Abstract
Background
Silver diamine fluoride (SDF) is used topically to prevent or arrest dental caries. The study aim was to characterize the kinetics of silver and fluoride following topical application of SDF.
Methods
Sixteen adults participated in a pharmacokinetics study following the application of 38% SDF to 5 teeth (about 50 μL, estimated 4 to 11 mg per subject). Serum and urine samples were collected over 24 hours following application and were analyzed for silver (Ag) and fluoride (F−).
Results
Ag serum peak was 0.67±0.49 ng/mL; median time to peak was 3 hr. The estimated elimination half-life of Ag was 46±26 hr. No Ag was recovered in urine. Baseline F− serum levels ranged from <10–50 ng/mL (<0.01–0.05 ppm) and fluctuated around baseline following SDF. The 24-hr urinary F− was 1.29±0.81 mg.
Conclusions
SDF was well tolerated in this study and no adverse events related to SDF were reported.
Practical Implications
This clinical study confirmed that topical application of 38% silver diamine fluoride, in growing use in the U.S., is safe and well tolerated in healthy adults.
Keywords: silver diamine fluoride, clinical study, healthy volunteers, dental
Introduction
Silver diamine fluoride (SDF) is a topical agent that has been increasingly studied as a nonrestorative treatment to prevent or arrest dental caries.1 SDF has been used in adults for dental desensitization2–3 and in the elderly for prevention or treatment of root caries.4–5 The majority of the SDF efficacy studies were performed in children. Studies have shown SDF to prevent new caries and arrest carious surfaces in the teeth of preschool and school-aged children,6–11 though one study reported that SDF was less effective in preventing caries in permanent first molars compared to ART glass-ionomer cement sealants.12
Where caries lesions are present, the majority of the SDF applied to the teeth is thought to be sequestered in the teeth.13 However, after topical application some of the the product may also be swallowed and some amount may be absorbed through the GI tract or excreted. Direct absorption from the oral mucosa is unlikely. Circulating levels of silver and fluoride would be expected be be proportional to the dose of silver and fluoride administered topically to the teeth. In addition, as fluoride is primarily excreted renally,14 absorbed fluoride can be collected in the urine. In contrast, silver is unlikely to be recovered in the urine as biliary excretion is the principle route of elimination of silver.15
Despite the numerous efficacy studies, only one study reported the time course of silver and fluoride concentrations following application of SDF. Vasquez and colleagues conducted a preliminary pharmacokinetics study in six adults in which 38% SDF was applied topically to the intact, non-carious buccal segments of three teeth in each participant.16 The maximum concentration and time to maximum concentration for serum silver and fluoride were determined in the four hours following application. Though peak serum concentrations of fluoride and silver were observed within 3 and 2.5 hrs respectively, the follow-up period was too short to adequately determine the serum exposure (i.e., area under the curve or AUC) or elimination half-life for fluoride and silver following SDF application. The aim of this study was to further characterize the kinetics of silver and fluoride over 24 hr following topical application of SDF in healthy adults.
Materials and Methods
The reporting guidelines for Clinical Pharmacokinetic Studies (ClinPK) are followed in this paper.17
Subject Recruitment
The study was conducted under IND 124808 from the U.S. Food and Drug Administration (FDA). The study was approved by the Western IRB (#20171249). We enrolled healthy adult participants from August, 2017 to September, 2017 from the greater Seattle, Washington USA region. Inclusion criteria were that participants must be aged 18 years or older, have at least 20 teeth, were healthy as determined by medical history by the attending clinician, and not be taking prescription or over-the-counter medications. Participants were excluded if they were pregnant, weighed less than 50 kg, had oral mucositis or ulcerative lesions, or known sensitivity to silver or fluoride.
Procedures
Following informed consent, basic demographics and health history were obtained. A brief visual examination was conducted to note any evidence of inflammatory or ulcerative changes to the gingival or other oral tissues. Participants were instructed to use non-fluoride toothpaste on the study day through the 24-hr blood draw. Fluoride-free collection tubes were used for blood collection. Prior to application of the SDF, baseline blood and urine samples were collected from each participant. Teeth were brushed with a soft toothbrush to remove debris and dried with gauze prior to application.
