Abstract
Integrated exposure to polycyclic aromatic hydrocarbons (PAHs) can be assessed through monitoring of urinary mono-hydroxylated PAHs (OH-PAHs). The aim of this study was to provide the first assessment of exposure to PAHs in a large sample of the population in Queensland, Australia including exposure to infant (0–4 years). De-identified urine specimens, obtained from a pathology laboratory, were stratified by age and sex, and pooled (n = 24 pools of 100) and OH-PAHs were measured by gas chromatography-isotope dilution-tandem mass spectrometry. Geometric mean (GM) concentrations ranged from 30 ng/L (4-hydroxyphenanthrene) to 9221 ng/L (1-naphthol). GM of 1-hydroxypyrene, the most commonly used PAH exposure biomarker, was 142 ng/L. The concentrations of OH-PAHs found in this study are consistent with those in developed countries and lower than those in developing countries. We observed no association between sex and OH-PAH concentrations. However, we observed lower urinary concentrations of all OH-PAHs in samples from infants (0–4 years), children (5–14 years) and the elderly (>60 year old) compared with samples from other age groups (15–29, 30–44 and 45–59 years) which may be attributed to age-dependent behaviour-specific exposure sources.
Keywords: OH-PAHs, Urinary metabolite, Biomonitoring, Infant, Exposure monitoring
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs), a class of hazardous pollutants, are produced predominantly during the incomplete combustion of organic materials naturally or by human activities (e.g., vehicular emissions, power plants, wood smoke, bush fires). Several PAHs have been classified as probable human carcinogens (IARC, 2010; Kim et al., 2013). Recent findings suggest a relationship between PAHs in placenta and the risk of neural tube defects, and with alteration of the immune system (Langlois et al., 2012; Walker et al., 2013). Other epidemiological studies also revealed associations between exposure to PAHs and childhood obesity and behavioural changes (Perera et al., 2014; Scinicariello and Buser, 2014).
Human exposure to PAHs may occur via inhalation, ingestion, and dermal absorption. The major sources of exposure to PAHs in non-occupational settings are smoking, grilled food and air pollution (Srogi, 2007). Biomonitoring provides an aggregate exposure estimate, integrating exposures from all sources and routes, including those that are hard to measure such as hand to mouth transfer in children (Sexton et al., 2004). Biomonitoring of PAH exposure has been carried out through the measurement of urinary mono-hydroxylated PAH (OH-PAH), a group of PAH metabolites (Jacob and Seidel, 2002), with 1-hydroxypyrene being the most commonly used PAH biomarker of exposure (Hansen et al., 2008).
There have been many biomonitoring studies using PAH metabolites for assessing PAH exposure, but these studies are typically limited to occupational settings, or specific sub-populations (Srogi, 2007). Fewer than 10 countries have conducted large-scale monitoring of PAH exposure in the general population (Bartolomé et al., 2015). Systematic monitoring of environmental pollutants in the general population provides the basis for future epidemiological studies to examine burden of disease or to provide evidence for the effectiveness of policies aiming at reducing chemical exposure (CDC, 2015; Health Canada, 2013; Toms et al., 2014; Toms et al., 2012). For example, US National Health and Nutrition Examination Survey (NHANES) data (2001–2006) for OH-PAHs have been used to investigate associations between exposure to PAHs and childhood obesity (Scinicariello and Buser, 2014). Although there have been some biomonitoring studies conducted in the general Australian population for other chemicals, including polyfluorinated alkyl substances and brominated flame retardants (Toms et al., 2014; Toms et al., 2012), to our knowledge there are no data on PAH exposure except for a small group of nine people (Thai et al., 2015).
While biomonitoring is regarded as the gold standard for exposure assessment (Sexton et al., 2004), measuring individual samples in general population studies involves substantial financial investment. Appropriately pooled biological samples can be used in cross-sectional studies to monitor temporal and spatial trends at the population level with considerably fewer resource requirements (Heffernan et al., 2014a; Toms et al., 2014). The aim of this study is to provide the first assessment of PAH exposure in a large subsection of the Australian population through the quantification of OH-PAH metabolites in pooled urine samples.
2. Materials and methods
2.1. Study population, sample collection and pooling protocol
De-identified urine samples were obtained from a community-based pathology laboratory from surplus stored specimens that had been collected and analysed as part of routine clinical pathology testing in Queensland, Australia. Urine samples were collected in sterile polyethylene specimen containers, refrigerated for up to three days, and then frozen. Descriptive information about each sample included donor’s sex, birthdate, and date of sample collection. Before pooling, samples were stratified by age (calculated from the birthdate and date of urine collection) and sex into six age strata: 0–4, 5–14, 15–29, 30–44, 45–59, and ≥60 years. The mean age of each pool was calculated from the average age of the individuals making up that pool. A total of 2400 individual samples were combined into 24 pools, with 100 individual samples contributing to each pool, with two replicate pools for each sex by age strata (Table 1). Samples were pooled based on volume, where each individual in the pool contributed the same volume (1 mL), thus the concentration measured in each pool is equivalent to the arithmetic mean of the concentration in each individual sample contributing to the pool (Caudill, 2010; Mary-Huard, 2007). To make the pools, individual urine samples were thawed, mixed well and aliquoted, after which the pooled sample was homogenised, divided into smaller aliquots and frozen until analysis. No measurements of creatinine or specific gravity were available. Sample collection and pooling occurred from November 2012 to November 2013. This work was approved by the University of Queensland ethics committee (approval number 2013000397). The involvement of the Centers for Disease Control and Prevention (CDC) laboratory (Atlanta, GA, USA) was determined not to constitute engagement in human subject research.
