Abstract
Sampling reservoir hosts over time and space is critical to detect epizootics, predict spillover and design interventions. However, because sampling is logistically difficult and expensive, researchers rarely perform spatio-temporal sampling of many reservoir hosts. Bats are reservoirs of many virulent zoonotic pathogens such as filoviruses and henipaviruses, yet the highly mobile nature of these animals has limited optimal sampling of bat populations. To quantify the frequency of temporal sampling and to characterize the geographical scope of bat virus research, we here collated data on filovirus and henipavirus prevalence and seroprevalence in wild bats. We used a phylogenetically controlled meta-analysis to next assess temporal and spatial variation in bat virus detection estimates. Our analysis shows that only one in four bat virus studies report data longitudinally, that sampling efforts cluster geographically (e.g. filovirus data are available across much of Africa and Asia but are absent from Latin America and Oceania), and that sampling designs and reporting practices may affect some viral detection estimates (e.g. filovirus seroprevalence). Within the limited number of longitudinal bat virus studies, we observed high heterogeneity in viral detection estimates that in turn reflected both spatial and temporal variation. This suggests that spatio-temporal sampling designs are important to understand how zoonotic viruses are maintained and spread within and across wild bat populations, which in turn could help predict and preempt risks of zoonotic viral spillover.
Keywords: Chiroptera, spillover, sampling design, zoonotic virus, phylogenetic meta-analysis, spatio-temporal
1. Introduction
Risks of pathogen spillover vary across time and space [1,2], in part because pathogen shedding from reservoir hosts is a dynamic spatio-temporal processes [3,4]. Metapopulation dynamics and other spatial processes characterize many reservoir hosts [5], where populations connectivity can determine the spatio-temporal distribution of a pathogen [6,7] and degree of spatial synchrony structuring infection dynamics [8]. Temporal pulses of shedding driven by seasonality in birth and climate are also common [9,10]. Understanding how infection prevalence in reservoir hosts varies over space and time is thus a critical need for predicting and managing zoonotic disease risks.
However, surveillance strategies often do not sample this underlying spatio-temporal process, as spatially and temporally explicit designs present logistical challenges when studying mobile and gregarious species [3,11,12]. For hosts such as birds and bats, surveillance is often opportunistic or relies on convenience sampling [13]. These non-probabilistic and often single sampling events cannot characterize spatial and temporal fluctuations in infection, can over- or under-represent times or locations of high prevalence, and can result in non-randomly missing data [3,14]. These challenges cannot be fixed with statistical modelling and can bias estimates of prevalence and epidemiological parameters such as the basic reproductive number [13,15].
Given a fixed cost, difficult decisions must be made about how to allocate sampling efforts. Sampling over space facilitates detecting geographical clusters of disease and predictive mapping [16,17], while sampling over time can identify periods of intensive pathogen shedding and enable inference about dominant transmission routes [18,19]. Researchers often treat this as a trade-off between sampling over either time or space, rather than allocating effort to both [20]. Implicit here is that the temporal component is constant over space or that the spatial component is constant over time, and such sampling designs result in no data to assess this assumption.
We here quantify the temporal and spatial data limitations for two taxa of high-profile viruses of bats: the family Filoviridae and genus Henipavirus. Bats have been widely studied as reservoirs for zoonotic pathogens and host more viruses with zoonotic potential than other mammals [21,22]. Some henipaviruses and filoviruses (e.g. Marburg virus) can be shed from bats into the environment [23,24] and can cause fatal disease in humans by environmental exposure or from contact with intermediate hosts such as horses, wild primates or pigs [25–30]. Many filo- and henipaviruses show variable dynamics in space and time, including shedding pulses from bats [6,25,31–33], which implies spatio-temporal sampling are likely necessary to capture viral dynamics in bats. Yet while past efforts have focused on bat virus discovery [34], determinants of reservoir status [35] and experimental mechanisms of viral transmission [36], spatio-temporal studies of the bat–virus dynamics are rare [37]. This limits understanding how viruses are maintained and spread within and across bat populations and impairs improving future sampling designs and ecological interventions [20,38].
