Abstract
Anxa2 is the most studied member of the calcium-mediated phospholipid-binding protein family annexins and is a biomarker in cancers. In this review, we listed clinical findings and confirmed the value of Anxa2 in early diagnosis and prognostic prediction due to its overexpression and adverse effect on the outcome in most tumors. Anxa2 plays a pivotal role in cancer cell proliferation, migration, invasion, metastasis, and treatment resistance. Improved understanding of its cancer-promoting function might make it an ideal target for cancer therapy. Here, we systematically summarized the mechanism of Anxa2 in regulating epithelial-mesenchymal transition (EMT), cytoskeleton dynamicity, cell cycle, apoptosis, angiogenesis, and immunology by using various tumor models. These data emphasize the potential of Anxa2 for targeted intervention in tumors. Altering Anxa2 expression, neutralizing the cell surface Anxa2, or inhibiting its activation, such as through Tyr23 phosphorylation, could be considered based on the regulatory mechanism of Anxa2 in tumor progression.
Keywords: Anxa2, cancer, diagnosis, prognosis, metastasis, treatment resistance
Introduction
The 36KD calcium-mediated protein Anxa2 is a member of the phospholipid-binding protein family annexins, which comprise a highly conserved C terminal domain with binding sites to calcium, negatively charged phospholipid, heparin, and F-actin, and a variable N terminal domain that is unique to each annexin. As the most studied member, the amino-terminal domain of Anxa2 contains S100A10 and t-PA binding sites, and post-transcriptional modification sites, including three important phosphorylation sites, that are crucial to its biological function1,2. Anxa2 is implicated in several pivotal cellular processes, such as cell proliferation, cell adhesion, cell motility, angiogenesis, and endocytosis. This protein abundantly expressed in various types of tumor, and thus is proposed as a potential marker for cancers3,4. Its critical role in cancer progression is involved in the complicated pathways of cancer cell invasion, metastasis, and treatment resistance. Further exploration of Anxa2 mechanisms in cancer progression might help establish it as an effective intervention and an ideal therapeutic target for certain cancers. In this review, we presented clinical evidence on the value of Anxa2 as a biomarker for cancer diagnosis and prognostic prediction and highlighted its molecular mechanism in promoting cancer progression. This article helps develop new insights into the intervention targets in cancers.
Diagnostic and prognostic value of Anxa2 in various types of cancer
The expression and prognostic effect of Anxa2 depend on the tumor type. In most cancers, Anxa2 overexpression indicates a poor outcome. A meta-analysis of 2321 patients with malignant tumors from 15 clinical studies showed that its elevated expression is correlated with poor prognosis in terms of overall survival rate (OS; HR = 1.56, 95% CI = 1.24 – 1.97, P < 0.001) and disease-free survival rate (DFS; HR = 1.47, 95% CI = 1.18 – 1.83, P < 0.001) 5. This result indicates the overall adverse prognostic value and the progression-promoting role of Anxa2 in malignant tumors. The clinical significance of Anxa2 in cancers is summarized in Table 1.
1.
The clinical significance of Anxa2 in cancers
| Tumor type | Sample | Sample size | Expression | Significance | Reference | |
| Breast cancer | Tissue | IDC* = 100
DCIS** = 20 Normal = 50 |
mRNA | Upregulated in IDC
Lymph node invasion |
6 | |
| Tissue | n = 20 | Protein | Upregulated in cancer | 7 | ||
| Tissue | n = 85 | Protein | Upregulated in invasive cancer
Advanced stage High histological grade High EGFR expression Poor OS and DFS |
8 | ||
| Blood
Tissue |
Cancer = 40
Normal = 40 n = 274 |
Protein | Upregulated in cancer
High histological grade High Ki-67 proliferation index P53 overexpression ER- subtype TNBC subtype |
9 | ||
| Blood
Tissue |
n = 36
n = 45 |
Protein | Upregulated in HER2- cancer
TNBC subtype |
10 | ||
| Blood
Tissue |
n = 101
n = 101 |
mRNA
Protein |
Upregulated on CTCs***
Upregulated in tumor with EMT CTCs ER- subtype Bcl-2- |
11 | ||
| Gynecologic tumors | ||||||
| Ovarian cancer | Tissue
Public database |
n = 109
n = 1604 |
Protein
mRNA |
Upregulated in cancer
Poor RFS and OS |
14 | |
| Tissue | n = 119 | Protein | Upregulated in cancer
Advanced FIGO stage High histologic grade Ascites Malignant tumor cells in peritoneal fluid Larger residual tumor size High Ki-67 expression Poor OS |
15 | ||
| Endometrial cancer | Tissue | Cancer = 84
Atypical hyperplasia = 30 Normal = 18 |
Protein | Upregulated in cancer
Advanced FIGO stage Poor differentiation Myometrial invasion Lymph node invasion Poor OS |
16 | |
| Tissue | n = 53 | Protein | Upregulated in lymph node metastasis cases | 17 | ||
| Tissue | n = 293 | Protein | Upregulated in recurrence cases
Upregulated in NEEC than EEC**** Poor RFS |
18 | ||
| Cervical cancer | Tissue | Cancer = 33
Normal = 17 |
Protein | Upregulated in cancer | 19 | |
| Tissue | Cancer = 336
Normal = 46 |
Protein | Upregulated in squamous cell carcinoma
Advanced stage Larger tumor size Stromal invasion depth Lymph node invasion Parametrial involvement Poor DFS and OS |
20 | ||
| Tissue | n = 68 | Protein | Upregulated in post-chemotherapy tissue
Overexpression in tumor cells in chemotherapy non-response group Stroma Overexpression correlates with poor PFS |
21 | ||
| Digestive system tumors | ||||||
| Pancreatic cancer | Tissue | Cancer = 21
Pancreatitis= 9 Normal = 10 Cancer = 51 Pancreatitis= 32 Normal = 30 |
mRNA
Protein |
Upregulated mRNA level in cancer than in normal tissues (non-significant elevation than in pancreatitis)
Cell surface redistribution of Anxa2 protein from PanIN-1 lesions to cancer |
22 | |
| Tissue | n = 78 | Protein | Predominant cancer cell surface location
High expression with TNC overexpression in advanced stage, hematogenous and peritoneal recurrence cases Poor DFS and OS |
23 | ||
| Tissue | n = 56 | Protein | High stromal expression correlates with poor DFS and OS | 24 | ||
| Tissue | n = 62 | Protein | Upregulated in cancer
Gemcitabine resistance Poor DFS and OS |
25 | ||
| Gastric cancer | Tissue | n = 126 | mRNA, protein | Upregulated in cancer
Advanced stage Lymph node metastasis Distant metastasis |
26 | |
| Tissue | Cancer = 51
Normal = 24 |
Protein | Upregulated in cancer
Advanced stage Lager tumor size Lymph node metastasis Poor differentiation |
27 | ||
| Hepatocellular cancer | Tissue | Cancer = 34
Hepatitis = 39 Normal = 5 |
mRNA, protein | Upregulated in cancer
(negative in normal, low expression in hepatitis, high expression in cancer) Poor differentiation of tumor |
28 | |
| Tissue
Blood |
Cancer = 224
Adjacent normal tissues = 224 Cancer = 175 Hepatitis = 23 Cirrhosis = 51 Benign tumors = 19 Normal = 49 |
Protein | Upregulated in cancer
Upregulated in serum of HCC patients than in hepatitis, cirrhosis, benign tumors and healthy groups Upregulated in serum in AFP-negative HCC patients |
30 | ||
| Blood | Cancer = 50
Chronic liver disease = 25 Normal = 15 |
Protein | Upregulated in cancer than in chronic disease and normal groups | 29 | ||
| Tissue | n = 105 | Protein | Poor RFS and OS | 31 | ||
| Colorectal cancer | Tissue | n = 105 | Protein | Histologic type
Lager tumor size Invasion depth Advanced stage Poor OS |
32 | |
| Tissue | Primary cancer = 59
Liver metastasis = 8 |
Protein | Upregulated in cancer
Marked increase in advanced tumor and metastatic lesions |
33 | ||
| Tissue | LNM***** = 45
Non-LNM = 45 |
Protein | Upregulated in lymph node metastasis cancer | 34 | ||
| Lung Cancer | Tissue | Cancer = 71
Normal = 20 |
Protein | Upregulated in cancer
Advanced stage Lymph node metastasis Poor OS |
35 | |
| Tissue
Public database |
Cancer = 152
Normal = 36 n = 2437 |
Protein
mRNA |
Upregulated in cancer
Advanced stage DDP resistance Poor OS |
36 | ||
| Tissue | n = 36 | Protein | Upregulated in squamous carcinomas | 37 | ||
| Head and neck tumors | ||||||
| Glioma | Tissue
Public database |
n = 89
n = 710 |
Protein
mRNA |
Upregulated in cancer
Overexpression in aggressive tumors Poor PFS and OS |
38 | |
| Tissue | Cancer = 130
Normal = 15 |
mRNA Protein | Upregulated in cancer
High histological grade Poor OS |
39 | ||
| Tissue
Public database |
Cancer = 99
Normal = 12 n = 923 |
mRNA | Anxa2 and its pseudogene upregulated in cancer
Poor OS |
41 | ||
| Pediatric neuroblastoma | Tissue | n = 42 | Protein | Advanced stage
More chemotherapy cycles Metastasis Poor OS |
42 | |
| Oral squamous cell carcinoma | Saliva | Cancer = 131
OPMD******= 233 Normal = 96 |
Protein | Upregulated in cancer | 44 | |
| Serum | Cancer = 126
Benign disease = 115 Normal = 158 |
Protein | Upregulated in cancer
Poor OS |
45 | ||
| Tissue | n = 30 | Protein | Upregulated in cancer
Well differentiation |
46 | ||
| Tissue | n = 106 | Protein | Larger tumor size
Less recurrence Well differentiation |
47 | ||
| Laryngeal cancer | Tissue | Cancer = 209
Normal = 88 |
Protein | Upregulated in cancer
Larger tumor size Advanced stage Lymph node metastasis Distant metastasis Poor OS |
48 | |
| Nasophary-ngeal cancer | Tissue | Primary cancer = 48
Metastatic Cancer = 13 |
Protein | Metastasis
Poor OS |
50 | |
| Genitourinary system tumors | ||||||
| Renal cancer | Tissue |
n = 47
