Abstract
As immunotherapy has gained increasing interest as a new foundation for cancer therapy, some atypical response patterns, such as pseudoprogression and hyperprogression, have garnered the attention of physicians. Pseudoprogression is a phenomenon in which an initial increase in tumor size is observed or new lesions appear, followed by a decrease in tumor burden; this phenomenon can benefit patients receiving immunotherapy but often leads to premature discontinuation of treatment owing to the false judgment of progression. Accurately recognizing pseudoprogression is also a challenge for physicians. Because of the extensive attention on pseudoprogression, significant progress has been made. Some new criteria for immunotherapy, such as irRC, iRECIST and imRECIST, were proposed to accurately evaluate the response to immunotherapy. Many new detection indexes, such as ctDNA and IL-8, have also been used to identify pseudoprogression. In this review, the definition, evaluation criteria, mechanism, monitoring, management and prognosis of pseudoprogression are summarized, and diagnostic and treatment processes for patients with progression but with a suspicion of pseudoprogression are proposed; these processes could be helpful for physicians in clinical practice and enhances the understanding of pseudoprogression.
Keywords: Cancer, immunotherapy, pseudoprogression, RECIST, ctDNA, IL-8
Introduction
Immunotherapy has changed the treatment status of cancer therapy, and several clinical trials have shown that immunotherapy can prolong the survival of patients with advanced cancer1-3. In 2011, the monoclonal antibody (mAb) ipilimumab, which targets cytotoxic T lymphocyte antigen 4 (CTLA-4), was approved for advanced melanoma, and, one by one, many immunotherapy agents have been approved for clinical use4,5. Compared to chemotherapy, immunotherapy exhibits a significant advantage in disease control and overall survival (OS) for cancer patients and has become the standard of care for melanoma, non-small cell lung cancer (NSCLC), head and neck squamous cell cancer (HNSCC) and so on1. For example, pembrolizumab, an antibody targeting programmed death ligand 1 (anti-PD-L1), was approved as a first-line therapy in NSCLC with PD-L1 expression on at least 50% of tumor cells and achieved a median progression-free survival (mPFS) 4.3 months longer than that achieved with platinum-based chemotherapy6,7. Durvalumab, another anti-PD-L1 mAb, has a 17.2-month mPFS for consolidation treatment after chemoradiotherapy in advanced NSCLC8.
Despite the dramatic achievement in immunotherapies, especially with anti-CTLA-4/PD-L1/PD-1 agents, some atypical response patterns, such as pseudoprogression and hyperprogression, have been described9-12. The early and precise recognition of pseudoprogression can prevent the premature discontinuation of immunotherapy and ensure a better benefit from immunotherapy. This review mainly discusses pseudoprogression in cancer immunotherapy and focuses on the mechanism, evaluation criteria, detection and prognosis of pseudoprogression. Diagnostic and treatment processes for patients who receive immunotherapy but meet progressive disease (PD) criteria are also proposed; these processes could be helpful for physicians to accurately distinguish pseudoprogression and true progression.
The definition of pseudoprogression after immunotherapy
Pseudoprogression is an unconventional response pattern that can occur in gliomas treated with chemoradiotherapy and in essentially all tumors treated with immunotherapy13,14. When solid tumors are treated with immunotherapy agents, pseudoprogression is the phenomenon in which an initial increase in tumor size occurs or new lesions appear, followed by a decrease in tumor burden; these changes can be confirmed by tumor biopsy or a continuous radiography scan14-17. According to the time at which the tumor shrinks, pseudoprogression is categorized as early and delayed pseudoprogression; the former is defined as a ≥ 25% increase in tumor burden at imaging assessment within 12 weeks from the start of immunotherapy but is not confirmed as PD per immune-related response criteria (irRC) at the next imaging assessment, whereas the latter is defined as a ≥ 25% increase in tumor burden at any imaging assessment after 12 weeks but is not confirmed as PD per irRC at the next imaging assessment18.
The incidence of immunotherapy pseudoprogression in clinical trials was 2.78%−9.69% for melanoma, 1.81%−5.77% for NSCLC, 2.86%−8.82% for renal cell carcinoma, 1.49%−7.14% for urothelial carcinoma, 11.11% uveal melanoma, 1.79% for HNSCC, 1.14% for Merkel cell carcinoma and 6.90% for mesothelioma (Table 1)18-44. Until now, the exact reasons for the variation in pseudoprogression incidence for different tumors were unclear. The variations of the definition for pseudoprogression across the literature are partly responsible for the variation in the reported incidences, and the clinical and biological characteristics of different tumors may also affect the incidence of pseudoprogression. The demographic characteristics and immunotherapy agents may also be involved in the variations of pseudoprogression. In addition, there are also some sites of pseudoprogression specific to the tumor type, such as brain metastasis pseudoprogression of lung cancer and renal cell carcinoma, after immunotherapy45,46. In addition to the common pattern of pseudoprogression, some unconventional pseudoprogression patterns also exist; for example, Ozaki et al.47 found that a patient with metastatic melanoma treated with nivolumab experienced early pseudoprogression on liver lesions and delayed pseudoprogression on peritoneal nodules. This phenomenon suggests that pseudoprogression can occur throughout the entire treatment process. Although there is work regarding the treatment of pseudoprogression with a single immunotherapy agent, there have been reports of two patients with lung cancer brain metastasis pseudoprogression after receiving the combination of nivolumab and ipilimumab; this phenomenon was confirmed by a continuous radiography scan48. This finding reminds us that more studies on pseudoprogression after combined immunotherapy are needed.
1.
