Abstract
Background
Indoor residual spraying (IRS) is widely used as a vector control measure, although there are conflicting findings of its effectiveness in reducing malaria incidence. The objective of this study was to estimate the effect of multiple IRS rounds on malaria incidence and hemoglobin levels in a cohort of children in rural southeastern Uganda.
Methods
The study was based upon a dynamic cohort of children aged 0.5–10 years enrolled from August 2011 to June 2017 in Nagongera Subcounty. Confirmed malaria infections and hemoglobin levels were recorded over time for each participant. After each of 4 rounds of IRS, malaria incidence, hemoglobin levels, and parasite density were evaluated and compared with pre-IRS levels. Analyses were carried out at the participant level while accounting for repeated measures and clustering by household.
Results
Incidence rate ratios comparing post-IRS to pre-IRS incidence rates for age groups 0–3, 3–5, and 5–11 were 0.108 (95% confidence interval [CI], .078–.149), 0.173 (95% CI, .136–.222), and 0.226 (95% CI, .187–.274), respectively. The mean hemoglobin levels significantly increased from 11.01 (pre-IRS) to 12.18 g/dL (post-IRS).
Conclusions
Our study supports the policy recommendation of IRS usage in a stable and perennial transmission area to rapidly reduce malaria transmission.
Keywords: children, malaria, indoor residual spraying, Uganda, hemoglobin
Continuous measures of malaria incidence over repeated rounds of indoor residual spraying is needed to better understand the dynamics between IRS and malaria. Our findings indicate that repeated IRS activity in a high-transmission setting significantly reduced malaria incidence in children.
Malaria remains a major cause of global illness and death despite significant gains due to enormous investments in malaria control activities. Indoor residual spraying (IRS) generally involves applying an insecticide to the walls of a house where the insecticide will kill the adult mosquitoes that rest on these surfaces, and, to be effective, IRS must be applied to a high proportion of households in the target area (>80%). Between 2015 and 2017, 68 countries reported IRS activities using various insecticide formulations, and 62 of these countries also reported insecticide-treated mosquito net (ITN) sales or distributions during the same time period [1]. Despite its widespread use and support as a key vector control measure, there are conflicting findings of IRS effectiveness [2–5]. The context in which IRS activity occurs influences its effectiveness, and these factors include malaria transmission intensity, IRS coverage and frequency, type of insecticide, and ITN coverage and usage [6]. Insecticide resistance and decreased residual activity are direct factors that challenge its effectiveness in killing mosquitoes [2, 4].
Having continuous measures of malaria incidence from defined populations over repeated rounds of spraying is crucial to better understand the dynamics between IRS and malaria. To the best of our knowledge, there is 1 randomized controlled trial in Sudan [7] and 1 cohort study in Uganda [8, 9] that have examined the influence of several applications (3 rounds and over) of IRS on the incidence of malaria over time with continual longitudinal data. One study in Zambia [10] used longitudinal data to estimate protective efficacy of different IRS formulations over multiple rounds of IRS, whereas different studies from Uganda [10], Mozambique [11], and Mali [12] used annual seroprevelance surveys or surveillance data to compare malaria risk throughout 3 to 5 rounds of spraying.
Uganda reintroduced IRS activity in 2006 in selected epidemic-prone or high-transmission areas, and nationwide campaigns have been conducted in 2013–2014 and 2017–2018, aiming to achieve universal ITN coverage. Recent studies have found significant protective effects of IRS activity on malaria incidence in Uganda [8, 9, 13, 14]. The objective of this study was to estimate the effect of repeated rounds of IRS on malaria incidence and hemoglobin levels in a cohort of children in Nagongera Subcounty, Uganda.