n = 13 |
mRNA
Protein |
Upregulated in cancer | 51 | |
| Tissue | Primary cancer = 18
Primary cancer = 154 Metastatic tumor = 24 |
mRNA
Protein |
Upregulated in cancer
Highly expressed in metastatic tumor Advanced stage Higher nuclear grade Poor MFS |
52 | ||
| Bladder cancer | Tissue | n = 60 | Protein | Upregulated in cancer
Advanced stage Higher expression in short term recurrence group |
53 | |
| Prostate cancer | Tissue | Cancer = 85
Benign hyperplasia = 40 |
Protein | Downregulated in cancer
Less metastasis Early stage Better OS |
54 | |
| Tissue | Cancer = 31
Benign hyperplasia = 12 Normal = 16 |
Protein | Downregulated in cancer | 55 | ||
| Tissue | n = 24 | Protein | High expression in relapse group | 56 | ||
| Tissue | n = 87 | Protein | Re-expression in poorly differentiated adenocarcinoma | 57 | ||
| Hematologic tumors | ||||||
| Acute promyelocytic leukemia | Blood
Bone marrow |
n = 14 | Protein | Upregulated in APL | 60 | |
| Acute myelogenous leukemia | Blood | n = 103 | Protein | Highest expression in M3 cells and downregulated after As2O3 treatment with hyperfibrinolysis reduction | 61 | |
| Acute lymphoblastic leukemia | Blood
Bone marrow |
Prednisolone-resistant = 24
Prednisolone-sensitive = 19 |
mRNA Protein | Higher expression and phosphorylation in prednisolone-resistant group | 62 | |
| * IDC = invasive ductal cancer; ** DCIS = ductal carcinoma in situ; *** CTC = circulating tumor cells; **** EEC = endometrioid endometrial carcinomas; ***** LNM = lymph node metastasis; ****** OPMD = oral potentially malignant disorders. | ||||||
Breast cancer
We previously detected the higher elevated expression of Anxa2 mRNA in primary breast carcinoma than in normal breast tissues and the positive correlation of Anxa2 mRNA expression with lymph node metastasis6. At the protein level, immunohistochemistry (IHC) results show the consistently increased Anxa2 expression in cancer tissues7 and the advanced pathological stage and high histological grade in Anxa2 high-expression group8. Survival analysis reveals substantially poor OS and DFS in patients with high Anxa2 expression8. Anxa2 could also be a secretory biomarker in the plasma samples of patients with breast cancer and thus is valuable for early diagnosis9,10. The triple negative (ER, PR, and HER2 negative) breast cancer (TNBC) is the most aggressive type usually detected with upregulated EGFR expression, and Anxa2 is reversely correlated with hormone receptors11 and HER2 expression12 but positively related with EGFR expression in breast cancer tissues8. In conformance with the clinical data, collective studies verified higher Anxa2 upregulation in highly aggressive breast cancer cell types, especially in TNBC cell lines, than in less aggressive types8,12,13.
Gynecologic tumors
Analysis based on the public ovarian cancer database reveals that the high Anxa2 mRNA level in stage III serous ovarian cancers is associated with reduced progression-free survival (PFS) and OS, and the strong stromal Anxa2 immunostaining in tissue samples is consistently and substantially associated with poor outcomes. Multivariate Cox regression identified stromal Anxa2 expression as an independent risk factor of OS14. In epithelial ovarian cancer (EOC) tissues, Western blotting and IHC show that Anxa2 overexpression increases with the intensifying pathological features. By contrast, Anxa2 has a reverse effect on EOC outcome and is an independent risk factor for prognosis according to multivariate analysis15.
IHC results revealed remarkably high Anxa2 expression in cancer tissues of endometrial carcinoma, and the expression level is positively correlated with FIGO stage, differentiation degree, and myometrial and lymph node invasion. Anxa2 is also an independent prognostic indicator for endometrial cancer16. Bioinformatics and proteomic approaches have been performed to determine an accurate model for lymph node invasion prediction and avoid the radical surgery in lymph nodes of patients with negative primary endometrial cancer; these methods revealed Anxa2 upregulation as a predictive factor in lymph metastasis endometrial cancer tissues17. Approximately 20% of patients with endometrial cancer die of recurrence, and a retrospective study in a cohort of 93 patients reported that Anxa2 effectively predicts disease recurrence, highlighting its clinical utility as a biomarker in endometrial cancer18.
Proteomic analysis identified Anxa2 as an up-regulated protein in squamous cervical cancer19. A study involving 336 cervical cancer cases showed a prominent Anxa2 expression in squamous cell carcinoma tissues that is positively correlated with advanced cancer phenotypes with high stage and large-sized tumors, lymphovascular space invasion, stromal invasion depth, lymph node metastasis, and parametrial involvement; the patients in high Anxa2 expression group have poor DFS and OS20. Moreover, the patients with cervical cancer and high Anxa2 expression have resistance to cisplatin-based neoadjuvant chemotherapy, confirming Anxa2 expression as an independent prognostic factor for decreased PFS21.
Digestive system tumors
The adverse effects of Anxa2 were mainly discovered in pancreatic ductal adenocarcinoma (PDA). The expression rate of Anxa2 is high in PDA tissues and increases in the highly aggressive tumor types22. Its overexpression in PDA cells is substantially correlated with distant metastasis, hematogenous/peritoneal recurrence, and worse outcome for patients23. High stromal Anxa2 expression rate (more than 80%) is remarkably related to reduced DFS and OS of patients with PDA, and is the only independent prognostic marker established by multivariate analysis24. Furthermore, high Anxa2 expression level predicts the rapid recurrence of PDA in patients who receive gemcitabine adjuvant chemotherapy25.
Anxa2 is overexpressed at mRNA and protein levels in gastric adenocarcinoma, and substantially correlated with lymph node and distant metastasis26. This consistent result is confirmed by another IHC exploration, which stated that Anxa2 expression rate is increased in gastric cancer tissues compared with that in adjacent tissues and is related to large tumor size, poor histological differentiation, and lymph node invasion27.
In hepatocellular carcinoma (HCC), Anxa2 is overexpressed at transcriptional and translational levels in tumor tissues and is barely detected in normal human liver or chronic hepatitis liver tissues. Moreover, Anxa2 is tyrosine-phosphorylated in HCC and is related to high histological grade, suggesting that phosphorylated Anxa2 is the effective form28,29. Anxa2 expression is remarkably increased in the serum of patients with HCC and has high titer in early stage and AFP-negative patients, indicating its value in early diagnosis, especially in AFP normal HCC30. This protein also has adverse effects on the prognosis of patients with HCC and is an independent prognostic factor for OS and RFS31.
Similar results were found in colorectal cancer (CRC). IHC analysis revealed that Anxa2 has elevated expression in CRC tissues, is correlated with tumor size, depth of invasion, pTNM stage, and thus is identified an independent factor of poor outcome of patients with CRC32,33. Proteomic approach showed that Anxa2 increases CRC lymph node invasion34.
Lung cancer
Non-small cell lung cancers (NSCLCs) constitute 80% of lung cancers. A cohort study for 72 cases showed Anxa2 overexpression in NSCLC tissues, especially in lymph node invasion cases, and the patients with high Anxa2 expression level have poor OS35. Prognostic analysis using a large online database from 1926 lung adenocarcinoma cases confirmed the negative prognostic value of Anxa2 mRNA expression36. Proteomic study identified Anxa2 as a substantially increased protein in squamous carcinomas37. Platinum, particularly cisplatin (DDP)-based chemotherapy, is an important treatment for patients with NSCLC. Among the patients with NSCLC who receive DDP-based chemotherapy, those with elevated Anxa2 expression have shorter OS compared with those with low Anxa2 expression, suggesting that Anxa2 plays a role in DDP resistance36.
Head and neck tumors
IHC staining on a series of 89 glioma cases disclosed that Anxa2 expression is higher in the most aggressive type, glioblastoma multiforme (GBM), than in the less aggressive tumors. Patients with high Anxa2 score have decreased PFS and OS, particularly in GBM, and the Anxa2 IHC score is an independent prognostic factor for PFS. The analysis based on 710 cases public database validated the consistent prognostic potential of Anxa2 at the mRNA level38. Another 130-case IHC study revealed higher Anxa2 protein levels in glioma tissues than in the normal brain samples and the same adverse prognostic function of Anxa2 on the survival of glioma patients39. Pseudogenes influence human cancer progression40. Anxa2 and its pseudogenes, Anxa2P1, Anxa2P2, and Anxa2P3, are remarkably upregulated in glioblastoma based on the analysis of GSE4290 and TCGA database and then validated in tissues by qRT-PCR. Anxa2, Anxa2P1, and Anxa2P2 are the independent risk factors of glioblastoma prognosis41. In pediatric neuroblastoma, strong Anxa2 expression is closely related to increased chemo cycles, tumor metastasis and poor survival, indicating the effect of Anxa2 in drug resistance42.
