Abstract:
Cardiac surgery results in a multifactorial systemic inflammatory response with inflammatory cytokines, such as interleukin-10 and 6 (IL-10 and IL-6), shown to have potential in the prediction of adverse outcomes including readmission or mortality. This study sought to measure the association between IL-6 and IL-10 levels and 1-year hospital readmission or mortality following cardiac surgery. Plasma biomarkers IL-6 and IL-10 were measured in 1,047 patients discharged alive after isolated coronary artery bypass graft surgery from eight medical centers participating in the Northern New England Cardiovascular Disease Study Group between 2004 and 2007. Readmission status and mortality were ascertained using Medicare, state all-payer claims, and the National Death Index. We evaluated the association between preoperative and postoperative cytokines and 1-year readmission or mortality using Kaplan–Meier estimates and Cox’s proportional hazards modeling, adjusting for covariates used in the Society of Thoracic Surgeons 30-day readmission model. The median follow-up time was 1 year. After adjustment, patients in the highest tertile of postoperative IL-6 values had a significantly increased risk of readmission or death within 1 year (HR: 1.38; 95% CI: 1.03–1.85), and an increased risk of death within 1 year of discharge (HR: 4.88; 95% CI: 1.26–18.85) compared with patients in the lowest tertile. However, postoperative IL-10 levels, although increasing through tertiles, were not found to be significantly associated independently with 1-year readmission or mortality (HR: 1.25; 95% CI: .93–1.69). Pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 may be postoperative markers of cardiac injury, and IL-6, specifically, shows promise in predicting readmission and mortality following cardiac surgery.
Keywords: CABG, cytokines, readmission, mortality, cardiac surgery
A sustained rise in national health care expenditures has led to increasing focus on hospital readmission rates as a target for improving the overall quality and cost of health care delivery in the United States. In a study of Medicare fee-for-service beneficiaries discharged from hospital in 2003 and 2004, researchers found that 19.6% of the patients were readmitted within 30 days, 34.0% within 90 days, and 56.1% within 365 days (1). Centers for Medicare and Medicaid Services (CMS) presently estimates that avoidable hospital readmissions account for $17.4 billion of the $102.6 billion Medicare budget (2).
Cardiac surgical revascularization using coronary artery bypass grafting (CABG) is relevant as it is a common surgical repair. Readmission rates following CABG are high and represent one of the most expensive procedural areas estimated at approximately $100,000 per case (3,4). Demographic changes including more elderly and morbid patients presenting with multivessel coronary disease necessitate in more complex interventional strategies (5,6).
Current readmission models using clinical factors from registries and claim data poorly predict readmission (7–10). Primary causes of 30-day readmission after CABG include pleural effusions, infection, heart failure, and angina (11). Many prediction models have identified these clinical variables as risk factors for readmission, but few have examined the association between novel biomarkers and general adult hospital readmission to improve model validity (12). However, recently published readmission models for pediatric cohorts have shown marked improvement in the last few years (13,14). The potential for biomarkers to enhance predictive models has been noted by the cardiothoracic community, specifically inflammatory biomarkers such as IL-6 (15).
Increasing interest has focused on the role of cytokines as mediators of metabolic, immunological, and endocrine responses to cardiac surgery (16,17). However, little is known about the cytokine response in patients who develop postoperative complications after CABG. Previous research suggests that cytokines participate in reperfusion injury and that reperfusion injury is a common consequence of CABG repair. IL-6 is a major pro-inflammatory mediator well characterized in the inflammatory response following cardiac surgery, including acute renal injury, and has been shown to be a superior marker in patients with renal disease compared with other markers (18,19). IL-10 has shown to be an anti-inflammatory cytokine responsible for regulating inflammation (20). As such, IL-6 has been suggested to intensify renal injury, whereas IL-10 has been shown to confer protective effect (21).
As patients readmitted after cardiac surgery are at a significantly greater risk of death (8,22), the goal of this study was to evaluate the relationship between inflammatory cytokines and 1-year hospital readmissions or death among patients undergoing isolated CABG.
MATERIALS AND METHODS
Northern New England (NNE) Biomarker Study
NNE Biomarker Study is an initiative designed to assess the role of biomarkers in cardiac surgery (23–27). This study uses a harmonized dataset from eight medical centers in Vermont, New Hampshire, and Maine in the NNE Cardiovascular Study Group. Patients undergoing CABG and/or valve surgery at any of participating hospitals were prospectively enrolled into the NNE Biomarker Study from 2004 to 2007 (n = 1,690). Our current study population was restricted to only those patients 1) for whom isolated CABG was the primary procedure (n = 1,690), 2) who were discharged alive, 3) who were included if they did have preoperative (n = 1,393) and postoperative (n = 1,047) IL-6 or IL-10 levels. The Committee for the Protection of Human Subjects at Dartmouth College approved this study.
Biomarker Data Collection and Primary Exposure: Inflammatory Biomarkers
Inflammatory cytokine biomarkers IL-6 and IL-10 were the main exposures of interest in this study. We collected biomarker samples preoperatively before incision at each participating site, and the postoperative samples (10 mL) were collected approximately 24 hours after patient surgery. IL-6 and IL-10 were analyzed using MSD multiplex assays (Meso Scale Diagnostics, LLC. Rockville, MD). See Supplemental Appendix for additional details.
