We validate and evaluate a new phenotypic assay, named the direct β-lactam inactivation method (dBLIM), for the rapid and simultaneous detection of carbapenemase or extended-spectrum-cephalosporinase activity directly from Enterobacterales (EB)-positive blood cultures (BCs). It originates from the carbapenem inactivation method (CIM), an inexpensive and highly sensitive assay for carbapenemase activity detection.
KEYWORDS: CIM test, ESBL detection, carbapenemase detection, blood culture
ABSTRACT
We validate and evaluate a new phenotypic assay, named the direct β-lactam inactivation method (dBLIM), for the rapid and simultaneous detection of carbapenemase or extended-spectrum-cephalosporinase activity directly from Enterobacterales (EB)-positive blood cultures (BCs). It originates from the carbapenem inactivation method (CIM), an inexpensive and highly sensitive assay for carbapenemase activity detection. dBLIM cutoff values to detect extended-spectrum β-lactamase (ESBL) and carbapenemase activities resulted in diameters of ≤12 mm for a 5-μg-cefotaxime disk and for a 10-μg-meropenem disk. dBLIM assessment was determined with both aerobic and anaerobic BC bottles spiked with 422 characterized EB strains, classifiable into the following 4 phenotypic groups: (i) ESBL/AmpC-type β-lactamase (ACBL)/carbapenemase (CARB)-nonproducing (np-ESBL/ACBL/CARB) EB (n = 116), (ii) ESBL-producing EB (n = 111), (iii) AmpC-β-lactamase-producing EB (n = 33), and (iv) carbapenemase-producing EB (n = 162). No false-positive results were obtained in any of the np-ESBL/ACBL/CARB EB, ESBL, and AmpC groups, demonstrating an overall assay specificity of 100%. There were no significant discrepancies in dBLIM performance between aerobic and anaerobic BCs across all groups, except with VIM-type carbapenemase-expressing EB. Interestingly, among BCs spiked with blaVIM-harboring EB, the sensitivity rates of the assay in anaerobic and aerobic bottles were 53.6% and 100%, respectively. In contrast, dBLIM performance was deemed excellent for the KPC, OXA-48, and NDM carbapenemase producers regardless of the type of bottle being tested, with a sensitivity rate ranging between 99% and 100%. Concerning the detection of the extended-spectrum cephalosporinases of the ESBL-producing and AmpC types, dBLIM sensitivities was 100% and 84 to 87%, respectively. dBLIM may be a cost-effective and highly robust phenotypic screening method for the reliable detection of carbapenemases or extended-spectrum cephalosporinases directly from BCs on the same day of bottle positivity detection.
INTRODUCTION
In recent years, the threat posed by multidrug-resistant Gram-negative (GN) bacteria has continued to grow worldwide, becoming a major concern among hospital- and community-acquired infections (1). The most common pathogens responsible for health care-associated infections are Enterobacterales (EB), treated mainly with β-lactams (2). However, the extensive use of β-lactams has led to the emergence and dissemination of resistance, the most common mechanism of which involves the hydrolysis of the β-lactam ring by β-lactamases, such as extended-spectrum β-lactamases (ESBLs), AmpC-type β-lactamases (ACBLs), and carbapenemases (CARBs) (3). Unfortunately, in the past 2 decades, only a few novel classes of antibiotics able to counter these drug-resistant bacteria have been put on the market. Thus, it is more important than ever to implement highly effective antimicrobial stewardship programs as well as devise new diagnostic methods for the rapid detection of antibiotic resistance.
In this scenario, the rapid detection of ESBL-producing EB (Ep-EB) and carbapenemase-producing EB (Cp-EB) associated with severe infections, especially bloodstream infections (BSIs), seems particularly relevant given that early treatment with effective antibiotics may not only improve patient outcome but also limit the spread of these highly resistant pathogens (4, 5). Although a number of genotypic diagnostic methods for the detection of β-lactamase-encoding genes in blood cultures (BCs) are currently available, they are expensive procedures requiring skilled personnel and are not able to identify all ESBL- or carbapenemase-encoding genes (6). Thus, during the last decade, several phenotype-based assays have been developed to detect ESBL or carbapenemase production in BCs, including colorimetric methods (7–13), immunochromatogenic assays (14–16), and β-lactam hydrolysis assays combined with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (17–20).
In this study, we validate and evaluate a new method, termed the direct β-lactam inactivation method (dBLIM), for the rapid detection of carbapenemase or ESBL activity directly from positive BC bottles. The dBLIM originates from the carbapenem inactivation method (CIM), first described in 2015 (21), an easy, inexpensive, and highly sensitive assay for carbapenemase activity detection from GN isolates. A modified version of CIM, called mCIM, is recommended by the Clinical and Laboratory Standards Institute (CLSI) for the detection of carbapenemases in EB (22–24). mCIM includes the use of an alternative incubation medium (tryptic soy broth versus water) and extension of the incubation time to 4 h, both of which were found to improve the assay’s sensitivity for certain carbapenemases. Furthermore, several CIM variants have recently been developed to improve the CIM diagnostic performance (25), time of response (26, 27), and carbapenemase class characterization (27–29). However, to date, there has been no study evaluating the effectiveness of CIM in detecting other hydrolyzing enzymes, such as ESBLs, and its direct application to clinical specimens, such as BC bottles.
Here, we provide experimental evidence attesting that the CIM variant dBLIM can simultaneously detect carbapenemase- or extended-spectrum-cephalosporinases-producing EB directly from positive BC bottles in less than 7 h.
MATERIALS AND METHODS
Direct β-lactam inactivation method.
