Abstract
Traditional active avoidance tasks have advanced the field of aversive learning and memory for decades and are useful for studying simple avoidance responses in isolation; however, these tasks have limited clinical relevance because they fail to model key features of clinical avoidance. In contrast, platform-mediated avoidance (PMA) more closely resembles clinical avoidance because the response i) is associated with an unambiguous safe location, ii) is not associated with an artificial termination of the warning signal, and iii) is associated with a decision-based appetitive cost. Recent findings on the neuronal circuits of PMA have confirmed that amygdala-striatal circuits are essential for avoidance. However, in PMA the prelimbic cortex facilitates the avoidance response early during the warning signal, perhaps through disinhibition of the striatum. Future studies on avoidance need to account for additional factors such as sex differences and social interactions that will advance our understanding of maladaptive avoidance contributing to neuropsychiatric disorders.
Keywords: Fear, amygdala, prelimbic cortex, striatum, accumbens
1. Introduction
The study of fear and aversive learning is entering an exciting period. Three decades of research using Pavlovian fear (threat) conditioning have uncovered many of the neuronal circuit mechanisms necessary to associate a sensory stimulus (e.g. an auditory tone) with an aversive outcome (e.g. an electrical foot-shock), and to respond appropriately (for reviews, see Johansen et al., 2011; Herry and Johansen, 2014; Duvarci and Pare, 2014; Giustino and Maren, 2015; Do Monte et al., 2016). Auditory fear conditioning is elegant in its simplicity and enables cellular studies in animals and translation to humans. The most easily measured conditioned response in rodents is freezing: an automatic, species-specific defense response provided by evolution to counter potential threats (Blanchard and Blanchard, 1972; Fanselow, 1994). However, behavioral responses to learned threats can also be complex and decision-based, such as halting foraging for food or actively avoiding cues or places associated with danger. Avoidance of danger and its competition with foraging are common problems for rodents in the wild. In humans, avoidance can be a coping strategy to reduce the likelihood of harmful encounters (e.g. moving to the sidewalk to avoid a potential threat from an oncoming car that is honking at you). Avoidance becomes maladaptive, however, in neuropsychiatric disorders by interfering with other goal-directed activities (American Psychiatric Association, 2013). Therefore, the development of appropriate animal models could give rise to new approaches for the treatment of these disorders (Milad and Quirk, 2012; Rodriguez-Romaguera and Quirk, 2017; Diehl et al., 2018c; Singewald and Holmes, 2019).
Compared to conditioned freezing, it will be more challenging to uncover the neural mechanisms of decision-based avoidance responses. Because fear conditioning is thought to represent the first stage of avoidance learning (Mowrer and Lamoreaux, 1946), our increased understanding of fear circuits should accelerate active avoidance research. For example, the roles of specific subdivisions of the prefrontal cortex, amygdala, and striatum in fear learning have been extensively researched (Pezze and Feldon, 2004; Kravitz and Kreitzer, 2012; Giustino and Maren, 2015; Duvarci and Pare, 2014). Indeed, a lack of knowledge about fear conditioning and habit learning may have periled earlier attempts to uncover active avoidance mechanisms (Wendler et al., 2014; LeDoux et al., 2017). In recent years, a number of groups interested in aversive learning are shifting their focus away from simple classical fear conditioning (Servatius et al., 2008; Amir et al., 2015; Pare and Quirk, 2017; Fadok et al., 2017; Burgos-Robles et al., 2017; Kim and Jung, 2018; Kyriazi, Headley and Pare, 2018; Cain, 2019). The objective of this review, therefore, is to compare current active avoidance paradigms, their neural mechanisms, and relation to psychiatric disorders. Future research on avoidance will need to employ tasks that are more relevant to clinical syndromes in humans.
2. Traditional active avoidance tasks and their limitations
A variety of tasks have been used to study active avoidance. Unlike passive avoidance, in which an individual can avoid danger without requiring an overt behavioral response, active avoidance requires an overt behavioral response. Here, we focus on signaled active avoidance, in which a conditioned warning signal (WS) such as a tone predicts the occurrence of an aversive unconditioned stimulus (US), usually a footshock (Mowrer, 1939; Kamin, Brimer and Black, 1963; Mowrer, 1960). The most commonly used active avoidance task is two-way shuttle avoidance. Rodents learn shuttle avoidance in two stages (Mowrer and Lamoreaux, 1946; Levis, 1989). Initially, they learn the tone-shock association, by exhibiting freezing to the tone. Next, animals run to the opposite chamber in response to the shock and quickly learn that they can terminate the WS and the shock by escaping into the adjacent chamber (Figure 1A). After further training, animals learn that shuttling in response to the onset of the WS (before shock occurs) terminates the WS and prevents shock delivery. Across days of training, conditioned freezing to the WS diminishes as rats learn that the avoidance response reliably prevents the occurrence of shock (Kamin, Brimer and Black, 1963). In other tasks, animals are required to run on a wheel (Bolles, Stokes and Younger, 1966; Gabriel, Saltwick and Miller, 1975) or press a lever (D’Amato and Schiff, 1964; Berger and Brush, 1975; Servatius et al., 2008) to terminate the tone and prevent the occurrence of shock.
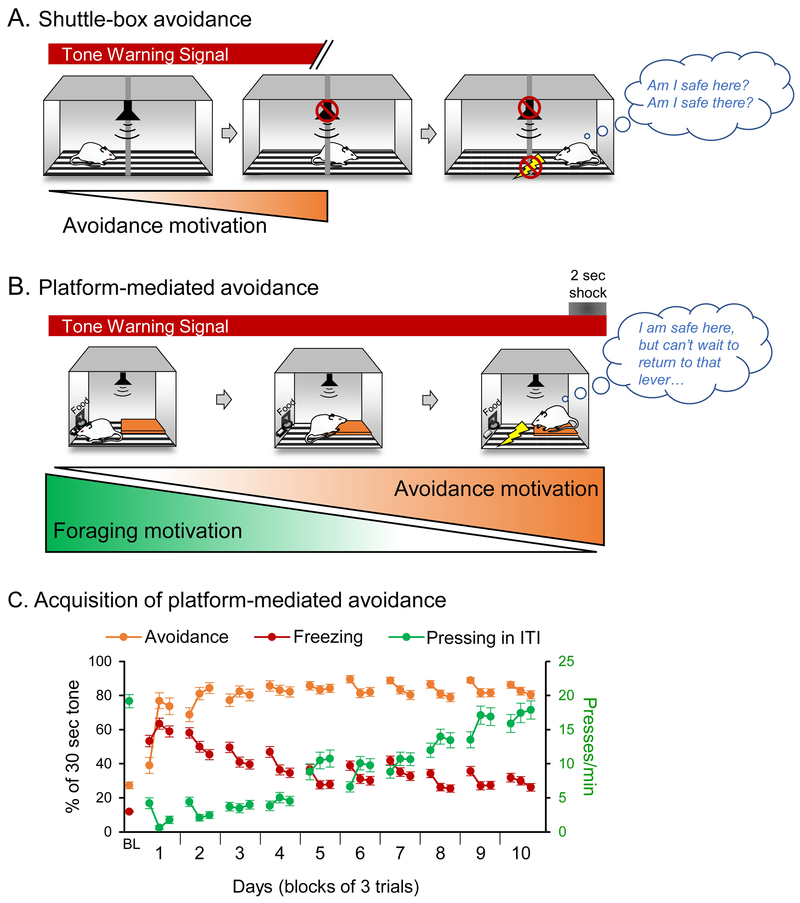
Figure 1. Comparison of shuttle-box avoidance and platform-mediated avoidance tasks.