The test product was aqueous silver diamine fluoride [Ag(NH3)2]F, CAS RN 33040–28-7, 38.3% to 43.2% in purified water 5.0 – 5.9% (w/v) fluoride; 24.4 −28.8% (w/v) silver (Advantage Arrest, Elevate Oral Care, LLC, West Palm Beach, FL, USA). SDF is identical to that used in a previous FDA-supervised clinical trial and the subject of a current Phase 3 randomized clinical trial.18 The nominal silver and fluoride concentrations in SDF are 2.5 mg/mL and 0.45 mg/mL, respectively. One drop (about 50 μL) was dispensed from the manufacturer’s multi-use bottle into a plastic dappen dish. The SDF solution was applied to the cervical area of the facial surfaces of 5 posterior teeth with a dental applicator brush. The amount applied was calculated as the difference in weights before and after application and estimated to be from 4 to 11 mg. For most of the subjects (75%), the amount applied was between 7 and 9 mg. The teeth were isolated with cotton rolls for one minute and then rinsed with water and high-volume evacuation.
Participants were not allowed to eat for at least 2 hr following application and were directly observed during the first 12 hr after application. Procedures were carried out in the University of Washington Medical Center, Institute of Translational Health Sciences, Translational Research Unit and the Regional Clinical Dental Research Center, School of Dentistry, University of Washington.
Sampling and Analytical Analysis
Blood samples (6 mL) were collected at baseline and 30 min, 1, 2, 3, 4, 6, 8, 12 and 24 hr following application of SDF. Participants collected urine for 24 hr following application of SDF and the total volume was recorded. Serum was obtained by centrifugation and serum and urine samples were frozen at −20°C until analysis. Blood and urine samples were analyzed by the Environmental Health Laboratory and Trace Organics Analysis Center at the University of Washington (Seattle WA, USA). Fluoride concentrations were determined by fluoride ion selective electrode.19 In brief, 950 μL of serum or urine was combined with 50 μL of buffer TISAB III with EDTA (Thermo Scientific Orion, Waltham, MA). Readings were taken using a fluoride ion selective electrode (Thermo Scientific Orion). mV readings were converted to fluoride concentrations using a 6-point calibration curve (R2 > 0.9992) and reported in increments of 10 ng/mL. The limit of detection for fluoride was 10 ng/mL (0.01 ppm) for both serum and urine. Silver concentrations were determined by inductively coupled plasma-mass spectrometry (ICP-MS) based on U.S. Environmental Protection Agency (EPA) 6020a Rev.1 2007.20 For each serum sample, 0.5 mL of serum was digested with 0.5 mL concentrated nitric acid using microwave-assisted digestion with a CEM MARS (CEM, Matthews, NC) (800 W, 10 min ramp to 90°C, held for 60 min). Following digestion, samples were diluted to 5 mL with a final concentration of 10% nitric acid. For each urine sample, 1 mL of urine was diluted with nitric acid to 5 mL with a final concentration of 10% nitric acid. Tb (10 ng/mL) was used as the internal standard for all samples. Prepared samples were analyzed using an Agilent 7900 ICP-MS (Agilent Technologies, Santa Clara, CA). Silver concentrations were estimated using a 6-point calibration curve (R2 = 1.0000 for urine and R2 = 0.9999 for serum). The limits of detection for silver were 0.1 ng/mL for serum and 0.05 ng/mL for urine.
Data Analysis
For the pharmacokinetic analysis, baseline serum silver and fluoride concentrations were assessed and when appropriate, subtracted from other timed serum concentrations to adjust for baseline levels. Pharmacokinetic parameters were estimated using Phoenix WinNonlin (Certara, Princeton, NJ, USA). The elimination rate constant (kelim) was determined by non-linear regression of the terminal slope with a minimum of 3 time points with decreasing concentrations over time observed after the peak concentration. Half-life (t1/2) was calculated as ln(2)/kelim. Two areas under the curve (AUC) were calculated. The AUC0–24 was calculated using a log-linear trapezoidal rule of serum silver concentrations from time 0 to 24 hr. For participants for whom an elimination rate constant was estimated, AUC0−∞ was calculated using a log-linear trapezoidal rule of serum concentrations and extrapolated to infinite time by C24 hr/kelim. The AUC0−∞ represents the total serum exposure to silver following application of SDF. The extrapolated AUC was calculated as (AUC0−∞ − AUC0–24 hr)/AUC0−∞ x 100. The total urinary recovery of fluoride and silver was determined by multiplying the concentration measured in a 24-hr urine sample by the total volume of urine of the 24-hr urine sample.