The incidence of pseudoprogression in published clinical trials and retrospective studies
| Author | Immunotherapy agents | No. of
evaluated patients |
No. of
pseudoprogression patients |
Rate (%) | Evaluation criteria |
| NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; HNSCC, head and neck squamous cell cancer. | |||||
| Melanoma | |||||
| Wolchok et al.19 | Ipilimumab | 227 | 22 | 9.69 | WHO/irRC |
| O'Day et al.20 | Ipilimumab | 155 | 12 | 7.74 | mWHO/irRC |
| Di Giacomo et al.21 | Ipilimumab | 27 | 2 | 7.41 | mWHO |
| Ribas et al.22 | Tremelimumab | 36 | 1 | 2.78 | RECIST v1.0 |
| Millward et al.23 | Tremelimumab and toll-like receptor-9 agonist | 16 | 1 | 6.25 | RECIST v1.0 |
| Topalian et al.24 | Nivolumab | 107 | 4 | 3.74 | RECIST v1.0 |
| Weber et al.25 | Nivolumab | 120 | 10 | 8.33 | RECIST v1.1 |
| Robert et al.26 | Nivolumab | 206 | 17 | 8.25 | RECIST v1.1 |
| Hodi et al.18 | Pembrolizumab | 327 | 24 | 7.34 | RECIST v1.1 |
| Nishino et al.27 | Pembrolizumab | 107 | 4 | 3.74 | irRECIST |
| NSCLC | |||||
| Gettinger et al.28 | Nivolumab | 129 | 6 | 4.65 | RECIST v1.0 |
| Borghaei et al.29 | Nivolumab | 287 | 16 | 5.57 | RECIST v1.1 |
| Gettinger et al.30 | Nivolumab | 52 | 3 | 5.77 | RECIST v1.1 |
| Fujimoto et al.31 | Nivolumab | 542 | 14 | 2.58 | RECIST v1.1 |
| Kim et al.32 | Anti PD-1/PD-L1/CTLA-4 antibody | 41 | 2 | 4.88 | RECIST v1.1/irRC |
| Katz et al.33 | Anti PD-L1 antibody | 166 | 3 | 1.81 | RECIST v1.1 |
| Tazdait et al.34 | Anti PD-L1/PD-1 antibody | 160 | 8 | 5.00 | RECIST v1.1 |
| RCC | |||||
| McDermott et al.35 | Nivolumab | 34 | 3 | 8.82 | RECIST v1.0 |
| McDermott et al.36 | Atezolizumab | 70 | 2 | 2.86 | RECIST v1.1/irRC |
| Urothelial carcinoma | |||||
| Powles et al.37 | Atezolizumab | 67 | 1 | 1.49 | RECIST v1.1 |
| Rosenberg et al.38 | Atezolizumab | 310 | 21 | 6.77 | RECIST v1.1 /imRECIST |
| Massard et al.39 | Durvalumab | 42 | 3 | 7.14 | RECIST v1.1 |
| Uveal melanoma | |||||
| Danielli et al.40 | Ipilimumab | 9 | 1 | 11.11 | mWHO |
| HNSCC | |||||
| Seiwert et al.41 | Pembrolizumab | 56 | 1 | 1.79 | RECIST v1.1 |
| Merkel cell carcinoma | |||||
| Kaufman et al.42 | Avelumab | 88 | 1 | 1.14 | RECIST v1.1 |
| Mesothelioma | |||||
| Calabro et al.43 | Tremelimumab | 29 | 2 | 6.90 | RECIST v1.1/irRC |
| Multiple cancer type | |||||
| Topalian et al.44 | Nivolumab | 236 | 8 | 3.39 | RECIST v1.0 |
Evaluation criteria of pseudoprogression after immunotherapy
Accurate response evaluation criteria are paramount for precise tumor treatment. In 1979, the WHO criteria were first proposed to evaluate the treatment response for tumors49. Then, response criteria for tumor treatment were proposed and repeatedly revised50-52. Owing to the wide use of immunotherapy in tumors, some atypical response patterns that cannot be described by the WHO criteria or RECIST guidelines were reported53. For an accurate identification of the response pattern, irRC, iRECIST and immune-modified response evaluation criteria in solid tumors (imRECIST) were proposed based on previous guidelines19,54,55.
irRC were first proposed in 2009 and are based on the WHO criteria and clinical observations with anti-CTLA-4 immunotherapy for advanced melanoma19. irRC, which follow the bidimensional measurement of WHO criteria, evaluate tumor burden by the sum of the two largest perpendicular diameters (SPD) in the first evaluation and the SPD of all index lesions and the index lesions of new, measurable lesions at every subsequent tumor assessment. The irPD criteria need additional confirmation by a repeat, consecutive assessment no less than 4 weeks from the date first documented. After the tumor response was evaluated simultaneously with the WHO criteria and irRC guidelines, 22 of 227 patients had PD per the WHO criteria but met an objective response per the irRC guidelines (5 had irPR, and 17 had irSD). This outcome can prevent the premature use of ipilimumab for individuals with an objective response and guarantees a benefit for patients treated with immunotherapy19. In addition to the bidimensional irRC criteria, unidimensional irRC criteria, proposed by Nishino et al., measure the longest diameter and obtain results that are highly concordant with bidimensional irRC criteria; furthermore, the unidimensional irRC criteria were more reproducible than bidimensional irRC criteria56.
iRECIST guidelines were developed in 2017 to provide a consistent framework for the management of data collected in clinical trials of immune-based therapy. They proposed two specific response patterns: unconfirmed progressive disease (iUPD) and confirmed progressive disease (iCPD). iUPD is defined as PD per the RECIST v1.1 criteria that is not confirmed at the follow-up imaging assessment within 4-8 weeks. iCPD is defined as the appearance of another new lesion or a further growth of the new lesion that appears at the most recent imaging assessment, target lesions and nontarget lesions that appear at the subsequent assessment within 4-8 weeks, or an increase of ≥ 5 mm in the target lesions. iCR, iPR and iSD were assigned based on the RECIST v1.1 criteria. iRECIST guidelines proposed a status of iUPD, which would allow continuation of treatment and follow-up more closely to better benefit patients54.
imRECIST guidelines were designed to better capture immunotherapy responses and were proposed in 2018 on the basis of the RECIST v1.1 and irRC guidelines, which defined PD as a ≥ 20% increase in the sum of the longest diameters (SLD) of the target lesions and new lesions compared with baseline/nadir (minimum recorded tumor burden), which can be negated by subsequent non-PD assessment ≥ 4 weeks from the date first documented. Patients are allowed to continue immunotherapy when they meet PD based on RECIST v1.1 or imRECIST guidelines and if they do not have any deterioration in performance status or signs or symptoms of unequivocal PD or PD at sensitive sites. This finding is also supported by the case report of Rebuzzi et al.57, which describes an advanced renal clear cell cancer patient who had a support performance status treated with nivolumab, upon which the patient achieved clinical benefit and exhibited a delayed radiological response after initial progression. Moreover, when imRECIST-defined PFS (imPFS) is analyzed, imRECIST PD or death is considered an event; however, imRECIST PD is not considered an imPFS event if the time point response at the subsequent scan (≥ 4 weeks later) is imRECIST SD/PR/CR. imRECIST PD followed by no additional assessment is considered an imPFS event. The same article also evaluated trials that used atezolizumab and found that patients with PD per the RECIST v1.1 guidelines alone had better OS than those with PD per both the RECIST v1.1 and imRECIST criteria; for example, a longer median OS of 4.4 months was observed in IMvigor210 patients with PD by RECIST v1.1 alone versus those with PD per both the RECIST v1.1 and imRECIST guidelines55.
Table 2 summarizes the response evaluation criteria mentioned above. Here, we refer to the WHO criteria and RECIST v1.1 guidelines as conventional evaluation criteria and consider irRC, iRECIST and imRECIST as immunotherapy evaluation criteria. Upon comparison of immunotherapy evaluation criteria with conventional evaluation criteria, two obvious differences were found. In the immunotherapy evaluation criteria, the new lesions do not always represent PD, and they are incorporated with the target and nontarget lesions to calculate the response pattern. In addition PD does not always indicate the discontinuation of treatment (there is a potential benefit from the treatment beyond PD, and PD must be confirmed by a repeat assessment no less than 4 weeks from the date first documented)19,50,52,54,55. Although several immunotherapy evaluation criteria have been proposed, the diagnosis of pseudoprogression is still based on the change in tumor burden, and immunotherapy evaluation criteria should be widely considered and used in clinical practice by physicians to correctly determine the response to immunotherapy.
2.