A. Typical behavior of rats during the shuttle-box avoidance task. Following the onset of the tone warning signal, rats learn to avoid by moving to the opposite side of the box, which both terminates the tone and prevents the generation of a footshock. Because rats must enter a place where they were previously shocked, no place is associated with safety. B. Typical behavior of rats during the platform-mediated avoidance task (PMA). Following the onset of the tone warning signal, rats learn to stop pressing the bar for sucrose pellets and move toward the platform, so that they are no longer in contact with the grid floor. Performing this avoidance response does not terminate the tone or prevent the generation of a footshock; rats must remain on the platform throughout the tone. In this way, rats are able to control their exposure to a shock, but not the presence of the shock or its predictive stimulus. While protecting them from shock, the platform also prevents rats’ access to sucrose reward. C. Acquisition of PMA across 10 days of training. As training proceeds, avoidance (time spent on platform) increases (orange lines), while freezing to the tone decreases (red lines). The rate of bar pressing during the inter-trial interval (ITI) increases back to its pre-conditioning rate (green lines). First data point shows the pre-conditioning baseline value (BL) to the first tone. All other data points are blocks of three tone trials. n=82 rats. All data shown as mean ± SEM. Modified from Bravo-Rivera et al. (2014).
While these paradigms have taught us much about active avoidance (Dinsmoor, 2001; LeDoux et al., 2017; Moscarello and Hartley, 2017; Cain, 2019), they are somewhat limited as models of naturally occurring avoidance. First, in none of these avoidance tasks (wheel-running, lever-pressing, and shuttle) is there a permanent safe location because rats both receive shock and avoid shock within the same location. For example, in the shuttle task, rats must enter a compartment in which it was shocked on the previous trial. Without a dedicated safe location, positional conflict can occur, leading to increased freezing. This can be a problem because conditioned freezing to the context (Bouton and Bolles, 1979; Kim and Fanselow, 1992) can interfere with expression of the instrumental response needed to avoid. This is more evident with shuttle avoidance, because the rat is required to move to a chamber where it was previously shocked. This can cause rats to move very slowly, or even freeze. In fact, approximately one quarter of rats fail to learn shuttle avoidance due to excessive freezing (Choi, Cain and LeDoux, 2010; Galatzer-Levy et al., 2014). Interestingly, freezing has the effect of masking avoidance because eliminating freezing by inactivating the central nucleus of the amygdala reveals the underlying avoidance behavior (Lazaro-Munoz, LeDoux and Cain, 2010). Thus, the circuits of the expression of conditioned freezing and the expression of conditioned avoidance appear to be separate.
A second limitation of these avoidance tasks is that there is no cost associated with performing the avoidance response. When danger is encountered in naturalistic settings, animals are usually seeking food, shelter, or mates (Kavaliers and Choleris, 2001). The interruption of these activities by avoidant behaviors constitutes a cost of avoidance. This phenomenon is clinically relevant because patients avoiding a perceived threat end up sacrificing activities that are otherwise rewarding or necessary for social functioning (Foa and Kozak, 1986). In the shuttle task, there is no cost to shuttle as this action does not require a sacrifice of any kind. A third limitation is that avoidance responses in these tasks often cause termination of the WS. The rat’s ability to terminate both the WS and the shock demonstrates considerable control over the environment, but is this realistic? In natural settings, an individual’s avoidance response does not eliminate the existence of a threat but decreases the likelihood of being harmed by that threat. For example, a rodent retreating to its burrow does not make the predator above disappear (it only obscures the view of the predator), in the same way that a person’s decision to avoid traffic does not make an oncoming car disappear. Because our ability to alter the existence of external threats is limited, the avoidance-triggered termination of the WS (and even the US) in these tasks does not reflect the conditions of naturally occurring avoidance. Some shuttle avoidance studies have employed a constant CS duration, which resolves this issue (Smith et al., 2002; Trigo et al., 2008; Carmona et al., 2014). Despite these limitations, shuttle avoidance and other traditional active avoidance tasks are useful for isolating avoidance circuits during low cost conditions which do not compete with motivated behaviors.
3. Platform-mediated avoidance is well-suited for studying clinical avoidance
To study active avoidance in a more realistic manner, we performed a simple modification of our auditory fear conditioning task, in which rats are exposed to tone-shock pairings while pressing a lever for sucrose pellets on a variable interval schedule of reinforcement (Estes and Skinner, 1941; Quirk et al., 2000). In platform-mediated avoidance (PMA), a 1 cm high platform is fixed in one corner of the behavioral chamber, located diagonally from the sucrose lever (Figure 1B; for task details, see Bravo-Rivera et al., 2014). Similar to other active avoidance tasks, rats initially learn the tone-shock association and then discover that they can escape the shock (final 2 s of 30 s tone) by moving onto the platform. Next, rats learn that they can completely avoid the shock if they move onto the platform prior to the shock onset (on average, 5 s after tone onset). Early in training, the level of freezing to the tone is high and the rate of lever pressing during the inter-trial intervals (ITIs) is low (Figure 1C). As rats progress through 10 days of training, freezing gradually decreases. Pressing during the tone remains low but pressing during the ITI gradually increases to pre-conditioning levels, reflecting rats’ learning that the threat is limited to the tone period. Thus, avoidance behavior in PMA consists of halting food seeking and approaching a safety zone.
PMA is distinct from other avoidance tasks, such as lever press and shuttle avoidance, in which the avoidance response does not terminate the WS. During PMA, the WS continues while the rat is waiting on the platform. Additionally, avoiding in PMA does not prevent the generation of a shock. By stepping onto the platform, rats have removed themselves from danger, in effect controlling their exposure to the shock rather than the existence of the shock or its predictive stimulus. This more closely resembles commonly encountered threats. Using the aforementioned example, the animal that hides in its burrow must wait to come out until the predator goes away. While it was originally proposed that the termination of the WS contributes to avoidance learning (Kamin, Brimer and Black, 1963; Keehn and Nakkash, 1959), our findings support the idea suggested by Bolles that this contribution is not essential (Bolles, Stokes and Younger, 1966). However, in the absence of WS termination, how does the rat in PMA know it needs to continue executing the avoidance during the tone? On some trials, rats fail to avoid the shock. In addition, some rats on the platform show “testing” behavior by placing their front paws onto the bars to confirm the presence of shock during the WS. This occurs on occasional trials toward the end of the 10 days of training and may be important for maintaining the avoidance response by reinforcing that the tone still predicts danger and also that the platform is a safe zone.
PMA bears some resemblance to early avoidance tasks in which rats were required to jump up to a safe platform. Rats could jump 25 cm up onto a shelf to avoid a shock (Maatsch, 1959) or jump 7.5 cm up onto to a shelf to avoid excessive heat (Henderson and Graham, 1979). In addition to requiring a jump, differences between these tasks and PMA include the absence of a predictive WS and the absence of a foraging cost. The cost of PMA mirrors clinical avoidance, which competes with goal-directed behaviors (Foa and Kozak, 1986; Breslau, 2001; Asmundson, Stapleton and Taylor, 2004). The motivation to press a lever for reward also serves to decrease freezing. Freezing is further reduced in PMA because rats do not enter an area in which they were previously shocked. Thus, the relatively low levels of freezing observed in PMA (~60% on day 1 to ~30% on day 10, see Figure 1C) do not prevent rats from moving to the platform. Consequently, unlike the shuttle task, almost all rats learn and express PMA (>95%).
PMA is not without its own limitations, however. Because it involves reward/avoidance conflict, PMA requires food restriction to generate reward seeking motivation. Recent studies have shown that food restriction can alter neural circuits of motivation (Nieh et al., 2015; Huang et al., 2016; Verma et al., 2016). Therefore, if a researcher wishes to study active avoidance under homeostatic conditions, then PMA may not be the optimal task. Moreover, although a strength of PMA is that freezing does not compete with avoidance, freezing-induced blockade of avoidance in the shuttle task could model non-adaptive responses to trauma often observed in PTSD.
