Abstract
Background
Comorbidities impact outcomes in heart failure with reduced ejection fraction (HFrEF). However, the effect of age on the impact of comorbidities on prognosis is not clearly understood.
Objectives
Examine whether age modifies the impact of key comorbidities on clinical outcomes for patients with HFrEF.
Methods
Cox proportional hazards models assessed interactions between age and comorbidities on the primary composite end point (all-cause mortality or hospitalization) and secondary endpoints in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) multicenter trial of 2,331 patients with HFrEF.
Results
Age didn’t significantly modify the effect of any comorbidity on the primary endpoint. However, age significantly modified the effect of body mass index (BMI) on all-cause mortality (interaction p=0.02). Among patients ≥70 years, there was a U-shaped relationship between BMI and 1-year mortality: BMI=20, 17.6%; BMI=30, 7.0%; and BMI=40, 11.0%. For patients <60 years, mortality increased non-significantly from 3.2% to 3.7% with increasing BMI. Age also modified the effect of depressive symptoms on all-cause mortality (interaction p=0.03). Among patients ≥70 years, 1-year mortality rate significantly increased from 7.8% for Beck Depression Inventory (BDI) score =5 to 15.6% for BDI = 20. For patients <60 years, mortality was non-significantly related to BDI. Cumulative comorbidity score was a stronger predictor than age for mortality/hospitalization.
Conclusions
In chronic HFrEF, age markedly altered the impact of BMI and depressive symptoms on all-cause mortality, with much higher risk in older patients, but was not as strong a predictor of mortality/hospitalizations as cumulative comorbidity score.
Keywords: heart failure with reduced ejection fraction, comorbidities, age, body mass index, depression
Condensed Abstract
The effect of age on the impact of comorbidities on clinical outcomes in patients with chronic, stable HFrEF is not well defined. This study demonstrates the importance of age and specific comorbidities on clinical outcomes in a large cohort of HFrEF patients. The effect of comorbidities on the primary endpoint was not modified by age. BMI was found to have a U-shaped relationship in older patients with HFrEF, with greater risk of mortality and hospitalization at the extremes of BMI. Depression also increased mortality in older patients with HFrEF. Cumulative comorbidity score was a strong predictor of mortality/hospitalizations.
Introduction
As the US population ages, prevalence of heart failure (HF) is increasing (1). Prognosis is poor, even in younger adults with HF, and it continues to worsen as patient age advances (2). Both cardiac and non-cardiac comorbidities are more common in older HF patients; and are associated with diminished quality of life, increased adverse events; and greater management complexity (3–5). The presence of multiple comorbid conditions has become increasingly recognized as relevant to chronic HF outcomes care (6), including greater risk of mortality and hospitalization (7). A recent study of hospitalized HF patients showed that the number of comorbidities also corresponded to longer hospitalization as well as greater in-hospital mortality, 30-day readmission and all-cause mortality (8). Though a component of increased risk of mortality and hospitalization is likely attributable to age itself, we hypothesized that certain comorbidities also contribute to vulnerability. To better understand the relationship between age and comorbidities on clinical endpoints in adults with chronic HF, we used data from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION). Using this population of ambulatory patients with chronic, stable HF and reduced ejection fraction (HFrEF), we evaluated whether age modifies the impact of specific comorbidities or a cumulative comorbidity score on outcomes.
Methods
Overview
The design, rationale and primary results of HF-ACTION have been published (9,10). In brief, HF-ACTION was a multicenter, randomized controlled trial designed to test the long-term efficacy and safety of exercise training in patients with chronic, stable HFrEF. Patients were recruited from 82 clinical centers in the United States, Canada and France; 2331 were enrolled and randomized to aerobic exercise training or usual care in the presence of guideline-directed medical therapy. The patient population included medically stable outpatients with HF and left ventricular ejection fraction (LVEF) ≤35% and New York Heart Association (NYHA) functional class II to IV symptoms with at least 6 weeks of guideline-based medical therapy. Exclusion criteria included inability to exercise, patients already exercising regularly or having experienced a major cardiovascular event in the past 6 weeks. Baseline exercise capacity was measured by cardiopulmonary exercise (CPX) test and 6-minute walk distance (6MWD). The exercise training consisted of 36 supervised sessions of aerobic exercise (treadmill walking or stationary biking) followed by unsupervised, home-based training five or more times per week for an additional two years. Median follow-up was 30 months. The institutional review board or ethics committee for each center approved the protocol, and patients provided written informed consent.
Definitions of Age Groups and Comorbidities
For the present analysis, age groups were defined as <60 years, 60–69 years and ≥70 years for descriptive purposes. Body mass index (BMI) and key comorbidities assessed at baseline in the HF-ACTION trial were analyzed, including ischemic heart disease, hypertension, peripheral vascular disease, atrial fibrillation and/or flutter (Afib), diabetes mellitus, chronic obstructive pulmonary disease (COPD), anemia, chronic kidney disease (CKD) and depression. Ascertainment of comorbidities was based on physician/coordinator assessment from a predetermined group of candidate comorbidities and medical history at the time of baseline evaluation. Comorbidity definitions are included in the Supplement.