Table 1.
Summary of pool characteristics and concentrations of OH-PAHs (ng/L) per strata. Each pool represents 100 individuals. Shaded rows are female pools.
| Pool # | Age strata (years) | Av. Age (years) | Urinary concentration (ng/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–NAP | 2–NAP | 2–FLU | 3–FLU | 9–FLU | 1–PHE | 2–PHE | 3–PHE | 4–PHE | 1–PYR | 1–NAP/2–NAP | ΣOH–PAHs | |||
| 1 | 0–4 | 2.93 | 2365 | 2804 | 111 | 51 | 196 | 113 | 29 | 54 | 18 | 130 | 0.84 | 5871 |
| 2 | 2.74 | 2453 | 3233 | 119 | 54 | 140 | 82 | 26 | 48 | 15 | 106 | 0.76 | 6276 | |
| 3 | 3.33 | 4002 | 2663 | 129 | 64 | 209 | 141 | 34 | 65 | 24 | 156 | 1.50 | 7487 | |
| 4 | 3.24 | 924 | 2698 | 95 | 50 | 135 | 83 | 28 | 52 | 15 | 115 | 0.34 | 4195 | |
| 5 | 5–14 | 8.83 | 1644 | 2838 | 185 | 89 | 239 | 123 | 53 | 82 | 21 | 164 | 0.58 | 5438 |
| 6 | 9.21 | 1227 | 3521 | 135 | 62 | 142 | 79 | 40 | 60 | 12 | 115 | 0.35 | 5393 | |
| 7 | 8.74 | 1654 | 2456 | 140 | 59 | 204 | 97 | 45 | 63 | 15 | 142 | 0.67 | 4875 | |
| 8 | 9.54 | 1615 | 3069 | 168 | 78 | 270 | 160 | 47 | 82 | 23 | 122 | 0.53 | 5634 | |
| 9 | 15–29 | 24.3 | 5490 | 5957 | 463 | 261 | 302 | 127 | 82 | 113 | 30 | 190 | 0.92 | 13,015 |
| 10 | 24.0 | 45,308 | 5461 | 501 | 276 | 328 | 152 | 88 | 117 | 32 | 235 | 8.30 | 52,498 | |
| 11 | 24.0 | 5683 | 8104 | 634 | 374 | 478 | 208 | 102 | 119 | 50 | 270 | 0.70 | 16,022 | |
| 12 | 23.4 | 21,957 | 6035 | 347 | 164 | 332 | 131 | 78 | 80 | 36 | 178 | 3.64 | 29,338 | |
| 13 | 30–44 | 37.8 | 30,136 | 6122 | 652 | 330 | 501 | 201 | 130 | 151 | 54 | 307 | 4.92 | 38,584 |
| 14 | 37.3 | 6660 | 7107 | 460 | 274 | 432 | 151 | 82 | 114 | 34 | 198 | 0.94 | 15,512 | |
| 15 | 36.7 | 115,397 | 7720 | 513 | 276 | 439 | 157 | 81 | 92 | 47 | 203 | 14.95 | 124,925 | |
| 16 | 36.8 | 3749 | 5192 | 390 | 234 | 312 | 147 | 68 | 86 | 39 | 170 | 0.72 | 10,387 | |
| 17 | 45–59 | 52.9 | 79,452 | 4955 | 458 | 259 | 358 | 130 | 77 | 87 | 34 | 115 | 16.04 | 85,925 |
| 18 | 53.2 | 24,661 | 5702 | 555 | 334 | 770 | 183 | 113 | 151 | 50 | 245 | 4.33 | 32,764 | |
| 19 | 53.3 | 375,182 | 6444 | 402 | 236 | 485 | 278 | 104 | 136 | 40 | 150 | 58.22 | 383,457 | |
| 20 | 53.0 | 50,690 | 5673 | 478 | 253 | 570 | 159 | 83 | 101 | 65 | 132 | 8.94 | 58,204 | |
| 21 | ≥60 | 73.7 | 20,562 | 3102 | 188 | 80 | 349 | 133 | 54 | 66 | 40 | 76 | 6.63 | 24,650 |
| 22 | 71.9 | 6077 | 2695 | 265 | 117 | 340 | 143 | 63 | 74 | 40 | 96 | 2.26 | 9910 | |
| 23 | 75.1 | 26,736 | 2196 | 110 | 46 | 197 | 91 | 40 | 43 | 22 | 57 | 12.18 | 29,538 | |
| 24 | 76.1 | 3677 | 2017 | 129 | 60 | 218 | 105 | 37 | 42 | 29 | 50 | 1.82 | 6364 | |
| Geometric mean | 9221 | 4104 | 261 | 132 | 299 | 134 | 60 | 81 | 30 | 142 | ||||
2.2. Urine analysis