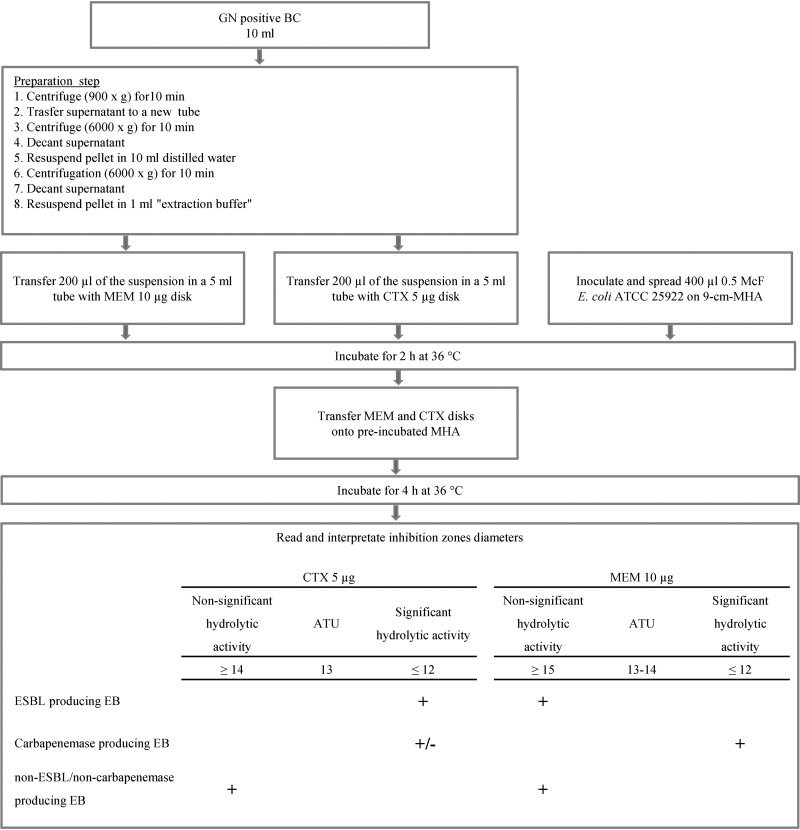
BC bottles positive for GN rods during a Gram staining examination were subjected to dBLIM analysis (Fig. 1). To this aim, bacteria were recovered from the BC fluid by stepwise centrifugation. In detail, 10 ml culture fluid was drawn from a BC bottle and centrifuged at 900 × g for 10 min to sediment blood cells. The supernatant was carefully transferred to an empty 15-ml tube and centrifuged at 6,000 × g for 10 min. The supernatant was then discarded, and the pellet was resuspended in 10 ml of distilled water. Subsequently, bacteria were pelleted by a second centrifugation at 6,000 × g for 10 min, and the supernatant was again discarded. One milliliter of extraction buffer (990 μl Tris HCl, 0.5 M, pH 7.5, plus a 10-μl ZnSO4, 0.1 M, water solution) was added to the bacterial pellet and mixed using a disposable pipette. After this first step, 400 μl of the obtained suspension was halved, and each half was transferred into a 5-ml tube containing either a 10-μg-meropenem (MEM) disk or a 5-μg-cefotaxime (CTX) disk (Oxoid Ltd., Hampshire, United Kingdom). The tubes were plugged and incubated for 2 h at 36°C. Simultaneously, 400 μl of a 0.5 McFarland standard Escherichia coli ATCC 25922 reference strain was inoculated onto a 9-cm-diameter Mueller-Hinton agar (MHA) plate and gently streaked onto the entire surface using a 10-μl disposable inoculation loop. The seeded plate was incubated in parallel with antibiotic mixtures for 2 h at 36°C. After incubation, the disks were removed from the suspensions using 10-μl disposable inoculation loops and directly placed on preincubated MHA plates, which were then incubated at 36°C. After 4 h, inhibition zone reading was performed manually from the front of the plate, with the lid removed, under reflected light. Inhibition zone diameters were measured to the nearest millimeter. Within the inhibition zone, faint growth areas with clear zone edges were ignored. Inhibition zones were interpreted using zone diameter breakpoints. For each antibiotic, the following three categories were defined: (i) significant hydrolytic activity, (ii) nonsignificant hydrolytic activity, and (iii) area of technical uncertainty (ATU), which corresponds to a zone diameter interval where the categorization is doubtful. ESBL producers were expected to exert a significant hydrolytic activity against CTX (cutoff ≤ 12 mm), whereas carbapenemase producers should at least hydrolyze MEM (cutoff ≤ 12 mm). Finally, ESBL/carbapenemase-nonproducing EB were expected to display no significant hydrolytic activity against both CTX and MEM.
FIG 1.
Direct β-lactam inactivation method. In the lowest box, the symbol + indicates the interpretative category for cefotaxime and meropenem disks required to define a β-lactamase-producing phenotype. The symbol +/– means that hydrolytic activity on cefotaxime among carbapenemase-producing Enterobacterales is not required to define the carbapenemase-producing phenotype (some OXA-type carbapenemases can hydrolyze cephalosporins weakly). Abbreviations: GN, Gram negative; BC, blood culture; MEM, meropenem; CTX, cefotaxime; 9-cm-MHA, 9-cm-diameter Mueller-Hinton agar plate; ATU, area of technical uncertainty; EB, Enterobacterales.
Characterization of EB strains.
In this study, we used EB clinical strains collected from different clinical specimens at the University Hospital Città della Salute e della Scienza di Torino from 2016 to 2019. These isolates were identified by MALDI-TOF MS (Bruker Daltonik GmbH, Bremen, Germany), and their antimicrobial susceptibility was determined by a commercially available microdilution method (MicroScan WalkAway 96 Plus; Beckman Coulter, Nyon, Switzerland). For data interpretation, EUCAST recommendations were followed (30).
A disk-based phenotypic method evaluating the inhibitory activity of clavulanate or cloxacillin toward extended-spectrum cephalosporinases (total ESBL + AmpC Confirm kit [Rosco, Taastrup, Denmark]) was used to identify ESBL or ACBL production if CTX and/or ceftazidime (CAZ) MICs were >1 mg/liter.
A commercial molecular assay (Xpert Carba-R; Cepheid, Sunnyvale, CA) was used to characterize carbapenemase producers when the MEM MIC was >0.125 mg/liter.
Spiked blood cultures.
We used BacT/Alert FA/FN Plus bottles (bioMérieux, Marcy l’Étoile, France). The clinical BC bottles that remained negative after 5 days of incubation were spiked and anonymized. Prior to inoculation in BC bottles, bacterial isolates from frozen stocks were cultured overnight on MacConkey agar plates at 36°C. BCs were inoculated with 0.5 ml of 103 CFU/ml sterile saline solution. Spiked BC bottles were incubated in the BacT/Alert Virtuo (bioMérieux) automated system until the flask was flagged as positive. Then, positive BCs were subjected to the dBLIM protocol and subcultured on solid medium to confirm the growth of the tested bacterial strain.
dBLIM validation.
dBLIM breakpoints together with interpretative criteria (Fig. 1) were validated using BC BacT/Alert FA Plus bottles spiked with 30 characterized EB isolates, 10 for each of the following three phenotypic categories: ESBL/AmpC-type-β-lactamase/carbapenemase-nonproducing (np-ESBL/ACBL/CARB) EB, ESBL-producing EB (Ep-EB), and carbapenemase-producing EB (Cp-EB) (KPC [n = 3], NDM [n = 2], VIM [n = 3], and OXA-48 [n = 2]). For Ep-EB and Cp-EB, we selected isolates for which MICs of cefotaxime and meropenem were relatively low (ranges, 1 to 8 mg/liter and 0.25 to 8 mg/ml, respectively). dBLIM was performed on the flagged-positive BCs, and results evaluating sensitivity and specificity in detecting ESBL and carbapenemase activity were analyzed.