Evaluation criteria for tumor response to treatment
| WHO | RECIST v1.1 | IrRC | IRECIST | ImRECIST | |
| Dimensional | Bidimensional/
unidimensional |
Unidimensional | Bidimensional | Unidimensional | Unidimensional |
| Target lesion | NA | Up to a maximum of two lesions per organ and five lesions total | Up to maximum five lesions per organ, 10 visceral lesions and 5 cutaneous target lesions | Per RECIST v1.1 | Per RECIST v1.1 |
| Nontarget lesion | NA | Contribute to the CR, PR, SD and PD | Nontarget progression does not define PD; can only contribute to defining CR (complete disappearance required) | Contribute to the CR, PR, SD and PD | Nontarget progression does not define PD; can only contribute to define CR (complete disappearance required) |
| New lesion | Always represent PD | Always represent PD | Do not define progression but preclude irCR | Represent iUPD and require a next imaging assessment to confirmation | New lesions are added to the total tumor burden along with the sum of the target lesions when measurable; when not measurable, they are not factored into the PD assessment |
| CR | Disappearance of all known lesions, determined by two observations not less than 4 weeks apart | Disappearance of all target and nontarget lesions and normalization of tumor marker level | Complete disappearance of all lesions (whether measurable or not, and no new lesions), confirmation by a repeat, consecutive assessment no less than 4 weeks from the date first documented | ICR: meet CR per RECIST v1.1 at first or at the next assessment within 4−8 weeks after iUPD | Per RECIST v1.1 |
| PR | 50% or more decrease in total tumor load of the lesions that have been measured to determine the effect of therapy by two observations not less than four weeks apart | At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Persistence of one or more nontarget lesion(s) and/or maintenance of tumor marker level above the normal limits | Decrease in tumor burden ≥ 50% relative to baseline confirmed by a consecutive assessment at least 4 weeks after first documentation | IPR: meet PR per RECIST v1.1 at first or at the next assessment within 4−8 weeks after iUPD | Per RECIST v1.1 |
| SD | No change (NC): A 50% decrease in total tumor size cannot be established nor has a 25% increase in the size of 1 or more measu-rable lesions have been demonstrated | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Persistence of one or more nontarget lesion(s) and/or maintenance of tumor marker level above the normal limits | Not meeting criteria for irCR or irPR, in absence of irPD | ISD: meet SD per RECIST v1.1 at first or at the next assessment within 4−8 weeks after iUPD | Per RECIST v1.1 |
| PD | 25% or more increase in the size of one or more measurable lesions or the appearance of new lesions | At least a 20% increase in the sum of diameters of target lesions. In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. Or unequivocal progression of existing nontarget lesions | Increase in tumor burden ≥ 25% relative to nadir (minimum recorded tumor burden) confirmed by a repeat, consecutive assessment no less than 4 weeks from the date first documented | IUPD: PD per RECIST v1.1 but has not been confirmed at the next assessment. ICPD: in the next imaging assessment, done at 4–8 weeks after iUPD, confirms additional new lesions or a further increase in new lesions, target lesions and nontarget lesions from iUPD (sum of measures increase in target lesions ≥ 5 mm, any increase for nontarget lesions) | ≥ 20% increase in SLD of target lesions and new lesions compared with baseline/nadir, can be negated by subsequent non-PD assessment ≥ 4 weeks from the date first documented; allows treatment beyond PD |
| CR, complete response; PR, partial response; imRECIST, immune-modified RECIST; irRC, immune-related response criteria; PD, progressive disease; iUPD, unconfirmed progressive disease; iCPD, unconfirmed progressive disease; RECIST, Response Evaluation Criteria In Solid Tumors; SLD, sum of longest diameters; nadir, minimum recorded tumor burden; NA, no application | |||||
Mechanism of pseudoprogression after immunotherapy
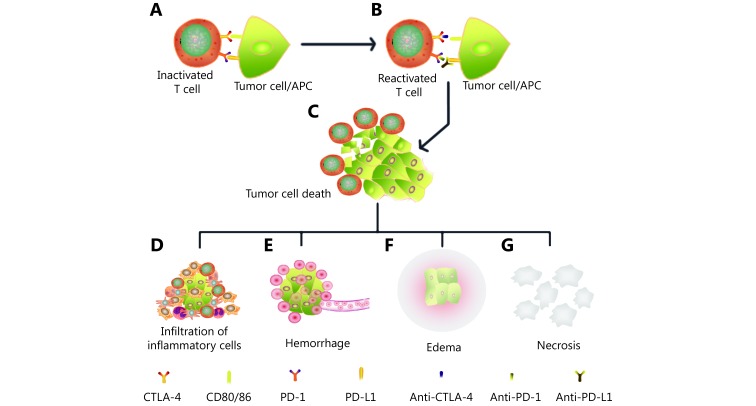
Although there are many studies on pseudoprogression, there is still no consensus on its exact molecular mechanism27,33. Cohen et al.58 described a patient with melanoma brain metastasis who was treated with pembrolizumab and presented a mental status change 11 days thereafter. Magnetic resonance imaging (MRI) of the brain showed the enlargement of central nervous system lesions with intense central enhancement and diffuse perilesional edema. The histologic evaluation of a resected left parietal-occipital lesion revealed isolated clusters of tumor cells surrounded by reactive astrocytosis, scattered inflammatory cells, and an abundance of microglial cells, which was consistent with the response to treatment rather than tumor growth. The patient passed away 3 weeks later due to a deterioration in performance status but was considered to exhibit pseudoprogression in the brain metastases58. Rocha et al. described a patient with stage IV squamous cell lung cancer with pseudoprogression who was treated with nivolumab and exhibited a discordant response (partial response in the central nervous system and stable lung disease) but a significant increase in liver lesions. Pathologic findings of the liver lesions revealed extensive areas of necrosis, no viable tumor cells, and the presence of lymphohistiocytic infiltrate. In the liver biopsy, the number of CD4-, CD8- and CD103-positive cells was increased, the ratio of CD4/CD8 T cells was decreased (from 1.25 to 0.875), and CD68 staining indicated a higher proportion of macrophages59. These authors showed the differential infiltration of inflammatory cells in individual patients, which may be dependent on the location of the lesions with pseudoprogression and the distribution of special inflammatory cells. Other cases also exhibited necrosis, hemorrhage, edema and immune cell infiltration in lesions with pseudoprogression16,17,60,61. As mentioned above, the major mechanism of pseudoprogression is the infiltration of immune cells, such as CD4 T cells, CD8 T cells and macrophagocytes; tumor necrosis; hemorrhage and edema. The purported mechanism of pseudoprogression after immunotherapy is summarized in Figure 1.
1.
The mechanism of pseudoprogression after immunotherapy. (A) T cells were inactivated by the PD-L1 and CTLA-4 presented by tumor cells or antigen-presenting cells (APCs). (B) T cells were reactivated after the administration of immune checkpoint inhibitors such as anti-PD-1/PD-L1/CTLA-4. (C) Activated T cells infiltrate tumor lesions and kill the tumor cell. (D) Antigens released by the death of tumor cells attract more infiltrating inflammatory cells. (E) Shrinking tumor tissues can cause vascular tears and hemorrhage in locoregional lesions. (F) The inflammatory response and hemorrhage cause the edema of lesions. (G) The necrotic byproducts of dead tumor cells cannot be absorbed immediately and accumulate in locoregional lesions. Inflammatory cell infiltration, hemorrhage, edema and necrosis enlarged the lesions in imageologic assessments and indicate pseudoprogression.