Pooled urine aliquots were shipped on dry ice to the CDC and analysed for ten OH-PAHs using a modification of the gas chromatography–isotope dilution-tandem mass spectrometry method described previously (Li et al., 2014). Briefly, urine samples (1 mL) were spiked with 13C-labelled internal standards and sodium acetate buffer containing β-glucuronidase/sulfatase to hydrolyse urinary conjugates overnight at 37 °C. The target analytes were then extracted through semi-automated liquid–liquid extraction using a Gilson 215 Liquid Handler (Gilson Inc., Middleton, WI, USA). The extracts were evaporated, and the target analytes were derivatised, separated, and quantified on an Agilent 7890 gas chromatograph coupled with an Agilent 7000B triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The 10 OH-PAHs determined were metabolites of naphthalene, 1-naphthol (1-NAP) and 2-naphthol (2-NAP); of fluorene, 2-hydroxyfluorene (2-FLU), 3-hydroxyfluorene (3-FLU), 9-hydroxyfluorene (9-FLU); of phenanthrene, 1-hydroxyphenanthrene (1-PHE), 2-hydroxyphenanthrene (2-PHE), 3-hydroxyphenanthrene (3-PHE), 4-hydroxyphenanthrene (4-PHE); and of pyrene, 1-hydroxypyrene (1-PYR). The limits of detection were 44 ng/L for 1-NAP, 40 ng/L for 2-NAP, and 10 ng/L for the remaining OH-PAHs. As described elsewhere (Li et al., 2014), analyses were subject to a series of quality control (QC) and quality assurance checks, including a multi-rule QC evaluation for each analytical run, and relative retention time and 13C-internal standard spiking accuracy checks for each analyte in each sample.
3. Results
The 10 PAH metabolites measured were detected in all samples. Individual results are presented in Table 1. The concentrations varied between different OH-PAHs with geometric means (GM) ranging from 30 ng/L (4-PHE) to 9221 ng/L (1-NAP). GM mean concentration of 1-PYR, the most commonly used PAH exposure biomarker, was 142 ng/L.
For each metabolite the range of concentrations among age groups was relatively small, with the ratio of maximum to minimum concentrations ranging from 4 to 8, except for 1-NAP for which concentrations varied widely (924 to 375,182 ng/L). The concentration of 1-NAP in two pooled samples (Pools 15 and 19, Table 1) was two orders of magnitude higher than the GM (9221 ng/L). The highest concentrations of 1-NAP were detected in the 45–59 years age strata (7900–375,000 ng/L, Fig. 1A).
Fig. 1.
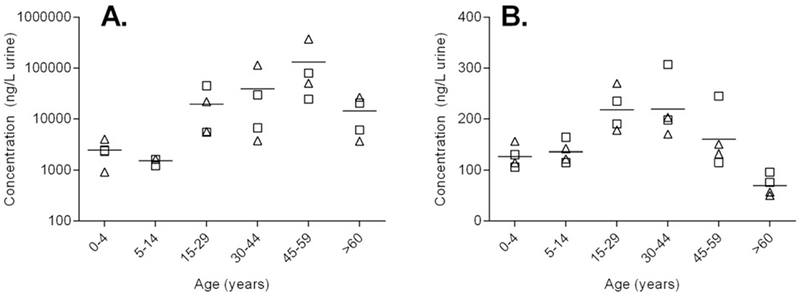
Urinary concentration versus age (years) for 1-NAP (A, ng/L) and 1-PYR (B, ng/L). Triangles denote female pools, squares denote male pools. Horizontal line indicates mean concentration of four pools in each age strata. Note log axis for 1-NAP.
Representative plots of concentration versus age are shown for 1-NAP and 1-PYR in Fig. 1. In general, concentrations of OH-PAHs appeared to be higher in the 15–59 years adolescents and adult demographic groups, with lower concentrations in infants (0–4 years), children (5–14 years) and the elderly (>60 years). Age-concentration profiles for the remaining metabolites can be viewed in the supplementary material (Fig. S1). Visual inspection of the data suggests a non-linear relationship between urinary concentration and age for the studied PAH metabolites. There appears to be no significant differences in concentration of male and female pools.