Results
Participants
Sixteen healthy adults participated in the study. The participant demographics are summarized in Table 1. No adverse events, determined by the investigators and medical monitor as related or possibly related to the application of the silver diamine fluoride, were observed (gingival inflammation or soft tissue changes) or reported by participants (nausea, vomiting, difficulty swallowing or breathing, swelling around the lips or skin of the face, itchiness around the lips or skin of the face, hives or rash, stomach ache or diarrhea).
Table 1.
Participant demographics in the study of the pharmacokinetics of 38% silver diamine fluoride applied topically to the teeth
| Characteristic | Healthy Adult Volunteers (N = 16) |
|---|---|
| Age (years) a | 41.4 ± 13.4 (18 to 57) |
| Male (n =) | 8 |
| Race (n =) | |
| Caucasian | 11 |
| Asian | 3 |
| More than one race | 2 |
| Ethnicity (n =) | |
| Not Hispanic or Latino | 15 |
| Unknown or not reported | 1 |
| SDF Applied (mg) a,b | 7 ± 2 (4 to 11) |
Mean ± standard deviation and (range) are provided for age and SDF applied.
SDF applied was calculated as the difference in weights before and after application.
Silver Pharmacokinetics
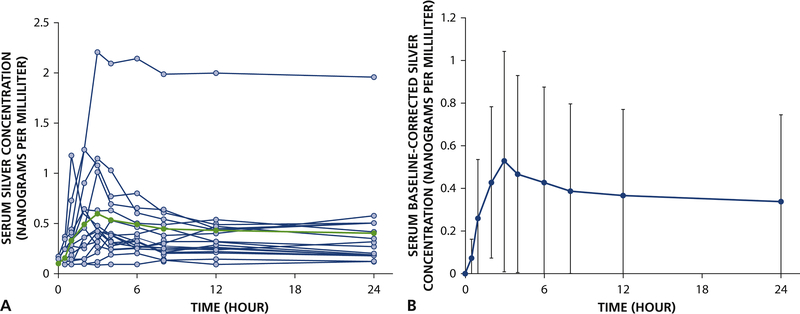
Individual and average serum silver vs. time curves are shown in Figure 1 and pharmacokinetic parameters are summarized in Table 2. The baseline serum silver concentrations ranged from less than detectable (<0.1 ng/mL) in 13 of 16 participants to 0.17 ng/mL (Participant 1). After correction for baseline levels, the peak baseline-corrected concentrations of silver was 0.67±0.49 ng/mL (range: 0.13 to 2.0 ng/mL) and the median peak time was 3 hrs after SDF application. For four participants, the peak concentrations were observed between 8 and 24 hours. The average estimated half-life of silver was 46 ± 26 hr in 11 of 16 participants. The terminal half-life was not estimated for participants with fewer than 3 decreasing concentrations following Cmax (n = 4) or concentrations that remained elevated and resulted in an imprecise estimate of half-life. The 24-hr exposure to silver (AUC0–24 hr) was 9.8 ± 9.0 ng·hr/mL. However, for those participants who had a calculated terminal half-life, the fraction of the total AUC extrapolated from the last measured concentration at 24 hr was large (64.9 ± 13.5%). No silver was recovered in the urine.
Figure 1.
Kinetics of silver following application of 38% silver diamine fluoride in healthy adults (n = 16). (A) Observed individual serum silver concentrations (open circles) with average serum silver concentrations (closed circles) and (B) baseline-corrected serum silver concentrations vs. time (average concentration ± standard deviation for each timepoint shown).
Table 2.
Summary of observed serum silver and fluoride concentrations and pharmacokinetic parameters and urinary recovery of fluoride (N=16) in a study of 38% silver diamine fluoride applied topically to the teeth of adults
| Parameters a | Silver | Fluoride |
|---|---|---|
| Observed Baseline (ng/mL) b | 0.15 ± 0.03 (<0.10 – 0.17) | 26 ± 12 (<10 – 50) |
| Tmax (hr) c | 5.3 ± 5.8 (1 – 24) | -- d |
| Cmax (ng/mL) e | 0.70 ± 0.52 (0.13 – 2.2) | -- |
| Baseline-corrected Cmax (ng/mL) | 0.67 ± 0.49 (0.13 – 2.0) | -- |
| t1/2 (hr) f | 46 ± 26 (19 – 110) | -- |
| AUC0–24 hr (ng·hr/mL) g | 9.8 ± 9.0 (2.3 – 41) | -- |
| AUC0-∞ (ng·hr/mL) h | 23.9 ± 5.8(13 – 31) | -- |
| Extrapolated AUC (%) i | 64.9 ± 13.5 (43.1 – 86.2) | -- |
| Baseline urinary concentration (ng/mL) j | <0.05 (<0.05 – 0.06) | 876 ± 469 (400 – 2090) |
| 24-hr urinary concentration (ng/mL) j | <0.05 (<0.05 – 0.06) | 373 ± 92 (200 – 530) |
| Total urinary recovery (mg) k | -- | 1.29 ± 0.81 (0.35 – 3.8) |
Average ± standard deviation (range)
Baseline serum silver concentrations were detectable in 3 of 16 participants; baseline serum fluoride concentrations were detectable in 13 of 16 participants. Values less than the lower limit of detection were not included in the calculation of the average and standard deviation (<0.1 ng/mL for silver and <10 ng/mL for fluoride, respectively).