We here systematically collated data on filo- and henipavirus prevalence and seroprevalence in wild bats to (i) quantify the frequency of temporal studies and (ii) assess the geographical scope of current research. We used phylogenetic meta-analysis to (iii) quantify how sampling designs and reporting practices may influence viral detection estimates. Single snapshots could miss pulses of viral shedding from bats, whereas pooling data over time could under- or overestimate viral presence [18,20]. Lastly, we (iv) characterized the degree of temporal and spatial variation in bat virus detection estimates.
2. Methods
To systematically identify studies quantifying the proportion of wild bats positive for filoviruses and henipaviruses using PCR or serology, we searched Web of Science, CAB Abstracts and PubMed (see electronic supplementary material, figure S1). Our dataset included 1177 records from 68 studies. Viruses included not only Hendra, Nipah, Ebola and Marburg virus but also Lloviu and Reston virus. We grouped viruses by taxa given our sample sizes and known issues of serological cross-reactivity [39,40].
From each study, we defined sampling subunits: a temporally defined sampling event of one bat species in one location per viral detection estimate. Each subunit is the lowest spatial, temporal and phylogenetic scale (of bats and their viruses) reported. We classified subunits into three sampling designs and reporting practices: one sampling event, multiple events or pooled events over time. Records of a single prevalence or seroprevalence estimate from a population sampled from a period less than or equal to one month were classified as single sampling events, whereas records of a population over multiple monthly time points were classified as spanning multiple events (i.e. a longitudinal study). For example, every monthly prevalence estimate per population of Pteropus lylei in Thailand would represent a unique subunit and be classified as longitudinal [41]. Records of a period longer than one month were classified as pooled events, where researchers may have sampled a population across more than one time point but reported data as a single viral detection estimate. A schematic of these categorizations is provided in figure 1a. One month was selected because this time frame was the lowest common temporal unit and because bat shedding of these viruses can occur within a month [36,42]. These data were reported for most records (1122/1177 subunits; three publications did not report these data and three additional publications did not always report such data for all records). For each subunit, we also recorded the bat species, virus taxon, coarse detection method (i.e. PCR or serology), number of bats sampled, proportion of bats positive, sampling time points, sampling location and country (recoded to the United Nations geoscheme for our descriptive analyses).
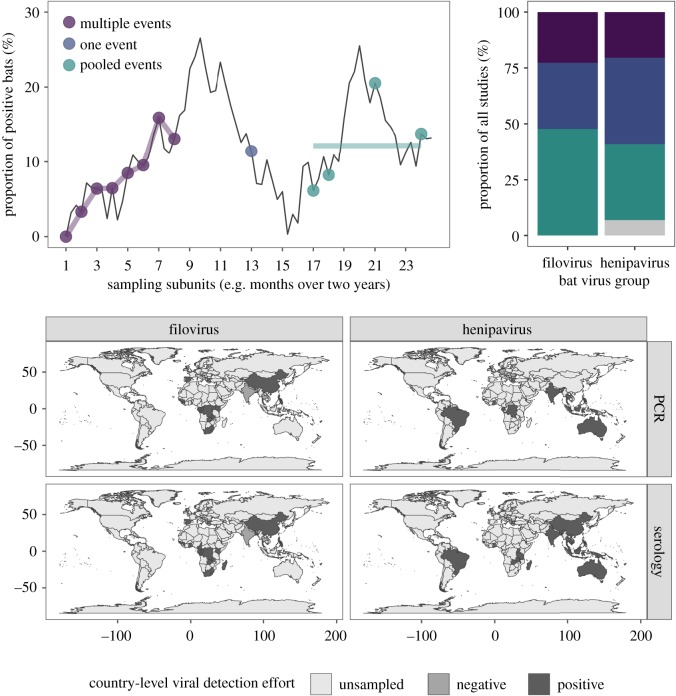
Figure 1.
