | 8 | 26 | |
| High | 0 | 1b | 0 | 3 | 7 | 0 | 0 | 1c | 2 | 8 | 11 | |
| Invalid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 1 | 8 | 22 | 17 | 0 | 1 | 7 | 22 | 18 | 48 | |
ID 120.
ID 153.
ID 152.
ID 142.
ID 144.
Three aliquots (ID 120, 152, and 153) showed discordant Xpert results in the OM-S group. Aliquot 120 showed a lower grade of positivity with OM-S than for day 0 without treatment, while aliquots 152 and 153 had a higher grade with OM-S than for untreated samples at day 0 and day 15.
OM-S aliquots had Xpert results concordant with those of untreated aliquots for 46/48 (95.8%) and 47/48 (97.9%) at day 0 and day 15, respectively.
Five aliquots (ID 152, 153, 120, 142, and 144) showed discordances in the ethanol group. Aliquots 120, 142, and 144 with added ethanol gave lower results than for untreated aliquots at both time points, while aliquots 152 and 153 reported high Xpert results with ethanol but low or very low results when untreated. Of 48 aliquots containing ethanol, 44 (91.7%) and 45 (93.8%) had results concordant with those of untreated aliquots tested at day 0 and day 15, respectively (Table 4).
Comparison of aliquots treated with OM-S and ethanol showed a concordance of 44/48 (91.7%) (Table 5). Two aliquots were positive with OM-S and negative with ethanol (ID 120 and 115), and two (ID 142 and 144) were highly positive with OM-S and exhibited low or very low results with ethanol.
TABLE 5.
Comparison of Xpert results for ETHD15 versus OMD15
| Treatmen | Result | No. of OMD15 samples with indicated result |
Total | ||||
|---|---|---|---|---|---|---|---|
| Negative | Very low | Low | Medium | High | |||
| ETH15 | Negative | 0 | 0 | 2a | 0 | 0 | 2 |
| Very low | 0 | 1 | 0 | 0 | 1b | 2 | |
| Low | 0 | 0 | 2 | 4 | 1c | 7 | |
| Medium | 0 | 0 | 2 | 17 | 7 | 26 | |
| High | 0 | 0 | 0 | 2 | 9 | 11 | |
| Invalid | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 1 | 6 | 23 | 18 | 48 | |
ID 115 and ID 120.
ID 142.
ID 144.
All aliquots gave rifampin-susceptible results except for ID 120, which was rifampin resistant for untreated aliquots at days 7 and 15 and with OM-S but was rifampin susceptible at day 0 and negative for the aliquot treated with ethanol.
Phase 2: assessment of the effect of OM-S on MGIT cultures.
Of 57 patients enrolled in phase 2 between October 2016 and August 2017, 1 patient was excluded because of insufficient sample volume. Of the 56 patients included, 33 (62%) provided one 10-ml sample and 23 (38%) collected two sputum samples, and none required a third sample. Of the 56 pooled samples, 8 were excluded because they were Sm−; the remaining 48 included 5 (10.4%) scanty positive, 18 (37.5%) 1+, 10 (20.8%) 2+, and 15 (31.2%) 3+ samples. All aliquots prepared from the same pooled sample showed either the same level of smear positivity or 1 grade level of difference, except for 4 cases (ID 206, 208, 230, and 236) (Table 6).
TABLE 6.
