Abstract
Inflammation elicited by infection or noninfectious insults during gestation induces proinflammatory cytokines that can shift the trajectory of development to alter offspring phenotype, promote adiposity, and increase susceptibility to metabolic disease in later life. In this study, we use mice to investigate the utility of a small molecule Toll-like receptor (TLR)4 antagonist (+)-naloxone, the nonopioid isomer of the opioid receptor antagonist (−)-naloxone, for mitigating altered fetal metabolic programming induced by a modest systemic inflammatory challenge in late gestation. In adult progeny exposed to lipopolysaccharide (LPS) challenge in utero, male but not female offspring exhibited elevated adipose tissue, reduced muscle mass, and elevated plasma leptin at 20 weeks of age. Effects were largely reversed by coadministration of (+)-naloxone following LPS. When given alone without LPS, (+)-naloxone elicited accelerated postweaning growth and elevated muscle and fat mass in adult male but not female offspring. LPS induced expression of inflammatory cytokines Il1a, Il1b, Il6, Tnf, and Il10 in fetal brain, placental, and uterine tissues, and (+)-naloxone suppressed LPS-induced cytokine expression. Fetal sex-specific regulation of cytokine expression was evident, with higher Il1a, Il1b, Il6, and Il10 induced by LPS in tissues associated with male fetuses, and greater suppression by (+)-naloxone of Il6 in females. These data demonstrate that modulating TLR4 signaling with (+)-naloxone provides protection from inflammatory diversion of fetal developmental programming in utero, associated with attenuation of gestational tissue cytokine expression in a fetal sex-specific manner. The results suggest that pharmacologic interventions targeting TLR4 warrant evaluation for attenuating developmental programming effects of fetal exposure to maternal inflammatory mediators.
Developmental events and exposures are critical in programming offspring phenotype and determining susceptibility to metabolic conditions in later life (1, 2). Infants exposed to elevated inflammatory cytokines in utero are at risk for increased adiposity, predisposing to obesity, cardiovascular disease, and type 2 diabetes (3, 4). Inflammatory cytokines can be triggered by bacterial or viral infection (4–6), or noninfectious drivers of meta-inflammation resulting from maternal obesity and gestational diabetes (7–9), poor nutrition, and/or exposures to environmental toxins (10, 11). Understanding the underlying mechanisms and molecular mediators is important to devise interventions in pregnancy to mitigate the impact on infant and child health (2, 12).
Detection of proinflammatory signals is mediated by sensing molecules called Toll-like receptors (TLRs) that bind microbial moieties (13) associated with infection, dysbiosis, or elevated gut permeability (14), as well as endogenous ligands known as “damage-associated molecular patterns” (DAMPs) released during tissue damage associated with injury, hypoxia, nutritional disruption, or metabolic dysfunction (15–17). The archetypal receptor of this detection system is TLR4, which is activated by bacterial lipopolysaccharide (LPS), as well as various DAMPs (16). TLR4 is extensively expressed in maternal immune cells and gestational tissues, including the fetal membranes (18, 19), placenta (20, 21), and uterus (22), and has been implicated as a key element by which inflammatory signals converge to impact fetal programming (23).
To investigate the fetal effects of exposure to inflammatory activation, rodent models using systemic administration of LPS during late gestation have been informative. Even modest inflammatory insults in utero can disturb fetal development and trigger metabolic phenotype changes with long-term health implications. In rats, offspring of dams administered low-dose LPS in pregnancy are disposed to central obesity and metabolic syndrome, particularly in male offspring (24), and fetal exposure to LPS-induced inflammatory cytokines TNF and IL-6 is implicated in mediating programming effects (25, 26). In mice, late gestation systemic administration of low-dose LPS caused fetal growth restriction and skeletal growth retardation (26), and surviving pups developed elevated fat deposition and blood lipids (27), as well as glucose intolerance and insulin resistance in adult male but not female offspring (28).
Mouse models show that TLR4 is required for LPS- and infection-induced in utero inflammation. Protection against fetal injury and preterm birth is afforded by spontaneous mutation in TLR4 (29, 30), use of anti-TLR4 neutralizing antibodies (31), and targeted knockout of TLR4 (32) or receptor adaptor proteins required for TLR signaling (33). Recently we reported the utility of a novel TLR4 antagonist, (+)-naloxone, to reduce inflammatory cytokines and protect against preterm birth induced by LPS (34). (+)-Naloxone is a TLR4 antagonist that is the nonopioid isomer of the opioid receptor antagonist (−)-naloxone (35), which has pharmacological and potentially clinical advantages over anti-TLR4 neutralizing antibodies (36). (+)-Naloxone is a small molecule that penetrates the blood–brain barrier (37) and may traverse placental membranes (38). (+)-Naloxone has similar anti-inflammatory properties to its chiral counterpart (−)-naloxone (39, 40), but unlike (−)-naloxone it does not have opioid receptor-binding activity, and it is a specific antagonist of TLR4 signaling (35). (+)-Naloxone binds the adaptor protein myeloid differentiation factor 2 (MD2) to prevent LPS engagement with TLR4 (41), leading to diminished nuclear factor κB activation and IL-1B, IL-6, and TNF synthesis (42–44).
These observations raise the question of whether TLR4 inhibition might comprise a useful strategy for targeted suppression of inflammation-induced fetal programming. We hypothesized that (+)-naloxone may be an effective intervention for counteracting adverse effects of TLR4-driven inflammation on fetal developmental programming. In this study, we show in a mouse model of LPS-induced systemic inflammation that (+)-naloxone can modulate fetal and placental cytokine expression in a fetal sex-specific manner, and that it acts to protect LPS-exposed fetuses, particularly males, from developing elevated fat deposition and plasma leptin in adulthood. Furthermore, attenuating TLR4 signaling with (+)-naloxone in the absence of LPS boosted postnatal growth of male offspring, implicating TLR4 sensing in the physiological mechanisms underpinning fetal developmental programming.
Materials and Methods
Mice
C57BL/6 (B6) mice were obtained from Laboratory Animal Services, University of Adelaide and maintained in the specific pathogen-free University of Adelaide Medical School Animal House, with a 12-hour light/12-hour dark cycle and room temperature of 20 ± 2°C. Food (SF02-004 nude mouse cubes, Specialty Feeds, Glen Forrest, WA, Australia; 10% fat and 20% protein) and water were provided ad libitum. Animals were used in accordance with the National Health and Medical Research Council Australian Code of Practice for the care and use of animals for scientific purposes. All experiments were approved by the University of Adelaide Animal Ethics Committee.
One to three virgin female mice of 8 to 12 weeks of age were housed with a proven fertile B6 male and checked daily between 8:00 am and 10:00 am for vaginal plugs, as evidence of mating. The morning of vaginal plug detection was designated gestational day (gd) 0.5. Following mating, females were removed from the male and housed individually.
Treatments and pregnancy outcomes
To analyze effects of LPS and (+)-naloxone on postnatal outcomes, pregnant mice were administered PBS or Salmonella typhimurium lipopolysaccharide (LPS, 20 μg/kg; Sigma-Aldrich, St. Louis, MO) in 200 μL of PBS IP, or PBS control, at 11:00 am on gd 16. Mice were then administered (+)-naloxone (60 mg/kg) in 100 μL of PBS plus 0.1% BSA IP, or vehicle control within 5 minutes of LPS injection, followed by a further three equivalent doses of (+)-naloxone or vehicle at 12-hour intervals on gd 17.0, 17.5, and 18.0. Perinatal outcomes for each dam, as well as postnatal growth and body morphometry at 20 weeks of age for all offspring, were analyzed as below. To analyze the effects of LPS and (+)-naloxone on gene expression, pregnant mice were administered LPS or PBS, and (+)-naloxone or vehicle as above, then euthanized 4 hours later for tissue collection and quantitative PCR (qPCR) analysis.
Progeny growth trajectory
Pregnant mice administered LPS and/or (+)-naloxone or vehicle as above were monitored at 12-hour intervals until the time of parturition. Gestation length and the number of viable pups were recorded. Pups were weighed at 12 to 24 hours after birth, 8 days and 21 days, when pups were weaned and housed in groups of one to four siblings according to sex. All progeny were weighed again at 4 weeks and then every 2 weeks until 20 weeks of age.
Autopsy and body morphometry analysis
At 20 weeks of age, progeny were anesthetized with avertin and bled by terminal heart puncture to recover at least 500 µL of blood. Mice were then euthanized by cervical dislocation, weighed, and autopsied for full-body composition analysis. Tissues likely to be susceptible to developmental growth perturbation (24–28, 45) were excised and weighed individually, including brain, heart, lungs (left and right), kidneys (left and right), liver, adrenal glands (left and right), quadriceps (left and right), triceps (left and right), biceps (left and right), gastrocnemius muscle (left and right), retroperitoneal fat, perirenal fat, epididymal fat (males, left and right) and parametrial fat (females), thymus, and spleen. Weights of bilateral tissues and organs were combined for each mouse. Total muscle weight was calculated by summing the weights of quadriceps, triceps, and biceps and gastrocnemius muscles. Total central fat weight was calculated by summing the weights of retroperitoneal fat, perirenal fat, and epididymal fat (for males) or parametrial fat (for females), and the muscle/central fat ratio was determined. Total central fat weight was subtracted from total body weight to calculate total lean weight. A relative weight for each tissue was calculated as a percentage of the total lean weight.
Mouse adipocytokine assays
Adiponectin and leptin were quantified by Luminex multiplex microbead assay (Merck Millipore, Bayswater, VIC, Australia), according to the manufacturer’s instructions. For adiponectin, serum samples were diluted 1 in 5000 in assay buffer, as recommended by the manufacturer, whereas for leptin, samples were tested neat. The minimum detectable threshold was 3.0 pg/mL and 4.2 pg/mL for adiponectin and leptin, respectively.
Cytokine, Tlr4, and Sry gene expression
Pregnant mice administered combinations of LPS and/or (+)-naloxone or vehicle as above were euthanized by cervical dislocation 4 hours after the first injections at 3:00 pm on gd 16 and the intact uterus of each female was removed. Two fetoplacental units were randomly selected and the adjacent uterine myometrium (between implantation sites), entire uterine decidua (at placental attachment site), placenta and fetal membranes, and fetal brain were dissected, then snap-frozen in liquid N2 and stored at −80°C.
