Abstract
Background
The phytochemical ingredients of berries have been used in the treatment of various bodily ailments; while their roles in preventing the severity of glaucoma are poorly understood. Hence, the present study was framed to investigate whether ethanolic extracts of Lycium barbarum exerts protection against the onset of glaucoma using cultured PC12 neuronal cells by modulating the expression of extracellular matrix proteins.
Material/Methods
In order to develop glaucoma like condition in cells, cultured PC12 cells were subjected to 50 and 100 mmHg hydrostatic pressure for 24 hours. The pressure exposed cells were analyzed for the expression of glaucoma markers such as ANGPTL7 and the expressions of extracellular matrix proteins in the presence and absence of L. barbarum, matrix metalloproteinase (MMP)-9 inhibitor, and latanoprost, a current drug for the treatment of glaucoma.
Results
PC12 cells exposed to hydrostatic pressures (50 and 100 mmHg) increased the expression of glaucoma marker, ANGPTL7. Moreover, results have demonstrated the significant changes in the expression of MMP-2, MMP-9, collagen I, and TGF-β at the gene level. In contrast, cells pretreated with L. barbarum extracts showed reduced expression of ANGPTL7 and extracellular matrix proteins compared to control. Furthermore, to elucidate the role of MMP-9 in the onset of glaucoma, cells were silenced using MMP-9 inhibitor along with L. barbarum demonstrated a significant reduction in the glaucoma marker ANGPTL7 while improving the expression of caveolin-1 expression in cells subjected to pressure.
Conclusions
The extract of L. barbarum protects the cells from intraocular pressure by activating caveolin-1 dependent pathway via inhibition of MMP-9 expression.
MeSH Keywords: Gene Expression; Glaucoma, Angle-Closure; Injections, Intraocular; Matrilin Proteins
Background
Nature has always provided with an extensive and infinite source of biologically active compounds. Meanwhile, looming major health complications become a threat to humankind. Amongst these, the development of glaucoma is a major cause of blindness. Reports have shown that glaucoma is the world’s second most important factor for irreversible blindness, and symptoms are often not noted until the disease worsens [1]. The major problem associated with glaucoma is intraocular pressure [2]. Many patients lose their vision, even when using therapies to lower intraocular pressure [3]. Recently, non-intraocular pressure strategies to improve retinal lesions in glaucoma have received attention, including novel treatment strategies [4]. Matrix metalloproteinases (MMPs) which belong to a metzincin superfamily consists of 23 MMPs. MMPs showed higher specificity and selectivity against different extracellular matrix proteins, growth factors, cytokines, and membrane proteins. MMPs are also significantly expressed in the trabecular mesh, retina, aqueous humor, and optic nerves of the glaucoma patient and in experimental animal glaucoma models; the MMP-9 has been well-characterized in the retina in apoptotic retinal ganglion cells and its increased expression is correlated with enhanced degradation of laminin in the retinal ganglion cell layer [5,6]. On the other hand, reports have shown that MMP-9 deficit showed neuroprotective properties in a glaucoma optic nerve ligation model [7]. The search for drugs showing little side effects and with beneficial effects on ocular disease treatment if ongoing; one study found anti-glaucoma effects of Lycium barbarum in an experimental rat model of ocular hypertension using an oral route of drug administration. These results demonstrated a significant reduction in the loss of retinal ganglion cells while no change in the ocular pressure [8]. Hence, in the present study, the effect of L. barbarum on the PC12 cells was attempted with the aim of targeting the extracellular matrix proteins.
L. barbarum, commonly referred to as goji berry, has been used in China and throughout Asia for medicinal properties as well as a functional food for at least 2000 years. L. barbarum has been used for anti-aging, beneficial effects on vision, improving the immune system, strengthening the body’s liver, kidney and lung activity, as well as antioxidant, anti-inflammatory, hepatoprotective effects and renal protection [9–14]. L. barbarum’s effect has been attributed to the presence of about 40% of the polysaccharide’s composition; studies in rats demonstrated a neuroprotective effect after ingestion of L. barbarum extract in experimental models of retinal ischemia and optic nerve section. The whole plant extract has also shown protection against oxidative and apoptotic effects in cultures of human lens epithelial cells [15,16]. A recent study has shown that the ethanolic extracts of L. barbarum exhibited significant protection on UVB-induced growth arrest of in human retinal pigment epithelial cells (ARPE-19) [17].