Clinical Endpoints
The primary endpoint of HF-ACTION was a composite of all-cause mortality or all-hospitalization. Secondary endpoints included all-cause mortality and the composite of cardiovascular mortality or HF hospitalization. Blinding was not possible due to the nature of the intervention; however, a blinded clinical events committee adjudicated events of death and cardiovascular hospitalization. Once a patient had a confirmed HF hospitalization, no future hospitalizations were adjudicated for that patient.
Statistical Analysis
All randomized patients (N = 2331) randomized were included in this analysis. Sociodemographic and clinical characteristics, comorbidities, exercise performance measures and clinical endpoints are presented by age group (<60, 60–69 and ≥70 years). Categorical variables are summarized as frequencies and percentages while continuous variables are described with median and quartiles. To test differences between age groups, the chi-square test was used for categorical variables while the Krushkal-Wallis test was used for continuous variables.
The relationships between age as a continuous variable and clinical endpoints were assessed using restricted cubic splines. A similar approach was used for comorbidities measured on a continuous scale. Age was truncated from below at age 60, i.e. all patients 60 and younger were assigned the same risk, and the estimated glomerular filtration rate (eGFR) was truncated from above at 90 mL/min/1.73m2.
The association of each comorbidity on primary and secondary clinical endpoints was assessed using Cox proportional hazard models with robust variance estimators to protect the type I error against departures from the proportional hazards assumption (11). The interaction of each comorbidity with age as a continuous variable was tested using the Wald test in each model over total follow-up. For categorical comorbidities with an interaction p-value ≤0.10, separate hazard ratios were calculated for age intervals (<60, 60–69, ≥70 years). For continuous comorbidities with an interaction p-value ≤0.10, descriptive spline plots with three knots (at the 10th, 50th, 90th percentiles of the continuous comorbidity) were drawn for the different age intervals. Linearity of non-monotonic splines was tested using the Wald test. We tested monotonicity using the Wald test for the corresponding slope coefficient. A cumulative comorbidity score was calculated as the sum of the nine comorbidities, excluding anemia because assessment was missing in 24% of participants. For the score, depression was dichotomized at a Beck inventory score = 19, which is the cutoff for moderate-to-severe depression (12). Chronic kidney disease was dichotomized at an eGFR = 60 mL/min/1.73m2, which is the cutoff for mild reduction in kidney function (13).
Analyses were performed with SAS version 9.4 or R version 3.4.2 (14) including the survival and rms packages. No imputation was performed. Statistical significance was defined as a 2-tailed p <0.05 with no correction for multiple comparisons.
Results
Table 1 provides baseline characteristics stratified by age group. The median age was 59 years. Male sex and white race were more prevalent with age. Median LVEF was not significantly different across age groups; however, older patients had significantly worse NYHA symptom class.
Table 1.
Baseline Characteristics Stratified by Age Group
| Overall | <60 years | 60–69 years | ≥70 years | p-value | |
|---|---|---|---|---|---|
| N = 2331 | N = 1214 | N = 640 | N = 477 | ||
| Age (years) | 59.3 (51.1 – 68.0) | 51.5 (44.8 – 56.1) | 64.4 (62.2 – 67.2) | 75.3 (72.5 – 78.7) | |
| Men | 1670 (71.6) | 821 (67.6) | 474 (74.1) | 375 (78.6) | < 0.0001 |
| White | 1426 (62.1) | 619 (52.0) | 434 (68.8) | 373 (78.5) | < 0.0001 |
| LVEF (%) | 24.7 (20.0 – 30.1) | 24.4 (20.0 – 30.0) | 24.8 (20.0 – 29.9) | 25.5 (20.6 – 30.7) | 0.06 |
| NYHA Class | 0.0009 | ||||
| II | 1477 (63.4) | 808 (66.6) | 393 (61.4) | 276 (57.9) | |
