Outbreaks of infections by Mycobacterium abscessus, particularly subspecies massiliense, are increasingly reported worldwide. Several multilocus sequence typing (MLST) protocols for grouping international outbreak strains have been developed but not yet directly compared.
KEYWORDS: multilocus sequencing, Mycobacterium abscessus
ABSTRACT
Outbreaks of infections by Mycobacterium abscessus, particularly subspecies massiliense, are increasingly reported worldwide. Several multilocus sequence typing (MLST) protocols for grouping international outbreak strains have been developed but not yet directly compared. Using the three-gene (hsp65, rpoB, and secA1), seven-gene (argH, cya, glpK, gnd, murC, pta, and purH) and thirteen-gene (all of the preceding genes plus gdhA, pgm, and pknA) MLST schemes, we identified 22, 38, and 40 unique sequence types (STs), respectively, among a total of 139 nonduplicated M. abscessus isolates. Among subspecies massiliense, three-gene MLST not only clustered all outbreak strains together (in 100% agreement with the seven-gene and thirteen-gene schemes), but it also distinguished between two new STs that would have been grouped together by the seven-gene MLST but were distinct by the thirteen-gene MLST owing to differences in hsp65, rpoB, and pknA. Here, we show that an abbreviated MLST may be useful for simultaneous identification of M. abscessus the subspecies level and screening M. abscessus subsp. massiliense isolates with outbreak potential.
INTRODUCTION
The Mycobacterium abscessus complex comprises three closely related genomospecies—M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii—that cannot be reliably discriminated by single gene sequencing (1–3). Previous studies have indicated great diversity within M. abscessus among cystic fibrosis patients, suggesting independent acquisitions from the environment (4, 5). However, suspicion of patient-to-patient transmission arose after two reports of respiratory outbreaks with M. abscessus subsp. massiliense at different cystic fibrosis centers across the Atlantic (6–8). One outbreak occurred in Seattle, WA, wherein the index case-patient and four additional patients were infected with nearly identical M. abscessus subsp. massiliense isolates with resistance to amikacin and clarithromycin and indistinguishable by repetitive unit sequence-based PCR (rep-PCR) patterns and pulsed-field gel electrophoresis (PFGE) analysis (9). The second outbreak occurred in Cambridge, United Kingdom, and involved 11 patients who all had M. abscessus subsp. massiliense infections sharing the same constitutive resistance to amikacin and clarithromycin, despite some individuals being naive to long-term macrolide or aminoglycoside therapy (6).
By whole-genome sequencing (WGS), isolates from these two cystic fibrosis centers were subsequently found to be highly related, belonging to sequence type 23 (ST23) and clonal cluster 3 (CC3) according to a multilocus sequence typing (MLST) protocol (7). Meanwhile, an epidemic of at least 2,032 postsurgical infections between 2004 and 2011 across Brazil was also due to M. abscessus subsp. massiliense ST23 (CC3), thereafter referred to as the “globally successful clone” (10, 11). Other outbreaks occurring after ultrasound-guided procedures, acupuncture, injections, dental, ophthalmological, cardiac, obstetric, and cosmetic surgeries due to M. abscessus continue to be reported worldwide (12–20).
However, outbreak investigations and comparisons of interrelatedness of outbreak strains by WGS and PFGE are too costly, lengthy, and labor-intensive for routine infection control surveillance. Hence, the aim of the present study was to identify a molecular typing method for M. abscessus that would be feasible in the context of epidemiology, postoutbreak surveillance, and the validation of new infection control measures. Although the optimal research method may be in evolution, such as 65-kDa heat shock protein analysis, matrix-assisted laser desorption ionization–time of flight (MALDI-TOF), next-generation sequencing, and WGS, we chose here to characterize MLST, since the MLST approach has been validated for determining subspecies of the M. abscessus complex (11, 21, 22). Three different MLST schemes, with three-gene, seven-gene, and thirteen-gene targets have been proposed in recent decades by different investigators for typing collections of clinical and environmental isolates of the M. abscessus complex, but these methods have not been directly compared (7, 11, 21).
The three-gene MLST scheme was developed in 2011 for the accurate determination of M. abscessus subspecies after the failure of single-gene targets, such as 16srRNA, to reliably distinguish between M. chelonae and M. abscessus, followed by the failure of rpoB, hsp65, and secA1 individual gene sequencing to reliably distinguish between subspecies due to the inferred horizontal transfer of genes between the closely related subspecies, especially from the more ancestral M. abscessus subsp. abscessus to the more recently emerged, M. abscessus subsp. massiliense (22–24). Shortly after publication, we used three-gene MLST to identify a clone of M. abscessus subsp. massiliense, TPE 101, that was determined to be identical by PFGE and rep-PCR as the cause of a multicenter outbreak of postprocedural infections related to the use of contaminated ultrasonography gel between 2010 and 2012 island-wide across Taiwan (12, 25).
The more usual seven-gene MLST scheme in bacterial taxonomy was developed to delineate microbial species within various taxonomic groups, including groups of highly recombinant bacteria, such as Neisseria spp., and to allow the assignment of unknown strains to species clusters over the Internet in a global collective electronic taxonomy (26). Typically, the seven-gene MLST scheme concatenated the sequences of between six to eight housekeeping genes that are present as a single copy within the genome and are not subject to selective pressure (26). For the M. abscessus complex, a seven-gene approach using the housekeeping genes argH, cya, glpK, gnd, murC, pta, and purH was published for nonoutbreak molecular epidemiological studies of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense in 2014 (with different numbering systems and protocols in two different databases (https://bigsdb.pasteur.fr/mycoabscessus/mycoabscessus.html and https://pubmlst.org/mabscessus/) (11, 27). Notably, no strains of M. abscessus subsp. bolletii were included in these MLST databases. According to the publication of this MLST scheme, we used the same set of primers and conditions to identify our TPE 101 outbreak strain as ST48 by the former Pasteur Institute’s system, which differed by only one of seven MLST loci (MurC gene) from ST23.
However, due to the recognized differences between mycobacteria and more rapidly evolving bacteria, investigators studying trans-Atlantic cystic fibrosis outbreaks proposed simultaneously in 2014 an extended 13-gene MLST approach (incorporating the loci cya, gdhA, argH, glpK, gnd, murC, pgm, pknA, pta, pur, rpoB, hsp65, and secA1) alongside WGS to better characterize strains similar to the Seattle and Papworth outbreak strains (7). As far as we know, the merits of increasing the number of loci in the MLST approach from three mycobacterium-specific genes, seven generic housekeeping genes, and three mycobacterial and ten housekeeping genes have not been directly compared. In this study, we sought to study the discriminatory power of these three MLST schemes in discerning isolates from the previous outbreak, clustering within the “globally successful clonal cluster 3” from sporadic clinical and environmental isolates.