According to recent studies, pseudoprogression can occur in metastatic lesions, such as those found in brain, liver, kidney, lung, pancreas, spleen and lymph node metastatic lesions of other primary tumors; it can also occur in primary lesions, such as lung cancer and renal cell cancer; and, in some patients, it can present with pleural effusion and ascites16,62-67. However, the exact details about the organ tendency of pseudoprogression are unknown. In addition, it is unclear whether there is organ tendency in sites with pseudoprogression; furthermore, if there is, it is unknown what drives organ tendency, the kinds of primary tumors, the immunogenicity of the organ or other factors. There is an urgent demand for accurate data on the incidence of pseudoprogression in every organ and in all kinds of tumors to guide clinical decisions and to identify pseudoprogression. We also want to know whether the different immunotherapy agents can influence the incidence of pseudoprogression, and, if there are, what are the factors that control these differences.
Although pseudoprogression has attracted much attention, only a few studies have focused on the clinical characteristics of patients who develop pseudoprogression. Nishino et al.27 reported that four patients with pseudoprogression were younger than the other patients (median age 46 years versus 63 years); moreover, the study also found lower tumor burden at baseline in patients with pseudoprogression than in the other patients. However, this study was not convincing enough because of the small sample size of patients with pseudoprogression27. In addition to the age of patients with pseudoprogression, other factors, such as sex, immune status and tumor characteristics, may be associated with pseudoprogression. We also seek to determine whether there is a difference in pseudoprogression with the use of first-line, second-line or further-line immunotherapy and whether the incidence of pseudoprogression is different when immunotherapy is combined with other treatments, such as chemotherapy, radiotherapy and targeted drug therapy.
How to distinguish pseudoprogression from real progression
Although many studies on pseudoprogression after immune checkpoint inhibitor therapy describe pseudoprogression in different kinds of tumors, until now, there has not been a uniform identification standard for pseudoprogression. New evaluation criteria, such as irRC, iRECIST, and imRECIST, were proposed to better identify pseudoprogression for tumor immunotherapy, but they all have some limitations; for example, there is a time delay for confirmation because these criteria need at least 4 weeks for identification after finding the first progression, which may lead to deterioration of the disease and the performance status of patient. Thus, the best opportunity for other treatments may be lost. The variability between the imaging method used, especially for small or new lesions, is also a limitation. There is a burning desire for the precise and timely diagnosis of pseudoprogression, and the next section discusses potential useful methods to identify pseudoprogression for immunotherapy in tumors.
Biopsies of enlarged lesions or new lesions
The combination of biopsy and histopathologic examination has always been the gold standard for tumor diagnosis and is also used to confirm pseudoprogression. Tabei et al.60 reported a patient with renal clear cell carcinoma treated with nivolumab who experienced enlarged pulmonary hila, para-aortic lymph nodes, subcutaneous lesions, and a new hepatic lesion. Histologic evaluation of excised subcutaneous lesions revealed extensive hemorrhaging in the tumor tissue and a large number of mature lymphocytes that infiltrated metastatic foci, leading to necrosis and/or cellular death; these factors were responsible for the enlarged lesions. This case was confirmed as pseudoprogression according to the histopathologic examination60. There are also other cases of progression that were confirmed as pseudoprogression by biopsy and histologic examination37,68,69. Although the combination of biopsy and histologic examination is the gold standard for pseudoprogression, there are also some limitations for its clinical use. First, biopsy is an invasive procedure that can result in serious complications, such as hemorrhage, infection and other specific complications according to the site of puncture. Second, poor compliance may cause a patient to refuse biopsy because of invasion or a fear of puncture; a poor performance status is also a contraindicatio for puncture. Third, although biopsy is a standard for pseudoprogression, there is also a possibility of puncture failure, which also differs according to the experience of the physician and the exact location of lesion in the patient. In addition, the appropriate time to perform a biopsy is also a challenge for physicians. The disadvantages mentioned above limit the use of biopsy.
Radiographic follow-up
Compared with biopsy, radiographic follow-up is a more common method for estimating pseudoprogression. Mamdani et al. reported a 79-year-old man with hepatocellular carcinoma who was treated with nivolumab. Baseline CT showed two liver lesions and no extrahepatic lesions, and the restaging CT assessment after 4 cycles of nivolumab showed an increase in the size of the liver lesions but no new lesions. The patient continued nivolumab treatment due to a lack of other effective treatments. After 8 cycles of nivolumab, physicians found a significant decline in tumor size and a continued decrease after 12 cycles. The patient remained stable until the last restaging (after 19 cycles; 9 months), which is a successful case of pseudoprogression that was monitored by radiographic follow-up70. There are also reports of some patients who experienced progression but were proven to have pseudoprogression via continuous immunotherapy and radiographic follow-up63,64,71. In addition to these case reports, some clinical trials selected patients who experienced PD after immunotherapy (presented with a better Karnofsky performance status (KPS) score, progression associated with the appearance of new lesions, normal lactate dehydrogenase concentrations, longer time to progression and a higher likelihood of disease control) to be treated beyond progression and found a decrease in target lesions at the follow-up; moreover, these patients who were treated beyond progression experienced longer OS than those not treated beyond progression, which also supports the use of continued immunotherapy in addition to standard radiographic follow-up72-75. To summarize the studies mentioned above, physicians usually continue treatment for patients who have a better KPS score and do not experience a deterioration in performance status while closely performing imaging follow-up. Clinical practice, the most common way to monitor pseudoprogression, offers several benefits. First, it is a convenient and noninvasive method for physicians and patients to monitor tumor evolution. Additionally, through continuous imaging, we can obtain an intuitive understanding of tumor growth kinetics. Second, in patients with pseudoprogression, continuous immunotherapy can achieve a better clinical benefit than discontinuing immunotherapy. Some shortcomings should not be ignored, such as continued immunotherapy can accelerate the progression when the patient experience a hyperprogression, which can also impede the use of effective treatment for those who are really progression. Some patients may also be worried about progression and ask to change treatment urgently, which exacerbates the difficulty of continuing immunotherapy. In summary, some disadvantages in continued immunotherapy and radiographic follow-up exist, but they also have incomparable advantages in the monitoring of pseudoprogression.
Imageologic examination
In addition to radiographic follow-up, there are also other radiographic examination methods that can be used to distinguish pseudoprogression. Serkova et al. summarized in vivo research and the clinical practice results of superparamagnetic iron oxide nanoparticles (SPIONs) in tumor-associated macrophage (TAM) imaging and concluded that SPIONs can serve as a T2-weighted contrast for MRI and be used to image TAMs76. Pseudoprogression mainly consists of inflammatory cells, necrotic tissue and edema. TAMs are sometimes a main component of tumor-infiltrating inflammatory cells59,77. The use of SPION T2-weighted contrast for MRI is a convenient way to image TAMs and identify inflammatory tissue. SPION T2-weighted contrast for MRI is believed to be an advantageous tool to identify inflammatory and enlarged tumor tissues, which can then be used to distinguish pseudoprogression and progression. This method can also be combined with positron emission tomography-computed tomography (PET-CT), as inflammatory tissue can sometimes contribute to the standardized uptake value (SUV) of PET-CT; therefore, perhaps a subtraction or other algorithms can be used to determine the SUV of PET-CT and of the MRI of SPION T2-weighted contrast. However, this is just speculation, and a large number of fundamental studies and clinical trials are urgently needed to confirm this hypothesis.
