Abstract
A growing body of research indicates that cities can support diverse bee communities. However, urbanization may disproportionately benefit exotic bees, potentially to the detriment of native species. We examined the influence of urbanization on exotic and native bees using two datasets from Michigan, USA. We found that urbanization positively influenced exotic—but not native—bee abundance and richness, and that this association could not be explained by proximity to international ports of entry, prevalence of exotic flora or urban warming. We found a negative relationship between native and exotic bee abundance at sites with high total bee abundance, suggesting that exotic bees may negatively affect native bee populations. These effects were not driven by the numerically dominant exotic honeybee, but rather by other exotic bees. Our findings complicate the emerging paradigm of cities as key sites for pollinator conservation.
Keywords: exotic bees, native bees, urbanization, urban ecology, conservation, urban agriculture
1. Introduction
Exotic species introductions have accelerated in recent decades alongside increases in international commerce [1–3]. Exotic species can cause biodiversity loss [4,5], biotic homogenization [6] and changes to ecosystem function [4]. Among the most widely established exotic species is the European honeybee—Apis mellifera Linn.—which has been introduced worldwide for pollination and honey production [7,8]. Other bee species have also been introduced beyond their native ranges, both purposefully and accidentally [9,10]. While exotic bees can provide important pollination services, they may also compete with native species for resources or spread pathogens to native species [6,10–13]. This is concerning given that native bee populations are declining in some areas [14–16]. To date, studies of the effects of exotic bees on native bees have focused primarily on eusocial exotics, and less so on solitary exotics (but see [10]).
Little is known about the ecological determinants of exotic bee colonization and spread. However, there are several ways urbanization could facilitate exotic bee establishment. First, exotic bee introductions often occur accidentally via international commerce [10]. As trading nodes with international ports of entry (IPOE), cities may be a frequent introduction point for exotic bees, thus increasing establishment via high propagule pressure [17]. Second, most exotic bees are cavity-nesting [9], presumably because this nesting strategy facilitates long-distance transport and introduction via the movement of occupied nest substrate [10]. Multiple studies have found increased prevalence of cavity-nesting bees with urbanization [18–20], likely because the built environment provides suitable nest sites for these species [18]. Thus, exotic bees may thrive in urban areas due to their nesting behaviour. Third, urban warming [21] may favour a suite of bee species different from that present in less urbanized areas [22]. This could allow for increased dominance of exotic species whose thermal tolerance better matches warmer urban conditions (e.g. [23]). Finally, exotic bees often show foraging preference for exotic flowering plants [11], and cities can be richer in exotic plants than their surroundings [24–26]. However, the abundance of managed species in cities, such as honeybees, is likely more related to management intensity, rather than preferred resource availability. Nonetheless, cities might support non-honeybee exotics via increased abundance of preferred floral resources.
Despite evidence that cities support exotic bees [27,28], limited research has shown that urbanization positively influences exotic bee populations (but see [29]), or explored the underlying drivers of this association. Recently, several authors have suggested that cities may be important sites for bee conservation [30–32]. Given this emerging paradigm, and the fact that cities are predicted to continue expanding worldwide [33], we urgently need to understand how urbanization influences the establishment and spread of exotic bees, and associated impacts on native bees.
In this study, we address the following questions:
-
(1)
Are exotic bee abundance and richness positively associated with increased urbanization?
-
(2)
Is the relationship between exotic bees and urbanization mediated by (i) proximity to registered IPOE, (ii) prevalence of exotic plants, (iii) urban warming and/or (iv) nesting strategy?
-
(3)
Do different exotic bee species demonstrate similar responses to urbanization?
-
(4)
Is there evidence for negative effects of exotic bees on native bees?
2. Material and methods
Our dataset was compiled from two field studies conducted within southeast Michigan, USA [34,35]. Bees were sampled at 41 farms and community gardens, with 26 sites sampled in 2014 and 15 in 2017. Monthly from June to August, we sampled bees using bowl traps and netting and conducted floral surveys. Data loggers at each site collected hourly temperature data. For details of survey methods, see electronic supplementary material.