MATERIALS AND METHODS
Mycobacterial isolates.
A total of 139 M. abscessus nonduplicated isolates were included in this study, comprising 121 clinical isolates, 16 environmental isolates, and 2 reference isolates (M. abscessus subsp. abscessus ATCC 19977 and M. abscessus subsp. massiliense BCRC 16916). Of these, 57 M. abscessus isolates were outbreak strains of M. abscessus subsp. massiliense ST48 (CC3), as reported previously, and 81 M. abscessus isolates were sporadic isolates that were confirmed to be unrelated to the outbreak by epidemiological investigation, PFGE, and rep-PCR (12, 25). Of the 121 clinical isolates, 65 were pulmonary isolates cultured from sputum (n = 61), bronchoalveolar lavage (n = 2), pleural effusion (n = 1) and biopsied lung tissue (n = 1), and 56 were extrapulmonary isolates cultured from the blood (n = 10), surgical wound (n = 21), cerebrospinal fluid (n = 1), ascites (n = 2), cornea (n = 5), endocervical swab (n = 1), biopsied lymph node (n = 2), ear (n = 4), and other skin and soft tissue (n = 10). Of the 16 environmental isolates, 13 were obtained from contaminated ultrasonography transmission gel (different batches and lot numbers) implicated in the nationwide outbreak, and 3 were obtained from routine infection control surveillance of hospital water at the National Taiwan University Hospital, a 2,500-bed teaching hospital in Taipei, Taiwan.
MLST.
As described previously, molecular typing of the M. abscessus isolates was done by concatenating the partial sequences of three genes (hsp65, rpoB, and secA1) according to the methods of Zelazny et al. and Cheng et al. (21, 25), seven genes (argH, cya, glpK, gnd, murC, pta, and purH) according to the primers and conditions pioneered by Macheras et al. and publicly available at http://bigsdb.pasteur.fr/mycoabscessus/mycoabscessus.html) (11, 25), and thirteen genes (the above-mentioned ten genes plus gdhA, pgm, and pknA) according to the method of Tettelin et al. (7). Subspecies determination was secondarily confirmed by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) (28).
Briefly, the mycobacterial strains were stored at –80°C in GermBank (Creative Life Sciences, New Taipei City, Taiwan). Prior to use, the strains were subcultured onto sheep blood agar at 30°C (Creative Life Sciences). Mycobacterial DNA was extracted using Tris-EDTA, lysozyme, and proteinase K (Uni-Onward Corp., New Taipei City, Taiwan). PCRs using the primers listed in the Table 1 were performed to amplify fragments of the thirteen genes. Sequences were analyzed for their similarity with sequences in the GenBank database using the Basic Local Alignment Tool (BLAST; https://www.ncbi.nlm.nih.gov/BLAST) and compared to type strains of M. abscessus subsp. abscessus (ATCC 19977) and M. abscessus subsp. massiliense (CIP 108297). Phylogenetic analysis conducted by the minimum-spanning tree algorithms based on the p distance of concatenated sequence data were performed using BioNumerics v6.6 (Applied Maths, Austin, TX).
TABLE 1.
Primers used to amplify each gene in the MLST schemes
| Gene locus | Primers, sequences (5′–3′) | Size (bp) | Analyzed fragment (5′–3′) | Reference |
|---|---|---|---|---|
| hsp65 | HSP65-F, ACCAACGATGGTGTGTCCAT; HSP65-R, CTTGTCGAACCGCATACCCT | 401 | CGCCAAGGAG…GAGCTCACCG | 21 |
| rpoB | RPOB-F, GGCAAGGTCACCCCGAAGGG; RPOB-R, AGCGGCTGCTGGGTGATCATC | 711 | TGARACCGAG…GBCCGTACTC | 21 |
| secA1 | SECA1-F, GACAGYGAGTGGATGGGYCGSGTGCACCG; SECA1-R, GCGGACGATGTARTCCTTGTCSCG | 466 | CTTCCTVGGS…RCTRTTCMMS | 21 |
| argH | ARGHF, GACGAGGGCGACAGCTTC; ARGHSR1, GTGCGCGAGCAGATGATG | 480 | GTGAGCACYAACGAAGGCTC…CGATCATGCCGGGCAAGACT | 11 |
| cya | ACF, GTGAAGCGGGCCAAGAAG; ACSR1, AACTGGGAGGCCAGGAGC | 510 | CTGGTGGGGTCCACCCAGTT…TKGCGCGCCCGCGTCACGGC | 11 |
| glpK | GLPKSF1, AATCTCACCGGCGGTGTC; GLPKSR2, GGACAGACCCACGATGGC | 534 | GTGACAAATGCCAGTCGCAC…TGTTCGCGCCGTACTGGCGR | 11 |
| gnd | GNDF, GTGACGTCGGAGTGGTTGG; GNDSR1, CTTCGCCTCAGGTCAGCTC | 480 | CARTTCRTTGAAGAYGTGCG…WCCGYAACGAAGTWGAGGCG | 11 |
| murC | MURCSF1, CGGACGAAAGCGACGGCT; MURCSR2, CCAAAACCCTGCTGAGCC | 537 | CCGAACCTGATCRTCGTSAC…AGGTGCGYACRGTGCTGCAG | 11 |
| pta | PTASF1, GATCGGGCGTCATGCCCT; PTASR2, ACGAGGCACTGCTCTCCC | 486 | GACGTMCTACTSGCCGTGGC…AAATCCGYTCCCGTGCYGCC | 11 |
| purH | PURHSF1, CGGAGGCTTCACCCTGGA; PURHSR2, CAGGCCACCGCTGATCTG | 549 | AAGGTTYTRGCTGCCAAGGC…GCAAGAAGAACGTGCGGCTG | 11 |
| gdhA | GDHAF, GTCAGTGCCCCGATCGCTGDHASR1, GGCTCTCGGAGTACGTCGA | 542 | GTCGACGGGDCMGAAGGGTC…GAGCTCCCCCGCCGTGTTYT | 7 |
| pgm | PGMSF1, CCATTTGAACCCGACCGG; PGMSR2, GTGCCAACGAGATCCTGCG | 559 | TACCTCGATCAGCGTCCGGC…TCACCGAGCGCCAGCCGTCG | 7 |
| pknA | PKNAF, CAGGTGGACCTCGGACATG; PKNASR1, AACCAGGCGCCCACCATC | 457 | CCGCCATAGCCGAGGATCTC…GCAGCCGGCGTCGCSCGGCT | 7 |
RESULTS
Of the 139 M. abscessus isolates characterized in the previously published outbreak and case-control studies, 54 belonged to the subspecies abscessus, and 85 belonged to the subspecies massiliense (Table 2). Figures 1 and 2 and Table 2 show the results for 139 isolates discriminated based on the three-gene, seven-gene, and thirteen-gene MLST schemes. The 54 M. abscessus subsp. abscessus isolates were grouped into 10 sequence types, MAB1 to MAB10 by the three-gene scheme and into 24 and 25 sequence types, respectively, by the seven-gene and thirteen-gene schemes. Forty isolates clustered together as determined by the three-gene scheme (MAB1). Of these 40 MAB1 isolates, 22, including the ATCC 19977 reference strain, belonged to ST1 according to the seven-gene MLST database of the Pasteur Institute (Table 3). These MAB1/ST1 isolates exhibited different PFGE/rep-PCR patterns and were not epidemiologically linked (published previously) (12, 25). One ST1 isolate based on seven-gene scheme did not fall into the MAB1 main cluster due to a difference in the internal sequencing of the secA1 gene alone (labeled MAB5 for the three-gene approach and ST1a for the thirteen-gene approach) (Table 4). This was the only additional sequence type gained from extending the MLST schemes from seven to thirteen genes (Fig. 1 and 2). For the remaining isolates, there was full agreement between the seven-gene and thirteen-gene schemes, i.e., the addition of gdhA, pgm, and pknA to the latter method did not increase its discriminatory power. Overall for M. abscessus subsp. abscessus, the agreement between the three-gene and seven-gene MLST schemes was 75%, the agreement between the three-gene and thirteen-gene MLST schemes was 77.5%, and the agreement between the seven-gene and thirteen-gene MLST schemes was 97.5%.