METHODS
Study Design
Nagongera is a rural area in eastern Uganda with historically high malaria transmission intensity [15]. Transmission is perennial with 2 annual peaks after the rainy seasons (March to May and October to November). A detailed description of the study protocol has been reported previously [8]. In brief, a dynamic cohort of children from randomly selected households began in August 2011 and continued through June 2017. All eligible children aged 0.5–10 years were enrolled from 100 households. At enrollment, written informed consent was provided by parents and/or guardians, and study participants were given an ITN. Cohort study participants received all medical care free of charge at a designated study clinic open every day and parents and/or guardians were encouraged to bring their children to the clinic any time they were ill. Children who presented with a documented fever (tympanic temperature ≥38.0°C) or history of fever in the previous 24 hours had blood obtained by finger prick for a thick blood smear. If the smear was positive for malaria parasites, the patient was diagnosed with malaria. Episodes of uncomplicated malaria were treated with artemether-lumefantrine, and episodes of complicated malaria or recurrent malaria were treated with quinine. Participants were also invited for routine visits to the study clinic every 90 days, with the frequency of routine visits increasing to every 30 days in December 2014. Hemoglobin levels were measured with each malaria diagnosis and at each routine visit using a portable spectrophotometer (HemoCue, Ängelholm, Sweden). The housing characteristics of cohort participants was assessed through 2 household surveys in 2013 and in 2016, which classified households as either having traditional or modern housing characteristics.
In Nagongera, the first round of IRS using the carbamate bendiocarb was delivered in December 2014–February 2015, a second round in June–July 2015, and a third round in November–December 2015. Actellic CS, an organophosphate, was used for a fourth round of spraying from June to July 2016. Data on the number of households targeted and the number that received IRS in Nagongera were obtained from the Ugandan National Malaria Control Program. From September 2012 through August 2014, the government of Uganda carried out a countrywide mass distribution of free ITNs. An estimated 21 million ITNs were distributed, with the goal of achieving universal coverage with at least 1 ITN for every 2 people. The ITNs were distributed during November 2013 in Nagongera. Estimates of monthly rainfall were obtained from the NASA's Precipitation Processing System [16].
Statistical Analysis
The primary outcome was malaria incidence, defined as the number of new episodes of malaria per person over person time at risk of the disease. New episodes of malaria were defined as any episode of laboratory-confirmed malaria not preceded by another episode of malaria in the prior 14 days. The analyses were performed using R version 3.4.2 (https://www.r-project.org/) with the cpt.meanvar function and the GENMOD and MIXED procedures in SAS software, version 9.4.
Changepoint Detection
We first conducted a changepoint detection analysis to identify the time points of significant changes in the weekly overall malaria incidence rate (IR) from August 2011 to June 2017. For each week in the study period, we calculated the overall IR by dividing the total number of incident malaria episodes in cohort participants by the total person-time in that week. We also recorded the percentage of households that had been sprayed up to that week in the IRS period (ie, household coverage of IRS). For this analysis, we used the cpt.meanvar function from the changepoint package [17] and explored a variety of penalty values and methods, retaining the most consistently identified changepoints with the proposed pruned exact linear time (PELT) algorithm.
Incidence Analysis
We then conducted an individual level analysis to assess the impact of IRS rounds on malaria incidence and hemoglobin levels, after adjusting for potential confounders. We divided the study into 5 distinct intervals: (0) pre-IRS; (1) between IRS 1 and 2 spraying rounds; (2) between IRS 2 and 3 spraying rounds; (3) between IRS 3 and 4 spraying rounds; (4) and post-IRS 4 spraying round, which was specified for each household using household specific IRS dates. We restricted the pre-IRS period from January 1, 2014 to the first spraying round (December 2014) versus using the time of study commencement (August 2011), given the length of the pre-IRS period and, consequently, to improve comparability between periods. We calculated each individual's incidence of malaria (number of malaria episodes) and person-time (number weeks) for each of the 5 intervals. The IRs (incidence/person-time) in each post-individualized rating-scale procedure (IRSP) interval 1–4 were compared with the pre-IRS interval IR through the IR ratio ([IRR] the post-IRSP rate divided by the pre-IRS rate). If participating households did not have their house sprayed during an IRS round (average of 5 households per round), the midpoint of the distribution of spraying dates for that round was used as a spray date to define periods for calculating IRs for the household's participants.