We assessed urbanization at each site as the proportion of developed land within each of four concentric buffers, using the National Land Cover 2011 database [36] following the approach described in [37] (see electronic supplementary material, table S1). Among the four radii, 500 m was the most predictive for exotic bee abundance and richness and was used in all subsequent analyses. We also measured the distance from each site to the nearest registered IPOE (electronic supplementary material).
Statistical analyses were conducted in R v. 3.5.1 [38]. Because honeybees occur mainly in managed colonies in the study region, we considered them separately from other exotic bees in all analyses. Since honeybees were treated differently in netting protocols between studies (electronic supplementary material), we used only trap data in analyses that included honeybees.
We tested the effect of urbanization on per-site exotic and native bee richness and abundance using generalized linear models (GLMs) fit with a Poisson distribution (negative binomial if data were overdispersed) and log-link function. Study year and proximity to IPOE were included as predictors in these models. We evaluated the relationship between urbanization and the abundance of four widespread non-honeybee exotics (species found at ≥10 sites) using the same framework. We were interested in whether urbanization disproportionately favoured exotics, so we evaluated proportional abundance and richness of exotic bees and exotic flowering plants. To do so, we included total abundance or richness as an offset in the model. We evaluated the effects of additional putative drivers using likelihood ratio tests and AICc.
To assess the effect of exotic floral resources on the bee fauna, we considered each monthly observation separately and used generalized linear mixed models (GLMMs) with site as a random effect and proportional richness or cover of exotic plants as a fixed effect. Total floral richness, urbanization and year were also included as predictors in these models (electronic supplementary material).
To test whether nesting strategy could account for the correlation between exotic bees and urbanization, we assessed the responses of native cavity-nesting and non-cavity-nesting bee abundance and richness to urbanization using GLMs.
We evaluated the effect of exotic bee abundance on native bee abundance using GLMMs with native bee abundance as the response variable; exotic bee abundance, urbanization and floral richness as fixed effects; and site as a random effect. Effects of exotic bees on natives are likely to be density-dependent [39], with stronger negative effects when total population density is higher [40]. Thus, we assessed the relationship between native and exotic bee abundance separately at sites with high versus low bee abundance (electronic supplementary material). To test the robustness of our findings, we considered a range of cut-offs for separating high-abundance from low-abundance sites [25–50 bees/sampling period for comparing non-honeybee exotics and natives; 10–40 bees/sampling period for comparing honeybees and natives (smaller numbers because only trap data are included; electronic supplementary material)], and, for each cut-off, fit models for both high- and low-abundance sites.
3. Results
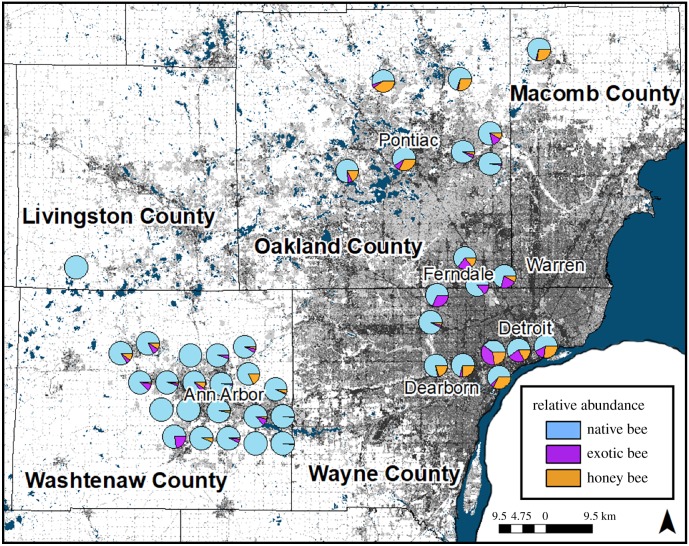
We found 14 exotic bee species, comprising 20% of all bee species collected [41]. The percentage of exotic bees collected per site ranged from 0 to 40% (mean ± s.d. = 16 ± 14%; figure 1), while exotic bee species richness ranged from 0 to 9 (mean ± s.d. = 3 ± 2) per site (electronic supplementary material, table S2). The European honeybee—the only actively managed bee species in the study area—was the most abundant exotic species, comprising 58% of all exotics, and occurring at 18 of 41 sites (30 of 41 when including netting data). Non-honeybee exotics belonged to four families: Colletidae (two species, 50% of individuals), Megachilidae (eight species, 34%), Andrenidae (one species, 11%) and Halictidae (two species, 5%) (electronic supplementary material, figures S1 and S2). Proportional abundance of exotic bees was higher in 2017 (29%) than 2014 (14%).