TABLE 2.
Source and subspecies distribution of 139 M. abscessus isolates included in this study
| Source | No. (%) of subspecies | No. (%) of M. abscessus strains |
|
|---|---|---|---|
| Subsp. abscessus | Subsp. massiliense | ||
| Clinical isolates | 121 (87.1) | ||
| Pulmonary | 65 (50.4) | 40 (61.5) | 25 (38.4) |
| Extrapulmonary | 56 (46.2) | 12 (21.4) | 44 (78.6) |
| Environmental isolates | 16 (11.5) | ||
| Ultrasonography gel | 13 (81.3) | 0 (0.0) | 16 (100) |
| Hospital water | 3 (18.7) | 1 (33.3) | 2 (66.7) |
| Reference standard isolatesa | 2 (1.4) | 1 (50.0) | 1 (50.0) |
| Total | 54 (38.8) | 85 (61.2) | |
M. abscessus subsp. abscessus ATCC 19977 and M. abscessus subsp. massiliense BCRC 16916.
FIG 1.
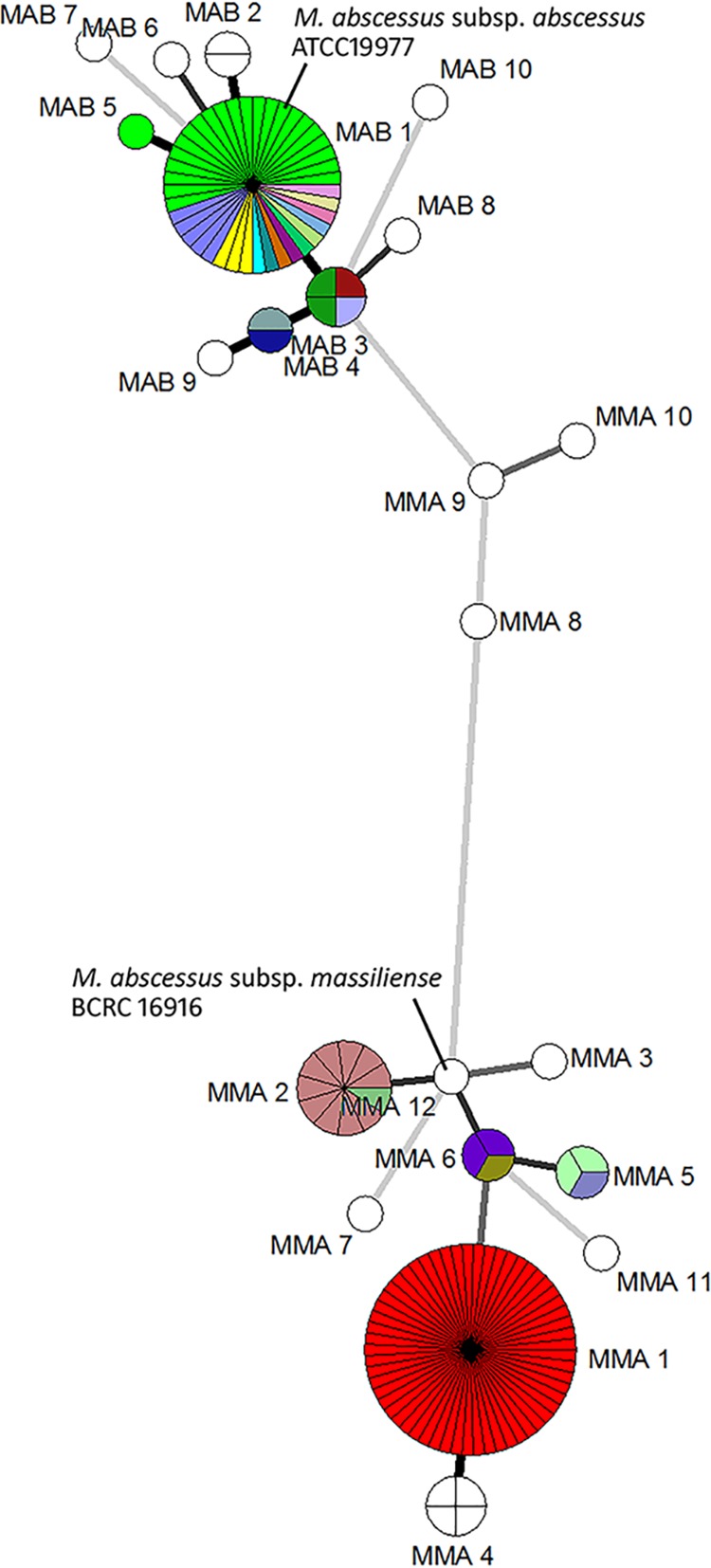
Minimum-spanning tree of 139 M. abscessus complex isolates based on the three-gene and seven-gene MLST schemes. Strains clustered together by the three-gene scheme was depicted as circles, segments within the circles depict more than one isolate clustered together by the seven-gene schemes. Different colors within one circle represent different sequence types determined by the seven-gene MLST that were indistinguishable by the three-gene MLST.