Oral squamous cell carcinoma (OSCC) accounts for more than 90% of oral cancer with more than 60% of patients at an advanced stage disease43. An oral cancer screening program in Taiwan collected 460 saliva samples from healthy, potential oral malignant disorder and OSCC subjects, and identified a four-protein panel with high sensitivity and specificity for early diagnosis of OSCCs; Anxa2 is included with high expression level44. The Anxa2 level in the serum of patients with OSCC is also higher than that in benign and healthy controls, and the patients in high Anxa2 level OSCC group have poor OS45. IHC detection revealed elevated Anxa2 expression in OSCC tissues, but its expression level is negatively correlated with the differentiation grades46,47.
Anxa2 was studied using differential proteomics and verified by IHC staining as a substantially upregulated protein in laryngeal carcinoma tissues compared with that in adjacent tissues, and the strong Anxa2 expression is highly correlated with aggressive pathological features and poor diagnosis48.
Nasopharyngeal carcinoma (NPC) is a high-risk head and neck cancer in Southeast China and Asia49. The NPC patients with high Anxa2 staining score have high metastasis rate and poor OS, and Anxa2 enhances the chemoradiotherapy resistance50.
Genitourinary system tumors
Renal cell carcinoma (RCC) is the most common renal tumor. Anxa2 is significantly elevated in RCC tissues compared with that in normal renal tissues51. Its expression rate is higher in metastasis clear-cell RCC (ccRCC) than in primary ccRCC, and the ccRCC patients with positive Anxa2 expression have a low 5-year metastasis-free rate52, suggesting the role of Anxa2 in RCC progression.
A study exploring the bladder cancer drug resistance mechanism detected high Anxa2 expression in short-term recurrence group and its association with the invasion depth of cancer53.
The expression level of Anxa2 in prostate cancer (PC) seems contrary to that in other cancers. Anxa2 downregulated in PC tissues and indicates good OS of patients with PC54,55. However, a study reported 100% relapse in Anxa2-positive patients with PC, but only 50% relapse in Anxa2-negative patients, and Anxa2 is mostly located in the cytoplasm of PC cells and in the plasma membrane of benign prostatic tissues56. Another study showed that Anxa2 is lost in moderately differentiated adenocarcinoma but retained in poorly differentiated adenocarcinomas, suggesting a complex regulation of AnxA2 in the prostate microenvironment57. Anxa2 also plays a key role in the bone metastasis of PC in animal models58,59. Therefore, the function of Anxa2 in PC needs further exploration.
Hematologic tumors
Bleeding could be the fatal complication in acute promyelocytic leukemia (APL), and Anxa2 is expressed at an abnormally high level in APL cells leading to hemorrhagic disorders60. In a research including 103 patients with acute myelogenous leukemia (AML) receiving As2O3 treatment, the Anxa2 protein expression level in leukemic cells was monitored by flow cytometry. The results indicate that As2O3 may reduce hyperfibrinolysis by downregulating Anxa2, which therefore could be a biomarker61.
MLL-rearranged infant acute lymphoblastic leukemia (ALL) (< 1 year of age) is frequently resistant to glucocorticoids. Gene-expression signature analysis through comparison of gene-expression profiles from prednisolone-resistant and -sensitive patients showed highly different Anxa2 gene expression. In addition, Anxa2 is overexpressed at the mRNA, protein, and phosphorylation levels in prednisolone-resistant MLL-rearranged ALL cells62.
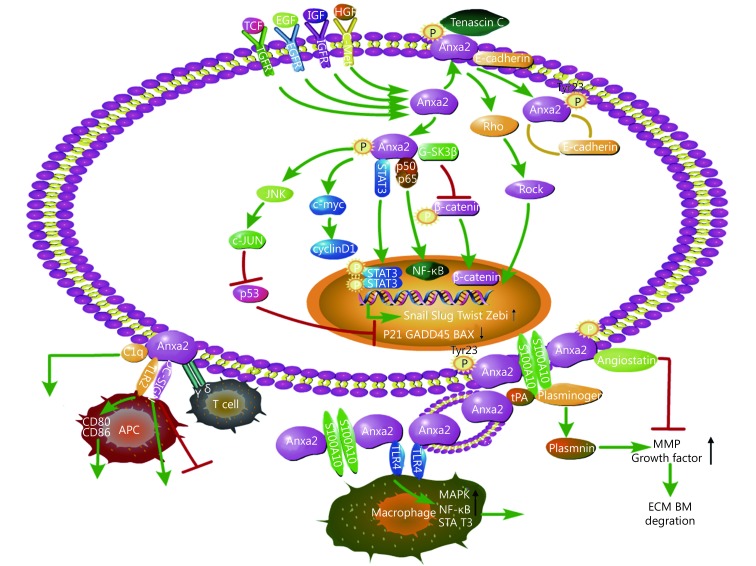
Novel insights on the mechanism of Anxa2 in promoting cancer progression
Anxa2 plays a critical role in EMT in various cancers
In the past two decades, epithelial-mesenchymal transition (EMT) has been illustrated as a key mechanism of metastasis in multiple cancer models. During EMT, stationary epithelial cells lose polarity with cell–cell junctions and acquire a mesenchymal morphology and the ability to migrate and invade63,64. We previously reported the negative correlation of Anxa2 and E-cadherin expression in breast cancer tissues, and the loss of E-cadherin is a fundamental event in EMT. We revealed that Anxa2 enhances STAT3 transcriptional activity by directly binding to it, increases the EMT driver Slug expression, and promotes EGF–induced EMT in breast cancer cells. The 23-tyrosine phosphorylation of Anxa2 is required for the EGF induced EMT model8. Circulating tumor cells (CTCs) are responsible for tumor dissemination, and Anxa2 stromal expression in tissues is related to CTCs with EMT phenotype in primary breast cancer patients, indicating that Anxa2 may play a role in EMT CTCs release11. Another evidence that Anxa2 regulates EGF–induced EMT is reported in CaSki cervical cancer cells, in which the viability and migration of the cells are elevated65.
The 23-tyrosine phosphorylation is essential for the cell–surface location of Anxa2, and mediates transforming growth factor β (TGFβ)–induced EMT in PDA cells through a Rho–mediated way66. Tenascin C (TNC) is one of the major extracellular matrix (ECM) components that can promote cancer cell invasion and metastasis. This protein directly binds to Anxa2 in PDA cells, and their interaction drives EMT in the pre-invasive pancreatic intraepithelial neoplasia (PanIN) cells and I, thereby inducing metastasis23.
IHC analysis revealed the negative correlation between Anxa2 and E-cadherin expression in CRC samples, especially in metastatic tumors. Silencing of Anxa2 expression inhibits TGFβ–induced EMT of CRC cells, and Anxa2 plays its mediating role through the Src/Anxa2/STAT3 signal pathway33. Anxa2 knock-down blocks TGFβ–induced Snail/Twist elevation and inhibits EMT in NPC cells, thus, reducing cell invasion, migration, and radio-chemo-resistance. These data confirm Anxa2 as a possible target of therapeutic intervention for NPC50.
In ovarian cancer cells, Anxa2 downregulation suppresses β-catenin expression and consequently inhibits EMT and, attenuates the proliferation and invasion of cancer cells67. Anxa2 also activates Wnt/β-catenin pathway in HCC cells. The long noncoding RNA IncRNA-MUF binds to Anxa2, then activates Wnt/β-catenin signaling and finally mediates EMT development in HCC cells68.
Proteomic analysis identified Anxa2 elevation in cancer-associated fibroblasts (CAFs) inducing tyrosine kinase inhibitors resistance in NSCLCs. CAFs secret growth factor HGF and IGF-1, increase the expression and tyrosine phosphorylation of Anxa2, and induce EMT and resistance to gefitinib of NSCLC cells69. Another research reported that Anxa2 increases the expression of an EMT-driver transcription factor, Snail, and then enhances NSCLC cell EMT and invasion by inhibiting p53 expression and its signaling35.
Anxa2 is a pivotal link in plasmin system and angiogenesis in tumors
ECM degradation is a key event of adjacent invasion of tumor cells into normal host tissues that promotes cellular migration, and is regulated by different proteolytic enzymes70. Among the proteases in this process, plasminogen/plasmin system plays an active role. Plasminogen is activated by plasminogen activators (PA) into plasmin, which facilitates the proteolytic cascade reaction, including the activation of matrix metalloproteases (MMPs), and leads to ECM and basement membrane (BM) degradation71. The degradation of ECM and BM is also a critical step in the initiation of angiogenesis, and angiogenic regulators sequestered in the matrix, such as vascular endothelial growth factor (VEGF), are released after ECM degradation by MMPs72,73. Published reports strongly support the effect of plasmin system in cancer neoangiogenesis, invasion and metastasis71.
Annexin A2 can exist as a heterotetrameric complex with S100A10 (p11) which is referred as Anxa2t and is an essential mediator in plasmin activation. On the cell membrane especially on the vascular endothelial cells (ECs), Anxa2 is associated with S100A10 through its N-terminal tail binding to the C-terminal region of the S100A1074. Anxa2 transports and anchors S100A10 to the extracellular surface of the plasma membrane, forms the Anxa2t as a receptor to plasminogen, PA, and tissue PA (tPA), and consequently stimulates the tPA-dependent conversion of plasminogen to plasmin2. Anxa2 exists as a monomeric form in the cytoplasm, and its binding to S100A10 leads to the translocation of the complex to the inner leaflet of the cells75. However, the exact mechanism of the Anxa2t translocation to the surface of the cytomembrane remains unclear. Several studies showed that the 23-tyrosine phosphorylation of Anxa2 is required for Anxa2 translocation76,77. Anxa2t has been illustrated to play a role in inflammation by recruiting macrophages and releasing inflammatory cytokines such as IL-1, IL-6 and tumor necrosis factor α (TNFα)78.
