Abstract
Background
Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen worldwide. Here, we determined the ability of a C. trachomatis recombinant major outer membrane protein (rMOMP) vaccine to elicit cross-serogroup protection.
Methods
Female C3H/HeN mice were vaccinated by mucosal and systemic routes with C. trachomatis serovar D (UW-3/Cx) rMOMP and challenged in the ovarian bursa with serovars D (UW-3/Cx), D (UCI-96/Cx), E (IOL-43), or F (N.I.1). CpG-1826 and Montanide ISA 720 were used as adjuvants.
Results
Immune responses following vaccination were more robust against the most closely related serovars. Following a genital challenge (as determined by number of mice with positive vaginal cultures, number of positive cultures, number of inclusion forming units recovered, and number of days with positive cultures) mice challenged with C. trachomatis serovars of the same complex were protected but not those challenged with serovar F (N.I.1) from a different subcomplex. Females were caged with male mice. Based on fertility rates, number of embryos, and hydrosalpinx formation, vaccinated mice were protected against challenges with serovars D (UW-3/Cx), D (UCI-96/Cx), and E (IOL-43) but not F (N.I.1).
Conclusions
This is the first subunit vaccine shown to protect mice against infection, pathology, and infertility caused by different C. trachomatis serovars.
Keywords: cross-serogroup, protection, vaccine, Chlamydia trachomatis, MOMP, mice
Female mice vaccinated with a Chlamydia trachomatis recombinant major outer membrane protein mounted robust immune responses and were cross-serogroup protected against a chlamydial genital challenge determined by rates of infection, shedding, upper genital tract pathology, and infertility.
Chlamydia trachomatis is the leading bacterial sexual transmitted infection in the world [1, 2]. Acute and chronic chlamydial infections in women may lead to long-term sequelae including pelvic inflammatory disease, chronic abdominal pain, ectopic pregnancy, and infertility [3–6]. In countries with poor sanitary conditions it produces trachoma [7]. Screening and antibiotics treatment programs have not resulted in a decline in infections. Likely as a result of the antibiotic treatment, patients have halted natural immune responses that facilitate reinfections [8, 9]. Thus, there is a need for a vaccine to protect against this pathogen [10–13].
Based on immunological and protection studies, 15 major C. trachomatis serovars have been identified [14, 15]. These serovars were classified into 2 major immunocomplexes: B (B, Ba, E, D, L1, and L2) and C (C, J, H, I, and A). Serovars G and F are related to the B complex while K and L3 are related to the C complex but bridge both the B and C complexes. In each complex there is a senior to junior relationship. The senior serovar, for example B in the B complex, protects against all the other serovars in the complex while the junior serovar, L2, elicits much limited cross-protection [15, 16]. When the sequence of C. trachomatis major outer membrane protein (MOMP) was determined, it was found to have variable domains (VD), regions of DNA unique to each serovar [17]. Phylogenetic analysis of the MOMP sequence supported the likelihood that the serovar/serocomplex protection elicited during the trachoma vaccine trials was due to MOMP [7, 18]. Therefore, we can hypothesize that a polyvalent vaccine formulated with the senior serovar of each complex will protect against all the individual serovars.
Here, we tested a vaccine formulated with the C. trachomatis serovar D (UW-3/Cx) rMOMP for its ability to induce protection against the homologous isolate, against a different isolate of serovar D (UCI-N96/Cx), and against 2 other serovars, E (IOL-43) and F (N.I.1). Our results showed robust protection against the homologous serovar, a different isolate of the same serovar, and against serovar E (IOL-43) from the same B-complex. No protection was induced against serovar F (N.I.1) from a different subcomplex. To our knowledge, this is the first time that cross-serogroup protection against C. trachomatis-induced infection, shedding, upper genital tract pathology, and infertility has been achieved with a subunit vaccine.
MATERIALS AND METHODS
Chlamydia trachomatis
C. trachomatis serovars D (UW-3/Cx), D (UCI-N96/Cx), E (IOL-43/GU), and F (strain N.I.1) were grown in HeLa-229 cells and elementary bodies (EBs) were purified as previously described [19]. Genomic DNA from serovar D (UW-3/Cx) was extracted and the momp gene was amplified, cloned, expressed, and purified as before [20–24] (Supplementary Figure 1). MOMP had less than 0.05 EU of lipopolysaccharide/mg of protein [25].
Immunization Protocols
Three-week-old female C3H/HeN (H-2k) mice (Charles River Laboratories; Wilmington, MA) were vaccinated twice by the colonic (10 µg protein/mouse/immunization) route, followed by 2 intramuscular (6.6 µg protein/mouse/immunization) plus subcutaneous (3.3 µg protein/mouse/immunization) immunizations [26–28]. Two adjuvants were used: CpG-1826 (10 µg mouse/immunization; Tri-Link BioTechnologies LLC, San Diego, CA) and Montanide ISA 720 VG (Seppic Inc., Fairfield, NJ) [27, 28]. Montanide was delivered only systemically.
Two negative controls received phosphate-buffered saline (PBS) with adjuvants or minimum essential medium eagle (MEM) intranasally. Positive-control mice were immunized intranasally with 1 × 106 inclusion forming units (IFU) of serovar D (UW-3/Cx). A fertility control group was only mated. The experiment was replicated. The University of California Irvine Institutional Animal Care and Use Committee approved the protocols.
Immunological Assays
Blood and vaginal washes were collected before immunization and the day before the challenge. EB and rMOMP were used as antigens and levels of antibodies were determined using an enzyme-linked immunosorbent assay (ELISA) [29]. The in vitro neutralization assay was performed as previously described using HeLa-229 cells [30]. Neutralization was defined as greater than or equal to 50% decrease in the number of IFU as compared to the control incubated with preimmunization sera. Serological assays were performed in 6 independent samples.