Furthermore, protocol validation assays were performed to analyze possible influential variables on dBLIM performance. Step by step, examined variables were as follows: the volume of BC broth (5 ml versus 10 ml), centrifugation steps (1st at 900 × g for 15 min and 2nd and 3rd at 5,000 × g for 15 min versus 1st at 900 × g for 10 min and 2nd and 3rd at 6,000 × g for 10 min), the presence versus absence of the washing step, the type of extraction buffer (H2O or Mueller-Hinton broth or Tris HCl solution versus zinc-supplemented Tris HCl solution), volume of extraction buffer (500 μl versus 1,000 μl), inoculum of the E. coli indicator strain (200 μl at 1 McFarland standard and traditional swab-based seeding versus 400 μl at a 0.5 McFarland standard and streaking using a disposable 10-μl inoculation loop), time of incubation of the antibiotic mixtures (2 h versus 3 h), time of reading of inhibition zones (6 h versus 18 h following indicator strain seeding), and, finally, the use of CAZ versus CTX as the antibiotic substrate for β-lactamase activity detection. dBLIM was then performed on 30 BC BacT/Alert FA Plus bottles spiked with the isolates used in the previous phase of dBLIM breakpoint validation. Results obtained according to each variable were analyzed to assess impact on diagnostic performance.
dBLIM assessment.
A total of 422 EB clinical strains other than those used in validation assays were used to inoculate both BC BacT/Alert FA and FN Plus bottles. Among isolates, 111/422 expressed the ESBL phenotype, 33/422 were ACBL producers, 162/422 were carbapenemase producers, and 116/422 were np-ESBL/ACBL/CARB EB. Collected Cp-EB strains harbored the following carbapenemase genes: blaKPC (n = 100), blaVIM (n = 46), blaOXA-48 (n = 9), blaNDM (n = 3), and two carbapenemase-encoding genes (n = 4; blaKPC/blaVIM [n = 2] and blaNDM/blaOXA-48 [n = 2]).
Flagged-positive BCs were subjected to the dBLIM assay to evaluate diagnostic sensitivity and specificity in detecting ESBL, ACBL, and carbapenemase activity. For ACBL activity detection, ESBL interpretative cutoff values were used.
The chi-square test was used to statistically analyze the results obtained in aerobic versus anaerobic BC bottles.
RESULTS
dBLIM validation.
Validation results are reported in Table 1. dBLIM breakpoints and interpretative criteria validation assays showed an excellent ability to differentiate np-ESBL/ACBL/CARB, ESBL, and carbapenemase EB phenotypes. Indeed, 100% sensitivity and specificity for both ESBL and carbapenemase detection were achieved.
TABLE 1.
Validation of the breakpoints for dBLIM result interpretation and evaluation of different variables of the dBLIM protocola
| Evaluation | Variable | Diam range (mm), mean, for the |
Evidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| np-ESBL/ACBL/CARB group with: |
ESBL group with: |
Carbapenemase group with: |
|||||||||
| MEM at 10 μg | CTX at 5 μg | CAZ at 10 μg | MEM at 10 μg | CTX at 5 μg | CAZ at 10 μg | MEM at 10 μg | CTX at 5 μg | CAZ at 10 μg | |||
| Validation of interpretative breakpoints | 17–20, 18.3 | 14–16, 15.2 | 15–19, 18.1 | 6–12, 7.3 | 6–12, 7.2 | 6–10, 6.5 | Breakpoints and interpretative criteriab allow one to correctly differentiate np-ESBL/ACBL/CARB, ESBL, and carbapenemase groups. This validation phase shows 100% sensitivity and specificity for both ESBL and carbapenemase detection. | ||||
| Validation of DBLIM steps and parameters | |||||||||||
| Vol of BC broth (ml) | 5 | 17–20, 18.5 | 14–16, 15.3 | 18–20, 18.6 | 6–12, 7.1 | 6–12, 7.3 | 6–12, 6.6 | No difference in diagnostic sensitivity and specificity. However, a 10-ml volume may ensure a higher sensitivity. | |||
| 10 | 18–20, 18.6 | 14–16, 15.2 | 17–19, 18.5 | 6–10, 6.9 | 6–10, 7 | 6–10, 6.4 | |||||
| Centrifugation step(s): time and speed of rotation | 1st: 900 × g for 15 min; 2nd and 3rd: 5,000 × g for 15 min | 17–19, 18.3 | 14–16, 15 | 18–20, 18.6 | 6–12, 7 | 6–12, 7.1 | 6–9,6.3 | No difference in diagnostic sensitivity and specificity. The second option allows a reduction of step preparation time. | |||
| 1st: 900 × g for 10 min; 2nd and 3rd: 6,000 × g for 10 min | 18–20, 18.5 | 14–15, 14.8 | 18–19, 18.4 | 6–11, 6.9 | 6–11, 7 | 6–10, 6.4 | |||||