Top: conceptual schematic of how different sampling designs and reporting practices (coloured points and lines) capture the underlying temporal dynamics of infection (black line), followed by observed proportions for studies of bat filoviruses and henipaviruses (grey shows the proportion of studies not reporting these data). Bottom: countries sampled for bat filoviruses and henipaviruses and where wild bats have been found positive through PCR or serology. (Online version in colour.)
We quantified the proportion of studies using each sampling and reporting design, both across all data and stratified by virus taxon. To assess how the frequency of longitudinal studies (i.e. those with repeated sampling) has changed over time, we fit a generalized additive model with the mgcv package in R and a smooth term for publication year [43]. We also calculated the duration of repeat sampling for these longitudinal studies. For studies that pooled data over time, we quantified days represented per subunit. To describe geographical biases in bat virus studies, we assessed sampling gaps according to the region (United Nations geoscheme). We used a χ2 test to assess if sampling designs and reporting practices were differently distributed across regions.
To assess the contribution of sampling designs and reporting practices to viral detection estimates and to quantify the degree of spatial and temporal variation in bat–virus interactions, we used the metafor package to calculate logit-transformed proportions and sampling variances and to fit hierarchical meta-analysis models [44,45]. To account for phylogenetic dependence, we included bat species as a random effect [46], for which the covariance structure used the phylogenetic correlation matrix; we obtained our phylogeny from the Open Tree of Life with the rotl and ape packages [47,48]. We excluded subunits that pooled data across or within bat genera (n = 102). As a small number of subunits (n = 14) pooled data across specified species in a genus, we randomly selected one species to retain these records. Our final dataset included 1075 subunits from 63 studies and 219 bat species (electronic supplementary material, figure S2). Our models also included subunit nested within the study as a random effect and weighting by sampling variances. To first assess heterogeneity among all viral detection estimates, we fit a random-effects model (REM; intercept only) and then stratified this analysis per viral taxon and detection method. We used restricted maximum likelihood to obtain unbiased estimates of the variance components, from which we derived I2 to quantify the contribution of true heterogeneity to the total variance in viral detection estimates [49]. We used these estimates to partition variance attributed to each random effect; in the case of bat species, we derived phylogenetic heritability (H2) as a measure of phylogenetic signal [46]. We used Cochran's Q to test if such heterogeneity was greater than expected by sampling error alone [50].
To next test how sampling designs and reporting practices may influence viral detection estimates (n = 1020), we fit a mixed-effects model (MEM) with the same random effects and an interaction between sampling design and reporting practices, detection method and virus taxon. We tested the significance of moderators using the Q test [44] and derived a pseudo-R2 as the proportional reduction in the summed variance components compared with those of an REM [51].
To test if viral detection estimates showed spatio-temporal variation, we fit models with the same random effects to our data subset reporting multiple events (n = 273). We fit a REM to quantify I2 for longitudinal studies. We then fit MEMs with location and month as univariate moderators to test if viral detection estimates varied across space and time. Because this subset of the data included many unique locations (n = 28) and months (n = 12), we did not use interaction terms and instead fit an additional set of MEMs to each viral taxon–detection method strata.
3. Results
Only 26% of bat virus studies reported data longitudinally (10 filo- and nine henipavirus studies; figure 1). However, the frequency of such studies has weakly increased over time (electronic supplementary material, figure S3, p = 0.1). Eleven studies reported sampling populations two to three times while 12 reported sampling populations over four times. The duration of longitudinal studies ranged from 150 days to over 10 years, on average spanning 2.5 years of repeat sampling (electronic supplementary material, figure S4). By contrast, half of our studies (n = 35) instead reported estimates across multiple time points as pooled proportions, which on average represented 643 days of temporally aggregated data (s.d. = 492; electronic supplementary material, figure S5).
Bat sampling showed geographical biases (figure 1 and table 1). Filovirus studies were conducted across much of Africa and Asia but not in Latin America and Oceania. PCR and serology have been used in the same region in most areas, but only one or the other have been used in Europe, Eastern and Middle Africa, and Eastern Asia for henipaviruses (table 1). Geography was also associated with sampling design and reporting (χ2 = 365, p = 0.001). Longitudinal data were only reported from Central, Eastern, Middle and Southern Africa for filoviruses and from Southeastern Asia, Eastern Africa and Oceania for henipaviruses (table 1).