Figure 1.
Map of study sites with proportional abundance of native and exotic bees. Greyscale represents degree of urbanization. Geographical location of nearby sites is offset to prevent overlap of pie charts. (Online version in colour.)
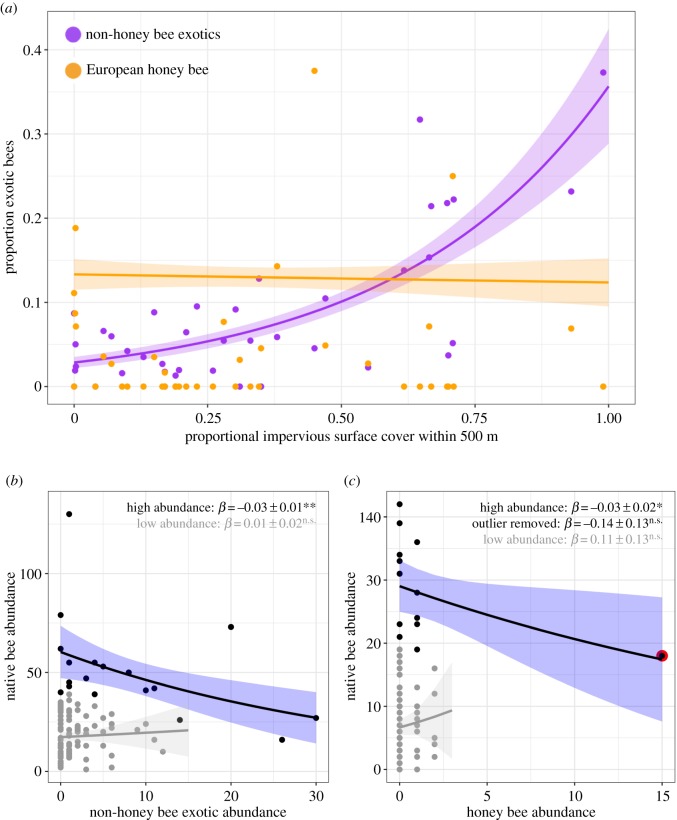
The absolute and proportional abundance and richness of exotic bees significantly increased with urbanization, while native and total bee abundance and richness were unaffected (figure 2a and table 1). Abundance of each widespread wild exotic showed a qualitatively similar response to urbanization, indicating that the overall relationship between urbanization and exotic bee abundance was not driven by a single species (table 1; electronic supplementary material, figure S3). However, honeybees showed no response to urbanization (figure 2a).
Figure 2.
(a) Relationship between urbanization and exotic bee proportional abundance. (b,c) Relationship between native and exotic bee abundance for (b) non-honeybee exotics and (c) honeybees. Grey points represent low-abundance sites; black points represent high-abundance sites (in (b), high abundance: ≥40 bees collected; in (c) ≥20 bees). Outlined point in (c) indicates outlier. Trendlines derived from GLMs. Significance codes: n.s.p > 0.05; *p < 0.05; **p < 0.01. (Online version in colour.)
Table 1.