Patient Follow-Up and Primary Outcomes
The primary endpoint of this study was 1-year readmission status or mortality after isolated CABG. A composite readmission/mortality outcome was used because it has been shown that CABG patients with elevated postoperative biomarker levels who are readmitted to the hospital are also at significantly increased risk of death (28,29). For a clearer understanding of risk, the outcomes were also modeled separately. Readmission status and all-cause mortality were obtained using Medicare in-patient claims; state all-payer in-patient claims using name, gender, social security number, date of birth, and zip code of residence at the time of surgery, and the National Death Index.
Statistical Analysis
Clinical and procedural characteristics were compared by the composite outcome of 1-year readmission or mortality status using descriptive statistics. Kaplan–Meier time-to-event methods and log-rank tests were conducted to compare the risk association between 1-year readmission (first readmission) and 1-year mortality and tertile- and median-defined biomarker categories. Time zero was defined as the date of discharge after CABG, and the follow-up period was predefined as 1 year. Baseline, operative, and postoperative dichotomous outcomes were compared using Pearson’s chi-square tests; continuous variables were compared with two-sample t test or Wilcoxon rank-sum tests. Patients were stratified by preoperative and postoperative biomarker levels with cubic splines, but as no biological cut point was uncovered, we additionally split the data above and below the population median and across biomarker tertiles to examine distinctly separated biomarker levels. Distinct outcomes were measured with competing risk regression. We elected to treat the biomarker levels as categorical rather than continuous variables because of the graded relationship of median and tertile categories and the incidence of readmission or mortality.
Multivariate Cox proportional hazard models were constructed to assess the relationship of biomarker levels and 1-year readmission or mortality, using biomarker median and tertile cut points. Adjustment was carried out using the Society of Thoracic Surgeons (STS) 30-day readmission model (30) with and without the STS long-term ASCERT mortality model. The STS 30-day model corrects for 21 different preoperative factors (30), and the ASCERT model corrects for eight additional factors (31) (Table S1 in Supplemental Appendix for all included risk factors). Missing data were imputed to the most common category of categorical variables and to the median or subgroup-specific median of continuous variables (30,32). All biomarker values less than the assay’s lower quantitative limit were assigned the lower limit of detection. All analyses were performed using STATA 15.1.619 (StataCorp LLC, College Station, TX).
RESULTS
Patient, clinical, and procedural characteristics by 1-year readmission or mortality are presented in Table 1. Of the 1,047 patients included in the study, 39% (n = 405) experienced 1-year readmission or mortality, whereas 61% (n = 642) did not experience an event. CABG patients who experienced 1-year readmission or mortality were significantly older (67.8 vs. 63.9 years; p < .001) and were more likely to have a history of atrial fibrillation, myocardial infarction, congestive heart failure, renal failure, chronic obstructive pulmonary disease (COPD), any vascular disease, and cerebrovascular disease than patients without an event. A significantly higher percentage of females comprised the event group (28.9% vs. 20.1%; p < .001).
Table 1.
Patient characteristics by readmission or death within 1 year from discharge.
| Readmitted or Dead | |||
|---|---|---|---|
| No (n = 642) | Yes (n = 405) | p Value | |
| Age (mean, SD) | 63.9 ± 9.9 | 67.8 ± 10.0 | <.001 |
| Female | 20.1% | 28.9% | <.001 |
| BMI (mean, SD) | 29.7 ± 5.4 | 29.8 ± 5.8 | .636 |
| BSA (mean, SD) | 2.0 ± .2 | 2.0 ± .3 | .534 |
| Smoker | 22.6% | 23.0% | .889 |
| Atrial fibrillation | 3.7% | 9.1% | <.001 |
| CHF | 7.7% | 13.9% | <.001 |
| Last preoperative serum creatinine (mean, SD) | 1.1 ± 1.1 | 1.2 ± .8 | .115 |
| Diabetes | 36.2% | 40.5% | .136 |
| Ejection fraction <40% | 9.8% | 12.0% | .221 |
| Hypertension | 80.2% | 82.9% | .242 |
| IABP preoperative | 3.9% | 3.7% | .900 |
| Prior MI | |||
| No | 57.9% | 51.4% | .132 |
| <24 hours preoperative | 1.5% | 1.7% | |
| >24 hours and <7 days preoperative | 19.1% | 19.3% | |
| >7 days and <365 days preoperative | 9.3% | 12.4% | |
| >365 days preoperative | 12.2% | 15.3% | |
| Vascular disease | 22.6% | 35.8% | <.001 |
| Unstable angina | 55.4% | 55.5% | .975 |
| COPD | 10.4% | 16.8% | .001 |
| Left main, ≥50% stenosis | 32.4% | 36.1% | .189 |
| Prior CABG | 1.7% | 2.8% | .227 |
| Prior PCI | 18.9% | 21.5% | .276 |
| Priority | |||
| Emergent | 2.3% | 1.2% | .385 |
| Urgent | 66.3% | 68.2% | |
| Nonurgent | 31.4% | 30.6% | |
| Received pRBC units | 31.9% | 45.9% | <.001 |
| Number of pRBC units given pre-op | |||
| 0 | 99.0% | 95.8% | .001 |
| 1 | * | * | |
| 2 | * | * | |
| ≥3 | * | * | |
BMI, body mass index; BSA, body surface area; CHF, coronary heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; PRBC, packed red blood cells.
*Indicates a suppressed or counter-suppressed value because of CMS policy.