Smear and culture results among OMNIgene and untreated samples
| Lab ID | Sm pooled sample | UN day 0 |
OM day 1 |
UN day 8 |
OM day 8 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Sm | Culture | Sm | Culture | Sm | Culture | Sm | Culture | ||
| 202 | 3+ | 2+ | MTC | 2+ | MTC | 2+ | MTC | 2+ | Negative |
| 203 | 2+ | 2+ | MTC | 3+ | MTC | 2+ | MTC | 2+ | MTC |
| 204 | 3+ | 3+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | Negative |
| 205 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 206 | 2+ | 3+ | MTC | 3+ | Negative | 2+ | MTC | 1+ | MTC |
| 207 | 1+ | 1+ | MTC | 1+ | MTC | 1+ | MTC | 1+ | MTC |
| 208 | 3+ | 1+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | Negative |
| 209 | Scanty | 1+ | MTC | 1+ | MTC | Scanty | MTC | 1+ | MTC |
| 210 | 3+ | 3+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | MTC |
| 211 | 2+ | 3+ | MTC | 2+ | Negative | 2+ | MTC | 2+ | MTC |
| 214 | 3+ | 2+ | MTC | 2+ | MTC | 3+ | MTC | 3+ | MTC |
| 215 | 3+ | 3+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | MTC |
| 216 | 2+ | 2+ | MTC | 2+ | MTC | 2+ | MTC | 2+ | Negative |
| 217 | 1+ | 2+ | MTC | 1+ | MTC | 1+ | MTC | 1+ | Negative |
| 218 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 219 | 1+ | 2+ | MTC | 1+ | MTC | 1+ | MTC | 1+ | Negative |
| 220 | 2+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | MTC |
| 221 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 223 | 3+ | 3+ | MTC | 3+ | Negative | 3+ | MTC | 3+ | NTM |
| 224 | 1+ | 1+ | MTC | 1+ | MTC | 1+ | MTC | 1+ | Negative |
| 225 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | MTC |
| 226 | 2+ | 2+ | MTC | 2+ | MTC | 1+ | MTC | 1+ | Negative |
| 227 | Scanty | Scanty | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 228 | 1+ | 1+ | Negative | 2+ | Negative | 1+ | MTC | 1+ | MTC |
| 229 | 3+ | 3+ | Negative | 3+ | Negative | 3+ | MTC | 2+ | MTC |
| 230 | 2+ | 2+ | MTC | 1+ | MTC | 3+ | MTC | 2+ | MTC |
| 234 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 235 | 1+ | 1+ | MTC | Scanty | Negative | 1+ | MTC | 1+ | MTC |
| 236 | 3+ | 1+ | MTC | 3+ | Negative | 3+ | MTC | 3+ | MTC |
| 237 | 3+ | 1+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | MTC |
| 240 | 1+ | 1+ | MTC | 1+ | MTC | 1+ | NTM | 1+ | Negative |
| 241 | Scanty | Scanty | MTB | Scanty | MTB | Scanty | MTB | Scanty | Negative |
| 242 | Scanty | Negative | Contaminated | Negative | Negative | Negative | Not done | Negative | |
| 243 | 1+ | 1+ | MTB | 2+ | Negative | 1+ | MTB | 1+ | Negative |
| 244 | 2+ | 2+ | MTC | 2+ | Negative | 2+ | MTC | 2+ | MTC |
| 245 | 2+ | 2+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 246 | 3+ | 3+ | MTC | 3+ | Negative | 3+ | MTC | 3+ | Negative |
| 248 | 3+ | 3+ | MTC | 3+ | Negative | 3+ | MTC | 3+ | Negative |
| 249 | 3+ | 2+ | MTC | 3+ | MTC | 3+ | MTC | 3+ | MTC |
| 250 | Scanty | Scanty | MTC | Scanty | MTC | 1+ | MTC | 1+ | MTC |
| 252 | 1+ | 1+ | Negative | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 253 | 3+ | 3+ | MTC | 3+ | Negative | 3+ | MTC | 3+ | Negative |
| 254 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | MTC | 1+ | Negative |
| 255 | 1+ | 1+ | MTC | 1+ | Negative | 1+ | Negative | 1+ | Negative |
| 256 | 3+ | 2+ | Negative | 1+ | Negative | 2+ | MTC | 1+ | Negative |
| 257 | 1+ | Scanty | Negative | Scanty | Negative | Scanty | MTC | Scanty | Negative |
| 260 | 1+ | 1+ | MTC | 1+ | MTC | 1+ | MTC | 1+ | MTC |
For sample ID 242, the untreated aliquot at day 0 was contaminated, the untreated aliquot at day 8 was not tested, and the other aliquots were smear and culture negative. One aliquot (ID 240, untreated day 8) was positive for nontuberculous mycobacteria (NTM).