Table 1.
Summary of the temporal and spatial limitations for bat filovirus and henipavirus prevalence and seroprevalence data. Some studies had multiple diagnostic methods, sampling designs and reporting methods. Diagnostic mismatch refers to geographical regions (United Nations geoscheme) where either PCR or serology have been used (but not together).
| longitudinal virus studies | geographical sampling gaps | diagnostic mismatch | regions with longitudinal data | ||
|---|---|---|---|---|---|
| filoviruses | PCR | 5/20 | Latin America, Oceania | Central Africa, Eastern Africa | |
| serology | 7/25 | Central Africa, Eastern Africa, Middle Africa, Southern Africa | |||
| henipaviruses | PCR | 4/13 | Eastern Africa, Southern Africa, Eastern Asia | Europe, Eastern Africa, Middle Africa, Eastern Asia | Southeastern Asia, Oceania |
| serology | 5/27 | Middle Africa, Southern Africa, Europe | Southeastern Asia, Oceania, Eastern Africa |
We observed significant heterogeneity across viral detection estimates (I2 = 0.90, Q1074 = 7115, p < 0.001). Bat species and study accounted for most variation ( H2 = 0.40; electronic supplementary material, table S1). We also found significant heterogeneity within each viral taxon–detection strata, although I2 and H2 values varied across these subsets (electronic supplementary material, table S1). Viral detection estimates for henipaviruses had much stronger phylogenetic signal than filoviruses.
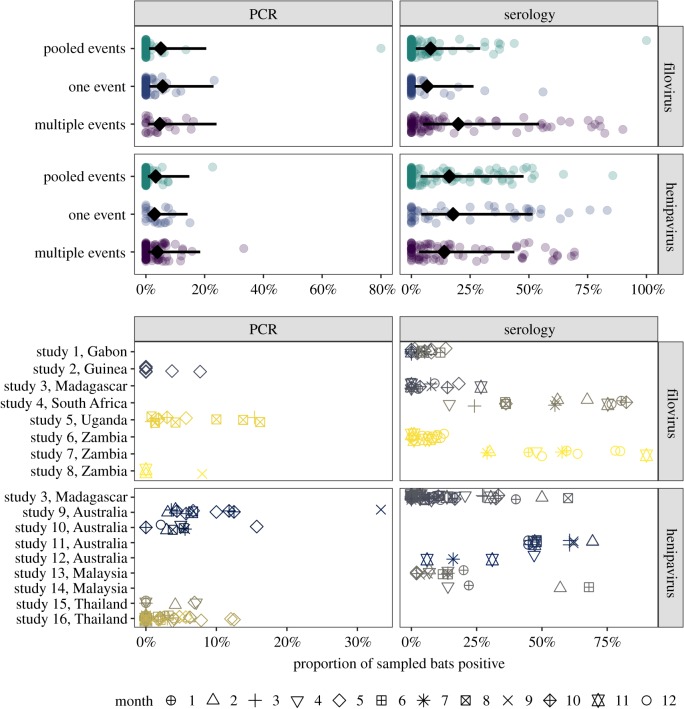
Our MEM showed that viral detection estimates varied with detection method and virus taxa (Q1 = 7.75, p < 0.01; seroprevalence was higher than prevalence, especially for henipaviruses) and that associations with sampling design and reporting weakly depended upon both virus taxa and detection method (three-way interaction: Q2 = 5.95, p = 0.05, R2 = 0.02; electronic supplementary material, table S2). A post hoc analysis with MEMs fit to each stratum showed sampling design and reporting were associated primarily with filovirus seroprevalence (Q2 = 10.84, p = 0.01, R2 = 0.11; figure 2), with longitudinal studies showing higher proportions of positive bats. Sampling design and reporting had no effects on henipavirus seroprevalence and weak effects on henipavirus prevalence (electronic supplementary material, table S3).
Figure 2.