A T-cell lymphoproliferative assay was performed the day before the challenge using nylon-purified splenic T cells (>95% purity) [31]. The mean counts per minute (cpm) were determined from triplicate culture wells. Levels of interferon-γ (IFN-γ) were assessed in supernatants from stimulated T cells as before (BD Biosciences, San Diego, CA) [31]. Samples from 4 different mice/group were assayed.
Intrabursal Challenge
Four weeks after the last immunization, mice were challenged in the left ovarian bursa [28]. The number of IFUs for the challenge was chosen to induce at least 80% infertility in the sham-immunized animals [28]. To synchronize the estrus cycle in diestrus, 4 days before the challenge, mice were treated subcutaneously with 2 mg/mouse of medroxyprogesterone acetate (MPA; Greenstone Ltd, Peapack, NJ) [28, 32]. Following the challenge vaginal swabs were cultured twice a week for the weeks 2 and 3, and once a week for the rest of the experiment. The number of IFU was determined in HeLa-229 monolayers [28, 30, 33]. The limit of detection was 2 IFU/culture.
Fertility Studies
Female mice were housed with male mice at 6 weeks following the intrabursal challenge [29, 31]. Fertility was determined based on the number of embryos in the left and right uterine horn. Hydrosalpinx formation was assed visually.
Statistics
The 2-tailed unpaired Student t test, the Fisher exact test, and the Mann-Whitney U test, with Prism version 6 (GraphPad Software Inc.), were employed to determine the statistical significance between groups.
RESULTS
Vaccine-Induced Humoral Responses
Following vaccination, serum and vaginal washes were collected before the intrabursal challenge and probed against C. trachomatis EB from the 4 isolates and against C. trachomatis serovar D (UW-3/Cx) rMOMP (Table 1, Supplementary Table 1, and Supplementary Figure 2). The IgG geometric mean titers (GMTs) were higher against the homologous than against the heterologous serovars and the lowest titers where against serovar F (N.I.1), the most distantly related serovar. For example, in mice vaccinated with D (UW-3/Cx)-rMOMP, the IgG GMT to EBs from the 4 C. trachomatis isolates were: D (UW-3/Cx) GMT, 12 800; D (UCI-N96/Cx) GMT, 6400; E (IOL-43) GMT, 3805; and F (N.I.1) GMT, 1131. Mice vaccinated with serovar D (UW-3/Cx) EBs also had high IgG GMT to D (UW-3/Cx) EB (GMT, 6400).
Table 1.
Serum Antibody Geometric Mean Titers the Day Before Chlamydia trachomatis Challenge Probed Against Serovar D (UW-3/Cx), D (UCI-N96/Cx), E (IOL-43), F (N.I.1) EB, and D (UW-3/Cx) rMOMP
| Vaccine | D (UW-3/Cx) EB | D (UCI-N96/Cx) EB | E (IOL-43) EB | F (N.I.1) EB | D (UW-3/Cx) rMOMP | |||
|---|---|---|---|---|---|---|---|---|
| IgG | IgG | IgG | IgG | IgG | IgG1 | IgG2a | IgG2a/IgG1 | |
| D (UW-3/Cx) rMOMP | 12 800a (12 800–12 800) | 6400 (3200–12 800) | 3805b (3200–6400) | 1131b,c,d (800–1600) | 819 200a (819 200– 819 200) | 22 400a (12 800–25 600) | 179 200a (102 400– 204 800) | 8 |
| PBS | <100 | <100 | <100 | <100 | <100 | <100 | <100 | … |
| D (UW-3/Cx) EB | 6400 (6400–6400) | NT | NT | NT | 1600 (1600–1600) | 200 (200–200) | 1600 (1600–1600) | 8 |
| MEM | <100 | NT | NT | NT | <100 | <100 | <100 | … |
Data are GMT (range).
Abbreviations: EB, elementary body; GMT, geometric mean titer; MEM, minimum essential medium eagle; NT, not tested; PBS, phosphate-buffered saline; rMOMP, recombinant major outer membrane protein.
a P < .05 by Student t test compared to D (UW-3/Cx) EB immunized.
b P < .05 by Student t test compared to D (UW-3/Cx) rMOMP-immunized group's anti-D(UW-3/Cx) EB GMT.
c P < .05 by Student t test compared to D (UW-3/Cx) rMOMP immunized group's anti-D(UCI-N96/Cx) EB GMT.
d P < .05 by Student t test compared to D (UW-3/Cx) rMOMP immunized group's anti-E(IOL-43) EB GMT.
Mice vaccinated with serovar D (UW-3/Cx) rMOMP had very high IgG GMT against D (UW-3/Cx)-rMOMP (GMT, 819 200) when compared to the control D (UW-3/Cx) EB vaccinated group (GMT, 1600; P < .05). The IgG2a/IgG1 ratio was 8 for both groups vaccinated with D (UW-3/Cx) rMOMP or EB.
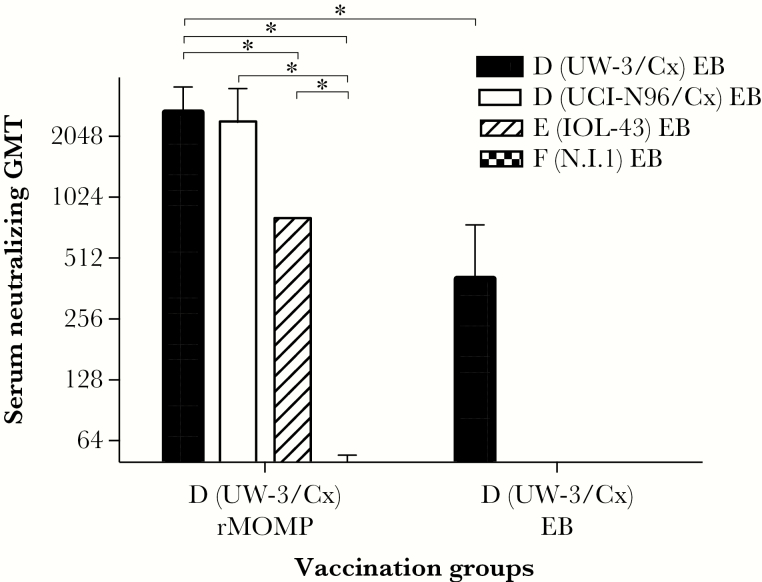
As shown in Figure 1, mice immunized with serovar D (UW-3/Cx) rMOMP had higher neutralizing GMT against the homologous serovar D (UW-3/Cx) (GMT, 2540) and D (UCI-N96/Cx) (GMT, 2263) than against a heterologous serovar E (IOL-43) (GMT, 800; P < .05). Serovar F (N.I.1) was not neutralized (GMT, <50). The positive control immunized with serovar D (UW-3/Cx) EB had a GMT of 317 against the homologous isolate.
