Abstract
Purpose of Review
This review examines associations between FGF-23 and cardiovascular disease.
Recent findings
FGF-23 is a hormone produced by osteocytes and osteoblasts which aids with phosphate excretion by the kidney and acts as a negative feedback regulator for activated vitamin D synthesis. Recent studies have found associations between elevated FGF-23 levels and a number of cardiovascular diseases including hypertension, left ventricular hypertrophy, endothelial dysfunction and cardiovascular events and mortality.
Summary
Recent studies have explored the possible effects of FGF-23 on the cardiovascular system. In animal and observational human studies, there is a link between elevated FGF-23 levels and multiple cardiovascular outcomes, including hypertension, left ventricular hypertrophy and cardiovascular events and mortality. Further studies are required to evaluate whether decreasing FGF-23 levels improves cardiovascular outcomes.
Keywords: FGF-23, Cardiovascular disease, vitamin D, Phosphorus, chronic kidney disease
Introduction
Fibroblast growth factor 23 (FGF-23), a ~32 kDa hormone secreted by osteocytes and osteoblasts, is a major regulator of vitamin D and phosphate homeostasis. In the kidney, FGF-23 most commonly binds to an FGF receptor and α-klotho together in order to have biologic activity. The Klotho null mouse and the FGF-23 null mouse exhibit a similar rapid aging phenotype, with hyperphosphatemia, increased 1,25 dihydroxyvitamin D levels, and ectopic calcification.1,2 High levels of FGF-23 produced by tumors or due to constant activation of FGF-23 receptors by missense gene mutations, can cause increased phosphate urinary excretion by downregulating NPT2a in the proximal tubule and hypophosphatemia.3–5 High levels of FGF-23 also suppress the renal expression of 1- α hydroxylase (CYP27B1) and increase activity of the 24-hydroxylase enzyme (CYP24A1),6 which together result in decreased levels of the active 1, 25-dihydroxyvitamin D, decreased phosphate absorption in the intestines and rickets or osteomalacia.7
FGF-23 has been associated with numerous different manifestations of cardiovascular disease (CVD). Animal studies reveal direct toxicities of an infusion of FGF-23 in the form of myocyte hypertrophy and left ventricular hypertrophy (LVH).8 Previous studies have linked LVH, cardiovascular and all-cause mortality to higher levels of FGF-23.8–10 Results of several studies reveal associations between higher FGF-23 and an increased risk of cardiovascular events, independent of kidney function or serum levels of other mineral metabolites.11–14 An independent risk of heart failure as well as cardiovascular death has been seen in stable ischemic heart disease patients with elevated levels of FGF-23 regardless of renal function.15 Interestingly, recent data also reveals associations between elevated FGF-23 levels and hypertension.16 The aim of this review is to present the most updated research on FGF-23 and its associations with CVD.
REGULATORS OF THE RELEASE OF FGF-23
There appear to be several key regulators of FGF-23 including 1, 25-dihydroxyvitamin D, parathyroid hormone (PTH), phosphate intake and possibly others. FGF-23 levels increase as GFR falls, likely due to the need for more phosphate excretion as nephrons fail. Therapy with activated vitamin D compounds, such as calcitriol, paricalcitol or doxercalciferol, cause FGF-23 elevations.17,18 Treatment with nutritional vitamin D compounds, such as cholecalciferol and ergocalciferol, has been shown to increase FGF-23 in some studies19–21 but not in others.22 Therefore, it is somewhat unclear at this time whether nutritional vitamin D affects FGF-23 levels. Dietary phosphate and PTH are other important regulator of FGF-23.23,24 Activated vitamin D, dietary phosphate and PTH are regulators that are physiologically consistent with the actions of FGF-23. Newer regulators have been described that need further exploration. In a pilot study of 20 patients with CKD, therapy with sodium bicarbonate elevated FGF-23 over a 6 week period.25 Experimental induction of a myocardial infarction in a rat model also led to elevation of FGF-23 levels, independent of changes in PTH and klotho.26 In addition, patients with acute decompensated heart failure had much higher levels of FGF-23 compared to controls but the excess FGF-23 was not of myocardial origin, suggesting that control of FGF-23 is more complicated than previously thought.27 Further research is needed to evaluate the mechanisms behind these observations.