Imafuku et al. described two patients treated with nivolumab who experienced pseudoprogression as identified by ultrasonography (US)78. Both patients experienced an increase in lesion size but a decrease in blood flow ratio and continued nivolumab immunotherapy, and the lesion sizes gradually decreased. Ultimately, both patients were confirmed to have pseudoprogression. We conclude that US is another way to distinguish pseudoprogression and progression because of its advantage in blood flow detection, as blood flow can represent tumor growth in several ways. However, there are some limitations to the use of US. First, US can only be used in some superficial or subcutaneous lesions; for example, subcutaneous metastasis or metastasis in the regional lymph node, liver, many deep organs or organs with air cavities cannot be well detected. Second, the reliability of US can differ depending on the operator. Regardless of these shortcomings, the convenience and economic impact of US may accelerate the use of US in evaluating superficial organs or subcutaneous lesions to identify pseudoprogression.
In addition to MRI and US, PET-CT may also be a potential way to identify pseudoprogression, as it has been widely used to evaluate the tumor response to immunotherapy and was proposed by PERCIMT evaluation criteria79-81. Additional studies are expected to propose a better evaluation criterion for the identification of pseudoprogression.
Circulating tumor DNA
Circulating tumor DNA (ctDNA) is derived from tumor DNA following the apoptosis and/or necrosis of cancer cells in tumor patients and includes a variable fraction of circulating cell-free DNA82,83. The detection of ctDNA can be used to estimate tumor burden, tumor mutational burden (TMB), microsatellite instability (MSI) and some rare mutations84-87. Lee et al.88 performed a study to detect the association between immunotherapy pseudoprogression and the level of ctDNA. The study reported PD in 29 of 125 melanoma patients treated with immunotherapy, 9 of whom were confirmed to have pseudoprogression. All 9 patients with pseudoprogression had a favorable ctDNA profile, whereas all but 2 patients with true progression had an unfavorable ctDNA profile. There is a high sensitivity and specificity of ctDNA to predict pseudoprogression88. Goldberg et al.89 also reported a study comparing longitudinal changes in ctDNA levels with radiographic tumor size in 28 metastatic NSCLC patients receiving immune checkpoint inhibitor therapy. They found a strong consistency between the ctDNA response and the optimal radiographic response. The median time to the initial response among patients who achieved a response in both categories was 24.5 days and 72.5 days by ctDNA and imaging, respectively89. There are also some studies on the level of ctDNA and the tumor immunotherapy response, which revealed good consistency between ctDNA and tumor burden90-92.
To summarize the studies mentioned above, we can conclude that ctDNA has the potential to serve as a biomarker to reflect tumor burden and assess the efficacy of immunotherapy. Moreover, ctDNA can be used to distinguish pseudoprogression from progression, with high sensitivity and specificity; therefore, the ctDNA level could better distinguish pseudoprogression and progression than radiographic follow-up in some ways. First, ctDNA is released from the apoptosis and/or necrosis of cancer cells, which can reflect tumor burden; this was proven by the study mentioned above. It could be better than radiographic follow-up because an enlarged lesion can sometimes contain infiltrating immune cells, necrotic tissue and edema. Second, ctDNA can distinguish pseudoprogression and progression earlier than radiographic imaging because it reflects tumor burden in real time, while radiographic imaging can detect an enlarged lesion that has shrunk before the radiographic follow-up, which is responsible for the longer time needed to confirm pseudoprogression. Third, the detection of ctDNA is convenient for physicians and patients, as it only requires the collection of venous blood. Notwithstanding the superiorities that exist, there are some shortcomings of ctDNA. First, the number of studies on ctDNA and pseudoprogression is limited; until now, only a few studies have revealed a relationship between the two. More studies are needed to establish criteria for the tumor response using ctDNA; for example, a 50% reduction in ctDNA from baseline or an undetectable ctDNA level, as mentioned above, can be regarded as a ctDNA response88,89. Second, at present, the detection of ctDNA depends on next-generation sequencing, which is expensive for clinical use; this requires the creation of an economical detection method. In brief, ctDNA can be a useful way to distinguish pseudoprogression from progression, but it still has some limitations.
Serum IL-8 levels
Interleukin-8 (IL-8) was originally described as a chemokine that attracts the infiltrate of polymorphonuclear inflammatory leukocytes, but it is now known to be mainly produced by tumor cells and to exert a pro- and antitumor role through the formation of neuroendocrine tumors (NETs) in tumor patients93,94. Sanmamed et al.95 evaluated the relationship between changes in the serum IL-8 level and the response of melanoma and NSCLC to immunotherapy and found that a change in the serum IL-8 level can correctly reflect the tumor response to immunotherapy. Early decreases in serum IL-8 levels were associated with longer OS in patients with melanoma or NSCLC. In addition, in this study, changes in serum IL-8 also correctly reflected true responses in three patients who experienced pseudoprogression. Although there was an increase in the target lesions during pseudoprogression, the serum IL-8 level was continuously decreased compared to that at baseline, and a patient with increased serum IL-8 levels eventually developed PD95. We conclude that the IL-8 level may be a biomarker for the true tumor response and has the potential to distinguish pseudoprogression and progression. When a patient presents enlarged target lesions but a decrease in serum IL-8 levels, pseudoprogression must be considered. IL-8 detection is convenient and economical, and additional studies are needed to optimize the diagnostic criteria for the tumor response to immunotherapy when changes in serum IL-8 level are used.
Performance status of the patient
There are different opinions about the patient’s performance status and pseudoprogression. Some researchers believe that pseudoprogression is not accompanied by a deterioration in the clinical or performance status with weight loss, fever, night sweats, and increased pain; in fact, they believe these symptoms reflect true progression10. Additionally, others have reported that patients with a good performance status experienced pseudoprogression63,65. However, there are many case reports in which patients with pseudoprogression experienced clinical deterioration, such as weight loss and dyspnea16,71,96. In summary, the performance status is believed to be a clinical manifestation that can be the result of the mass effect caused by enlarged lesions or some effusion, which can be explained by either PD or enlarged lesions caused by the infiltration of inflammatory cells and/or the presence of necrotic tissue. The performance status could be a reference for clinical decisions but should not be a major factor in the determination of pseudoprogression. Perhaps there are some pseudoprogression-specific clinical manifestations we have not yet discovered, and additional studies are needed to better comprehend and manage patients with pseudoprogression.