| III | 831 (35.6) | 399 (32.9) | 241 (37.7) | 191 (40.0) | |
| IV | 23 (1.0) | 7 (0.6) | 6 (0.9) | 10 (2.1) | |
| Body Mass Index (kg/m2) | 29.9 (26.0 – 35.1) | 31.9 (27.1 – 37.6) | 29.3 (26.0 – 33.4) | 27.3 (24.1 – 30.5) | < 0.0001 |
| Atrial Fibrillation / Flutter | 488 (20.9) | 178 (14.7) | 139 (21.7) | 171 (35.9) | < 0.0001 |
| Ischemic Heart Disease | 1197 (51.4) | 456 (37.6) | 390 (61.9) | 345 (72.3) | < 0.0001 |
| Hypertension | 1426/ 2320 (61.5) | 687 / 1210 (56.8) | 409 / 635 (64.4) | 330 / 475 (69.5) | < 0.0001 |
| Peripheral Vascular Disease | 157 (6.8) | 43 / 1208 (3.6) | 57 / 637 (9.0) | 57 / 475 (12.0) | < 0.0001 |
| Diabetes | 748 (32.1) | 352 (29.0) | 246 (38.4) | 150 (31.5) | 0.0002 |
| COPD | 249 (10.8) | 89 / 1202 (7.4) | 89 / 636 (14.0) | 71 / 473 (15.0) | < 0.0001 |
| Anemia | 515 / 1763 (29.2) | 229 / 913 (25.1) | 143 / 485 (29.5) | 143 / 365 (39.2) | < 0.0001 |
| Chronic Kidney Disease (eGFR < 60) | 806 / 2091 (38.6) | 255 / 1084 (23.5) | 272 / 571 (47.6) | 279 / 436 (64.0) | < 0.0001 |
| Severe/Moderate Depressive Symptoms (Beck index ≥ 19) | 388 / 2322 (16.7) | 258 / 1210 (21.3) | 89 / 635 (14.0) | 41 (8.6) | < 0.0001 |
| Six Minute Walking Distance (m) | 371 (299 – 435) | 390 (317 – 452) | 366 (305 – 427) | 324 (244 – 388) | < 0.0001 |
| CPX Duration (min) | 9.6 (6.9 – 12.0) | 10.3 (8.0 – 13.0) | 9.2 (6.7 – 11.6) | 7.7 (5.6 – 10.2) | < 0.0001 |
| Beta Blocker | 2203 (94.5) | 1163 (95.8) | 604 (94.4) | 436 (91.4) | 0.0017 |
| Loop Diuretic | 1816 (77.9) | 947 (78.0) | 505 (78.9) | 364 (76.3) | 0.58 |
| ACE Inhibitor | 1736 (74.5) | 950 (78.3) | 461 (72.0) | 325 (68.1) | <0.0001 |
| ARB | 544 (23.3) | 255 (21.0) | 164 (25.6) | 125 (26.2) | 0.02 |
| MRA | 1051 (45.1) | 617 (50.8) | 258 (40.3) | 176 (36.9) | <0.0001 |
Presented as median (IQR) or N (%). P-values computed by one-way analysis of variance for continuous variables and chi-square for categorical. LVEF= Left ventricular ejection fraction; NYHA= New York Heart Association; COPD= Chronic Obstruction Pulmonary Disease; eGFR=estimated Glomerular Filtration Rate; CPX= Cardiopulmonary Exercise Test; ACE=Angiotensin converting enzyme; ARB=Angiotensin receptor blocker; MRA=Mineralocorticoid receptor antagonist
The prevalence of ischemic heart disease, hypertension, peripheral vascular disease, Afib, COPD, anemia, and CKD were all greater in older groups (all p<0.0001). Median BMI was 29.9 and was significantly lower in older groups. Moderate or severe depression was less prevalent in the older groups.
Baseline CPX test duration and 6MWD were both significantly lower in the older groups. With increased age, a slightly lower percentage of patients were on beta-blockers at the time of randomization; yet, the overall use was very high (>90%). ACE inhibitor and MRA use were higher in younger patients while ARB use was higher in older patients.
Clinical Outcomes
In Table 2, outcome measures stratified by age are reported as one-year event rates. The one-year rate for the primary endpoint of all-cause mortality or all-cause hospitalization was 39.2% in patients <60, 41.9% in those 60–69 and 49.0% in those ≥70 years (p<0.0001). For the key secondary endpoint of all-cause mortality, the one-year rates were 3.4% for patients <60 compared to 4.3% in the 60–69 group and 9.6% in those ≥70, respectively (p<0.0001). Cardiovascular mortality or HF hospitalization at one-year was highest in patients ≥70 years at 20.0% (p<0.0001).
Table 2.
One-Year Outcomes Stratified by Age Group
| Overall | <60 years | 60–69 years | ≥70 years | p-value* | |
|---|---|---|---|---|---|
| All-Cause Mortality or All-Cause Hospitalization | 41.9 (39.9–43.9) | 39.2 (36.3–41.8) | 41.9 (37.9–45.6) | 49.0 (44.2–53.4) | < 0.0001 |
| All-Cause Mortality | 4.9 (4.0–5.8) | 3.4 (2.4–4.5) | 4.3 (2.7–5.8) | 9.6 (6.9–12.3) | < 0.0001 |
| Cardiovascular mortality or heart failure hospitalization | 15.2 (13.7–16.7) | 14.5 (12.5–16.5) | 13.0 (10.3–15.6) | 20.0 (16.2–23.5) | < 0.0001 |
Presented as KM% (95% CI) at 1 year.