FGF-23 AND CHRONIC KIDNEY DISEASE
FGF-23 levels increase as CKD progresses and elevated FGF-23 levels are often the first mineral metabolic marker that changes in CKD.28 Alongside this change, renal klotho levels are also seen to decrease in patients with CKD prior to FGF-23 elevation.29 As the kidneys fail, they become less able to excrete the body’s phosphate load. Elevated phosphate levels have been associated with early mortality and cardiovascular disease in patients with kidney disease and in the general population.30–32 As kidney disease progresses and the body is less able to excrete phosphate, FGF-23 levels increase to aid the body by increasing excretion of phosphate by the kidney but this increase appears to be, in the long-term, maladaptive.33
Much of the early work on associations between elevated FGF-23 and cardiovascular events and mortality was performed in patients with kidney disease.10,34 In most studies of mortality and cardiovascular events, the magnitude of the effect of FGF‐23 was larger than, and independent of, serum phosphate.12,28,34,35 Phosphate and FGF-23 have been hypothesized to have distinct effects on the cardiovascular system, with elevated FGF-23 directly promoting cardiac remodeling and hyperphosphatemia directly promoting arterial injury36,37, although other studies have suggested a synergism between FGF-23 and phosphate.38 In addition to elevated cardiovascular risk, elevated FGF-23 levels have been associated with a faster progression to end-stage renal disease.12,28
FGF-23 AND HYPERTENSION
Recently, elevated FGF-23 levels have been associated with increased plasma volume and hypertension in animal and human studies.16,39 Higher sodium intake is a fairly well-established independent risk factor on elevations in blood pressure.40–42 In mouse models, infusion of FGF-23 was found to increase sodium reabsorption.39 Mice lacking the FGF-23 gene excreted more sodium in their urine, whereas mice with elevated FGF-23 levels had higher plasma volumes, hypertension and cardiac hypertrophy.39 This study suggested that FGF-23 stimulates sodium re-absorption and volume expansion through Na-Cl co-transporter (NCC) in the distal convoluted tubule.39 In addition, a high phosphate diet in the 5/6 nephrectomized rat model increased FGF-23 levels and angiotensin-converting enzyme expression, possibly leading to hypertension through direct activation of the renin-angiotensin system.43
Several patient and epidemiologic studies also show an association between high FGF-23 levels and hypertension. In a small study of 42 pediatric hypertensive patients, urinary FGF-23/creatinine ratios were higher than in healthy children.44 The positive correlation of FGF-23 was seen with systolic but not diastolic blood pressure.44 In the Atherosclerosis Risk in Communities (ARIC) study, among 7,948 middle-aged individuals without hypertension at baseline, higher FGF-23 levels were associated with the development of hypertension over a median follow-up of 5.9 years. After adjustment for demographics, behaviors and clinical factors (including kidney function), the final hazard ratio for incident hypertension was 1.21 (95% confidence interval: 1.08, 1.35) for the highest decile of FGF-23 compared with the lowest.16 Adjustment for serum PTH, calcium and 25-hydroxyvitamin D levels did not alter the association. Thus, both animal models and epidemiological studies suggest that elevated FGF-23 levels may play a role in hypertension.