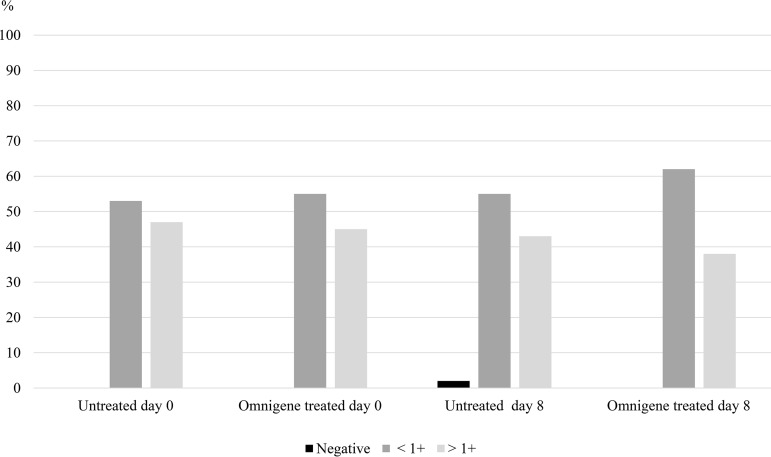
As shown in Fig. 2, the culture positivity across smear categories at different time points was uniformly distributed.
FIG 2.
Culture positivity for all aliquots by smear grade.
MGIT performance for untreated specimens.
At day 0, 41/47 (87.2%) untreated aliquots had MTC culture-positive results, compared to 44/46 (95.7%) at day 8.
Effect of OM-S specimen treatment on MGIT performance.
Untreated and OM-S-treated aliquots were compared at day 0 and day 8. Of the 41 untreated aliquots with MTC at day 0, only 18 (43.9%) treated with OM-S had MTC at day 8 (Table 7). Similarly, among 44 MTC+ untreated cultures at day 8, merely 20 (45.5%) were positive among OM-S-treated aliquots on the same date (Table 7). In addition, among 21 MTC+ OM-S-treated aliquots at day 0, only 11 (52.4%) were positive among OM-S-treated aliquots at day 8 (Table 7).
TABLE 7.
Comparison of culture results of OMD8 with UND0, UND8, and OMD0 samplesa
| Treatment | Result | No. of samples with indicated result |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UND0 |
UND8 |
OMD0 |
||||||||||
| Neg | MTC | NTM | Cont | Neg | MTC | NTM | ND | Neg | MTC | NTM | ||
| OMD8 | Neg | 3 | 22 | 0 | 1 | 1 | 23 | 1 | 1 | 16 | 10 | 0 |
| MTC | 2 | 18 | 0 | 0 | 0 | 20 | 0 | 0 | 9 | 11 | 0 | |
| NTM | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Total | 5 | 41 | 0 | 1 | 1 | 44 | 1 | 1 | 26 | 21 | 0 | |
Cont, culture contaminated; Neg, culture negative; NTM, nontuberculous mycobacteria.
As determined by comparing OM-S-treated aliquots at day 0 and day 8, there were 11 MTC+ cultures at both time points, 10 at day 0, and 9 at day 8 alone (Table 7).
Finally, among 41 untreated aliquots MTC+ at day 0, only 21 (51.2%) were positive among OM-S-treated aliquots on the same date (Table 8).
TABLE 8.
Comparison of culture results of UND0 with UND8 and OMD0 samples
| Sample type | Result | No. of samples with indicated result |
||||||
|---|---|---|---|---|---|---|---|---|
| UND8 |
OMD0 |
|||||||
| Neg | MTC | NTM | ND | Neg | MTC | NTM | ||
| Untreated, day 0 | Neg | 0 | 5 | 0 | 0 | 5 | 0 | 0 |
| MTC | 1 | 39 | 1 | 0 | 20 | 21 | 0 | |
| Cont | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| Total | 1 | 44 | 1 | 1 | 26 | 21 | 0 | |
Time to culture positivity for OM-S-treated and untreated samples at different time points.