Log-rank p-value over entire follow-up (median=2.5 years)
Interaction Between Age and Comorbidities
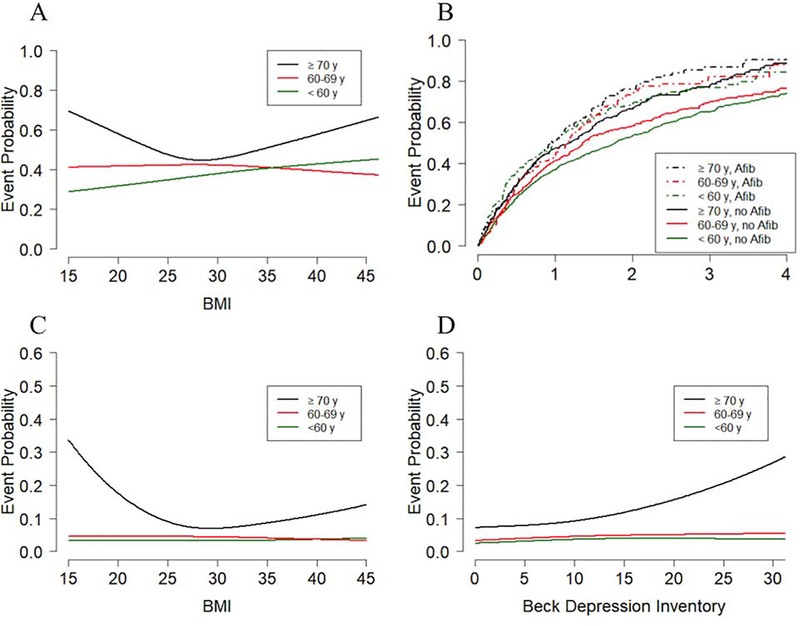
Table 3 shows interaction analyses for the primary endpoint by comorbidity status. None of the specified comorbidities had significant interactions with age although there were trends for BMI (interaction p-value=0.10) and Afib (interaction p-value=0.07). For BMI (Figure 1A) there was a significant (p=0.009) U-shaped relationship between BMI and the one-year primary endpoint rate in patients ≥70 years: a BMI=20 corresponded to a rate of 58%, a BMI=30, a rate of 45%, and a BMI=40, a rate of 58%. In contrast, for patients aged 60–69 years, as BMI increased from 20 to 40, the primary event rate non-significantly changed from 42% to 39% (p=0.56 for the BMI slope coefficient). For patients aged <60 years, the event rate increased from 32% to 43% (p=0.0006 for the BMI slope coefficient). The relationship between age and Afib on the primary outcome is shown in Figure 1B. As seen in Table 3, the relative impact of Afib on outcome was least prominent in the group ≥70 years.
Table 3.
Interaction between age and comorbidities for all-cause mortality or all-cause hospitalization
| Comorbidity | HR represents | Age-adjusted HR# (95% CI) | Age-comorbidity Interaction p-value |
|---|---|---|---|
| Body Mass Index | Refer to Figure 1 | 0.10 | |
| Atrial Fibrillation / Atrial Flutter | Yes vs. No | ≤60 y: 1.50 (1.24 – 1.82) 60–69 y: 1.32 (1.06–1.63) ≥70 y: 1.21 (0.98 – 1.49) |
0.07 |
| Ischemic Heart Disease | Yes vs. No | 1.15 (1.03 – 1.27) | 0.63 |
| Hypertension (History) | Yes vs. No | 1.21 (1.09 – 1.34) | 0.19 |
| Peripheral Vascular Disease | Yes vs. No | 1.41 (1.17 – 1.70) | 0.34 |
| Diabetes (History) | Yes vs. No | 1.25 (1.13 – 1.39) | 0.13 |
| COPD | Yes vs. No | 1.28 (1.10 – 1.48) | 0.59 |
| Anemia | Yes vs. No | 1.40 (1.23 – 1.58) | 0.61 |
| eGFR (truncated at 90) | 5 unit decrease | 1.05 (1.03 – 1.06) | 0.83 |
| Beck Depression Inventory | 5 unit increase | 1.09 (1.06 – 1.12) | 0.34 |
| Comorbidity score | Per comorbidity | 1.18 (1.14–1.22) | 0.50 |
Hazard ratio adjusted for age. Age truncated from below at 60 years.
Figure 1. Interaction of age and comorbidities on of all-cause mortality or all-hospitalization and all-cause mortality.
Displayed are curves for event probability for patients aged <60, 60–69 or ≥70 y. A) One-year probability of all-cause mortality or all-cause hospitalization by BMI and age. B) Kaplan-Meier event curves for all-cause mortality or all-cause hospitalization categorized by the presence or absence of Afib and age group. C) One-year all-cause mortality by BMI and age. D) One-year all-cause mortality by Beck Depression Inventory and age.
For the secondary outcome of all-cause mortality (Table 4), age had significant interactions with BMI, hypertension and depression. For BMI (Figure 1C) there was a significant (p=0.0001) U-shaped relationship between BMI and one-year mortality for patients ≥70 years: BMI=20, 17.6%, BMI=30, 7.0%, and BMI=40, 11.0%. In contrast, the mortality rate was essentially flat across BMI for the 2 younger groups. Higher levels of depressive symptoms as measured by the Beck Depression Inventory (BDI) were associated with greater mortality risk in older patients (interaction p-value=0.03, Figure 1D); for patients ≥70 years, the rate increased from 7.8% for a BDI score=5 to 15.6% for BDI=20 (p=0.0002 for the BDI slope coefficient). In contrast, depressive symptoms were unrelated to mortality in the 2 younger groups.
Table 4.