FGF-23 LEVELS AND SUBCLINICAL ATHEROSCLEROSIS
Associations have been found between high FGF-23 levels and subclinical atherosclerosis including coronary artery calcification and carotid artery intima-media thickness. A study of 282 African American patients with type 2 diabetes mellitus but without significant kidney disease showed an association of higher FGF-23 levels with coronary artery calcified atherosclerotic plaque, but not carotid artery or aorto-illiac calcified plaque.45 A different study in different ethnic groups (68% Hispanic) did show an association between higher FGF-23 levels and carotid calcification.46 Similar associations between high FGF-23 levels and coronary artery calcification scores were seen in a study of 150 patients with CKD stages 3-5D in China47 and in a study of 142 French patients.48 The study from China also found that patients with the highest FGF-23 levels and the most coronary calcification had the highest risk of all-cause mortality.47 In a study done on children with stage 2 CKD and hypertension, findings were consistent in showing that higher FGF-23 levels were associated with arterial wall stiffness.49 Higher FGF-23 levels are also associated with higher carotid artery intima-media thickness, another marker of subclinical atherosclerosis, in peritoneal dialysis patients.50
The mechanisms for association between FGF-23 and subclinical atherosclerosis remain unclear. If elevated FGF-23 levels are associated with hypertension and hypertension is a risk for atherosclerosis, elevated FGF-23 levels could be associated with atherosclerosis and vascular dysfunction through hypertension. Another hypothesis suggests that FGF-23 enhances phosphate induced vascular calcification.38 A study using rat aortas showed that FGF-23 may enhance vascular calcification by causing aortic cells to differentiate into osteoblastic cells.38
FGF-23 LEVELS AND LEFT VENTRICULAR HYPERTROPHY (LVH)
The correlation between FGF- 23 and LVH has been well established through animal studies8,51,52 and epidemiologic and patient studies.53 A series of experiments has established that infusion of FGF-23 results in LVH through apparently a blood pressure independent mechanism,8 that the receptor responsible for the hypertrophy is the FGF Receptor 4,51 and that treatment with an FGF receptor blocker can lead to regression of LVH.52 Other authors have found that phosphate loading and Klotho deficiency, in addition to FGF-23, may also play a role in cardiac remodeling.54
Multiple epidemiological and patient studies have shown an association between high FGF-23 levels and LVH. In a study of 162 patients with CKD, higher FGF-23 levels were associated with higher odds of LVH.10 In a study of 3070 participants of the CRIC study (Chronic Renal Insufficiency Cohort), higher FGF-23 levels at baseline were associated with decrease ejection fraction, and higher mean left ventricular mass index after one year of follow-up.8 The previous studies were in patients with kidney disease, but the association between higher FGF-23 levels and LVH and cardiovascular events has also been shown in the Cardiovascular Health Study, a community-based cohort study.53,55 These epidemiologic investigations cannot distinguish whether FGF-23 acts directly on the myocardium or via elevations in blood pressure to cause left ventricular hypertrophy. Interestingly, due to these effects of FGF-23, it is interesting to evaluate studies of vitamin D therapy, such as the PRIMO trial.56 The primary hypothesis of the PRIMO trial was that therapy with activated vitamin D compounds would improve LVH in patients with kidney disease. This hypothesis was not proven by the trial and one potential explanation is that the activated vitamin D compound made the FGF-23 levels increase which then potentially led to LVH through the FGF-23 pathway.
FGF-23 LEVELS AND ATRIAL FIBRILLATION
Evaluating the potential association between FGF-23 and atrial fibrillation has yielded conflicting results.57,58 Several mechanisms have been proposed including direct effects of FGF-23 on the atrial myocardium and the induction of atrial enlargement leading to fibrillation of the atria.59–61 Analysis of data in one study demonstrated a tight connection between higher FGF-23 levels and incident atrial fibrillation in CKD patients, which was reinforced in a pooled meta-analysis showing higher FGF-23 levels as an independent predictor of atrial fibrillation risk (OR 1.35, 95% CI 1.09 to 169).60,62
FGF-23 LEVELS AND CARDIOVASCULAR EVENTS AND MORTALITY
Other studies have shown associations between high FGF-23 levels and coronary and other ischemic cardiovascular events. In a study of 704 patients with coronary artery disease, higher FGF-23 levels were associated with a combined outcome of acute coronary syndrome, stroke or transient ischemic attack, heart failure and death in patients with T2DM at baseline.63 An analysis of the Cardiovascular Health Study revealed that participants with higher FGF-23 levels were more likely to experience non-sudden cardiac death (cardiovascular deaths that were not sudden such as myocardial infarctions and strokes).64 Among dialysis patients, higher FGF-23 levels have been associated with cardiovascular mortality and all-cause mortality.34,35 Higher baseline FGF-23 levels were associated with incident heart failure in the CRIC cohort.9 High levels of FGF-23 were also shown to be associated with an increased risk of mortality, regardless of kidney function, in patients with heart failure with reduced ejection fraction.50 Thus, higher FGF-23 levels have been associated with multiple different cardiovascular events in multiple different populations.