FIG 2.
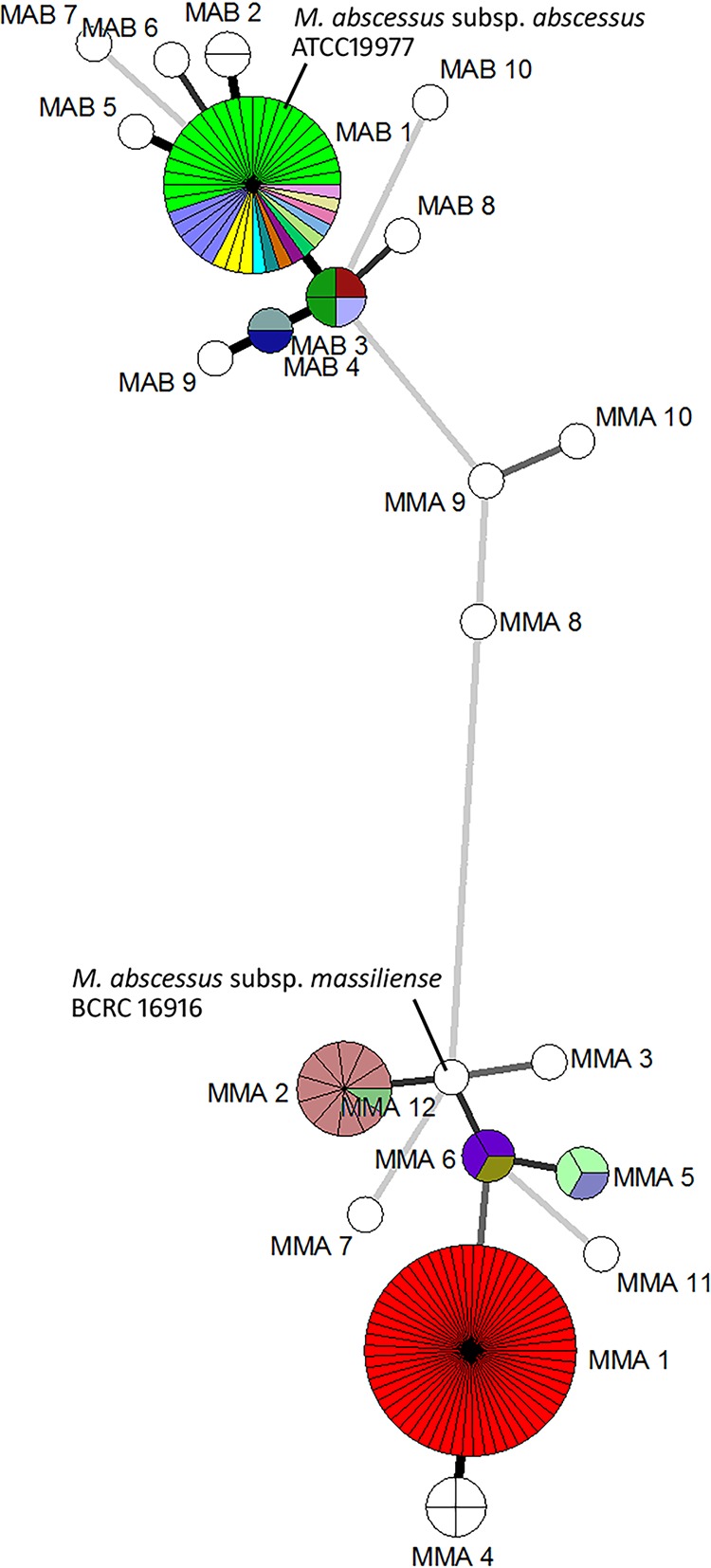
Minimum-spanning tree of 139 M. abscessus complex isolates based on the three-gene and thirteen-gene MLST schemes. Strains clustered together by the three-gene scheme was depicted as circles, segments within the circles depict more than one isolate clustered together by the thirteen-gene schemes. Different colors within one circle represent different sequence types determined by the thirteen-gene MLST that were indistinguishable by the three-gene MLST.
TABLE 3.
Comparison of three-, seven-, and thirteen-gene MLST schemes for 139 M. abscessus isolates
| Subspecies (n) | Subspecies and sequence type detection (n) |
||
|---|---|---|---|
| Three-gene MLST | Seven-gene MLST | Thirteen-gene MLST | |
| M. abscessus subsp. abscessus (54) | MAB1 (40) | ST1 (22) | ST1 (22) |
| ST22 (1) | ST22 (1) | ||
| ST40 (1) | ST40 (1) | ||
| ST63 (3) | ST63 (3) | ||
| ST127 (5) | ST127 (5) | ||
| ST276c (1) | ST276c (1) | ||
| ST280c (1) | ST280c (1) | ||
| ST272c (1) | ST272s (1) | ||
| ST289c (1) | ST289c (1) | ||
| ST277c (1) | ST277c (1) | ||
| ST283c (1) | ST283c (1) | ||
| ST284c (1) | ST284c (1) | ||
| ST288c (1) | ST288c (1) | ||
| MAB2 (2) | ST126 (2) | ST126 (2) | |
| MAB3 (4) | ST33 (1) | ST33 (1) | |
| ST49 (2) | ST49 (2) | ||
| ST286c (1) | ST286c (1) | ||
| MAB4 (2) | ST137 (1) | ST137 (1) | |
| ST278c (1) | ST278c (1) | ||
| MAB5 (1) | ST1a (1) | ST1aa (1) | |
| MAB6 (1) | ST61 (1) | ST61 (1) | |
| MAB7 (1) | ST282c (1) | ST282c (1) | |
| MAB8 (1) | ST281c (1) | ST281c (1) | |
| MAB9 (1) | ST290c (1) | ST290c (1) | |
| MAB10 (1) | ST274c (1) | ST274c (1) | |
| M. abscessus subsp. massiliense (85) | MMA1b (57) | ST48b (57) | ST48b (57) |
| MMA2 (11) | ST117 (10) | ST117 (10) | |
| ST275c (1) | ST275c (1) | ||
| MMA3 (1) | ST271c (1) | ST271c (1) | |
| MMA4 (4) | ST23 (4) | ST23 (4) | |
| MMA5 (3) | ST176 (2) | ST176 (2) | |
| ST287c (1) | ST287c (1) | ||
| MMA6 (3) | ST115 (2) | ST115 (2) | |
| ST285c (1) | ST285c (1) | ||
| MMA7 (1) | ST279c (1) | ST279c (1) | |
| MMA8 (1) | ST34 (1) | ST34 (1) | |
| MMA9 (1) | ST273c (1) | ST273c (1) | |
| MMA10 (1) | ST291c (1) | ST291c (1) | |
| MMA11 (1) | ST279a ,c (1) | ST279aa (1) | |
| MMA12 (1) | ST37 (1) | ST37 (1) | |
Denotes where typing results obtained by seven- and thirteen-gene MLST were not in agreement.