Effects of potential drivers of native and exotic bee abundance and richness. Dash indicates that the predictor was not included in the best model (determined by AICc). %DE, per cent of null deviance explained; DF, degrees of freedom; ΔAICc, difference in AICc between full model and the best-fitting model, which omits non-significant predictors. Significance codes are indicated by asterisk. Italic text represents significant predictors at p < 0.05.
| predictors of interest |
covariates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| response variable | urbanization (β ± s.e.) | proximity to international port of entry (β ± s.e.) | proportional richness of exotic plants (β ± s.e.) | proportional bloom cover of exotic plants (β ± s.e.) | total plant richness (β ± s.e.) | year (β ± s.e.) | %DE | DF | ΔAICc |
| (a) GLMs | |||||||||
| abundance | |||||||||
| non-honeybee exotic | 0.80 ± 0.21*** | –0.11 ± 0.21 | — | — | — | 1.03 ± 0.28*** | 52.2 | 37 | 2.35 |
| proportion non-honeybee exotic | 0.98 ± 0.09*** | –0.34 ± 0.09*** | — | — | — | — | 63.6 | 38 | 0.00 |
| honeybee | 0.10 ± 0.42 | –0.31 ± 0.41 | — | — | — | — | 0.0 | 38 | 3.52 |
| Hylaeus hyalinatus | 0.70 ± 0.29* | 0.53 ± 0.32 | — | — | — | — | 46.8 | 38 | 0.34 |
| H. leptocephalus | 2.07 ± 0.52*** | –0.84 ± 0.51 | — | — | — | — | 55.6 | 38 | 0.35 |
| Anthidium manicatum | 1.19 ± 0.59* | –0.07 ± 0.62 | — | — | — | — | 24.8 | 38 | 2.45 |
| Megachile rotundata | 1.51 ± 0.45*** | –0.61 ± 0.45 | — | — | — | — | 38.6 | 38 | 0.69 |
| exotic cavity-nesting | 0.81 ± 0.21*** | 0.05 ± 0.21 | — | — | — | 1.66 ± 0.27*** | 68.4 | 37 | 2.55 |
| native overall | –0.35 ± 0.25 | — | — | — | — | 0.37 ± 0.14** | 17.6 | 38 | 0.58 |
| native cavity-nesting | 0.27 ± 0.14 | — | — | — | — | 2.92 ± 0.33*** | 73.7 | 38 | 0.35 |
| native non-cavity-nesting | –0.01 ± 0.07 | — | — | — | — | 0.30 ± 0.14* | 9.7 | 38 | 2.42 |
| richness | |||||||||
| exotic | 0.42 ± 0.16* | –0.11 ± 0.16 | — | — | — | 0.78 ± 0.21*** | 42.4 | 37 | 2.01 |
| proportion exotic | 0.49 ± 0.16** | 0.19 ± 0.16 | — | — | — | — | 30.5 | 38 | 0.95 |
| exotic cavity-nesting | 0.59 ± 0.22** | –0.10 ± 0.21 | — | — | — | 1.26 ± 0.27*** | 55.4 | 37 | 2.24 |
| native overall | –0.18 ± 0.16 | — | — | — | — | 0.34 ± 0.09*** | 27.6 | 38 | 1.24 |
| native cavity-nesting | 0.11 ± 0.11 | — | — | — | — | 2.24 ± 0.30*** | 70.0 | 38 | 1.30 |
| native non-cavity-nesting | –0.02 ± 0.04 | — | — | — | — | 0.26 ± 0.09** | 17.6 | 38 | 2.20 |
| total | –0.04 ± 0.16 | — | — | — | — | 0.40 ± 0.09 | 33.8 | 38 | 2.39 |
| (b) GLMMs | |||||||||
| non-honeybee exotic abundance | 0.66 ± 0.13*** | — | 0.11 ± 0.14 | 0.01 ± 0.11 | 0.39 ± 0.08*** | 0.76 ± 0.29** | 10.4 | 106 | 3.59 |
| H. hyalinatus abundance | 1.02 ± 0.20*** | — | –0.23 ± 0.24 | 0.09 ± 0.21 | 0.59 ± 0.13*** | — | 15.5 | 107 | 3.48 |
| H. leptocephalus abundance | 1.59 ± 0.40*** | — | 0.13 ± 0.40 | –0.02 ± 0.31 | — | — | 9.8 | 108 | 4.22 |
| A. manicatum abundance | 0.84 ± 0.42* | — | –0.27 ± 0.50 | 0.34 ± 0.39 | 0.75 ± 0.28** | — | 10.8 | 107 | 3.60 |
| M. rotundata abundance | 1.01 ± 0.32** | — | 0.08 ± 0.41 | 0.15 ± 0.35 | — | — | 7.4 | 108 | 3.89 |
| non-honeybee exotic richness | 0.39 ± 0.09*** | — | 0.11 ± 0.15 | –0.23 ± 0.13 | 0.25 ± 0.10* | 0.71 ± 0.21*** | 11.6 | 106 | 1.30 |
*p < 0.05; **p < 0.01; ***p < 0.001.