POSSIBLE TREATMENT STRATEGIES FOR ELEVATED FGF-23 LEVELS
Low phosphate diets and the use of phosphate binders should theoretically lower FGF-23 levels. However, small clinical trials have not consistently shown this.65,66 Eating a plant based diet compared to eating a meat diet with the same nutrient content for one week led to lower phosphate and FGF-23 levels.67 Treatment with cincalcet in hemodialysis patients with secondary hyperparathyroidism lowered FGF-23 levels and this lowering of FGF-23 was associated with lower rates of cardiovascular events in a re-analysis of the EVOLVE clinical trial.68 Further research is required to evaluate whether lowering FGF-23 levels is associated with lower rates of cardiovascular events consistently.
CONCLUSION
FGF-23 is a bone-derived hormone that we believe primarily acts as a negative feedback on the activation of vitamin D and increases phosphate excretion, thereby decreasing phosphate levels. FGF-23, the FGF receptor and the obligate co-receptor α-klotho work in concert to effect FGF-23 actions. Low α-klotho levels have also been associated with cardiovascular disease.69 Elevations of FGF-23 occur early in the course of chronic kidney disease, likely due to several factors, and help to control phosphate levels. Elevated FGF-23 levels have been associated with several different forms of cardiovascular disease including hypertension, subclinical atherosclerotic disease, left ventricular hypertrophy, atrial fibrillation and other cardiovascular events (Figure). The next step in our understanding of FGF-23 pathophysiology is ascertaining whether decreasing FGF-23 levels leads to improved outcomes in patients. Table 1.
Fig 1.
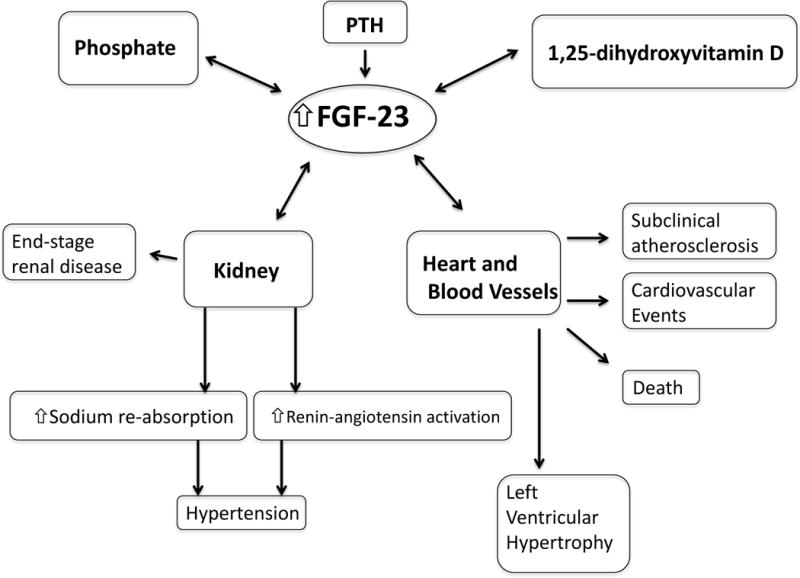
Highlights of the associations described in the review. Phosphate, PTH and 1,25-dihydroxyvitamin D lead to increased FGF-23 levels. Elevated FGF-23 levels may lead to hypertension through effects in the kidney and cardiovascular diseases and mortality.
Table 1. Determinants of FGF-23 levels.
Putative factors whose effect is either unclear or novel are designated with a question mark.
| Positive Regulators | Negative Regulators |
|---|---|
| Dietary Phosphate23 | Cinacalcet68 |
| Activated vitamin D (calcitriol, paricalcitol, doxercalciferol)17,18 | Phosphate binders?65,66 |
| Nutritional vitamin D (ergocalciferol, cholecalciferol)?19–22 | Low phosphate diet?65,66 |
| PTH24 | Plant based diet?67 |
| Sodium bicarbonate?25 | |
| Experimental myocardial infarction?26 | |
| Acute decompensated heart failure?27 |
KEY POINTS.
- FGF-23 is a hormone produced by osteocytes and osteoblasts which acts to increase phosphate excretion by the kidney and decrease 1,25-dihydroxyvitamin D levels as a negative feedback.