Tmax: time of peak serum concentration
Not determined
Cmax: observed peak serum concentration
t1/2: terminal elimination half-life (determined for 11 of 16 participants for silver)
AUC0–24 hr: area under the curve from time 0 to 24 hr
AUC0-∞: area under the curve from time 0 to ∞ (n = 11, calculated for participants with a terminal elimination half-life estimate for silver)
Extrapolated AUC: (AUC0-∞ – AUC0–24 hr)/AUC0-∞ × 100
Median and range reported for silver (limit of detection <0.05 ng/mL for urinary silver); average and standard deviation reported for fluoride.
Total urinary recovery: (24 hr urine concentration) × (24 hr urine volume)
Fluoride pharmacokinetics
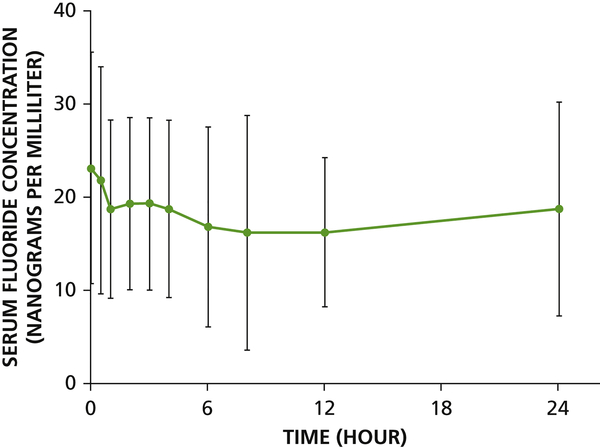
Individual and average serum fluoride vs. time curves are shown in Figure 2 and mean baseline fluoride concentrations and 24-hr urinary excretion are summarized in Table 2. At baseline, serum fluoride concentrations ranged from <10 to 50 ng/mL (<0.01 to 0.05 ppm). After application of SDF, fluoride concentrations were relatively invariant during the 24 hr sampling period for all participants. The amount of fluoride recovered in urine over 24 hr ranged from 0.35 to 3.8 mg (Table 2).
Figure 2.
Kinetics of fluoride following application of 38% silver diamine fluoride in healthy adults (n = 16). (A) Mean observed serum fluoride concentrations vs. time (average concentration ± standard deviation for each timepoint shown). Limit of detection was 10 ng/mL (0.01 ppm) for serum fluoride.
Discussion
Limited data have been published describing the kinetics of SDF, despite SDF being marketed and used in several countries. In the study participants, serum silver concentrations ranged from 0.13 to 2.2 ng/mL which was typically observed 3 hr post-application. In contrast, Vasquez et al.16 reported peak serum silver concentrations of 3.02 to 29.02 ng/mL. Though the time to peak concentrations was comparable between studies, the peak serum silver concentrations were substantially higher in the Vasquez study.16 The SDF doses between the studies did not differ (this study: estimated 4 to 11 mg; Vasquez et al.: 7.57 mg). Moreover, since silver is not renally excreted, we are unable to confirm the dose by comparing the amount recovered in the 24-hr urine samples. Although silver is reportedly excreted in the bile,21 including fecal collection in the study design would pose participant recruitment challenges due to the very long half-life of silver, analytical difficulties, and computational issues as we would not be able to differentiate between unabsorbed swallowed silver from the SDF application vs. silver that was excreted in the bile following absorption. We cannot account for differences in the silver concentrations between the two studies. In addition, Participant #1 had elevated serum silver concentrations compared to the other participants, even after correction for baseline silver concentrations.