Cavity-nesting bees comprised 6% of native bees and 57% of non-honeybee exotics. More urbanized sites supported more exotic and native cavity-nesting bees, although this relationship was much stronger for exotics. While exotic cavity-nester richness was positively related to urbanization, native cavity-nester richness was not (table 1).
Urbanization was positively related to proximity to the nearest IPOE (LM: R2 = 0.53, F1,39 = 45.8, p < 0.001). When both proximity to IPOE and urbanization were included as model predictors, only urbanization had a significant effect on exotic bee richness and abundance and including both measures increased AICc. However, proportional non-honeybee exotic abundance was negatively related to proximity to IPOE (table 1). Similarly, urbanization and minimum temperature were positively related (LM: R2 = 0.32, F1,39 = 17.9, p < 0.001), but minimum temperature had no independent effect on exotic bee richness or abundance (electronic supplementary material, table S3).
The positive relationships between urbanization and exotic bee richness and abundance were not mediated by exotic plants. Neither raw nor proportional richness or cover of exotic plants were correlated with urbanization (electronic supplementary material, table S5). Moreover, while exotic bee abundance and richness were positively related to total floral resource availability, they were not influenced by proportional richness or cover of exotic plants (table 1).
Exotic bee abundance had no effect on native bee abundance (β = 0.01 ± 0.02, p = 0.39). However, at high-abundance sites, there was a significant negative relationship between non-honeybee exotic and native bee abundance not seen at low-abundance sites (figure 2b; electronic supplementary material, table S5). The relationship between honeybee abundance and native bee abundance was qualitatively similar; however, the negative relationship between honeybees and native bees was generated by a single observation (figure 2c; electronic supplementary material, table S6) and should be interpreted with caution. The negative relationship between native and wild exotic bee abundance at high-abundance sites was not due to correlation between exotic abundance and any other measured driver of native bee abundance (electronic supplementary material, table S6).
4. Discussion
Urbanization alters the composition of biotic communities by creating a matrix of habitats distinct from natural ecosystems [42,43]. In this study, urbanization correlated with increased prevalence of exotic bees, via increases in exotic bee abundance and richness rather than declines in native bees. The association between urbanization and exotic bees was not mediated by exotic floral resource availability, proximity to IPOE or urban warming.
The lack of relationship between exotic bees and exotic plant prevalence contradicts other studies suggesting that exotic bees preferentially visit exotic plants [44–46]. However, 96% of exotic bees we collected were from generalist species; their success in their introduced range may derive from the ability to feed on a wide range of plants [47]. Because we did not assess bee diet, our findings do not demonstrate that exotic bees do not prefer exotic plants. They do, however, indicate that the success of exotic bees in cities is not due to increased abundance of exotic floral resources.
Most of the exotic bees we collected nest in cavities; the additional nesting substrate provided by urbanization may facilitate these species [18]. Indeed, the abundance of native cavity-nesting bees also increased with urbanization, though this response was weaker than that of exotic bees, suggesting that nesting preferences alone cannot account for exotic bee success in cities. In sum, the increased prevalence of exotic bees in cities is largely attributable to trait-matching between exotics and urban environments (e.g. cavity-nesting habit). We found no evidence that proximity to IPOE increases exotic bee abundance or richness, but the limited scope of our study does not allow us to definitively evaluate the role of propagule pressure in exotic bee success in cities.
