Abstract
Collective decision-making is predicted to be more egalitarian in conditions where the costs of group fission are higher. Here, we ask whether Trinidadian guppies (Poecilia reticulata) living in high or low predation environments, and thereby facing differential group fission costs, make collective decisions in line with this prediction. Using a classic decision-making scenario, we found that fish from high predation environments switched their positions within groups more frequently than fish from low predation environments. Because the relative positions individuals adopt in moving groups can influence their contribution towards group decisions, increased positional switching appears to support the prediction of more evenly distributed decision-making in populations where group fission costs are higher. In an agent-based model, we further identified that more frequent, asynchronous updating of individuals' positions could explain increased positional switching, as was observed in fish from high predation environments. Our results are consistent with theoretical predictions about the structure of collective decision-making and the adaptability of social decision-rules in the face of different environmental contexts.
Keywords: consensus, coordination, information, Poecilia reticulata
1. Introduction
Collective decisions involve individuals in groups combining their own imperfect estimates of the world around them to reach consensuses about travel directions, activities or choices while, at the same time, remaining cohesive [1]. In many cases, if animals are to benefit from such information sharing, they should distribute decision-making evenly between group members [1]. However, because conflict exists in groups, where individuals have to balance the need for social cohesion with that of their own goal-oriented behaviour [1–4], some individuals may disproportionally influence the decision-making process, through either active or passive mechanisms.
Theoretical models suggest that the degree to which decision-making is shared between group members is influenced by both environmental and social conditions [5,6]. In environments where the benefits of remaining with other group members outweigh any potential ‘consensus costs’, that is, costs of following others' decisions, then equally shared decision-making is more likely to evolve [7,8]. Unshared decision-making, on the other hand, is more likely to evolve when consensus costs are relatively high compared with the benefits of social cohesion [7,8]. Importantly, under both these scenarios, the observed outcome of decision-making can often be the same, where groups remain cohesive despite consensus being reached by relatively shared or unshared decision-making processes.
Investigating these theoretical predictions requires an experimental system where either the consensus costs or group cohesion costs differ between populations, and the degree to which decisions are shared or unshared can be approximated. The Trinidadian guppy (Poecilia reticulata) offers one such system. Populations of guppies in the Northern Mountain range of Trinidad have been exposed to either relatively high or relatively low levels of predation over both their evolutionary and ontogenetic histories [9,10]. Because group cohesion significantly reduces predation risk [11,12], this system offers an opportunity to assess whether group decision-making appears more or less shared between group members in populations where the costs of group fragmentation differ. Here, we give groups of guppies a classic decision-making paradigm [13,14], where groups choose to swim down one of two arms of a Y-maze. We tested multiple group sizes to assess whether the patterns observed were robust to differences in group size. Because positions at the front of groups are more conducive of leadership, and in many animal groups information flows from the front to the back of groups, [15–17], positional changes within groups appear to be informative about who is disproportionally influencing the decision-making process [13,18]. We therefore calculated the number of times individuals switched positions within the group before they reached a decision, with increased positional switching acting as a proxy for more distributed decision-making. Furthermore, using a simple one-dimensional model, we explored how differences in how individuals moved might result in different amounts of positional switching within groups.
2. Material and methods
(a). Experimental methods
Adult female guppies (P. reticulata) were caught from four locations with high predation risk (Arima, Lower Guanapo, Lower Lopinot and Tacarigua rivers) and four locations with low predation risk (Paria, Upper Guanapo, Upper Lopinot and Upper Turure rivers) in July 2013. High predation sites contain Crenicichla frenata, Hoplias malabaricus or Aequidens pulcher which prey on adult guppies, whereas these predators are largely absent from low predation sites, although low predation sites do contain Rivulus hartii which prey on juvenile guppies [9,18].