Interaction between age and comorbidities for all-cause mortality
| Comorbidity | HR represents | Age-adjusted HR# (95% CI) | Age-comorbidity Interaction p-value |
|---|---|---|---|
| Body Mass Index | Refer to Figure 1C | 0.03 | |
| Atrial Fibrillation / Atrial Flutter | Yes vs. No | 1.68 (1.34 – 2.09) | 0.23 |
| Ischemic Heart Disease | Yes vs. No | 1.16 (0.94 – 1.43) | 0.63 |
| Hypertension (History) | Yes vs. No | <60 y: 1.05 (0.76 – 1.45) 60–69 y: 1.02 (0.67 – 1.53) ≥70 y: 0.77 (0.53 – 1.10) |
0.10 |
| Peripheral Vascular Disease | Yes vs. No | 1.75 (1.28 – 2.41) | 0.37 |
| Diabetes (History) | Yes vs. No | 1.32 (1.08 – 1.62) | 0.56 |
| COPD | Yes vs. No | 1.73 (1.32 – 2.26) | 0.39 |
| Anemia | Yes vs. No | 1.39 (1.10 – 1.76) | 0.79 |
| eGFR (truncated at 90) | 5 unit decrease | 1.10 (1.07 – 1.13) | 0.20 |
| Beck Depression Inventory | 5 unit increase | <60 y: 1.05 (0.97 – 1.13) 60–69 y: 1.08 (0.97 – 1.20) ≥70 y: 1.27 (1.12 – 1.43) |
0.03 |
| Comorbidity score | Per comorbidity | 1.21 (1.13–1.30) | 0.30 |
Hazard ratio for each comorbidity adjusted for age. Age truncated from below at 60 years.
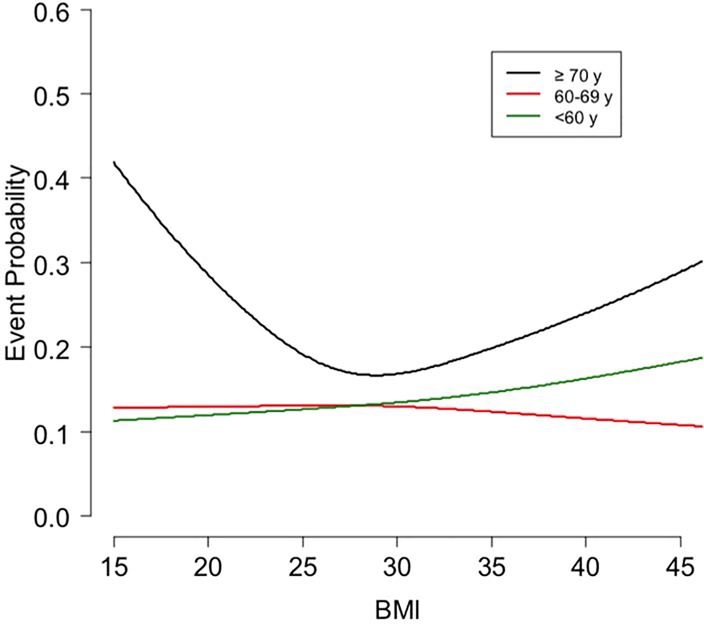
For the secondary endpoint of cardiovascular mortality and HF hospitalization (Supplementary Table 1), there were significant interactions between age and BMI, ischemic heart disease, and kidney function. For the relationship between BMI and the one-year endpoint (Figure 2) there was a significant (p=0.005) U-shaped relationship in patients ≥70 years: BMI=20, 29%, BMI=30, 17%, and BMI=40, 24%. In contrast, for patients aged 60–69 years, as BMI increased from 20 to 40, the primary event rate non-significantly changed from 13.0% to 11.6% (p=0.57 for the BMI slope coefficient). For patients aged <60 years, the event rate increased from 12.0% to 16.33% (p=0.002 for the BMI slope coefficient).
Figure 2. One-Year Cardiovascular mortality or HF hospitalization by BMI and age.
Displayed is the one-year event probability of the secondary outcome of cardiovascular mortality or HF hospitalization by BMI and age. Shown are curves of event probability by BMI with each curve representing patients aged <60, 60–69 or ≥70 y.
Cumulative Comorbidity Risk Model
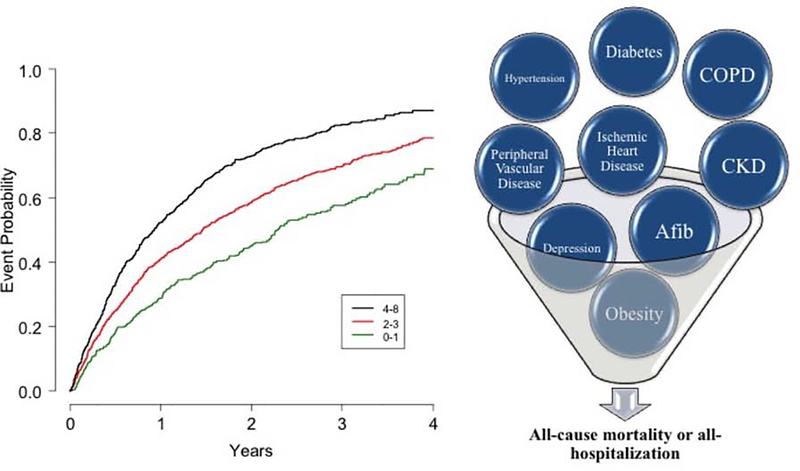
There were no significant differential associations between age and the cumulative comorbidity score for any of the clinical endpoints. Table 5A shows the model for impact of the cumulative comorbidity score, age, gender and CPX duration on the primary composite endpoint. CPX-determined exercise duration was the strongest predictor of the primary outcome; for every additional minute, there was a 9% lower risk for the primary endpoint. Age had a non-significant HR= 1.01 per 5 years. The c-index for the model including comorbidity score, age, sex and CPX duration was 0.619. Removing CPX duration led to a fairly large reduction in the model c-index to 0.577. The Central Illustration shows the probability of the primary outcome stratified by number of comorbidities, with greater risk in patients with 4–8 comorbidities, followed by those with 2–3 and 0–1, respectively (logrank p<0.0001). Supplementary Figure 2A displays the addition of age group to the number of comorbidities showing that the number of comorbidities drives the risk of the primary outcome (p=0.0003 for age category; p<0.0001 for 0–3 vs. 4–8 comorbidities).