In summary, shuttle avoidance may be more appropriate for studying basic principles of active avoidance that can be directly compared with decades of prior research, whereas PMA more closely resembles real life avoidance by adding a decision-based cost contingency. During PMA: 1) avoidance competes with foraging behavior, 2) avoidance does not artificially terminate the WS or the US, 3) rats are not required to move toward (or remain in) a place where they were previously shocked. Moreover, once learned, the levels of freezing do not compete with expression of PMA, disambiguating the effects of experimental manipulations on the circuitry of avoidance vs. the circuitry of conditioned fear.
4. Neural circuitry of active avoidance: amygdala, striatum, and prefrontal cortex
The amygdala is essential for acquiring the tone-shock association (LeDoux, 2000; Maren and Quirk, 2004; Maren, 2005; Ponnusamy, Poulos and Fanselow, 2007; Johansen et al., 2011; Lüthi and Lüscher, 2014; Sears, Schiff and LeDoux, 2014; Do Monte et al., 2016). Consequently, the amygdala is essential for both the learning and initial expression of active avoidance. Both pre-training (Lazaro-Munoz, LeDoux and Cain, 2010) and post-training (Choi, Cain and LeDoux, 2010) lesions of the basolateral amygdala (BLA) impaired shuttle avoidance, and pharmacological inactivation of BLA impaired expression of PMA (Bravo-Rivera et al., 2014; see Figure 2A). Increased activity in the amygdala has been correlated with expression of active avoidance, as demonstrated with the activity marker cFos in the shuttle task (Martinez et al., 2013), lever press task (Jiao et al., 2015), and PMA (Bravo-Rivera et al., 2015; Martínez-Rivera et al., 2018), as well as with unit recording in the wheel-running task (Poremba and Gabriel, 1999; Maren, Poremba and Gabriel, 1991). Dopamine signaling in the ventral striatum (VS) is required for shuttle avoidance (Darvas, Fadok and Palmiter, 2011) and notably, a crossed-inactivation study has implicated amygdala projections to VS in shuttle avoidance (Ramirez et al., 2015). The VS is thought to inhibit the substantia nigra pars reticulata in order to execute the avoidance response (Hormigo, Vega-Flores and Castro-Alamancos, 2016).
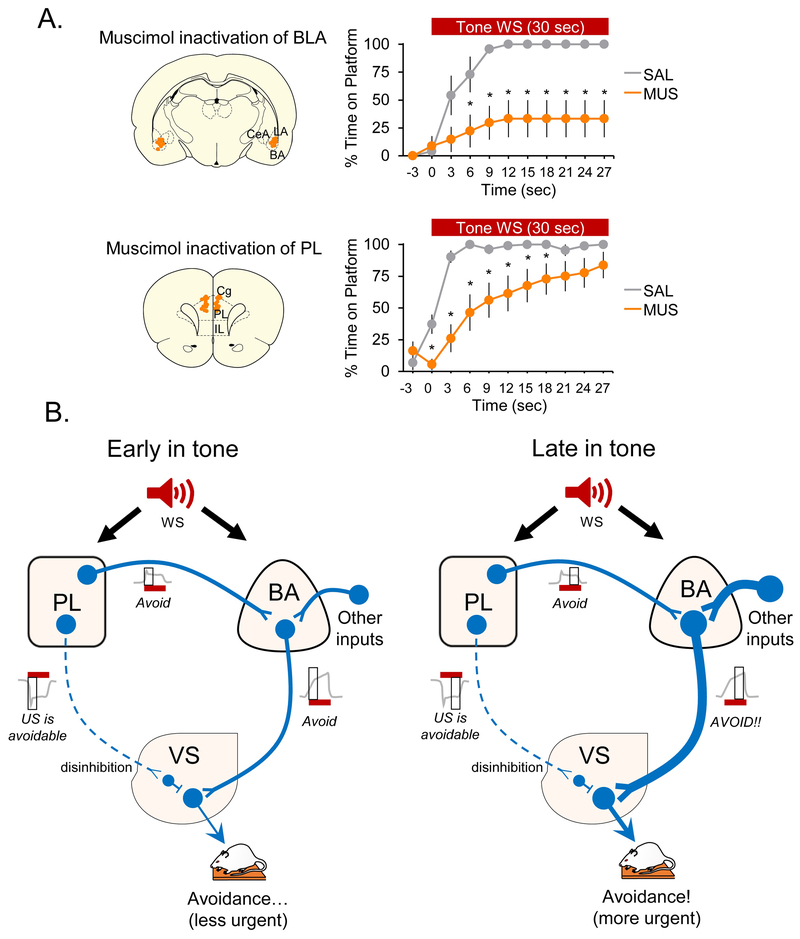
Figure 2. Proposed circuitry of platform-mediated avoidance at different timepoints during the tone.
A. (Left) Coronal schematics of BA (top) and PL (bottom) inactivations by infusing muscimol (MUS) or saline (SAL), showing location of cannula tips. (Right) Time spent on platform (in 3 sec bins) during the tone revealed that BLA inactivation blocked avoidance in MUS rats (top) across the entire tone compared to SAL controls (rptd meas ANOVA, post hoc Tukey). Data from Bravo-Rivera et al. (2014). PL inactivation significantly impaired in avoidance early, but not late, in the tone (bottom), in MUS rats compared to SAL controls (rptd meas ANOVA, post hoc Tukey). Modified from Diehl et al. (2018). B. (Left) Early during the tone, inhibitory responses are observed in PL neurons (dashed axon), which likely project to inhibitory interneurons in the VS. Inhibitory responses in PL would promote avoidance by disinhibiting the response of VS output neurons to BA inputs. PL neurons also show excitatory responses at platform mounting, which could project to BA neurons and facilitate avoidance. (Right) Late in the tone, as avoidance becomes more urgent, PL disinhibition of VS is maintained and BA excitation of VS increases in response to other inputs (thick axon), thereby over-riding inhibition from PL. Thus, expression of urgent avoidance (when shock is imminent) occurs independently of PL. Grey trace is hypothetical neural activity with black box indicating activity signal during the tone. All data shown as mean ±SEM. *p<0.05. Abbreviations: BA – basal amygdala; BLA – basolateral amygdala; CeA – central nucleus of the amygdala; IL – infralimbic cortex; LA – lateral amygdala; PL – prelimbic cortex; VS – ventral striatum.
Apparent differences in neural circuitry between the shuttle task and PMA arise, however, in the recruitment of the prefrontal cortex. In shuttle avoidance, inactivation of the infralimbic cortex (IL), but not the prelimbic cortex (PL), impaired shuttle avoidance (Moscarello and LeDoux, 2013). In PMA, inactivation of these structures had the opposite effects: impairment with PL inactivation, but no impairment with IL in activation (Bravo-Rivera et al., 2014). The discrepancy with IL could be due to the differing effects of freezing behavior in the two tasks. IL inactivation increased freezing in both tasks (Moscarello and LeDoux, 2013; Bravo-Rivera et al., 2014), but only in the shuttle task did this interfere with expression of avoidance; rats in PMA were still able to step onto the platform. Thus, while IL may be essential for reducing freezing in avoidance, it is not essential for avoidance itself. What is less clear is why PL is necessary for PMA but not shuttle avoidance. One possibility is the difference in timing of lesion (pre-training in shuttle and post-training in PMA). Another possibility is the presence of conflict between avoidance and foraging in PMA, which has been proposed to recruit PL during a motivational conflict task (Illescas-Huerta et al., 2018) as well as cost-benefit decision-making task (Friedman et al., 2015). We have recently suggested that PL may be more modulatory than essential. Inactivating PL impaired the expression of PMA early in the tone (when avoidance is less urgent), but not late in the tone (when avoidance is more urgent; Figure 2A) (Diehl et al., 2018a). In contrast, inactivation of BLA impaired avoidance throughout the tone (Figure 2A; Bravo-Rivera et al., 2014).