- FGF-23 levels are higher in patients with kidney disease and may be the first biomarker of chronic kidney disease-bone mineral disorder.
- High FGF-23 levels have been associated with multiple adverse cardiovascular outcomes including hypertension, left ventricular hypertrophy, subclinical atherosclerosis and cardiovascular events and mortality.
- Studies are needed to evaluate whether decreasing FGF-23 levels in humans may decrease cardiovascular events, especially in the setting of kidney disease.
Acknowledgments
None
Financial support: None
Dr. Melamed is supported by grant R01 DK102952 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
None of the authors report any conflicts of interest.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Nakatani T, Sarraj B, Ohnishi M, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23(2):433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 6.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87(11):4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 8.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. Journal of the American Society of Nephrology: JASN. 2014;25(2):349–360. doi: 10.1681/ASN.2013050465. This analysis using 3,860 participants with kidney disease in the CRIC study shows that higher FGF-23 levels at baseline were associated with cardiovascular events including heart failure over 3.7 years of follow-up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Annals of internal medicine. 2010;152(10):640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. Journal of the American Society of Nephrology: JASN. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano C, Hamano T, Fujii N, et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50(6):1266–1274. doi: 10.1016/j.bone.2012.02.634. [DOI] [PubMed] [Google Scholar]
- 14.Arnlov J, Carlsson AC, Sundstrom J, et al. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clinical journal of the American Society of Nephrology: CJASN. 2013;8(5):781–786. doi: 10.2215/CJN.09570912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Udell JA, Morrow DA, Jarolim P, et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. Journal of the American College of Cardiology. 2014;63(22):2421–2428. doi: 10.1016/j.jacc.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Fyfe-Johnson AL, Alonso A, Selvin E, et al. Serum fibroblast growth factor-23 and incident hypertension: the atherosclerosis risk in communities study. J Hypertens. 2016 doi: 10.1097/HJH.0000000000000936. This analysis of the ARIC study among almost 8,000 community dwelling individuals showed that higher FGF-23 levels were associated with incident hypertension over 5.9 years of follow-up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesseling-Perry K, Pereira RC, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79(1):112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 18*.Mizobuchi M, Ogata H, Yamazaki-Nakazawa A, et al. Cardiac effect of vitamin D receptor modulators in uremic rats. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.03.028. This study compared the effects of paricalcitol to VS-105, a novel vitamin D receptor activator, on uremic rats and showed that paricalcitol increased FGF-23 levels while VS-105 did not. [DOI] [PubMed] [Google Scholar]
- 19.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol. 2012;7(4):624–631. doi: 10.2215/CJN.10030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner C, Dalton N, Inaoui R, Fogelman I, Fraser WD, Hampson G. Effect of a 300 000-IU loading dose of ergocalciferol (Vitamin D2) on circulating 1,25(OH)2-vitamin D and fibroblast growth factor-23 (FGF-23) in vitamin D insufficiency. J Clin Endocrinol Metab. 2013;98(2):550–556. doi: 10.1210/jc.2012-2790. [DOI] [PubMed] [Google Scholar]
- 21.Alshayeb H, Showkat A, Wall BM, Gyamlani GG, David V, Quarles LD. Activation of FGF-23 mediated vitamin D degradative pathways by cholecalciferol. J Clin Endocrinol Metab. 2014;99(10):E1830–1837. doi: 10.1210/jc.2014-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhagatwala J, Zhu H, Parikh SJ, et al. Dose and time responses of vitamin D biomarkers to monthly vitamin D3 supplementation in overweight/obese African Americans with suboptimal vitamin d status: a placebo controlled randomized clinical trial. BMC Obes. 2015;2:27. doi: 10.1186/s40608-015-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006;21(8):1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 24.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299(4):F882–889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Melamed ML, Hostetter TH, et al. Effect of oral sodium bicarbonate on fibroblast growth factor-23 in patients with chronic kidney disease: a pilot study. BMC Nephrol. 2016;17(1):114. doi: 10.1186/s12882-016-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Andrukhova O, Slavic S, Odorfer KI, Erben RG. Experimental Myocardial Infarction Upregulates Circulating Fibroblast Growth Factor-23. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2015;30(10):1831–1839. doi: 10.1002/jbmr.2527. This study in mice and rats showed elevations in FGF-23 levels after induction of myocardial infarction in the animals. This suggests that cardiovascular dysfunction may lead to higher FGF-23 levels, the opposite of most current evidence which suggests that high FGF-23 levels may lead to cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Andersen IA, Huntley BK, Sandberg SS, Heublein DM, Burnett JC., Jr Elevation of circulating but not myocardial FGF23 in human acute decompensated heart failure. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31(5):767–772. doi: 10.1093/ndt/gfv398. This study in 21 patients with acute decompensated heart failure and 19 healthy controls showed that circulating FGF-23 levels were much higher in the patients with heart failure but that FGF-23 immunostaining in the myocardium was similar between the two groups. [DOI] [PubMed] [Google Scholar]
- 28.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. Jama. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimamura Y, Hamada K, Inoue K, et al. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol. 2012;16(5):722–729. doi: 10.1007/s10157-012-0621-7. [DOI] [PubMed] [Google Scholar]
- 30.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology: JASN. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 31.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. Jama. 2011;305(11):1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 32.Bai W, Li J, Liu J. Serum phosphorus, cardiovascular and all-cause mortality in the general population: A meta-analysis. Clin Chim Acta. 2016 doi: 10.1016/j.cca.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off” hypothesis. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(9):1710–1716. doi: 10.2215/CJN.02640310. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Scialla JJ, Parekh RS, Eustace JA, et al. Race, Mineral Homeostasis and Mortality in Patients with End-Stage Renal Disease on Dialysis. Am J Nephrol. 2015;42(1):25–34. doi: 10.1159/000438999. This study in incident dialysis patients showed higher phosphate and FGF-23 levels were associated with all-cause mortality more strongly in African-American than in whites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf M. Mineral (Mal)Adaptation to Kidney Disease–Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1875–1885. doi: 10.2215/CJN.04430415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney international. 2013;83(6):1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimbo R, Kawakami-Mori F, Mu S, et al. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney international. 2014;85(5):1103–1111. doi: 10.1038/ki.2013.332. [DOI] [PubMed] [Google Scholar]
- 39.Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO molecular medicine. 2014;6(6):744–759. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ: British Medical Journal. 1988;297(6644):319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott P, Stamler J, Nichols R, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. Bmj. 1996;312(7041):1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 43.Eraranta A, Riutta A, Fan M, et al. Dietary phosphate binding and loading alter kidney angiotensin-converting enzyme mRNA and protein content in 5/6 nephrectomized rats. Am J Nephrol. 2012;35(5):401–408. doi: 10.1159/000337942. [DOI] [PubMed] [Google Scholar]
- 44.Zajac M, Rybi-Szuminska A, Wasilewska A. Urine fibroblast growth factor 23 levels in hypertensive children and adolescents. Croatian medical journal. 2015;56(4):344–350. doi: 10.3325/cmj.2015.56.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Freedman BI, Divers J, Russell GB, et al. Plasma FGF23 and Calcified Atherosclerotic Plaque in African Americans with Type 2 Diabetes Mellitus. Am J Nephrol. 2015;42(6):391–401. doi: 10.1159/000443241. This observational study showed that higher FGF-23 levels were associated with coronary artery calcification in African-Americans with type 2 diabetes mellitus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Shah NH, Dong C, Elkind MS, et al. Fibroblast Growth Factor 23 Is Associated With Carotid Plaque Presence and Area: The Northern Manhattan Study. Arterioscler Thromb Vasc Biol. 2015;35(9):2048–2053. doi: 10.1161/ATVBAHA.115.305945. This observational study showed that higher FGF-23 levels were associated with carotid atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Yan J, Zhu M, Ni Z. Fibroblast growth factor 23 predicts coronary calcification and poor prognosis in patients with chronic kidney disease stages 3-5D. Annals of clinical and laboratory science. 2015;45(1):17–22. [PubMed] [Google Scholar]
- 48.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23(7):2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 49.Tasdemir M, Eroglu AG, Canpolat N, et al. Cardiovascular alterations do exist in children with stage-2 chronic kidney disease. Clinical and experimental nephrology. 2016 doi: 10.1007/s10157-016-1234-3. [DOI] [PubMed] [Google Scholar]