Table 5A.
Cox Model for all-cause mortality or all-cause hospitalization
| Parameter | HR represents | HR (95% CI) | Chi-square | P-value |
|---|---|---|---|---|
| Comorbidity score | per comorbidity | 1.09 (1.05–1.13) | 20.6 | <0.0001 |
| Age* | per 5 years | 1.01 (0.98–1.05) | 0.51 | 0.48 |
| Sex | if female | 0.85 (0.75–0.96) | 7.2 | 0.007 |
| CPX duration | per minute | 0.91 (0.90–0.93) | 110 | <0.0001 |
Central Illustration. Impact of cumulative number of comorbidities on all-cause mortality or all-hospitalization.
Patients with chronic stable HFrEF who had a greater number of cumulative comorbidities had greater risk of adverse clinical outcomes with 9% greater risk per comorbidity. Displayed are Kaplan-Meier event curves for the primary outcome of all-cause mortality or all-cause hospitalization categorized by number of comorbidities. Patients were split into 0–1 (n=385), 2–3 (n=969), and 4–8 (n=713) comorbidities.
For the secondary outcome of all-cause mortality (Table 5B), CPX duration was the strongest predictor; for every additional minute there was a 16% lower risk. The comorbidity score had a non-significant HR of 1.06 per comorbidity, but gender was highly significant with female sex associated with 48% less risk. Age was significantly associated with a 14% greater risk for every 5 years. The c-index for the model including age, sex, comorbidity score, and CPX duration was 0.707. The c-index for the model which omits exercise duration was 0.637. Table 5C shows the model for the secondary endpoint of cardiovascular mortality or heart failure hospitalization with CPX again as the strongest predictor.
Table 5B.
Cox Model for all-cause mortality
| Parameter | HR represents | HR (95% CI) | Chi-square | P-value |
|---|---|---|---|---|
| Comorbidity score | per comorbidity | 1.06 (0.98–1.14) | 2.3 | 0.12 |
| Age* | per 5 years | 1.14 (1.06–1.22) | 13.8 | 0.0002 |
| Sex | if female | 0.52 (0.40–0.69) | 21.6 | <0.0001 |
| CPX duration | per minute | 0.84 (0.81–0.87) | 97 | <0.0001 |
Table 5C.
Cox Model for heart failure hospitalization or cardiovascular mortality
| Parameter | HR represents | HR (95% CI) | Chi-square | P-value |
|---|---|---|---|---|
| Comorbidity score | per comorbidity | 1.14 (1.08–1.20) | 20.9 | <0.0001 |
| Age* | per 5 years | 1.02 (0.96–1.08) | 0.43 | 0.51 |
| Sex | if female | 0.75 (0.63–0.90) | 9.2 | 0.002 |
| CPX duration | per minute | 0.87 (0.84–0.89) | 121 | <0.0001 |
Truncated from below at 60 years.
CPX=Cardiopulmonary Exercise
Discussion
Older patients with HFrEF have higher rates of adverse clinical outcomes, and have far more comorbidities than younger individuals. We examined the impact of age and specific comorbidities on clinical outcomes in a large cohort of chronic, stable HFrEF patients. In models including CPX duration, sex, and the cumulative comorbidity score, age remained an independent predictor of all-cause mortality, but not of the composite primary endpoint of all-cause mortality or hospitalization. Conversely, the cumulative comorbidity score was an independent predictor of all-cause mortality or hospitalization, but not of mortality. Age significantly impacted the effect of BMI and depression on outcomes. In older patients with HFrEF, there was a U-shaped relationship between BMI and risk of all-cause mortality, and presence of depression also contributed to a greater risk. For older patients the extremes of BMI, ischemic heart disease and lower eGFR contributed a greater risk of cardiovascular mortality or HF hospitalization. These results provide key insights regarding the interrelationships between comorbidities and HF outcomes (7,8) and provide an evidence-based rationale to prioritizing attention to specific comorbidities in the management of HF patients.
The present data suggest that the impact of comorbidities on outcomes differs depending on age. Specifically, there were significant differences in the relation between BMI or depression and all-cause mortality depending on age. Our data reinforce growing awareness and insights regarding the management of HF patients inherently prone to multimorbidity and complexity of care, particularly in older individuals. While multimorbidity is a challenge of older adults in general, HF patients may be particularly vulnerable, as comorbid diseases contribute to HF pathophysiology, and also have the potential to overwhelm limited cardiovascular reserves (15).