| Washing step | No | 16–18, 17.4 | 6–15, 11.7 | 15–19, 17.3 | 6, 6 | 6–12 6.6 | 6, 6 | Three false-positive ESBLs among the np-ESBL/ACBL/CARB group. The absence of a washing step is associated with smaller inhibition zone diameters and a reduction of specificity for ESBL detection. | |||
| Yes | 17–20, 18.6 | 14–16, 15.1 | 18–20, 18.7 | 6–12, 7.1 | 6–12, 7.1 | 6–8, 6.2 | |||||
| Extraction buffer | H2O | 18–19, 18.4 | 15–16, 15.6 | 18–19, 18.6 | 6–11, 7.2 | 6–18, 12.2 | 6–16, 10.6 | No difference in diagnostic sensitivity and specificity for ESBL activity detection. Metallo-β-lactamase activity is not detected using H2O or Tris HCl. Levels of MEM hydrolysis on cation-adjusted Mueller-Hinton broth and Tris HCl-Zn were similar for all carbapenemase classes. | |||
| Cation-adjusted Mueller-Hinton broth | 18–20, 18.6 | 14–16, 14.9 | 17–20, 18.3 | 6–11, 7.2 | 6–12, 7.2 | 6–15, 10.2 | |||||
| Tris HCl, 0.5 M | 17–20, 18.5 | 14–15, 14.7 | 17–20, 18.3 | 6–10, 7.1 | 6–17, 11.3 | 6–14, 9.7 | |||||
| Tris HCl, 0.5 M, + ZnSO4, 0.1 M (990 + 10 μl) | 18–19, 18.3 | 14–16, 14.8 | 18–19, 18.1 | 6–9, 6.5 | 6–12, 7 | 6, 6 | |||||
| Vol of extraction buffer (μl) | 500 | 18–20, 18.7 | 14–16, 15.2 | 18–20, 18.8 | 6–11, 7 | 6–12, 7.2 | 6–12, 6.6 | No difference in diagnostic sensitivity and specificity. A 1,000-μl volume can allow one to test more than 2 antibiotic disks. | |||
| 1,000 | 18–20, 18.6 | 14–16, 15.1 | 17–19, 18.5 | 6–10, 6.9 | 6–11, 7.1 | 6–10, 6.4 | |||||
| Inoculum of E. coli ATCC 25922(μl) on 9-cm-diam MHA disks | 200 at 1 McF suspension and streaking using a sterile swab | 18–20, 18.5 | 14–16, 15.4 | 18–20, 18.6 | 6–12, 7.2 | 6–12, 7.1 | 6–9, 6.4 | No difference in diagnostic sensitivity and specificity. The second option eases the reading of inhibition zone diameters. | |||
| 400 at a 0.5 McF suspension and streaking using a 10-μl inoculation loop | 18–19, 18.3 | 14–16, 15.2 | 18–20, 18.5 | 6–11, 7.1 | 6–10, 7 | 6–10, 6.4 | |||||
| Length of incubation (h) of bacterial suspensions with antibiotic disks | 2 | 18–20, 18.7 | 14–16, 15.2 | 18–20, 18.8 | 6–12, 7.1 | 6–11, 7.1 | 6–9, 6.3 | No difference in diagnostic sensitivity and specificity. Inhibition zones were small with 3 h of incubation; therefore, using the validated breakpoints, a 2-h incubation may ensure a higher specificity. | |||
| 3 | 16–17, 16.4 | 13–15, 13.9 | 15–17, 16.8 | 6, 6 | 6, 6 | 6, 6 | |||||
| Time (h) to reading of inhibition zones | 6 | 18–19, 18.5 | 14–16, 15.4 | 18–20, 18.7 | 6–11, 7 | 6–12, 7.2 | 6–10, 6.4 | No difference in diagnostic sensitivity and specificity and no significant difference in inhibition zone diameters. | |||
| 18 | 18–20, 18.8 | 15–16, 15.5 | 17–20, 18.8 | 6–12, 7.1 | 6–12, 7.2 | 6–11, 6.4 | |||||
| Evaluation of CAZ for β-lactamase activity detection | 18–19, 18.2 | 14–16, 15.4 | 14–16, 14.6 | 17–19, 18.5 | 6–11, 7 | 6–15 12.8 | 6–11, 7 | 6–9, 6.4 | 6–14, 9.8 | There is overlap of CAZ inhibition zone diameters between the ESBL and np-ESBL/ACBL/CARB groups (5 of 10 ESBL-producing isolates). CAZ is not effectively in vitro hydrolyzed by ESBL enzymes. Therefore, it is not an appropriate substrate for β-lactamase activity detection. | |
Bold and underlined values denote relevant differences between examined variables. Abbreviations: np-ESBL/ACBL/CARB, extended-spectrum-β-lactamase/AmpC-type-β-lactamase/carbapenemase-nonproducing; MEM, meropenem; CTX, cefotaxime; BC, blood culture; EB, Enterobacterales; ATU, area of technical uncertainty; McF, McFarland standard. Ten BCs were tested for each group and each method.
Breakpoints and interpretation criteria were as follows. For np-ESBL/ACBL/CARB EB, the MEM diameter was ≥15 mm and the CTX diameter was ≥14 mm; for ESBL-producing EB, the MEM diameter was ≥15 mm and the CTX diameter was ≤12; and for carbapenemase-producing EB, the MEM diameter was ≤12. For disks with ATUs, the MEM diameter was 13 to 14 and the CTX diameter was 13.
Furthermore, protocol validation assays carefully analyzed different parameters of dBLIM to prove the optimal analytic performance of the method. The key factor improving diagnostic sensitivity for metallo-β-lactamases was the use of a zinc-supplemented Tris HCl 0.5 M solution or cation-adjusted Mueller-Hinton broth as extraction buffer, whereas CTX showed higher sensitivity in ESBL detection. The washing step was essential to ensure a high diagnostic specificity in ESBL detection. Of note, there were no significant discrepancies between the readings of inhibition zone diameters performed at 6 h and 18 h of incubation.