More clues about the role of PL in active avoidance are suggested by unit recording and optogenetic findings (Diehl et al., 2018a). Following PMA training, PL neurons exhibited robust inhibitory responses to the tone. Opposing these inhibitory responses with optogenetic stimulation delayed the expression of avoidance or blocked it entirely (Diehl et al., 2018a). Inhibitory responses in PL were observed in rats trained in PMA, but not those trained in auditory fear conditioning, suggesting that avoidance was signaled by PL inhibition. Interestingly, inhibitory responses in PL were observed whether or not the rat avoided on a given trial, suggesting that PL inhibition signals the avoidability of the shock, perhaps acting as a “discriminative stimulus” (Cain, 2019) that lets the rat know it has the option to avoid. Given the robust projection from PL to VS (Sesack et al., 1989; Ding, Gabbott and Totterdell, 2001; Vertes, 2004; Gabbott et al., 2005) including those onto inhibitory interneurons (Berke, 2011), we suggest that PL inhibitory responses to the WS serve to disinhibit the driving of VS output neurons by basal amygdala (BA) inputs, hence promoting avoidance (Figure 2B). Excitatory responses in PL were correlated with platform entry (Diehl et al., 2018a), which is consistent with cFos studies showing that increased PL activity correlates with avoidance behavior (Martinez et al., 2013; Bravo-Rivera et al., 2015). These excitatory responses are likely transmitted to BA rather than to VS, as indicated by a recent retrograde labeling study (Martínez-Rivera et al., 2018).
In summary, it appears that PL is important for avoiding early during the tone, when the rat must weigh avoidance against foraging to decide if (and when) it will initiate platform approach. PL promotes avoidance during this period through inhibitory responses that disinhibit VS responses to BA inputs. PL also promotes avoidance by exciting BA neurons that may, in turn, excite VS outputs. As the tone progresses and shock becomes imminent, BA excitation of VS increases so that disinhibition by PL is no longer necessary (Figure 2B). Thus, while BA inputs to VS are necessary throughout the tone, PL inputs to VS are only necessary early in the tone, when decisions are made regarding a more ambiguous threat. Experiments employing optogenetics are currently underway to test this circuit (Diehl et al., 2018b).
5. Modeling maladaptive avoidance with PMA
Excessive avoidance can be maladaptive due to missed opportunities for reward (Lovibond and Shanks, 2002; Vervliet and Indekeu, 2015), and missed opportunities for extinction of trauma-associated cues (Foa and Kozak, 1986). Excessive avoidance can occur when the individual is unable to learn that a previous threat is no longer dangerous (extinction deficits), or is unable to recall this learning (Bravo-Rivera et al., 2015; Vervliet and Indekeu, 2015). Excessive avoidance is a hallmark of anxiety disorders such as PTSD and OCD (Breslau, 2001; Asmundson, Stapleton and Taylor, 2004; McGuire et al., 2012), but is also observed in phobias (Eaton, Bienvenu and Miloyan, 2018), depression (Trew, 2011) and autism (Madipakkam et al., 2017). In contrast to excessive fear, the neural mechanisms of excessive avoidance are not well-understood, and animal models such as PMA may lead researchers toward improved treatments for patients suffering from neuropsychiatric disorders (Bravo-Rivera et al., 2015; Rodriguez-Romaguera and Quirk, 2017; Diehl et al., 2018c; Cain, 2019).
Persistent avoidance related to extinction deficits has been studied in the PMA task using the activity marker cFos (Bravo-Rivera et al., 2015). Rats failing to extinguish avoidance after two days of extinction training had excessive activity in BLA, VS, and PL, consistent with the proposed circuit of avoidance expression shown in Figure 2B. Rats showing persistent avoidance also exhibited reduced activity in IL consistent with impaired extinction of the tone-shock association. Unlike extinction of fear, extinction of active avoidance involves two components: extinction of the tone-shock association and extinction of the avoidance behavior. To distinguish between these two, Bravo-Rivera, et al. (2015) also assessed extinction of avoidance with the platform removed, so that the tone-shock association could be extinguished in the absence of the option to avoid. After rats extinguished their conditioned fear responses (measured by suppression of bar pressing), the platform was returned in a subsequent test session. Rats showing persistent avoidance under these conditions had excessive activity only in PL and VS; BLA and IL showed a normal fear extinction profile. Thus, PL-VS connections appear to support maladaptive avoidance independently of tone-shock association circuits. Furthermore, unlike extinction of conditioned fear, PL plays a key role in the extinction of PMA, as BDNFergic inputs from the ventral hippocampus to PL are both necessary for, and activated by, extinction training (Rosas-Vidal et al., 2018).
In order to understand maladaptive avoidance with regard to OCD, the PMA task has been modified to study extinction-based treatment of avoidance-related compulsions in OCD, known as exposure-with-response-prevention (ERP; Rachman, Hodgson and Marks, 1971; Foa et al., 2005; Simpson et al., 2008; Franklin and Foa, 2011). In ERP therapy, patients are exposed to triggers for their compulsions but are prevented from expressing their compulsions, in order to extinguish the compulsive behavior. Similar to PTSD, ERP is only effective for a subset of patients (Foa et al., 2005; Simpson et al., 2008; Foa et al., 2013). ERP was modelled in the PMA task by preventing rats’ access to the platform during extinction (extinction-with-response-prevention, or Ext-RP; Rodriguez-Romaguera et al., 2016). After several days of Ext-RP training, when the platform was again accessible, approximately 25% of rats showed persistent avoidance. Persistent avoidance could be eliminated by either pharmacologically inactivating the orbitofrontal cortex or applying deep brain stimulation (DBS) to the VS, a rodent homologue of effective sites of DBS for the treatment of OCD in humans (Rodriguez-Romaguera et al., 2016). In agreement with human studies of OCD (Evans, Lewis and lobst, 2004; Menzies et al., 2008; Milad and Rauch, 2012), persistent avoidance in PMA is associated with hyperactive orbitofrontal areas.
A poor clinical response to exposure-based therapies in OCD may reflect the repetitive nature of compulsions in this disorder. It has been suggested that OCD patients are prone to exhibit habit learning that drives the repetition of avoidance behaviors that are no longer necessary (Gillan et al., 2015). Consistent with this, extending PMA conditioning from 8 days to 20 days impairs Ext-RP, and augments the percentage of rats showing persistent avoidance (Martínez-Rivera et al., 2018). cFos immunohistochemistry revealed marked differences in patterns of avoidance-related neuronal activity in trained vs over-trained groups, suggesting possible mechanisms by which individuals fail ERP therapy. Thus, the circuits of goal-directed avoidance may differ from the circuits of the more habitual avoidance associated with OCD.
6. Active avoidance in humans
Human studies examining active avoidance are emerging, often comparing avoidance training with extinction of fear. There is a long-standing idea that conditioned avoidance is resistant to extinction (Solomon and Wynne, 1954; Rescorla, 2003; Lovibond et al., 2009). In support of this, it was shown that avoidance behaviors with little cost can persist following Pavlovian extinction (Vervliet and Indekeu, 2015), similar to rodents (Bravo-Rivera et al., 2015; Rodriguez-Romaguera et al., 2016). Avoidance training can even impair subsequent extinction training (Rattel et al., 2017). Indeed, high levels of avoidance can prevent extinction from occurring (“protection from extinction effect”) which can account for the high prevalence of avoidance symptoms in anxiety disorders. However, a recent study comparing subjects with avoidance training to subjects with extinction training (as a “yoked” control group) found that avoidance produced longer-lasting reductions in fear that continued through re-conditioning (Boeke et al., 2017), suggesting that learning “controllability” may be clinically beneficial (Maier, 2015).