Tissues were homogenized using ceramic beads in TRIzol (Ambion, Carlsbad, CA), and RNA was precipitated using isopropanol and ethanol, then DNase treated using an Ambion DNA-free™ kit according to the manufacturer’s instructions. RNA purity and concentration were determined by A260 and A280 (NanoDrop, Wilmington DE), and RNA integrity was verified by denaturing agarose electrophoresis. First-strand cDNA was reverse transcribed from 2 μg of extracted RNA with SuperScript III (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions, with 200 ng of random sequence oligohexamers (GeneWorks, Adelaide, SA, Australia) and 500 ng of oligo(dT)18 (Proligo, Lismore, NSW, Australia) at 52°C for 1 hour. Transcripts for cytokines Il1a, Il1b, Il6, Il10, and Tnf, all implicated previously in mechanisms underpinning fetal programming (25, 26, 45), as well as Tlr4 and Actb were quantified in all cDNAs, and the Y-chromosome transcript Sry was measured in fetal brain to determine fetal sex. Each reaction contained 2 µL of cDNA (10 ng/µL) and 18 µL of master mix consisting of Power SYBR® Green PCR master mix (Life Technologies), 0.5 to 1 μM 5′ and 3′ primers (46), and RNase-free water. PCR reactions were 10 minutes at 95°C followed by 40 cycles of 95°C for 15 seconds and 60°C for 45 seconds, using a Rotor-Gene 6000 (Corbett Life Sciences, Sydney, NSW, Australia). PCR product integrity was confirmed by high-resolution melt analysis. Housekeeping gene Actb expression was unchanged by treatments (data not shown). Data were normalized to Actb expression and expressed as ΔΔCT using the formula mRNA level = Log2(CtActb− Cttarget gene) (47).
Statistical analysis
All statistical analysis was conducted using SPSS for Windows, version 20.0 software (SPSS, Chicago, IL). Data were tested for normality using a Shapiro–Wilk test. ANOVA and post hoc t tests were used when data were normally distributed. Kruskal–Wallis and Mann–Whitney U tests were used when data were not normally distributed. Categorical data (viable birth rate, offspring sex ratio) were assessed by a χ2 test. Gestation length and litter size data expressed as mean ± SEM were analyzed by two-way ANOVA and a post hoc Sidak t test. Body allometry is expressed as estimated marginal means ± SEM and was analyzed by mixed model linear repeated measures ANOVA and a post hoc Sidak t test, with dam as subject and litter size as covariate. Growth curve data were also analyzed by area under the curve analysis in R studio. qPCR and adipocytokine data are mean ± SEM analyzed by one-way ANOVA and a post hoc Sidak t test. Linear mixed model analysis was also used to estimate effects of fetal sex, as well as interaction with (+)-naloxone, on body morphometry and cytokine expression. Differences between treatments and impact of effects were considered significant when P < 0.05. For body allometry data, male and female tissues are reported separately, as linear mixed model analysis showed offspring sex and interaction of sex with LPS to be main effects for most parameters (P < 0.05).
Results
In utero LPS and (+)-naloxone modulate perinatal outcomes and postnatal survival
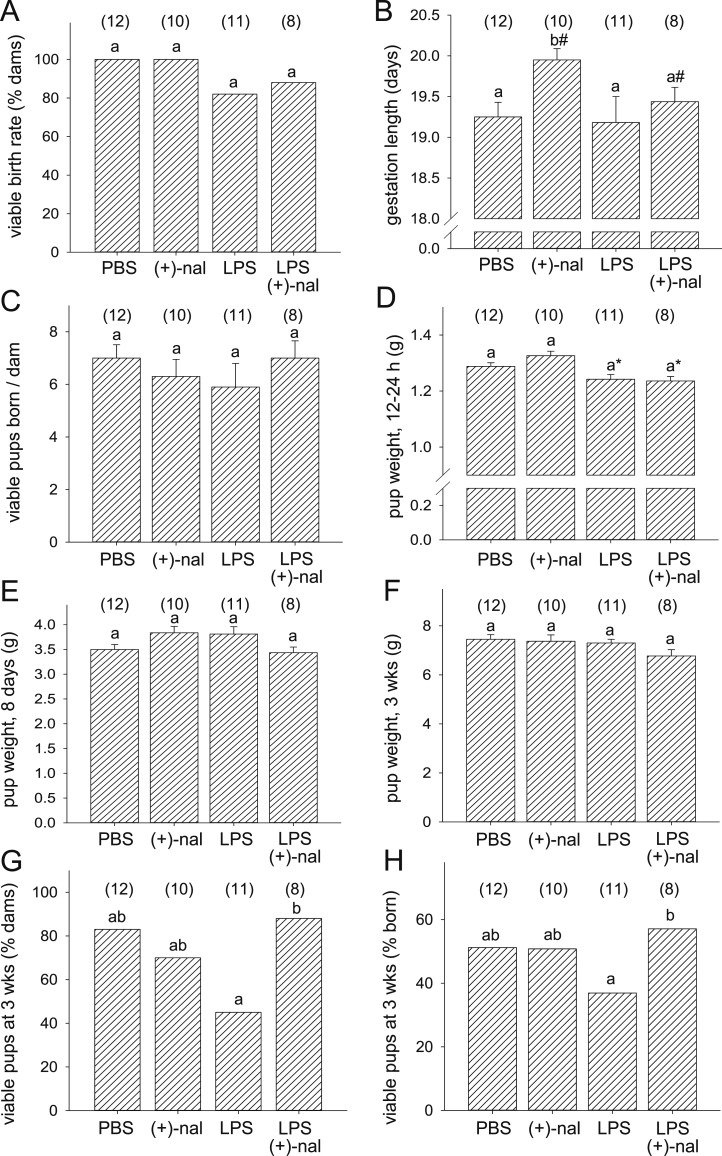
To investigate the effect of (+)-naloxone on LPS-induced fetal programming, pregnant B6 mice were administered LPS with or without (+)-naloxone, (+)-naloxone alone, or PBS control. LPS was given at 20 μg/kg, half the dose required to reliably elicit fetal death and preterm birth in B6 mice (48, 49) (data not shown). The proportion of pregnant mice that delivered viable pups, as well as the number of viable pups born per dam, was unchanged by treatments compared with PBS controls (Fig. 1A and 1C). (+)-Naloxone alone increased average gestation length by 13 hours (41 dams, P = 0.036, one-way ANOVA) (Fig. 1B), consistent with our earlier report (32). LPS reduced pup weight at birth (P < 0.001, mixed model ANOVA), but the effect was lost when data were normalized to gestation length (Fig. 1D). Treatments did not impact offspring weight at 8 days or 3 weeks (Fig. 1E and 1F). A higher proportion of dams retained viable pups at 3 weeks, and a higher proportion of pups born survived to 3 weeks, when dams were administered (+)-naloxone after LPS, compared with LPS alone (Fig. 1G and 1H). The ratio of male to female pups surviving to weaning was not changed relative to PBS control after administration of LPS and/or (+)-naloxone (data not shown).
Figure 1.
Effect of (+)-naloxone with and without LPS challenge on gestation length, litter size, and early postnatal growth. Pregnant B6 mice were administered LPS or PBS IP on gd 16.5, followed by (+)-naloxone or PBS IP on gd 16.5, 17.0, 17.5, and 18.0, then allowed to progress to birth. (A) Number of dams with viable pups, (B) gestation length, (C) number of viable pups born per dam, (D) pup weight at 12 to 24 h, (E) pup weight at 8 d, (F) pup weight at 3 wk, (G) percentage of dams with viable pups at 3 wk, and (H) percentage of pups born surviving at 3 wk are shown. Data are (A, G, and H) percentage analyzed by χ2 test or (B–F) mean ± SEM analyzed by two-way ANOVA. Number of pregnant dams per group is shown in parentheses. a,bDifferent letters indicate differences between groups (P < 0.05). *P < 0.05, effect of LPS; #P < 0.05, effect of (+)-naloxone.
In utero LPS and (+)-naloxone attenuate adult offspring body allometry in a sex-specific manner
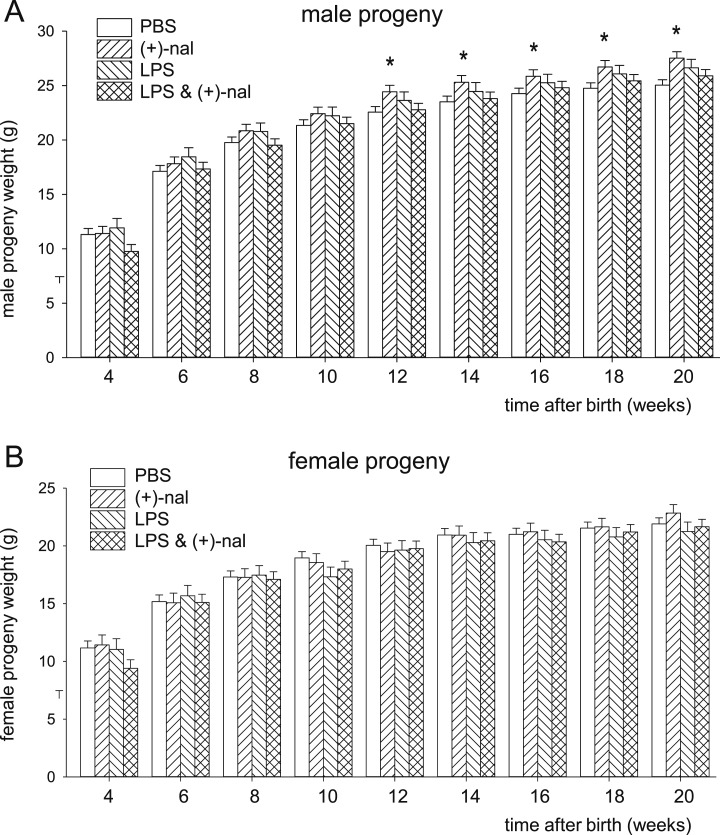
Assessment of postnatal growth curve data by area under the curve analysis identified effects of offspring sex (135 pups, P < 0.001, one-way ANOVA), (+)-naloxone (P = 0.019), and an interaction of (+)-naloxone with LPS treatment (P = 0.012) that was most evident in male offspring. Mixed model ANOVA showed in male progeny of dams administered LPS alone, or LPS and (+)-naloxone, that growth trajectories were unchanged compared with control progeny (Fig. 2A). Males exposed to (+)-naloxone without LPS exhibited a larger body mass as adults, first evident at 12 weeks and reaching a 10% increase at 20 weeks compared with controls (Fig. 2A). There was no effect of LPS or (+)-naloxone on growth trajectory in female progeny (Fig. 2B).
Figure 2.