The plasminogen/plasmin system regulates angiogenesis and promotes breast cancer progression79. IHC analysis of breast cancer samples revealed heightened Anxa2 staining in ECs, tumor cells, and perivascular stromal cells7, and the expression of Anxa2 on the surface of the cancer cells is positively correlated with the secretion of tPA and the expression and location of neovascular biomarker CD10513. An in vitro study showed much higher expression level of Anxa2 in aggressive breast cancer MDA-MB-231 cell lines compared with that in noninvasive MCF-7 cell lines. Anxa2 is required in the conversion of plasminogen to plasmin in MDA-MB-231 cells and promotes cellular invasion and migration, whereas MCF-7 cells fail to convert plasminogen to plasmin7. Inhibition of Anxa2 using anti-Anxa2 antibody blocks the angiogenesis on 3D matrigel cultures by eliciting EC death due to apoptosis. In a xenograft model, the anti-Anxa2 antibody inhibits the growth of human breast tumor at least partly by attenuating the neoangiogenic activity of the tumor80. Tumor-derived exosomes can promote tumor growth, metastasis and drug resistance. Proteomic research indicated that Anxa2 is abundant in the exosomes81. In vitro endothelial tube formation assay, endothelial cell migration and invasion study, and in vivo matrigel plug assay using mouse model, produced consistent results that exosomal-Anxa2 promotes angiogenesis. Further investigation revealed that exosomal-Anxa2 acts as the cell surface Anxa2 through the activation of plasminogen in a tPA dependent manner82.
Anxa2 is overexpressed in high-grade human gliomas and tumor cells surrounding dilated vessels in glioblastoma tissues83. This protein also promotes glioma progression by enhancing angiogenesis in the animal model. In the glioma mouse model, the knockdown of Anxa2 by siRNA decreases blood vessel density within the tumor, alters the tumor vascular pattern and blocks tumor growth84. Another rat xenograft model using two glioma cell lines with different expression levels of Anxa2 exhibited greater tumor angiogenesis in the high-Anxa2 expression group than in the low-Anxa2 expression group. Anxa2 silencing in high-Anxa2 expression cells decreases the angiogenic activity, whereas Anxa2 overexpression in low-Anxa2 expression cells increases the angiogenesis83.
In APL, Anxa2 is explored at an abnormally high expression level and increases the production of plasmin leading to hyperfibrinolysis and bleeding. Anxa2 knockdown reduces tPA-dependent plasmin production, whereas its upregulation stimulates plasmin generation85. Another report showed that promyelocytic leukemia/retinoic acid receptor α fusion protein increases Anxa2 transcription and accumulation of S100A10, thus consequently promoting plasmin generation and invasive potential86.
In addition to binding to S100A10, Anxa2 also forms a complex with S100A4, another member of S100 family, and plays a similar role to Anxa2t in facilitating the tPA-dependent plasmin activation on the EC surface87. S100A4 is upregulated in cancer cells and affects pro-angiogenesis during tumor progression and, therefore, may be a substitutional protein for S100A10 as the binding partner of Anxa2 under certain circumstances88,89.
Cell surface Anxa2 is also a receptor for angiostatin90, which inhibits neovascularization and angiogenesis-dependent tumor growth and metastasis91,92 and regulates plasminogen activation by competitively binding to Anxa2. These data suggest Anxa2 as a crucial link in the regulation of plasmin system, angiogenesis, and tumor progression.
Crucial functions of Anxa2 on the dynamics of cytoskeleton and tumor cell motility
Dynamic remodeling of the actin cytoskeleton is essential for tumor cell spreading, migration, adhesion, and membrane traffic system, such as endocytosis and phagocytosis1. Monomeric Anxa2 may regulate the growth of newly formed actin filament by capping the growing free-barbed end of the filament, and can interact with G-actin93,94. Anxa2t binds to bundle filamentous actin (F-actin) through the C-terminal of Anxa295, and this binding capability is decreased by the Tyr23 phosphorylation of Anxa293. The binding of Anxa2 to actin requires calcium mediation94,96. Anxa2 plays a critical role in maintaining the plasticity of the dynamic membrane actin cytoskeleton.
In endocytosis, Anxa2 is present in clathrin-coasted vesicles (CCV), the basic unit in the first step of endocytosis97. This protein is associated with early endosomes and affects their location by binding to cholesterol-rich domains without S100A10 interaction98. Moreover, Anxa2 helps late endosomes to mature99. Invagination of CCV needs a burst of actin polymerization, and actin remodeling is required in early and late endosomes. Anxa2 mediates these structures by regulating actin rearrangement100,101. Tyr23 phosphorylation is necessary for endosome transportation and early-to-late endosome maturation99. In phagocytosis, Anxa2 accumulates on the actin-rich phagosomes during outer segment internalization, and its regulatory form is Tyr23– phosphorylated Anxa2102. A study that screened 172 endocytosis and actin regulators identified Anxa2 as the mediator of EGFR endocytosis in TNBC cells, that facilitates EFGR endocytosis by inactivating actin severing protein cofilin103. Although this article explored the negative function of Anxa2 in EGFR signaling and cell metastasis as opposed to other articles, it suggests the novel function of Anxa2 in cancer progression by regulating membrane traffic. High-risk human papillomaviruses (HPVs) are closely associated with several cancers, and Anxa2t is crucial for oncogenic HPV endocytosis, capsid disassembly, and virion degradation prevention. Anxa2 forms a complex with the HPV trafficking mediator CD63104, and the small molecular inhibitors of Anxa2t block 100% of the high oncogenic HPV16 infection mainly by blocking the entry of the virus into epithelial cells105.
Anxa2 also recruits and regulates Rho GTPases and subsequently controls actin remodeling and cell to cell adhesion106. Tyr23 Phosphorylation results in Anxa2 accumulation in actin remodeling sites and triggers the Rho/ROCK signaling, leading to cell detachment and migration107. Another study found that Tyr23- phosphorylated Anxa2 controls the activation of cofilin and enhances cell scattering and branching morphogenesis108. Anxa2 is overexpressed in HCC cells. Its depletion blocks the expression of F-actin, β-tubulin, and Lamin B, inhibits cell pseudopodia/filopodia formation, and suppresses HCC cell motility109. A similar phenomenon was found in gastric cancer cells, in which F-actin and β-tubulin expression, pseudopodia/filopodia and cells motility are impaired after Anxa2 depletion110. Another study on HCC cell motility showed that Tyr23-phosphorylated Anxa2 mediates Rho/ROCK signaling and improves the amoeboid movement of HCC cells111. In addition, cell migration is partly a result of their link to ECM through focal adhesions, and the GBM cells with high Anxa2 expression exhibit focal adhesion-like structures in cytoskeletal fibers. The inhibition of Anxa2 leads to the redistribution of F-actin, complete loss of focal adhesions, and attenuation of GBM cell dissemination38.
Anxa2 also has an instrumental function in tumor cell cytomembrane repair. Tumor cells have unstable cytomembrane with reduced stiffness, which enhances their migrative and invasive abilities112. However, the elevated motility of metastatic cells increases the danger of cytomembrane damage; therefore, an enhanced cytomembrane repair is essential for cancer cell survival. The regulation of the cortical actin cytoskeleton is one of the fundamental mechanisms of membrane repair. Cortical actin is depolymerized around the wounded edges at the injury site to increase the membrane relaxation and prevent further damage on the cytomembrane113. Anxa2 and S100A11 accumulate at the repair site, thus allowing the buildup of new F-actin around the injury site; metastatic cancer cells enhance their cytomembrane repair by upregulating Anxa2 and S100A11114,115.
Anxa2 regulates cell cycle and apoptosis and then enhances cancer cell proliferation
Anxa2 improves cancer cell proliferation in multiple types of cancer models35,38,116,117. An early study found that its expression at mRNA or protein level changes during the cell cycle, and peaks during the S phase118. The periodic correlation of Anxa2 expression and cell cycle suggests its regulatory role in cell cycle. As a DNA binding and primer recognition protein, Anxa2 is involved in DNA replication and cell cycle progression119-121. Genotoxic agents can induce its nuclear accumulation whereas its depletion causes DNA repair-related genes to increase, indicating its mitigating role for DNA damage122. In tumors, Anxa2 silencing induces the gastric cancer SGC-7901 cell cycle arrest at G1 phase and reduces cell proliferation110. Similarly, in GBM cells, Anxa2 knockdown arrests the cell cycle at the S-G2/M phase and apparently impairs the GBM cell proliferation. Further gene ontology analysis showed that Anxa2 regulates the gene clustering in cell cycle, DNA replication, and chromosome segregation, indicating its modulatory function in GBM proliferation38.
Anxa2 is also an RNA binding protein that binds to the 3’UTR of the c-myc mRNA, leading to its perinuclear location and cytoskeleton association, and facilitates c-myc protein synthesis123. C-myc is a well-known proto-oncogene crucial to cell proliferation and apoptosis. We previously reported that Anxa2 upregulation in breast cancer MCF-7 cells increases S+G2/M phase cells and cell proliferation. The cell cycle related proteins, c-myc and cyclin D1, are markedly regulated by Anxa2117. Cyclin D1 is the downstream factor of c-myc and the critical regulator for G1 to S phase transition. In other two breast cancer cell lines, T47D and MDA-MB-231, Anxa2 downregulation attenuates cell proliferation and retards G1 to S phase transition. This finding is attributed to the decreased levels of cyclin D1. We also gave evidence that Anxa2 mediates cyclin D1 transcription by regulating the phosphorylation of the transcription factor STAT3124.