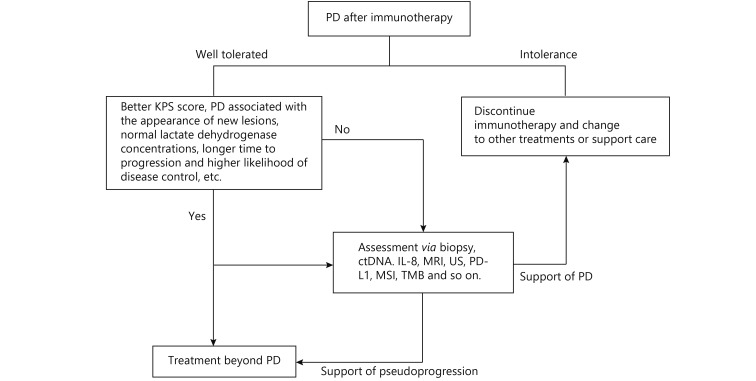
As mentioned above, there are many methods used to distinguish pseudoprogression and progression, and we summarize the advantages and disadvantages of these methods in Table 3. Among them, biopsy of enlarged lesions is a standard method; radiographic follow-up is the most commonly used method; ctDNA and IL-8 detection has the advantage of convenience; imageologic examination, such as MRI, PET-CT and US, has the potential to identify pseudoprogression; and the patient performance status is a reference for clinical decision making. In addition to these methods being mentioned for the identification of pseudoprogression, there are also some factors that should be considered when making clinical decisions, including TMB, PD-L1 expression, MSI, the T cell invigoration to tumor burden ratio, and changes in tumor biomarkers, such as CEA, CA-125, and CA-19997-101. In our opinion, due to the lack of enough information on pseudoprogression, all the biomarkers used to predict the tumor response to immunotherapy can be used as references when determining whether a patient has PD or pseudoprogression, and a diagnosis and treatment process for PD after immunotherapy can be determined according to the methods mentioned above (Figure 2).
3.
Advantages and disadvantages of methods to diagnose pseudoprogression after immunotherapy
| Methods to identify pseudoprogression | Type of tumor | Advantages | Disadvantages |
| NSCLC, non-small cell lung cancer; IL-8, interleukin-8; US, ultrasonography. | |||
| Biopsies of enlarged lesions or new lesions | Enlarged visible lesions that can be biopsied | Can provide the histopathology for the physician to judge the evolution of tumor and guide the clinical practice | Invasive procedure and may be refused by the patient. The biopsy tissue does not represent the whole lesion sometimes |
| Radiographic follow-up | All kinds of tumors with measurable lesions | Convenient and noninvasive and can avoid a premature discontinued immunotherapy for pseudoprogression | Can accelerate progression if the patient experiences hyperprogression; can impede the use of effective treatments for those who are experiencing true progression |
| SPION T2-weighted contrast MRI + PET-CT | Suitable for all kinds of tumors (theoretically) | Can distinguish inflammatory cell infiltration from the enlarged tumor tissue | Theoretical method; no clinical studies have been performed to prove its effectiveness |
| US | Superficial or subcutaneous lesions | Convenient and economical, and can distinguish blood flow volume in the lesions | Reliability can differ depending on the operator |
| Circulating tumor DNA | Melanoma | Consistent with tumor burden and reflects the dynamic change of the tumor | No current criteria for the diagnosis of pseudoprogression; high costs limit clinical use |
| Serum IL-8 levels | Tumor cells of patients; positively correlate with tumor burden | Convenient and economical, the dynamic monitor of IL-8 can reflect the change in tumor burden | Theoretical methods; no published clinical studies prove the correlation between IL-8 and pseudoprogression |
2.
The diagnosis and treatment process for patients with progressive disease with suspicion of pseudoprogression.
Prognosis of pseudoprogression
Only a few studies have focused on prognosis, but they have not reached a consensus about the prognosis of pseudoprogression; several studies reported better OS in patients with pseudoprogression than those with PD27,28,31,34. Tazdait et al.34 described 20 of 160 NSCLC patients treated with immunotherapy who experienced an atypical response; among them, 8 experienced pseudoprogression, and they observed longer OS in patients with pseudoprogression than in those with confirmed PD (9.8 months versus 6.1 months). Fujimoto et al.31 reported that 3% (14/542) of NSCLC patients treated with nivolumab experienced pseudoprogression and that these patients had significantly longer OS than those with PD (not reached versus 6.4 months, respectively) and longer OS than patients who responded (not reached versus 20.1 months), although the difference was not significant. Kurra et al. retrospectively assessed 365 tumor patients treated with anti-PD1, anti-PD-L1 and anti-CTLA4 and found that the 1-year OS was 58%, 82%, 33% and 81% for patients with SD, PR, PD and pseudoprogression when evaluated with irRC. This finding supports a better prognosis of pseudoprogression than SD and PD102. Upon reviewing the mechanism of pseudoprogression, we know that pseudoprogression can be caused by immune cell infiltration, necrosis, hemorrhage and edema; among these, immune cell infiltration indicates a good response to immunotherapy9. Therefore, by estimating immune cell infiltration, we can infer that pseudoprogression may represent a better response to immunotherapy than other response patterns and that a better response pattern also portends a better prognosis. Based on the studies and mechanisms mentioned above, patients with pseudoprogression have a better prognosis than those with true progression or stable disease, but whether it is better than that in patients who achieve a PR or CR after immunotherapy is not clear. We expect that further studies will provide more information about the prognosis and propose better management strategies for patients with pseudoprogression.
Conclusions
As it is an uncommon phenomenon, pseudoprogression is an atypical response that exists not only in tumors treated with immunotherapy but also in malignant gliomas treated with chemoradiotherapy and in tumors treated with tyrosine kinase inhibitors13,103. As a clinical phenomenon, pseudoprogression is a challenge for both physicians and patients. In this article, we mainly introduce the definition, evaluation criteria, mechanism, recognition and prognosis of tumor pseudoprogression after immunotherapy. To date, research on pseudoprogression remains limited, and there is a lack clear knowledge of the relationship between clinical and tumor characteristics. What is responsible for the different incidences of pseudoprogression for different tumors is still not clear. The identification of pseudoprogression is a vital issue for clinical physicians. Although some progress has been made, consistent criteria for monitoring pseudoprogression have not been established, and comprehensive evaluation criteria is required before implementation in clinical practice. As observed in several studies, the prognosis of patients with pseudoprogression is better than that of patients with true PD or SD, but whether it is better than that in patients who achieve a PR or CR to immunotherapy is not clear. Thus far, the exact mechanism(s) of pseudoprogression are unclear, although it is known that enlarged lesions of pseudoprogression consist of infiltrating immune cells, hemorrhage, necrosis and edematous tissue. However, the specific molecular mechanisms involved in pseudoprogression are unclear, as MDM2/MDM4 amplification is involved in hyperprogression after immunotherapy104. It is possible that specific molecules in the infiltrating immune cells exist and can be activated more easily by immunotherapy.
When assessing the response via radiographic follow-up, a patient can present an enlarged lesion that shrinks at the follow-up assessment, but it is unknown whether the enlarged lesion consists of immune cell infiltrate and necrotic tissue or only enlarged tumor tissue (which is true progression) that shows a response at the following assessment. The composition of the enlarged lesions cannot be assessed except by biopsy and histologic examination. It is possible that some patients experience true progression but respond later, although we do not have such a report; therefore, it is difficult to determine whether a shrunken lesion represents pseudoprogression or true progression with a delayed response. Considering the mechanism of pseudoprogression, the infiltration of immune cells can reflect the activation of immune cells, and hemorrhage, necrosis and edematous tissue can also reflect a response to treatment; however, there is no report on whether any difference (e.g., in incidence or prognosis) between pseudoprogression and true progression with a delayed response exists.