The observed negative correlation between native and exotic bees at sites supporting high total bee abundance—but not at low-abundance sites—suggests density-dependent effects of exotics on natives. Negative effects of exotic bees on natives may be due to competition for food or nest sites [9,48,49], or apparent competition mediated by shared pathogens [9,50]. Intriguingly, we found that wild exotic bee abundance accounted for more deviance in native bee abundance than did honeybee abundance. The strength of the relationship between wild exotic and native bee abundance is surprising, given that (1) studies measuring the effect of exotic honeybees on native bees rarely demonstrate population-level consequences [12] and (2) effects of non-eusocial exotic bees on native bees are understudied. Alternatively, environmental filtering, operating differently on native versus exotic bees, may be responsible for the observed relationship. Conclusively determining whether the observed relationship indicates that wild exotic bees exert more influence than honeybees on natives, or results from collinearity with some unassessed driver of bee abundance requires further study.
Recent findings that cities can maintain diverse bee communities [31,32,37,51] has increased interest in cities as targets for bee conservation [30–32]. While promoting bee-friendly management of urban land is vital to protecting pollinators, this study highlights the need to think critically about the bee communities supported by urban environments. While urbanization can increase bee beta-diversity by supporting a different suite of species from those found outside cities, this may come at a cost to native species. Our research suggests that, globally, urbanization may homogenize bee communities by increasing the dominance of a small number of cosmopolitan, synanthropic species.
Supplementary Material
Acknowledgements
Thanks to undergraduate assistants and technicians who helped collect and process data, and to gardeners and land managers who generously allowed us to conduct research on their properties. Helpful comments from three anonymous reviewers improved this manuscript.
Ethics
No permits or ethics committee approvals were required for this research. We received permission from all landowners and managers to conduct research.
Data accessibility
Data and R scripts are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.9kf08rj [41].
Authors' contributions
All authors jointly conceived of this paper and participated in data collection. G.F. conducted statistical analyses; C.J.W. conducted geographical analyses; G.F. and C.J.W. drafted the manuscript. All authors revised and approved the manuscript and are accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Foundation for Food and Agricultural Research's New Innovator Award (grant no. FFAR 430876); Oakland University; University of Michigan.
References
- 1.Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288. ( 10.1016/j.ecolecon.2004.10.002) [DOI] [Google Scholar]
- 2.Hulme PE. 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18. ( 10.1111/j.1365-2664.2008.01600.x) [DOI] [Google Scholar]
- 3.Seebens H, et al. 2015. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 21, 4128–4140. ( 10.1111/gcb.13021) [DOI] [PubMed] [Google Scholar]
- 4.Simberloff D, et al. 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28, 58–66. ( 10.1016/j.tree.2012.07.013) [DOI] [PubMed] [Google Scholar]
- 5.Clavero M, Garciaberthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110 ( 10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 6.Qian H, Ricklefs RE. 2006. The role of exotic species in homogenizing the North American flora. Ecol. Lett. 9, 1293–1298. ( 10.1111/j.1461-0248.2006.00982.x) [DOI] [PubMed] [Google Scholar]
- 7.Moritz RFA, Härtel S, Neumann P. 2005. Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Écoscience 12, 289–301. ( 10.2980/i1195-6860-12-3-289.1) [DOI] [Google Scholar]