dBLIM assessment.
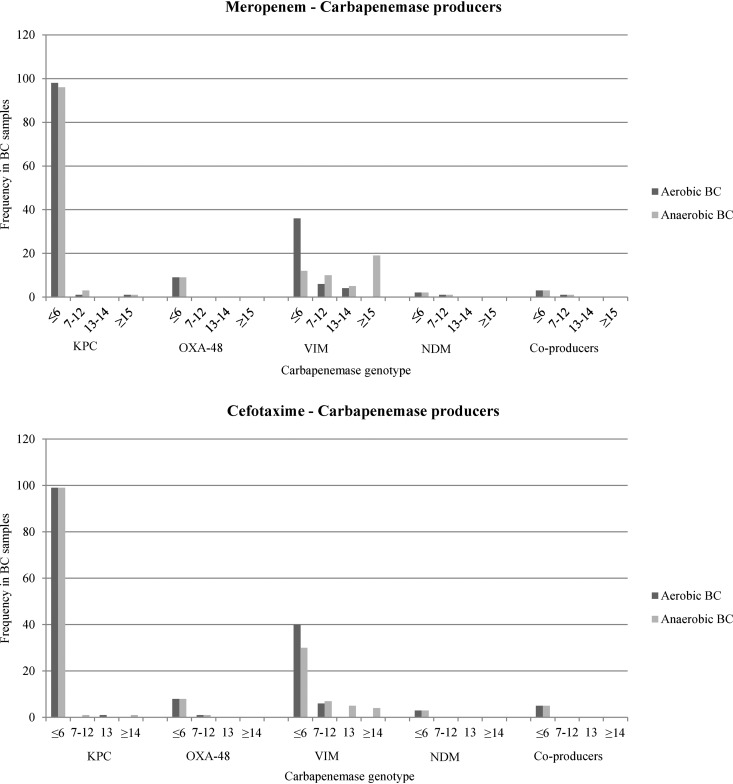
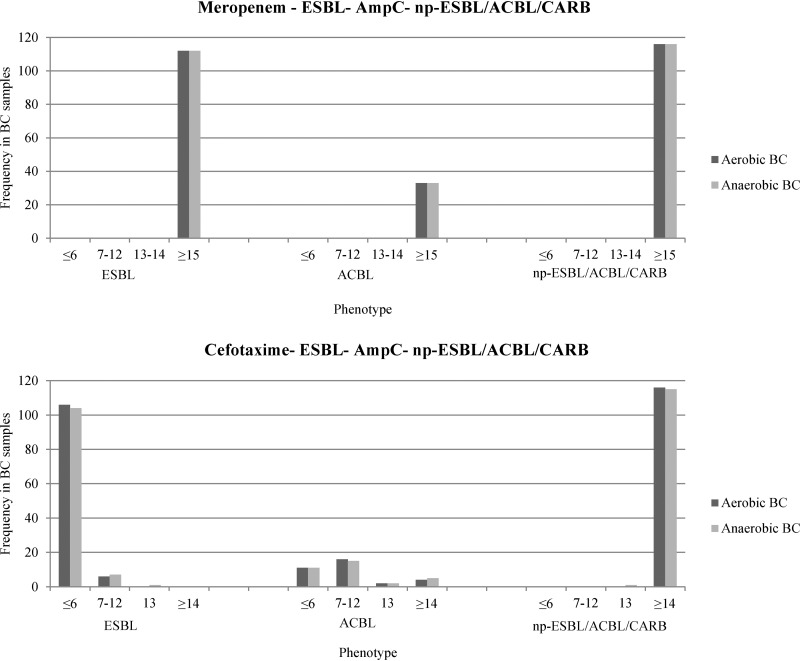
Sensitivity, specificity, and indeterminate dBLIM result rates for each phenotypic group and for aerobic and anaerobic bottles are shown in Table 2. No false-positive results were obtained in any of the np-ESBL/ACBL/CARB, ESBL, and AmpC groups, demonstrating an overall specificity of 100%. Furthermore, we obtained only indeterminate results in 15/844 executed tests (1.8%). Moreover, there were no significant dBLIM performance discrepancies between aerobic and anaerobic BCs across all groups, except with VIM-expressing EB (P < 0.001). Interestingly, among BCs spiked with blaVIM-harboring EB, the sensitivity rate in anaerobic bottles was 53.6%, while that in aerobic bottles was 100%. In contrast, dBLIM performance in detecting KPC, OXA-48, and NDM was excellent under either condition, with a sensitivity rate ranging between 99% and 100%. With regard to the detection of extended-spectrum-cephalosporinase activity, dBLIM had a sensitivity for ESBL and ACBL of 100% and 84 to 87%, respectively.
TABLE 2.
Performance of dBLIM for the detection of extended-spectrum cephalosporinases and carbapenemase productiona
| Enzyme status of EB | Species | No. of isolates | dBLIM (aerobic BC) |
dBLIM (anaerobic BC) |
||||
|---|---|---|---|---|---|---|---|---|
| ATU (%) | % sensitivity | % specificity | ATU (%) | % sensitivity | % specificity | |||
| ES/ACBL/CARB-nonproducing | All | 116 | 1 | 100 | 0 | 100 | ||
| E. coli | 45 | 0 | 100 | 0 | 100 | |||
| Klebsiella pneumoniae | 20 | 0 | 100 | 0 | 100 | |||
| Citrobacter spp. | 13 | 0 | 100 | 0 | 100 | |||
| Proteus spp. | 12 | 0 | 100 | 0 | 100 | |||
| Enterobacter spp. | 8 | 0 | 100 | 0 | 100 | |||
| Morganella morganii | 7 | 0 | 100 | 0 | 100 | |||
| Other species | 11 | 1 | 100 | 0 | 100 | |||
| ESBL | All | 111 | 0 | 100 | 100 | 1 | 100 | 100 |
| E. coli | 57 | 0 | 100 | 100 | 1 | 100 | 100 | |
| K. pneumoniae | 36 | 0 | 100 | 100 | 0 | 100 | 100 | |
| Proteus mirabilis | 14 | 0 | 100 | 100 | 0 | 100 | 100 | |
| Other species | 4 | 0 | 100 | 100 | 0 | 100 | 100 | |
| ACBL | All | 33 | 2 | 87.1 | 100 | 2 | 83.9 | 100 |
| Enterobacter spp. | 16 | 1 | 80 | 100 | 0 | 68.7 | 100 | |
| Citrobacter freundii | 7 | 0 | 100 | 100 | 0 | 100 | 100 | |
| P. mirabilis | 4 | 1 | 100 | 100 | 1 | 100 | 100 | |
| Other species | 6 | 0 | 83.3 | 100 | 1 | 100 | 100 | |
| Carbapenemases | All | 162 | 4 | 99.4 | 5 | 87.3 | ||
| blaKPC | All | 100 | 0 | 99 | 0 | 99 | ||
| K. pneumoniae | 95 | 0 | 98.9 | 0 | 98.9 | |||
| E. coli | 2 | 0 | 100 | 0 | 100 | |||
| Klebsiella oxytoca | 2 | 0 | 100 | 0 | 100 | |||
| Serratia marcescens | 1 | 0 | 100 | 0 | 100 | |||
| blaOXA-48 | All | 9 | 0 | 100 | 0 | 100 | ||
| E. coli | 5 | 0 | 100 | 0 | 100 | |||
| K. pneumoniae | 4 | 0 | 100 | 0 | 100 | |||
| blaVIM | All | 46 | 4 | 100 | 5 | 53.6 | ||
| Enterobacter cloacae | 26 | 4 | 100 | 3 | 34.8 | |||
| K. pneumoniae | 9 | 0 | 100 | 0 | 100 | |||
| E. coli | 4 | 0 | 100 | 0 | 25 | |||
| C. freundii | 3 | 0 | 100 | 2 | 100 | |||
| Citrobacter farmeri | 2 | 0 | 100 | 0 | 100 | |||
| Providencia spp. | 2 | 0 | 100 | 0 | 50 | |||
| blaNDM | K. pneumoniae | 3 | 0 | 100 | 0 | 100 | ||
| Coproducers | K. pneumoniae KPC/VIM | 2 | 0 | 100 | 0 | 100 | ||
| K. pneumoniae NDM/OXA-48 | 1 | 0 | 100 | 0 | 100 | |||
| E. coli NDM/OXA-48 | 1 | 0 | 100 | 0 | 100 | |||
| Total | 422 | 7 (1.6) | 98.3 | 100 | 8 (1.9) | 91.6 | 100 | |
Breakpoints and interpretation criteria were as follows. For ES/ACBL/CARB-nonproducing EB, the MEM diameter was ≥15 mm and the CTX diameter was ≥14; for ESBL/ACBL-producing EB, the MEM diameter was ≥15 and the CTX diameter was ≤12; for carbapenemase-producing EB, the MEM diameter was ≤12 mm; and for disks with ATUs, the MEM diameter was 13 to 14 and the CTX diameter was 13 mm. Abbreviations: BC, blood culture; ATU, area of technical uncertainty; ES/ACBL/CARB, extended-spectrum-β-lactamase/AmpC-type-β-lactamase/carbapenemase; EB, Enterobacterales.