Although there has been great progress in tumor pseudoprogression after immunotherapy, many problems also urgently need to be solved, and we expect further studies to elucidate the mechanism of pseudoprogression and provide better management for patients with pseudoprogression.
Acknowledgements
This work was support by the National Key Research and Development Program of China (Grant No. 2018YFC1313201).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Hui Zhu, Email: drzhuh@126.com.
Jinming Yu, Email: sdyujinming@163.com.
References
- 1.Ribas A, Wolchok JD Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balar AV, Weber JS PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol, Immunother. 2017;66:551–64. doi: 10.1007/s00262-017-1954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley RS, June CH, Langer R, Mitchell MJ Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui RN, Csőszi T, Fülöp A, et al Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.NCCN clinical practice guidelines in oncology non-small cell lung cancer version 5.[Accessed June 7, 2019]. <a href="https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf">https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf</a>.
- 8.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui RN, et al Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 9.Wang QH, Gao J, Wu X Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125–35. doi: 10.1016/j.intimp.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Baxi SS, Dunn LA, Burtness BA Amidst the excitement: a cautionary tale of immunotherapy, pseudoprogression and head and neck squamous cell carcinoma. Oral Oncol. 2016;62:147–8. doi: 10.1016/j.oraloncology.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Onesti CE, Frères P, Jerusalem G Atypical patterns of response to immune checkpoint inhibitors: interpreting pseudoprogression and hyperprogression in decision making for patients' treatment. J Thorac Dis. 2019;11:35–8. doi: 10.21037/jtd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanjanapan Y, Day D, Wang L, Al-Sawaihey H, Abbas E, Namini A, et al Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer. 2019;125:1341–9. doi: 10.1002/cncr.31999. [DOI] [PubMed] [Google Scholar]
- 13.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 14.Reckamp KL Real-world pseudoprogression: an uncommon phenomenon. J Thorac Oncol. 2018;13:880–2. doi: 10.1016/j.jtho.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Nishino M Pseudoprogression and measurement variability. J Clin Oncol. 2016;34:3480–1. doi: 10.1200/JCO.2016.67.6759. [DOI] [PubMed] [Google Scholar]
- 16.Hochmair MJ, Schwab S, Burghuber OC, Krenbek D, Prosch H Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer. 2017;113:4–6. doi: 10.1016/j.lungcan.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol, Immunother. 2009;58:1297–306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al Evaluation of immune-related response criteria and recist v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 20.O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 21.Di Giacomo AM, Danielli R, Calabrò L, Bertocci E, Nannicini C, Giannarelli D, et al Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol, Immunother. 2011;60:467–77. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Chesney JA, Gordon MS, Abernethy AP, Logan TF, Lawson DH, et al Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:236. doi: 10.1186/1479-5876-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millward M, Underhill C, Lobb S, McBurnie J, Meech S J, Gomez-Navarro J, et al Phase I study of tremelimumab (CP-675206) plus PF-3512676(CPG 7909) in patients with melanoma or advanced solid tumours. Br J Cancer. 2013;108:1998–2004. doi: 10.1038/bjc.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 26.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 27.Nishino M, Giobbie-Hurder A, Manos MP, Bailey N, Buchbinder EI, Ott PA, et al Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res. 2017;23:4671–9. doi: 10.1158/1078-0432.CCR-17-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980–7. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, et al Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol. 2019;14:468–74. doi: 10.1016/j.jtho.2018.10.167. [DOI] [PubMed] [Google Scholar]
- 32.Kim HK, Heo MH, Lee HS, Sun JM, Lee SH, Ahn JS, et al Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother Pharmacol. 2017;80:591–8. doi: 10.1007/s00280-017-3396-4. [DOI] [PubMed] [Google Scholar]
- 33.Katz SI, Hammer M, Bagley SJ, Aggarwal C, Bauml JM, Thompson JC, et al Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:978–86. doi: 10.1016/j.jtho.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari F, et al Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 35.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013–20. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al Atezolizumab, an Anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 37.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al Safety and Efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–25. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol, Immunother. 2012;61:41–8. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabro L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, et al Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3:301–9. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 44.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doherty MK, Jao K, Shepherd FA, Hazrati LN, Leighl NB Central nervous system pseudoprogression in a patient treated with PD-1 checkpoint inhibitor. J Thorac Oncol. 2015;10:e100–1. doi: 10.1097/JTO.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 46.Lewis GD, Jonasch E, Shah AY, Fuller GN, Farach AM, Butler EB, et al Renal cell carcinoma brain metastasis with pseudoprogression and radiation necrosis on nivolumab after previous treatment with stereotactic radiosurgery: an illustrative case report and review of the literature. Pract Radiat Oncol. 2018;8:e262–e5. doi: 10.1016/j.prro.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki Y, Shindoh J, Miura Y, Nakajima H, Oki R, Uchiyama M, et al Serial pseudoprogression of metastatic malignant melanoma in a patient treated with nivolumab: a case report. BMC Cancer. 2017;17:778. doi: 10.1186/s12885-017-3785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melian M, Lorente D, Aparici F, Juan O Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac Cancer. 2018;9:1770–3. doi: 10.1111/tca.2018.9.issue-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoogstraten B, Miller AB, Staquet M, Winkler A. WHO handbook for reporting results of cancer treatment. WHO Offset Publication no.48, Geneva: WHO, 1979.