Effect of (+)-naloxone and LPS challenge on postnatal growth trajectory in progeny. Pregnant B6 mice were administered LPS or PBS IP on gd 16.5, followed by (+)-naloxone or PBS IP on gd 16.5, 17.0, 17.5, and 18.0, then allowed to deliver. (A) Growth trajectory of male progeny and (B) female progeny at 4 wk and every subsequent 2 wk until 20 wk of age. n = 14 to 24 male progeny and n = 8 to 21 female progeny per group. Data are the estimated marginal means ± SEM, analyzed by a mixed model linear repeated measures ANOVA and post hoc Sidak t test. *P < 0.05 compared with PBS control.
When adult offspring allometry was analyzed at 20 weeks, maternal LPS treatment was seen to cause changes in adult male progeny, which were partly alleviated by (+)-naloxone. LPS caused a 15% decrease in quadriceps muscle weight and a 36% increase in epididymal fat, contributing to a 20% decrease in the muscle/central fat ratio compared with control male progeny (84 offspring from dams, P < 0.05, mixed model ANOVA) (Table 1). Loss of muscle and gain of fat was also reflected in relative weight data (Table 2). Coadministration of (+)-naloxone normalized the muscle/central fat ratio, largely through increasing muscle mass to be comparable with controls. The elevated epididymal fat elicited by LPS exposure was moderately reduced by (+)-naloxone to be not different from the control group; however, perirenal fat remained high (Tables 1 and 2).
Table 1.
Effect of Maternal LPS and (+)-Naloxone Treatment on Body Morphometry (Absolute Weight) in Male and Female Progeny at 20 wk
| Absolute Weight | PBS | (+)-Naloxone | LPS | LPS/(+)-Naloxone |
|---|---|---|---|---|
| Male progeny | ||||
| N, pups (dams) | 24 (10) | 21 (7) | 14 (5) | 25 (7) |
| Total body weight, g* | 25.0 ± 0.5a | 27.5 ± 0.6b | 26.6 ± 0.8a,b | 25.9 ± 0.6a |
| Lean body weight, g | 24.4 ± 0.4a | 27.1 ± 0.4b | 24.9 ± 0.5a | 25.6 ± 0.4a,b |
| Total muscle, mg§ | 748 ± 19a,b | 831 ± 20c | 692 ± 25b | 817 ± 20a,c |
| Total central fat, mg^ | 734 ± 34a | 902 ± 36b | 864 ± 46a,b | 840 ± 37a,b |
| Muscle/central fat ratio | 1.05 ± 0.04a | 0.95 ± 0.04a,b | 0.84 ± 0.06b | 1.00 ± 0.05a,b |
| Quadriceps, mg# | 325 ± 9a | 365 ± 10b | 277 ± 12c | 358 ± 10a,b |
| Triceps, mg# | 195 ± 6a,b | 218 ± 7a | 188 ± 8b | 216 ± 7a,b |
| Biceps, mg# | 31.7 ± 2.7 | 29.5 ± 2.9 | 34.2 ± 3.7 | 29.3 ± 3.0 |
| Gastrocnemius muscle, mg | 197 ± 7 | 218 ± 8 | 193 ± 10 | 215 ± 8.0 |
| Retroperitoneal fat, mg | 405 ± 21a | 498 ± 23b | 420 ± 29a,b | 434 ± 23a,b |
| Perirenal fat, mg | 53.4 ± 4.7a | 69.8 ± 6.4a,b | 70.5 ± 5.1a,b | 72.7 ± 5.2b |
| Epididymal fat, mg | 276 ± 18a | 334 ± 19a,b | 374 ± 24b | 333 ± 19a,b |
| Female progeny | ||||
| N, pups (dams) | 21 (10) | 8 (5) | 9 (4) | 13 (7) |
| Total body weight, g* | 21.9 ± 0.5 | 22.8 ± 0.7 | 21.4 ± 0.8 | 21.7 ± 0.6 |
| Lean body weight, g | 21.0 ± 3.8 | 22.0 ± 6.2 | 20.3 ± 5.8 | 20.7 ± 0.5 |
| Total muscle, mg§ | 587 ± 15a,b | 661 ± 24a | 541 ± 23b | 608 ± 20a,b |
| Total central fat, mg^ | 716 ± 34 | 618 ± 55 | 650 ± 52 | 628 ± 45 |
| Muscle/central fat ratio | 0.87 ± 0.05 | 1.13 ± 0.09 | 0.88 ± 0.08 | 0.99 ± 0.07 |
| Quadriceps, mg# | 252 ± 8a,b | 293 ± 13a | 231 ± 12b | 261 ± 11a,b |
| Triceps, mg# | 149 ± 5a | 164 ± 7a | 122 ± 7b | 156 ± 6a |
| Biceps, mg# | 25.6 ± 1.6 | 25.9 ± 2.7 | 29.8 ± 2.5 | 25.5 ± 2.2 |
| Gastrocnemius muscle, mg | 162 ± 7 | 179 ± 11.4 | 158 ± 11 | 166 ± 9 |
| Retroperitoneal fat, mg | 388 ± 14 | 396 ± 23.1 | 359 ± 22 | 375 ± 19 |
| Perirenal fat, mg | 39.2 ± 2.9 | 42.4 ± 4.4 | 32.5 ± 4.7 | 39.8 ± 3.9 |
| Parametrial fat, mg | 288 ± 26 | 190 ± 42 | 249 ± 39 | 213 ± 34 |
Different superscript letters (a, b, and c) indicate differences between treatment groups (P < 0.05). The number of progeny in each group is indicated, with the number of pregnant dams in parentheses. Other organs are shown in an online repository (46).
Data are estimated marginal mean ± SEM absolute weights in 20-wk-old male and female progeny of dams administered LPS and/or (+)-naloxone in late gestation. Effect of treatment group was analyzed by mixed model linear repeated measures ANOVA, using dam as subject and litter size as covariate.
Total muscle is the sum of all muscles measured.
Total central fat is the sum of all fat depots measured.
Combined weight of left and right organs.
Table 2.
Effect of Maternal LPS and (+)-Naloxone Treatment on Body Composition (Relative) in Male and Female Progeny at 20 wk
| Relative Weight | PBS | (+)-Naloxone | LPS | LPS/(+)-Naloxone |
|---|---|---|---|---|
| Male progeny | ||||
| N, pups (dams) | 24 (10) | 21 (7) | 14 (5) | 25 (7) |
| Total muscle, %§* | 3.59 ± 0.09a,b | 3.99 ± 0.01c | 3.32 ± 0.012b | 3.93 ± 0.10a,c |
| Total central fat, %^ | 3.52 ± 0.17a | 4.28 ± 0.22b | 4.32 ± 0.18b | 3.98 ± 0.16a,b |
| Quadriceps, %# | 1.56 ± 0.04a | 1.75 ± 0.05b | 1.33 ± 0.06c | 1.72 ± 0.05a,b |
| Triceps, %# | 0.93 ± 0.03a,b | 1.05 ± 0.03a | 0.90 ± 0.04b | 1.04 ± 0.03a,b |
| Biceps, %# | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.02 | 0.14 ± 0.01 |
| Gastrocnemius muscle, % | 0.94 ± 0.03 | 1.05 ± 0.04 | 0.93 ± 0.05 | 1.03 ± 0.04 |
| Retroperitoneal fat, % | 1.95 ± 0.10a | 2.39 ± 0.11b | 2.02 ± 0.14a,b | 2.08 ± 0.11a,b |
| Perirenal fat, % | 0.26 ± 0.02a | 0.34 ± 0.02a,b | 0.34 ± 0.03a,b | 0.35 ± 0.02b |
| Epididymal fat, % | 1.33 ± 0.09a | 1.60 ± 0.09a,b | 1.80 ± 0.12b | 1.60 ± 0.09a,b |
| Female progeny | ||||
| N, pups (dams) | 21 (10) | 8 (5) | 9 (4) | 13 (7) |
| Total muscle, %§* | 2.82 ± 0.07a,b | 3.18 ± 0.12a | 2.60 ± 0.11b | 2.92 ± 0.10a,b |
| Total central fat, %^ | 3.43 ± 0.16 | 2.96 ± 0.26 | 3.11 ± 0.25 | 2.95 ± 0.21 |
| Quadriceps, %# | 1.21 ± 0.04a,b | 1.41 ± 0.06a | 1.11 ± 0.06b | 1.25 ± 0.05a,b |
| Triceps, %# | 0.72 ± 0.02a | 0.78 ± 0.04a | 0.59 ± 0.03b | 0.75 ± 0.03a |
| Biceps, %# | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 |
| Gastrocnemius muscle, % | 0.78 ± 0.03 | 0.86 ± 0.05 | 0.76 ± 0.05 | 0.80 ± 0.04 |
| Retroperitoneal fat, % | 1.87 ± 0.07 | 1.90 ± 0.11 | 1.72 ± 0.10 | 1.80 ± 0.09 |
| Perirenal fat, % | 0.19 ± 0.01 | 0.15 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.02 |
| Parametrial fat, % | 1.38 ± 0.12 | 0.91 ± 0.20 | 1.20 ± 0.19 | 1.02 ± 0.16 |
Different superscript letters (a, b, and c) indicate differences between treatment groups (P < 0.05). The number of progeny in each group is indicated, with the number of pregnant dams in parentheses. Other organs are shown in an online repository (46).
Data are estimated marginal mean ± SEM relative weights in 20-wk-old male and female progeny of dams administered LPS and/or (+)-naloxone in late gestation. Effect of treatment group was analyzed by mixed model linear repeated measures ANOVA, using dam as subject, and litter size as covariate.
Total muscle is the sum of all muscles measured.
Total central fat is the sum of all fat depots measured.
Combined weight of left and right organs.
Autopsy data showed an impact of dam (+)-naloxone treatment on male offspring allometry in the absence of LPS, with elevated muscle and central fat deposits contributing to increased total and lean body weight, compared with control progeny (Table 1). Increases of 24% and 33% in absolute thymus and spleen weight compared with controls were retained relative to elevated body weight in progeny exposed to (+)-naloxone (46). The absolute and relative weights of other organs (brain, heart, lungs, kidneys, liver, adrenal glands) were unaffected by maternal LPS and/or (+)-naloxone treatment (46).
Female offspring were less impacted by dam treatments than were males in most parameters measured at autopsy. LPS administration caused an 18% decrease in absolute triceps weight, which was corrected by coadministration of (+)-naloxone (51 offspring from 26 dams, P < 0.05, mixed model ANOVA) (Table 1). (+)-Naloxone alone did not change muscle or fat composition (Tables 1 and 2) or weight of other organs compared with control female offspring (46).