The total concentration of OH-PAHs (ΣOH-PAHs — sum of all measured metabolites) among pools ranged from 4195 to 383,457 ng/L with the combined concentration of 1-NAP and 2-NAP accounting for 82–99% of ΣOH-PAHs value (Table 1). Contribution of metabolites from each parent PAH, e.g. 1-NAP and 2-NAP for naphthalene, to ΣOH-PAHs was naphthalene > fluorene > phenanthrene > pyrene, and was similar to the profile reported for the US population (Li et al., 2008; CDC, 2015).
4. Discussion
4.1. Concentration and age/sex trends
This study presents the first OH-PAH biomonitoring data for infants <3 years and together with the Canadian Health Measures Survey (CHMS) provides some of the first data on PAH exposures in young children (3–6 years) ( Health Canada, 2013). Infants and young children are often excluded from biomonitoring campaigns due to logistical challenges with sample collection and recruitment. For example, NHANES in the USA only measured urinary OH-PAHs in participants > 6 years (CDC, 2015); other studies (e.g., in Korea) did not include infants and children (Sul et al., 2012).
Urinary concentrations of all OH-PAHs measured were lower in infants (0–4 years), children (5–14 years) and the elderly (>60 years) than in adolescent and adult(15–29; 30–44; 45–59 years) demographic groups (Fig. 1). Similar age-dependent profiles have been reported in CHMS II (Health Canada, 2013). The lower concentrations at these ages may reflect lower exposure to PAHs and thus potentially reduce the risk of adverse health effects among infants and the elderly, two population groups who are more susceptible than adults to negative impacts following pollutant exposure (Perera et al., 2014).
The non-linear relationship between OH-PAH concentration and age suggests adult-specific behaviours or exposure sources to PAHs that are not experienced by the other age groups. For example, smoking rates are higher in adolescents and adults than among the other age groups in Australia (ABS, 2013). Because smoking is a major source of PAH exposure among the non-occupationally exposed general population (Srogi, 2007), the different smoking rates by age groups may have contributed to the age-dependent profile of OH-PAH concentrations seen in this study. Regrettably, we could not assess the actual impact of smoking on the measured concentrations of OH-PAHs because measurement of urinary cotinine (nicotine’s major metabolite) for individual specimens was unavailable.
We observed no association between sex and OH-PAH urinary concentrations. This finding is consistent with studies from Israel, Spain and the United States that reported no difference in OH-PHEs and OH-PYR among males and females (Levine et al., 2015; Bartolomé et al., 2015; CDC, 2015). However, in Korea, males had significantly higher OH-PAH urinary concentrations than females, likely due to higher incidence of smoking among Korean males (Sul et al., 2012). In Australia, the proportion of smokers among males (20.4%) and females (16.3%) is similar (ABS, 2013), and hence sex-trends with smoking status are unlikely to impact the relative distribution of OH-PAH concentrations between the two sexes.
4.2. Comparison with international data
With the exception of 1-NAP, the GM concentrations of urinary OH-PAH measured in this Australian population are comparable with those reported in other developed countries including the USA (CDC, 2015) and Canada (Health Canada, 2013), but are several times lower than in populations in developing countries such as Afghanistan, China, India, and Vietnam mainly for OH-PHEs (1, 2 and 3-PHE) and 1-PYR (Guo et al., 2013; Hemat et al., 2012) (Table 2). These differences are likely a reflection of lower exposure to PAHs in ambient air in Australia than in those relatively more polluted developing countries where emission of PAHs from industry and traffic contributes substantially to air pollution (Hopke et al., 2008).
Table 2.
Concentrations of urinary OH-PAHs in selected populations (geometric mean or median) (ng/L).