The tumor suppressor p53 is the main driver of stress response in human cells, that can elicit DNA repair, apoptosis, cell cycle arrest, and metabolic homeostasis maintenance125,126. Anxa2 plays a role in p53 signaling pathway according to NSCLC studies. In NSCLC cell lines, Anxa2 silencing increases p53 expression, leads to cell cycle arrest at G2 phase, and inhibits cell proliferation35. In a further research in NSCLC cell models, the mechanism of Anxa2 regulating the p53 expression revealed that Anxa2 mediates c-Jun stabilization through the phosphorylated activation of JNK and the consequent suppression of p53 expression at a transcriptional level127. Knockdown of Anxa2 increases the lung cancer cell apoptosis, and apoptotic stimulation causes its cleavage128. A negative correlation exists between Anxa2 and p53 expression in human lung cancer cells, and alteration of Anxa2 expression levels affects the apoptosis induced by enhancing p53 expression129. In addition, Anxa2 is upregulated in cisplatin-resistant NSCLC cells and enhances the cisplatin induced cell apoptosis. The mechanism revealed that the expression of p53 and its downstream apoptotic genes, p21, GADD45 and BAX, are suppressed by Anxa2 through its activated role on JNK/c-Jun signaling36.
Anxa2 is involved in tumor immunology
Anxa2 is involved in both innate and adapt immunities. The complement system serves as an integral part of innate immunity and contributes to clearance of immune complexes and dying cells. Anxa2 has been demonstrated as the new ligand on apoptotic cells for C1q, the initiator of the classical complement pathway130. It is phosphorylated on serine residues by PKCs after T cell antigen receptor (TCR) stimulation, suggesting its potential role in signal transduction in lymphocytes131.
As to tumors, hypoxia could enhance the immune activity of lysates from murine glioma cells in glioblastoma vaccination model through danger-associated molecular pattern (DAMP), and Anxa2 monomer, which is overexpressed by murine glioma cells under low oxygen situation, has been identified as one DAMP. Anxa2 binds to toll-like receptor 2 (TLR2) on antigen presenting cell (APC) inducing murine and human dendritic cells (DC) maturation and increasing antigen cross-presentation by MHC class I molecules, then enhances the activation of CD8+ T cells. These data bring a novel approach to increase the efficiency of tumor vaccine in glioma using Anxa2 as an adjuvant132. Similar hypoxia-induced Anxa2 membrane expression in glioblastoma cell line is proved to activate γδ T cells. Anxa2 is the direct ligand of γδ TCR and mandatory for recognition of glioblastoma cells by γδ T cells and enhances the proliferation of γδ T cell subset in peripheral blood mononuclear cells. It could act as an oxidative stress signal for adaptive stress surveillance133. Besides, soluble Anxa2t is also a mediator of macrophage activation through TLR4, enhancing the secretion of inflammatory cytokines and phagocytosis78,134. These data suggest the immunology mediation role of Anxa2 in tumor under some stress situation.
Anxa2 has also been reported to mediate immunology suppression in cancers. It is abundantly expressed in nasopharyngeal carcinoma (NPC), and is the ligand on NPC cells for DC-SIGN, a kind of C-type lectin receptor on DC. It activates DC-SIGN then inhibits DC maturation and IL-12 production, and promotes the immunosuppressive IL-10 production instead135. Besides the significant role of Anxa2 in oncogenic HPV infection through mediating the virus endocytosis and trafficking in epithelial cells104, Anxa2t also interacts with HPV16 then induces the suppression of the maturation of Langerhans cells (LC), the APC of the epithelium, which is manifested as attenuated secretion of Th1-associated cyokines and surface expression of MHC class II molecules136.
In a phase II study of a GM-CSF secreting whole cell PDA vaccine trial, Anxa2 was identified as the antigen by proteomics in patients who had post-vaccination cellular immune responses and prolonged DFS and OS. Meanwhile, IHC confirmed the elevated cell-surface Anxa2 expression in these patients. These data suggest that Anxa2 may serve as a new candidate for PDA tumor-associated antigen and biomarker for an immunologic target66.
Effect of Anxa2 in cancer treatment resistance and prospect of targeting Anxa2 in cancer
Some researchers have also confirmed the role of Anxa2 in the therapeutic resistance of cancers. Anxa2 is involved in the cisplatin and vincristine resistance in gastric cancer cells137. Mycoplasma membrane protein P37 can interact with Anxa2 of the HCC cells and induce mycoplasma-associated multidrug resistance of HCC138, and as mentioned above, Anxa2 mediates the cisplatin resistance in NSCLC via regulating p53 pathway36. Anxa2 also regulates NF-κB signaling as a study showed two ginsenosides inhibit the interaction between Anxa2 and NF-κB then increase the apoptosis in cancer cells139, and it is remarkably upregulated in chemo-resistant neuroblastoma cell lines and induces drug resistance through NF-κB activation and nuclear translocation42. EGFR expression is elevated in TNBC, but TNBC patients cannot benefit from EGFR-targeted therapy. Anxa2 mediates the tyrosine kinase inhibitor gefitinib resistance in breast cancer cells140. These data suggest that the treatment targeting Anxa2 could be a synergist in cancer therapy. Intervention targeting Anxa2 in tumors is anticipated. Some attempts using anti-Anxa2 antibody have shown the inhibitory effects in cancer models. Anti-Anxa2 antibody blocks the TNBC and Herceptin-resistance breast cancer cell proliferation and migration by impairing the EGFR signaling141. In the breast cancer xenograft mouse model, anti-Anxa2 antibody attenuates neoangiogenesis and inhibits the growth of tumor80. After long- term observation, treatments with anti-Anxa2 antibody show good control rates of the breast cancer growth and no detectable adverse effects in the pre-clinical mouse model142.
Conclusions
Based on the collective studies shown above, Anxa2 could be a good biomarker for early diagnosis of some cancers, because it can be easily detected as elevated in blood or saliva samples. It is very valuable in the prediction of cancer prognosis due to the high positive correlation of Anxa2 overexpression and the poor outcome of various cancers. In this review, we systematically summarized the mechanism of Anxa2 in regulating cancer cell proliferation, motility, migration, invasion and cancer angiogenesis and immunity, emphasizing its strong promoting function on cancer progression (Figure 1). Although some studies showed good results of targeting Anxa2 in cancer in vitro or in animal models, no in vivo clinical trial targeting Anxa2 in human tumors has been reported by now. It needs further investigation to develop efficient interventions targeting Anxa2 and achieve the ideal anti-tumor effect for clinical use. Alteration of Anxa2 expression, neutralization of the cell surface Anxa2, or inhibition of its activation for example the Tyr23 phosphorylation, could be considered based on the regulatory functions of Anxa2 in tumors.
1.
The mechanism of Anxa2 in promoting cancer progression. Anxa2 is involved in multiple signal pathways and regulates epithelial-mesenchymal transition (EMT), cytoskeleton dynamicity, cell cycle, apoptosis, angiogenesis immunology and treatment resistance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81702992).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Ruifang Niu, Email: rniu@tmu.edu.cn.
Liang Wang, Email: miaochang@sina.com.