Additional patient characteristics by preoperative and postoperative cytokine levels (continuous and categorical) are summarized in Supplemental Tables 5–8. Patients in the highest postoperative tertile of IL-6 (tertile 3: 37.6–1,548 pg/mL) and IL-10 (tertile 3: 1.26–140 pg/mL) were significantly more likely to have a longer hospital stay (p < .001). Patients in the lowest tertile of preoperative IL-6 (tertile 1: .19–1.06 pg/mL) and postoperative Il-10 (.1–.66 pg/mL) were the least likely to suffer from congestive heart failure (p < .001).
Preoperative and Postoperative IL-6 and 1-Year Readmission or Mortality
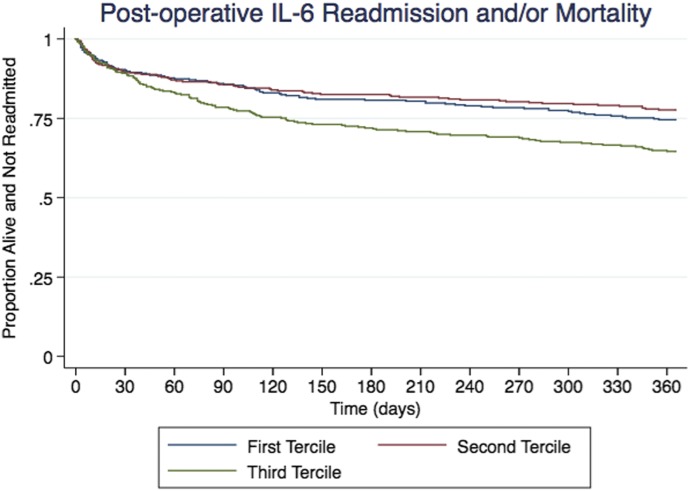
Biomarker summary statistics can be viewed in Table S2 in the Supplementary Appendix. Unadjusted and adjusted IL-6 levels, when stratified by tertiles, correlated with risk of readmission and mortality. Patients in the highest tertile of postoperative IL-6 values had significantly higher risk of readmission or death within 1 year of discharge than patients in the lowest tertile (HR: 1.38; 95% CI: 1.03–1.85). Patients in the highest tertile of postoperative IL-6 had significantly higher risk of death within 1 year of discharge (HR: 4.88; 95% CI: 1.23–19.30) (Table 2 and Figure 1).
Table 2.
Cox and competing-risk regression model results for postoperative cytokine biomarker levels and readmission or mortality.*
| Adjusted* HR (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Readmission | Mortality | Combined | |||||||
| Marker | Readmission | Mortality | Combined | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| IL-6 (pg/mL) | Log continuous | 1.26 (1.07–1.48) | 1.70 (1.25–2.32) | 1.28 (1.09–1.49) | 1.24 (1.04–1.48) | 1.22 (1.02–1.46) | 1.62 (1.08–2.43) | 1.76 (1.12–2.77) | 1.25 (1.05–1.48) | 1.22 (1.03–1.45) |
| Tertile 1 (.33–20.10) | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Tertile 2 (20.30–37.50) | .84 (.61–1.14) | 2.30 (.59–8.91) | .87 (.64–1.19) | .79 (.57–1.08) | .78 (.56–1.08) | 2.63 (.57–12.2) | 2.66 (.51–13.71) | .83 (.60–1.14) | .81 (.59–1.12) | |
| Tertile 3 (37.60–1,548.00) | 1.45 (1.10–1.90) | 4.58 (1.32–15.9) | 1.48 (1.12–1.94) | 1.36 (1.01–1.82) | 1.29 (.95–1.74) | 4.88 (1.23–19.3) | 5.93 (1.29–27.31) | 1.38 (1.03-1.85) | 1.31 (.97–1.77) | |
| Below median | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Above median >28.40 | 1.31 (1.03–1.65) | 2.33 (.97–5.63) | 1.33 (1.05–1.67) | 1.22 (.95–1.56) | 1.15 (.89–1.48) | 1.88 (.71–4.95) | 1.91 (.71–5.15) | 1.22 (.95–1.55) | 1.15 (.89–1.47) | |
| IL-10 (pg/mL) | Log continuous | 1.26 (1.10–1.46) | 1.48 (.96–2.27) | 1.26 (1.09–1.45) | 1.18 (1.01–1.38) | 1.23 (1.05–1.44) | 1.09 (.58–2.03) | 1.05 (.58–1.91) | 1.16 (1.00–1.36) | 1.21 (1.03–1.41) |
| Tertile 1 (.10–.66) | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Tertile 2 (.66–1.25) | 1.26 (.93–1.71) | 1.12 (.41–3.10) | 1.23 (.92–1.65) | 1.09 (.80–1.49) | 1.13 (.82–1.55) | .56 (.18–1.79) | .61 (.19–1.95) | 1.06 (.78–1.43) | 1.09 (.80–1.49) | |
| Tertile 3 (1.26–140.00) | 1.52 (1.14–2.05) | 1.25 (.47–3.36) | 1.47 (1.11–1.96) | 1.32 (.98–1.80) | 1.40 (1.03–1.91) | .53 (.15–1.87) | .57 (.16–2.05) | 1.25 (.93–1.69) | 1.32 (.97–1.78) | |
| Below median | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Above median >.92 | 1.31 (1.04–1.66) | 1.37 (.61–3.09) | 1.28 (1.01–1.61) | 1.20 (.93–1.53) | 1.25 (.97–1.61) | .81 (.34–1.97) | .78 (.31–1.91) | 1.15 (.90–1.46) | 1.20 (.98–1.77) | |
Model 1: Adjusted for STS 30-day readmission model (factors: ejection fraction, preoperative atrial fibrillation, prior myocardial infarction, age, unstable angina, congestive heart failure, renal function, status, gender, reoperation, chronic lung disease, diabetes, IABP or inotropes, immunosuppressive treatment, peripheral vascular disease, body surface area, cerebrovascular disease, hypertension, percutaneous coronary intervention ≤6 hours before surgery, left main disease, and surgery date).