In this study, cultured PC12 neuronal cells were used and the modulations in the expression of extracellular matrix proteins were elucidated. Here, we hypothesized that the extract of L. barbarum protects the cells from intraocular pressure by activating the caveolin-1 dependent pathway via inhibition of MMP-9 expression.
Material and Methods
Chemicals
Dulbecco’s modified Eagle’s Medium (DMEM), fetal bovine serum, penicillin, streptomycin, MMP-9 inhibitor [(2R)-2-[(4-biphenylylsulfonyl) amino]-3-phenylpropionic acid] and latanoprost were obtained from Sigma Aldrich, USA. Antibodies such as ANGPTL7 antibody caveolin-1 antibody, β-actin, and HRP conjugated secondary antibodies were obtained from Santa Cruz, CA, USA. SYBR green master mix was obtained from BioRad, USA. First-strand cDNA Synthesis Kit was from Takara PrimeScript™, USA. Primers were obtained from Eurofins MWG Operon, USA. All other reagents used were analytical grade.
Induction of glaucoma
In order to develop glaucoma-like symptoms, the cells were subjected to stable hydrostatic pressure using the in-house designed, modified pressure chamber as described in a previous publication [18]. The chamber inlet was joined through a low-pressure 2-stage regulator to a gas tank, and the needle valve gas-pressure controllers contributed constant hydrostatic pressure varying from 0 to 220 mmHg. The hydrostatic pressure in the chamber was examined and regulated by means of a pressure gauge directly linked to the inlet circuit near the chamber, and the controlled gas flow was performed with the help of a ball-type flow gauge regulated with an outlet needle valve.
Preparation of L. barbarum extract
L. barbarum fruits were purchased from the local market and botanically identified and verified at the Medicinal Plant Research Laboratory. The aqueous extract was prepared by grinding and pulverizing the fruits and extracted using 20% ethanolic solution for 3 hours in a reflux extraction apparatus followed by filtration through filter paper. The resulting filtrate was lyophilized to produce a powder by the freeze-drying procedure. The obtained powdered extract of L. barbarum was stored in the cold until being used for the experiment. All other chemicals used in the present investigation are analytical reagent grade.
Cell culture
PC12 cells were received from American Type Culture Collection (ATCC) and cultured as suggested by ATCC. The cells were grown in T75 flasks with Dulbecco’s modified Eagle’s Medium (DMEM) containing fetal bovine serum (10%), penicillin (100 units/mL) and streptomycin (50 μg/mL). Cultures were incubated at 37°C in a humidified incubator containing 95% air and 5% CO2. For differentiation, cells were dissociated and plated on poly-D-lysine coated in differentiating DMEM containing 100 ng/mL of NGF with growth medium for various experiments. In order to elucidate the effect of L. barbarum, the cells were then exposed to pressure with or without L. barbarum extract. Moreover, to identify the signaling mechanism, the cells were treated with known MMP-9 inhibitor and latanoprost, a current drug for the treatment of glaucoma solubilized in dimethyl sulfoxide (DMSO) and diluted in phosphate-buffered saline (PBS) (0.05% DMSO).
Experimental studies
Cells treated with drugs were tested for the expression of altered mitochondrial enzymes using the citrate synthase, malate dehydrogenase, succinyl-CoA synthetase, aconitase, 2-oxo glutarate dehydrogenase, succinic dehydrogenase, and fumarase using Abcam enzyme assay kits as per the manufacturer’s instructions (Abcam, USA).