Specifically, expected IRs for each interval and IRRs adjusted for confounders were estimated using generalized estimating equations based on a negative binomial distribution with a compound symmetry covariance structure where each household was considered a cluster and children were nested within a given household. The log of person-weeks of at-risk individuals was included as an offset. Compound symmetry was chosen because it does not assume equal spacing of measurements and is efficient in terms of power requirements for estimation [18].
As a first step, we estimated the unadjusted IRs and IRRs of the IRS spraying episode at each period using a model without covariates. We then added the variables: sex, number children per household, proportion of days in the interval with rain, age, and age by interval interactions. Age was defined as the youngest recorded age since the start of the study period (January 1, 2014). We analyzed the data separately for the age strata at baseline: 0- to 3-year-olds, 3- to 5-year-olds, and 5- to 11-year-olds given model goodness of fit assessed by the quasi-likelihood under the independence model criterion (QICu). As previously mentioned, bednet use was captured at each clinic visit but was not included in the model because there was no variation in the responses (all responses were positive for bednet use at each clinic visit for all participants). In addition to using the QICu to compare fit, we assessed the similarity between the empirical and model-based covariance matrices and tested the adequacy of the log link [19].
We conducted sensitivity analyses to examine any effect modification by household size, the significance of housing type of each household, the choice of rainfall measure, and any potential implications using the restricted pre-IRS period (January 1, 2014 onwards) versus the unrestricted pre-IRS period (August 19, 2011 onwards). To assess any effect modification of household size on IRs, we stratified the analysis into small (3 children or less) and large households (4 children or more). We also examined the importance of housing type (traditional vs modern) and categorized households as either having traditional housing, based upon having traditional housing at both 2013 and 2016 survey points, as modern, based upon having modern housing at both survey points, or as having improved housing, for households that had traditional housing in 2013 but changed to modern housing in 2016. Models with and without the housing type variable were compared to determine its significance. We assessed the effect of IRS spraying rounds having cumulative rainfall with a 2-month lag as a covariate instead of proportion of days it rained. Finally, we compared IRRs using the entire baseline period to the restricted baseline period of January 1, 2014 onwards.
Hemoglobin Levels
Mean hemoglobin levels (g/dL) for each time period were estimated using a linear mixed-effect repeated measures model adjusted for age and sex and assuming a compound symmetry covariance matrix. We calculated hemoglobin levels for incident episodes as well as for all measures (incident and routine) over time. For the hemoglobin measures through time, we assumed a compound symmetry covariance structure, which accounted for the clustering of children within households. The Bayesian Information Criterion (BIC) was used to compare model goodness of fit. Quantile-quantile plots (QQ-plots) of the residuals were used to look for any substantial departures from normality.
Parasite Density
A secondary outcome was parasite density (µL), which was measured at each malaria diagnosed and at every routine visit. Similar to the analysis for hemoglobin levels, mean parasite density at each time period was estimated using a linear mixed-effects model of the log-transformed parasite density model, assuming within subject and household clustering, a compound symmetry covariance structure, and adjustment for age and sex. The geometric means are presented to give results on the original scale. Parasite density was analyzed separately for incident and asymptomatic episodes. The BIC was used to compare goodness of fit, and a QQ-plot was used to identify important departures from normal.
RESULTS
There were 384 children from 107 households in the study from August 19, 2011 through to June 30, 2017, which was reduced to 319 children when the pre-IRS period was restricted to January 1, 2014 with a total of 802 person years (Table 1). There were 4 rounds of spraying included in the study period, which began on December 5, 2014 (Figure 1). Since January 1, 2014, there were 1166 incident episodes of malaria and an average of 1.5 episodes per year per person (Table 1). The IR of malaria decreased from 3.25 to 0.62 episodes per person year after the implementation of IRS. Furthermore, the mean hemoglobin levels (g/dL) increased from 11.2 to 12.1.
Table 1.