Fish were transported back to the University of the West Indies, St Augustine Campus, where they were housed in 120 cm diameter circular holding pools (approx. 90 fish per pool) in an outdoor enclosure that was shaded between 08.00 and 14.00 h (when trials were run). Water depth in the pools was maintained between 10 and 13 cm, and the pools were emptied, rinsed and refilled between stocking fish from different populations. We suspended a clear polythene sheet over the housing pools and test arena throughout the study to stop rain falling in the pools.
For each trial, groups of two, four or eight fish with approximately the same body length were caught from the housing pools and placed into a 15 × 15 cm transparent plastic box at the end of the stem of a Y-maze (stem 15 cm wide, 71 cm long; figure 1a). Following 2 min of acclimation, the box was remotely lifted, allowing the shoals to explore the novel maze environment. Groups swam down the stem of the Y-maze before deciding to swim into the left or right arm of the maze. Trials were filmed with a Canon 550D DSLR camera mounted 1.25 m above the maze at 25 fps and a resolution of 1920 × 1080 pixels. We tested group sizes of two (n = 77), four (n = 76) or eight (n = 77) fish, with each group size being tested once in a block of three trials, and the order of testing randomized within each block. Fish were never used in more than one trial. We used automated tracking software [19] to track the positions and orientations of fish as they made a decision. In particular, we measured the number of times the group did not reach a consensus (defined when at least two group members chose different arms of the Y-maze to swim down), the mean speed of fish, their cohesion (median distance of group members to the group's centroid), the number of times they switched position (see Results) and the number of movement decisions fish made per second (see Results). All measures were calculated from the time a fish entered the blue region in figure 1a until a fish crossed into one of the arms of the Y-maze (dashed white lines in figure 1a). Group cohesion was only measured during times when all group members were simultaneously tracked. All measures were analysed using linear or generalized linear mixed models (see electronic supplementary material for further details). All models included predation regime (high or low), group size and the mean body size of fish (standard length measured from stills in the videos) in each group as fixed effects. As expected, fish from high predation populations were significantly smaller (2.02 ± 0.48 cm, mean ± s.d.) than fish from low predation populations (2.29 ± 0.36 cm, mean ± s.d.; linear mixed model, likelihood ratio test (LRT): 28.97, p < 0.001), making body size an important covariate in our models. Population was included as a random effect in all models. The significance of each term within the models was tested using LRTs to compare models with and without the term of interest. All statistical analyses were carried out in R v.3.1.2, and data are available in the electronic supplementary material.
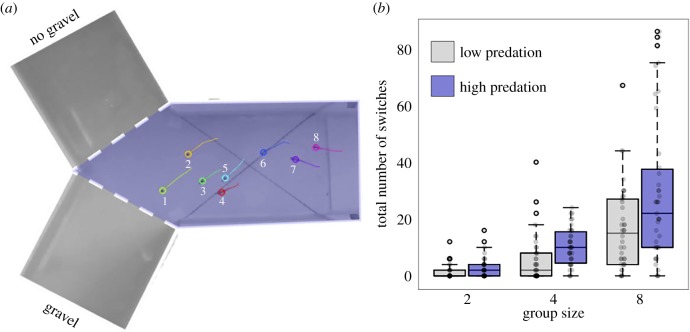
Figure 1.
(a) The experimental Y-maze. Tracking is superimposed on a frame for one of the trials of eight fish. The numbers next to each fish represent their positional ranks within the group on that frame. The left arm of the Y-maze contained a gravel patch (off-screen), while the right arm contained no patch. This was designed to create an asymmetric choice. (b) Boxplots of the total number of times individuals switched position in the group. Raw data points are shown as grey circles. The central line on each box depicts the median, and the top and bottom edges of each box represent the 25th and 75th percentiles. Whiskers extend to data points not considered outliers.
3. Results
The proportion of groups that split apart during the decision-making process did not differ between the two predation regimes (LRT = 1.61, p = 0.20; only 33/231 groups split). Furthermore, fish from the different predation regimes did not differ in their median swim speeds as they made these decisions (LRT = 0.42, p = 0.52). Groups of fish from high and low predation environments, therefore, made similarly fast and cohesive decisions.