Top: influence of sampling designs and reporting practices on virus detection estimates. Points show proportions of positive bats per subunit; lines and diamonds display back-transformed predicted means and 95% confidence intervals from the MEM. Some overlap in 95% confidence intervals does not imply lack of statistical difference in mean estimates [52]. Bottom: spatio-temporal variation in viral detection estimates for longitudinal studies. Points represent subunit virus detection estimates and are coloured by locations and shaped by month. (Online version in colour.)
We also detected high variation in viral detection estimates across longitudinal studies (Q272 = 2866, p < 0.0001, I2 = 0.94; figure 2). Study contributed more to residual variance than phylogeny ( ). Across these data, location did not predict viral detection estimates (Q27 = 17.67, p = 0.91). Yet MEMs fit to each stratum showed high spatial heterogeneity for all data strata except filovirus prevalence, with location explaining up to 76% of the variation in viral detection estimates (electronic supplementary material, table S4). Month also had little predictive power across all longitudinal data (Q11 = 6.95, p = 0.80), but separate MEMs revealed that time explained up to 37% of the variation in filovirus seroprevalence and henipavirus prevalence (electronic supplementary material, table S5).
4. Discussion
Our study provides a systematic synthesis of prevalence and seroprevalence for bat filoviruses and henipaviruses that can guide future sampling. Only one in four studies reported longitudinal data, although the use of such approaches is increasing. Half of the studies instead pooled data over time (and space). Geographical limitations were also evident, especially for where longitudinal studies have been conducted. This was especially evident for filoviruses; although the absence of studies in Latin America and Oceania may reflect the lack of reported human cases, bat reservoirs are predicted to occur in both regions [35]. Many studies also used either PCR or serology, although using both may improve statistical inference about how zoonotic pathogens persist in hosts [18].
We found generally weak evidence that variation in sampling design and reporting affected viral detection estimates, although filovirus seroprevalence tended to be greatest from longitudinal studies. Serological surveys of Marburg and Ebola virus have found strong temporal dynamics that may reflect seasonality in bat reproduction or food availability [31,53,54]. Detection estimates could be higher with repeated sampling, as such studies are more likely to detect shedding pulses and pooling of data could increase zeros in the numerator (underestimating seroprevalence). The lack of a similar pattern for filovirus PCR data could result from the low prevalence and be biased by zero inflation. However, our low R2, alongside high contributions of bat phylogeny and study random effects, suggests other aspects of bat ecology (e.g. seasonal birth [31,55]) or study idiosyncrasies (e.g. assay type, lethal versus live sampling, serological cut-offs [39,40]) likely play more critical roles in shaping viral detection estimates. The high H2 for henipaviruses in particular also suggests that cladistic or trait-based analyses of viral shedding could be useful for guiding surveillance [35,56]. However, given at least some potential for sampling design and reporting practices to affect viral detection estimates, we encourage researchers to publish data at the lowest spatial, temporal and phylogenetic scale associated with sampling and to provide data at such scales to facilitate these future analyses.
Lastly, our analysis of longitudinal studies found significant spatial and temporal variation in some bat virus data. This implies spatio-temporal sampling is likely important to make inference about bat virus spillover. Although sampling over space and time is challenging, especially for highly mobile animals like bats, sampling can be informed by spatio-temporal variation in prevalence and seroprevalence and analyses of spatio-temporal autocorrelation [20,57]. Greater variation over space can require more fine-scale spatial sampling, and greater variation over time can require more fine-scale temporal sampling. Spatio-temporal designs, such as stratified random sampling or rotating panels, can help capture spatial and temporal variation in virus shedding while also addressing some logistical challenges [13,58,59]. The increased use of such approaches, especially in the understudied regions identified from our analysis, will help improve understanding bat virus dynamics and how spillover risk varies over time and space.
Supplementary Material
Acknowledgements
We thank Megan Higgs and anonymous reviewers for helpful comments on this manuscript.
Data accessibility
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.kkwh70s18 [60].