Western blotting
Control and experimental cells exposed to drugs and plant extract were scrapped from the culture plates and sonicated to release the proteins. The extracted protein samples were mixed with 4× Laemmli sodium dodecyl sulfate (SDS) sample buffer, with reducing agents and ran in SDS-polyacrylamide gels. After the completion of electrophoresis, the proteins were blotted onto polyvinylidene difluoride (PVDF) membrane, and blocked in PBS/1% bovine serum albumin (BSA) for 1 hour, and probed with goat anti-human ANGPTL7 antibody (1: 250 dilution), caveolin-1 antibody (1: 100 dilution) and β-actin for endogenous control at 4°C. At the end of incubation, the membrane was washed and probed for the corresponding HRP labeled secondary antibody for 1 hour at room temperature and washed followed by visualized using ECL chemiluminescence system and the band intensities were determined using NIH ImageJ software.
Real-time quantitative polymerase chain reaction (RT-qPCR)
At the end of the experimental period, total RNA was extracted, and the first-strand cDNA synthesis was proceeded with 1 μg of total RNA according to the manufacturer’s instruction. Real-time quantitative polymerase chain reaction (RT-qPCR) was carried out using the SYBR green kit on the Bio-Rad real-time PCR system. The forward (F) and reverse (R) primers used were:
MMP-2: F-5′-CTATTCTGTCAGCACTTTGG-3′;
R-5′-CAGACTTTGGTTCTCCAACTT-3′,
MMP-9: F-5′-AGTTTGGTGTCGCGGAGCAC-3′;
R-5′-TACATGAGCGCTTCCGGCAC-3′,
Collagen 1: F-5′-CCCACGTAGGTGTCCTAAAGT-3′;
R5′-CCGTGGTGCTAAAATAATAAA-3′,
TGF-β: F-5′-TGGAAGTGGATCCACGCGCCCAAGG-3′;
R-5′-GCAGGAGCGCACGATCATGTTGGAC-3′,
GAPDH: F-5′-TCCACCACCCTGTTGCTGTAGC-3′;
R-5′-TGGAAAGCTGTGGCGTGATG-3.
The following reaction conditions such as 95°C for 3 minutes, followed by 35 cycles at 95°C for 15 seconds, annealing and extension for the 30 seconds was followed for qPCR analysis. The reaction mixture that devoid of template cDNA was used as negative controls. The comparative threshold (Ct) values were determined with qPCR software, and the gene expression changes were quantified by the 2−ΔΔCt methods. The gene expression was normalized to GAPDH values, an endogenous control within each sample and relative to positive and negative controls.
Statistical analysis
The results are indicated as means±standard error (SE). The statistical significance was assessed using GraphPad Prism tool (GraphPad Software, San Diego, CA, USA) as a one-way analysis of variance (ANOVA). Significant was the P-value of <0.05.
Results
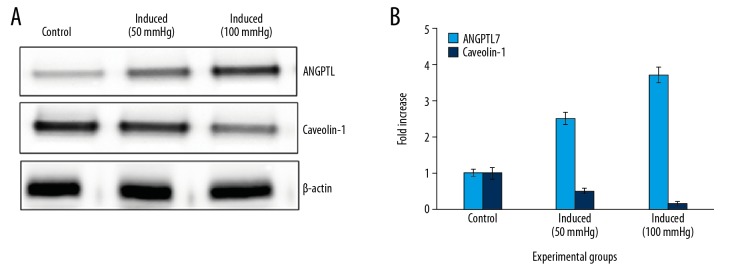
To evaluate the protective functions of L. barbarum extracts against the onset of glaucoma, PC12 neuronal cells were grown under controlled pressure conditions, and the extracellular matrix protein expressions were tested. The hydrostatic pressure-induced glaucoma like symptoms was initially confirmed in the cells through the elucidation of ANGPTL7 protein expression. The results demonstrated a significant (P<0.01) increase in the expression of ANGPTL7 is a pressure dose-dependent manner (Figure 1). Hence, the work was preceded with the 50 mmHg hydrostatic pressures to illuminate the protective role of L. barbarum. Additionally, cells exposed to hydrostatic pressures (50 mmHg) altered (P<0.01) the mitochondrial enzymes compared to control. While the cells pre-treated with extracts of L. barbarum restored the mitochondrial enzymes (Table 1).