For MMA1/ST48, all 57 outbreak isolates shared the same PFGE and rep-PCR patterns. Of the 57 isolates, 51 were obtained from contaminated ultrasonography or patients with documented exposure to invasive procedures following ultrasonography. Two isolates were from hospital water supplying two bronchoscopic units, three were from three patients’ sputum, and one was from a wound without documented exposure to contaminated ultrasonography gel.
New STs from this study that were submitted to the Pasteur Institute and assigned a new sequence number (ST271 to ST291).
TABLE 4.
Key divergent loci for strains clustered together by the three-gene schemea
| MAB or MMA ST | Locus no. |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hsp65 | rpoB | secA | argH | cya | glpK | gnd | murC | pta | purH | gdhA | pgm | pknA | |
| MAB1 ST1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 2 | 6 |
| MAB5 ST1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 2 | 6 |
| MAB1 ST22 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 5 | 15 | 1 | 15 | 7 | 7 |
| MAB1 ST40 | 1 | 1 | 1 | 3 | 11 | 1 | 3 | 3 | 18 | 3 | 11 | 9 | 8 |
| MAB1 ST63 | 1 | 1 | 1 | 3 | 11 | 1 | 3 | 3 | 3 | 3 | 11 | 5 | 8 |
| MAB1 ST127 | 1 | 1 | 1 | 3 | 23 | 1 | 3 | 3 | 3 | 3 | 11 | 5 | 8 |
| MAB1 ST276 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 3 | 12 | 35 | 10 | 5 | 7 |
| MAB1 ST280 | 1 | 1 | 1 | 3 | 14 | 7 | 3 | 3 | 6 | 36 | 11 | 5 | 8 |
| MAB1 ST272 | 1 | 1 | 1 | 18 | 1 | 1 | 8 | 5 | 1 | 1 | 11 | 2 | 7 |
| MAB1 ST289 | 1 | 1 | 1 | 18 | 32 | 1 | 3 | 5 | 1 | 1 | 17 | 9 | 7 |
| MAB1 ST277 | 1 | 1 | 1 | 20 | 12 | 1 | 8 | 3 | 37 | 9 | 16 | 2 | 7 |
| MAB1 ST283 | 1 | 1 | 1 | 18 | 32 | 1 | 1 | 5 | 1 | 1 | 17 | 9 | 7 |
| MAB1 ST284 | 1 | 1 | 1 | 3 | 11 | 1 | 3 | 3 | 3 | 20 | 11 | 5 | 8 |
| MAB1 ST288 | 1 | 1 | 1 | 3 | 11 | 1 | 3 | 3 | 32 | 3 | 11 | 5 | 8 |
| MAB3 ST33 | 1 | 1 | 2 | 7 | 5 | 1 | 8 | 5 | 6 | 1 | 10 | 6 | 7 |
| MAB3 ST49 | 1 | 1 | 2 | 3 | 11 | 1 | 3 | 3 | 5 | 10 | 11 | 8 | 8 |
| MAB3 ST286 | 1 | 1 | 2 | 38 | 1 | 1 | 3 | 5 | 1 | 1 | 12 | 7 | 7 |
| MAB4 ST137 | 1 | 2 | 2 | 3 | 12 | 1 | 3 | 5 | 1 | 10 | 9 | 2 | 7 |
| MAB4 ST278 | 1 | 2 | 2 | 13 | 12 | 1 | 3 | 3 | 3 | 9 | 11 | 7 | 7 |
| MMA2 ST117 | m1 | m2 | m2 | 11 | 14 | 4 | 24 | 6 | 2 | 16 | 2 | 1 | 1 |
| MMA2 ST275 | m1 | m2 | m2 | 11 | 14 | 30 | 24 | 6 | 2 | 16 | 2 | 1 | 1 |
| MMA5 ST176 | m2 | m4 | m1 | 21 | 13 | 4 | 10 | 6 | 11 | 7 | 4 | 1 | 3 |
| MMA5 ST287 | m2 | m4 | m1 | 21 | 13 | 4 | 10 | 8 | 11 | 7 | 4 | 1 | 3 |
| MMA6 ST115 | m1 | m4 | m2 | 24 | 20 | 1 | 9 | 8 | 8 | 27 | 3 | 2 | 2 |
| MMA6 ST285 | m1 | m4 | m2 | 24 | 20 | 1 | 9 | 6 | 8 | 27 | 3 | 2 | 2 |
| MMA9 ST273 | m1 | 1 | m2 | 11 | 20 | 1 | 9 | 8 | 8 | 27 | 3 | 2 | 2 |
| MMA11 ST273a | 1 | m4 | m2 | 11 | 20 | 1 | 9 | 8 | 8 | 27 | 3 | 2 | 1 |
The maximum sequence divergence observed in the pta, purH, and gdhA genes for M. abscessus subsp. abscessus and in the murC, purH, and gdhA genes for M. abscessus subsp. massiliense. The least sequence divergence was observed in the glpK gene for M. abscessus subsp. abscessus and in the argH, cya, and pgm genes for M. abscessus subsp. massiliense. A lowercase “m”—e.g., m1, m2, m2, m4, etc.—denotes allelic type numbering for M. abscessus subsp. massiliense.
The 85 M. abscessus subsp. massiliense isolates were grouped into 12 sequence types, MMA1 to MMA12 by the three-gene scheme and consisted of 14 and 15 sequence types, respectively, as determined by the seven-gene and thirteen-gene schemes. Of 85 isolates evaluated, 57 isolates belonged to MMA1/ST48, with 100% agreement between the three MLST schemes (Table 3). Clear epidemiological links to the 2010-2012 outbreak by case definition of contaminated ultrasonography gel or invasive procedures were established for 51 of these MMA1/ST48 isolates (as published previously) (12, 25, 29). The remaining six MMA1/ST48 isolates exhibited identical PFGE patterns but did not fit the case definition of the contaminated ultrasonography gel or invasive procedures (Table 3).