Characteristics of Children in Cohort From January 1, 2014 to June 30, 2017
| Characteristic | All Years | Pre-IRS | Post-IRS | P Valuea |
|---|---|---|---|---|
| Person-years of observation | 801.5 | 546.8 | 254.8 | |
| Number of participating children | 319 | 274 | 286 | |
| Proportion female | 46.3 | 43.8 | 47.0 | .59 |
| Mean age at episode (SD) | 5.2 (2.6) | 5.0 (2.5) | 6.3 (2.5) | <.0001 |
| Total number of incident episodes of malaria | 1166 | 828 | 338 | |
| Median episodes per person year (IQR) | 1.5 (2.1) | 2.1 (3.97) | 0.40 (0.96) | <.0001 |
| Mean hemoglobin (SD; g/dL) | 11.7 (0.9) | 11.2 (1.1) | 12.1 (0.8) | <.0001 |
Abbreviations: IRS, indoor residual spraying; IQR, interquartile range; SD, standard deviation;
a P value is for the difference between pre-IRS and post-IRS values.
Figure 1.
Cohort participants over time and incident episodes of malaria from August 2011 to June 2017. Each line represents a cohort participant and each incident malaria episode is demarcated. IRS, indoor residual spraying.
There was a drastic drop in the weekly overall IR after the first round of IRS with the largest drop occurring on week 9 within the first IRS round, coinciding with 100% household coverage (Figure 2). Significant decreases in incidence occurred on week 6 for IRS 2 and IRS 4, associated with 96.5% and 100% household coverage, with no significant changes in trend mean noted for IRS 3. Significant resurgences of incidence were detected beginning at week 24 for IRS 1, week 32 for IRS 3, and week 44 for IRS 4.
Figure 2.
Weekly overall incidence rate across all cohort participants by week from August 2011 to June 2017. January 1, 2014 is indicated by a dotted line. Significant decreases in the trend were detected from weeks 6–9 after an indoor residual spraying (IRS) round began, whereas significant increases in the trend were detected from weeks 24–44 across the various spraying rounds.
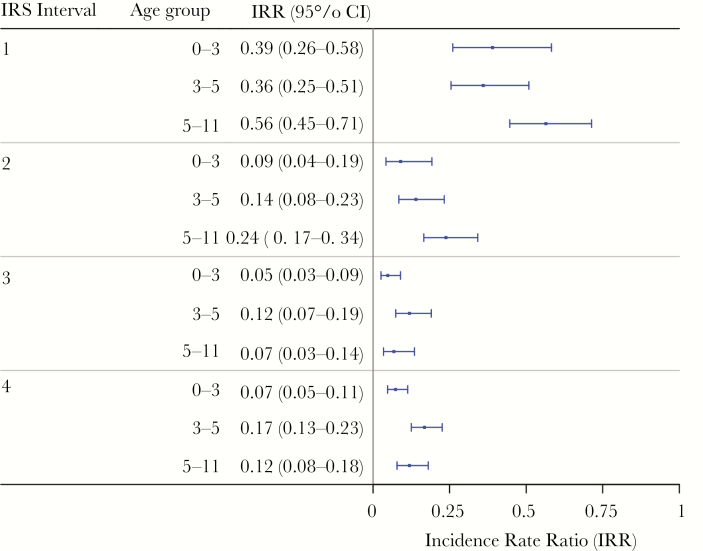
Based on the individual analysis, after adjusting for age, sex, rainfall, and number of children in the household and accounting for overdispersion, the estimated IR during the pre-IRS period was 0.064 (95% confidence interval [CI], .058–.070) and the estimated IR post-IRS was 0.011 (95% CI, .097–.012) malaria episodes per person per week, which were stratified by age group (Table 2). For each round of IRS, there were incremental reductions of malaria incidence, with the lowest mean malaria incidence occurring after IRS 3 and before IRS 4. Incidence rate ratios comparing pre-IRS versus post-IRS IRs for age groups 0–3, 3–5, and 5–11 were 0.108 (95% CI, .078–.149), 0.173 (95% CI, .136–.222), and 0.226 (95% CI, .187–.274), respectively (Figure 3). All pairwise comparisons for the IRRs are available in Supplemental Tables 1–3.
Table 2.