We next investigated whether individuals within groups from different predation regimes contributed to the consensus decisions more or less equally. To measure this, fish were ranked from 1 to n as they swam down the stem of the Y-maze (shaded blue region in figure 1a), with fish at the front of the group given a ranking of 1 and the fish at the back of the group, n (figure 1a). We then calculated the number of times these ranks changed in the times leading up to the final decision (when the first fish crossed a dashed line in figure 1a). Note that if a pair of fish switched their positions, this was counted as two switches, and we controlled for potential differences in cohesion between the populations by including cohesion as a covariate in the models. Fish from high predation environments switched position more often than fish from low predation environments (LRT = 5.12, p = 0.024; figure 1b), and as expected, larger groups also made more switches than smaller groups (LRT = 122.8, p < 0.001; figure 1b). These effects were also observed when considering only switches that occurred at the front position of the group (predation: LRT = 7.07, p < 0.01; group size: LRT = 20.28, p < 0.001).
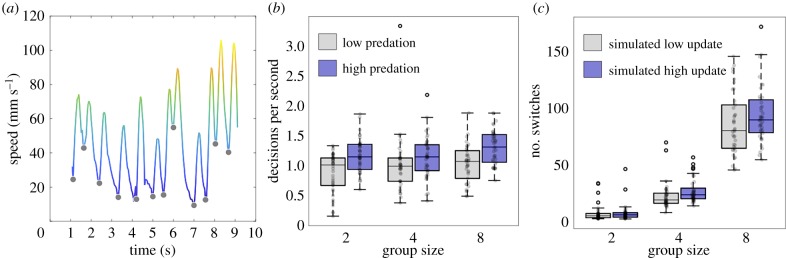
We then investigated the potential mechanism for how fish from high-predation environments made more switches in positions than fish from low predation environments. Guppies, as in many other species of fish, move with intermittent changes in speed, which can be thought of as movement decisions [20]. We identified the number of movement decisions that fish made per second by identifying the times when fish's speeds were at a minimum (see grey markers in figure 2a). After controlling for the effects of median speed (LRT = 197.6, p < 0.001) and body size (LRT = 22.1, p < 0.001), fish from high predation environments still made more decisions per second than fish from low predation environments (LRT = 4.31, p = 0.038; figure 2b).
Figure 2.
(a) Example speed profile of a fish as it moved through the Y-maze. Grey markers represent times when the speed profile has local minima, indicating times immediately before the fish made a decision to move. (b) Boxplot of the number of decisions fish made per second as a function of group size and low (grey) or high (blue) predation environments. (c) Results of the simulation where each point represents the average switches a group made out of 100 simulation runs. Simulations were given two update frequencies: low (grey) or high (blue), respectively, matching the update frequency of fish from low or high predation environments. The central line on each box depicts the median, and the top and bottom edges of each box represent the 25th and 75th percentiles. Whiskers extend to data points not considered outliers.
To test whether differences in the rate at which fish updated their position could explain differential switching behaviour between the populations, we built a simple one-dimensional self-propelled particle model capturing the dynamics of guppies' movements. On each time step, agents updated their position along an one-dimensional world with a probability, p, that was determined by the mean update frequency of fish in either low (p = 0.0368) or high (p = 0.0463) predation environments (figure 2b). If fish updated their position, they moved for a uniformly randomly determined distance in the range, 0–d (where d > 0). The only social interaction we implemented was an attraction rule to neighbours behind a focal individual, that is, if the focal individual was in front of its closest follower by more than d, it did not update its position. One hundred simulation runs were performed for the same relative number of time steps it took fish to make the decision for each experimental trial (n = 231 × 100). This simple model captured the switching rates observed in the experimental trials, with agents with higher update probabilities switching position more often than agents with low update probabilities (figure 2c).
4. Discussion
Groups of fish from high predation environments switched positions more often, and made more movement decisions per second, than fish from low predation environments. In a simple agent-based model, the increased frequency of asynchronous movement decisions was associated with this increased positional switching. These results are consistent with theoretical predictions that collective decision-making is more equally shared between group members in environments where the costs of group fission are higher [7,8].