Authors' contributions
D.J.B., D.E.C., A.D.W. and R.K.P. designed the study, D.E.C. collected data and D.J.B. analysed data. All authors contributed to writing the manuscript. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The authors were supported by the National Science Foundation (grant no. DEB-1716698), the Defense Advanced Research Projects Agency (Young Faculty Award D16AP00113 and PREEMPT award D18AC0003) and USDA National Institute of Food and Agriculture (Hatch project 1015891). The content of the information does not necessarily reflect the position or the policy of the U.S. government, and no official endorsement should be inferred.
References
- 1.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502–510. ( 10.1038/nrmicro.2017.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt JP, Park AW, Kramer AM, Han BA, Alexander LW, Drake JM. 2017. Spatiotemporal fluctuations and triggers of Ebola virus spillover. Emerg. Infect. Dis. 23, 415–422. ( 10.3201/eid2303.160101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoye BJ, Munster VJ, Nishiura H, Klaassen M, Fouchier RAM. 2010. Surveillance of wild birds for avian influenza virus. Emerg. Infect. Dis. 16, 1827–1834. ( 10.3201/eid1612.100589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plowright RK, et al. 2015. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B 282, 20142124 ( 10.1098/rspb.2014.2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergara PM, Saravia-Zepeda A, Castro-Reyes N, Simonetti JA. 2016. Is metapopulation patch occupancy in nature well predicted by the Levins model? Popul. Ecol. 58, 335–343. ( 10.1007/s10144-016-0550-5) [DOI] [Google Scholar]
- 6.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess G. 1996. Disease in metapopulation models: implications for conservation. Ecology 77, 1617–1632. ( 10.2307/2265556) [DOI] [Google Scholar]
- 8.Bjørnstad ON, Ims RA, Lambin X. 1999. Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends Ecol. Evol. 14, 427–432. ( 10.1016/S0169-5347(99)01677-8) [DOI] [PubMed] [Google Scholar]
- 9.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484. ( 10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 10.Peel AJ, Pulliam JRC, Luis AD, Plowright RK, O'Shea TJ, Hayman DTS, Wood JLN, Webb CT, Restif O. 2014. The effect of seasonal birth pulses on pathogen persistence in wild mammal populations. Proc. R. Soc. B 281, 20132962 ( 10.1098/rspb.2013.2962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuiken T, Leighton FA, Fouchier RAM, LeDuc JW, Peiris JSM, Schudel A, Stöhr K, Osterhaus ADME. 2005. Pathogen surveillance in animals. Science 309, 1680–1681. ( 10.1126/science.1113310) [DOI] [PubMed] [Google Scholar]
- 12.Stallknecht DE. 2007. Impediments to wildlife disease surveillance, research, and diagnostics. In Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission, pp. 445–461. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 13.Nusser SM, Clark WR, Otis DL, Huang L. 2008. Sampling considerations for disease surveillance in wildlife populations. J. Wildl. Manag. 72, 52–60. ( 10.2193/2007-317) [DOI] [Google Scholar]
- 14.Venette RC, Moon RD, Hutchison WD. 2002. Strategies and statistics of sampling for rare individuals. Annu. Rev. Entomol. 47, 143–174. ( 10.1146/annurev.ento.47.091201.145147) [DOI] [PubMed] [Google Scholar]
- 15.Hayman DTS, Suu-Ire R, Breed AC, McEachern JA, Wang L, Wood JLN, Cunningham AA. 2008. Evidence of henipavirus infection in West African fruit bats. PLoS ONE 3, e2739 ( 10.1371/journal.pone.0002739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson AT. 2006. Ecologic niche modeling and spatial patterns of disease transmission. Emerg. Infect. Dis. 12, 1822–1826. ( 10.3201/eid1212.060373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J-F, Stein A, Gao B-B, Ge Y. 2012. A review of spatial sampling. Spat. Stat. 2, 1–14. ( 10.1016/j.spasta.2012.08.001) [DOI] [Google Scholar]