Estimated Malaria Incidence Rates Pre- and Post-IRSa,b
| Age Group | Pre-IRS IR (95% CI) | Post-IRS IR (95% CI) |
|---|---|---|
| 0–3 years | 0.089 (.075–.106) | 0.010 (.007–.013) |
| 3–5 years | 0.084 (.071–.099) | 0.014 (.012–.018) |
| 5–11 years | 0.045 (.040–.052) | 0.010 (.008–.012) |
Abbreviations: CI, confidence interval; IR, incidence rates; IRS, indoor residual spraying.
aResults are based on the restricted baseline period of January 1, 2014 onwards.
bThe IRs were adjusted for sex, number of children in household, and the proportion of days in the interval that it rained. See Supplemental Tables 4–6 for coefficients of adjusted results.
Figure 3.
Adjusted incidence rate ratio (IRR) comparing post-indoor residual spraying (IRS) incidence to pre-IRS weekly incidence stratified by age group. All age groups experienced significant reductions in malaria incidence with the youngest age group (0–3 year olds) having the largest reductions. These results are based on the restricted baseline period of January 1, 2014 onwards. CI, confidence interval.
The findings of the sensitivity analyses found that household size (smaller vs larger) was not an effect modifier, the type of housing (traditional vs modern) was not a significant confounder across the study period, and adjusting for cumulative rainfall with a 2-month lag instead of proportion of rainy days at each interval yielded similar results to the primary analysis with the exception of IRS 1 versus pre-IRS having a nonstatistically significant IRR with cumulative rainfall (although proportion of rainy days was better for model fit). Finally, the results did not significantly differ with the use of the complete pre-IRS period compared with the restricted pre-IRS period from January 1, 2014 onwards.
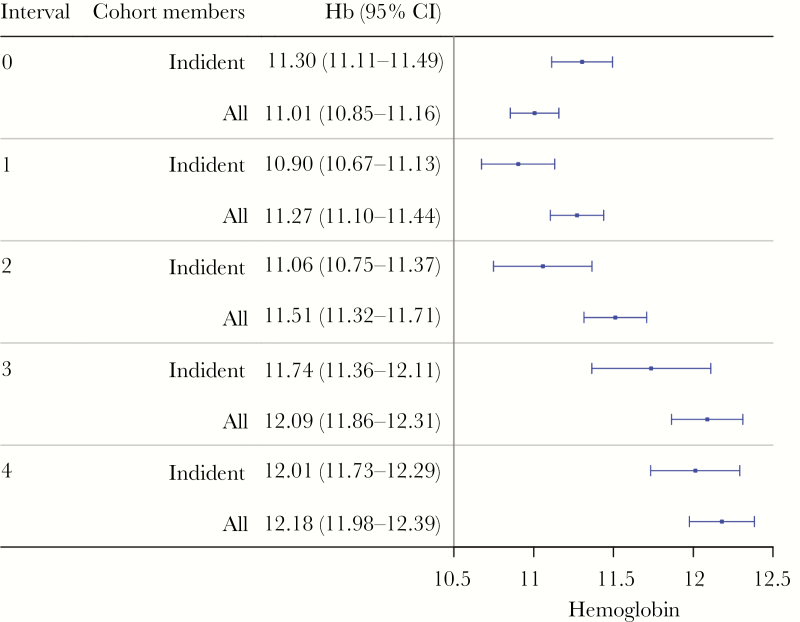
Hemoglobin levels were normally distributed in the study participants, and, on average, there were 38.9 (standard deviation = 22.13) hemoglobin measurements per week. Hemoglobin levels were 11.01 (95% CI, 10.85–11.16) g/dL before IRS activity and incrementally rose after each spraying period (Figure 4). Age was significantly and positively associated with hemoglobin levels. Parasite density was significantly lower in asymptomatic infections compared with incident infections and decreased with IRS activity (Table 3), whereas for incident episodes of malaria, the parasite density was considerably higher during the third and fourth round of IRS. Age was statistically and negatively associated with parasite density levels.
Figure 4.