References
- 1.Grieve AG, Moss SE, Hayes MJ Annexin A2 at the interface of actin and membrane dynamics: a focus on its roles in endocytosis and cell polarization. Int J Cell Biol. 2012;2012:852430. doi: 10.1155/2012/852430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharadwaj A, Bydoun M, Holloway R, Waisman D Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259–305. doi: 10.3390/ijms14036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokman NA, Ween MP, Oehler MK, Ricciardelli C The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4:199–208. doi: 10.1007/s12307-011-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen MV, Høgdall CK, Jochumsen KM, Høgdall EVS Annexin A2 and cancer: a systematic review. Int J Oncol. 2018;52:5–18. doi: 10.3892/ijo.2017.4197. [DOI] [PubMed] [Google Scholar]
- 5.Liu XY, Ma D, Jing XQ, Wang BR, Yang WP, Qiu WH Overexpression of ANXA2 predicts adverse outcomes of patients with malignant tumors: a systematic review and meta-analysis. Med Oncol. 2015;32:392. doi: 10.1007/s12032-014-0392-y. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Zhang HC, Wang ZY, Yu M, Tian R, Ji W, et al P-glycoprotein associates with Anxa2 and promotes invasion in multidrug resistant breast cancer cells. Biochem Pharmacol. 2014;87:292–302. doi: 10.1016/j.bcp.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol. 2006;81:146–56. doi: 10.1016/j.yexmp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Yuan J, Zhang J, Tian R, Ji W, Zhou Y, et al Anxa2 binds to STAT3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget. 2015;6:30975–92. doi: 10.18632/oncotarget.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon YR, Kim SY, Lee EJ, Kim YN, Noh DY, Park SY, et al Identification of annexin II as a novel secretory biomarker for breast cancer. Proteomics. 2013;13:3145–56. doi: 10.1002/pmic.201300127. [DOI] [PubMed] [Google Scholar]
- 10.Shetty P, Patil VS, Mohan R, D'souza LC, Bargale A, Patil BR, et al Annexin A2 and its downstream IL-6 and HB-EGF as secretory biomarkers in the differential diagnosis of Her-2 negative breast cancer. Ann Clin Biochem. 2017;54:463–71. doi: 10.1177/0004563216665867. [DOI] [PubMed] [Google Scholar]
- 11.Bystricky B, Cierna Z, Sieberova G, Janega P, Karaba M, Minarik G, et al Relationship between circulating tumor cells and annexin A2 in early breast cancer patients. Anticancer Res. 2017;37:2727–34. doi: 10.21873/anticanres.11624. [DOI] [PubMed] [Google Scholar]
- 12.Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS One. 2012;7:e44299. doi: 10.1371/journal.pone.0044299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma M, Ownbey RT, Sharma MC Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol. 2010;88:278–86. doi: 10.1016/j.yexmp.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Lokman NA, Pyragius CE, Ruszkiewicz A, Oehler MK, Ricciardelli C Annexin A2 and S100A10 are independent predictors of serous ovarian cancer outcome. Transl Res. 2016;171:83–95. doi: 10.1016/j.trsl.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Chen C, Hua MH, Xi QH, Liu R, Yang SY, et al Annexin A2 plays a critical role in epithelial ovarian cancer. Arch Gynecol Obstet. 2015;292:175–82. doi: 10.1007/s00404-014-3598-5. [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Gao YP, Li X, Cai MB, Wang HM, Zhuang HY, et al Expression and clinical significance of annexin A2 and human epididymis protein 4 in endometrial carcinoma. J Exp Clin Cancer Res. 2015;34:96. doi: 10.1186/s13046-015-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal P, Klingler-Hoffmann M, Arentz G, Winderbaum L, Kaur G, Anderson L, et al Annexin A2 and alpha actinin 4 expression correlates with metastatic potential of primary endometrial cancer. Biochim Biophys Acta Proteins Proteom. 2017;1865:846–57. doi: 10.1016/j.bbapap.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Alconada L, Santacana M, Garcia-Sanz P, Muinelo-Romay L, Colas E, Mirantes C, et al Annexin-A2 as predictor biomarker of recurrent disease in endometrial cancer. Int J Cancer. 2015;136:1863–73. doi: 10.1002/ijc.29213. [DOI] [PubMed] [Google Scholar]
- 19.Bae SM, Min HJ, Ding GH, Kwak SY, Cho YL, Nam KH, et al Protein expression profile using two-dimensional gel analysis in squamous cervical cancer patients. Cancer Res Treat. 2006;38:99–107. doi: 10.4143/crt.2006.38.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi CH, Chung JY, Chung EJ, Sears JD, Lee JW, Bae DS, et al Prognostic significance of annexin A2 and annexin A4 expression in patients with cervical cancer. BMC Cancer. 2016;16:448. doi: 10.1186/s12885-016-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin LY, Shen Q, Ding SD, Jiang WX, Jiang LL, Zhu XQ Immunohistochemical expression of Annexin A2 and S100A proteins in patients with bulky stage IB-IIA cervical cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;126:140–6. doi: 10.1016/j.ygyno.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, et al Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208:673–85. doi: 10.1002/path.1935. [DOI] [PubMed] [Google Scholar]
- 23.Yoneura N, Takano S, Yoshitomi H, Nakata Y, Shimazaki R, Kagawa S, et al Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer. Int J Mol Med. 2018;42:821–30. doi: 10.3892/ijmm.2018.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy AG, Foley K, Rucki AA, Xia T, Jaffee EM, Huang CY, et al Stromal Annexin A2 expression is predictive of decreased survival in pancreatic cancer. Oncotarget. 2017;8:106405–14. doi: 10.18632/oncotarget.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takano S, Togawa A, Yoshitomi H, Shida T, Kimura F, Shimizu H, et al Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy. Ann Surg Oncol. 2008;15:3157–68. doi: 10.1245/s10434-008-0061-5. [DOI] [PubMed] [Google Scholar]
- 26.Han YH, Ye J, Dong Y, Xu ZP, Du Q Expression and significance of annexin A2 in patients with gastric adenocarcinoma and the association with E-cadherin. Exp Ther Med. 2015;10:549–54. doi: 10.3892/etm.2015.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han F, Shrestha S, Huang H, Lv HY, Nie C, Lin L, et al Expression of annexin II in gastric carcinoma and its role in gastric cancer metastasis. World J Gastroenterol. 2017;23:7009–15. doi: 10.3748/wjg.v23.i38.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, et al Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33:1157–63. [PubMed] [Google Scholar]
- 29.Shaker MK, Abdel Fattah HI, Sabbour GS, Montasser IF, Abdelhakam SM, El Hadidy E, et al Annexin A2 as a biomarker for hepatocellular carcinoma in Egyptian patients. World J Hepatol. 2017;9:469–76. doi: 10.4254/wjh.v9.i9.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun YL, Gao GZ, Cai JQ, Wang YL, Qu XH, He LD, et al Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis. 2013;34:595–604. doi: 10.1093/carcin/bgs372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai DS, Wu C, Yang LX, Zhang C, Zhang PF, He YZ, et al UBAP2 negatively regulates the invasion of hepatocellular carcinoma cell by ubiquitinating and degradating Annexin A2. Oncotarget. 2016;7:32946–55. doi: 10.18632/oncotarget.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, et al Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92:1419–26. doi: 10.1002/1097-0142(20010915)92:6<1419::AID-CNCR1465>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Rocha MR, Barcellos-de-Souza P, Sousa-Squiavinato ACM, Fernandes PV, de Oliveira IM, Boroni M, et al Annexin A2 overexpression associates with colorectal cancer invasiveness and TGF-ß induced epithelial mesenchymal transition via Src/ANXA2/STAT3. Sci Rep. 2018;8:11285. doi: 10.1038/s41598-018-29703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei HP, Zhu H, Zeng S, Li YX, Yang HX, Shen LF, et al Proteome analysis and tissue microarray for profiling protein markers associated with lymph node metastasis in colorectal cancer. J Proteome Res. 2007;6:2495–501. doi: 10.1021/pr060644r. [DOI] [PubMed] [Google Scholar]
- 35.Wu MH, Sun YQ, Xu FP, Liang YQ, Liu H, Yi YM Annexin A2 silencing inhibits proliferation and epithelial-to-mesenchymal transition through p53-dependent pathway in NSCLCs. J Cancer. 2019;10:1077–85. doi: 10.7150/jca.29440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng XM, Liu H, Zhang ZJ, Gu YX, Qiu HS, He ZM Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36:123. doi: 10.1186/s13046-017-0594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agababaoglu I, Önen A, Demir AB, Aktas S, Altun Z, Ersoz H, et al Chaperonin (HSP60) and annexin-2 are candidate biomarkers for non-small cell lung carcinoma. Medicine (Baltimore) 2017;96:e5903. doi: 10.1097/MD.0000000000005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maule F, Bresolin S, Rampazzo E, Boso D, Della Puppa A, Esposito G, et al Annexin 2A sustains glioblastoma cell dissemination and proliferation. Oncotarget. 2016;7:54632–49. doi: 10.18632/oncotarget.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao HS, Yu B, Yan YH, Shen JH, Zhao SH, Zhu JH, et al Correlation of expression levels of ANXA2, PGAM1, and CALR with glioma grade and prognosis. J Neurosurg. 2013;118:846–53. doi: 10.3171/2012.9.JNS112134. [DOI] [PubMed] [Google Scholar]
- 40.Lu XJ, Gao AM, Ji LJ, Xu J Pseudogene in cancer: real functions and promising signature. J Med Genet. 2015;52:17–24. doi: 10.1136/jmedgenet-2014-102785. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Zou HC, Shao YY, Mei Y, Cheng Y, Hu DL, et al Pseudogenes of annexin A2, novel prognosis biomarkers for diffuse gliomas. Oncotarget. 2017;8:106962–75. doi: 10.18632/oncotarget.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Chen K, Cai YH, Cai YX, Yuan XJ, Wang LF, et al Annexin A2 could enhance multidrug resistance by regulating NF-κB signaling pathway in pediatric neuroblastoma. J Exp Clin Cancer Res. 2017;36:111. doi: 10.1186/s13046-017-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingen MW, Kalmar JR, Karrison T, Speight PM Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu JS, Chen YT, Chiang WF, Hsiao YC, Chu LJ, See LC, et al Saliva protein biomarkers to detect oral squamous cell carcinoma in a high-risk population in Taiwan. Proc Natl Acad Sci USA. 2016;113:11549–54. doi: 10.1073/pnas.1612368113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Gao CH, Zhang SH, Fang GQ Serum annexin A2 level is associated with diagnosis and prognosis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75:1081–87. doi: 10.1016/j.joms.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 46.Zhong LP, Wei KJ, Yang X, Zhang L, Zhou XJ, Pan HY, et al Increased expression of Annexin A2 in oral squamous cell carcinoma. Arch Oral Biol. 2009;54:17–25. doi: 10.1016/j.archoralbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigo JP, Lequerica-Fernández P, Rosado P, Allonca E, García-Pedrero JM, de Vicente JC Clinical significance of annexin A2 downregulation in oral squamous cell carcinoma. Head Neck. 2011;33:1708–14. doi: 10.1002/hed.21661. [DOI] [PubMed] [Google Scholar]