| Australiaa | US 2007–08b | US 2011–12b | Canadac | Koread | Spaine | UKf | Germanyg | Israelh | Chinai | Vietnami | Japani | Indiai | Malaysiai | Kuwaiti | Afghanistanj | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 24 × 100 | 2581 | 2487 | 2422 | 4702 | 954 | 85 | 963 | 243 | 84 | 23 | 34 | 38 | 29 | 38 | 55 |
| 1-NAP | 9221 | 2580 | 1670 | 1500 | n/a | n/a | n/a | n/a | n/a | 528 | 642 | 266 | 1110 | 263 | 1410 | |
| 2-NAP | 4104 | 3830 | 4140 | 3800 | 3840 | n/a | n/a | n/a | n/a | 2270 | 4900 | 3250 | 3780 | 1550 | 7330 | |
| 2-FLU | 261 | 303 | 240 | 270 | n/a | n/a | 170 | n/a | n/a | 893 | 473 | 207 | 346 | 112 | 448 | |
| 3-FLU | 132 | 116 | 93.6 | 96 | n/a | n/a | 280 | n/a | n/a | |||||||
| 9-FLU | 299 | 337 | 245 | 160 | n/a | n/a | 180 | n/a | n/a | |||||||
| 1-PHE | 134 | 139 | 126 | 150 | n/a | 200 | 220 | n/a | 190 | 323 | 157 | 65 | 256 | 36 | 89 | 1310 |
| 2- PHE | 60 | 63.6 | 61 | 67 | n/a | 140 | n/a | 95 | 387 | 201 | 121 | 289 | 43 | 137 | ||
| 3- PHE | 81 | 97.6 | 62 | 97 | n/a | 220 | n/a | 130 | 217 | 279 | 77 | 154 | 33 | 163 | 1147 | |
| 4- PHE | 30 | 29.3 | 21 | 25 | n/a | n/a | n/a | 29 | 33 | 32 | 10 | 27 | 6 | 0 | 119 | |
| 1-PYR | 142 | 118 | 111 | 110 | 150 | 180 | n/a | 140 | 172 | 378 | 463 | 75 | 424 | 65 | 220 | 1646 |
4.2.1. Metabolite of pyrene
1-PYR is the most commonly used OH-PAH biomarker, and has been used to compare exposure to PAHs between occupational and non-occupational populations (Srogi, 2007; Hansen et al., 2008). Geometric mean concentrations of 1-PYR measured in this Australian population (142 ng/L) are comparable with those reported in other developed countries including the USA (111 ng/L, n = 2487) (CDC, 2015), Germany (140 ng/L, n = 963) (Wilhelm et al., 2008), Canada (110 ng/L, n = 2422) and Korea (150 ng/L, n = 4702) (Table 2), but considerably lower than those reported in developing countries such as China (378 ng/L, n = 84), India (424 ng/L, n = 38) and Vietnam (463 ng/L, n = 23) (Guo et al., 2013). Of interest, 1-PYR concentration in this study is significantly lower (more than 10 times) than among people in developing countries who were regularly exposed to biomass fuel burning (Hemat et al., 2012; Li et al., 2011; Riojas-Rodriguez et al., 2011) or traffic pollution (Wertheim et al., 2012); concentrations of 1-hydroxypyrene were as high as 1646 ng/L for biomass burning (Hemat et al., 2012) or 1020 ng/g creatinine (~1000 ng/L) for traffic pollution (Wertheim et al., 2012).
4.2.2. Metabolites of naphthalene
Concentrations of 1-NAP varied widely—up to two orders of magnitude (Table 1). Of note, 10 of the 24 pooled samples had 1-NAP concentrations >20,000 ng/L; the highest concentration was 375,182 ng/L. Those values are near or higher than the 95th concentrations reported in CHMS II (15,000 ng/L, n = 2522) (Health Canada, 2013) and the US NHANES 2011–2012 (22,100 ng/L, n = 2492) (CDC, 2015). Unlike 1-NAP, the concentration profile of 2-NAP presented a narrow range of less than one order of magnitude. This profile is also seen for the other OH-PAH metabolites. The GM value of 2-NAP in this study (4100 ng/L) coincided with the value reported for the United States NHANES 2011–2012 survey cycle (4100 ng/L) (CDC, 2015). It is also comparable to the values reported in Canada (3830 ng/L, n = 2422) (Health Canada, 2013), Korea (3840 ng/L, n = 4702) (Sul et al., 2012), India (3780 ng/L, n = 38), Japan (3250 ng/L, n = 34) and Vietnam (4900 ng/L, n = 23) (Guo et al., 2013) (Table 2).
While urinary concentrations of 1-NAP and 2-NAP both arise from exposure to naphthalene, 1-NAP is also a major urinary metabolite of carbaryl (1-naphthyl-N-methylcarbamate), a broad-spectrum carbamate insecticide that can be used in domestic, gardening and agricultural settings, excluding fruits and vegetables in home gardens (APVMA, 2014). On the other hand, 2-NAP is a unique biomarker for naphthalene, and has been used in many studies to assess exposure to naphthalene. Meeker et al. (2007) suggested the use of 1-NAP/2-NAP ratio to evaluate the contribution of carbaryl exposure, where a ratio >2 would indicate sources other than naphthalene contributing to the 1-NAP urinary concentration. In this study, 11 pools had ratios of 1-NAP/2-NAP ranging from 2.26 to 16.04; ten of the pools also had 1-NAP concentrations >20,000 ng/L (Table 1). It is therefore probable that exposure to sources other than naphthalene contributed to the measured 1-NAP concentrations in some pools.