At day 0, the mean time to detection was 10.4 days (SD, 1.1) among untreated aliquots, compared to 18.2 days (SD, 2.5) for OM-S-treated aliquots, with a P value of 0.003. Correspondingly, it was 10.9 days (SD, 1.2) at day 8 among untreated aliquots, compared to 25.5 days (SD, 3.0) for OM-S-treated aliquots (P value < 0.001).
DISCUSSION
OM-S has been proposed as a sample preservative prior to testing with Xpert and culture but so far has not been endorsed by the WHO (17). This study adds more evidence of accuracy on the use of this reagent to preserve samples for delayed testing. The study also provides data on Xpert and MGIT performance on samples kept beyond the recommended 3 days at RT without preservative. Samples treated with OM-S can be stored for up to 30 days at RT prior to Xpert testing. Our choice to limit the delay to a maximum of 15 days was based on the assumption that the benefit of this test is to provide early diagnosis and would be compromised if results are available beyond this time frame.
Overall, all aliquots gave Xpert-positive results except for 4 aliquots: 2 scanty positive or Sm− aliquots treated with ethanol, which gave negative results, and 2 smear grade 1+ (untreated) aliquots processed on day 7, which gave error codes 2008 and 5007. These errors are reported by Cepheid as related mainly to high pressure and probe check control failure, so they are due mainly to specimen handling rather than RT preservation (18). Surprisingly, long sample storage at RT without a preservative did not alter the Xpert performance over 15 days. Only 2 aliquots showed an Xpert quantitative result discordant for more than one grade. These results suggest that mycobacterial organisms in Sm+ samples may not significantly degrade by storage beyond the recommended 3 days.
There was good concordance between OM-S-treated aliquots and untreated samples tested at days 0 and 15. This shows that OM-S does not alter the Xpert performance on specimens stored up to 15 days at RT, compared to testing at day 0, which is considered the best practice. It also demonstrates that the reagent did not improve MTC detection after long storage compared to that in untreated samples. At the same time points, ethanol performance was lower, with 5 discordant results. However, with the exception of two aliquots that were either Sm− or scanty positive and Xpert negative, all aliquots treated with ethanol gave positive results.
In the OM-S and ethanol comparisons, all discordances (results above one grade difference) occurred in 5 samples (ID 120, 142, 144, 152, and 153). Higher dilution of sediments treated with OM-S or ethanol unlikely contributed to these discordances, as all aliquots showed consistent smear grades, and in two cases, the lower Xpert grade was observed in the untreated, less diluted sediments.
For ID 120, the same aliquot showed discordance with the rifampin result: Xpert positive and rifampin susceptible at day 0 untreated but resistant for extended untreated aliquots at days 7 and 15 and ethanol and OM-S both at day 15. This could be due to a clerical error from the laboratory, but other explanations cannot be excluded, such as heteroresistance or a false-susceptible result due to low mycobacterial load, as reported by others (19, 20). However, this discrepancy was not further investigated.
Other studies have reported a good performance of Xpert with OM-S-treated compared to untreated samples, but the samples were always processed on the day of collection (9, 12). In addition, our study showed that similar performances can be obtained beyond the recommended time with OM-S-treated and untreated samples until 15 days. Although Xpert testing should be performed as soon as the sample is collected to allow rapid treatment initiation, these results are very important for remote settings where Xpert can be performed only after prolonged transport collection.
The culture positivity rate was unexpectedly lower for fresh untreated samples than for samples left untreated and processed after 8 days. The reason for these results remains unexplained, as aliquots had equivalent smear grades. MGIT performance was much lower for samples treated with OM-S than for untreated samples (50%). The poor concordance at day 0 indicates a negative effect on bacterial growth of MTC by the OM-S treatment regardless of time of exposure. DNA Genotek has recently released a revised protocol that includes OM-S neutralization with buffer before inoculation. This procedure should be further investigated.
The negative effect of OM-S on mycobacterial recovery on MGIT has been reported in other studies (13, 14). The incompatibility between the reagent and culture, however, has been mainly reported for the MGIT system (7, 13, 16). One study reported poor recovery of MTC across both MGIT and Lowenstein-Jensen (LJ) media (13). One study reported improved results in MGIT cultures using samples treated with OM-S for up to 3 weeks, with the only concerns being about a delay in MGIT results (15).