In utero LPS and (+)-naloxone attenuate adult offspring adipocytokines in a sex-specific manner
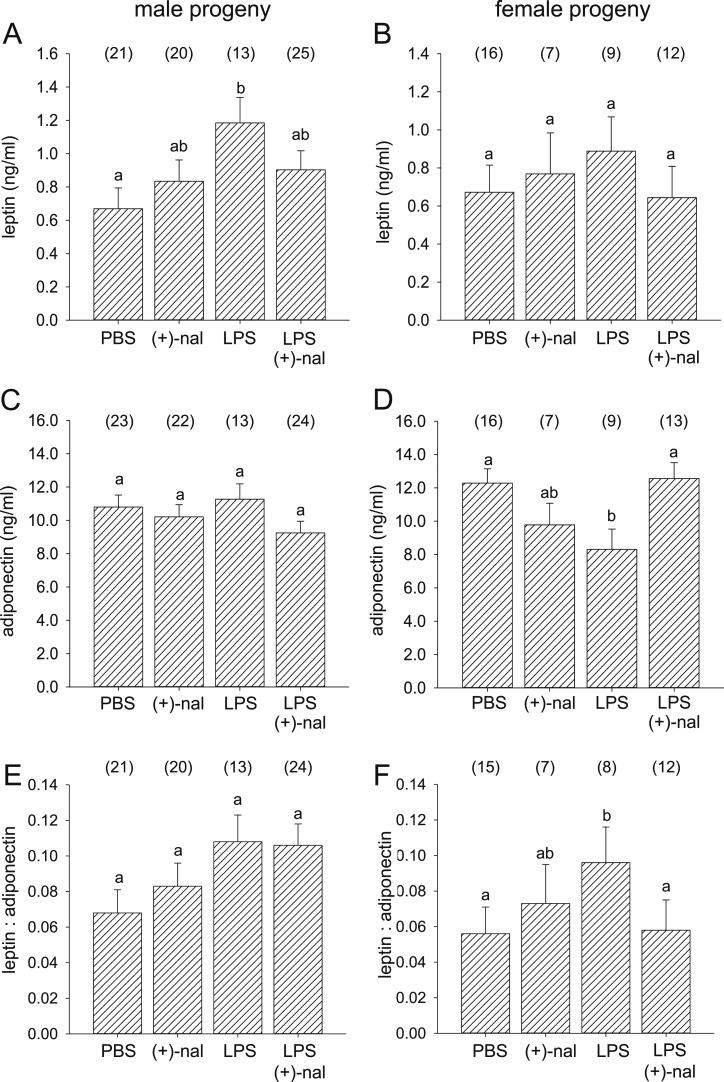
Adiponectin and leptin were measured by microbead assay in plasma from male and female progeny at 20 weeks of age. Plasma leptin but not adiponectin was altered in offspring after maternal LPS treatment (131 offspring, P = 0.004, univariate ANOVA). Sexually dimorphic effects of treatments were observed for adipocytokines. LPS caused a greater increase in plasma leptin of male than female offspring, and the effect in males was reversed by coadministration of (+)-naloxone (Fig. 3A and 3B). Plasma adiponectin was decreased in female offspring but not changed in males after maternal LPS treatment, and the effect in females was reversed by coadministration of (+)-naloxone (Fig. 3C and 3D). An inverse association was seen in the leptin/adiponectin ratio, which increased in female but not male offspring after LPS treatment, and was reversed in females by coadministration of (+)-naloxone (Fig. 3E and 3F).
Figure 3.
Effect of (+)-naloxone exposure in utero with and without LPS challenge on adipocytokines in adult offspring. (A and C) Leptin and (B and D) adiponectin were quantified by microbead assay in serum, and (E and F) the leptin/adiponectin ratio was calculated for (A, C, and E) male and (B, D, and F) female offspring of B6 dams administered LPS or PBS IP on gd 16.5, followed by (+)-naloxone or PBS IP on gd 16.5, 17.0, 17.5, and 18.0, at 20 wk of age. Data are mean ± SEM, analyzed by ANOVA and post hoc Sidak t test. The number of offspring per group is shown in parentheses. a,b,cDifferent letters indicate differences between groups (P < 0.05).
LPS and (+)-naloxone regulate cytokine expression in fetal, placental, and uterine tissues
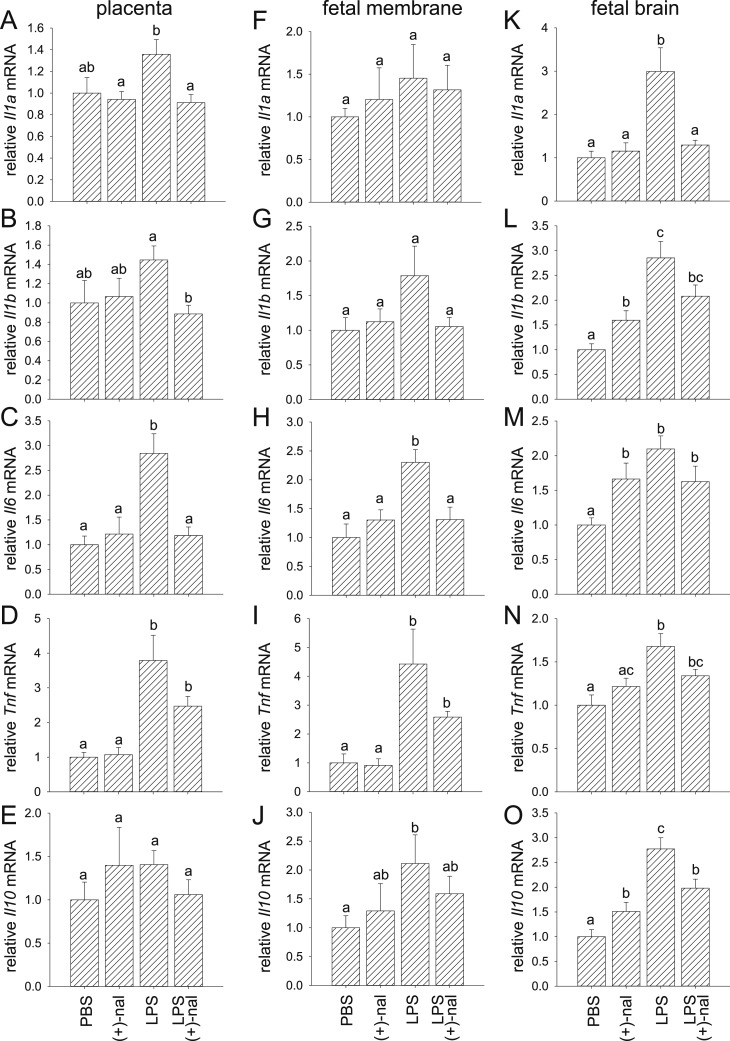
To examine whether proinflammatory cytokines induced by IP injection of LPS are attenuated by (+)-naloxone, cytokine mRNA transcripts were quantified by qPCR in placenta, fetal membranes, and fetal brain, as well as maternal decidua and myometrium collected 4 hours after LPS administration with or without concurrent (+)-naloxone. LPS induced 2.8- and 3.8-fold increases in placental Il6 and Tnf expression, respectively (Fig. 4C and 4D), 2.3-, 4.4-, and 2.1-fold increases in fetal membrane Il6, Tnf, and Il10 expression, respectively (Fig. 4H–4J), and 3.0-, 2.9-, 2.1-, 1.7-, and 2.8-fold increases in fetal brain Il1a, Il1b, Il6, Tnf, and Il10, respectively (Fig. 4K–4O), compared with tissues from control mice given PBS (64 fetal units from 32 dams, P < 0.05, ANOVA). Administration of (+)-naloxone reduced LPS-driven expression of Il1a, Il1b, and Il6 in placenta (Fig. 4A–4C), Il6 in fetal membrane (Fig. 4H), and Il1a and Il10 in the fetal brain (Fig. 4K and 4O). In each case expression was reduced to control levels, other than Il10 in the fetal brain, which remained elevated relative to control (Fig. 4O).
Figure 4.
Effect of (+)-naloxone on proinflammatory cytokine expression induced by LPS challenge in placenta and fetal membranes. Pregnant B6 mice were administered LPS or PBS IP on gd 16.5, followed by (+)-naloxone or PBS IP, and 4 h later placenta and fetal membranes were recovered from two implantation sites. Relative expression of (A, F, and K) Il1a, (B, G, and L), Il1b, (C, H, and M) Il6, (D, I, and N) Tnf, and (E, J, and O) Il10 mRNAs were determined in (A–E) placenta, (F–J) fetal membranes, and (K–O) fetal brain by qPCR and normalized to Actb. Data are mean ± SEM relative gene expression of n = 14 to 16 tissues from n = 8 dams per group and were analyzed by one-way ANOVA and a post hoc Sidak t test. a,b,cDifferent letters indicate differences between groups (P < 0.05).