The distribution of inhibition zone diameters is reported in Fig. 2 and 3. Overall, the absence of hydrolytic activity was associated with a median inhibition zone diameter of 18 mm for MEM (range, 15 to 20 mm) and 16 mm for CTX (range, 14 to 17 mm). All Cp-EB samples found positive by dBLIM revealed significant hydrolytic activity against both MEM and CTX. Among the Cp-EB group, 270/294 positive dBLIM tests (91.8%) showed no inhibition for both antibiotics. Likewise, a high rate of complete hydrolysis of CTX was achieved in BCs spiked with ESBL-expressing EB (210/224, 93.7%). No correlation was found between antibiotic MICs and dBLIM inhibition zone diameters in both the carbapenemase and the extended-spectrum-cephalosporinase group (see Table S1 in the supplemental material).
FIG 2.
Distribution of dBLIM inhibition zone diameters among blood cultures spiked with carbapenemase-producing Enterobacterales. Breakpoints and interpretation criteria are as follows: for ES/ACBL/CARB-nonproducing EB, the MEM diameter was >15 mm and the CTX diameter was >14 mm; for ESBL/ACBL-producing EB, the MEM diameter was >15 mm and the CTX diameter was ≤12 mm; for carbapenemase-producing EB, the MEM diameter was ≤12 mm; and for disks with ATUs, the MEM diameter was 13 to 14 mm and the CTX diameter was 13 mm. Abbreviations: BC, blood culture; np-ESBL/ACBL/CARB, extended-spectrum-β-lactamase/AmpC-type-β-lactamase/carbapenemase-nonproducing; EB, Enterobacterales; ATU, area of technical uncertainty.
FIG 3.
Distribution of dBLIM inhibition zone diameters among blood cultures spiked with ESBL-producing Enterobacterales, AmpC-type-β-lactamase-producing Enterobacterales, and extended-spectrum-β-lactamase/AmpC-type-β-lactamase/carbapenemase-nonproducing Enterobacterales. Breakpoints and interpretation criteria were as follows: for ES/ACBL/CARB-nonproducing EB, the MEM diameter was >15 mm and the CTX diameter was >14 mm; for ESBL/ACBL-producing EB, the MEM diameter was >15 mm and the CTX diameter was ≤12 mm; for carbapenemase-producing EB, the MEM diameter was ≤12 mm; and for disks with ATUs, the MEM diameter was 13 to 14 mm and the CTX diameter was 13 mm. Abbreviations: BC, blood culture; ESBL, extended-spectrum-β-lactamase; ACBL, AmpC-type β-lactamase; np-ESBL/ACBL/CARB, extended-spectrum-β-lactamase/AmpC-type-β-lactamase/carbapenemase-nonproducing; EB, Enterobacterales; ATU, area of technical uncertainty.
DISCUSSION
Rapid detection of resistance mechanisms is a challenge that must be overcome in order to optimize antimicrobial management and infection control practices.
In this study, we validated and evaluated a new phenotypic approach for prompt identification of carbapenemases or extended-spectrum-cephalosporinase production directly from positive BC bottles. Our method originates from the CIM test, a simple and accurate assay recently developed to detect carbapenemase production in pure cultures of GN bacterial isolates. Various studies demonstrated a higher sensitivity of the CIM test and its improved variants over those of traditional colorimetric methods, especially for the detection of low-activity carbapenemases (26, 31, 32). As recently highlighted by Humphries (33), the interest of the clinical microbiology community regarding CIM-based tests is shown by the many articles posted on this topic since 2015. dBLIM represents a relevant evolution of CIM-based tests, as it is characterized by a wide clinical diagnostic utility. Indeed, our findings indicate that this new screening protocol is capable of detecting carbapenemases or extended-spectrum-cephalosporinases directly from EB-positive BCs. This means that dBLIM may allow a prompt prediction of the probable resistance phenotype on the same day of positive BC processing, with potentially positive effects on antimicrobial management and infection control measures. Furthermore, given that reading and interpretation of results are feasible within 6 to 18 h of incubation, dBLIM may be implemented in laboratories with different opening times and with BC bottles flagged at different times of the workday. Nevertheless, the overall impact of dBLIM is expected to be higher in laboratories operating on a 24-h/7-day regimen.
Compared to previous CIM-based tests, dBLIM requires an initial step to harvest the bacterial pellet from BC broth by three stepwise centrifugations. It requires Tris HCl solution as an extraction buffer, as with CIMTris (34), but with a zinc sulfate supplement. The zinc requirement for metallo-β-lactamase activity, proved by validation assays, is probably due to the bacterial growth in BC broth, a medium containing cation-chelating anticoagulants.
Similarly to the original CIM (21), dBLIM requires 2 h of incubation time of tested bacteria with antibiotic mixtures. CIM and mCIM tests enable the detection of carbapenemases, with optimal performance within 18 to 24 h. This lengthy delay is due mainly to the long incubation time of MHA culture. Detection in 8 h was suggested by Van der Zwaluw et al., but the delay required for the reading and interpretation of the test results remains debated (21, 35). In the CIMplus variant recently proposed by Caméléna et al. (27), the limit of culture incubation time is overcome with introduction of the early incubation of E. coli, which allows the start of bacterial growth on the MHA surface and consequently adds 2 h of growth within the total 8-h time frame. In dBLIM, we used the early incubation of E. coli culture, and in addition, we inoculated a higher E. coli load than used in all traditional disk diffusion methods. This choice, together with the direct streaking of the suspension using a disposable 10-μl inoculation loop, allowed us to obtain a homogeneous and evident bacterial growth in 6 h. Increasing the inoculated E. coli load warranted the validation of new dBLIM-specific interpretation cutoff values for carbapenemase detection. Furthermore, we had to identify cutoff values for ESBL activity detection. In this context, our data showed that CTX is more sensitive than CAZ in detecting the hydrolytic activities of ESBLs, as previously reported by other authors (19, 36).