Ten of the eleven isolates grouped together in the second largest subspecies massiliense group (MMA2) by the three-gene scheme belonged to ST117 based on the seven-gene and thirteen-gene schemes (Fig. 1 and 2). The remaining isolate differed from ST117 in the glpK loci (Tables 2 and 3). The reference strain, M. abscessus subsp. massiliense BCRC 16916, was typed as MMA12/ST37 and was closely related to MMA2/ST117 (Fig. 1 and 2).
The overall rates of agreement between the three-gene and seven-gene MLST schemes, the three-gene and thirteen-gene MLST schemes, and the 7-gene and 13-gene MLST schemes for M. abscessus subsp. massiliense were 95.3, 96.5, and 98.8%, respectively. Two isolates of a new sequence type (ST273) based on the seven-gene scheme were discriminated into two clones by the three-gene scheme (MMA9 and MMA11) and by the thirteen-gene scheme (ST273 and ST273a) owing to single nucleotide polymorphisms (SNPs) in hsp65, rpoB, and pknA (Table 4).
In addition, the hot spots for genetic variation within the internal sequences of the 10 housekeeping genes differed between subspecies (Table 4). For subspecies abscessus, maximum sequence divergence was observed at the pta, purH, and gdhA loci (11 different allelic types). In contrast, the glpK loci were highly conserved, and 53 of 54 subspecies abscessus strains had the same allelic type for glpK loci. For subspecies massiliense, there were fewer sequence divergences overall, the greatest being for purH loci (seven allelic types), followed by gdhA loci (six allelic types). Unlike the subspecies abscessus, there was also significant variation at the murC loci (six allelic types) and for the glpK and pknA loci (five allelic types each).
In summary, among the subspecies abscessus, the three-gene scheme was only modestly discriminative compared to the standard seven-gene MLST (agreement rates of 75%); however, for the subspecies massiliense with identical PFGE and outbreak potential, the three-gene scheme yielded very high agreement rates with the standard seven-gene scheme (95.3%) and with the extended 13-gene scheme (96.5%).
DISCUSSION
Most experts now recommend identifying M. abscessus complex isolates to the subspecies level due to differences in antimicrobial susceptibility and prognosis (30). However, there is even greater pressure on clinical laboratories to fully identify M. abscessus subsp. massiliense following the emergence of a globally successful clone, ST23 or CC3, causing outbreaks among cystic fibrosis patients and soft tissue infections in Brazilian patients, as well as a closely related clone, ST48 (also CC3), causing outbreaks among Taiwanese patients following invasive procedures (7, 8, 12, 25). Despite the emphasis in recent guidelines on the necessity of screening all isolates of subspecies massiliense recovered from patients with cystic fibrosis for relatedness to outbreak strains in an effort to prevent future outbreaks and patient-to-patient transmission, there has been little practical advice on how to do so within the limits of clinical rather than research facilities (31).
Our study is the first to compare the use of three different MLST schemes for a very well-characterized collection of M. abscessus isolates, notably including both outbreak and sporadic isolates. We showed that for the newly emerged subspecies massiliense, with potentially higher virulence and transmissibility (7, 8, 32), a three-gene MLST using partial sequences of the hsp65, rpoB, and secA1 genes (1,578 bp) was sufficiently discriminatory. This mycobacterium-specific three-gene MLST scheme identified subspecies and accurately delineated dominant clusters to >95% agreement with the seven-gene MLST (3,576 bp) and thirteen-gene MLST (6,712 bp) schemes.
A limitation of this study is the lack of WGS for full genomic comparison. However, the clonality of the isolates clustered together by the three-gene MLST had previously been validated by PFGE and rep-PCR using a DiversiLab mycobacterium typing kit (12, 25). Another limitation of this study is the lack of M. abscessus subsp. bolletii isolates; hence, we cannot make any comparisons for this subspecies. However, in most clinical reports, M. abscessus subsp. bolletii appears to be less frequently encountered as a human pathogen, and this subspecies is not included in the publicly available MLST databases, nor has it been implicated in outbreaks (27, 33, 34). Misidentification of subspecies using the three-gene scheme might have led to the absence of M. abscessus subsp. bolletii; however, we attempted to exclude this possibility by cross-checking subspecies identity by using MALDI-TOF (28). As the latter technique is fine-tuned, this limitation will become less prominent in the future.
The sensitivity and specificity of the three-gene scheme for simultaneous subtyping and screening of M. abscessus subsp. massiliense cannot be established by this preliminary study. Validation using larger collections of known outbreak versus sporadic collections is warranted. However, our collection was sufficiently diverse, including 21 novel sequence types submitted to the Pasteur Institute, each were recently assigned a new number from ST271 to ST291.
The three-gene MLST approach may offer time, costs, and labor savings over the seven-gene MLST scheme. In addition, accurate determination of subspecies (either by three-gene MLST scheme or by MALDI-TOF-MS) is required as an initial step before sequences can be compared to the publicly available MLST database at the Institute of Pasteur. Due to the minimal gains of extending the MLST scheme from seven to thirteen genes (only two additional sequence types were identified without a significant impact on the phylogenetic tree), our study supports the omission of gdhA, pgm, and pknA from current MLST schemes for M. abscessus. For confirmation of clonality or dominant clusters, the thirteen-gene MLST did not perform better than the seven-gene MLST. This may be due to extensive horizonal gene transfer of genomic blocks of housekeeping genes through distributive conjugal transfer (35). Taking into account the slow mutation rate of M. abscessus, only WGS or PFGE are recognized as having sufficient resolution to confirm an outbreak or person-to-person transmission.
Tettlin et al. previously identified signature SNPs in rpoB and secA1 genes that were typical but not exclusive to the globally successful clonal cluster of subspecies massiliense outbreak strains (4). By using the rpoB gene MAB_3869c from the subspecies abscessus type strain described in the BRA-00 outbreak from Brazil, they showed that the Seattle and Papworth cystic fibrosis isolates carried two-rpoB-SNP signature (C→T at position 2569 and T→C at position 2760) and a secA1 SNP signature (G→T substitution at position 820) by using the secA1 gene MAB_3580c from the M. abscessus subsp. abscessus type strain). Similar to our findings wherein MMA1 by the three-gene MLST clustered all outbreak strains but also included six strains without clear epidemiological links but exhibiting the same PFGE patterns, the SNPs described for rpoB and secA1 were not 100% specific markers for the outbreak strains and were found in four unrelated M. abscessus subsp. massiliense strains. Hence, we are in agreement over the value of the rpoB and secA1 genes over other housekeeping genes for first-level identification of newly isolated strains as possibly being related to cystic fibrosis clusters or soft tissue outbreak strains, to be confirmed by a second assay.