Model 2: Adjusted for STS 30-day readmission model and STS long-term ASCERT mortality model (factors: height, BMI, creatinine, mean aortic gradient, number of prior cardiovascular operations, number of diseased vessels, moderate/severe tricuspid, aortic, or mitral insufficiencies [31]).
Significance p < .05 is in bold.
*Full cohort.
**Reference value (1.00).
Figure 1.
Kaplan–Meier curves for survival based on postoperative IL-6 tertiles. Patients with elevated postoperative IL-6 levels were more likely to suffer readmission or mortality after cardiac surgery.
Preoperative and Postoperative IL-10 and 1-Year Readmission or Mortality
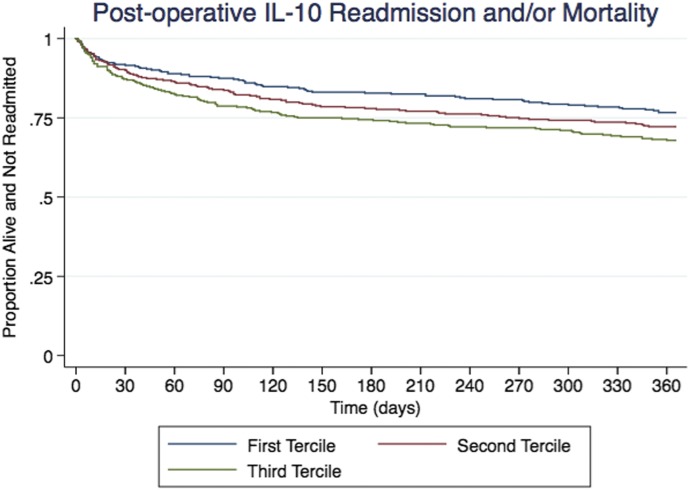
Biomarker summary statistics can be viewed in Table S2 in the Supplementary Appendix. Unadjusted postoperative IL-10 levels suggest an association of higher risk of readmission for patients in the highest tertile, compared with patients in the lowest tertile (HR: 1.5; 95% CI: 1.14–2.04). After adjustment of the STS 30-day model and the STS long-term ASCERT mortality model, patients in the highest tertile had a significantly higher risk of readmission within 1 year of discharge (HR: 1.40; 95% CI: 1.03–1.91). Although nonsignificant, mortality and combined readmission or mortality at 1 year did vary in the positive direction, but because of the variability in the IL-10 sample, an association cannot be clearly interpreted across postoperative IL-10 tertiles (Table 2 and Figure 2).
Figure 2.
Kaplan–Meier curves for survival based on postoperative IL-10 tertiles. In our study, postoperative IL-10 was not associated with higher risk of readmission or mortality after cardiac surgery.
DISCUSSION
In our multicenter cohort of adults undergoing CABG, we have demonstrated that elevated levels of postoperative IL-6 are associated with a higher risk of readmission and mortality. We found that patients in the third tertile of postoperative IL-6 levels had 1.3 times the adjusted odds of readmission than patients in the first tertile. Patients in the third tertile of postoperative IL-6 also had 4.88 times the adjusted odds of mortality than the first tertile. We did not find a strong association between postoperative IL-10 and readmission or mortality after cardiac surgery. Our findings suggest that postoperative levels of IL-6 are associated with readmission and mortality and could potentially be used to identify patients at the highest risk of developing these outcomes after cardiac surgery; therefore, at least IL-6 may facilitate strategies of personalized medicine in patients undergoing cardiac surgery. Personalized medicine is an emerging practice of medicine and progress in biomarker technology, coupled with the companion clinical diagnostic laboratory tests, and continues to advance this field, where individualized and customized treatment appropriate for each individual patient defines the standard of care (33).
Cardiac surgery with cardiopulmonary bypass (CPB) is a complex pathophysiologic environment in which exposure to nonphysiologic surfaces in the pump circuit, hemolysis, and systemic, myocardial, and cerebral ischemia/reperfusion combine to create oxidative stress, reactive oxygen species formation, peroxynitrite formation, cytokine release, excitotoxicity, complement cascade activation, matrix metalloproteinase activation, and macrophage activation (34,35). IL-6 and IL-10 are widely recognized as playing a role in mediating the systemic inflammatory response to cardiac surgery and CPB (36). IL-6 is one of the main pro-inflammatory cytokines, whereas IL-10 has anti-inflammatory and immunomodulatory functions regulating lymphocyte activity (21). The functions of IL-6 and IL-10 may explain the differential outcomes observed in this study, such that high pro-inflammatory cytokine IL-6 was associated with all endpoints, whereas high anti-inflammatory cytokine IL-10 was not associated with any endpoints after adjustment.