Figure 1.
In vitro Chlamydia trachomatis neutralization titers. Serum samples collected the day before the genital challenge from mice immunized with D (UW-3/Cx) MOMP or EB were tested for their ability to neutralize in vitro EB from the 4 C. trachomatis isolates used to challenge the mice. Abbreviations: EB, elementary body; GMT, geometric mean titer; MOMP, major outer membrane protein. * P < .05.
Vaginal IgG GMT in mice vaccinated with serovar D (UW-3/Cx) rMOMP were positive only in vaginal washes against EB from D (UW-3/Cx) (GMT, 28) and D (UCI-N96/Cx) (GMT, 12) (Supplementary Table 1). Like in serum, a major difference in IgG GMT was found in mice vaccinated with D (UW-3/Cx) rMOMP when tested against D (UW-3/Cx) rMOMP (GMT, 2153) versus mice vaccinated with D (UW-3/Cx) EBs (GMT, 14; P < .05). The highest IgA GMT was detected in mice vaccinated with D (UW-3/Cx) rMOMP probed with E (IOL-43) EB (GMT, 13). When probed with D (UW-3/Cx) rMOMP, mice vaccinated with D (UW-3/Cx) rMOMP had an IgA GMT of 80 while those immunized with D (UW-3/Cx) EB had a GMT of 40.
Thus, the results of the ELISA and neutralizing antibody titers in serum correlated with the known immunological and protective relationships among C. trachomatis serovars. Antibodies reacted strongly with the homologous serovars and the heterologous serovars of the same complex but reacted weakly with a serovar of another subcomplex.
Cell-Mediated Immune Responses Elicited by Vaccination
To determine C. trachomatis-specific T-cell memory responses elicited by vaccination, mice immunized with serovar D (UW-3/Cx) rMOMP were euthanized and their spleens collected the day before the intrabursal challenge. T cells were stimulated with D (UW-3/Cx), D (UCI-N96/Cx), E (IOL-43), or F (N.I.1) EB. Mice immunized with D (UW-3/Cx) rMOMP had a Δcpm of 4348 when stimulated with D (UW-3/Cx) EB and 4796 when stimulated with D (UCI-N96/Cx) EB (P < .05; Table 2). The weakest proliferative responses were against the most distantly related serovar F (N.I.1) (Δcpm, 2134; P < .05). The Δcpm in control animals immunized with D (UW-3/Cx) EB was 8319. All groups vaccinated with D (UW-3/Cx) rMOMP had significant proliferative T-cell responses when compared to their negative PBS control groups (P < .05).
Table 2.
T-Cell Proliferative Responses and IFN-γ Production From the Day Before Challenge When Stimulated With Serovar D (UW-3/Cx), D (UCI-N96/Cx), E (IOL-43), and F (N.I.1) EB
| Vaccine | D (UW-3/Cx) EB | D (UCI-N96/Cx) EB | E (IOL-43) EB | F (N.I.1) EB | ||||
|---|---|---|---|---|---|---|---|---|
| Δ cpma | IFN-γ, pg/mL | Δ cpma | IFN-γ, pg/mL | Δ cpma | IFN-γ, pg/mL | Δ cpma | IFN-γ, pg/mL | |
| D (UW-3/Cx) rMOMP | 4348 ± 18b,c,d | 3496 ± 489b,d | 4796 ± 396b,c | 3182 ± 634b | 3970 ± 560b,c | 2711 ± 717b | 2134 ± 235b | 2137 ± 746b |
| PBS | 1029 ± 126 | <15 | 363 ± 133 | <15 | 739 ± 100 | <15 | 856 ± 134 | <15 |
| D (UW-3/Cx) EB | 8319 ± 1267e | 4870 ± 192e | NT | NT | NT | NT | NT | NT |
| MEM | 964 ± 192 | <15 | NT | NT | NT | NT | NT | NT |
Data are mean ± 1 SE.
Abbreviations: IFN-γ, interferon-γ; Δ cpm, change in counts per minute; EB, elementary body; MEM, minimum essential medium eagle; NT, not tested; PBS, phosphate-buffered saline; rMOMP, recombinant major outer membrane protein.
aΔ cpm, difference in counts per minute between EB-stimulated and medium-stimulated T cells. EB were added at a ratio of 0.5:1 to Antigen presenting cells + T cells.
b P < .05 by Student t test compared to PBS immunized group.
c P < .05 by Student t test compared to D (UW-3/Cx) rMOMP immunized group stimulated with F (N.I.1) EB.
d P < .05 by Student t test compared to D (UW-3/Cx) EB immunized group stimulated with D (UW-3/Cx) EB.
e P < .05 by Student t test compared to MEM immunized group.
T cells from the serovar D (UW-3/Cx) rMOMP immunized group produced progressively decreasing levels of IFN-γ when stimulated with EB from D (UW-3/Cx), 3496 pg/mL; D (UCI-N96/Cx), 3182 pg/mL; E (IOL-43), 2711 pg/mL, or F (N.I.1), 2137 pg/mL. The differences, however, were not statistically significant (P > .05; Table 2). T cells from the D (UW-3/Cx) EB-immunized group also produced similar levels of IFN-γ when stimulated with EB from D (UW-3/Cx) (4870 pg/mL).