Emerging neuroimaging studies of human active avoidance parallel rodent studies. Avoidance activates both the amygdala and the ventral striatum, and their activities are correlated (Delgado et al., 2009; Levita, Hoskin and Champi, 2012), consistent with BA activation of VS in rodents. The vmPFC, which is the human homologue of rodent IL (Bicks et al., 2015; Heilbronner et al., 2016) is also activated during avoidance (Boeke et al., 2017; Wendt et al., 2017), consistent with IL reduction of conditioned fear responses (Moscarello and LeDoux, 2013; Bravo-Rivera et al., 2014). Avoidance-related activity of the dorsal anterior cingulate (dACC), a human homologue of rodent PL (Bicks et al., 2015; Heilbronner et al., 2016), was correlated with activity in both striatum and amygdala, consistent with prefrontal modulation of the amygdala-striatal circuit. It is interesting to note that avoidance symptoms in PTSD correlate with resting state activity of dACC (Marin et al., 2016). To better model clinical avoidance, future studies of human avoidance should include an appetitive cost of avoidance which could recruit dACC (Collins et al., 2014).
7. Future directions for active avoidance
Future tasks including a cost of avoidance will need to consider the magnitude of the cost vs. the magnitude of the competing reward. Increasing the cost of avoidance can reduce the decision to avoid (Rattel et al., 2017) or facilitate the pursuit of conflicting reward (Friedman et al., 2015). The cost of avoidance can increase when it interferes with a time-limited access to reward. In the PMA task, foraging-avoidance competition is minimal because sucrose is also available during the ITI when the tone is off. Restricting sucrose access to the period of the tone maximizes this conflict and reveals different “strategies” for conflict resolution. Preliminary data from our group show that rats exhibit one of three strategies under these conditions: 1) they remain on the platform throughout the tone, 2) they remain at the sucrose lever throughout the tone, or 3) they divide their time, spending the early part of the tone at the lever and the later part of the tone at the platform, the latter of which is the most prevalent phenotype (Bravo-Rivera, Rubio-Arzola and Quirk, 2016). These three subgroups can be useful for understanding maladaptive behaviors as well as reveal distinct patterns of activation within avoidance and reward circuits, as has been shown in previous studies on conflict (Burgos-Robles et al., 2017).
Future avoidance studies will need to assess sex differences, given that females show more avoidance following a traumatic event than males (Kessler et al., 1995). Prior studies have found that males acquire active avoidance more quickly than females (Gray and Lalljee, 1974; Farr et al., 1995; Beck et al., 2011; Yokota et al., 2017; but see ; Denti and Epstein, 1972; Rubio et al., 1999). In contrast, females extinguish the avoidance response more quickly than males but only when training is combined with a safety-associated cue in rodents (Beck et al., 2011; Radell et al., 2015) and in humans (Sheynin et al., 2014). This may be at odds with clinical findings showing that females have an increased risk of developing an anxiety disorder (Bangasser et al., 2018; Altemus, Sarvaiya and Neill Epperson, 2014). Therefore, future studies should address the extent to which the addition of safety cues during extinction training would reduce avoidance symptoms in males vs. females.
Thus far, the study of active avoidance has been investigated in the absence of any social factors. How might the presence of social cues alter the learning or expression of avoidance? Only one animal study has addressed social factors in active avoidance. Augmenting social interactions with pair-housing facilitated the learning of extinction of avoidance (Smith et al., 2016). Other behavioral paradigms have examined social factors in fear learning (Olsson and Phelps, 2007; Debiec and Olsson, 2017), decision making (Tremblay, Sharika and Platt, 2017), and addiction (Heilig et al., 2016). For example, addicted rats will abstain from taking drugs in order to interact with another rat (Venniro et al., 2018). Such studies demonstrate the power social interactions can have over behavior. It would be interesting to examine whether positive or negative types of social interactions facilitate or impair the acquisition of avoidance, respectively. From a clinical perspective, understanding how social interactions impact maladaptive avoidance could lead to beneficial treatments, given that positive social support systems can alleviate symptoms of anxiety (Boscarino, 1995; Brewin, Andrews and Valentine, 2000; Platt, Keyes and Koenen, 2014), PTSD (Arnberg et al., 2012), and depression (McGuire et al., 2018).
Highlights:
The field of active avoidance has used the same task in rodents for over 80 years, without updates.
Platform-mediated avoidance (PMA) models aspects of clinical avoidance that classical tasks do not.
Prelimbic cortex promotes PMA by disinhibiting BA-responsive neurons in VS.
Acknowledgements:
We acknowledge support by NIH grants R37-MH058883 and P50-MH106435 to GJQ, and the University of Puerto Rico President’s Office. We thank Hector Bravo-Rivera for assistance with figures and Christopher K. Cain for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- Altemus M, Sarvaiya N and Neill Epperson C (2014) ‘Sex differences in anxiety and depression clinical perspectives’, Front Neuroendocrinol, 35(3), pp. 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders : DSM-5. 5th edn. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Amir A, Lee SC, Headley DB, Herzallah MM and Pare D (2015) ‘Amygdala Signaling during Foraging in a Hazardous Environment’, J Neurosci, 35(38), pp. 12994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg FK, Hultman CM, Michel PO and Lundin T (2012) ‘Social support moderates posttraumatic stress and general distress after disaster’, J Trauma Stress, 25(6), pp. 721–7. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Stapleton JA and Taylor S (2004) ‘Are avoidance and numbing distinct PTSD symptom clusters?’, J Trauma Stress, 17(6), pp. 467–75. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Telenson AM and Salvatore M (2018) ‘Sex differences in stress regulation of arousal and cognition’, Physiol Behav, 187, pp. 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Jiao X, Ricart TM, Myers CE, Minor TR, Pang KC and Servatius RJ (2011) ‘Vulnerability factors in anxiety: Strain and sex differences in the use of signals associated with non-threat during the acquisition and extinction of active-avoidance behavior’, Prog Neuropsychopharmacol Biol Psychiatry, 35(7), pp. 1659–70. [DOI] [PubMed] [Google Scholar]
- Berger DF and Brush FR (1975) ‘Rapid acquisition of discrete-trial lever-press avoidance: effects of signal-shock interval’, J Exp Anal Behav, 24(2), pp. 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2011) ‘Functional properties of striatal fast-spiking interneurons’, Front Syst Neurosci, 5, pp. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S and Morishita H (2015) ‘Prefrontal Cortex and Social Cognition in Mouse and Man’, Front Psychol, 6, pp. 1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC and Blanchard RJ (1972) ‘Innate and conditioned reactions to threat in rats with amygdaloid lesions’, J. Comp Physiol Psychol, 81(2), pp. 281–290. [DOI] [PubMed] [Google Scholar]
- Boeke EA, Moscarello JM, LeDoux JE, Phelps EA and Hartley CA (2017) ‘Active Avoidance: Neural Mechanisms and Attenuation of Pavlovian Conditioned Responding’, J Neurosci, 37(18), pp. 4808–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Stokes LW and Younger MS (1966) ‘Does CS termination reinforce avoidance behavior?’, Journal of comparative and physiological psychology, 62(2), pp. 201–7. [DOI] [PubMed] [Google Scholar]