- 18.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O. 2016. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir-host populations. PLoS Negl. Trop. Dis. 10, e0004796 ( 10.1371/journal.pntd.0004796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restif O, et al. 2012. Model-guided fieldwork: practical guidelines for multidisciplinary research on wildlife ecological and epidemiological dynamics. Ecol. Lett. 15, 1083–1094. ( 10.1111/j.1461-0248.2012.01836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plowright RK, Becker DJ, McCallum H, Manlove KR. 2019. Sampling to elucidate the dynamics of infections in reservoir hosts. Phil. Trans. R. Soc. B 374, 20180336 ( 10.1098/rstb.2018.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280, 20122753 ( 10.1098/rspb.2012.2753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. 2017. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650. ( 10.1038/nature22975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton DJ, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Westbury HA, Halpin K, Daniels PW.. 2007. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 136, 266–272. ( 10.1016/j.jcpa.2007.03.002) [DOI] [PubMed] [Google Scholar]
- 24.Amman BR, et al. 2015. Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus). J. Wildl. Dis. 51, 113–124. ( 10.7589/2014-08-198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amman BR, et al. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8, e1002877 ( 10.1371/journal.ppat.1002877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane R, Becker N, Field H. 2011. Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS ONE 6, e28374 ( 10.1371/journal.pone.0028374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luby SP, et al. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 12, 1888–1894. ( 10.3201/eid1212.060732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulliam JRC, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9, 89–101. ( 10.1098/rsif.2011.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glennon EE, Restif O, Sbarbaro SR, Garnier R, Cunningham AA, Suu-Ire RD, Osei-Amponsah R, Wood JLN, Peel AJ. 2018. Domesticated animals as hosts of henipaviruses and filoviruses: a systematic review. Vet. J. 233, 25–34. ( 10.1016/j.tvjl.2017.12.024) [DOI] [PubMed] [Google Scholar]
- 30.Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Délicat A, Yaba P, Nkoghe D, Gonzalez J-P, Leroy EM. 2005. The natural history of Ebola virus in Africa. Microbes Infect. 7, 1005–1014. ( 10.1016/j.micinf.2005.04.006) [DOI] [PubMed] [Google Scholar]
- 31.Brook CE, et al. 2019. Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats. J. Anim. Ecol. 88, 1001–1016. ( 10.1111/1365-2656.12985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pourrut X, Délicat A, Rollin PE, Ksiazek TG, Gonzalez J-P, Leroy EM. 2007. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J. Infect. Dis. 196, S176–S183. ( 10.1086/520541) [DOI] [PubMed] [Google Scholar]
- 33.Páez DJ, Giles J, McCallum H, Field H, Jordan D, Peel AJ, Plowright RK. 2017. Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiol. Infect. 145, 3143–3153. ( 10.1017/S0950268817002138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler JF, et al. 2012. Bats host major mammalian paramyxoviruses. Nat. Commun. 3, 796 ( 10.1038/ncomms1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han BA, Schmidt JP, Alexander LW, Bowden SE, Hayman DT, Drake JM. 2016. Undiscovered bat hosts of filoviruses. PLOS Negl. Trop. Dis. 10, e0004815 ( 10.1371/journal.pntd.0004815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuh AJ, et al. 2017. Modelling filovirus maintenance in nature by experimental transmission of Marburg virus between Egyptian rousette bats. Nat. Commun. 8, 14446 ( 10.1038/ncomms14446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayman DTS, Bowen RA, Cryan PM, McCracken GF, O'Shea TJ, Peel AJ, Gilbert A, Webb CT, Wood JLN. 2013. Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses Public Health 60, 2–21. ( 10.1111/zph.12000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokolow SH, et al. 2019. Ecological interventions to prevent and manage zoonotic pathogen spillover. Phil. Trans. R. Soc. B 374, 20180342 ( 10.1098/rstb.2018.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peel AJ, et al. 2013. Use of cross-reactive serological assays for detecting novel pathogens in wildlife: assessing an appropriate cutoff for henipavirus assays in African bats. J. Virol. Methods 193, 295–303. ( 10.1016/j.jviromet.2013.06.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert AT, et al. 2013. Deciphering serology to understand the ecology of infectious diseases in wildlife. Ecohealth 10, 298–313. ( 10.1007/s10393-013-0856-0) [DOI] [PubMed] [Google Scholar]