These results demonstrate that cell-mediated immune responses were more robust against the homologous than against distantly related serovars.
C. trachomatis Vaginal Cultures
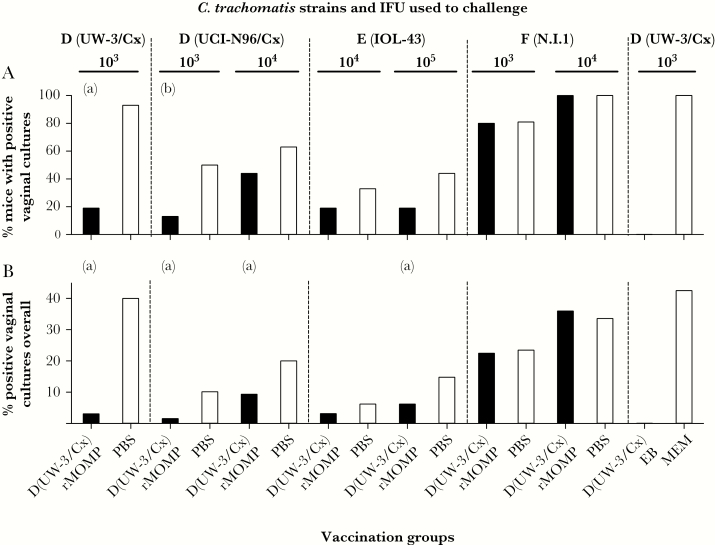
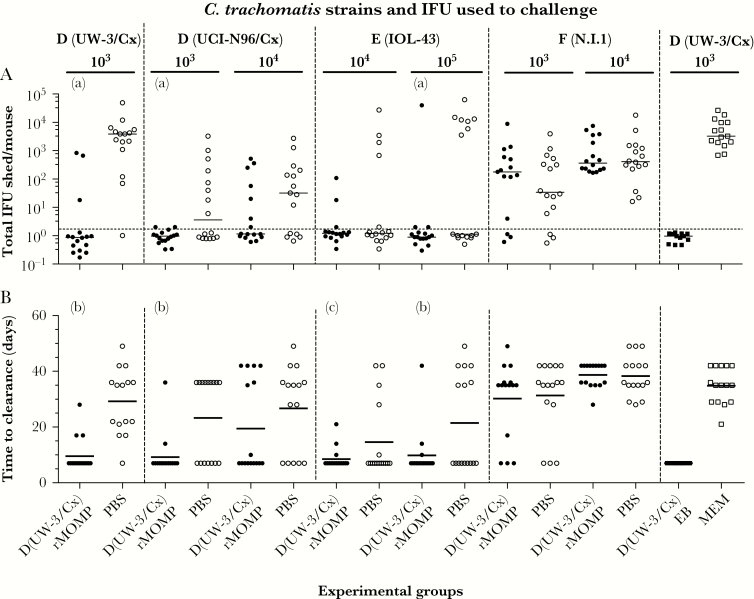
Four weeks after the last immunization, mice were challenged in the left ovarian bursa with various dosages (103, 104, or 105 IFUs) of 4 different C. trachomatis serovars. Protection against the intrabursal challenge was evaluated based on the number of mice with positive vaginal cultures, number of positive cultures, number of IFU recovered, and mean number of days to clearance (Figure 2, Figure 3, Supplementary Figure 3, and Supplementary Table 2).
Figure 2.
Chlamydia trachomatis vaginal cultures over the 6 weeks of testing. A, Percentage of mice with positive vaginal cultures. B, Percentage of positive vaginal cultures in the 6 weeks of testing. aP < .05 by Fisher exact test compared to own PBS control. bP < .1 by Fisher exact test compared to own PBS control. Abbreviations: EB, elementary body; IFU, inclusion forming unit; MEM, minimum essential medium eagle; PBS, phosphate-buffered saline; rMOMP, recombinant major outer membrane protein.
Figure 3.
Chlamydia trachomatis vaginal cultures over the 6 weeks of testing. A, Total C. trachomatis IFU shed/mouse. The horizontal lines correspond to the median IFU shed/mouse. B, Time to clearance. The horizontal lines correspond to the mean number of days to clearance. aP < .05 by Mann-Whitney test compared to own PBS control.
b P < .05 by Student t test compared to own PBS control. cP < .1 by Student t test compared to own PBS control. Abbreviations: EB, elementary body; IFU, inclusion forming unit; MEM, minimum essential medium eagle; PBS, phosphate-buffered saline; rMOMP, recombinant major outer membrane protein.
The best protected groups were those challenged with the homologous or the heterologous serovars from the same complex. For example, over the 6-week period, of the mice challenged with 103 IFU of D (UW-3/Cx), only 19% (3/16) of the D (UW-3/Cx) rMOMP-vaccinated mice shed compared to 93% (14/15) of the PBS-immunized group (P < .05; Figure 2A and Supplementary Table 2). When challenged with 103 IFUs of D (UCI-N96/Cx), 13% (2/16) of the rMOMP-vaccinated mice were culture positive versus 50% (8/16) of the controls, approaching statistical significance (P = .057). In contrast, of the mice challenged with 103 IFUs of F (N.I.1.), 80% (12/15) of the rMOMP-vaccinated mice were culture positive versus 81% (13/16) of the controls (P > .05). Mice immunized intranasally with 104 IFU of D (UW-3/Cx) were solidly protected against a challenge with the homologous serovar (0% positive, 0/12). All MEM control mice had positive cultures (100%, 15/15).