- 50.Miller AB, Hoogstraten B, Staquet M, Winkler A Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 51.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 52.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Nishino M Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a "common language" for the new arena of cancer treatment. J Immunother Cancer. 2016;4:30. doi: 10.1186/s40425-016-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Schwartz LH, et al iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al Immune-Modified Response Evaluation Criteria in Solid Tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–8. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 56.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–43. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rebuzzi SE, Bregni G, Grassi M, Damiani A, Buscaglia M, Buti S, et al Immunotherapy beyond progression in advanced renal cell carcinoma: a case report and review of the literature. Immunotherapy. 2018;10:1123–32. doi: 10.2217/imt-2018-0042. [DOI] [PubMed] [Google Scholar]
- 58.Cohen JV, Alomari AK, Vortmeyer AO, Jilaveanu LB, Goldberg SB, Mahajan A, et al Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4:179–82. doi: 10.1158/2326-6066.CIR-15-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocha P, Hardy-Werbin M, Naranjo D, Taus Á, Rodrigo M, Zuccarino F, et al CD103+CD8+ lymphocytes characterize the immune infiltration in a case with pseudoprogression in squamous NSCLC. J Thorac Oncol. 2018;13:e193–6. doi: 10.1016/j.jtho.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Tabei T, Tsuura Y, Kobayashi K Pseudoprogression: a case of metastatic renal clear cell carcinoma treated with nivolumab. Pathol Int. 2018;68:627–9. doi: 10.1111/pin.v68.11. [DOI] [PubMed] [Google Scholar]
- 61.Tanizaki J, Hayashi H, Kimura M, Tanaka K, Takeda M, Shimizu S, et al Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44–8. doi: 10.1016/j.lungcan.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Swami U, Smith M, Zhang J Central nervous system pseudoprogression with nivolumab in a patient with squamous cell lung cancer followed by prolonged response. J Thorac Oncol. 2018;13:e183–4. doi: 10.1016/j.jtho.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Yoshimura A, Takumi C, Tsuji T, Hamashima R, Shiotsu S, Yuba T, et al Pulmonary pleomorphic carcinoma with pseudoprogression during nivolumab therapy and the usefulness of tumor markers: a case report. Clin Case Rep. 2018;6:1338–41. doi: 10.1002/ccr3.2018.6.issue-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong AS, Thian YL, Kapur J, Leong CN, Kee P, Lee CT, et al Pushing the limits of immune-related response: a case of "extreme pseudoprogression". Cancer Immunol, Immunother. 2018;67:1105–11. doi: 10.1007/s00262-018-2167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chae YK, Wang S, Nimeiri H, Kalyan A, Giles FJ Pseudoprogression in microsatellite instability-high colorectal cancer during treatment with combination T cell mediated immunotherapy: a case report and literature review. Oncotarget. 2017;8:57889–97. doi: 10.18632/oncotarget.18361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu GH, Chen T, Li RH, Zhu L, Liu DY, Ding ZY Well-controlled pleural effusion indicated pseudoprogression after immunotherapy in lung cancer: a case report. Thorac Cancer. 2018;9:1190–3. doi: 10.1111/tca.2018.9.issue-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sweis RF, Zha YY, Pass L, Heiss B, Chongsuwat T, Luke JL, et al Pseudoprogression manifesting as recurrent ascites with anti-PD-1 immunotherapy in urothelial bladder cancer. J Immunother Cancer. 2018;6:24. doi: 10.1186/s40425-018-0334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curioni-Fontecedro A, Ickenberg C, Franzen D, Rogler G, Burger IA, van den Broek M Diffuse pseudoprogression in a patient with metastatic non-small-cell lung cancer treated with Nivolumab. Ann Oncol. 2017;28:2040–1. doi: 10.1093/annonc/mdx233. [DOI] [PubMed] [Google Scholar]
- 69.Izumida T, Kawagishi Y, Tsuji H Pseudoprogression in lung adenocarcinoma during treatment with nivolumab. BMJ Case Rep. 2017:2017. doi: 10.1136/bcr-2017-219919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mamdani H, Wu H, O'Neil BH, Sehdev A Excellent response to Anti-PD-1 therapy in a patient with hepatocellular carcinoma: case report and review of literature. Discov Med. 2017;23:331–6. [PubMed] [Google Scholar]
- 71.Yoshida T, Furuta H, Hida T Risk of tumor flare after nivolumab treatment in patients with irradiated field recurrence. Med Oncol. 2017;34:34. doi: 10.1007/s12032-017-0895-4. [DOI] [PubMed] [Google Scholar]
- 72.Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, et al Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3:1511–9. doi: 10.1001/jamaoncol.2017.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, et al Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229–39. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2:1179–86. doi: 10.1001/jamaoncol.2016.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, et al Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72:368–76. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 76.Serkova NJ Nanoparticle-based magnetic resonance imaging on tumor-associated macrophages and inflammation. Front Immunol. 2017;8:590. doi: 10.3389/fimmu.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;379:1976–86. doi: 10.1056/NEJMc1812159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imafuku K, Hata H, Kitamura S, Yanagi T, Shimizu H Ultrasonographic findings can identify 'pseudoprogression' under nivolumab therapy. Br J Dermatol. 2017;177:1726–31. doi: 10.1111/bjd.15198. [DOI] [PubMed] [Google Scholar]
- 79.Sachpekidis C, Larribere L, Pan LY, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42:386–96. doi: 10.1007/s00259-014-2944-y. [DOI] [PubMed] [Google Scholar]
- 80.Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab . Eur J Nucl Med Mol Imaging. 2018;45:376–83. doi: 10.1007/s00259-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 81.Dimitrakopoulou-Strauss A Monitoring of patients with metastatic melanoma treated with immune checkpoint inhibitors using PET-CT. Cancer Immunol, Immunother. 2019;68:813–22. doi: 10.1007/s00262-018-2229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin . Cell. 2016;164:57–68. doi: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun K, Jiang PY, Chan KCA, Wong J, Cheng YKY, Liang RHS, et al Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci USA. 2015;112:E5503–12. doi: 10.1073/pnas.1508736112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cabel L, Proudhon C, Romano E, Girard N, Lantz O, Stern MH, et al Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol. 2018;15:639–50. doi: 10.1038/s41571-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 85.Siravegna G, Marsoni S, Siena S, Bardelli A Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 86.Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 87.Alix-Panabières C, Pantel K Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–91. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Long GV, Menzies AM, Lo S, Guminski A, Whitbourne K, et al Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018;4:717–21. doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, et al Early assessment of lung cancer immunotherapy response via circulating tumor DNA . Clin Cancer Res. 2018;24:1872–80. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guibert N, Mazieres J, Delaunay M, Casanova A, Farella M, Keller L, et al Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma. Oncotarget. 2017;8:38056–60. doi: 10.18632/oncotarget.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen TJ, Goodman AM, Kato S, Ellison CK, Daniels GA, Kin L, et al Genome-wide sequencing of cell-free DNA identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol Cancer Therapeut. 2019;18:448–58. doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]
- 92.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2:42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez-Aparicio M, Alfaro C Influence of interleukin-8 and Neutrophil Extracellular Trap (NET) formation in the tumor microenvironment: is there a pathogenic role? Journal of Immunology Research. 2019;2019:6252138. doi: 10.1155/2019/6252138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. doi: 10.1016/j.ctrv.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al Changes in serum interleukin-8(IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988–95. doi: 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barnet MB, Zielinski RR, Warby A, Lewis CR, Kao S Pseudoprogression associated with clinical deterioration and worsening quality of life in malignant pleural mesothelioma. J Thorac Oncol. 2018;13:e1–2. doi: 10.1016/S1556-0864(18)33440-3. [DOI] [PubMed] [Google Scholar]
- 97.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 99.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 100.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–5. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuo ML, Chen HX, Zhang TZ, Yang X, Zhong J, Wang YY, et al The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer Biomark. 2018;22:467–76. doi: 10.3233/CBM-171089. [DOI] [PubMed] [Google Scholar]
- 102.Kurra V, Sullivan RJ, Gainor JF, Hodi FS, Gandhi L, Sadow CA, et al Pseudoprogression in cancer immunotherapy: rates, time course and patient outcomes. J Clin Oncol. 2016;34:6580. doi: 10.1200/JCO.2016.34.15_suppl.6580. [DOI] [Google Scholar]
- 103.Kelly PJ, Weiss SE, Sher DJ, Perez-Atayde AR, Dal Cin P, Choueiri TK Sunitinib-induced pseudoprogression after whole-brain radiotherapy for metastatic renal cell carcinoma. J Clin Oncol. 2010;28:e433–5. doi: 10.1200/JCO.2009.26.8193. [DOI] [PubMed] [Google Scholar]
- 104.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]