- 8.Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo L. 2016. Positive and negative impacts of non-native bee species around the world. Insects 7, 69 ( 10.3390/insects7040069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cane J. 2003. Exotic non-social bees (Hymenoptera: Apoidea) in North America: ecological implications. In For non-native crops, whence pollinators of the future? (eds Strickler K, Cane JH), pp. 113–126. Lanham, MD: Entomological Society of America. [Google Scholar]
- 11.Goulson D. 2003. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 34, 1–26. ( 10.1146/annurev.ecolsys.34.011802.132355) [DOI] [Google Scholar]
- 12.Mallinger RE, Gaines-Day HR, Gratton C. 2017. Do managed bees have negative effects on wild bees? A systematic review of the literature. PLoS ONE 12, e0189268 ( 10.1371/journal.pone.0189268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdovinos FS, Berlow EL, De Espanés PM, Ramos-jiliberto R, Vázquez DP, Martinez ND. 2018. Species traits and network structure predict the success and impacts of pollinator invasions. Nat. Commun. 9, 2153 ( 10.1038/s41467-018-04593-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biesmeijer JC. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 15.Carvalheiro LG, et al. 2013. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 16, 870–878. ( 10.1111/ele.12121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkle LA, Marlin JC, Knight TM. 2013. Plant–pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615. ( 10.1126/science.1232728) [DOI] [PubMed] [Google Scholar]
- 17.Lockwood JL, Cassey P, Blackburn T. 2005. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 20, 223–228. ( 10.1016/j.tree.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 18.Cane JH, Minckley RL, Kervin LJ, Roulston TH, Neal M. 2006. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 16, 632–644. ( 10.1890/1051-0761) [DOI] [PubMed] [Google Scholar]
- 19.Fortel L, Henry M, Guilbaud L, Guirao AL, Kuhlmann M, Mouret H, Rollin O, Vaissière BE. 2014. Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 9, e104679 ( 10.1371/journal.pone.0104679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitch GM. 2017. Urbanization-mediated context dependence in the effect of floral neighborhood on pollinator visitation. Oecologia 185, 713–723. ( 10.1007/s00442-017-3982-5) [DOI] [PubMed] [Google Scholar]
- 21.Oke TR. 1973. City size and the urban heat island. Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 22.Hamblin AL, Youngsteadt E, López-Uribe MM, Frank SD. 2017. Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biol. Lett. 13, 20170125 ( 10.1098/rsbl.2017.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strange JP, Koch JB, Gonzalez VH, Nemelka L, Griswold T. 2011. Global invasion by Anthidium manicatum (Linnaeus) (Hymenoptera: Megachilidae): assessing potential distribution in North America and beyond. Biol. Invasions 13, 2115–2133. ( 10.1007/s10530-011-0030-y) [DOI] [Google Scholar]
- 24.Pysek P. 1998. Alien and native species in Central European urban floras: a quantitative comparison. J. Biogeogr. 25, 155–163. ( 10.1046/j.1365-2699.1998.251177.x) [DOI] [Google Scholar]
- 25.Zerbe S, Maurer U, Schmitz S, Sukopp H. 2003. Biodiversity in Berlin and its potential for nature conservation. Landsc. Urban Plan. 62, 139–148. ( 10.1016/S0169-2046(02)00145-7) [DOI] [Google Scholar]
- 26.Aronson MFJ, Handel SN, La Puma IP, Clemants SE. 2015. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 18, 31–45. ( 10.1007/s11252-014-0382-z) [DOI] [Google Scholar]
- 27.Normandin É, Vereecken NJ, Buddle CM, Fournier V. 2017. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 5, e3051 ( 10.7717/peerj.3051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteson KC, Ascher JS, Langellotto GA. 2008. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 101, 140–150. ( 10.1603/0013-8746(2008)101[140:BRAAIN]2.0.CO;2) [DOI] [Google Scholar]
- 29.Sivakoff F, Prajzner S, Gardiner M. 2018. Unique bee communities within vacant lots and urban farms result from variation in surrounding urbanization intensity. Sustainability 10, 1926 ( 10.3390/su10061926) [DOI] [Google Scholar]
- 30.Hall DM, et al. 2017. The city as a refuge for insect pollinators. Conserv. Biol. 31, 24–29. ( 10.1111/cobi.12840) [DOI] [PubMed] [Google Scholar]