- 50**.Koller L, Kleber ME, Brandenburg VM, et al. Fibroblast Growth Factor 23 Is an Independent and Specific Predictor of Mortality in Patients With Heart Failure and Reduced Ejection Fraction. Circ Heart Fail. 2015;8(6):1059–1067. doi: 10.1161/CIRCHEARTFAILURE.115.002341. This study of 980 patients with heart failure showed that elevated FGF-23 levels were associated with an increased risk of mortality in those with reduced ejection fraction but not in those with preserved ejection fraction. [DOI] [PubMed] [Google Scholar]
- 51**.Grabner A, Amaral AP, Schramm K, et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015;22(6):1020–1032. doi: 10.1016/j.cmet.2015.09.002. In this study the investigators establish that the cardiac FGF receptor 4 leads to left ventricular hypertrophy through knock-out mice that do not develop LVH and knock-in mice that develop LVH spontaneously. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Marco GS, Reuter S, Kentrup D, et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(11):2028–2035. doi: 10.1093/ndt/gfu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jovanovich A, Ix JH, Gottdiener J, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231(1):114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. Journal of the American Society of Nephrology: JASN. 2015;26(6):1290–1302. doi: 10.1681/ASN.2014050465. In this study using mouse models of cardiac remodeling, the authors show that low klotho levels lead to cardiac remodeling and fibrosis and the FGF-23 also contributes to this in low klotho states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60(3):200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 57.Alonso A, Misialek JR, Eckfeldt JH, et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. Journal of the American Heart Association. 2014;3(5):e001082. doi: 10.1161/JAHA.114.001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seiler S, Cremers B, Rebling NM, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. European heart journal. 2011;32(21):2688–2696. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 59.Nowak A, Friedrich B, Artunc F, et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One. 2014;9(7):e100688. doi: 10.1371/journal.pone.0100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathew JS, Sachs MC, Katz R, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) Circulation. 2014;130(4):298–307. doi: 10.1161/CIRCULATIONAHA.113.005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratanapo S, Kittanamongkolchai W, Srivali N, Ahmed S, Cheungpasitporn W, Chongnarungsin D. The role of fibroblast growth factor-23 in left atrial volume. Am Heart J. 2013;165(5):e21. doi: 10.1016/j.ahj.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 62*.Meng L, Yang Y, Zhang Z, Li G, Liu T. Predictive value of circulating fibroblast growth factor-23 on atrial fibrillation: A meta-analysis. International journal of cardiology. 2016;210:68–71. doi: 10.1016/j.ijcard.2016.02.100. This is a meta-analysis evaluating the associations between FGF-23 levels and atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 63.Tunon J, Fernandez-Fernandez B, Carda R, et al. Circulating fibroblast growth factor-23 plasma levels predict adverse cardiovascular outcomes in coronary artery disease patients with diabetes mellitus. Diabetes Metab Res Rev. 2016 doi: 10.1002/dmrr.2787. [DOI] [PubMed] [Google Scholar]
- 64*.Deo R, Katz R, de Boer IH, et al. Fibroblast Growth Factor 23 and Sudden Versus Non-sudden Cardiac Death: The Cardiovascular Health Study. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2014.10.025. This analysis of over 3,000 participants in the Cardiovascular Health Study showed that elevated FGF-23 levels were associated with non-sudden cardiac death, such as death from myocardial infarction or stroke, but not sudden cardiac death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isakova T, Barchi-Chung A, Enfield G, et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clinical journal of the American Society of Nephrology: CJASN. 2013;8(6):1009–1018. doi: 10.2215/CJN.09250912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(2):584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(2):257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68**.Moe SM, Chertow GM, Parfrey PS, et al. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation. 2015;132(1):27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. This analysis of the EVOLVE trial showed that cincalcet decreased FGF-23 levels when compared to placebo and that participants with decreases in FGF-23 had lower risks of cardiovascular events. [DOI] [PubMed] [Google Scholar]
- 69.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33(2):118–129. doi: 10.1016/j.semnephrol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