Oscillations in speed and switching of positions are thought to break visual occlusion between group members, thereby facilitating the more efficient spread of information through groups [21]. Mechanisms that promote the likelihood that multiple individuals contribute towards detecting and sharing information about potential sources of risk, therefore, might be favoured in environments where those threats are higher. Indeed, such mechanisms could allow the collective pooling of information and the emergence of swarm intelligence [22], especially when information collected by group members is uncorrelated [23,24]. While, in our model, increased asynchronous movements could explain increased positional switching, more frequent movements are also likely to be coupled with increased energetic requirements. This may explain why increased positional switching may not be adopted in environments where information sharing might be less important, such as when predation risk is relatively lower.
While we interpret our results in the context of decision-making, it is important to consider other mechanisms that could contribute towards increased positional switching in high compared with low predation environments. Higher sensitivity to risk [25], swimming performance [26] or trade-offs in occupying rewarding yet risky positions in groups [12] may contribute towards increased positional switching in high compared with low predation environments. While these factors are not mutually exclusive from more or less distributed decision-making processes, future work should attempt to control for these factors when investigating the importance of positional switching during decision-making. Our work suggests, however, that populations have intrinsic differences in the degree to which decision-making is shared between group members, and this could be ultimately shaped by differences in the ecological conditions that these populations experience.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Kharran Deonarinesingh and Dr Matthew Edenbrow for support and helpful discussions, and Dr Kurvers and two anonymous reviewers for their critiques of our work.
Ethics
All procedures were approved by the University of Bristol Ethical Review Group (UIN 13/028).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org.10.5061/dryad.nvx0k6dn6 [27].
Authors' contributions
J.E.H.-R., A.S.I.W. and C.C.I. contributed to the conception and design of experiments. A.S.I.W. and C.C.I. contributed to the acquisition of data. J.E.H.-R., A.S.I.W., I.W.R. and C.C.I. contributed to the analysis and interpretation of data. All authors contributed intellectual content to drafting the article and revising it critically. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
The authors declare they have no conflicts of interest.
Funding
This work was supported by the Natural Environment Research Council grants nos NE/K009370/1 and NE/P012639/1 awarded to C.C.I. and a Swedish Research Council grant no. 2018-04076 awarded to J.E.H.-R.
References
- 1.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 2.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155 ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 3.Conradt L, List C. 2008. Group decisions in humans and animals: a survey. Phil. Trans. R. Soc. B 364, 719–742. ( 10.1098/rstb.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan PA, Gosetto I, Jenkins ER, Barnes I, Ioannou CC. 2018. Regulation between personality traits: individual social tendencies modulate whether boldness and leadership are correlated. Proc. R. Soc. B 285, 20180829 ( 10.1098/rspb.2018.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone RA, Manica A. 2011. Evolution of personality differences in leadership. Proc. Natl Acad. Sci. USA 108, 8373–8378. ( 10.1073/pnas.1102191108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf M, Van Doorn GS, Weissing FJ. 2008. Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl Acad. Sci. USA 105, 15 825–15 830. ( 10.1073/pnas.0805473105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conradt L, Roper TJ. 2008. Conflicts of interest and the evolution of decision sharing. Phil. Trans. R. Soc. B 364, 807–819. ( 10.1098/rstb.2008.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conradt L. 2011. Models in animal collective decision-making: information uncertainty and conflicting preferences. Interface Focus 2, 226–240. ( 10.1098/rsfs.2011.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Seghers BH. 1974. Schooling behavior in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution 28, 486–489. ( 10.1111/j.1558-5646.1974.tb00774.x) [DOI] [PubMed] [Google Scholar]