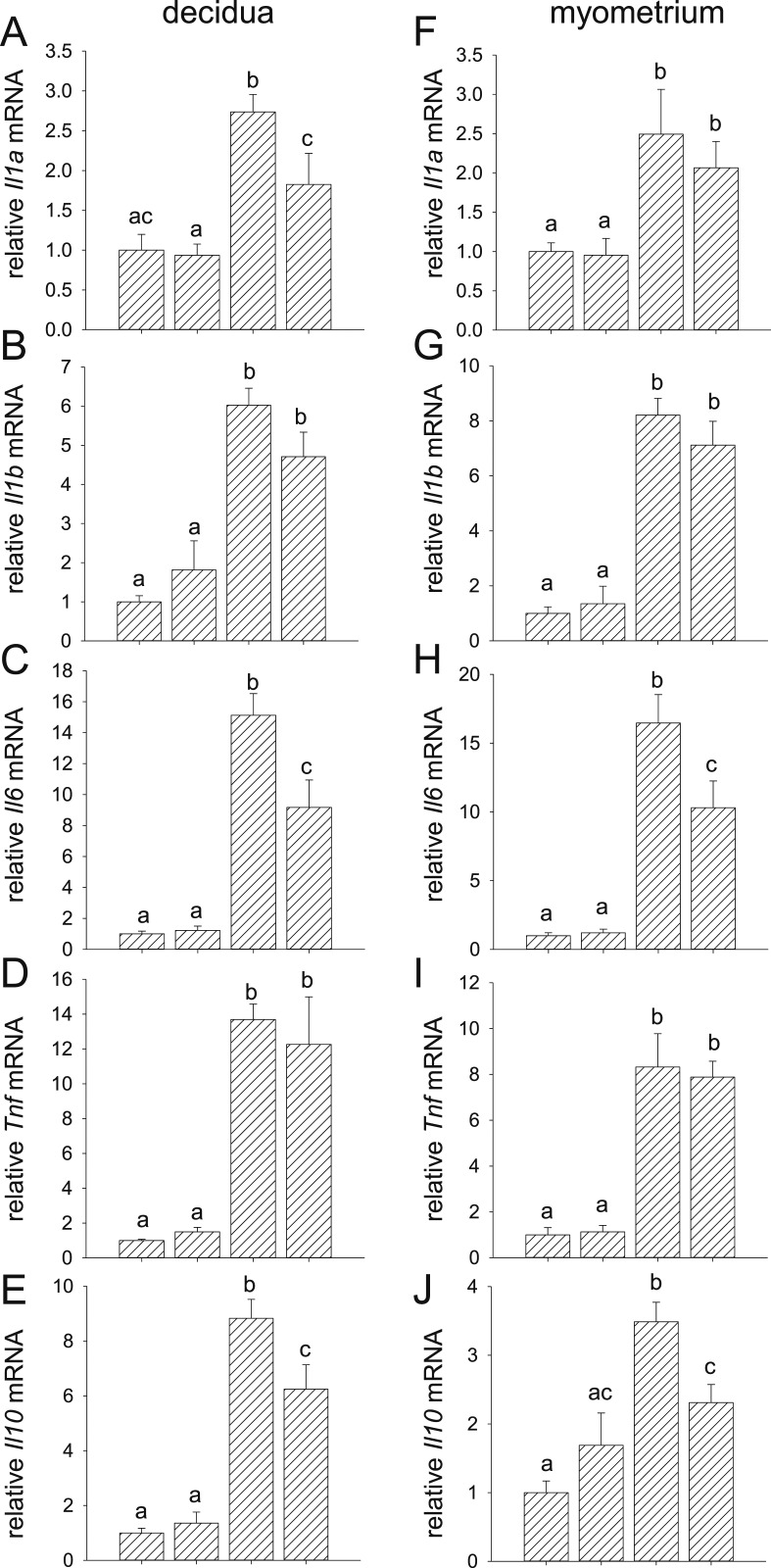
In the uterine decidua and myometrium, LPS induced 2.7-, 6.0-, 15.1-, 13.7-, and 8.8-fold increases in decidual Il1a, Il1b, Il6, Tnf, and Il10 expression, respectively (Fig. 5A–5E), and 2.5-, 8.2-, 16.5-, 8.3, and 3.5-fold increases in myometrial Il1a, Il1b, Il6, Tnf, and Il10, respectively (Fig. 5F–5J), compared with tissues from control mice given PBS. Administration of (+)-naloxone reduced LPS-driven expression of Il1a, Il6, and Il10 in the decidua (Fig. 5A, 5C, and 5E) and Il6 and Il10 in the myometrium (Fig. 5H and 5J), although in each case expression remained elevated over control levels. These data show that proinflammatory cytokines induction by LPS in fetal, placental, and uterine tissues is attenuated by (+)-naloxone.
Figure 5.
Effect of (+)-naloxone on proinflammatory cytokine expression induced by LPS challenge in uterine decidua and myometrium. Pregnant B6 mice were administered LPS or PBS on gd 16.5, followed by (+)-naloxone or PBS IP, and 4 h later uterine decidua and myometrium were recovered from two implantation sites. Relative expression of (A and F) Il1a, (B and G) Il1b, (C and H) Il6, (D and I) Tnf, and (E and J) Il10 mRNAs were determined in (A–E) decidua and (F–J) myometrium by qPCR and normalized to Actb. Data are mean ± SEM relative gene expression of n = 14 to 16 tissues from n = 8 dams per group and were analyzed by one-way ANOVA and a post hoc Sidak t test. a,b,cDifferent letters indicate differences between groups (P < 0.05).
LPS and (+)-naloxone regulate cytokine expression in a fetal sex-specific manner
To examine whether fetal sex is a determinant of in utero responsiveness to LPS and (+)-naloxone, tissues were classified as associated with male or female fetuses on the basis of diagnostic qPCR for Sry expression in fetal brain. Initially we examined whether Tlr4 expression was impacted by fetal sex. Tlr4 expression in fetal, placental, or uterine tissues was unchanged by LPS or (+)-naloxone other than in fetal membranes and fetal brain, where expression was elevated after LPS and (+)-naloxone administration, respectively (50) (31 male and 33 female fetal units from 32 dams, P < 0.05, ANOVA). There was no effect of fetal sex on Tlr4 expression in any tissue (46).
Because Tlr4 expression level did not account for differential responsiveness of male and female tissues, the effect of sex on cytokine expression was examined. In tissues from dams given LPS, fetal brain Il10 and myometrial Il1a, Il1b, Il6, and Il10 expressions were attenuated by fetal sex, with higher expression in tissue associated with male than female fetuses (all P < 0.05) (Table 3). In some tissues, there was an interaction between fetal sex and (+)-naloxone. Decidual Il6 was more responsive to (+)-naloxone suppression in female implantation sites (P = 0.030). For placental Il6 and fetal brain Il1b, there was a trend toward a greater effect of (+)-naloxone in females (P = 0.072 and P = 0.054, respectively), whereas myometrial Tnf trended toward higher responsiveness to (+)-naloxone in males (P = 0.055) (Table 3).
Table 3.
Effect of Fetal Sex and (+)-Naloxone Treatment on Cytokine Expression in Fetal and Gestational Tissues after LPS Administration
| Sexa | (+)-naloxonea | Sex–(+)-Naloxone Interactiona | LPS | LPS + (+)-Naloxone | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| Placenta (n) | 10 | 6 | 5 | 11 | |||
| IL-1A | NS | 0.024 | NS | 1.43 ± 0.20 | 1.24 ± 0.14 | 1.00 ± 0.06 | 0.87 ± 0.11 |
| IL-1B | NS | 0.005 | NS | 1.35 ± 0.18 | 1.60 ± 0.25 | 1.01 ± 0.12 | 0.83 ± 0.12b |
| IL-6 | NS | 0.001 | 0.072 | 2.49 ± 0.51 | 3.42 ± 0.61 | 1.69 ± 0.96 | 0.96 ± 0.15b |
| TNF | NS | NS | NS | 3.85 ± 1.16 | 3.69 ± 0.39 | 3.33 ± 0.41 | 2.08 ± 0.31 |
| IL-10 | NS | NS | NS | 1.42 ± 0.23 | 1.38 ± 0.23 | 1.19 ± 0.16 | 1.00 ± 0.24 |
| Fetal membrane (n) | 10 | 6 | 5 | 11 | |||
| IL-1A | NS | NS | NS | 1.58 ± 0.62 | 1.24 ± 0.24 | 1.31 ± 0.59 | 1.29 ± 0.34 |
| IL-1B | NS | NS | NS | 2.23 ± 0.65 | 1.06 ± 0.18 | 1.15 ± 0.35 | 1.02 ± 0.13 |
| IL-6 | NS | 0.05 | NS | 2.30 ± 0.24 | 2.31 ± 0.45 | 1.16 ± 0.33 | 1.38 ± 0.28 |
| TNF | NS | NS | NS | 5.28 ± 1.90 | 3.01 ± 0.45 | 2.58 ± 0.46 | 2.59 ± 0.21 |
| IL-10 | NS | NS | NS | 2.28 ± 0.78 | 1.84 ± 0.36 | 1.11 ± 0.21 | 1.81 ± 0.42 |
| Fetal brain (n) | 10 | 6 | 5 | 11 | |||
| IL-1A | NS | 0.020 | NS | 3.40 ± 0.84 | 2.31 ± 0.31 | 1.72 ± 0.20 | 1.10 ± 0.08 |
| IL-1B | NS | 0.096 | 0.054 | 2.56 ± 0.29 | 3.35 ± 0.75 | 2.68 ± 0.49 | 1.81 ± 0.21c |
| IL-6 | NS | NS | NS | 2.12 ± 0.14 | 2.07 ± 0.47 | 1.99 ± 0.34 | 1.46 ± 0.28 |
| TNF | NS | NS | NS | 1.67 ± 0.20 | 1.70 ± 0.24 | 1.42 ± 0.12 | 1.31 ± 0.10 |
| IL-10 | 0.015 | NS | NS | 3.20 ± 0.47 | 2.06 ± 0.27 | 2.53 ± 0.38 | 1.73 ± 0.16 |
| Decidua (n) | 10 | 6 | 5 | 11 | |||
| IL-1A | NS | 0.090 | NS | 2.71 ± 0.31 | 2.64 ± 0.32 | 2.18 ± 0.97 | 1.63 ± 0.33 |
| IL-1B | NS | NS | NS | 6.08 ± 0.61 | 5.93 ± 0.62 | 6.01 ± 1.38 | 3.99 ± 0.56 |
| IL-6 | NS | 0.025 | 0.030 | 13.83 ± 1.63 | 17.28 ± 2.52 | 13.67 ± 3.95 | 6.92 ± 1.48b |
| TNF | NS | NS | NS | 13.77 ± 1.20 | 13.57 ± 1.43 | 17.76 ± 6.91 | 9.21 ± 1.50 |
| IL-10 | NS | 0.042 | NS | 8.21 ± 0.52 | 9.86 ± 1.64 | 7.38 ± 1.53 | 5.68 ± 1.11b |
| PTGS2 | 0.055 | NS | 0.035 | 4.41 ± 1.03 | 4.67 ± 0.92 | 7.50 ± 2.58 | 2.30 ± 0.50 |
| Myometrium (n) | 10 | 6 | 5 | 11 | |||
| IL-1A | 0.042 | NS | NS | 3.00 ± 0.88 | 1.65 ± 0.18 | 3.10 ± 0.74 | 1.59 ± 0.27 |
| IL-1B | 0.035 | NS | NS | 8.94 ± 0.81 | 7.00 ± 0.72 | 9.04 ± 2.23 | 6.24 ± 0.70 |
| IL-6 | 0.009 | NS | NS | 19.00 ± 2.84 | 12.24 ± 2.06 | 16.16 ± 3.01 | 7.65 ± 2.08 |
| TNF | NS | NS | 0.055 | 9.81 ± 2.22 | 5.83 ± 0.43 | 6.10 ± 0.76 | 8.69 ± 0.85 |
| IL-10 | 0.002 | 0.031 | NS | 3.99 ± 0.37 | 2.65 ± 0.13d | 3.07 ± 0.49 | 1.97 ± 0.26 |
| PTGS2 | 0.093 | NS | NS | 12.11 ± 4.01 | 5.68 ± 1.12 | 6.41 ± 1.33 | 2.96 ± 0.99 |
Abbreviation: NS, not significant.
Main effects of sex, (+)-naloxone, and sex–(+)-naloxone interaction were determined by linear mixed model analysis, and P values are reported when P < 0.10.
P < 0.05, LPS + (+)-naloxone vs LPS alone, within same sex by ANOVA.