Interestingly, there are a few studies in the literature reporting similarly high ratios of 1-NAP/2-NAP. Zhang et al. (2014) report 1-NAP/2-NAP ratio in the range of 10–20 in a Chinese population. In that study, the average 1-NAP concentration in participants was >50,000 ng/L after ingestion of various food including rice, cabbage, braised chicken, pork chops, and grilled lamb; the average concentration of 2-NAP was 2000 ng/L in males and 5000 ng/L in females. No information is available about exposure to other chemicals but all participants lived and worked on campus at Peking University during the study (Zhang et al., 2014). High 1-NAP concentrations and 1-NAP/2-NAP ratios were also reported in a controlled dietary exposure study on excretion profile and half-lives of OH-PAHs among nine persons who consumed barbecued chicken (Li et al., 2012). The higher than expected 1-NAP/2-NAP ratios (91–442) in three participants, attributed to exposure to carbaryl, led to the recommendation of using 2-NAP, not 1-NAP, as the biomarker to naphthalene exposure in future biomonitoring studies (Li et al., 2012). Further, a commercially available Standard Reference Material for urinary OH-PAHs in non-smokers also had a 1-NAP/2-NAP ratio of > 150, with the certified value of 1-NAP being 140 times higher than the GM value for US non-smokers (Schantz et al., 2015). To explain these findings the authors suggested that sources other than naphthalene (e.g., carbaryl) may have contributed to the urinary concentrations of 1-NAP. The poor correlation between 1-NAP and other OH-PAHs as shown in Table 3 provides further evidence that a non-PAH exposure source may have contributed to the relatively high 1-NAP urinary concentrations measured in this study.
Table 3.
Correlation coefficients (r) among urinary concentrations of OH-PAHs.
| 2-NAP | 2-FLU | 3-FLU | 9-FLU | 1-PHE | 2-PHE | 3-PHE | 4-PHE | 1-PYR | |
|---|---|---|---|---|---|---|---|---|---|
| 1-NAP | 0.366 | 0.239 | 0.252 | 0.341 | 0.689 | 0.388 | 0.396 | 0.277 | 0.077 |
| 2-NAP | 0.903* | 0.912 | 0.703 | 0.662 | 0.827 | 0.782 | 0.683 | 0.750 | |
| 2-FLU | 0.991 | 0.822 | 0.691 | 0.946 | 0.889 | 0.813 | 0.807 | ||
| 3-FLU | 0.822 | 0.697 | 0.928 | 0.891 | 0.782 | 0.800 | |||
| 9-FLU | 0.748 | 0.868 | 0.843 | 0.881 | 0.592 | ||||
| 1-PHE | 0.796 | 0.818 | 0.692 | 0.569 | |||||
| 2-PHE | 0.950 | 0.805 | 0.781 | ||||||
| 3-PHE | 0.692 | 0.826 | |||||||
| 4-PHE | 0.493 |
The numbers in bold indicate statistical significance (p < 0.001).
4.2.3. Other PAH metabolites
In biomonitoring studies on PAH exposures, the metabolites of fluorene and phenanthrene are not measured as frequently as 1-PYR, 1-NAP and 2-NAP. Fluorene and phenanthrene metabolites were included in a number of population exposure studies including NHANES 2001–02 (for OH-PHEs) and NHANES 2003–04 (for OH-FLUs) in the United States (CDC, 2015), and later in the United Kingdom (Aquilina et al., 2010), Canada (Health Canada, 2013) and some Asian countries (Guo et al., 2013; Yang et al., 2015). Studies in Afghanistan, Israel, and Spain only included OH-PHEs (Bartolomé et al., 2015; Hemat et al., 2012; Levine et al., 2015).
The concentrations of OH-FLUs and OH-PHEs in the above-mentioned studies usually correlate with the 1-PYR concentration. Such correlations (OH-FLUs and OH-PHEs with 1-PYR) were demonstrated again in this study (Table 3), which supports the use of 1-PYR as a representative biomarker for human exposure to PAHs (Hansen et al., 2008; Wilhelm et al., 2008). Moreover, information on other OH-PAHs provides a broad assessment of the exposure to PAHs.
4.3. Limitations
The study population consisted of samples of convenience collected during the course of routine pathology testing and creatinine or specific gravity measures were not available. The samples are not statistically representative of the Australian population as a whole but of Queensland, where exposures to PAHs are likely to be similar to those of the general Australian population. Pooled pathology urine specimens have been used successfully in previous studies to measure urinary bisphenol A, another ubiquitous environmental chemical (Heffernan et al., 2013; Heffernan et al., 2014b), and a discussion of the opportunities and limitations of using pooled samples for biomonitoring has been published recently (Heffernan et al., 2014a).
5. Conclusions
This study provides the first data on PAH exposure of a large Australian population including infants. With the exception of 1-NAP, concentrations of urinary OH-PAHs in this study are comparable to the concentrations reported in similar populations from developed countries, and lower than those reported from selected developing countries. The relatively high urinary concentration of 1-NAP in some samples suggests that alternate exposure sources other than exposure to naphthalene also exist. While there were no differences in concentrations of OH-PAHs by sex, adolescent and adult groups (15–49 years) had higher urinary concentrations of OH-PAHs than the young and the elderly, most likely reflective of behaviour-specific exposure sources, such as smoking. This study can serve as a valuable reference for future studies to evaluate temporal trends in PAH exposures.