- Boscarino JA (1995) ‘Post-traumatic stress and associated disorders among Vietnam veterans: the significance of combat exposure and social support’, J Trauma Stress, 8(2), pp. 317–36. [DOI] [PubMed] [Google Scholar]
- Bouton ME and Bolles RC (1979) ‘Role of conditioned contextual stimuli in reinstatement of extinguished fear’, J. Exp. Psychol. Anim Behav. Process, 5(4), pp. 368–378. [DOI] [PubMed] [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F and Quirk GJ (2014) ‘Neural structures mediating expression and extinction of platform-mediated avoidance’, J Neurosci, 34(29), pp. 9736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Montesinos-Cartagena M and Quirk GJ (2015) ‘Persistent active avoidance correlates with activity in prelimbic cortex and ventral striatum’, Front Behav Neurosci, 9, pp. 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera H, Rubio-Arzola P and Quirk GJ ‘A modification of the platform-mediated avoidance task to study food-avoidance conflict’. Society for Neuroscience, San Diego, CA, 2016. [Google Scholar]
- Breslau N (2001) ‘Outcomes of posttraumatic stress disorder’, J. Clin. Psychiatry, 62 Suppl 17, pp. 55–59. [PubMed] [Google Scholar]
- Brewin CR, Andrews B and Valentine JD (2000) ‘Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults’, J Consult Clin Psychol, 68(5), pp. 748–66. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A and Tye KM (2017) ‘Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment’, Nat Neurosci, 20(6), pp. 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK (2019) ‘Avoidance problems reconsidered’, Current Opinion in Behavioral Sciences, 26, pp. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona GN, Nishimura T, Schindler CW, Panlilio LV and Notkins AL (2014) ‘The dense core vesicle protein IA-2, but not IA-2β, is required for active avoidance learning’, Neuroscience, 269, pp. 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Cain CK and LeDoux JE (2010) ‘The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats’, Learning & memory, 17(3), pp. 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KA, Mendelsohn A, Cain CK and Schiller D (2014) ‘Taking action in the face of threat: neural synchronization predicts adaptive coping’, J Neurosci, 34(44), pp. 14733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato MR and Schiff D (1964) ‘LONG-TERM DISCRIMINATED AVOIDANCE PERFORMANCE IN THE RAT’, J Comp Physiol Psychol, 57, pp. 123–6. [DOI] [PubMed] [Google Scholar]
- Darvas M, Fadok JP and Palmiter RD (2011) ‘Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response’, Learning & memory, 18(3), pp. 136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J and Olsson A (2017) ‘Social Fear Learning: from Animal Models to Human Function’, Trends Cogn Sci, 21(7), pp. 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE and Phelps EA (2009) ‘Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning’, Frontiers in behavioral neuroscience, 3, pp. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denti A and Epstein A (1972) ‘Sex differences in the acquisition of two kinds of avoidance behavior in rats’, Physiol Behav, 8(4), pp. 611–5. [DOI] [PubMed] [Google Scholar]
- Diehl MM, Bravo-Rivera C, Rodriguez-Romaguera J, Pagan-Rivera PA, Burgos-Robles A, Roman-Ortiz C and Quirk GJ (2018a) ‘Active avoidance requires inhibitory signaling in the rodent prelimbic prefrontal cortex’, Elite, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MM, Iravedra-Garcia JM, Gonzalez-Diaz FN, Moran-Sierra J and Quirk GJ ‘Interrogating the projections of rostral prelimbic cortex that drive active avoidance’. Society for Neuroscience, San Diego, CA, 2018. [Google Scholar]
- Diehl MM, Lempert KM, Parr AC, Ballard I, Steele VR and Smith DV (2018c) ‘Toward an integrative perspective on the neural mechanisms underlying persistent maladaptive behaviors’, Eur J Neurosci, 48(3), pp. 1870–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DC, Gabbott PL and Totterdell S (2001) ‘Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat’, Brain Res, 917(1), pp. 81–9. [DOI] [PubMed] [Google Scholar]
- Dinsmoor JA (2001) ‘Stimuli inevitably generated by behavior that avoids electric shock are inherently reinforcing’, J Exp Anal Behav, 75(3), pp. 311–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Monte FH, Quirk GJ, Li B and Penzo MA (2016) ‘Retrieving fear memories, as time goes by...’, Mol Psychiatry, 21(8), pp. 1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S and Pare D (2014) ‘Amygdala microcircuits controlling learned fear’, Neuron, 82(5), pp. 966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Bienvenu OJ and Miloyan B (2018) ‘Specific phobias’, Lancet Psychiatry, 5(8), pp. 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK and Skinner BF (1941) ‘Some quantitative properties of anxiety.’, Journal of Experimental Psychology, 29(5), pp. 390–400. [Google Scholar]
- Evans DW, Lewis MD and lobst E (2004) ‘The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder’, Brain Cogn, 55(1), pp. 220–34. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P and Lüthi A (2017) ‘A competitive inhibitory circuit for selection of active and passive fear responses’, Nature, 542(7639), pp. 96–100. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1994) ‘Neural organization of the defensive behavior system responsible for fear’, Psychon Bull Rev, 1(4), pp. 429–38. [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT and Morley JE (1995) ‘Effect of ovarian steroids on footshock avoidance learning and retention in female mice’, Physiol Behav, 58(4), pp. 715–23. [DOI] [PubMed] [Google Scholar]
- Foa EB and Kozak MJ (1986) ‘Emotional processing of fear: exposure to corrective information’, Psychol Bull, 99(1), pp. 20–35. [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Huppert JD, Kjernisted K, Rowan V, Schmidt AB, Simpson HB and Tu X (2005) ‘Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder’, Am J Psychiatry, 162(1), pp. 151–61. [DOI] [PubMed] [Google Scholar]
- Foa EB, Simpson HB, Liebowitz MR, Powers MB, Rosenfield D, Cahill SP, Campeas R, Franklin M, Hahn CG, Hembree EA, Huppert JD, Schmidt AB, Vermes D and Williams MT (2013) ‘Six-month follow-up of a randomized controlled trial augmenting serotonin reuptake inhibitor treatment with exposure and ritual prevention for obsessive-compulsive disorder’, J Clin Psychiatry, 74(5), pp. 464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin ME and Foa EB (2011) ‘Treatment of obsessive compulsive disorder’, Annu Rev Clin Psychol, 7, pp. 229–43. [DOI] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, Riad MH and Graybiel AM (2015) ‘A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict’, Cell, 161(6), pp. 1320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P and Busby SJ (2005) ‘Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers’, J. Comp Neurol, 492(2), pp. 145–177. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Saltwick SE and Miller JD (1975) ‘Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus’, Science, 189(4208), pp. 1108–9. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Moscarello J, Blessing EM, Klein J, Cain CK and LeDoux JE (2014) ‘Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues’, Front Syst Neurosci, 8, pp. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Apergis-Schoute AM, Morein-Zamir S, Urcelay GP, Sule A, Fineberg NA, Sahakian BJ and Robbins TW (2015) ‘Functional neuroimaging of avoidance habits in obsessive-compulsive disorder’, Am J Psychiatry, 172(3), pp. 284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF and Maren S (2015) ‘The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear’, Front Behav Neurosci, 9, pp. 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA and Lalljee B (1974) ‘Sex differences in emotional behaviour in the rat: correlation between open-field defecation and active avoidance’, Anim Behav, 22(4), pp. 856–61. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ and Haber SN (2016) ‘Circuit-Based Corticostriatal Homologies Between Rat and Primate’, Biol Psychiatry, 80(7), pp. 509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA and Shaham Y (2016) ‘Time to connect: bringing social context into addiction neuroscience’, Nat Rev Neurosci, 17(9), pp. 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RW and Graham J (1979) ‘Avoidance of heat by rats: Effects of thermal context on rapidity of extinction’, Learning and Motivation, 10(3), pp. 351–363. [Google Scholar]