From mice vaccinated with D (UW-3/Cx) rMOMP and challenged with 103 IFUs of D (UW-3/Cx), C. trachomatis was recovered from 3% (4/128) of all the vaginal cultures collected, compared to 40% (48/120) of the PBS-immunized group (P < .05) (Figure 2B and Supplementary Table 2). When challenged with 103 or 104 IFUs of D (UCI-N96/Cx), 2% (2/128) and 9% (12/128), respectively, of the cultures were positive in the D (UW-3/Cx) rMOMP-vaccinated groups versus 10% (13/128) and 20% (24/120) in the PBS-immunized mice, respectively (P < .05). Protection against a challenge with 105 IFUs of E (IOL-43) was also elicited by vaccination with D (UW-3/Cx) rMOMP when compared with the PBS controls (6% [8/128] positive cultures versus 15% [19/128], respectively; P < .05). No protection was achieved against a challenge with 103 or 104 IFUs of C. trachomatis serovar F (N.I.1).
The total number of IFUs recovered per mouse during the 6 weeks was significantly lower for mice vaccinated with serovar D (UW-3/Cx) rMOMP and challenged with 103 IFU of D (UW-3/Cx), 103 IFUs of D (UCI-N96/Cx), or with 105 IFUs of E (IOL-43) when compared to the PBS controls: median 0 (range, 0–836) versus 3866 (range, 0–48 946); median 0 (range, 0–2) versus 3 (range, 0–3267); and median 0 (range, 0–39 847) versus 0 (range, 0–62 997), respectively (P < .05; Figure 3A and Supplementary Table 2). None of the other vaccinated groups had significant decreases in the total number of IFU shed. The controls vaccinated with EB had no positive vaginal cultures while the PBS control shed median 3273 (range, 688–26 510) IFU/mouse.
The length of time of shedding was calculated from the time of challenge until the first negative vaginal culture (first day of culture: day 7 postchallenge) (Figure 3B and Supplementary Table 2). This parameter was significantly shorter in the D (UW-3/Cx) rMOMP-immunized mice versus PBS controls when challenged with 103 IFU D (UW-3/Cx) (10 days ± 1 vs 29 days ± 3), 103 IFU D (UCI-N96/Cx) (9 days ± 2 vs 23 days ± 4), or with 105 IFU E (IOL-43) (10 days ± 2 vs 22 days ± 4) (P < .05). In addition, the decrease in the length of shedding approached statistical significance between immunized and control mice in the 104 IFU E (IOL-43) challenged pair (9 days ± 1 versus 15 days ± 3; P < .1). No protection was observed in the 2 groups challenged with C. trachomatis serovar F (N.I.1). The EB-immunized group shed for a shorter amount of time than the MEM group (7 days ± 0 versus 35 days ± 2; P < .05).
In summary, based on the number of mice with positive vaginal cultures, number of positive vaginal cultures, number of IFU recovered, and days to clearance, C. trachomatis rMOMP elicited protective immune responses against genital challenges with various dosages of serovars of the same complex but not against a serovar from a different subcomplex.
Fertility Studies
To determine the ability of serovar D (UW-3/Cx) rMOMP to protect against upper genital tract pathology and infertility, vaccinated mice challenged in the left ovarian bursa were caged with males and the outcome of the mating evaluated (Table 3). Based on the number of fertile mice, embryos in the left uterine horn, and hydrosalpinges, mice challenged with 103 IFU of D (UW-3/Cx), 103 or 104 IFU of D (UCI-N96/Cx), or 104 IFU of E (IOL-43) were protected. For example, mice vaccinated with D (UW-3/Cx) rMOMP were protected against challenges with 103 IFU of D (UW-3/Cx) (38% [6/16] vs 7% [1/15]; P = .08), 103 IFU of D (UCI-N96/Cx) (56% [9/16] vs 6% [1/16)]), and 104 IFU of E (IOL-43) (63% [10/16] vs 20% [3/15]) when compared to their respective PBS controls (P < .05). Serovar D (UW-3/Cx) rMOMP-immunized groups challenged with higher doses were protected but the differences were not significant: 104 IFU of D (UCI-N96/Cx) (31% [5/16] vs 7% [1/15]) or 105 IFU of E (IOL-43) (38% [6/16] vs 13% [2/16]). None of the groups challenged with serovar F (N.I.1) were protected against infertility. Mice immunized with D (UW-3/Cx) EB had fertility rates similar to the fertility group (P > .05). No protection was observed in the MEM group.
Table 3.
Fertility of Mice Immunized With Chlamydia trachomatis Serovar D (UW-3/Cx) rMOMP or Controls and Challenged With C. trachomatis Serovars
| Vaccine | Challenge | No. Fertile Micea/ Total No. Mice (%) | No. Embryos in Uterine Horn, Mean ± SD | Total No. Hydrosalpinges/ No. Uterine Horns (%) | |||
|---|---|---|---|---|---|---|---|
| Serovar | IFU/Mouse | Leftb | Right | Both | |||
| D (UW-3/Cx) rMOMP | D (UW-3/Cx) | 103 | 6/16c,d (38) | 1.2 ± 0.5g | 3.0 ± 0g | 4.2 ± 0.9 g | 2/32e (6) |
| PBS | D (UW-3/Cx) | 103 | 1/15 (7) | 0.2 ± 0.2 | 0.5 ± 0.3 | 0.7 ± 0.5 | 22/30 (73) |