Other studies have reported no significant difference between untreated and OM-S-treated smear-positive remnant samples with MGIT at day 8 (17, 23). Although there was a difference in study design, our study used fresh samples, while FIND evaluation used sediments; this is unlikely to have caused such a difference in the results.
There was only one contaminant on untreated samples at day 0. Previous studies have shown that OM-S-treated samples have a lower contamination rate than untreated counterparts (10, 12–15, 23). In our study, only one contaminant in the untreated group may not explain much about the contribution of OM-S in reducing contamination compared to standard decontamination.
Finally, we observed a substantial delay in days to positivity between untreated and OM-S-treated samples at both time points. Previous studies have also noted delayed culture growth in samples treated with OM-S (13, 15, 21, 22). This raises further concerns about the utility of OM-S in its current procedure and the compatibility with MGIT cultures.
Limitations.
This study had a few limitations. This was a proof-of-concept study, and aliquots were stored in a controlled research laboratory and not in the type of setting in which the protocols would actually be applied.
We used only known Sm+ samples, and therefore, we could not demonstrate the effect of the reagent in Sm− samples tested on Xpert and in MGIT liquid media. More evaluation is needed, especially among smear-negative, Xpert-positive samples in a high-TB-HIV context.
Conclusion.
In this proof-of-concept study, we have shown that there is no advantage in using OM-S reagent, or ethanol, for smear-positive sputum stored at RT up to 15 days, as Xpert performance remains high even after such delays. This study brings reassuring data regarding the possibility of using Xpert on transported sputum samples without a cold chain, which is common practice in high-burden and limited-resource countries. On the other hand, this study does not support the use of OM-S for delayed culture processing, unless additional evaluation of the revised protocol gives more promising results.
ACKNOWLEDGMENTS
We express our sincere appreciation to the study participants. We also thank the nurses, laboratory, and data personnel of Epicentre that participated in data collection and analysis. We also thank the following members of Médecins Sans Frontières for their constructive contributions during the development of this study: Martina Casenghi (EGPAF), Cathy Hewison (MSF, France), Francis Varaine (MSF, France), and Bouke de Jong (ITM, Belgium).
We appreciate Médecins Sans Frontières for funding.
We declare that we have no conflict of interest.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.WHO. 2017. Global tuberculosis report 2018. Pharmacol Rep 69:683–690.28549307 [Google Scholar]
- 2.WHO. 2014. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’: practical considerations. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 3.WHO. 2011. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF. Policy statement. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.Cepheid. 2009. Operator manual test Xpert MTB/RIF. Cepheid, Sunnyvale, CA. [Google Scholar]
- 5.Williams DL, Gillis TP, Dupree WG. 1995. Ethanol fixation of sputum sediments for DNA-based detection of Mycobacterium tuberculosis. J Clin Microbiol 33:1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramasivan CN, Narayana ASL, Prabhakar R, Rajagopal MS, Somasundaram PR, Tripathy SP. 1983. Effect of storage of sputum specimens at room temperature on smear and culture results. Tubercle 64:119–124. doi: 10.1016/0041-3879(83)90036-3. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqi SH, Rüsch-Gerdes S. 2006. MGIT™ procedure manual for BACTEC™ MGIT 960™ TB system. Foundation for Innovative New Diagnostics, Geneva, Switzerland. [Google Scholar]
- 8.DNA Genotek. 2018. Omigene sputum flyer, p 1–2. DNA Genotek, Kanata, Canada. [Google Scholar]