The finding that the highest tertile of IL-6 is associated with a greater risk of rehospitalization or mortality after cardiac surgery is consistent with previous studies. IL-6 functions as a pro-inflammatory cytokine which increases in response to trauma, such as surgery. Numerous studies have demonstrated an association with elevated IL-6 levels and cardiac events, including incidence of heart failure, unstable angina, and functional status outcomes for patients after cardiac surgery (37–39). Whether IL-6 indicates a specific response to surgery or overall chronic/systemic inflammation is unknown, but evidence indicates that elevated IL-6 is a predictor of negative surgical outcomes.
As an anti-inflammatory cytokine, high IL-10 levels could indicate several disparate underlying processes either directly related or unrelated to the CABG procedure of interest. Although reduced IL-10 measures are associated with some chronic diseases, the role of IL-10 in infection at the cellular level is not well understood (20,21,28,40–42). For example, Anguera et al. (2014) showed that elevated serum IL-10 reduced the risk of cardiovascular events in a cohort (n = 127) of patients with unstable angina, although serum IL-10 was not significantly elevated in a separate study of angina patients compared with healthy patients (43,44). Moreover, IL-10 had an anti-fibrotic effect in mouse models of heart failure (45). On the other hand, IL-10 is cleared by the kidneys, such that poor kidney function is associated with elevated IL-10 concentrations (21). Therefore, elevated IL-10 could indicate either strong immune functioning or impaired renal functioning, which is a well-established predictor of negative outcomes after cardiovascular surgery. In our study, we found that postoperative elevated IL-10 was positively associated with readmission before adjustment. The positive relationship observed between IL-10 and readmissions was not significant after adjustment for STS variables, which included unstable angina, renal failure, and COPD, and therefore the unadjusted risk association of IL-10 may be only indirectly indicating readmission with renal function, unstable angina, or COPD as possible primary factors Although this study adds information to the current literature base on IL-10 in translational sciences, more efforts at the basic and epidemiological level are needed to better characterize its role in the inflammatory response.
The relationship between cytokine IL-10 and cardiac surgery outcomes is not well understood. To date, this is the first study of postoperative IL-10 and risk of readmission or mortality for adults following cardiac surgery. Previous research has shown IL-10 has prognostic utility in adverse cardiac events including acute myocarditis, early renal dysfunction, heart failure, and unstable angina (43,46,47). We have not been able to demonstrate the predictive utility of IL-10 for readmission or mortality at 1 year after isolated CABG (28,41,43,44).
Recently, other biomarkers have been assessed for their ability to predict readmission and mortality, both individually and collectively, and to enhance current prediction models. Urinary biomarkers were shown to be unable to predicting readmission after kidney injury, and the author suggested that the outcomes may have been associated with cardiovascular or hemodynamic processes and not directly related to the kidneys (12). A 2018 study by Brown et al. (48) revealed that a panel of combined urinary, inflammatory, and cardiac biomarkers showed low generalizability for readmission and mortality prediction, with an Area Under the Receiver Operating Characteristics (AUROC) curve of .74 in the derivation cohort, but only a .48 in the validation cohort. Jacobs and colleagues did display biomarker predictive ability when they looked at a multivariate response of biomarkers in the same population of patients as our cohort. They found that postoperative levels of galectin-3 and n-terminal prohormone of brain natriuretic peptide actually were significantly associated with readmission after CABG surgery, with hazard ratios at 1.40 and 1.42, respectively, greater than the median (49). IL-10, although seemingly related to the outcomes, does not show the same significant clinical power in predicting risk outcome as either of the cardiac biomarkers. By contrast, our analysis shows a stronger association between the third tertile of IL-6 and all outcome measures including readmission, mortality, and the combined outcome, in unadjusted and adjusted models, suggesting that IL-6 could enhance the prediction of outcomes beyond comorbidities and other markers.
Strengths and Limitations
The NNE Biomarker Study was a large, multicenter, regional investigation that prospectively enrolled patients across multiple hospitals, ensuring the generalizability of its subsequent findings. Furthermore, we conducted sensitivity analyses to account for competing risks and missing values to judge the accuracy of our main findings. Our data are from 2004 to 2007, when the prevalence of heart disease was slightly higher than that in current data (221.6 vs. 168.5 age-adjusted deaths per 100,000 people). Although age-adjusted mortality rates have declined in recent years, heart disease remains the leading cause of death in the United States (50); therefore, updated cardiac biorepositories are needed to address the current patient demographics as well as recently discovered biomarkers. We did not focus on preoperative biomarker measurements in our study. Biomarkers IL-6 and IL-10 are present at very low concentrations preoperatively, and their levels increase by several folds in patients who experience adverse cardiac events after surgery. It is possible that some of these patients have underlying pathology and chronically elevated biomarker levels, and that this underlying pathology is related to their higher rate of 1-year readmission or death. In addition to this, more statistical power is needed to uncover any possible nonlinear association between postoperative IL-6 and IL-10 with the primary outcomes as there is seemingly a nonlinear decrease in the risk of readmission in the second tertile of IL-6 and a drop in mortality risk, although nonsignificant, for IL-10.
In our study, risk adjustment was performed using the STS 30-day readmission model (32), which likely reflects perioperative problems and complications. By contrast, readmissions at 1 year are more likely a reflection of underlying chronic disease processes. Regardless, the finding of increased risk for mortality or readmission at 1 year is important whether the increased risk is related to perioperative problems or underlying chronic disease.