Although Tettelin et al. suggested that partial sequencing of rpoB and secA1 genes, should be followed by thirteen-target MLST analysis to rule out isolates as belonging to these two cystic fibrosis clusters, we have shown that a second assay based on another MLST approach with more routine loci targets did not outperform the first approach with mycobacterium-specific targets. Further studies are needed to elucidate a more appropriate second confirmatory assay that offers labor, time, and cost-savings over WGS and PFGE.
In conclusion, this preliminary study supports the utility of the three-gene MLST based on the partial sequences of the hsp65, rpoB, and secA1 genes as a screening tool for the routine microbiology laboratory struggling to implement the recommendations of recent guidelines recognizing the outbreak and person-to-person transmission potential of M. abscessus subsp. massiliense. The next step would require development of a publicly available three-gene MLST database to corroborate previously identified signature SNPs and to identify new patterns. With our collective efforts, accurate identification of M. abscessus subsp. massiliense with potentially higher transmissibility might soon fall within the scope of clinical practice in many more parts of the world.
ACKNOWLEDGMENTS
We thank Po-ren Hsueh and the Department of Laboratory Medicine, National Taiwan University Hospital, for storage and access to the mycobacterial isolates.
A.C. designed the study, analyzed the results, wrote the manuscript. H.-Y.S. provided critical analysis and review of the manuscript. Y.-T.T. conducted the experiments and analyzed the results. S.-Y.C. collected the mycobacterial isolates and helped execute the study. U.-I.W. helped collect mycobacterial isolates and execution of the study. P.-R.H. helped collect and analyze the mycobacterial isolates and critically reviewed the manuscript. W.-H.S. provided technical expertise, critique, and review of the manuscript. Y.-C.C. conceived the study, coordinated the infection prevention and control program and the clinical and laboratory research teams, provided technical expertise, critique, funding, and research assistance, and reviewed the manuscript. S.-C.C. provided technical expertise, critique, and review of the manuscript.
This study was funded by the Taiwan Ministry of Science and Technology (105-2628-B-002-019-MY3) and the Taiwan Ministry of Health and Welfare (MOHW108-TDU-B-211-133002).
REFERENCES
- 1.Adekambi T, Sassi M, van Ingen J, Drancourt M. 2017. Reinstating Mycobacterium massiliense and Mycobacterium bolletii as species of the Mycobacterium abscessus complex. Int J Syst Evol Microbiol 67:2726–2730. doi: 10.1099/ijsem.0.002011. [DOI] [PubMed] [Google Scholar]
- 2.Sassi M, Drancourt M. 2014. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 15:359. doi: 10.1186/1471-2164-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macheras E, Roux AL, Ripoll F, Sivadon-Tardy V, Gutierrez C, Gaillard JL, Heym B. 2009. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J Clin Microbiol 47:2596–2600. doi: 10.1128/JCM.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bange FC, Brown BA, Smaczny C, Wallace RJ Jr, Bottger EC. 2001. Lack of transmission of Mycobacterium abscessus among patients with cystic fibrosis attending a single clinic. Clin Infect Dis 32:1648–1650. doi: 10.1086/320525. [DOI] [PubMed] [Google Scholar]
- 5.Harris KA, Underwood A, Kenna DT, Brooks A, Kavaliunaite E, Kapatai G, Tewolde R, Aurora P, Dixon G. 2015. Whole-genome sequencing and epidemiological analysis do not provide evidence for cross-transmission of Mycobacterium abscessus in a cohort of pediatric cystic fibrosis patients. Clin Infect Dis 60:1007–1016. doi: 10.1093/cid/ciu967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tettelin H, Davidson RM, Agrawal S, Aitken ML, Shallom S, Hasan NA, Strong M, de Moura VC, De Groote MA, Duarte RS, Hine E, Parankush S, Su Q, Daugherty SC, Fraser CM, Brown-Elliott BA, Wallace RJ Jr, Holland SM, Sampaio EP, Olivier KN, Jackson M, Zelazny AM. 2014. High-level relatedness among Mycobacterium abscessus subsp. massiliense strains from widely separated outbreaks. Emerg Infect Dis 20:364–371. doi: 10.3201/eid2003.131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, McNulty S, Wallace RJ Jr. 2012. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 185:231–232. doi: 10.1164/ajrccm.185.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Nunes LDS, Baethgen LF, Ribeiro MO, Cardoso CM, de Paris F, De David SMM, da Silva MG, Duarte RS, Barth AL. 2014. Outbreaks due to Mycobacterium abscessus subsp. bolletii in southern Brazil: persistence of a single clone from 2007 to 2011. J Med Microbiol 63:1288–1293. doi: 10.1099/jmm.0.074906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macheras E, Konjek J, Roux AL, Thiberge JM, Bastian S, Leao SC, Palaci M, Sivadon-Tardy V, Gutierrez C, Richter E, Rusch-Gerdes S, Pfyffer GE, Bodmer T, Jarlier V, Cambau E, Brisse S, Caro V, Rastogi N, Gaillard JL, Heym B. 2014. Multilocus sequence typing scheme for the Mycobacterium abscessus complex. Res Microbiol 165:82–90. doi: 10.1016/j.resmic.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Cheng A, Sheng WH, Huang YC, Sun HY, Tsai YT, Chen ML, Liu YC, Chuang YC, Huang SC, Chang CI, Chang LY, Huang WC, Hsueh PR, Hung CC, Chen YC, Chang SC. 2016. Prolonged postprocedural outbreak of Mycobacterium massiliense infections associated with ultrasound transmission gel. Clin Microbiol Infect 22:382.e1–382.e11. doi: 10.1016/j.cmi.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Koh SJ, Song T, Kang YA, Choi JW, Chang KJ, Chu CS, Jeong JG, Lee JY, Song MK, Sung HY, Kang YH, Yim JJ. 2010. An outbreak of skin and soft tissue infection caused by Mycobacterium abscessus following acupuncture. Clin Microbiol Infect 16:895–901. doi: 10.1111/j.1469-0691.2009.03026.x. [DOI] [PubMed] [Google Scholar]