- 11.Ioannou CC, Guttal V, Couzin ID. 2012. Predatory fish select for coordinated collective motion in virtual prey. Science 337, 1212–1215. ( 10.1126/science.1218919) [DOI] [PubMed] [Google Scholar]
- 12.Ioannou CC, Rocque F, Herbert-Read JE, Duffield C, Firth JA. 2019. Predators attacking virtual prey reveal the costs and benefits of leadership. Proc. Natl Acad. Sci. USA 116, 8925–8930. ( 10.1073/pnas.1816323116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns AL, Herbert-Read JE, Morrell LJ, Ward AJ. 2012. Consistency of leadership in shoals of mosquitofish (Gambusia holbrooki) in novel and in familiar environments. PLoS ONE 7, e36567 ( 10.1371/journal.pone.0036567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward AJ, Herbert-Read JE, Sumpter DJ, Krause J. 2011. Fast and accurate decisions through collective vigilance in fish shoals. Proc. Natl Acad. Sci. USA 108, 2312–2315. ( 10.1073/pnas.1007102108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettit B, Ákos Z, Vicsek T, Biro D. 2015. Speed determines leadership and leadership determines learning during pigeon flocking. Curr. Biol. 25, 3132–3137. ( 10.1016/j.cub.2015.10.044) [DOI] [PubMed] [Google Scholar]
- 16.Nagy M, Akos Z, Biro D, Vicsek T. 2010. Hierarchical group dynamics in pigeon flocks. Nature 464, 890 ( 10.1038/nature08891) [DOI] [PubMed] [Google Scholar]
- 17.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJ, Ward AJ. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–18 731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannou CC, Ramnarine IW, Torney CJ. 2017. High-predation habitats affect the social dynamics of collective exploration in a shoaling fish. Sci. Adv. 3, e1602682. ( 10.1126/sciadv.1602682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. 2009. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451 ( 10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert-Read JE, et al. 2017. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B 284, 20171126 ( 10.1098/rspb.2017.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain DT, Couzin ID, Leonard NE. 2015. Coordinated speed oscillations in schooling killifish enrich social communication. J. Nonlinear Sci. 25, 1077–1109. ( 10.1007/s00332-015-9263-8) [DOI] [Google Scholar]
- 22.Ioannou CC. 2017. Swarm intelligence in fish? The difficulty in demonstrating distributed and self-organised collective intelligence in (some) animal groups. Behav. Process. 141, 141–151. ( 10.1016/j.beproc.2016.10.005) [DOI] [PubMed] [Google Scholar]
- 23.Kao AB, Couzin ID. 2014. Decision accuracy in complex environments is often maximized by small group sizes. Proc. R. Soc. B 281, 20133305 ( 10.1098/rspb.2013.3305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao AB, Couzin ID. 2019. Modular structure within groups causes information loss but can improve decision accuracy. Phil. Trans. R. Soc. B 374, 20180378 ( 10.1098/rstb.2018.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botham MS, Hayward RK, Morrell LJ, Croft DP, Ward JR, Ramnarine I, Krause J. 2008. Risk-sensitive antipredator behavior in the Trinidadian guppy, Poecilia reticulata. Ecology 89, 3174–3185. ( 10.1890/07-0490.1) [DOI] [PubMed] [Google Scholar]
- 26.Svendsen JC, Banet AI, Christensen RH, Steffensen JF, Aarestrup K. 2013. Effects of intraspecific variation in reproductive traits, pectoral fin use and burst swimming on metabolic rates and swimming performance in the Trinidadian guppy (Poecilia reticulata). J. Exp. Biol. 216, 3564–3574. ( 10.1242/jeb.083089) [DOI] [PubMed] [Google Scholar]
- 27.Herbert-Read J, Wade A, Ramnarine I, Ioannou C. 2019. Data from: Collective decision-making appears more egalitarian in populations where group fission costs are higher, v3 Dryad Digital Repository. ( 10.5061/dryad.nvx0k6dn6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Herbert-Read J, Wade A, Ramnarine I, Ioannou C. 2019. Data from: Collective decision-making appears more egalitarian in populations where group fission costs are higher, v3 Dryad Digital Repository. ( 10.5061/dryad.nvx0k6dn6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org.10.5061/dryad.nvx0k6dn6 [27].