| D (UW-3/Cx) rMOMP | D (UCI-N96/Cx) | 103 | 9/16e (56) | 2.3 ± 0.6 g | 2.4 ± 0.5 | 4.7 ± 0.9 | 2/32 (6) |
| PBS | D (UCI-N96/Cx) | 103 | 1/16 (6) | 0.2 ± 0.2 | 2.9 ± 0.6 | 3.1 ± 0.7 | 6/32 (19) |
| D (UW-3/Cx) rMOMP | D (UCI-N96/Cx) | 104 | 5/16f (31) | 1.1 ± 0.5 h | 3.0 ± 0.6 g | 4.1 ± 0.9 g | 4/32e (13) |
| PBS | D (UCI-N96/Cx) | 104 | 1/15 (7) | 0.1 ± 0.1 | 0.5 ± 0.5 | 0.7 ± 0.5 | 13/30 (43) |
| D (UW-3/Cx) rMOMP | E (IOL-43) | 104 | 10/16e (63) | 2.2 ± 0.5 g | 3.4 ± 0.7 | 5.7 ± 1.1 | 2/32e (6) |
| PBS | E (IOL-43) | 104 | 3/15 (20) | 0.4 ± 0.2 | 3.5 ± 0.5 | 3.9 ± 0.6 | 9/30 (30) |
| D (UW-3/Cx) rMOMP | E (IOL-43) | 105 | 6/16d (38) | 1.2 ± 0.4 | 1.1 ± 0.4 | 2.5 ± 0.8 | 3/32c (9) |
| PBS | E (IOL-43) | 105 | 2/16 (13) | 0.3 ± 0.2 | 1.9 ± 0.6 | 2.2 ± 0.7 | 10/32 (31) |
| D (UW-3/Cx) rMOMP | F (N.I.1) | 103 | 3/15f,j (20) | 0.6 ± 0.4 i | 2.3 ± 0.7 | 2.9 ± 0.9 i | 5/30d (17) |
| PBS | F (N.I.1) | 103 | 0/16 (0) | 0.0 ± 0.0 | 2.8 ± 0.6 | 2.8 ± 0.6 | 11/32 (34) |
| D (UW-3/Cx) rMOMP | F (N.I.1) | 104 | 0/16f (0) | 0.0 ± 0.0 i | 0.9 ± 0.5 i | 0.9 ± 0.5 i | 4/32 (13) |
| PBS | F (N.I.1) | 104 | 0/16 (0) | 0.0 ± 0.0 | 1.5 ± 0.6 | 1.5 ± 0.6 | 8/32 (25) |
| D (UW-3/Cx) EB | D (UW-3/Cx) | 103 | 9/12 (75) | 2.0 ± 0.6 | 3.1 ± 0.5 | 5.1 ± 1.0 | 0/24 (0) |
| MEM | D (UW-3/Cx) | 103 | 0/15 (0) | 0.0 ± 0.0 | 0.9 ± 0.4 | 0.9 ± 0.4 | 18/30 (60) |
| Fertility control | … | … | 11/15 (73) | 2.6 ± 0.7 | 2.3 ± 0.5 | 4.9 ± 1.0 | 0/30 (0) |
Abbreviations: IFU, inclusion forming units; MEM, minimum essential medium eagle; PBS, phosphate-buffered saline; rMOMP, recombinant major outer membrane protein.
aFertility was defined as at least 1 embryo present in the any uterine horn.
bMice were challenged in the left ovarian bursa.
c P < .1 by Fisher exact test compared to PBS immunized group challenged with the same serovar.
d P < .1 by Fisher exact test compared to fertility control.
e P < .05 by Fisher exact test compared to PBS immunized group challenged with the same serovar.
f P < .05 by Fisher exact test compared to fertility control.
g P < .05 by Student t test compared to PBS immunized group challenged with the same serovar.
h P < .1 by Student t test compared to PBS immunized group challenged with the same serovar.
i P < .05 by Student t test compared to fertility control.
jD(UW-3/Cx) rMOMP immunized and challenged with F(N.I.1) 103 group lost 1 mouse during first mating.
The number of embryos in the left (challenged side) and the right (control) uterine horns were counted. As determined by the number of embryos in the left uterine horn, mice vaccinated with D (UW-3/Cx) rMOMP were protected against challenges with 103 IFU of D (UW-3/Cx) (1.2 ± 0.5 vs 0.2 ± 0.2), 103 IFU of D (UCI-N96/Cx) (2.3 ± 0.6 vs 0.2 ± 0.2), and 104 IFU of E (IOL-43) (2.2 ± 0.5 vs 0.4 ± 0.2) (P < .05) and approached significance when challenged with 104 IFU of D (UCI-N96/Cx) (1.1 ± 0.5 vs 0.1 ± 0.1) (P = .06). No protection was obtained against challenges with serovar F (N.I.1).
Although mice were challenged in the left uterine horn, groups infected with 103 IFU of D (UW-3/Cx) and 104 IFU of D (UCI-N96/Cx) had significant decreases in number of embryos in the right uterine horn indicating that C. trachomatis migrated to that side. These 2 groups of mice were also protected when the total number of embryos in both uterine horns were analyzed following challenge with 103 IFU of D (UW-3/Cx) (4.2 ± 0.9 vs 0.7 ± 0.5) and 104 IFU of D (UCI-N96/Cx) (4.1 ± 0.9 vs. 0.7 ± 0.5) (P < .05). The number of embryos in these groups was not significantly different from mice vaccinated with D (UW-3/Cx) EB or the fertility control group (P > .05). Mice challenged with 104 IFU of F (N.I.1) also had decreases in the number of embryos in the right uterine horn but vaccination with D (UW-3/Cx) rMOMP was not protective.
Fewer hydrosalpinges were found in the D (UW-3/Cx) rMOMP-immunized mice compared to their respective PBS controls when challenged with 103 IFU of D (UW-3/Cx) (6.3% [2/32] vs 73.3% [22/30]), 104 IFU of D (UCI-N96/Cx) (12.5% [4/32] vs 43.3% [13/30]), or 104 IFU of E (IOL-43) (6.3% [2/32] vs 30.0% [9/30]) (P < .05), and approached significance when challenge with 105 IFU E (IOL-43) (9.3% [3/32] vs 31.3% [10/32]) (P = .06). Mice immunized with D (UW-3/Cx) EB and the fertility control group had no hydrosalpinx.
Summarizing, vaccination with C. trachomatis rMOMP protected mice against infertility and hydrosalpinx following an ovarian bursa challenge with the homologous serovar, a different isolate of the same serovar, and a heterologous serovar of the same complex. No protection was observed against a serovar from a different subcomplex.