In our study, performing dBLIM on spiked BCs using clinical bottles that remained negative after 5 days of incubation allowed us to create conditions that closely mirror those occurring in daily clinical routine, including several other factors, such as variability of blood composition among different patients or the presence of drugs and/or antibiotics.
Our findings show an excellent performance of dBLIM in detecting ESBLs, which are the most common mediators of EB resistance to third-generation cephalosporins worldwide. A lower sensitivity of 84 to 87%, with 100% specificity, was instead achieved for ACBL-producing EB, in good agreement with previous studies using hydrolysis assays based on MALDI-TOF MS technology (20).
The performance of dBLIM was also rated as excellent in the detection of KPC, OXA-48, and NDM enzymes under both aerobic and anaerobic conditions. An interesting finding was the decreased diagnostic sensitivity for the detection of VIM activity in anaerobic versus aerobic bottles (53.6% and 100%, respectively), raising the hypothesis that this enzyme may be downregulated, inactivated, or rapidly degraded in anaerobic bottles. Previous studies have shown high sensitivity values when metallo-β-lactamases are detected directly from BCs by colorimetric methods (8–11), immunochromatographic tests (14, 16), or MALDI-TOF-based hydrolysis assays (17, 18), even though these screening were performed only on spiked aerobic BC bottles. More recently, Cointe et al. have reported the low reproducibility of the RESIST-4 O.K.N.V. immunochromatographic assay performed on spiked BCs to detect NDM and VIM carbapenemases using both aerobic and anaerobic bottles. In this regard, repeating the test on 4-h subcultures improved the detection of NDM- and VIM-type enzymes from 28 to 75% to 100% (16). Likewise, high sensitivity has been reported using the colorimetric Carba NP test on subcultures from both aerobic and anaerobic bottles after 3 h of incubation in brain heart infusion (7). Considering the dBLIM low-detection capacity for VIM-type carbapenemases and probably for all other metallo-β-lactamase types in anaerobic bottles, it is advisable to perform this test under aerobic conditions. Conversely, the test might be used indifferently for aerobic or anaerobic bottles in geographical areas where metallo-β-lactamases are not present. This study has some limitations. We did not provide a molecular characterization of ESBLs or ACBLs among extended-spectrum-cephalosporinase-producing EB isolates or sequence typing of all bacterial strains used. Second, bacterial strains expressing carbapenemases other than those of the KPC or VIM type are limited (i.e., blaOXA-48, blaNDM) or not evaluated (i.e., blaIMP, blaGES, etc.).
Further studies are needed to assess the dBLIM in other epidemiological contexts and to evaluate its ability to detect carbapenemase types other than those described in this study, including those produced by nonfermenting GN species. Further studies are also warranted to clarify the low performance of the dBLIM and probably of all other direct assays in detecting metallo-β-lactamase in anaerobic BC bottles. Evolutions of dBLIM, for example, adding a combination of antibiotics (MEM or CTX) with specific inhibitors of β-lactamases, such as EDTA and phenylboronic acid, to identify metalloenzymes and class A carbapenemases, respectively, or cloxacillin and clavulanic acid to differentiate ACBL from ESBL, are desirable.
In conclusion, our findings indicate that the dBLIM is a highly robust phenotypic method for detecting carbapenemases or extended-spectrum cephalosporinases directly from BCs on the same day of bottle positivity detection. As it requires no specialized equipment and reagents, it can easily be used by the majority of clinical microbiology laboratories as part of their BC daily routine in conjunction with fast bacterial identification methods.
Supplementary Material
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector; it was not supported by external funding.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2017. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016–2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 3.Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, Spencer J. 2019. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol 43:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco G, Boattini M, Iannaccone M, Sidoti F, Cavallo R, Costa C. 2019. Detection of antibiotic resistance genes from blood cultures: performance assessment and potential impact on antibiotic therapy management. J Hosp Infect 102:465–469. doi: 10.1016/j.jhin.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Verroken A, Defourny L, Le Polain de Waroux O, Belkhir L, Laterre PF, Delmée M, Glupczynski Y. 2016. Correction: clinical impact of MALDI-TOF MS identification and rapid susceptibility testing on adequate antimicrobial treatment in sepsis with positive blood cultures. PLoS One 11:e0160537. doi: 10.1371/journal.pone.0160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rood IGH, Li Q. 2017. Review: molecular detection of extended spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae in a clinical setting. Diagn Microbiol Infect Dis 89:245–250. doi: 10.1016/j.diagmicrobio.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Dortet L, Bréchard L, Poirel L, Nordmann P. 2014. Rapid detection of carbapenemase-producing Enterobacteriaceae from blood cultures. Clin Microbiol Infect 20:340–344. doi: 10.1111/1469-0691.12318. [DOI] [PubMed] [Google Scholar]
- 8.Lima-Morales D, Ávila H, Soldi T, Dalmolin TV, Lutz L, Aquino V, Zavascki AP, Barth AL. 2018. Rapid detection of carbapenemase production directly from blood culture by colorimetric methods: evaluation in a routine microbiology laboratory. J Clin Microbiol 56:e00325-18. doi: 10.1128/JCM.00325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier M, Hamprecht A. 2019. Rapid detection of carbapenemases directly from positive blood cultures by the β-CARBA test. Eur J Clin Microbiol Infect Dis 38:259–264. doi: 10.1007/s10096-018-3422-4. [DOI] [PubMed] [Google Scholar]
- 10.Nastro M, Ayora M, García S, Vay C, Famiglietti Á, Rodriguez CH. 2017. Rapid Blue-Carba test: reduction in the detection time of carbapenemases performed from a 4-hour bacterial lawn. J Chemother 29:150–153. doi: 10.1080/1120009X.2016.1201272. [DOI] [PubMed] [Google Scholar]