- 48.Luo S, Xie CB, Wu P, He J, Tang YY, Xu J, et al Annexin A2 is an independent prognostic biomarker for evaluating the malignant progression of laryngeal cancer. Exp Ther Med. 2017;14:6113–18. doi: 10.3892/etm.2017.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CY, Lin YS, Chen CH, Chen YJ Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma. J Biomed Sci. 2018;25:30. doi: 10.1186/s12929-018-0430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CY, Lin YS, Chen CL, Chao PZ, Chiou JF, Kuo CC, et al Targeting annexin A2 reduces tumorigenesis and therapeutic resistance of nasopharyngeal carcinoma. Oncotarget. 2015;6:26946–59. doi: 10.18632/oncotarget.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domoto T, Miyama Y, Suzuki H, Teratani T, Arai K, Sugiyama T, et al Evaluation of S100A10, annexin II and B-FABP expression as markers for renal cell carcinoma. Cancer Sci. 2007;98:77–82. doi: 10.1111/j.1349-7006.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohno Y, Izumi M, Kawamura T, Nishimura T, Mukai K, Tachibana M Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br J Cancer. 2009;101:287–94. doi: 10.1038/sj.bjc.6605128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu HH, Zhao J, Zhang M Expression of annexin A2 and Its correlation with drug resistance and recurrence of bladder cancer. Technol Cancer Res Treat. 2016;15:NP61-8. doi: 10.1177/1533034615606322. [DOI] [PubMed] [Google Scholar]
- 54.Ding T, Yang LY, Wang YH, Yuan JB, Chen TD, Cai XJ Down-regulation of annexin II in prostate cancer is associated with Gleason score, recurrence, metastasis and poor prognosis. Mol Med Rep. 2010;3:781–7. doi: 10.3892/mmr.2010.332. [DOI] [PubMed] [Google Scholar]
- 55.Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, et al Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–4. [PubMed] [Google Scholar]
- 56.Inokuchi J, Narula N, Yee DS, Skarecky DW, Lau A, Ornstein DK, et al Annexin A2 positively contributes to the malignant phenotype and secretion of IL-6 in DU145 prostate cancer cells. Int J Cancer. 2009;124:68–74. doi: 10.1002/ijc.23928. [DOI] [PubMed] [Google Scholar]
- 57.Yee DS, Narula N, Ramzy I, Boker J, Ahlering TE, Skarecky DW, et al Reduced annexin II protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Arch Pathol Lab Med. 2007;131:902–8. doi: 10.5858/2007-131-902-RAIPEI. [DOI] [PubMed] [Google Scholar]
- 58.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, et al Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105:370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung Y, Wang JC, Lee E, McGee S, Berry JE, Yumoto K, et al Annexin 2-CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res. 2015;13:197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- 61.Zhang XH, Hu Y, Bao L, Jiang Q, Yang LH, Lu XJ, et al Arsenic trioxide downregulates the expression of annexin II in bone marrow cells from patients with acute myelogenous leukemia. Chin Med J (Engl) 2009;122:1969–73. [PubMed] [Google Scholar]
- 62.Spijkers-Hagelstein JAP, Mimoso Pinhanços S, Schneider P, Pieters R, Stam RW Src kinase-induced phosphorylation of annexin A2 mediates glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia. 2013;27:1063–71. doi: 10.1038/leu.2012.372. [DOI] [PubMed] [Google Scholar]
- 63.Thiery JP, Acloque H, Huang RY, Nieto MA Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Chaffer CL, Weinberg RA A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 65.Cui L, Song J, Wu LT, Cheng LH, Chen AJ, Wang YL, et al Role of Annexin A2 in the EGF-induced epithelial-mesenchymal transition in human CaSki cells. Oncol Lett. 2017;13:377–83. doi: 10.3892/ol.2016.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng L, Foley K, Huang LQ, Leubner A, Mo GL, Olino K, et al Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Li HY, Ban ZY, Nai MM, Yang L, Chen Y, et al Annexin A2 inhibition suppresses ovarian cancer progression via regulating β-catenin/EMT. Oncol Rep. 2017;37:3643–50. doi: 10.3892/or.2017.5578. [DOI] [PubMed] [Google Scholar]
- 68.Yan XL, Zhang DD, Wu W, Wu SH, Qian JF, Hao YJ, et al Mesenchymal Stem Cells Promote Hepatocarcinogenesis via lncRNA-MUF Interaction with ANXA2 and miR-34a. Cancer Res. 2017;77:6704–16. doi: 10.1158/0008-5472.CAN-17-1915. [DOI] [PubMed] [Google Scholar]
- 69.Yi YM, Zeng SS, Wang ZT, Wu MH, Ma YH, Ye XX, et al Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Pepper MS Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86:346–55. doi: 10.1055/s-0037-1616232. [DOI] [PubMed] [Google Scholar]
- 71.Sharma MC, Sharma M The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des. 2007;13:3568–75. doi: 10.2174/138161207782794167. [DOI] [PubMed] [Google Scholar]
- 72.Eble JA, Niland S The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15:1385–400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 73.McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, et al Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863–8. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Réty S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, et al The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat Struct Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- 75.Zobiack N, Gerke V, Rescher U Complex formation and submembranous localization of annexin 2 and S100A10 in live HepG2 cells. FEBS Lett. 2001;500:137–40. doi: 10.1016/S0014-5793(01)02604-7. [DOI] [PubMed] [Google Scholar]
- 76.Deora AB, Kreitzer G, Jacovina AT, Hajjar KA An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem. 2004;279:43411–8. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- 77.Valapala M, Maji S, Borejdo J, Vishwanatha JK Cell surface translocation of annexin A2 facilitates glutamate-induced extracellular proteolysis. J Biol Chem. 2014;289:15915–26. doi: 10.1074/jbc.M113.511550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swisher JFA, Burton N, Bacot SM, Vogel SN, Feldman GM Annexin A2 tetramer activates human and murine macrophages through TLR4. Blood. 2010;115:549–58. doi: 10.1182/blood-2009-06-226944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephens RW, Brünner N, Jänicke F, Schmitt M The urokinase plasminogen activator system as a target for prognostic studies in breast cancer. Breast Cancer Res Treat. 1998;52:99–111. doi: 10.1023/A:1006115218786. [DOI] [PubMed] [Google Scholar]
- 80.Sharma M, Blackman MR, Sharma MC Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp Mol Pathol. 2012;92:175–84. doi: 10.1016/j.yexmp.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Simpson RJ, Kalra H, Mathivanan S ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1:18374. doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al Exosomal annexin ii promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15:93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Onishi M, Ichikawa T, Kurozumi K, Inoue S, Maruo T, Otani Y, et al Annexin A2 regulates angiogenesis and invasion phenotypes of malignant glioma. Brain Tumor Pathol. 2015;32:184–94. doi: 10.1007/s10014-015-0216-6. [DOI] [PubMed] [Google Scholar]
- 84.Zhai HY, Acharya S, Gravanis I, Mehmood S, Seidman RJ, Shroyer KR, et al Annexin A2 promotes glioma cell invasion and tumor progression. J Neurosci. 2011;31:14346–60. doi: 10.1523/JNEUROSCI.3299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu YH, Wang ZY, Jiang M, Dai L, Zhang W, Wu DP, et al The expression of annexin II and its role in the fibrinolytic activity in acute promyelocytic leukemia. Leuk Res. 2011;35:879–84. doi: 10.1016/j.leukres.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Huang D, Yang Y, Sun J, Dong XR, Wang J, Liu HC, et al Annexin A2-S100A10 heterotetramer is upregulated by PML/RARα fusion protein and promotes plasminogen-dependent fibrinolysis and matrix invasion in acute promyelocytic leukemia. Front Med. 2017;11:410–22. doi: 10.1007/s11684-017-0527-6. [DOI] [PubMed] [Google Scholar]
- 87.Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, et al Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–41. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 88.Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, et al Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002;160:45–50. doi: 10.1016/S0002-9440(10)64347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davies BR, O'Donnell M, Durkan GC, Rudland PS, Barraclough R, Neal DE, et al Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J Pathol. 2002;196:292–9. doi: 10.1002/path.1051. [DOI] [PubMed] [Google Scholar]
- 90.Tuszynski GP, Sharma MR, Rothman VL, Sharma MC Angiostatin binds to tyrosine kinase substrate annexin II through the lysine-binding domain in endothelial cells. Microvasc Res. 2002;64:448–62. doi: 10.1006/mvre.2002.2444. [DOI] [PubMed] [Google Scholar]
- 91.Ji WR, Castellino FJ, Chang Y, Deford ME, Gray H, Villarreal X, et al Characterization of kringle domains of angiostatin as antagonists of endothelial cell migration, an important process in angiogenesis. FASEB J. 1998;12:1731–8. doi: 10.1096/fasebj.12.15.1731. [DOI] [PubMed] [Google Scholar]
- 92.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–28. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 93.Hubaishy I, Jones PG, Bjorge J, Bellagamba C, Fitzpatrick S, Fujita DJ, et al Modulation of annexin II tetramer by tyrosine phosphorylation. Biochemistry. 1995;34:14527–34. doi: 10.1021/bi00044a031. [DOI] [PubMed] [Google Scholar]
- 94.Hayes MJ, Shao DM, Bailly M, Moss SE Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–26. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones PG, Moore GJ, Waisman DM A nonapeptide to the putative F-actin binding site of annexin-II tetramer inhibits its calcium-dependent activation of actin filament bundling. J Biol Chem. 1992;267:13993–7. [PubMed] [Google Scholar]