DISCUSSION
Here we demonstrated that vaccination with C. trachomatis serovar D (UW-3/Cx) rMOMP elicits robust humoral and cellular immune responses against the homologous serovar, a different isolate of the same serovar, and a heterologous serovar of the same immunocomplex. Weak responses were induced against a serovar from a different subcomplex. Following a genital challenge, vaccinated mice were protected against shedding when challenged with serovars of the same complex as the vaccine antigen but not from a different subcomplex. Vaccinated and C. trachomatis-challenged mice were mated and, as determined by fertility and hydrosalpinx formation, immunized mice were significantly protected against serovars of the same immunocomplex but not from a different subcomplex. This is the first time that cross-serogroup protection against infection, vaginal shedding, upper genital tract pathology, and infertility has been achieved with a subunit C. trachomatis vaccine.
Attempts to elicit cross-serovar C. trachomatis protection in mice are limited. This is in part due to the lack of long-term sequelae, specifically tubal pathology and infertility, induced by C. trachomatis in mice following a vaginal challenge [32, 34]. Yang et al [35] showed that mice pretreated with MPA and infected vaginally with 105 IFU of serovar D (UW-3/Cx; D-LC) have a short low-burden of infection, with minimal inflammatory responses and no seroconversion. Tuffrey et al [36] were the first to induce infertility by pretreating mice with MPA and challenging them in the ovarian bursae with C. trachomatis serovar F (N.I.1). Tuffrey et al [37] were also the first to attempt to elicit cross-serovar protection in mice with a subunit vaccine. In C3H mice immunized with a truncated rMOMP from C. trachomatis serovar L1 and challenged in the uterine horn with serovar F (N.I.1), there was a reduction in salpingitis and vaginal shedding but no protection against infertility. Olsen et al [38, 39] constructed a chimeric antigen (Hirep1) using the variable domain 4 (VD4) of MOMP and its surrounding constant domains (CD) from C. trachomatis serovars D, E, and F. C3H/HeN mice immunized with Hirep1, using the adjuvant CAFO1, were protected against vaginal challenges with C. trachomatis serovars D, E, or F, as determined by the number of IFUs in vaginal swabs and inflammatory responses in the upper genital tract. However, no protection against hydrosalpinx or infertility was reported.
A difficulty when characterizing C. trachomatis cross-serovar protection is that the in vitro infectivity and the in vivo virulence of a serovar could be different [28]. Even more, within the same serovar different isolates may have various levels of virulence [28]. For example, 103 IFU of C. trachomatis serovar D isolate X versus isolate Y may or may not result in the same rate of infection, shedding, upper genital tract pathology, and/or infertility. This requires careful planning of the experiments and interpretation of results. To address this issue, and the lack of pathogenicity of the C. trachomatis serovars following a vaginal infection, we tested the ability of several C. trachomatis isolates to induce upper genital tract pathology and infertility following an intrabursal challenge in C3H/HeN mice [28]. We were particularly interested in identifying isolates from serovars D, E, and F because they are the most frequent serovars causing human genital infections [40]. Based on those results, here, we used C. trachomatis isolates and challenge doses that induced at least 80% infertility in naive C3H/HeN mice [28]. High, rather than low, infectious doses in humans, like in mice, result in upper genital tract pathology [41, 42]. In most human infections, the number of C. trachomatis IFU transmitted is lower than some of the challenge dosages tested here and therefore, likely, a rMOMP vaccine will be protective for most exposed patients [42–44].
One limitation of this study is the treatment of mice with MPA before the intrabursal challenge. Tuffrey and Taylor-Robinson [45] demonstrated the need for pretreating mice with MPA for a C. trachomatis infection to elicit upper genital tract pathology. They postulated that by blocking the estrus cycle in diestrus MPA enhances the local infection. The major effect of MPA is likely a switch from Th1 to Th2 immune responses [46–49]. Protection against a chlamydial infection depends on CD4+ Th1 cells. Therefore, switching the immune response to Th2 before the challenge counteracts vaccine-induced protection.
The need to challenge mice in the ovarian bursa, or in the uterus, to induce upper genital tract pathology with the C. trachomatis serovars is also a limitation. However, it could be argued that direct inoculation in the ovarian bursa may be a more strictive approach for testing the protective effect of a vaccine than a vaginal challenge because C. trachomatis is directly inoculated at the site were upper genital tract pathology occurs [29]. Nevertheless, we realize that immune responses in the lower and upper genital tract of mice are different and this may affect protection depending on site of challenge [50].
In conclusion, immunization with C. trachomatis serovar D rMOMP protects mice against an intrabursal challenge with the homologous serovar, a different strain of same serovar, and a heterologous serovar from the same complex. No protection was observed against a serovar from a different subcomplex. Thus, a polyvalent vaccine formulated with rMOMP from the senior C. trachomatis serovar of each immunocomplex should elicit broad cross-serovar protection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number AI092129).