P < 0.10, LPS + (+)-naloxone vs LPS alone, within same sex by ANOVA.
P < 0.01 male vs female, within treatment group by ANOVA.
Baseline cytokine expression and regulation by (+)-naloxone in the absence of LPS was also affected by fetal sex. Placental and myometrial Tnf expression was higher in tissues associated with female than male fetuses (P < 0.05) (Table 4). Interaction between fetal sex and (+)-naloxone was evident for decidual Il6 (P = 0.004), which was induced by (+)-naloxone in decidua associated with male but not female fetuses. A trend toward interaction between fetal sex and (+)-naloxone was seen for fetal membrane Tnf (P = 0.100) and myometrial Il1a (P = 0.072), where (+)-naloxone suppressed expression in tissues associated with males more than with females, and in fetal brain Il1a, Il1b, and Il6 (all P < 0.100), which were each induced by (+)-naloxone more highly in females than in males (Table 4).
Table 4.
Effect of Fetal Sex and (+)-Naloxone Treatment on Cytokine Expression in Fetal and Gestational Tissues, Without LPS Administration
| Sexa | (+)-Naloxonea | Sex–(+)-Naloxone Interactiona | PBS | PBS + Naloxone | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| Placenta (n) | 12 | 4 | 4 | 12 | |||
| IL-1A | NS | NS | NS | 0.91 ± 0.16 | 1.26 ± 0.30 | 0.92 ± 0.09 | 0.95 ± 0.10 |
| IL-1B | NS | NS | NS | 0.74 ± 0.12 | 1.77 ± 1.10 | 1.05 ± 0.08 | 1.07 ± 0.27 |
| IL-6 | NS | NS | NS | 0.93 ± 0.21 | 1.29 ± 0.15 | 0.57 ± 0.06 | 1.45 ± 0.45 |
| TNF | 0.028 | NS | NS | 0.84 ± 0.09 | 1.48 ± 0.44 | 0.62 ± 0.03 | 1.23 ± 0.27 |
| IL-10 | NS | NS | NS | 1.11 ± 0.26 | 0.66 ± 0.16 | 1.44 ± 0.78 | 1.38 ± 0.56 |
| Fetal membrane (n) | 12 | 4 | 4 | 12 | |||
| IL-1A | NS | NS | NS | 1.01 ± 0.12 | 0.95 ± 0.21 | 0.68 ± 0.12 | 1.37 ± 0.49 |
| IL-1B | NS | NS | NS | 0.84 ± 0.14 | 1.49 ± 0.59 | 1.31 ± 0.07 | 1.04 ± 0.25 |
| IL-6 | NS | NS | NS | 0.99 ± 0.26 | 1.03 ± 0.56 | 0.93 ± 0.20 | 1.43 ± 0.21 |
| TNF | NS | NS | 0.101 | 1.15 ± 0.40 | 0.56 ± 0.27 | 0.25 ± 0.09 | 1.14 ± 0.27 |
| IL-10 | NS | NS | NS | 0.79 ± 0.17 | 1.64 ± 0.61 | 1.14 ± 0.56 | 1.35 ± 0.63 |
| Fetal brain (n) | 12 | 4 | 4 | 12 | |||
| IL-1A | NS | NS | 0.060 | 1.16 ± 0.18 | 0.52 ± 0.14 | 0.82 ± 0.24 | 1.26 ± 0.23 |
| IL-1B | NS | 0.045 | 0.091 | 1.07 ± 0.12 | 0.80 ± 0.30 | 1.15 ± 0.12 | 1.75 ± 0.24 |
| IL-6 | NS | 0.078 | 0.053 | 1.06 ± 0.11 | 0.82 ± 0.22 | 1.01 ± 0.31 | 1.88 ± 0.26 |
| TNF | NS | NS | NS | 1.08 ± 0.14 | 0.75 ± 0.15 | 1.07 ± 0.16 | 1.27 ± 0.11 |
| IL-10 | NS | NS | NS | 1.01 ± 0.18 | 0.99 ± 0.26 | 0.99 ± 0.05 | 1.68 ± 0.23 |
| Decidua (n) | 12 | 4 | 4 | 12 | |||
| IL-1A | NS | NS | NS | 1.06 ± 0.26 | 0.82 ± 0.18 | 0.57 ± 0.08 | 1.06 ± 0.17 |
| IL-1B | NS | NS | NS | 0.96 ± 0.19 | 1.12 ± 0.31 | 1.09 ± 0.41 | 2.06 ± 0.98 |
| IL-6 | NS | NS | 0.004 | 0.80 ± 0.16 | 1.59 ± 0.45 | 2.24 ± 0.95 | 0.88 ± 0.13 |
| TNF | NS | NS | NS | 0.96 ± 0.07 | 1.11 ± 0.19 | 0.99 ± 0.19 | 1.67 ± 0.32 |
| IL-10 | NS | NS | NS | 0.85 ± 0.20 | 1.43 ± 0.29 | 0.97 ± 0.42 | 1.49 ± 0.52 |
| PTGS2 | NS | NS | NS | 1.04 ± 0.22 | 0.87 ± 0.23 | 0.81 ± 0.09 | 0.99 ± 0.11 |
| Myometrium (n) | 12 | 4 | 4 | 12 | |||
| IL-1A | NS | NS | 0.072 | 1.03 ± 0.14 | 0.90 ± 0.19 | 0.32 ± 0.11 | 1.17 ± 0.26 |
| IL-1B | NS | NS | NS | 1.17 ± 0.30 | 0.50 ± 0.06 | 0.39 ± 0.09 | 1.67 ± 0.83 |
| IL-6 | NS | NS | NS | 1.16 ± 0.27 | 0.51 ± 0.03 | 1.29 ± 0.38 | 1.18 ± 0.34 |
| TNF | 0.051 | NS | NS | 0.79 ± 0.33 | 1.62 ± 0.79 | 0.30 ± 0.12 | 1.41 ± 0.34 |
| IL-10 | NS | NS | NS | 0.95 ± 0.18 | 1.16 ± 0.46 | 1.53 ± 0.34 | 1.74 ± 0.63 |
| PTGS2 | NS | NS | NS | 1.18 ± 0.28 | 0.46 ± 0.06 | 1.20 ± 0.39 | 1.42 ± 0.15 |
Abbreviation: NS, not significant.
Effects of sex, (+)-naloxone, and sex–(+)-naloxone interaction were determined by linear mixed model analysis, and P values are reported when P < 0.10.
Discussion
Fetal exposure to inflammatory insults in utero can modulate the course of development, eliciting adaptive responses that modify phenotype to cause elevated susceptibility to metabolic diseases in offspring (3, 4). Maternal TLR4 signaling is implicated in integrating short-term or chronic inflammatory signals from maternal microbial, nutritional, and environmental triggers to mediate a range of effects on fetal metabolic, neurologic, and immune system development (7, 23, 51). In this study we demonstrate that administration of a novel small molecule TLR4 antagonist, (+)-naloxone, is an effective treatment to mitigate the adverse impact of inflammation-induced fetal programming. In particular, coadministration of (+)-naloxone in the 48 hours following LPS challenge in late gestation was found to be effective in reducing the impact of inflammation on increased adiposity, reduced muscle, and elevated leptin in adult male offspring. The differential effects of LPS and (+)-naloxone on male and female offspring were linked with, and likely explained by, different degrees of proinflammatory cytokine induction and modulation depending on fetal sex.
Our data show that fetal exposure to LPS in late gestation programed altered development, even though the LPS insult was modest. The programming effects were strongest in male offspring, causing elevated central fat deposition and reduced muscle mass associated with elevated leptin in adulthood. The current results share features with outcomes of similar studies in rodent models and provide new insight. In rats, LPS administered on days 8, 10, and 12 of gestation resulted in increased adipose tissue deposition, insulin resistance, and high serum levels of leptin in adult male but not female offspring (24). A similar LPS administration protocol in rats showed increased adiposity and adipocyte hypertrophy, plus elevated blood pressure and altered renin angiotensin system activation in offspring, although sex-specific effects were not reported (52). In mice, male offspring were shown to be more susceptible than females to glucose intolerance and insulin resistance after LPS administration to pregnant dams on gestation days 15 and 17, but the study did not measure adiposity, muscle mass, or leptin (28). Others report a comparable maternal LPS exposure model in mice, in which female offspring exhibit elevated adiposity, but no change in insulin sensitivity (53).
LPS acts through TLR4 ligation to cause elevated synthesis of proinflammatory TNF, IL-6, IL-1B, and other cytokines in maternal and fetal tissues (34, 45, 48). TNF, IL-1, and IL-6 are implicated in attenuating fetal metabolic programming, as administering these mediators to pregnant dams can recapitulate effects on offspring adiposity, insulin synthesis and sensitivity, and locomotor activity (25, 26, 54). Suppression of TLR4-induced cytokines is therefore inferred to be the key mechanism by which (+)-naloxone reverses inflammation-induced changes to development. Gene expression analysis showed that (+)-naloxone treatment inhibited placental Il1a, Il1b, and Il6, fetal membrane Il6, and fetal brain Il1a, Il1b, and Il6, as well as the inflammation-resolving cytokine Il10. (+)-Naloxone was also effective in maternal tissues, suppressing Il1a, Il6, and Il10 in decidua, as well as Il6 and Il10 in myometrium. These results are consistent with previous studies showing that (+)-naloxone suppresses proinflammatory cytokines, causing fetal loss and preterm delivery after induction by LPS or heat-euthanized Escherichia coli (34). Our data imply that (+)-naloxone protects the developing fetus from cytokines synthesized locally in fetal tissues, or transported across the placenta from maternal tissues. Elevated inflammatory cytokines in maternal and placental tissues can also impact the fetus through indirect effects on placental vascular integrity, transport function, and nutrient supply (51, 55). It seems likely that (+)-naloxone also protects placental function from inflammatory damage, although this requires formal evaluation.
The sexually dimorphic impact of LPS on offspring phenotype was associated with, and likely caused by, a greater proinflammatory response in male fetuses after maternal LPS challenge, with higher fetal brain Il10 and myometrial Il1a, Il1b, Il6, and Il10 expression induced in implantation sites of male than female fetuses. To our knowledge, there are no previous studies investigating sex-specific responses of placental or uterine tissues to LPS in mice. In humans, male placental trophoblast cells treated with LPS produce more TNF than do trophoblasts from female placentae (56). The fetal brain is particularly susceptible to inflammatory injury, and several previous studies in rodent models report a greater impact of fetal inflammatory injury on male offspring (57), linked with estrogen- and testosterone-modulated inflammatory cytokine expression in microglia and astrocytes, notably including elevated IL-10 expression in male primary astrocytes (58).