Supplementary Material
Acknowledgments
The authors wish to thank Soumini Vijayasarathy, Andrew Banks, Beatrix Fletcher, Nhung Dang and the staff at Sullivan Nicolaides Pathology Taringa for assistance with sample collection and pooling. We also gratefully acknowledge US CDC staff for technical assistance in chemical analysis and statistical analysis. PT is partly funded by a UQ Postdoctoral Fellowship and a QUT VC Research Fellowship. LMLT is partly funded by an ARC DECRA (DE120100161). JFM is funded by an ARC Future Fellowship. The authors would like to thank the Australian Government Department of the Environment for their financial support. The authors declare no conflict of interest. Entox is a joint venture of the University of Queensland and the Queensland Department of Health.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2015.11.019.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US CDC or the views of the Australian Department of the Environment.
References
- ABS (2013) 4125.0 — Gender Indicators, Australia, Jan 2013 — Smoking. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4125.0~Jan%202013~Main%20Features~Smoking~3320 (last accessed 4 July 2015).
- APVMA (2014) Carbaryl Chemical Review. http://apvma.gov.au/node/12416 (last acessed 18 November 2015).
- Aquilina NJ, Delgado-Saborit JM, Meddings C, Baker S, Harrison RM, Jacob Iii P, Wilson M, Yu L, Duan M, Benowitz NL, 2010. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ. Int 36 (7), 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomé M, Ramos JJ, Cutanda F, Huetos O, Esteban M, Ruiz-Moraga M, Calvo E, Pérez-Gómez B, González O, Castaño A, 2015. Urinary polycyclic aromatic hydrocarbon metabolites levels in a representative sample of the Spanish adult population: the BIOAMBIENT.ES project. Chemosphere 135, 436–446. [DOI] [PubMed] [Google Scholar]
- Caudill SP, 2010. Characterizing populations of individuals using pooled samples.Journal of Exposure Science and Environmental Epidemiology 20, 29–37. [DOI] [PubMed] [Google Scholar]
- CDC (2015) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. http://www.cdc.gov/exposurereport/ (last accessed 4 March 2015).
- Guo Y, Senthilkumar K, Alomirah H, Moon HB, Minh TB, Mohd MA, Nakata H, Kannan K, 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ. Sci. Technol 47 (6), 2932–2938. [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Mathiesen L, Pedersen M, Knudsen LE, 2008. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies—a review. Int. J. Hyg. Environ. Health 211, 471–503. [DOI] [PubMed] [Google Scholar]
- Health Canada (2013) Second Report on Human Biomonitoring of Environmental Chemicals in Canada. www.healthcanada.gc.ca/biomonitoring (last accessed 4 May 2015).
- Heffernan AL, Aylward LL, Toms LM, Eaglesham G, Hobson P, Sly PD, et al. , 2013. Age-related trends in urinary excretion of bisphenol A in Australian children and adults: evidence from a pooled sample study using samples of convenience. Journal of Toxicology and Environmental Health Part A 76 (18), 1039–1055. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Aylward LL, Toms LM, Sly PD, Macleod M, Mueller JF, 2014a. Pooled biological specimens for human biomonitoring of environmental chemicals: opportunities and limitations.Journal of Exposure Science & Environmental Epidemiology 24 (3), 225–232. [DOI] [PubMed] [Google Scholar]
- Heffernan A, Sly P, Toms L, Hobson P, Mueller J, 2014b. Bisphenol A exposure is not associated with area-level socioeconomic index in Australian children using pooled urine samples. Environ. Sci. Pollut Res. Int 21 (15), 9344–9355. [DOI] [PubMed] [Google Scholar]
- Hemat H, Wittsiepe J, Wilhelm M, Muller J, Goen T, 2012. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J. Expos. Sci. Environ. Epidemiol 22 (1), 46–51. [DOI] [PubMed] [Google Scholar]
- Hopke PK, Cohen DD, Begum BA, Biswas SK, Ni B, Pandit GG, Santoso M, Chung Y-S, Davy P, Markwitz A, Waheed S, Siddique N, Santos FL, Pabroa PCB, Seneviratne MCS, Wimolwattanapun W, Bunprapob S, Vuong TB, Duy Hien P, Markowicz A, 2008. Urban air quality in the Asian region. Sci. Total Environ 404 (1), 103–112. [DOI] [PubMed] [Google Scholar]
- IARC, 2010. IARC monographs on the evaluation of carcinogenic risks to humans. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures vol. 92 International Agency for Research on Cancer, Lyon. [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Seidel A, 2002. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J. Chromatogr. B 778, 31–47. [DOI] [PubMed] [Google Scholar]
- Kim K-H, Jahan SA, Kabir E, Brown RJC, 2013. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int 60, 71–80. [DOI] [PubMed] [Google Scholar]