These data from the HF-ACTION trial of a large cohort of chronic, stable HFrEF patients highlight the characteristics and multiple comorbidities of older patients with HF, in whom the disease is most prevalent (16). Older patients had higher NYHA class and much greater prevalence of nearly all major comorbidities. This is consistent with prior studies describing the large burden of comorbidities in older patients with HF (4,17) and extends previous observations by demonstrating the effect of individual comorbidities across age groups. In our population, the proportion of patients with diabetes was greatest in patients aged 60–69 compared to those <60 or ≥70 years. Moderate or severe depressive symptoms were less prevalent in the older age groups, and BMI was significantly lower with age. These age-specific prevalence estimates provide a detailed characterization of comorbidities across age groups in patients with chronic, stable HFrEF.
Our results also provide insight into the interrelationship between the complexity of BMI and HF outcomes (18,19). Our data are consistent with prior studies showing greater rates of adverse clinical outcomes in HF patients with both very low and very high BMI (20–22). This U-shaped relationship in older patients held across all three clinical outcomes we examined. These data are similar to findings in patients with HF with preserved ejection fraction (HFpEF), in whom a U-shaped relationship for BMI and clinical outcomes was also found (23). Our data extend these prior findings by showing that older age significantly magnifies the relationship between BMI and poor outcomes, but provide contrast to the obesity paradox as BMI was not protective for younger HFrEF patients who have higher risk with increasing BMI. Obesity not only increases risk of incident HF, but also increases the risk of developing further comorbidities, adding complexity to the U-shape (24). A systematic review found improved outcomes in obese patients with HF compared to their normal weight counterparts, but patients with extreme obesity (BMI >40) had worse outcomes (25). The results of this study extend the existing literature showing increased risk in elderly patients with chronic, stable HFrEF at the extremes of BMI (26,27).
Depression is common in older adults and its prevalence and severity increases with age (28). In patients with HF it is under-diagnosed and under-treated (27,29). Studies have reported rates of major depression in hospitalized patients with HF to be 17–37% (30). For some patients, HF symptoms and other comorbidities may worsen depression, in turn leading to further inactivity and reduced medication adherence. The data for the older patients in this study are consistent with previous findings showing that a history of depression correlates with greater all-cause mortality in patients with HFrEF (31). In a prospective cohort study of 662 patients hospitalized with HF, depression was an independent risk factor for all-cause mortality over up to twenty years of follow-up (32). This study expands upon the previously available data showing that older age magnifies the relationship of depression with adverse outcomes in patients with chronic HFrEF.
Frailty was not directly measured in our study but is certainly relevant to the care of HF patients and there is overlap with certain comorbidities (33). Consideration should be given to the overlap between abnormal BMI and frailty as well as that fact that items within the BDI may be shared with depression, heart failure symptoms and frailty. Frailty has been associated with a number of comorbidities, though some patients were still at risk for frailty in the absence of comorbidities and the authors suggested sarcopenia as a potential contributor (34). These prior data and the findings of the current study, suggest that all patients with heart failure, particularly those at the extremes of BMI and with symptoms of depression, be screened for frailty. Particularly, patients over 70 may benefit from a comprehensive geriatric assessment.
Our model that included cumulative comorbidity score, age, gender and CPX duration showed that for every additional comorbidity, there was an average of 9% greater risk of the primary endpoint of all-cause mortality or hospitalization. This is similar to data from the Get with the Guidelines registry, which described increased risk of in-hospital mortality and 30-day mortality with additional comorbidities in hospitalized patients with HF (8). Consistent with a previously published model from the HF-ACTION trial, shorter exercise duration was a strong predictor of increased risk (31). Since age was strongly negatively correlated with CPX duration, age did not contribute significant additional prognostic value for the primary endpoint in that model. The finding that cumulative comorbidity score was not independently predictive of all-cause mortality is consistent with data from the Kungsholmen Project where 2 or more comorbidities were associated with disability but not mortality (35).
The current study has some limitations. Ascertainment of comorbidities was based on physician/coordinator assessment from a predetermined group of candidate comorbidities. These data are hypothesis generating and need validation in other datasets to support broader generalizability. Comorbidities such as cognitive dysfunction and a complete assessment of frailty are relevant to the elderly HF population, but were not measured in this study. Patients may under-report depressive symptoms, and change in depressive symptoms was not tracked over time. There was likely treatment heterogeneity for depression, and data were not stratified by modality of depression treatment. In our model for predicting the primary outcome, the c-index is lower than those published in previous models, likely related to the challenges in predicting hospitalization and the limited number of covariates we included compared to prior studies with more robust c-indexes (36,37).
Conclusion
In older HFrEF patients, BMI and depressive symptoms appear to have disproportionately greater impact, and the cumulative effect of comorbidities is a very important predictor of all-cause mortality or all-cause hospitalization. Specifically, in older patients, BMI displayed a U-shaped curve on outcomes, with greater risk at the extremes of low and high BMI; higher depression score was associated with greater risk of all-cause mortality. These findings highlight that the comorbidity profiles of patients with HF are typically more severe in older adults and that specific comorbidities may have differential prognostic significance as a function of age. Future studies are needed to determine if outcomes in older patients with HFrEF can be improved by treating these specific comorbidities.
Supplementary Material
Perspectives.
Core Clinical Competencies
In older patients with chronic, stable HFrEF there was a U-shaped relationship between BMI and increased mortality, and there was a significant impact of depression on mortality. A cumulative comorbidity score was a stronger predictor than age for all-cause mortality or hospitalization.