Sex-specific differences in TLR4 pathways are one potential mechanism of greater cytokine induction in male tissues. In humans, placenta from male fetuses is reported to express higher TLR4 than female placenta (56). However, this was not recapitulated in mice in the current study, where we identified similar Tlr4 expression in placental, fetal, and uterine tissues associated with male and female fetuses. Other sex-dependent differences in regulation of TLR4 signaling or transcriptional response could conceivably explain males being more susceptible to proinflammatory insult during gestation. Tissue-resident or blood-borne immune cells express high levels of TLR4, and in humans umbilical vein blood cells from male fetuses respond to LPS with greater cytokine production than do female fetuses (59). It was surprising that maternal decidual and myometrial tissue exhibited higher cytokine expression when associated with male fetuses. This may be because male fetuses evoke a stronger immune response than do females due to expression of male histocompatibility antigens such as HY, resulting in phenotype differences in decidual and placental immune cells (60, 61). In humans, a greater capacity for cytokine synthesis is reflected in higher placental macrophage numbers in male compared with female fetuses (62). Differential responsiveness to LPS is also seen in peripheral blood immune cells of pregnant women, with higher cytokine production when a male fetus is present (63).
(+)-Naloxone was effective in reversing some but not all of the sexually dimorphic effects of LPS on offspring phenotype. In male progeny, (+)-naloxone protected from quadriceps muscle loss and increased plasma leptin, and partially reversed increased epididymal fat. In females, (+)-naloxone protected against LPS-induced reduced muscle mass, reduced adiponectin, and an elevated leptin/adiponectin ratio [a measure of metabolic syndrome and diabetes risk (64)]. Consistent with sex-specific effects, (+)-naloxone modulation of LPS-induced cytokines was different for tissues associated with male and female fetuses. An interaction between fetal sex and (+)-naloxone was most notable for decidual Il6, which was more responsive to (+)-naloxone suppression in female implantation sites, with a similar trend for placental Il6 and fetal brain Il1b. Although not statistically significant, myometrial Tnf showed an opposite trend, with a potentially greater effect of (+)-naloxone in tissues associated with a male fetus.
The relatively greater adverse impact of LPS challenge on male as opposed to female fetal developmental programming supports emerging views that female placental and fetal tissues exhibit greater developmental plasticity to accommodate adversity during early life, whereas males trade off faster growth and smaller placental investment for less reserve capacity (62, 65, 66). The current results indicate that females can better sense and adjust to a TLR4-mediated inflammatory insult, as has been demonstrated for a range of dietary, hypoxic, and inflammatory challenges in animal models and humans (62, 66). This ability to accommodate a programming insult often confers survival benefit to females, but it can be associated with long-term constraints on function after birth (62, 66). In contrast, male fetuses often suffer more extensive damage or higher rates of fetal loss, with surviving fetuses experiencing a more debilitating legacy of in utero injury. The current observations add to evidence that “boys live dangerously in the womb” (65, 67), with their greater predisposition to inflammatory cytokine induction making them more vulnerable to LPS and presumably other TLR4-mediated insults, consistent with a higher risk strategy than females where the same challenge induced a lower cytokine response that, at least for IL-6, was more readily attenuated by TLR4 signaling inhibition.
A surprising observation was the effect of (+)-naloxone alone, without LPS challenge, on postnatal growth trajectory in male offspring. Accelerated growth was associated with increased muscle and fat mass at 20 weeks. The mechanism underlying this growth-promoting effect is unclear and requires further investigation. It seems possible that (+)-naloxone antagonizes actions of endogenous TLR4 ligands, which have a physiological role in attenuating late gestation fetal development, to impact postnatal growth rates and adult body morphology. A wide range of DAMPs that are ligands for TLR4 are produced in the placenta, fetus, and uterus (68). Their accumulation in late gestation is thought to influence the timing of parturition and birth through initiating TLR-induced proinflammatory responses (68, 69). Our observations raise the prospect of a physiological pathway that modulates fetal programming of postnatal growth potential via fetal or maternal DAMPs, endogenous TLR4 signaling inhibitors in amniotic fluid (70), or microbial agents in gestational tissues (71). Given the male-specific effect of (+)-naloxone on programming postnatal growth, it may be that a TLR4-mediated sensing mechanism contributes to the difference in growth strategy distinguishing male and female fetuses (62, 65, 66).
The small molecule TLR4 antagonist (+)-naloxone blocks LPS-induced TLR4-mediated signaling in a variety of nonpregnancy models, including the reduction of nuclear factor κB, TNF, and IL-1B in immune cells (42, 43). (+)-Naloxone has attractive pharmacological properties that are favorable for potential use of this drug in clinical or animal production settings. These include the capacity to penetrate placental tissue (38) and the established safety profile of closely related (−)-naloxone in pregnancy and infants (72, 73). In humans, (−)-naloxone is approved for use in pregnancy, and no negative neonatal outcomes are reported (72, 73). Given the lack of opioid receptor activity of (+)-naloxone owing to the stereoselectivity of opioid receptors (41), (+)-naloxone has the advantage over (−)-naloxone of not interfering with endogenous opioid signaling.
It is well recognized that the greatest opportunity for interventions to mitigate early life programming of metabolic disease is before birth (1, 2). Various anti-inflammatory agents, including biologics such as IL-1 receptor antagonist (45) and other cytokine inhibitors (74), are demonstrated in preclinical studies to modulate fetal inflammatory injury and are considered capable of improving fetal programming outcomes. Further studies are warranted to investigate the value of (+)-naloxone as a pharmacological intervention for protecting the fetus from inflammatory injury and altered developmental programming, when there is a rationale and reasonable expectation that treatment could alleviate long-term adverse consequences (75). Male infants are at particular risk in humans, experiencing higher rates of prematurity and stillbirth (76, 77), often associated with greater inflammatory infiltrates and damage to placental function, whereas females appear relatively protected (78, 79). This is clearly evident in maternal asthma, where males respond more severely than females to acute severe exacerbation (62).
Targeting TLR4 to mitigate transmission of maternal adiposity to offspring might be worthy of consideration given the likely involvement of elevated circulating LPS in driving meta-inflammation (14), as well as the demonstration that TLR4 is necessary for obesity-induced inflammation (16, 80). Clearly, however, the ultimate benefit of any prebirth intervention on later life body mass index and related markers of adiposity will depend on multiple factors, including the nature and timing of the in utero insult and postbirth events (81, 82).
Progressing TLR4 inhibitors toward possible clinical application requires further preclinical experiments to investigate long-term consequences for offspring, focusing not just on metabolism but also neurocognitive and immune function, which similar to metabolic dysfunction are also vulnerable to fetal programming (1, 2). Consideration of safety for fetal health and long-term development must be paramount. It will next be important to determine whether administration of (+)-naloxone is efficacious in the event of delayed administration after an acute inflammatory exposure, and with a wider range of proinflammatory triggers, in particular in the setting of chronic meta-inflammation.
Acknowledgments
Financial Support: This work was supported by project and fellowship grants from the National Health and Medical Research Council of Australia (Grants APP1026178 and APP465423), the Canadian Institutes of Health Research (Grant 2011-09-15), and the Australian Research Council (Grant DP110100297). A portion of this work was supported by National Institutes of Health intramural research programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism.
Author Contributions: P.Y.C. completed experiments, analyzed data, prepared figures, and assisted with drafting the manuscript; C.D. completed experiments and analyzed data; D.J.S. completed experiments and analyzed data; M.R.H contributed to experimental design, interpretation of data, and writing the manuscript; K.C.R. provided (+)-naloxone; L.M.M. contributed to analyzing data and writing the manuscript; and S.A.R. devised and oversaw the study, analyzed and interpreted data, prepared the manuscript, and secured funding.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- B6
C57BL/6
- DAMP
damage-associated molecular pattern
- gd
gestational day
- LPS
lipopolysaccharide
- qPCR
quantitative PCR
- TLR
Toll-like receptor
References and Notes
- 1. Ozanne SE, Constância M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3(7):539–546. [DOI] [PubMed] [Google Scholar]
- 2. Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199–205. [DOI] [PubMed] [Google Scholar]
- 3. Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW. Maternal inflammation during pregnancy and childhood adiposity. Obesity (Silver Spring). 2016;24(6):1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui LL, Lam HS, Leung GM, Schooling CM. Late prematurity and adiposity in adolescents: evidence from “Children of 1997” birth cohort. Obesity (Silver Spring). 2015;23(11):2309–2314. [DOI] [PubMed] [Google Scholar]
- 5. Doyle LW, Faber B, Callanan C, Ford GW, Davis NM. Extremely low birth weight and body size in early adulthood. Arch Dis Child. 2004;89(4):347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res. 2006;60(6):751–758. [DOI] [PubMed] [Google Scholar]
- 7. Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal obesity, inflammation, and developmental programming. BioMed Res Int. 2014;2014:418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reynolds CM, Vickers MH, Harrison CJ, Segovia SA, Gray C. High fat and/or high salt intake during pregnancy alters maternal meta-inflammation and offspring growth and metabolic profiles. Physiol Rep. 2014;2(8):e12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vohr BR, Poggi Davis E, Wanke CA, Krebs NF. Neurodevelopment: the impact of nutrition and inflammation during preconception and pregnancy in low-resource settings. Pediatrics. 2017;139(Suppl 1):S38–S49. [DOI] [PubMed] [Google Scholar]
- 11. Ferguson KK, Chin HB. Environmental chemicals and preterm birth: biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4(1):56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353(17):1848–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. [DOI] [PubMed] [Google Scholar]
- 14. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 15. Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Könner AC, Brüning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22(1):16–23. [DOI] [PubMed] [Google Scholar]