Adjusted mean hemoglobin levels per indoor residual spraying (IRS) interval for incident and all cohort members. There were significant increases in hemoglobin levels in both groups, when comparing pre-IRS levels to IRS 4. These results are based on the restricted baseline period of January 1, 2014 onwards. CI, confidence interval.
Table 3.
Mean Parasite Density (µL) in Children With Asymptomatic Parasitemia and Incident Malaria Episodesa
| IRS Period | Asymptomaticb Parasitemia (µL) (95% CI) | Incident Episodes of Malariac (95% CI) |
|---|---|---|
| Pre-IRS | 1042 (792–1371) | 8352 (6527–10 688) |
| Post-IRS | 872 (699–1088) | 6814 (5290–8777) |
| IRS 1 | 1328 (971–1816) | 4919 (3481–6951) |
| IRS 2 | 675 (461–988) | 3512 (2138–5768) |
| IRS 3 | 561 (351–895) | 16 852 (9119–31 140) |
| IRS 4 | 709 (460–1093) | 13 858 (8928–21 511) |
Abbreviations: CI, confidence interval; IRS, indoor residual spraying.
aResults are based on the restricted baseline period of January 1, 2014 onwards.
bAsymptomatic defined as nonfebrile at routine visit with parasite density >0.
cIncident malaria defined as any new episode of laboratory-confirmed malaria not preceded by another episode of malaria in the prior 14 days.
DISCUSSION
Our study demonstrated that repeated IRS activity in a high-transmission setting in Uganda with high ITN coverage had a significant impact on malaria incidence and hemoglobin levels in children. The number of incident malaria episodes per person week after the implementation of IRS was reduced by 83%, and hemoglobin levels significantly increased by 10.6% by IRS round 4. In general, the largest reductions in malaria occurred within the first 6 to 9 weeks of IRS commencement with a rebounding of incidence occurring several weeks after the spraying round was completed.
The 2010 Cochrane review found that the evidence suggested IRS effectiveness in certain contexts although the evidence was very limited [3]. Since this review, there have been several studies that have examined the effectiveness of IRS relative to ITN coverage or to non-IRS areas or time periods. Four randomized control trials examined the combined benefit of ITN and IRS with the trials from Benin [5] and the Gambia [2], demonstrating little benefit of IRS, and the Tanzanian [4] and Sudanese [7] trials, showing significant reductions in malaria incidence with the addition of IRS. Two observational studies were conducted in Kenya with modest [20] and significant [21] reductions in malaria incidence in IRS districts compared with ITN only, respectively.
Low hemoglobin levels and the presence of anemia (hemoglobin level <11 g/dL) are common features in children in high-transmission settings, which also could be further aggravated by poor nutrition and intestinal helminths [22]. Small yet significant gains in hemoglobin levels were achieved during the IRS spraying period, which is critical for development, growth, and immune function. Despite decreases in parasite densities among individuals with asymptomatic infections, our results suggest significant increases in parasite densities among individuals with incident malaria infections after the third and fourth rounds of IRS. This finding may reflect some waning of antiparasite immunity in the population after 2 years of low exposure [23].
There are several limitations of the study, which may have influenced study findings. The main limitation of our study is that this was an observational study without an external control group [24]. During the study period, there were no changes to malaria control practice in Nagongera Subcounty such as the distribution of intermittent preventive treatment for children, improving the uptake of intermittent preventive treatment for pregnant women, or any vector habitat reduction campaigns, outside of the universal bednet campaign and IRS activities. One potential important residual confounder is ITN use, which was captured at clinic visits and based upon self-report from the parents or guardians of study children. All cohort children were reported to having slept under bednets throughout the study period, but it is known that this indicator is not an accurate reflection of actual use [25]. Actual ITN use may not have been constant during IRS, which was not captured in our study. In an ongoing cohort study in the same study area, reported use of ITNs the prior night among children 0.5–10 years of age was 96.4% when assessed at the study clinic but only 47.2% when assessed at home (G. D., written personal communication, July 29, 2019). Thus, it appears that ITN use may have been markedly overreported when assessed by study clinicians at the clinic. In addition, it should be noted that there were moderate to high levels of resistance found for pyrethroids (deltamethrin and permethrin) in the study area during the study period, which may have contributed to the limited changes in malaria incidence after the distribution of pyrethroid-based ITNs, as previously assessed [8].