Potential conflicts of interest. All authors: no reported conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10:e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. Atlanta: US Department of Health and Human Services, 2018:1–168. [Google Scholar]
- 3. Møller BR, Weström L, Ahrons S, et al. Chlamydia trachomatis infection of the Fallopian tubes. Histological findings in two patients. Br J Vener Dis 1979; 55:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201(Suppl 2):S134–55. [DOI] [PubMed] [Google Scholar]
- 5. Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19:185–92. [PubMed] [Google Scholar]
- 6. Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med 2015; 372:2039–48. [DOI] [PubMed] [Google Scholar]
- 7. Taylor HR. Trachoma: a blinding scourge from the Bronze Age to the twenty-first century. Victoria, Australia: Haddington Press, 2008. [Google Scholar]
- 8. Götz H, Lindback J, Ripa T, Arneborn M, Ramsted K, Ekdahl K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis 2002; 34:28–34. [DOI] [PubMed] [Google Scholar]
- 9. Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis 2005; 192:1836–44. [DOI] [PubMed] [Google Scholar]
- 10. Zhong G, Brunham RC, de la Maza LM, Darville T, Deal C. National Institute of Allergy and Infectious Diseases workshop report: “Chlamydia vaccines: The way forward” [published online ahead of print 31 October, 2017]. Vaccine 2017; doi: 10.1016/j.vaccine.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 11. de la Maza LM, Zhong G, Brunham RC. Update on Chlamydia trachomatis vaccinology. Clin Vaccine Immunol 2017; 24:pii: e00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips S, Quigley BL, Timms P. Seventy years of chlamydia vaccine research - limitations of the past and directions for the future. Front Microbiol 2019; 10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gottlieb SL, Deal CD, Giersing B, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine 2016; 34:2939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang SP, Grayston JT. Classification of trachoma virus strains by protection of mice from toxic death. J Immunol 1963; 90:849–56. [PubMed] [Google Scholar]
- 15. Wang S-P, Grayston J. Microimmunofluorescence serology of Chlamydia trachomatis. In: Peterson EM, de la Maza LM, ed. Medical Virology. Vol. 3. New York: Elsevier Science, 1984. [Google Scholar]
- 16. Wang SP, Grayston JT. A potency test for trachoma vaccine utilizing the mouse toxicity prevention test. Am J Ophthalmol 1967; 63(Suppl):1443–54. [DOI] [PubMed] [Google Scholar]
- 17. Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol 1987; 169:3879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitch WM, Peterson EM, de la Maza LM. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol Biol Evol 1993; 10:892–913. [DOI] [PubMed] [Google Scholar]
- 19. Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun 1981; 31:1161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 2009; 27:5020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tifrea DF, Pal S, Popot JL, Cocco MJ, de la Maza LM. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J Immunol 2014; 192:5201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fielder TJ, Pal S, Peterson EM, de la Maza LM. Sequence of the gene encoding the major outer membrane protein of the mouse pneumonitis biovar of Chlamydia trachomatis. Gene 1991; 106:137–8. [DOI] [PubMed] [Google Scholar]
- 23. Marston FA. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J 1986; 240:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qi HL, Tai JY, Blake MS. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun 1994; 62:2432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987; 166:368–79. [DOI] [PubMed] [Google Scholar]
- 26. McConnell EL, Basit AW, Murdan S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, and serum IgG compared to oral administration. Vaccine 2008; 26:639–46. [DOI] [PubMed] [Google Scholar]
- 27. Carmichael JR, Pal S, Tifrea D, de la Maza LM. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine 2011; 29:5276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carmichael JR, Tifrea D, Pal S, de la Maza LM. Differences in infectivity and induction of infertility: a comparative study of Chlamydia trachomatis strains in the murine model. Microbes Infect 2013; 15:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun 2005; 73:8153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun 1988; 56:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun 1994; 62:3354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol 1986; 67:605–16. [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson EM, You JZ, Motin V, de la Maza LM. Intranasal immunization with Chlamydia trachomatis, serovar E, protects from a subsequent vaginal challenge with the homologous serovar. Vaccine 1999; 17:2901–7. [DOI] [PubMed] [Google Scholar]
- 34. Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol (Oxford) 1990; 71:403–10. [PMC free article] [PubMed] [Google Scholar]
- 35. Yang C, Whitmire WM, Sturdevant GL, Bock K, Moore I, Caldwell HD. Infection of hysterectomized mice with Chlamydia muridarum and Chlamydia trachomatis. Infect Immun 2017; 85:pii: e00197-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tuffrey M, Falder P, Gale J, Quinn R, Taylor-Robinson D. Infertility in mice infected genitally with a human strain of Chlamydia trachomatis. J Reprod Fertil 1986; 78:251–60. [DOI] [PubMed] [Google Scholar]
- 37. Tuffrey M, Alexander F, Conlan W, Woods C, Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol 1992; 138:1707–15. [DOI] [PubMed] [Google Scholar]
- 38. Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the major outer membrane protein. J Infect Dis 2015; 212:978–89. [DOI] [PubMed] [Google Scholar]
- 39. Olsen AW, Lorenzen EK, Rosenkrands I, Follmann F, Andersen P. Protective effect of vaccine promoted neutralizing antibodies against the intracellular pathogen Chlamydia trachomatis. Front Immunol 2017; 8:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Batteiger BE, Lennington W, Newhall WJ, Katz BP, Morrison HT, Jones RB. Correlation of infecting serovar and local inflammation in genital chlamydial infections. J Infect Dis 1989; 160:332–6. [DOI] [PubMed] [Google Scholar]
- 41. Pal S, Peterson EM, de la Maza LM. Susceptibility of mice to vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect Immun 2001; 69:5203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geisler WM, Suchland RJ, Whittington WL, Stamm WE. Quantitative culture of Chlamydia trachomatis: relationship of inclusion-forming units produced in culture to clinical manifestations and acute inflammation in urogenital disease. J Infect Dis 2001; 184:1350–4. [DOI] [PubMed] [Google Scholar]
- 43. Geisler WM, Suchland RJ, Rockey DD, Stamm WE. Epidemiology and clinical manifestations of unique Chlamydia trachomatis isolates that occupy nonfusogenic inclusions. J Infect Dis 2001; 184:879–84. [DOI] [PubMed] [Google Scholar]
- 44. Vodstrcil LA, McIver R, Huston WM, Tabrizi SN, Timms P, Hocking JS. The Epidemiology of Chlamydia trachomatis organism load during genital infection: a systematic review. J Infect Dis 2015; 211:1628–45. [DOI] [PubMed] [Google Scholar]
- 45. Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Letters 1981; 12:111–5. [Google Scholar]
- 46. Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 2003; 38:13–22. [DOI] [PubMed] [Google Scholar]
- 47. Kaushic C, Murdin AD, Underdown BJ, Wira CR. Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect Immun 1998; 66:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol 2003; 77:9845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 2013; 154:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maxion HK, Kelly KA. Chemokine expression patterns differ within anatomically distinct regions of the genital tract during Chlamydia trachomatis infection. Infect Immun 2002; 70:1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.