Translational Outlook
The greater role of BMI and depression on prognosis in older adults with HFrEF warrants additional studies to determine whether modifying these factors favorably affects the prognosis of these vulnerable patients.
Acknowledgments
Funding: The HF-ACTION () trial was funded by the National Heart, Lung and Blood Institute. Additional support was provided by U01HL064265.
Disclosures: DWK receives research support from the National Institutes of Health R01AG18917, R01AG045551, P30-AG21331, and U24-AG059624, and the Kermit G. Phillips II Endowed Chair; reports stock ownership from Gilead, personal fees from Corvia Medical, Relypsa, AbbVie, Akros, St. Luke’s Hospital, Kansas City, MO, Merck, Boehringer-Ingelheim, DCRI and CinRx; grants and personal fees from Novartis, Bayer; and Astra-Zeneca. DEF is supported by NIA R01 AG060499–01, R01AG058883, R01AG051376, R01AG053952, P30AG024827 and NIH UO1AR071130. RJM receives research support from the National Institutes of Health (U01HL125511–01A1, U10HL110312 and R01AG045551–01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold/American Regent, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations List
- 6MWD
6-min walk distance
- BMI
body mass index
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- CPX
cardiopulmonary exercise test
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
Footnotes
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
Trial Registration: clinicaltrials.gov Identifier:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin 2017;13:417–426. [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 3.Mentz RJ, Kelly JP, von Lueder TG et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunstein JB, Anderson GF, Gerstenblith G et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003;42:1226–33. [DOI] [PubMed] [Google Scholar]
- 5.Mentz RJ, Felker GM. Noncardiac comorbidities and acute heart failure patients. Heart Fail Clin 2013;9:359–67, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 7.Iorio A, Senni M, Barbati G et al. Prevalence and prognostic impact of non-cardiac comorbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail 2018. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Zhao X, Hammill BG et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail 2018;11:e004646. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whellan DJ, O’Connor CM, Lee KL et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J 2007;153:201–11. [DOI] [PubMed] [Google Scholar]
- 11.Lin DY WL. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association 1989;84:1074–8. [Google Scholar]
- 12.Beck AT SR, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 1988;Volume 8, Issue 1:Pages 77–100. [Google Scholar]
- 13.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 2013;3(1):1–150. [Google Scholar]
- 14.R Core Team (2017) R: A language and environment for statistical computing Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 15.Forman DE, Maurer MS, Boyd C et al. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol 2018;71:2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzman DW, Gardin JM, Gottdiener JS et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001;87:413–9. [DOI] [PubMed] [Google Scholar]
- 17.van Deursen VM, Urso R, Laroche C et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16:103–11. [DOI] [PubMed] [Google Scholar]
- 18.Zamboni M, Rossi AP, Corzato F, Bambace C, Mazzali G, Fantin F. Sarcopenia, cachexia and congestive heart failure in the elderly. Endocr Metab Immune Disord Drug Targets 2013;13:58–67. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, De Schutter A, Alpert MA, Mehra MR, Milani RV, Ventura HO. Obesity paradox, cachexia, frailty, and heart failure. Heart Fail Clin 2014;10:319–26. [DOI] [PubMed] [Google Scholar]
- 20.Khaled S, Matahen R. Obesity paradox in heart failure patients - Female gender characteristics-KAMC-single center experience. Egypt Heart J 2017;69:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murad K, Goff DC Jr., TM Morgan et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The Cardiovascular Health Study. JACC Heart Fail 2015;3:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenchaiah S, Pocock SJ, Wang D et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;116:627–36. [DOI] [PubMed] [Google Scholar]
- 23.Haass M, Kitzman DW, Anand IS et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 2011;4:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alagiakrishnan K, Banach M, Ahmed A, Aronow WS. Complex relationship of obesity and obesity paradox in heart failure - higher risk of developing heart failure and better outcomes in established heart failure. Ann Med 2016;48:603–613. [DOI] [PubMed] [Google Scholar]
- 25.Oga EA, Eseyin OR. The obesity paradox and heart failure: A systematic review of a decade of evidence. J Obes 2016;2016:9040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vest AR, Chan M, Deswal A et al. Nutrition, obesity and cachexia in patients with heart failure: A Consensus Statement from the HFSA Scientific Statements Committee. J Card Fail 2019. [DOI] [PubMed] [Google Scholar]
- 27.Reeves GR, Whellan DJ, Duncan P et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok RM, Reynolds CF 3rd.,Management of depression in older adults: A Review. JAMA 2017;317:2114–2122. [DOI] [PubMed] [Google Scholar]
- 29.Reeves GR, Whellan DJ, O’Connor CM et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: The REHAB-HF Pilot Study. JACC Heart Fail 2017;5:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003;65:119–28. [DOI] [PubMed] [Google Scholar]
- 31.Adelborg K, Schmidt M, Sundboll J et al. Mortality risk among heart failure patients with depression: A nationwide population-based cohort study. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedland KE, Hesseler MJ, Carney RM et al. Major depression and long-term survival of patients with heart failure. Psychosom Med 2016;78:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev 2012;17:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 35.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 2009;265:288–95. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor CM, Whellan DJ, Wojdyla D et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail 2012;5:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahimi K, Bennett D, Conrad N et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.