We also lacked information on when housing characteristics changed, which could have resulted in confounding. Data on house construction were collected at 2 time points, although having multiple time points would improve the analysis and allow a more accurate determination of whether housing type modifies the effect of IRS on malaria incidence. There is an increasing body of research that demonstrates the importance of housing design for malaria transmission, and future work should further examine this association in rural eastern Uganda, as well in other rural and urban settings [26]. We also controlled for the effect of rainfall on incidence using interval-specific measures (eg, proportion of rainy days and cumulative total), which may not have captured more dynamic temporal influences of rainfall on incidence. This includes potential synergistic effects of rainfall and reduced residual efficacy of the insecticide, which would be better quantified with repeated measures of residual efficacy. Despite these limitations, the dose-response relationship, after adjusting for confounders, seen between the IRS rounds and malaria incidence provides critical support for our findings.
Understanding why IRS is effective in certain settings is paramount and requires more complete information on the context and intervention operations, which would improve our understanding of the underlying conditions that contribute towards its success or failure. There also needs to be further examination of how household size and housing characteristics modify the effects of IRS, which could then be used to inform targeted interventions. Crucially, there needs to be more cost-effective insecticides available for country vector control programs [27]. There has been a significant downscaling of several country IRS programs due to the substantial increase in insecticide cost, first caused by increased use of carbamates, followed by replacement with organophosphates. Finally, there needs to be complimentary research investigating the feasibility and effectiveness of environmental management, community mobilization, as well as housing improvements to sustainably reduce the malaria burden in endemic countries given the environmental and health concerns with the repeated use of insecticides [28, 29].
CONCLUSIONS
Our study supports the policy recommendation of IRS usage in a stable and perennial transmission area to rapidly reduce transmission, and it may be most effective in areas where ITN coverage or use is low and/or pyrethroid resistance is high [8]. The significant reductions in incidence coincided with high household coverage of IRS and the speed of completing a spraying round increased with each round, which was likely due to improvements in operational efficiency. The rebounding of malaria incidence occurred when there were delays between spraying, which was also observed in Northern Uganda when IRS was discontinued and resulted in a rapid increase in malaria morbidity to pre-IRS levels [9]. Asymptomatic infections can also act as an important reservoir of infection, which can reinitiate transmission and resurgence of malaria in low- and high-transmission settings [30, 31]. This work supports the recommendation of regular application of IRS in high-transmission settings with close monitoring for emerging resistance, until evidence is available on the best methods to maintain reductions in incidence through complementary approaches [32].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study team and the Makerere University-UCSF Research Collaboration and the Infectious Diseases Research Collaboration for administrative and technical support. We are grateful to the study participants who participated in this study and their families.
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases as part of the International Centers of Excellent for Malaria Research program (U19AI089674; to G. D.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: American Society of Tropical Medicine and Hygiene 67th Annual Meeting, October 28–November 1, 2018, New Orleans, LA.
References
- 1. World Health Organization. World malaria report 2018. Available at: https://www.who.int/malaria/publications/world-malaria-report-2018/en/. Accessed 22 March 2018.