Figure 1.
Western blot analysis of ANGPTL and caveolin proteins in the control and pressure of exposed cells. (A) Representative western blots showing the protein expression. (B) The densitometry analysis of proteins normalized to β-actin expression. Values are expressed as means±standard error (SE) (n=6 in each group). Statistical significance expressed as * P<0.05; ** P<0.01 compared to respective control. LB – Lycium barbarum; LP – latanoprost.
Table 1.
The activities of mitochondrial enzymes in control and the experimental group of cells are represented.
| Enzymes (IU/min/mg protein) | Control | Induced | Lycium barbarum treated |
|---|---|---|---|
| Citrate synthase | 2.27±0.24 | 1.17±0.16 | 2.03±0.65 |
| Aconitase | 0.073±0.0035 | 0.019±0.0020 | 0.064±0.0039 |
| Succinyl-coa synthetase | 0.031±0.0073 | 0.012±0.0061 | 0.030±0.0066 |
| Succinic dehydrogenase | 0.004±0.0012 | 0.002±0.0018 | 0.0039±0.0014 |
| Fumarase | 0.078±0.45 | 0.044±0.29 | 0.072±0.77 |
| Malate dehydrogenase | 1.76±0.16 | 1.04±0.17 | 1.72±0.11 |
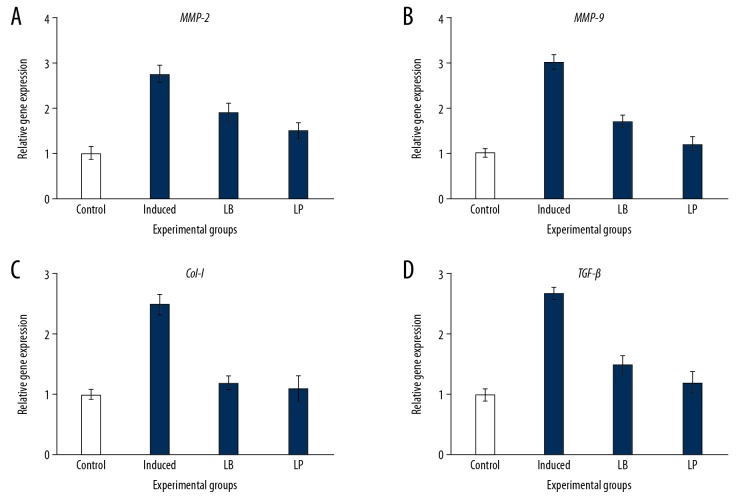
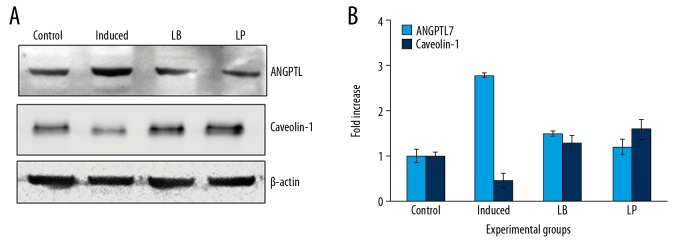
It is well documented that the induction of pressure directly alters the mediators of the extracellular matrix proteins which directly contribute to the fibrotic responses in cells. Hence in the present study, the expression of extracellular matrix proteins was illuminated in cells exposed to hydrostatic pressures. The increased (P<0.01) transcripts level of MMP-2, MMP-9, TGF-β1, and Collagen I genes was observed in pressure-induced cells compared to control (Figure 2). Further, the expression of caveolin-1 was elucidated in cells treated with or without extracts of L. barbarum and latanoprost, a current drug for the treatment of glaucoma. The western blotting analysis of ANGPTL7 and caveolin-1 demonstrated astonishing data. Cells treated with L. barbarum and latanoprost prevented the expression of ANGPTL7 compared to control while the expression of caveolin-1 has been increased to 2-fold on treatment with the plant extract showed the defensive role of L. barbarum against glaucoma (Figure 3). Moreover, it was speculated and found from the results that MMP-9 plays a major role in glaucoma aggression; hence, to elucidate the mechanism, the cells were treated with MMP-9 inhibitor.