Both this study and its predecessor16 applied a clinically relevant amount of SDF to intact non-carious buccal segments. Because there was no absorption into caries lesions, a greater proportion of the applied dose was likely swallowed and the results from the analyses of blood and urine are therefore higher than might be expected when treating dental caries.
A major limitation of this study is that the sampling time following application of SDF was inadequate to properly characterize the elimination of silver. The total extrapolated AUC was quite high (range: 43 to 85%) and the estimated terminal half-life (46 ± 26 hr) was longer than the duration of the study. Moreover, the terminal half-life could not be estimated in five of the 16 participants. The estimated pharmacokinetic parameters of silver may be imprecise due to the short duration of the study compared the half-life of silver in these adult subjects. A study duration between four to seven days, with the inclusion of 48, 72, 120, and 168 hr sampling timepoints, might be necessary to fully capture the pharmacokinetics of silver and the return of serum silver concentrations to baseline levels.
Due to the serum concentrations near the detection limit of the assay for fluoride, possibly low amount of fluoride ingested from SDF, and inherent variability in fluoride from diet, there was no discernible peak for serum fluoride. The fluctuations in serum fluoride concentrations appear to be unrelated to application of SDF. Over the 24-hr period, fluoride concentrations ranged from <10 to 60 ng/mL which was similar to the results reported by Vasquez et al.16 In the current study, the average 24-hr urinary recovery of fluoride was 1.29 ± 0.81 mg, which is within reported 24-hr fluoride urinary excretion in adults aged 18 to 75 years summarized by Villa et al. (range 0.49 to 7.52 mg).22
No adverse effects were observed and none of the participants reported any adverse effects that were deemed to be related to the application of the SDF. Similarly, no serious adverse events were observed in children 24 to 72 mo of age receiving SDF in a short-term randomized clinical trial.18 In the pediatric study, the adverse event rate did not differ between the SDF treatment and placebo group. Most commonly, diarrhea or stomachache were reported and were mild in severity. Moreover, Duangthip et al. reported no serious adverse events in 799 children aged 3 to 4 years treated with SDF at 38% and 12% when applied annually or biannually over 18 months.23
The low dosage applied topically and frequency of treatment mediates concerns about risks. It is important, nevertheless, to understand the disposition of silver and fluoride following SDF application in both adults and also children. It would be challenging ethically and practically to conduct a pharmacokinetic study in children due to the intensive and long duration of blood sampling required to monitor silver concentrations. Moreover, the variability in serum silver concentrations seen in adults suggests that it would be difficult to reduce the number of blood samples that would need to be taken in children. These issues are not unique to SDF, but to drug development in general. Increasingly, the use of “quantitative clinical pharmacology” (model-based drug development) has been used to predict drug disposition and efficacy in children and adults (reviewed by Mehrotra et al.24). Bachler et al. developed a physiologically-based pharmacokinetic model for ionic silver and silver nanoparticles in rats and adult males.25 Further work will need to be done to determine whether the proposed physiologically-based pharmacokinetic model for ionic silver could be adapted for pediatric silver disposition, though it might be impossible to validate the model as no data exist for silver pharmacokinetics in children.
Following application of SDF in healthy adult volunteers, the circulating levels of silver peaked at 3 hrs and slowly declined in 24 hr in the majority of participants, though some participants had a delayed peak serum silver concentration that was observed between 6 and 24 hr. The average half-life of silver was approximately 2 days though a study but a longer duration of sampling would need to be conducted to provide a more precise estimate of half-life and serum exposure to silver. The EPA lifetime lowest allowable effect level (LOAEL) for silver exposure is 1 g total dose, which is well over 400-fold the maximum amount of silver applied in this study.26 Serum fluoride levels fluctuated within normal ranges for the duration of the study and the excretion of fluoride in these adult participants was comparable to literature values. Results from this study support the continued development of topical SDF as a safe and efficacious treatment.
Acknowledgements
This work was supported, in part, by internal gift funds from the University of Washington Department of Oral Health Sciences. Elevate Oral Care, LLC donated the test agent. The authors acknowledge the contributions of the staff of the ITHS Translational Research Unit, University of Washington Medical Center. ITHS is supported by grants UL1 TR002319, KL2 TR002317, and TL1 TR002318 from the NIH National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA). The authors acknowledge the contributions of Mary K Hagstrom in carrying out the clinical aspects of the research.
Footnotes
Declaration of Conflicting Interests:
PM is a director of Advantage Silver Dental Arrest, LLC. The other authors declare no conflict of interest.