Given our combined readmission and mortality endpoint, we performed additional analyses using a harmonized dataset of the STS 30-day readmission model and the STS ASCERT long-term mortality model (Model 2; Table 2) (32). The ASCERT model is less parsimonious than the STS 30-day readmission model, but it does consider all potential confounders. We were unable to include eight covariates that are included in the ASCERT model because of the lack of available data in the NNE dataset (Supplementary Table S4).
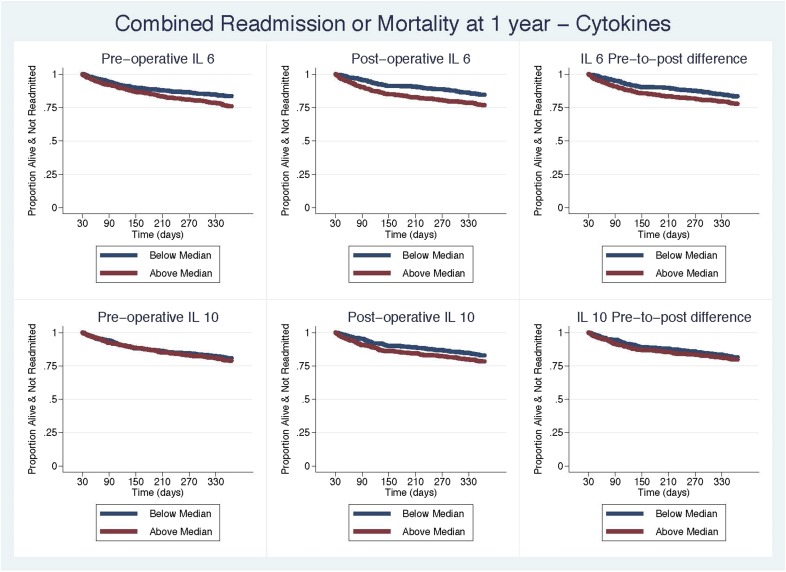
To evaluate the early vs. long-term effect of the biomarkers on readmission, we repeated our analyses only evaluating the biomarkers on readmission and mortality from 30 to 365 days, adjusted by the combined STS readmission and long-term mortality models. In the left censored analyses, we observed a significant association with postoperative IL-6 measurements after adjustment (Table 3 and Figure 3). We did not observe a change with hazard of readmission or mortality with IL-10. This brings us to conclude that elevated postoperative measurements of IL-6 provide important risk information for readmission or mortality at 1 year, whereas IL-10 does not.
Table 3.
Cox and competing-risk regression model results for postoperative cytokine biomarker levels and left-censored to 30–365 days readmission or mortality.
| Adjusted* HR (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Readmission | Mortality | Combined | |||||||
| Marker | Readmission | Mortality | Combined | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| IL-6 | Log continuous | 1.49 (1.15–1.92) | 1.88 (1.32–2.69) | 1.33 (1.10–1.61) | 1.13 (.82–1.54) | 1.10 (.80–1.50) | 2.07 (1.26–3.41) | 2.65 (1.52–4.61) | 1.24 (1.00–1.54) | 1.24 (1.00–1.54) |
| Tertile 1 (.33–20.10) | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Tertile 2 (20.30–37.50) | .69 (.46–1.05) | 1.98 (.36–10.81) | .75 (.50–1.12) | .63 (.41–.96) | .62 (.40–.94) | 2.03 (.33–12.37) | 2.58 (.3–22.28) | .68 (.45–1.02) | .66 (.43–1.00) | |
| Tertile 3 (37.60–1,548.00) | 1.71 (1.21–2.40) | 4.92 (1.08–22.51) | 1.75 (1.24–2.45) | 1.54 (1.05–2.27) | 1.50 (1.01–2.22) | 6.40 (1.26–32.48) | 11.03 (1.51–80.75) | 1.57 (1.07–2.30) | 1.52 (1.03–2.24) | |
| Below median | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Above median >28.40 | 1.57 (1.16–2.13) | 2.89 (.93–8.96) | 1.61 (1.19–2.17) | 1.42 (1.03–1.96) | 1.36 (.98–1.89) | 2.82 (.77–10.30) | 3.29 (.66–16.45) | 1.43 (1.03–1.96) | 1.36 (.98–1.88) | |
| IL-10 | Log continuous | 1.27 (1.06–1.52) | 1.65 (1.07–2.55) | 1.27 (1.06–1.51) | 1.18 (.96–1.44) | 1.25 (1.01–1.53) | 1.41 (.69–2.88) | 1.31 (.58–2.97) | 1.17 (.95–1.43) | 1.23 (1.00–1.51) |
| Tertile 1 (.10–.66) | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Tertile 2 (.66–1.25) | 1.22 (.84–1.77) | 2.29 (.59–8.85) | 1.26 (.87–1.82) | 1.00 (.67–1.48) | 1.06 (.71–1.61) | 1.27 (.29–5.55) | 1.21 (.27–5.43) | 1.01 (.68–1.49) | 1.08 (.72–1.62) | |
| Tertile 3 (1.26–140.00) | 1.44 (1.00–2.07) | 1.95 (.49–7.79) | 1.43 (1.00–2.06) | 1.24 (.85–1.80) | 1.32 (.90–1.95) | .87 (.14–5.24) | .85 (.12–5.94) | 1.20 (.83–1.75) | 1.28 (.87–1.87) | |
| Below median | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Above median >.92 | 1.34 (1.00–1.81) | 2.16 (.75–6.22) | 1.32 (.98–1.77) | 1.24 (.91–1.69) | 1.30 (.95–1.79) | 1.46 (.44–4.8) | 1.29 (.40–4.10) | 1.20 (.89–1.64) | 1.26 (.93–1.73) | |
Bold text indicates statistically significant hazard ratios.