- 14.Jung SY, Kim BG, Kwon D, Park JH, Youn SK, Jeon S, Um HY, Kwon KE, Kim HJ, Jung HJ, Choi E, Park BJ. 2015. An outbreak of joint and cutaneous infections caused by non-tuberculous mycobacteria after corticosteroid injection. Int J Infect Dis 36:62–69. doi: 10.1016/j.ijid.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Hatzenbuehler LA, Tobin-D’Angelo M, Drenzek C, Peralta G, Cranmer LC, Anderson EJ, Milla SS, Abramowicz S, Yi J, Hilinski J, Rajan R, Whitley MK, Gower V, Berkowitz F, Shapiro CA, Williams JK, Harmon P, Shane AL. 2017. Pediatric dental clinic-associated outbreak of Mycobacterium abscessus infection. J Pediatric Infect Dis Soc 6:e116–e122. doi: 10.1093/jpids/pix065. [DOI] [PubMed] [Google Scholar]
- 16.Baker AW, Lewis SS, Alexander BD, Chen LF, Wallace RJ Jr, Brown-Elliott BA, Isaacs PJ, Pickett LC, Patel CB, Smith PK, Reynolds JM, Engel J, Wolfe CR, Milano CA, Schroder JN, Davis RD, Hartwig MG, Stout JE, Strittholt N, Maziarz EK, Saullo JH, Hazen KC, Walczak RJ Jr, Vasireddy R, Vasireddy S, McKnight CM, Anderson DJ, Sexton DJ. 2017. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 64:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsao SM, Liu KS, Liao HH, Huang TL, Shen GH, Tsao TC, Lee YT. 2014. The clinical management of cesarean section-acquired Mycobacterium abscessus surgical site infections. J Infect Dev Ctries 8:184–192. doi: 10.3855/jidc.3821. [DOI] [PubMed] [Google Scholar]
- 18.Höfling-Lima AL, de Freitas D, Sampaio JLM, Leão SC, Contarini P. 2005. In vitro activity of fluoroquinolones against Mycobacterium abscessus and Mycobacterium chelonae causing infectious keratitis after LASIK in Brazil. Cornea 24:730–734. doi: 10.1097/01.ico.0000154411.07315.0a. [DOI] [PubMed] [Google Scholar]
- 19.Hung JH, Huang YH, Chang TC, Tseng SH, Shih MH, Wu JJ, Huang FC. 2016. A cluster of endophthalmitis caused by Mycobacterium abscessus after cataract surgery. J Microbiol Immunol Infect 49:799–803. doi: 10.1016/j.jmii.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Cai SS, Chopra K, Lifchez SD. 2016. Management of Mycobacterium abscessus infection after medical tourism in cosmetic surgery and a review of literature. Ann Plast Surg 77:678–682. doi: 10.1097/SAP.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 21.Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, Conlan S, McNulty S, Brown-Elliott BA, Wallace RJ Jr, Olivier KN, Holland SM, Sampaio EP. 2009. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol 47:1985–1995. doi: 10.1128/JCM.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macheras E, Roux AL, Bastian S, Leao SC, Palaci M, Sivadon-Tardy V, Gutierrez C, Richter E, Rusch-Gerdes S, Pfyffer G, Bodmer T, Cambau E, Gaillard JL, Heym B. 2011. Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol 49:491–499. doi: 10.1128/JCM.01274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leao SC, Tortoli E, Viana-Niero C, Ueki SY, Lima KV, Lopes ML, Yubero J, Menendez MC, Garcia MJ. 2009. Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J Clin Microbiol 47:2691–2698. doi: 10.1128/JCM.00808-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leao SC, Tortoli E, Euzeby JP, Garcia MJ. 2011. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov., and emended description of Mycobacterium abscessus. Int J Syst Evol Microbiol 61:2311–2313. doi: 10.1099/ijs.0.023770-0. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Liu YC, Chen ML, Hung CC, Tsai YT, Sheng WH, Liao CH, Hsueh PR, Chen YC, Chang SC. 2013. Extrapulmonary infections caused by a dominant strain of Mycobacterium massiliense (Mycobacterium abscessus subspecies bolletii). Clin Microbiol Infect 19:E473–E482. doi: 10.1111/1469-0691.12261. [DOI] [PubMed] [Google Scholar]
- 26.Fraser C, Alm EJ, Polz MF, Spratt BG, Hanage WP. 2009. The bacterial species challenge: making sense of genetic and ecological diversity. Science 323:741–746. doi: 10.1126/science.1159388. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, Kang YA, Bae IK, Yim JJ, Park MS, Kim YS, Kim SK, Chang J, Jeong SH. 2013. Standardization of multilocus sequence typing scheme for Mycobacterium abscessus and Mycobacterium massiliense. Diagn Microbiol Infect Dis 77:143–149. doi: 10.1016/j.diagmicrobio.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Kehrmann J, Wessel S, Murali R, Hampel A, Bange FC, Buer J, Mosel F. 2016. Principal component analysis of MALDI TOF MS mass spectra separates M. abscessus (sensu stricto) from M. massiliense isolates. BMC Microbiol 16:24. doi: 10.1186/s12866-016-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng A, Sun HY, Tsai YT, Wu UI, Chuang YC, Wang JT, Sheng WH, Hsueh PR, Chen YC, Chang SC. 2018. In vitro evaluation of povidone-iodine and chlorhexidine against outbreak and nonoutbreak strains of Mycobacterium abscessus using standard quantitative suspension and carrier testing. Antimicrob Agents Chemother 62:e01364-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 31.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society Guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). BMJ Open Respir Res 4:e000242. doi: 10.1136/bmjresp-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang S, Gibbs S, Henao-Tamayo M, Shanley CA, McDonnell G, Duarte RS, Ordway DJ, Jackson M. 2011. Increased virulence of an epidemic strain of Mycobacterium massiliense in mice. PLoS One 6:e24726. doi: 10.1371/journal.pone.0024726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tortoli E, Kohl TA, Brown-Elliott BA, Trovato A, Leao SC, Garcia MJ, Vasireddy S, Turenne CY, Griffith DE, Philley JV, Baldan R, Campana S, Cariani L, Colombo C, Taccetti G, Teri A, Niemann S, Wallace RJ Jr, Cirillo DM. 2016. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus, and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int J Syst Evol Microbiol 66:4471–4479. doi: 10.1099/ijsem.0.001376. [DOI] [PubMed] [Google Scholar]
- 34.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 35.Sapriel G, Konjek J, Orgeur M, Bouri L, Frezal L, Roux AL, Dumas E, Brosch R, Bouchier C, Brisse S, Vandenbogaert M, Thiberge JM, Caro V, Ngeow YF, Tan JL, Herrmann JL, Gaillard JL, Heym B, Wirth T. 2016. Genome-wide mosaicism within Mycobacterium abscessus: evolutionary and epidemiological implications. BMC Genomics 17:118. doi: 10.1186/s12864-016-2448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