Figure 2.
Expression analysis of extracellular matrix proteins in control and experimental group of cells respectively. (A–D). Represents the mRNA expression of matrix proteins such as MMP-2, MMP-9, Collagen-I, TGF-β normalized to GAPDH expression. Values are expressed as means±standard error (SE) (n=6 in each group). Statistical significance expressed as * P<0.05; ** P<0.01 compared to control group. LB – Lycium barbarum; LP – latanoprost.
Figure 3.
Western blot analysis of ANGPTL and caveolin proteins in the control and experimental group of cells. (A) Representative western blots showing the protein expression. (B) The densitometry analysis of proteins normalized to β-actin expression. Values are expressed as means±standard error (SE) (n=6 in each group). Statistical significance expressed as * P<0.05; ** P<0.01 compared to respective control. LB – Lycium barbarum; LP – latanoprost.
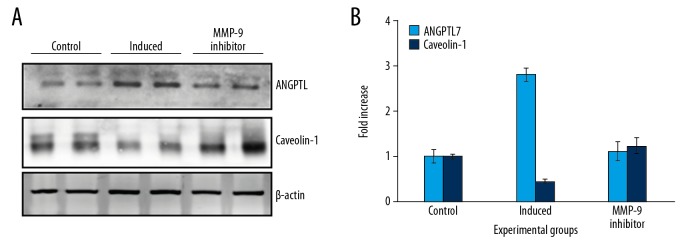
The results of the experiment showed that on inhibition of MMP-9 using the synthetic chemical, the expression of ANGPTL7 was reduced to the level equivalent (P<0.01) to control cells (Figure 4). In addition, the expression of caveolin-1 was also maintained in cells is correlated with the control cell and in cells pre-treated with extracts of L. barbarum. Thus, the results of the study demonstrated the attenuated (P<0.01) expression of glaucoma marker ANGPTL7 on MMP-9 inhibition supports the view that activation of MMP-9 contributes early to glaucomatous symptoms in optic cells via ANGPTL7 dependent pathway.
Figure 4.
Western blot analysis of ANGPTL and caveolin proteins in the control and the experimental group of cells. (A) Representative western blots showing the protein expression. (B) The densitometry analysis of proteins normalized to β-actin expression. Values are expressed as means±standard error (SE) (n=6 in each group). Statistical significance expressed as * P<0.05; ** P<0.01 compared to respective control.
Discussion
Glaucoma, the world’s second leading cause of blindness, is accompanied by the loss of stomatous retinal ganglion cells and high intraocular pressure is considered as crucial factor for glaucoma [2]. It has been reported that the extracts of L. barbarum exert dose-dependent alleviation of dry eye disease symptoms [19]. It was also suggested that the polysaccharides and betaine found in the L. barbarum extracts alleviate the oxidative stress and inflammation-mediated dry eye disease symptoms [20]. In accordance with our study, several previous reports showed that L. barbarum extracts have the potential to protect against oxidative stresses which is the major cause for the disturbance in retinal cells. For instance, the supplementation of L. barbarum polysaccharides in the high-fat mice liver oxidative injury, potentially improved the antioxidant status (enhanced the antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and total antioxidant capacity (TAOC) as well as antiperoxidative (lower malondialdehyde [MDA] level) status [21]. Similarly, latanoprost and L. barbarum polysaccharides exert protection to liver against oxidative injury and allergy through enhancement of GSH-Px, SOD, and suppression of the malondialdehyde (MDA) and inflammatory cytokine such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [22].