Model 1: Adjusted for STS 30-day readmission model.
Model 2: Adjusted for STS 30-day readmission model and STS long-term ASCERT mortality model.
*Full cohort.
**Reference value (1.00).
Figure 3.
Kaplan–Meier curves for survival based on median levels of postoperative IL-6 and IL-10. We also evaluated the association with readmission or mortality, adjusting for the STS 30-day readmission model and the STS long-term ASCERT mortality model and left censored the cohort at 30 days.
Conclusions and Future Directions
We find that patients in the highest tertile of postoperative IL-6 were significantly more likely to suffer readmission and/or mortality after cardiac surgery. However, we did not find sufficient evidence that postoperative IL-10 was associated with readmission and/or mortality after cardiac surgery. Future research may investigate molecular and clinical explanations for these findings, either with single time-point or multiple time-point measures. Additional research should aim to create and target new cardiac biorepositories for updated analysis.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by National Heart, Lung, and Blood Institute of the National Institutes of Health R01 HL119664 (Dr. Brown, PI). Dr. Donald Likosky is supported in part by grant R01HS022535 from the Agency for Healthcare Research and Quality. This research was supported by the National Heart Lung and Blood Institute R01HL119664 (PI: Brown). All authors are research staff or investigators on the grant.
REFERENCES
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. [DOI] [PubMed] [Google Scholar]
- 2.Stone J, Hoffman GJ. Medicare hospital readmissions: Issues, policy options and PPACA. Congressional Research Service, Washington, DC; 2010:1–37. [Google Scholar]
- 3.Hannan EL, Zhong Y, Krumholz H, et al. 30-day readmission for patients undergoing percutaneous coronary interventions in New York state. JACC Cardiovasc Interv. 2011;4:1335–42. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan JM, Gray HH, Clague JC, et al. Coronary arterial surgery in the elderly: Its effect in the relief of angina. Int J Cardiol. 1989;23:327–33. [DOI] [PubMed] [Google Scholar]
- 6.Kremneva LV, Suplotov SN. Risk factors and in-hospital outcomes of acute kidney injury that developed after coronary artery bypass grafting in patients with stable angina. Ter Arkh. 2018;90:48–52. [DOI] [PubMed] [Google Scholar]
- 7.Ferraris VA, Ferraris SP, Harmon RC, et al. Risk factors for early hospital readmission after cardiac operations. J Thorac Cardiovasc Surg. 2001;122:278–86. [DOI] [PubMed] [Google Scholar]
- 8.Litmathe J, Kurt M, Feindt P, et al. Predictors and outcome of ICU readmission after cardiac surgery. Thorac Cardiovasc Surg. 2009;57:391–4. [DOI] [PubMed] [Google Scholar]
- 9.Rockx MA, Fox SA, Stitt LW, et al. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg. 2004;47:34–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Tam DY, Fang J, Tran A, et al. A clinical risk scoring tool to predict readmission after cardiac surgery: An Ontario administrative and clinical population database study. Can J Cardiol. 2018;34:1655–64. [DOI] [PubMed] [Google Scholar]
- 11.Magnus PC, Chaisson K, Kramer RS, et al. Causes of 30-day readmission after cardiac surgery in Northern New England. Circulation. 2011;124(Suppl 21):A13474. [Google Scholar]
- 12.Brown JR, Thiessen-Philbrook H, Goodrich CA, et al. Are urinary biomarkers better than acute kidney injury duration for predicting readmission? Ann Thorac Surg. 2019;107:1699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JR, Stabler ME, Parker DM, et al. Biomarkers improve prediction of 30-day unplanned readmission or mortality after paediatric congenital heart surgery. Cardiol Young. 2019;29:1051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker DM, Everett AD, Stabler ME, et al. Biomarkers associated with 30-day readmission and mortality after pediatric congenital heart surgery. J Card Surg. 2019;34:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preeshagul IR, Van Besien K, Mark TM. Controversies in multiple myeloma: To transplant or not? Curr Hematol Malig Rep. 2014;9:360–7. [DOI] [PubMed] [Google Scholar]
- 16.Frantz S, Bauersachs J, Kelly RA. Innate immunity and the heart. Curr Pharm Des. 2005;11:1279–90. [DOI] [PubMed] [Google Scholar]
- 17.Tarnok A, Emmrich F. Immune consequences of pediatric and adult cardiovascular surgery: Report of the 7th Leipzig workshop. Cytometry B Clin Cytom. 2003;54:54–7. [DOI] [PubMed] [Google Scholar]
- 18.Jones SA, Fraser DJ, Fielding CA, et al. Interleukin-6 in renal disease and therapy. Nephrol Dial Transplant. 2015;30:564–74. [DOI] [PubMed] [Google Scholar]
- 19.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–60. [DOI] [PubMed] [Google Scholar]
- 21.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005;67:1216–33. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JP, Schreiner G, Wang Y, et al. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of medicare patients. J Am Coll Cardiol. 2009;54:903–7. [DOI] [PubMed] [Google Scholar]