- Herry C and Johansen JP (2014) ‘Encoding of fear learning and memory in distributed neuronal circuits’, Nat Neurosci, 17(12), pp. 1644–54. [DOI] [PubMed] [Google Scholar]
- Hormigo S, Vega-Flores G and Castro-Alamancos MA (2016) ‘Basal Ganglia Output Controls Active Avoidance Behavior’, J Neurosci, 36(40), pp. 10274–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chou D, Yeh CM and Hsu KS (2016) ‘Acute food deprivation enhances fear extinction but inhibits long-term depression in the lateral amygdala via ghrelin signaling’, Neuropharmacology, 101, pp. 36–45. [DOI] [PubMed] [Google Scholar]
- Illescas-Huerta E, L R-L, Ordonez-Sierra R and Sotres-Bayon F ‘Prelimbic prefrontal cortex is necessary to face threats during a motivational conflict guided by learned, but not innate, stimuli’. Society for Neuroscience, San Diego, CA. [Google Scholar]
- Jiao X, Beck KD, Myers CE, Servatius RJ and Pang KC (2015) ‘Altered activity of the medial prefrontal cortex and amygdala during acquisition and extinction of an active avoidance task’, Front Behav Neurosci, 9, pp. 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE and LeDoux JE (2011) ‘Molecular mechanisms of fear learning and memory’, Cell, 147(3), pp. 509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ, Brimer CJ and Black AH (1963) ‘Conditioned suppression as a monitor of fear of the CS in the course of avoidance training’, Journal of comparative and physiological psychology, 56, pp. 497–501. [DOI] [PubMed] [Google Scholar]
- Kavaliers M and Choleris E (2001) ‘Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences’, Neurosci Biobehav Rev, 25(7–8), pp. 577–86. [DOI] [PubMed] [Google Scholar]
- Keehn JD and Nakkash S (1959) ‘Effect of a signal contingent upon an avoidance response’, Nature, 184(Suppl 8), pp. 566–8. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M and Nelson CB (1995) ‘Posttraumatic stress disorder in the National Comorbidity Survey’, Arch Gen Psychiatry, 52(12), pp. 1048–60. [DOI] [PubMed] [Google Scholar]
- Kim JJ and Fanselow MS (1992) ‘Modality-specific retrograde amnesia of fear’, Science, 256(5057), pp. 675–677. [DOI] [PubMed] [Google Scholar]
- Kim JJ and Jung MW (2018) ‘Fear paradigms: The times they are a-changin”, Curr Opin Behav Sci, 24, pp. 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV and Kreitzer AC (2012) ‘Striatal mechanisms underlying movement, reinforcement, and punishment’, Physiology (Bethesda), 27(3), pp. 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi P, Headley DB and Pare D (2018) ‘Multi-dimensional Coding by Basolateral Amygdala Neurons’, Neuron, 99(6), pp. 1315–1328.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Munoz G, LeDoux JE and Cain CK (2010) ‘Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes’, Biological psychiatry, 67(12), pp. 1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) ‘Emotion circuits in the brain’, Annu. Rev. Neurosci, 23, pp. 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Moscarello J, Sears R and Campese V (2017) ‘The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm’, Mol Psychiatry, 22(1), pp. 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis DJ (1989) ‘The case for a return to a two-factor theory of avoidance: the failure of non-fear interpretations’, in Klein SB and Mowrer RR (eds.) Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. Hillsdale, NJ, USA: Lawrence Erlbaum, pp. 227–277. [Google Scholar]
- Levita L, Hoskin R and Champi S (2012) ‘Avoidance of harm and anxiety: a role for the nucleus accumbens’, Neuroimage, 62(1), pp. 189–98. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Mitchell CJ, Minard E, Brady A and Menzies RG (2009) ‘Safety behaviours preserve threat beliefs: Protection from extinction of human fear conditioning by an avoidance response’, Behav Res Ther, 47(8), pp. 716–20. [DOI] [PubMed] [Google Scholar]
- Lovibond PF and Shanks DR (2002) ‘The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications’, J. Exp. Psychol. Anim Behav. Process, 28(1), pp. 3–26. [PubMed] [Google Scholar]
- Lüthi A and Lüscher C (2014) ‘Pathological circuit function underlying addiction and anxiety disorders’, Nat Neurosci, 17(12), pp. 1635–43. [DOI] [PubMed] [Google Scholar]
- Maatsch JL (1959) ‘Learning and fixation after a single shock trial’, J Comp Physiol Psychol, 52, pp. 408–10. [DOI] [PubMed] [Google Scholar]
- Madipakkam AR, Rothkirch M, Dziobek I and Sterzer P (2017) ‘Unconscious avoidance of eye contact in autism spectrum disorder’, Sci Rep, 7(1), pp. 13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF (2015) ‘Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network’, Neurobiol Stress, 1, pp. 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2005) ‘Synaptic mechanisms of associative memory in the amygdala’, Neuron, 47(6), pp. 783–6. [DOI] [PubMed] [Google Scholar]
- Maren S, Poremba A and Gabriel M (1991) ‘Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits’, Brain Res., 549(2), pp. 311–316. [DOI] [PubMed] [Google Scholar]
- Maren S and Quirk GJ (2004) ‘Neuronal signalling of fear memory’, Nat. Rev. Neurosci, 5(11), pp. 844–852. [DOI] [PubMed] [Google Scholar]
- Marin MF, Song H, VanElzakker MB, Staples-Bradley LK, Linnman C, Pace-Schott EF, Lasko NB, Shin LM and Milad MR (2016) ‘Association of Resting Metabolism in the Fear Neural Network With Extinction Recall Activations and Clinical Measures in Trauma-Exposed Individuals’, Am J Psychiatry, 173(9), pp. 930–8. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Gupta N, Lazaro-Munoz G, Sears RM, Kim S, Moscarello JM, LeDoux JE and Cain CK (2013) ‘Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex’, Learning & memory, 20(8), pp. 446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rivera FJ, Perez-Torres JE, Huertas-Perez CI, Sanchez-Navarro MJ, Velazquez-Diaz CD and Quirk GJ ‘The role of orbital/insular outputs on a rodent model of persistent avoidance’. Society for Neuroscience, San Diego, CA, 2018. [Google Scholar]
- Martinez-Rivera FJ, Bravo-Rivera C, Velazquez-Diaz CD, Montesinos-Cartagena M and Quirk GJ (2018) ‘Prefrontal circuits signaling active avoidance retrieval and extinction’, Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AP, Gauthier JM, Anderson LM, Hollingsworth DW, Tracy M, Galea S and Coffey SF (2018) ‘Social Support Moderates Effects of Natural Disaster Exposure on Depression and Posttraumatic Stress Disorder Symptoms: Effects for Displaced and Nondisplaced Residents’, J Trauma Stress, 31(2), pp. 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Storch EA, Lewin AB, Price LH, Rasmussen SA and Goodman WK (2012) ‘The role of avoidance in the phenomenology of obsessive-compulsive disorder’, Compr Psychiatry, 53(2), pp. 187–94. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ and Bullmore ET (2008) ‘Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited’, Neurosci Biobehav Rev, 32(3), pp. 525–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR and Quirk GJ (2012) ‘Fear extinction as a model for translational neuroscience: ten years of progress’, Annu Rev Psychol, 63, pp. 129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR and Rauch SL (2012) ‘Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways’, Trends Cogn Sci, 16(1), pp. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello JM and Hartley CA (2017) ‘Agency and the Calibration of Motivated Behavior’, Trends Cogn Sci, 21(10), pp. 725–735. [DOI] [PubMed] [Google Scholar]