- 11.Fernández J, Rodríguez-Lucas C, Fernández-Suárez J, Vazquez F, Rodicio MR. 2016. Identification of Enterobacteriaceae and detection of carbapenemases from positive blood cultures by combination of MALDI-TOF MS and Carba NP performed after four hour subculture in Mueller Hinton. J Microbiol Methods 129:133–135. doi: 10.1016/j.mimet.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Hasso M, Porter V, Simor AE. 2017. Evaluation of the β-Lacta test for detection of extended-spectrum-β-lactamase (ESBL)-producing organisms directly from positive blood cultures by use of smudge plates. J Clin Microbiol 55:3560–3562. doi: 10.1128/JCM.01354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizrahi A, Amzalag J, Couzigou C, Péan De Ponfilly G, Pilmis B, Le Monnier A. 2018. Clinical impact of rapid bacterial identification by MALDI-TOF MS combined with the beta-LACTA™ test on early antibiotic adaptation by an antimicrobial stewardship team in bloodstream infections. Infect Dis (Lond) 50:668–677. doi: 10.1080/23744235.2018.1458147. [DOI] [PubMed] [Google Scholar]
- 14.Hamprecht A, Vehreschild JJ, Seifert H, Saleh A. 2018. Rapid detection of NDM, KPC and OXA-48 carbapenemases directly from positive blood cultures using a new multiplex immunochromatographic assay. PLoS One 13:e0204157. doi: 10.1371/journal.pone.0204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wareham DW, Shah R, Betts JW, Phee LM, Momin MH. 2016. Evaluation of an immunochromatographic lateral flow assay (OXA-48 K-SeT) for rapid detection of OXA-48-like carbapenemases in Enterobacteriaceae. J Clin Microbiol 54:471–473. doi: 10.1128/JCM.02900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cointe A, Bonacorsi S, Truong J, Hobson C, Doit C, Monjault A, Bidet P, Birgy A. 2018. Detection of carbapenemase-producing Enterobacteriaceae in positive blood culture using an immunochromatographic RESIST-4 O.K.N.V. Antimicrob Agents Chemother 62:e01828-18. doi: 10.1128/AAC.01828-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalhaes CG, Cayô R, Visconde MF, Barone T, Frigatto EA, Okamoto D, Assis DM, Juliano L, Machado AM, Gales AC. 2014. Detection of carbapenemase activity directly from blood culture vials using MALDI-TOF MS: a quick answer for the right decision. J Antimicrob Chemother 69:2132–2136. doi: 10.1093/jac/dku094. [DOI] [PubMed] [Google Scholar]
- 18.Hoyos-Mallecot Y, Riazzo C, Miranda-Casas C, Rojo-Martín MD, Gutiérrez-Fernández J, Navarro-Marí JM. 2014. Rapid detection and identification of strains carrying carbapenemases directly from positive blood cultures using MALDI-TOF MS. J Microbiol Methods 105:98–101. doi: 10.1016/j.mimet.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Oviaño M, Fernández B, Fernández A, Barba MJ, Mouriño C, Bou G. 2014. Rapid detection of Enterobacteriaceae producing extended-spectrum-β-lactamases directly from positive blood cultures by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Clin Microbiol Infect 20:1146–1157. doi: 10.1111/1469-0691.12729. [DOI] [PubMed] [Google Scholar]
- 20.Jung JS, Popp C, Sparbier K, Lange C, Kostrzewa M, Schubert S. 2014. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for rapid detection of β-lactam resistance in Enterobacteriaceae derived from blood cultures. J Clin Microbiol 52:924–930. doi: 10.1128/JCM.02691-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. 2015. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One 10:e0123690. doi: 10.1371/journal.pone.0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Ferraro MJ, Thomson RB, Jenkins SG, Limbago BM, Das S. 2017. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 55:2321–2333. doi: 10.1128/JCM.00193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI M100-S20 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing—28th ed. CLSI M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Liu M, Song Q, Wu L, Li M, Chen Z, Kang M, Xie Y. 2018. Triton X-100 and increased volume of test bacteria in the carbapenem inactivation method enhanced the detection of carbapenemase-producing Acinetobacter baumannii complex isolates. J Clin Microbiol 56:e01982-17. doi: 10.1128/JCM.01982-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntean MM, Muntean AA, Gauthier L, Creton E, Cotellon G, Popa MI, Bonnin RA, Naas T. 2018. Evaluation of the rapid carbapenem inactivation method (rCIM): a phenotypic screening test for carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:900–908. doi: 10.1093/jac/dkx519. [DOI] [PubMed] [Google Scholar]
- 27.Caméléna F, Cointe A, Mathy V, Hobson C, Doit C, Bercot B, Decré D, Podglajen I, Dortet L, Monjault A, Bidet P, Bonacorsi S, Birgy A. 2018. Within-a-day detection and rapid characterization of carbapenemase by use of a new carbapenem inactivation method-based test, CIMplus. J Clin Microbiol 56:e00137-18. doi: 10.1128/JCM.00137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K, Kashiwa M, Arai K, Nagano N, Saito R. 2017. Evaluation of the modified carbapenem inactivation method and sodium mercaptoacetate-combination method for the detection of metallo-β-lactamase production by carbapenemase-producing Enterobacteriaceae. J Microbiol Methods 132:112–115. doi: 10.1016/j.mimet.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Sfeir MM, Hayden JA, Fauntleroy KA, Mazur C, Johnson JK, Simner PJ, Das S, Satlin MJ, Jenkins SG, Westblade LF. 2019. EDTA-modified carbapenem inactivation method: a phenotypic method for detecting metallo-β-lactamase-producing Enterobacteriaceae. J Clin Microbiol 57:e01757-18. doi: 10.1128/JCM.01757-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EUCAST. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0 http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 31.Baeza LL, Pfennigwerth N, Greissl C, Göttig S, Saleh A, Stelzer Y, Gatermann SG, Hamprecht A. 2019. Comparison of five methods for detection of carbapenemases in Enterobacterales with proposal of a new algorithm. Clin Microbiol Infect 25:1286.e9–1286.e15. doi: 10.1016/j.cmi.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Madkour LA, Soliman MS, Hassan DM, Soliman NS, ElMahdy YA. 2017. Detection of carbapenemase-producers: evaluating the performance of the carbapenem inactivation method and Carba NP test versus multiplex PCR. J Glob Antimicrob Resist 9:10–14. doi: 10.1016/j.jgar.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Humphries RM. 2019. CIM City: the game continues for a better carbapenemase test. J Clin Microbiol 57:e00353-19. doi: 10.1128/JCM.00353-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uechi K, Tada T, Shimada K, Kuwahara-Arai K, Arakaki M, Tome T, Nakasone I, Maeda S, Kirikae T, Fujita J. 2017. A modified carbapenem inactivation method, CIMTris, for carbapenemase production in Acinetobacter and Pseudomonas species. J Clin Microbiol 55:3405–3410. doi: 10.1128/JCM.00893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier L, Bonnin RA, Dortet L, Naas T. 2017. Retrospective and prospective evaluation of the carbapenem inactivation method for the detection of carbapenemase-producing Enterobacteriaceae. PLoS One 12:e0170769. doi: 10.1371/journal.pone.0170769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oviaño M, Gómara M, Barba MJ, Revillo MJ, Barbeyto LP, Bou G. 2017. Towards the early detection of β-lactamase-producing Enterobacteriaceae by MALDI-TOF MS analysis. J Antimicrob Chemother 72:2259–2262. doi: 10.1093/jac/dkx127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.