- 23.Brown JR, Furnary AP, Mackenzie TA, et al. Does tight glucose control prevent myocardial injury and inflammation? J Extra Corpor Technol. 2011;43:144–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JR, Hernandez F Jr, Klemperer JD, et al. Cardiac troponin T levels in on- and off-pump coronary artery bypass surgery. Heart Surg Forum. 2007;10:E42–6. [DOI] [PubMed] [Google Scholar]
- 25.Brown JR, Landis RC, Chaisson K, et al. Preoperative white blood cell count and risk of 30-day readmission after cardiac surgery. Int J Inflam. 2013;2013:781024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JR, MacKenzie TA, Dacey LJ, et al. Using biomarkers to improve the preoperative prediction of death in coronary artery bypass graft patients. J Extra Corpor Technol. 2010;42:293–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JR, Parikh CR, Ross CS, et al. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg. 2014;97:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. J Immunol. 2008;180:5771–7. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg JH, Whitlock R, Zhang WR, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahian DM, O’brien SM, Filardo G, et al. The society of thoracic Surgeons 2008 cardiac surgery risk models: Part 1—Coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(Suppl 1):S2–S22. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster TS, Schill MR, Greenberg JW, et al. Long-term survival prediction for coronary artery bypass grafting: Validation of the ASCERT model compared with the society of thoracic surgeons predicted risk of mortality. Ann Thorac Surg. 2018;105:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahian DM, He X, O’Brien SM, et al. Development of a clinical registry-based 30-day readmission measure for coronary artery bypass grafting surgery. Circulation. 2014;130:399–409. [DOI] [PubMed] [Google Scholar]
- 33.Ong FS, Das K, Wang J, et al. Personalized medicine and pharmacogenetic biomarkers: Progress in molecular oncology testing. Expert Rev Mol Diagn. 2012;12:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corral-Velez V, Lopez-Delgado JC, Betancur-Zambrano NL, et al. The inflammatory response in cardiac surgery: An overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets. 2015;13:367–70. [DOI] [PubMed] [Google Scholar]
- 35.Larmann J, Theilmeier G. Inflammatory response to cardiac surgery: Cardiopulmonary bypass versus non-cardiopulmonary bypass surgery. Best Pract Res Clin Anaesthesiol. 2004;18:425–38. [DOI] [PubMed] [Google Scholar]
- 36.Chew MS, Brandslund I, Brix-Christensen V, et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: A descriptive study. Anesthesiology. 2001;94:745–53; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 37.Askevold ET, Gullestad L, Dahl CP, et al. Interleukin-6 signaling, soluble glycoprotein 130, and inflammation in heart failure. Curr Heart Fail Rep. 2014;11:146–55. [DOI] [PubMed] [Google Scholar]
- 38.DiMaria-Ghalili RA, Sullivan-Marx EM, Compher C. Inflammation, functional status, and weight loss during recovery from cardiac surgery in older adults: A pilot study. Biol Res Nurs. 2014;16:344–52. [DOI] [PubMed] [Google Scholar]
- 39.Rallidis L, Zolindaki M, Pentzeridis P, et al. Raised concentrations of macrophage colony stimulating factor in severe unstable angina beyond the acute phase are strongly predictive of long term outcome. Heart. 2004;90:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher P, Lowe G, Fitzgerald T, et al. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niemand C, Nimmesgern A, Haan S, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. [DOI] [PubMed] [Google Scholar]
- 42.Takanashi S, Hasegawa Y, Kanehira Y, et al. Interleukin‐10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. 1999;14:309–14. [DOI] [PubMed] [Google Scholar]
- 43.Anguera I, Miranda-Guardiola F, Bosch X, et al. Elevation of serum levels of the anti-inflammatory cytokine interleukin-10 and decreased risk of coronary events in patients with unstable angina. Am Heart J. 2002;144:811–7. [DOI] [PubMed] [Google Scholar]
- 44.Waehre T, Halvorsen B, Damås J, et al. Inflammatory imbalance between IL‐10 and TNFα in unstable angina potential plaque stabilizing effects of IL‐10. Eur J Clin Invest. 2002;32:803–10. [DOI] [PubMed] [Google Scholar]
- 45.Verma SK, Girikipathi VN, Krishnamurthy P, et al. Interleukin-10 inhibits bone marrow fibroblast progenitor cell-mediated cardiac fibrosis in pressure-overloaded myocardium. Circulation. 2017;136:940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalikias GK, Tziakas DN, Kaski JC, et al. Interleukin-18/interleukin-10 ratio is an independent predictor of recurrent coronary events during a 1-year follow-up in patients with acute coronary syndrome. Int J Cardiol. 2007;117:333–9. [DOI] [PubMed] [Google Scholar]
- 47.Izumi T, Nishii M. Diagnostic and prognostic biomarkers in acute myocarditis. Herz. 2012;37:627–31. [DOI] [PubMed] [Google Scholar]
- 48.Brown JR, Jacobs JP, Alam SS, et al. Utility of biomarkers to improve prediction of readmission or mortality after cardiac surgery. Ann Thorac Surg. 2018;106:1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs JP, Alam SS, Owens SL, et al. The association between novel biomarkers and 1-year readmission or mortality after cardiac surgery. Ann Thorac Surg. 2018;106:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. 2017. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.