- Moscarello JM and LeDoux JE (2013) ‘Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions’, J Neurosci, 33(9), pp. 3815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH (1939) ‘A stimulus-response analysis of anxiety and its role as a reinforcing agent.’, Psychological Review, 46(6), pp. 553–565. [Google Scholar]
- Mowrer OH (1960) Learning theory and behavior. 2 edn.: Wiley, 1960. [Google Scholar]
- Mowrer OH and Lamoreaux RR (1946) ‘Fear as an intervening variable in avoidance conditioning’, J Comp Psychol, 39, pp. 29–50. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP and Tye KM (2015) ‘Decoding neural circuits that control compulsive sucrose seeking’, Cell, 160(3), pp. 528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A and Phelps EA (2007) ‘Social learning of fear’, Nat Neurosci, 10(9), pp. 1095–102. [DOI] [PubMed] [Google Scholar]
- Pare D and Quirk GJ (2017) ‘When scientific paradigms lead to tunnel vision: lessons from the study of fear’, 2, pp. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA and Feldon J (2004) ‘Mesolimbic dopaminergic pathways in fear conditioning’, Prog Neurobiol, 74(5), pp. 301–20. [DOI] [PubMed] [Google Scholar]
- Platt J, Keyes KM and Koenen KC (2014) ‘Size of the social network versus quality of social support: which is more protective against PTSD?’, Soc Psychiatry Psychiatr Epidemiol, 49(8), pp. 1279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM and Fanselow MS (2007) ‘Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning’, Neuroscience, 147(4), pp. 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A and Gabriel M (1999) ‘Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits’, The Journal of neuroscience : the official journal of the Society for Neuroscience, 19(21), pp. 9635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL and Lebron K (2000) ‘The role of ventromedial prefrontal cortex in the recovery of extinguished fear’, J Neurosci, 20(16), pp. 6225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S, Hodgson R and Marks IM (1971) ‘The treatment of chronic obsessive-compulsive neurosis’, Behav Res Ther, 9(3), pp. 237–47. [DOI] [PubMed] [Google Scholar]
- Radell ML, Beck KD, Pang KC and Myers CE (2015) ‘Using signals associated with safety in avoidance learning: computational model of sex differences’, PeerJ, 3, pp. e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Moscarello JM, LeDoux JE and Sears RM (2015) ‘Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit’, J Neurosci, 35(8), pp. 3470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattel JA, Miedl SF, Blechert J and Wilhelm FH (2017) ‘Higher threat avoidance costs reduce avoidance behaviour which in turn promotes fear extinction in humans’, Behav Res Ther, 96, pp. 37–46. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (2003) ‘Protection from extinction’, Learn Behav, 31(2), pp. 124–32. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Greenberg BD, Rasmussen SA and Quirk GJ (2016) ‘An Avoidance-Based Rodent Model of Exposure With Response Prevention Therapy for Obsessive-Compulsive Disorder’, Biol Psychiatry, 80(7), pp. 534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J and Quirk GJ (2017) ‘Extinction of conditioned fear and avoidance: Relevance for OCD’, in Pittenger C (ed.) Obsessive Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment.: Oxford University Press. [Google Scholar]
- Rosas-Vidal LE, Lozada-Miranda V, Cantres-Rosario Y, Vega-Medina A, Melendez L and Quirk GJ (2018) ‘Alteration of BDNF in the medial prefrontal cortex and the ventral hippocampus impairs extinction of avoidance’, Neuropsychopharmacology, 43(13), pp. 2636–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio S, Miranda R, Cuesta M, Begaga A, Santin L and Arias J (1999) ‘Active avoidance conditioning in rats: Absence of sex difference and estrous effect’, Psicothema, 11(3), pp. 655–661. [Google Scholar]
- Sears RM, Schiff HC and LeDoux JE (2014) ‘Molecular mechanisms of threat learning in the lateral nucleus of the amygdala’, Prog Mol Biol Transl Sci, 122, pp. 263–304. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Jiao X, Beck KD, Pang KC and Minor TR (2008) ‘Rapid avoidance acquisition in Wistar-Kyoto rats’, Behav Brain Res, 192(2), pp. 191–7. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH and Bunney BS (1989) ‘Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin’, J Comp Neurol, 290(2), pp. 213–42. [DOI] [PubMed] [Google Scholar]
- Sheynin J, Beck KD, Servatius RJ and Myers CE (2014) ‘Acquisition and extinction of human avoidance behavior: attenuating effect of safety signals and associations with anxiety vulnerabilities’, Front Behav Neurosci, 8, pp. 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Ledley DR, Huppert JD, Cahill S, Vermes D, Schmidt AB, Hembree E, Franklin M, Campeas R, Hahn CG and Petkova E (2008) ‘A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder’, Am J Psychiatry, 165(5), pp. 621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N and Holmes A (2019) ‘Rodent models of impaired fear extinction’, Psychopharmacology (Berl), 236(1), pp. 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IM, Pang KC, Servatius RJ, Jiao X and Beck KD (2016) ‘Paired-housing selectively facilitates within-session extinction of avoidance behavior, and increases c-Fos expression in the medial prefrontal cortex, in anxiety vulnerable Wistar-Kyoto rats’, Physiol Behav, 164(Pt A), pp. 198–206. [DOI] [PubMed] [Google Scholar]
- Smith JW, Fetsko LA, Xu R and Wang Y (2002) ‘Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning’, Neuroscience, 113(4), pp. 755–65. [DOI] [PubMed] [Google Scholar]
- Solomon RL and Wynne LC (1954) ‘Traumatic avoidance learning: the principles of anxiety conservation and partial irreversibility’, Psychol Rev, 61(6), pp. 353–85. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Sharika KM and Platt ML (2017) ‘Social Decision-Making and the Brain: A Comparative Perspective’, Trends Cogn Sci, 21(4), pp. 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew JL (2011) ‘Exploring the roles of approach and avoidance in depression: an integrative model’, Clin Psychol Rev, 31(7), pp. 1156–68. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Cabrero-Castel A, Berrendero F, Maldonado R and Robledo P (2008) ‘MDMA modifies active avoidance learning and recall in mice’, Psychopharmacology (Berl), 197(3), pp. 391–400. [DOI] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH and Shaham Y (2018) ‘Volitional social interaction prevents drug addiction in rat models’, Nat Neurosci, 21(11), pp. 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Wood J, Lach G, Herzog H, Sperk G and Tasan R (2016) ‘Hunger Promotes Fear Extinction by Activation of an Amygdala Microcircuit’, Neuropsychopharmacology, 41(2), pp. 431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) ‘Differential projections of the infralimbic and prelimbic cortex in the rat’, Synapse, 51(1), pp. 32–58. [DOI] [PubMed] [Google Scholar]
- Vervliet B and Indekeu E (2015) ‘Low-Cost Avoidance Behaviors are Resistant to Fear Extinction in Humans’, Front Behav Neurosci, 9, pp. 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler E, Gaspar JC, Ferreira TL, Barbiero JK, Andreatini R, Vital MA, Blaha CD, Winn P and Da Cunha C (2014) ‘The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses’, Neurobiol Learn Mem, 109, pp. 27–36. [DOI] [PubMed] [Google Scholar]
- Wendt J, Low A, Weymar M, Lotze M and Hamm AO (2017) ‘Active avoidance and attentive freezing in the face of approaching threat’, Neuroimage, 158, pp. 196–204. [DOI] [PubMed] [Google Scholar]
- Yokota S, Suzuki Y, Hamami K, Harada A and Komai S (2017) ‘Sex differences in avoidance behavior after perceiving potential risk in mice’, Behav Brain Funct, 13(1), pp. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]