- 9.Maharjan B, Kelly-Cirino CD, Weirich A, Curry PS, Hoffman H, Avsar K, Shrestha B. 2016. Evaluation of OMNIgene W SPUTUM-stabilised sputum for long-term transport and Xpert W MTB/RIF testing in Nepal. Int J Tuberc Lung Dis 20:1661–1667. doi: 10.5588/ijtld.16.0421. [DOI] [PubMed] [Google Scholar]
- 10.Affolabi D, Sanoussi N, Sossou A, Nys T, Bodi O, Esse M, Houeto S, Massou F, de Jong BC, Rigouts L. 2018. Performance of OMNIgene·SPUTUM (DNA Genotek) and cetylpyridinium chloride for sputum storage prior to mycobacterial culture. J Med Microbiol 67:798–805. doi: 10.1099/jmm.0.000745. [DOI] [PubMed] [Google Scholar]
- 11.Maharjan B, Shrestha B, Weirich A, Stewart A, Kelly-Cirino CD. 2016. A novel sputum transport solution eliminates cold chain and supports routine tuberculosis testing in Nepal. J Epidemiol Glob Health 6:257–265. doi: 10.1016/j.jegh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly-Cirino CD, Musisi E, Byanyima P, Kaswabuli S, Andama A, Sessolo A, Sanyu I, Zawedde J, Curry PS, Huang L. 2017. Investigation of OMNIgene·SPUTUM performance in delayed tuberculosis testing by smear, culture, and Xpert MTB/RIF assays in Uganda. J Epidemiol Glob Health 7:103–109. doi: 10.1016/j.jegh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zallet J, Olaru ID, Witt A, Vock P, Kalsdorf B, Andres S, Hillemann D, Kranzer K. 2018. Evaluation of OMNIgene SPUTUM reagent for mycobacterial culture. Int J Tuberc Lung Dis 22:945–949. doi: 10.5588/ijtld.17.0020. [DOI] [PubMed] [Google Scholar]
- 14.Azam K, Cadir N, Madeira C, Gillespie SH, Sabiiti W. 2018. OMNIgene SPUTUM suppresses contaminants while maintaining Mycobacterium tuberculosis viability and obviates cold-chain transport. ERJ Open Res 4:00074–2017. doi: 10.1183/23120541.00074-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagliani E, Alagna R, Tafaj S, Hafizi H, Cirillo DM. 2017. Evaluation of Mycobacterium tuberculosis viability in OMNIgene-SPUTUM reagent upon multi-day transport at ambient temperature. BMC Infect Dis 17:663. doi: 10.1186/s12879-017-2756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rider HL, Van Deun A, Kam KM, Kim SJ, Chonde TM, Trébucq A, Urbanczik R. 2007. Priorities for tuberculosis bacteriology services in low-income countries, 2nd ed International Union Against Tuberculosis and Lung Disease, Paris, France. [Google Scholar]
- 17.World Health Organization. 2017. Technical Expert Group meeting report. Commercial products for preserving clinical specimens for the diagnosis of tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 18.Cepheid. 2012. GeneXpert system; operational manual. 301–0045–Rev C version 4 Cepheid, Sunnyvale, CA. [Google Scholar]
- 19.Van Rie A, Mellet K, John MA, Scott L, Page-Shipp L, Dansey H, Victor T, Warren R. 2012. False-positive rifampicin resistance on Xpert® MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis 16:206–208. doi: 10.5588/ijtld.11.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly JD, Lin SYG, Barry PM, Keh C, Higashi J, Metcalfe JZ. 2014. Xpert MTB/RIF false detection of rifampin-resistant tuberculosis from prior infection. Am J Respir Crit Care Med 190:1316–1318. doi: 10.1164/rccm.201408-1500LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly-Cirino CD, Curry PS, Marola JL, Helstrom NK, Salfinger M. 2016. Novel multi-day sputum transport reagent works with routine tuberculosis tests and eliminates need for cold chain: preliminary study of compatibility with the Xpert® MTB/RIF assay. Diagn Microbiol Infect Dis 86:273–276. doi: 10.1016/j.diagmicrobio.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 22.WHO. 2011. Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 23.Asandem DA, Asante-Poku A, Asare P, Aboagye SY, Stephen OW, Danso E, Klevor PM, Hayibor KM, Yeboah-Manu D. 2018. OMNIgene SPUTUM: a good transport and decontaminating reagent for tuberculosis testing. Int J Mycobacteriol 7:222–227. doi: 10.4103/ijmy.ijmy_102_18. [DOI] [PubMed] [Google Scholar]