L. barbarum polysaccharides protect human lens epithelial cells from H2O2-mediated apoptosis by regulating the production of ROS, loss of mitochondrial potential, Bcl-2 family and antioxidant enzyme activity and decreasing cellular senescence [23]. All these studies suggested that the extracts of L. barbarum could provide protection against oxidative stress through the induction of antioxidant enzymes and reduced the peroxidation (lower MDA level). Moreover, L. barbarum polysaccharide extracts provide protection in the retina after ischemia-reperfusion through enhanced the Nrf2 and HO-1 expression [24]. Therefore, in the present study, the PC12 neuronal cells exposed to hydrostatic pressures can restore the activity of mitochondrial enzymes might be due to the induction of antioxidant enzymes and reduction of MDA levels.
MMPs belong to the zinc-dependent endopeptidase family perform extracellular matrix and basement membranes proteolytic destruction. They are critical for invasion and metastasis of tumors [25]. The well-studied MMPs, such as MMP-2 and MMP-9, are mostly associated with the cancer invasion and metastasis [26]. In line with our study, the elevated intraocular pressure was also shown to stimulate the MMP-9 matrix activation that leads to retinal dysfunction. In addition, the protein expression of MMP1, MMP9, MMP12, and IL-1β was higher in the aqueous humor collected from patients. Furthermore, the upregulation of MMPs is recognized as a risk factor for the formation of primary open angle glaucoma corroborates the present hypothesis on the involvement of MMPs in glaucoma development [27].
It was also reported that the enhanced expression of MMP9 leads to the loss of retinal ganglion cells [7]. While cells pre-treated with extracts of L. barbarum prevented the expression of extracellular matrix proteins demonstrate that the possible mechanism by which the plant drug exerts the protective mechanism is by inhibiting the MMP pathway. In accordance with our study, the fungus derived theissenolactone C exhibits retino-protective activity through the suppression of MMP-9 expression in intraocular pressure-induced ischemia/reperfusion injury model of glaucoma [28]. The theissenolactone C extracted from the fungi exhibited retino-protective activity by inhibition of MMP-9 expression in intraocular pressure-induced glaucoma in ischemia/reperfusion model [28].
Conclusions
The present study aimed at investigating the protective role of L. barbarum extract on glaucoma using in vitro grown PC12 neuronal cells by modulating the expression of extracellular matrix proteins. Glaucoma like symptoms was induced in PC12 cells through hydrostatic pressure. The results demonstrated the expression of ANGPTL7, MMP-2, MMP-9, collagen I, and TGF-β were upregulated at the transcript level in the pressure-induced cells, whereas, their expression was found to be lower in the cells pretreated with L. barbarum extract, with improved expression of caveolin-1 and mitochondrial enzymes in cells subjected to pressure. From these research findings, we concluded that the L. barbarum extract protects cells against intraocular pressure through stimulation of caveolin-1 dependent pathway via inhibition of MMP-9 expression. Further studies are required to find out the role of L. barbarum in the prevention of glaucoma in the animal model and their role in the restoration of mitochondrial enzymes in glaucoma induced animals.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Calkins DJ, Horner PJ. The cell and molecular biology of glaucoma: Axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012;53:2482–84. doi: 10.1167/iovs.12-9483i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern DL, Grosskreutz CL. Glaucomatous optic neuropathy: Mechanisms of disease. Ophthalmol Clin North Am. 2002;15:61–68. doi: 10.1016/s0896-1549(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Guymer C, Wood JP, Chidlow G, et al. Neuroprotection in glaucoma: Recent advances and clinical translation. Clin Exp Ophthalmol. 2019;47(1):88–105. doi: 10.1111/ceo.13336. [DOI] [PubMed] [Google Scholar]
- 5.De Groef L, Van Hove I, Dekeyster E, et al. MMPs in the neuroretina and optic nerve: Modulators of glaucoma pathogenesis and repair? Invest Ophthalmol Vis Sci. 2014;55:1953–64. doi: 10.1167/iovs.13-13630. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Huang W, Zhang X, et al. Chemokine (C-C motif) ligand 2 and chemokine (C-C motif) ligand 7 in angle-closure glaucoma. Acta Ophthalmol. 2015;94:e220–24. doi: 10.1111/aos.12696. [DOI] [PubMed] [Google Scholar]