- 96.Ikebuchi NW, Waisman DM Calcium-dependent regulation of actin filament bundling by lipocortin-85. J Biol Chem. 1990;265:3392–400. [PubMed] [Google Scholar]
- 97.Turpin E, Russo-Marie F, Dubois T, de Paillerets C, Alfsen A, Bomsel M In adrenocortical tissue, annexins II and VI are attached to clathrin coated vesicles in a calcium-independent manner. Biochim Biophys Acta Mol Cell Res. 1998;1402:115–30. doi: 10.1016/S0167-4889(97)00151-1. [DOI] [PubMed] [Google Scholar]
- 98.Harder T, Kellner R, Parton RG, Gruenberg J Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell. 1997;8:533–45. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morel E, Gruenberg J Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. J Biol Chem. 2009;284:1604–11. doi: 10.1074/jbc.M806499200. [DOI] [PubMed] [Google Scholar]
- 100.Zobiack N, Rescher U, Ludwig C, Zeuschner D, Gerke V The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell. 2003;14:4896–908. doi: 10.1091/mbc.e03-06-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morel E, Parton RG, Gruenberg J Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16:445–57. doi: 10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, et al Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell. 2009;20:3896–904. doi: 10.1091/mbc.e08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Graauw M, Cao L, Winkel L, van Miltenburg MHAM, le Dévédec SE, Klop M, et al Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33:2610–9. doi: 10.1038/onc.2013.219. [DOI] [PubMed] [Google Scholar]
- 104.Taylor JR, Fernandez DJ, Thornton SM, Skeate JG, Lühen KP, Da Silva DM, et al Heterotetrameric annexin A2/S100A10(A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci Rep. 2018;8:11642. doi: 10.1038/s41598-018-30051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woodham AW, Taylor JR, Jimenez AI, Skeate JG, Schmidt T, Brand HE, et al Small molecule inhibitors of the annexin A2 heterotetramer prevent human papillomavirus type 16 infection. J Antimicrob Chemother. 2015;70:1686–90. doi: 10.1093/jac/dkv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hall A Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 107.Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci. 2008;121:2177–85. doi: 10.1242/jcs.028415. [DOI] [PubMed] [Google Scholar]
- 108.de Graauw M, Tijdens I, Smeets MB, Hensbergen PJ, Deelder AM, van de Water B Annexin A2 phosphorylation mediates cell scattering and branching morphogenesis via cofilin Activation. Mol Cell Biol. 2008;28:1029–40. doi: 10.1128/MCB.01247-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi HY, Xiao L, Duan W, He HM, Ma LL, Da MM, et al ANXA2 enhances the progression of hepatocellular carcinoma via remodeling the cell motility associated structures. Micron. 2016;85:26–33. doi: 10.1016/j.micron.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 110.Sun MY, Xing RH, Gao XJ, Yu X, He HM, Gao N, et al ANXA2 regulates the behavior of SGC-7901 cells. Asian Pac J Cancer Prev. 2013;14:6007–12. doi: 10.7314/APJCP.2013.14.10.6007. [DOI] [PubMed] [Google Scholar]
- 111.Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, et al HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54:2012–24. doi: 10.1002/hep.24592. [DOI] [PubMed] [Google Scholar]
- 112.Swaminathan V, Mythreye K, O'Brien ET, Berchuck A, Blobe GC, Superfine R Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075–80. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N An actin barrier to resealing. J Cell Sci. 2001;114:3487–94. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 114.Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, et al S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun. 2014;5:3795. doi: 10.1038/ncomms4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jaiswal JK, Nylandsted J S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle. 2015;14:502–9. doi: 10.1080/15384101.2014.995495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bao HY, Jiang M, Zhu MQ, Sheng F, Ruan J, Ruan CG Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. Int J Hematol. 2009;90:177–85. doi: 10.1007/s12185-009-0356-8. [DOI] [PubMed] [Google Scholar]
- 117.Wu B, Zhang F, Yu M, Zhao P, Ji W, Zhang H, et al Up-regulation of Anxa2 gene promotes proliferation and invasion of breast cancer MCF-7 cells. Cell Prolif. 2012;45:189–98. doi: 10.1111/j.1365-2184.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiang Y, Schneiderman MH, Vishwanatha JK Annexin II expression is regulated during mammalian cell cycle. Cancer Res. 1993;53:6017–21. [PubMed] [Google Scholar]
- 119.Vishwanatha JK, Kumble S Involvement of annexin II in DNA replication: evidence from cell-free extracts of Xenopus eggs. J Cell Sci. 1993;105:533–40. doi: 10.1242/jcs.105.2.533. [DOI] [PubMed] [Google Scholar]
- 120.Kumble KD, Iversen PL, Vishwanatha JK The role of primer recognition proteins in DNA replication: inhibition of cellular proliferation by antisense oligodeoxyribonucleotides. J Cell Sci. 1992;101:35–41. doi: 10.1242/jcs.101.1.35. [DOI] [PubMed] [Google Scholar]
- 121.Chiang Y, Rizzino A, Sibenaller ZA, Wold MS, Vishwanatha JK Specific down-regulation of annexin II expression in human cells interferes with cell proliferation. Mol Cell Biochem. 1999;199:139–47. doi: 10.1023/A:1006942128672. [DOI] [PubMed] [Google Scholar]
- 122.Madureira PA, Hill R, Lee PWK, Waisman DM Genotoxic agents promote the nuclear accumulation of annexin A2: role of annexin A2 in mitigating DNA damage. PLoS One. 2012;7:e50591. doi: 10.1371/journal.pone.0050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mickleburgh I, Burtle B, Hollås H, Campbell G, Chrzanowska-Lightowlers Z, Vedeler A, et al Annexin A2 binds to the localization signal in the 3' untranslated region of c-myc mRNA. FEBS J. 2005;272:413–21. doi: 10.1111/j.1742-4658.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- 124.Zhang F, Wang ZY, Yuan J, Wei XY, Tian R, Niu RF RNAi-mediated silencing of Anxa2 inhibits breast cancer cell proliferation by downregulating cyclin D1 in STAT3-dependent pathway. Breast Cancer Res Treat. 2015;153:263–75. doi: 10.1007/s10549-015-3529-6. [DOI] [PubMed] [Google Scholar]
- 125.Horn HF, Vousden KH Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–16. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 126.Walerych D, Napoli M, Collavin L, Del Sal G The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis. 2012;33:2007–17. doi: 10.1093/carcin/bgs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang CY, Chen CL, Tseng YL, Fang YT, Lin YS, Su WC, et al Annexin A2 silencing induces G2 arrest of non-small cell lung cancer cells through p53-dependent and -independent mechanisms. J Biol Chem. 2012;287:32512–24. doi: 10.1074/jbc.M112.351957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang CY, Lin YS, Su WC, Chen CL, Lin CF Glycogen synthase kinase-3 and Omi/HtrA2 induce annexin A2 cleavage followed by cell cycle inhibition and apoptosis. Mol Biol Cell. 2009;20:4153–61. doi: 10.1091/mbc.e09-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen F, et al Involvement of Annexin A2 in p53 induced apoptosis in lung cancer. Mol Cell Biochem. 2008;309:117–23. doi: 10.1007/s11010-007-9649-5. [DOI] [PubMed] [Google Scholar]
- 130.Martin M, Leffler J, Blom AM Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. J Biol Chem. 2012;287:33733–44. doi: 10.1074/jbc.M112.341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dubois T, Oudinet JP, Russo-Marie F, Rothhut B In vivo and in vitro phosphorylation of annexin II in T cells: potential regulation by annexin V. Biochem J. 1995;310:243–8. doi: 10.1042/bj3100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andersen BM, Xia JZ, Epstein AL, Ohlfest JR, Chen W, Blazar BR, et al Monomeric annexin A2 is an oxygen-regulated toll-like receptor 2 ligand and adjuvant. J Immunother Cancer. 2016;4:11. doi: 10.1186/s40425-016-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, et al Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci USA. 2017;114:3163–68. doi: 10.1073/pnas.1621052114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swisher JFA, Khatri U, Feldman GM Annexin A2 is a soluble mediator of macrophage activation. J Leukoc Biol. 2007;82:1174–84. doi: 10.1189/jlb.0307154. [DOI] [PubMed] [Google Scholar]
- 135.Chao PZ, Hsieh MS, Cheng CW, Hsu TJ, Lin YT, Lai CH, et al Dendritic cells respond to nasopharygeal carcinoma cells through annexin A2-recognizing DC-SIGN. Oncotarget. 2015;6:159–70. doi: 10.18632/oncotarget.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woodham AW, Raff AB, Raff LM, Da Silva DM, Yan L, Skeate JG, et al Inhibition of Langerhans cell maturation by human papillomavirus type 16: a novel role for the annexin A2 heterotetramer in immune suppression. J Immunol. 2014;192:4748–57. doi: 10.4049/jimmunol.1303190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bao J, Xu Y, Wang QY, Zhang JP, Li ZJ, Li DY, et al miR-101 alleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030–37. doi: 10.1016/j.biopha.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 138.Liu DY, Hu Y, Guo Y, Zhu Z, Lu BZ, Wang XL, et al Mycoplasma-associated multidrug resistance of hepatocarcinoma cells requires the interaction of P37 and Annexin A2. PLoS One. 2017;12:e0184578. doi: 10.1371/journal.pone.0184578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang YS, Li H, Li Y, Zhu HY, Jin YH Identification of natural compounds targeting Annexin A2 with an anti-cancer effect. Protein Cell. 2018;9:568–79. doi: 10.1007/s13238-018-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y, Bi JJ, Zhu HT, Shi M, Zeng XL ANXA2 could act as a moderator of EGFR-directed therapy resistance in triple negative breast cancer. Biosci Biotechnol Biochem. 2018;82:1733–41. doi: 10.1080/09168451.2018.1484275. [DOI] [PubMed] [Google Scholar]
- 141.Chaudhary P, Thamake SI, Shetty P, Vishwanatha JK Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by Annexin A2 antibodies. Br J Cancer. 2014;111:2328–41. doi: 10.1038/bjc.2014.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma MC, Tuszynski GP, Blackman MR, Sharma M Long-term efficacy and downstream mechanism of anti-annexinA2 monoclonal antibody (anti-ANX A2 mAb) in a pre-clinical model of aggressive human breast cancer. Cancer Lett. 2016;373:27–35. doi: 10.1016/j.canlet.2016.01.013. [DOI] [PubMed] [Google Scholar]