- 17. Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. 2011;117(3):151–164. [DOI] [PubMed] [Google Scholar]
- 18. Harju K, Ojaniemi M, Rounioja S, Glumoff V, Paananen R, Vuolteenaho R, Hallman M. Expression of Toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr Res. 2005;57(5 Pt 1):644–648. [DOI] [PubMed] [Google Scholar]
- 19. Moço NP, Martin LF, Pereira AC, Polettini J, Peraçoli JC, Coelho KI, da Silva MG. Gene expression and protein localization of TLR-1, -2, -4 and -6 in amniochorion membranes of pregnancies complicated by histologic chorioamnionitis. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):12–17. [DOI] [PubMed] [Google Scholar]
- 20. Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173(7):4286–4296. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez JM, Xu H, Ofori E, Elovitz MA. Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am J Obstet Gynecol. 2007;197(3):296.e1–6. [DOI] [PubMed] [Google Scholar]
- 22. Sheldon IM, Roberts MH. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS One. 2010;5(9):e12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013;131(1):23–30. [DOI] [PubMed] [Google Scholar]
- 24. Nilsson C, Larsson BM, Jennische E, Eriksson E, Björntorp P, York DA, Holmäng A. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology. 2001;142(6):2622–2630. [DOI] [PubMed] [Google Scholar]
- 25. Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, Björntorp P, Albertsson Wikland K, Holmäng A. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281(2):E326–E334. [DOI] [PubMed] [Google Scholar]
- 26. Xu DX, Chen YH, Wang H, Zhao L, Wang JP, Wei W. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol Lett. 2006;163(1):20–29. [DOI] [PubMed] [Google Scholar]
- 27. Qin S, Chen X, Gao M, Zhou J, Li X. Prenatal exposure to lipopolysaccharide induces PTX3 expression and results in obesity in mouse offspring. Inflammation. 2017;40(6):1847–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao M, Yuan L, Yuan MM, Huang LL, Su C, Chen YH, Yang YY, Hu Y, Xu DX. Maternal lipopolysaccharide exposure results in glucose metabolism disorders and sex hormone imbalance in male offspring. Mol Cell Endocrinol. 2018;474:272–283. [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of Toll-like receptor 4. Biol Reprod. 2003;69(6):1957–1963. [DOI] [PubMed] [Google Scholar]
- 30. Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163(5):2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod. 2010;16(4):267–272. [DOI] [PubMed] [Google Scholar]
- 32. Wahid HH, Dorian CL, Chin PY, Hutchinson MR, Rice KC, Olson DM, Moldenhauer LM, Robertson SA. Toll-like receptor 4 is an essential upstream regulator of on-time parturition and perinatal viability in mice. Endocrinology. 2015;156(10):3828–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli–induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200(1):93.e1–8. [DOI] [PubMed] [Google Scholar]
- 34. Chin PY, Dorian CL, Hutchinson MR, Olson DM, Rice KC, Moldenhauer LM, Robertson SA. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci Rep. 2016;6(1):36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robertson SA, Wahid HH, Chin PY, Hutchinson MR, Moldenhauer LM, Keelan JA. Toll-like receptor-4: a new target for preterm labour pharmacotherapies? Curr Pharm Des. 2018;24(9):960–973. [DOI] [PubMed] [Google Scholar]
- 37. Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398–401. [DOI] [PubMed] [Google Scholar]
- 38. Dailey PA, Brookshire GL, Shnider SM, Abboud TK, Kotelko DM, Noueihid R, Thigpen JW, Khoo SS, Raya JA, Foutz SE, Brizgys R, Goebelsmann U, Lo MW. The effects of naloxone associated with the intrathecal use of morphine in labor. Anesth Analg. 1985;64(7):658–666. [PubMed] [Google Scholar]
- 39. Miller RR, Menke JA, Hansen NB, Zwick DL, Bickers RG, Nowicki PT. The effect of naloxone on the hemodynamics of the newborn piglet with septic shock. Pediatr Res. 1986;20(8):707–710. [DOI] [PubMed] [Google Scholar]
- 40. Law WR, Ferguson JL. Naloxone alters organ perfusion during endotoxin shock in conscious rats. Am J Physiol. 1988;255(5 Pt 2):H1106–H1113. [DOI] [PubMed] [Google Scholar]
- 41. Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng W, Li Y, Hou X, Zhang N, Ma J, Ding F, Li F, Miao Z, Zhang Y, Qi Q, Li G, Shen Y, Liu J, Huang W, Wang Y. HSP60 is involved in the neuroprotective effects of naloxone. Mol Med Rep. 2014;10(4):2172–2176. [DOI] [PubMed] [Google Scholar]
- 43. Jiang X, Ni Y, Liu T, Zhang M, Ren H, Xu G. Inhibition of LPS-induced retinal microglia activation by naloxone does not prevent photoreceptor death. Inflammation. 2013;36(1):42–52. [DOI] [PubMed] [Google Scholar]
- 44. Wang TY, Su NY, Shih PC, Tsai PS, Huang CJ. Anti-inflammation effects of naloxone involve phosphoinositide 3-kinase delta and gamma. J Surg Res. 2014;192(2):599–606. [DOI] [PubMed] [Google Scholar]
- 45. Nadeau-Vallée M, Chin PY, Belarbi L, Brien ME, Pundir S, Berryer MH, Beaudry-Richard A, Madaan A, Sharkey DJ, Lupien-Meilleur A, Hou X, Quiniou C, Beaulac A, Boufaied I, Boudreault A, Carbonaro A, Doan ND, Joyal JS, Lubell WD, Olson DM, Robertson SA, Girard S, Chemtob S. Antenatal suppression of IL-1 protects against inflammation-induced fetal injury and improves neonatal and developmental outcomes in mice. J Immunol. 2017;198(5):2047–2062. [DOI] [PubMed] [Google Scholar]
- 46. Chin PY, Dorian C, Sharkey DJ, Hutchinson MR, Rice KC, Moldenhauer LM, Robertson SA. Data from: Toll-like receptor-4 antagonist (+)-naloxone confers sexually dimorphic protection from inflammation-induced fetal programming in mice. figshare 2019. Deposited 21 June 2019. 10.25909/5d0c35fb15657. [DOI] [PMC free article] [PubMed]
- 47. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 48. Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177(7):4888–4896. [DOI] [PubMed] [Google Scholar]
- 49. Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010;151(8):3996–4006. [DOI] [PubMed] [Google Scholar]
- 50. Chin PY, Dorian C, Sharkey DJ, Hutchinson MR, Rice KC, Moldenhauer LM, Robertson SA. Data from: Toll-like receptor-4 antagonist (+)-naloxone confers sexually dimorphic protection from inflammation-induced fetal programming in mice. figshare 2019. Deposited 20 June 2019. 10.25909/5d0b22d61cf97. [DOI] [PMC free article] [PubMed]
- 51. Dimasuay KG, Boeuf P, Powell TL, Jansson T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol. 2016;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao M, Zhang X, Chen X, Mi C, Tang Y, Zhou J, Li X. Prenatal exposure to lipopolysaccharide results in local RAS activation in the adipose tissue of rat offspring. PLoS One. 2014;9(10):e111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu XJ, Wang BW, Zhao M, Zhang C, Chen YH, Hu CQ, Zhao H, Wang H, Chen X, Tao FB, Xu DX. Effects of maternal LPS exposure during pregnancy on metabolic phenotypes in female offspring. PLoS One. 2014;9(12):e114780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lambin S, van Bree R, Vergote I, Verhaeghe J. Chronic tumor necrosis factor-α infusion in gravid C57BL6/J mice accelerates adipose tissue development in female offspring. J Soc Gynecol Investig. 2006;13(8):558–565. [DOI] [PubMed] [Google Scholar]
- 55. Burton GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33(Suppl):S23–S27. [DOI] [PubMed] [Google Scholar]
- 56. Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, Bocking AD. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200(5):532.e1–8. [DOI] [PubMed] [Google Scholar]
- 57. Ardalan M, Chumak T, Vexler Z, Mallard C. Sex-dependent effects of perinatal inflammation on the brain: implication for neuro-psychiatric disorders. Int J Mol Sci. 2019;20(9):E2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chistyakov DV, Azbukina NV, Astakhova AA, Goriainov SV, Chistyakov VV, Sergeeva MG. Sex-mediated differences in LPS induced alterations of TNFα, IL-10 expression, and prostaglandin synthesis in primary astrocytes. Int J Mol Sci. 2018;19(9):E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim-Fine S, Regnault TR, Lee JS, Gimbel SA, Greenspoon JA, Fairbairn J, Summers K, de Vrijer B. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Matern Fetal Neonatal Med. 2012;25(11):2470–2474. [DOI] [PubMed] [Google Scholar]
- 60. Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, Kilby MD. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol. 2017;199(10):3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kieffer TEC, Laskewitz A, Faas MM, Scherjon SA, Erwich JJHM, Gordijn SJ, Prins JR. Lower FOXP3 mRNA expression in first-trimester decidual tissue from uncomplicated term pregnancies with a male fetus. J Immunol Res. 2018;2018:1950879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- 63. Mitchell AM, Palettas M, Christian LM. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav Immun. 2017;60:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18(1):37–45. [DOI] [PubMed] [Google Scholar]
- 65. Sandman CA, Glynn LM, Davis EP. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145(1):R1–R13. [DOI] [PubMed] [Google Scholar]
- 67. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22(3):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154(1):483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nadeau-Vallée M, Obari D, Palacios J, Brien ME, Duval C, Chemtob S, Girard S. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152(6):R277–R292. [DOI] [PubMed] [Google Scholar]
- 70. Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, Neal MD, Yazji I, Jia H, Lin J, Branca MF, Ma C, Prindle T, Grant Z, Shah S, Slagle D II, Paredes J, Ozolek J, Gittes GK, Hackam DJ. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109(28):11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McGuire W, Fowlie PW. Naloxone for narcotic exposed newborn infants: systematic review. Arch Dis Child Fetal Neonatal Ed. 2003;88(4):F308–F311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Debelak K, Morrone WR, O’Grady KE, Jones HE. Buprenorphine + naloxone in the treatment of opioid dependence during pregnancy-initial patient care and outcome data. Am J Addict. 2013;22(3):252–254. [DOI] [PubMed] [Google Scholar]
- 74. Disdier C, Chen X, Kim JE, Threlkeld SW, Stonestreet BS. Anti-cytokine therapy to attenuate ischemic-reperfusion associated brain injury in the perinatal period. Brain Sci. 2018;8(6):E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Taguchi A, Yamashita A, Kawana K, Nagamatsu T, Furuya H, Inoue E, Osuga Y, Fujii T. Recent progress in therapeutics for inflammation-associated preterm birth: a review. Reprod Sci. 2017;24(1):7–18. [DOI] [PubMed] [Google Scholar]
- 76. Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30. [DOI] [PubMed] [Google Scholar]
- 77. Engel PJ, Smith R, Brinsmead MW, Bowe SJ, Clifton VL. Male sex and pre-existing diabetes are independent risk factors for stillbirth. Aust N Z J Obstet Gynaecol. 2008;48(4):375–383. [DOI] [PubMed] [Google Scholar]
- 78. Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11β-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R510–R514. [DOI] [PubMed] [Google Scholar]
- 79. Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):547–550. [DOI] [PubMed] [Google Scholar]
- 80. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hui LL, Wong MY, Leung GM, Schooling CM. The association of infant growth patterns with adiposity in adolescence: prospective observations from Hong Kong’s “Children of 1997” birth cohort. Paediatr Perinat Epidemiol. 2015;29(4):326–334. [DOI] [PubMed] [Google Scholar]