Disclosure. Dr. Milgrom is a director of Advantage Silver Dental Arrest, LLC. Drs. Lin and Rothen did not report any discloaures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yvonne S. Lin, Department of Pharmaceutics, University of Washington, Seattle, WA.
Marilynn L. Rothen, Department of Oral Health Sciences, University of Washington, Seattle, WA.
Peter Milgrom, Department of Oral Health Sciences, University of Washington, Seattle, WA.
References
- 1.Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. JADA. 2018;149(10):837–849.e19. [DOI] [PubMed] [Google Scholar]
- 2.Castillo JL, Rivera S, Aparicio T, et al. The short-term effects of diammine silver fluoride on tooth sensitivity: a randomized controlled trial. J Dent Res. 2011;90:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig GG, Knight GM, McIntyre JM. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust Dent J. 2012;57:308–311. [DOI] [PubMed] [Google Scholar]
- 4.Tan HP, Lo EC, Dyson JE, et al. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89:1086–1090. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, McGrath C, Lo EC, et al. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 2013;47:284–290. [DOI] [PubMed] [Google Scholar]
- 6.Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770. [DOI] [PubMed] [Google Scholar]
- 7.Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84:721–724. [DOI] [PubMed] [Google Scholar]
- 8.Braga MM, Mendes FM, De Benedetto MS, et al. Effect of silver diammine fluoride on incipient caries lesions in erupting permanent first molars: a pilot study. J Dent Child (Chic). 2009;76:28–33. [PubMed] [Google Scholar]
- 9.Yee R, Holmgren C, Mulder J, et al. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 2009;88:644–647. [DOI] [PubMed] [Google Scholar]
- 10.Liu BY, Lo EC, Chu CH, et al. Randomized trial on fluorides and sealants for fissure caries prevention. J Dent Res. 2012;91:753–758. [DOI] [PubMed] [Google Scholar]
- 11.Zhi QH, Lo EC, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent 2012;40:962–967. [DOI] [PubMed] [Google Scholar]
- 12.Monse B, Heinrich-Weltzien R, Mulder J, et al. Caries preventive efficacy of silver diammine fluoride (SDF) and ART sealants in a school-based daily fluoride toothbrushing program in the Philippines. BMC Oral Health. 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet. J Den Res. 2009;88:116–125. [DOI] [PubMed] [Google Scholar]
- 14.Spencer H, Lewin I, Wistrowski E, et al. Fluoride metabolism in man. Am J Med. 1970;49:807–813. [DOI] [PubMed] [Google Scholar]
- 15.Lansdown AB. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci. 2010;2010;910686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health. 2012;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanji S, Hayes M, Ling A, et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin Pharmacokinet. 2015;54:783–795. [DOI] [PubMed] [Google Scholar]
- 18.Milgrom P, Horst JA, Ludwig S, et al. Topical silver diamine fluoride for dental caries arrest in preschool children: A randomized controlled trial and microbiological analysis of caries associated microbes and resistance gene expression. J Dent. 2018;68:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Mier EA, Cury JA, Heilman JR, et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 2011;45:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Environmental Protection Agency. Method 6020A. Inductively Coupled Plasma – Mass Spectrometry. Revision 1. January, 1998. [URL: https://www.epa.gov/sites/production/files/2015-07/documents/epa-6020a.pdf] [Accessed October 3, 2018].
- 21.Klaassen CD. Biliary excretion of silver in the rat, rabbit, and dog. Toxicol Appl Pharmacol. 1979;50:49–55. [DOI] [PubMed] [Google Scholar]
- 22.Villa A, Anabalon M, Zohouri V, et al. Relationships between fluoride intake, urinary fluoride excretion and fluoride retention in children and adults: An analysis of available data. Caries Res. 2010;44:60–68. [DOI] [PubMed] [Google Scholar]
- 23.Duangthip D, Fung MHT, Wong MCM, et al. Adverse effects of silver diamine fluoride treatment among preschool children. J Dent Res. 2018;97:395–401. [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra N, Bhattaram A, Earp JC, et al. Role of quantitative clinical pharmacology in pediatric approval and labeling. Drug Metab Dispos. 2016;44:924. [DOI] [PubMed] [Google Scholar]
- 25.Bachler G, von Goetz N, Hungerbuhler K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int J Nanomedicine. 2013;8:3365–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATSDR: Toxicological profile for silver (CAS#:7440–22-4), 1990. [http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=539&tid=97.] [Accessed July 27, 2018].