- 7.Chintala SK, Zhang X, Austin JS, et al. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem. 2002;277:47461–68. doi: 10.1074/jbc.M204824200. [DOI] [PubMed] [Google Scholar]
- 8.Chan HC, Chang RC, Koon-Ching IA, et al. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203(1):269–73. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Adams M, Wiedenmann M, Tittel G, et al. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phytochem Anal. 2006;17(5):279–83. doi: 10.1002/pca.915. [DOI] [PubMed] [Google Scholar]
- 10.Du LQ, Wang HZ, Huang FC, et al. Genetic transformation of Lycium barbarum L. via A. tumefaciens. Sci China B. 1994;37(3):286–92. [PubMed] [Google Scholar]
- 11.Hai-Yang G, Ping S, Li JI, et al. Therapeutic effects of Lycium barbarum polysaccharide (LBP) on mitomycin C (MMC)-induced myelosuppressive mice. J Exp Ther Oncol. 2004;4(3):181–87. [PubMed] [Google Scholar]
- 12.Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28(5):643–52. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J, Zhou ZW, Sheng HP, et al. An evidence – based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2014;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing X, Liu F, Xiao J, So KF. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med. 2016;18:253–63. doi: 10.1007/s12017-016-8393-y. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Exp Ophthalmol. 2017;45(7):717–29. doi: 10.1111/ceo.12950. [DOI] [PubMed] [Google Scholar]
- 16.Li HY, Ruan YW, Kau PW, et al. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015;24(3):403–17. doi: 10.3727/096368915X686896. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh FC, Hung CT, Cheng KC, et al. Protective effects of Lycium barbarum extracts on UVB-induced damage in human retinal pigment epithelial cells accompanied by attenuating ROS and DNA damage. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/4814928. 4814928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Ju WK, Crowston JG, et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007;48(10):45800–9. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 19.Chien KJ, Horng CT, Huang YS, et al. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol Med Rep. 2018;17:809–18. doi: 10.3892/mmr.2017.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I. Betaine is a positive regulator of mitochondrial respiration. Biochem Biophys Res Commun. 2015;456:621–25. doi: 10.1016/j.bbrc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Wua HT, He XJ, Hong YK, et al. Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int J Biol Macromolecules. 2010;46:540–43. doi: 10.1016/j.ijbiomac.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Sun L, Yang Y, et al. Preventive and therapeutic effects of pigment and polysaccharides in Lycium barbarum on alcohol-induced fatty liver disease in mice, CyTA. J Food. 2018;16(1):938–49. [Google Scholar]
- 23.Qi B, Ji Q, Wen Y, et al. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress–induced apoptosis and senescence. PLoS One. 2014;9(10):e110275. doi: 10.1371/journal.pone.0110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He M, Pan H, Chang RC, et al. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9(1):e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabeshima K, Inoue T, Shimao Y, et al. Matrix metalloproteinases in tumor invasion role for cell migration. Pathol Int. 2002;52:255–64. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 26.Khasigov PZ, Podobed OV, Gracheva TS, et al. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry (Mosc) 2003;68:711–17. doi: 10.1023/a:1025051214001. [DOI] [PubMed] [Google Scholar]
- 27.Markiewicz L, Pytel D, Mucha B, et al. Altered expression levels of MMP1, MMP9, MMP12, TIMP1, and IL-1β as a risk factor for the elevated IOP and optic nerve head damage in the primary open-angle glaucoma patients. Biomed Res Int. 2015;2015 doi: 10.1155/2015/812503. 812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin FL, Cheng YW, Yua M, et al. The fungus-derived retino-protectant theissenolactone C improves glaucoma-like injury mediated by MMP-9 inhibition. Phytomedicine. 2019;56:207–14. doi: 10.1016/j.phymed.2018.11.002. [DOI] [PubMed] [Google Scholar]