- 41.Wacharapluesadee S, Boongird K, Wanghongsa S, Ratanasetyuth N, Supavonwong P, Saengsen D, Gongal GN, Hemachudha T. 2009. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: evidence for seasonal preference in disease transmission. Vector-Borne Zoonotic Dis. 10, 183–190. ( 10.1089/vbz.2008.0105) [DOI] [PubMed] [Google Scholar]
- 42.Halpin K, et al. 2011. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 85, 946–951. ( 10.4269/ajtmh.2011.10-0567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood S. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: CRC press. [Google Scholar]
- 44.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 45.Konstantopoulos S. 2011. Fixed effects and variance components estimation in three-level meta-analysis. Res. Synth. Methods 2, 61–76. ( 10.1002/jrsm.35) [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Santos ES. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 47.Michonneau F, Brown JW, Winter DJ. 2016. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 7, 1476–1481. ( 10.1111/2041-210X.12593) [DOI] [Google Scholar]
- 48.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 49.Senior AM, Grueber CE, Kamiya T, Lagisz M, O'Dwyer K, Santos ESA, Nakagawa S. 2016. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97, 3293–3299. ( 10.1002/ecy.1591) [DOI] [PubMed] [Google Scholar]
- 50.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. 2011. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 51.López-López JA, Marín-Martínez F, Sánchez-Meca J, Noortgate W, Viechtbauer W. 2014. Estimation of the predictive power of the model in mixed-effects meta-regression: a simulation study. Br. J. Math. Stat. Psychol. 67, 30–48. ( 10.1111/bmsp.12002) [DOI] [PubMed] [Google Scholar]
- 52.Cumming G, Finch S. 2005. Inference by eye: confidence intervals and how to read pictures of data. Am. Psychol. 60, 170 ( 10.1037/0003-066X.60.2.170) [DOI] [PubMed] [Google Scholar]
- 53.Changula K, et al. 2018. Seroprevalence of filovirus infection of Rousettus aegyptiacus bats in Zambia. J. Infect. Dis. 218, S312–S317. ( 10.1093/infdis/jix636) [DOI] [PubMed] [Google Scholar]
- 54.Pawęska JT, van Vuren PJ, Kemp A, Storm N, Grobbelaar AA, Wiley MR, Palacios G, Markotter W.. 2018. Marburg virus infection in Egyptian rousette bats, South Africa, 2013–2014. Emerg. Infect. Dis. 24, 1134 ( 10.3201/eid2406.172165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P, Foley JE. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. B 275, 861–869. ( 10.1098/rspb.2007.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Washburne AD, Crowley DE, Becker DJ, Olival KJ, Taylor M, Munster VJ, Plowright RK. 2018. Taxonomic patterns in the zoonotic potential of mammalian viruses. PeerJ 6, e5979 ( 10.7717/peerj.5979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cressie N, Wikle CK. 2015. Statistics for spatio-temporal data. New York, NY: John Wiley & Sons. [Google Scholar]
- 58.Yoccoz NG, Nichols JD, Boulinier T. 2001. Monitoring of biological diversity in space and time. Trends Ecol. Evol. 16, 446–453. ( 10.1016/S0169-5347(01)02205-4) [DOI] [Google Scholar]
- 59.Urquhart NS, Kincaid TM. 1999. Designs for detecting trend from repeated surveys of ecological resources. J. Agric. Biol. Environ. Stat. 4, 404–414. ( 10.2307/1400498) [DOI] [Google Scholar]
- 60.Becker DJ, Crowley DE, Washburne AD, Plowright RK.2019. Data from: Temporal and spatial limitations in global surveillance for bat filoviruses and henipaviruses. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Becker DJ, Crowley DE, Washburne AD, Plowright RK.2019. Data from: Temporal and spatial limitations in global surveillance for bat filoviruses and henipaviruses. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.kkwh70s18 [60].