- 31.Baldock KCR, et al. 2019. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 3, 363–373. ( 10.1038/s41559-018-0769-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldock KCR, et al. 2015. Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 282, 20142849 ( 10.1098/rspb.2014.2849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seto KC, Guneralp B, Hutyra LR. 2012. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl Acad. Sci. USA 109, 16 083–16 088. ( 10.1073/pnas.1211658109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitch G, Glaum P, Simao M-C, Vaidya C, Matthijs J, Iuliano B, Perfecto I. 2019. Changes in adult sex ratio in wild bee communities are linked to urbanization. Sci. Rep. 9, 3767 ( 10.1038/s41598-019-39601-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson MA, Carper AL, Wilson CJ, Scott VL, Gibbs J. 2019. Geographic biases in bee research limits understanding of species distribution and response to anthropogenic disturbance. Front. Ecol. Evol. 7, 1–8. ( 10.3389/fevo.2019.00194) [DOI] [Google Scholar]
- 36.Homer CG, et al. 2015. Completion of the 2011 National Land Cover Database for the conterminous United States—representing a decade of land cover change information. Photogramm. Eng. Remote Sensing 81, 345–354. [Google Scholar]
- 37.Glaum P, Simao M-C, Vaidya C, Fitch G, Iulinao B. 2017. Big city Bombus: using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. R. Soc. open sci. 4, 170156 ( 10.1098/rsos.170156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 39.Thomson D. 2004. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology 85, 458–470. ( 10.1890/02-0626) [DOI] [Google Scholar]
- 40.Adams ES, Tschinkel WR. 1995. Density-dependent competition in fire ants: effects on colony survivorship and size variation. J. Anim. Ecol. 64, 315 ( 10.2307/5893) [DOI] [Google Scholar]
- 41.Fitch G, Wilson CJ, Glaum P, Vaidya C, Simao M-C, Jamieson MA. 2019. Data from: Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities Dryad Digital Repository. ( 10.5061/dryad.9kf08rj) [DOI] [PMC free article] [PubMed]
- 42.McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. ( 10.1016/j.biocon.2005.09.005) [DOI] [Google Scholar]
- 43.Pickett STA, et al. 2011. Urban ecological systems: scientific foundations and a decade of progress. J. Environ. Manage. 92, 331–362. ( 10.1016/j.jenvman.2010.08.022) [DOI] [PubMed] [Google Scholar]
- 44.MacIvor JS, Ruttan A, Salehi B. 2015. Exotics on exotics: pollen analysis of urban bees visiting Sedum on a green roof. Urban Ecosyst. 18, 419–430. ( 10.1007/s11252-014-0408-6) [DOI] [Google Scholar]
- 45.Martins KT, Normandin É, Ascher JS. 2017. Hylaeus communis (Hymenoptera: Colletidae), a new exotic bee for North America with generalist foraging and habitat preferences. Can. Entomol. 149, 377–390. ( 10.4039/tce.2016.62) [DOI] [Google Scholar]
- 46.Soper J, Beggs J. 2013. Assessing the impact of an introduced bee, Anthidium manicatum, on pollinator communities in New Zealand. New Zeal. J. Bot. 51, 213–228. ( 10.1080/0028825X.2013.793202) [DOI] [Google Scholar]
- 47.Gibbs J, Sheffield CS. 2009. Rapid range expansion of the wool-carder bee, Anthidium manicatum (Linnaeus) (Hymenoptera: Megachilidae), in North America. J. Kansas Entomol. Soc. 82, 21–29. ( 10.2317/JKES805.27.1) [DOI] [Google Scholar]
- 48.Torné-Noguera A, Rodrigo A, Osorio S, Bosch J. 2016. Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 17, 199–209. ( 10.1016/j.baae.2015.11.004) [DOI] [Google Scholar]
- 49.Cane JH, Tepedino VJ. 2017. Gauging the effect of honey bee pollen collection on native bee communities. Conserv. Lett. 10, 205–210. ( 10.1111/conl.12263) [DOI] [Google Scholar]
- 50.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaluza BF, Wallace H, Heard TA, Klein A-M, Leonhardt SD. 2016. Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 6, 1304–1316. ( 10.1002/ece3.1941) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fitch G, Wilson CJ, Glaum P, Vaidya C, Simao M-C, Jamieson MA. 2019. Data from: Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities Dryad Digital Repository. ( 10.5061/dryad.9kf08rj) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R scripts are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.9kf08rj [41].