- 2. Pinder M, Jawara M, Jarju LBS, et al. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet 2015; 385:1436–46. [DOI] [PubMed] [Google Scholar]
- 3. Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev 2010; CD006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. West PA, Protopopoff N, Wright A, et al. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med 2014; 11:e1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corbel V, Akogbeto M, Damien GB, et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis 2012; 12:617–26. [DOI] [PubMed] [Google Scholar]
- 6. Okumu FO, Moore SJ. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J 2011; 10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kafy HT, Ismail BA, Mnzava AP, et al. Impact of insecticide resistance in Anopheles arabiensison malaria incidence and prevalence in Sudan and the costs of mitigation. Proc Natl Acad Sci U S A 2017; 114:11267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katureebe A, Zinszer K, Arinaitwe E, et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med 2016; 13:e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raouf S, Mpimbaza A, Kigozi R, et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin Infect Dis 2017; 65:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamainza B, Sikaala CH, Moonga HB, et al. Incremental impact upon malaria transmission of supplementing pyrethroid-impregnated long-lasting insecticidal nets with indoor residual spraying using pyrethroids or the organophosphate, pirimiphos methyl. Malar J 2016; 15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharp BL, Kleinschmidt I, Streat E, et al. Seven years of regional malaria control collaboration Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg 2007; 76:42–7. [PMC free article] [PubMed] [Google Scholar]
- 12. Wagman J, Gogue C, Tynuv K, et al. An observational analysis of the impact of indoor residual spraying with non-pyrethroid insecticides on the incidence of malaria in Ségou Region, Mali: 2012–2015. Malar J 2018; 17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kigozi R, Baxi SM, Gasasira A, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One 2012; 7:e42857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steinhardt LC, Yeka A, Nasr S, et al. The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda. Am J Trop Med Hyg 2013; 88:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talisuna AO, Noor AM, Okui AP, Snow RW. The past, present and future use of epidemiological intelligence to plan malaria vector control and parasite prevention in Uganda. Malar J 2015; 14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Aeronautics and Space Administration. Precipitation and Processing System. Available at: https://pps.gsfc.nasa.gov/. Accessed 14 November 2018.
- 17. Killick R, Eckley I. Changepoint: an R package for changepoint analysis. J Stat Soft 2014; 58:1–19. [Google Scholar]
- 18. Lu K, Mehrotra DV. Specification of covariance structure in longitudinal data analysis for randomized clinical trials. Stat Med 2010; 29:474–88. [DOI] [PubMed] [Google Scholar]
- 19. Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics 2002; 58:1–12. [DOI] [PubMed] [Google Scholar]
- 20. Zhou G, Afrane YA, Dixit A, et al. Modest additive effects of integrated vector control measures on malaria prevalence and transmission in western Kenya. Malar J 2013; 12:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gimnig JE, Otieno P, Were V, et al. The effect of indoor residual spraying on the prevalence of malaria parasite infection, clinical malaria and anemia in an area of perennial transmission and moderate coverage of insecticide treated nets in Western Kenya. PLoS One 2016; 11:e0145282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedman JF, Kwena AM, Mirel LB, et al. Malaria and nutritional status among pre-school children: results from cross-sectional surveys in western Kenya. Am J Trop Med Hyg 2005; 73:698–704. [PubMed] [Google Scholar]
- 23. Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, et al. Quantification of anti-parasite and anti-disease immunity to malaria as a function of age and exposure. Elife 2018; 7:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson AL, Boelaert M, Kleinschmidt I, et al. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol 2015; 31:380–90. [DOI] [PubMed] [Google Scholar]
- 25. Zinszer K, Stone J, Mpaata JC, et al. Success and failure: a firsthand look into Uganda's most recent bednet distribution campaign. Lancet Infect Dis 2017; 17:251–3. [DOI] [PubMed] [Google Scholar]
- 26. Rek JC, Alegana V, Arinaitwe E, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health 2018; 2:e83–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oxborough RM. Trends in US President's Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J 2016; 15:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eskenazi B, Chevrier J, Rosas LG, et al. The Pine River statement: human health consequences of DDT use. Environ Health Perspect 2009; 117:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen MRH, Jørs E, Lander F, et al. Neurological deficits after long-term Pyrethroid exposure. Environ Health Insights 2017; 11:117863021770062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 2013; 11:623–39. [DOI] [PubMed] [Google Scholar]
- 31. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nature 2014; 12:833–40. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization. Indoor residual spraying: an operational manual for IRS for malaria transmission control and elimination 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/177242/9789241508940_eng.pdf;jsessionid=F169E0D1EFFE8161A0B041EDBE03536B?sequence=1. Accessed 17 November 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.