- Langlois PH, Hoyt AT, Lupo PJ, Lawson CC, Waters MA, Desrosiers TA, et al. , 2012. Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of neural tube defect-affected pregnancies. Birth Defects Research Part A-Clinical and Molecular Teratology 94, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Berman T, Goldsmith R, Göen T, Spungen J, Novack L, Amitai Y, Shohat T, Grotto I, 2015. Urinary concentrations of polycyclic aromatic hydrocarbons in Israeli adults: demographic and life-style predictors. Int. J. Hyg. Environ. Health 218 (1), 123–131. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. , 2008. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res 107, 320–331. [DOI] [PubMed] [Google Scholar]
- Li Z, Sjödin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP, 2011. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ. Int 37 (7), 1157–1163. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A, 2012. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol 25 (7), 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Trinidad D, Pittman E, Hilton D, Hubbard K, Carmichael H, Parker J, Calafat A, Sjödin A, 2014. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal. Bioanal. Chem 406 (13), 3119–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary-Huard T, 2007. Biases induced by pooling samples in microarray experiments. Bioinformatics 23, i313–i318. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R, 2007. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study.J. Expos. Sci. Environ. Epidemiol 17 (4), 314–320. [DOI] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S, Rauh V, 2014. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, Masera O, Li Z, Romanoff L, Sjödin A, Rojas-Bracho L, Needham LL, Romieu I, 2011. Impact of the improved Patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural Mexican women. Environ. Health Perspect 119 (9), 1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz M, Benner B Jr., Heckert NA, Sander L, Sharpless K, Vander Pol S, Vasquez Y, Villegas M, Wise S, Alwis KU, Blount B, Calafat A, Li Z, Silva M, Ye X, Gaudreau É, Patterson D Jr., Sjödin A, 2015. Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Anal. Bioanal. Chem 407 (11), 2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, 2014. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006). Environ. Health Perspect 122 (3), 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Needham L, Pirkle J, 2004. Human biomonitoring of environmental chemicals. Am. Sci 92 (1), 38–45. [Google Scholar]
- Srogi K, 2007. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ. Chem. Lett 5 (4), 169–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul D, Ahn R, Im H, Oh E, Kim JH, Kim JG, Kim P, Kim H-A, Park WY, Son BS, Shin D, Shim A-S, Yang W, Yu S-D, Lee K-H, Lee KJ, Lee S. d., Lee JW, Lee CK, Jang B-K, Choi K, Han D-H, Hwang M-Y, Lee JH, 2012. Korea National Survey for Environmental Pollutants in the human body 2008: 1-hydroxypyrene, 2-naphthol, and cotinine in urine of the Korean population. Environ. Res 118, 25–30. [DOI] [PubMed] [Google Scholar]
- Thai PK, Li Z, Sjödin A, Fox A, Diep NB, Binh TT, Mueller JF, 2015. Biomonitoring of polycyclic aromatic hydrocarbons exposure in small groups of residents in Brisbane, Australia and Hanoi, Vietnam, and those travelling between the two cities. Chemosphere 139, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LML, Guerra P, Eljarrat E, Barceló D, Harden FA, Hobson P, Sjodin A, Ryan E, Mueller JF, 2012. Brominated flame retardants in the Australian population: 1993–2009. Chemosphere 89 (4), 398–403. [DOI] [PubMed] [Google Scholar]
- Toms LML, Thompson J, Rotander A, Hobson P, Calafat AM, Kato K, Ye X, Broomhall S, Harden F, Mueller JF, 2014. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ. Int 71, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AI, Kohli A, Syed A, Eisen EA, Noth EM, Pratt B, et al. , 2013. Exposure to polycyclic aromatic hydrocarbons is associated with higher levels of total IgE, decreased function of t regulatory cells and an increase of asthma occurrence in children. J. Allergy Clin. Immunol 131, Ab54. [Google Scholar]
- Wertheim HF, Ngoc DM, Wolbers M, Binh TT, Hi NTT, Loan NQ, Tú PT, Sjodin A, Romanoff L, Li Z, Mueller JF, Kennedy K, Farrar J, Stepniewska K, Horby P, Fox A, Bao ND, 2012. Studying the effectiveness of activated carbon R95 respirators in reducing the inhalation of combustion by-products in Hanoi, Vietnam: a demonstration study. Environmental Health: A Global Access Science Source 11 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Hardt J, Schulz C, Angerer J, 2008. New reference value and the back-ground exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: basis for validation of human biomonitoring data in environmental medicine. Int. J. Hyg. Environ. Health 211 (3–4), 447–453. [DOI] [PubMed] [Google Scholar]
- Yang Q, Qiu X, Li R, Ma J, Li K, Li G, 2015. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environ. Sci. Pollut. Res 22 (3), 1760–1769. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding J, Shen G, Zhong J, Wang C, Wei S, Chen C, Chen Y, Lu Y, Shen H, Li W, Huang Y, Chen H, Su S, Lin N, Wang X, Liu W, Tao S, 2014. Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites — a controlled case study in Beijing, China. Environ. Pollut 184 (0), 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


