Abstract
Voluntary exercise increases stress resistance by modulating stress-responsive neurocircuitry, including brainstem serotonergic systems. However, it remains unknown how exercise produces adaptations to serotonergic systems. Recruitment of serotonergic systems during repeated, daily exercise could contribute to the adaptations in serotonergic systems following exercise, but whether repeated voluntary exercise recruits serotonergic systems is unknown. In this study, we investigated the effects of six weeks of voluntary or forced exercise on rat brain serotonergic systems. Specifically, we analyzed c-Fos and FosB/ΔFosB as markers of acute and chronic cellular activation, respectively, in combination with tryptophan hydroxylase, a marker of serotonergic neurons, within subregions of the dorsal raphe nucleus using immunohistochemical staining. Compared to sedentary controls, rats exposed to repeated forced exercise, but not repeated voluntary exercise, displayed decreased c-Fos expression in serotonergic neurons in the rostral dorsal portion of the dorsal raphe nucleus (DRD) and increased c-Fos expression in serotonergic neurons in the caudal DR (DRC), and interfascicular part of the dorsal raphe nucleus (DRI) during the active phase of the diurnal activity rhythm. Similarly, increases in c-Fos expression in serotonergic neurons in the DRC, DRI, and ventral portion of the dorsal raphe nucleus (DRV) were observed in rats exposed to repeated forced exercise, compared to rats exposed to repeated voluntary exercise. Six weeks of forced exercise, relative to the sedentary control condition, also increased FosB/ΔFosB expression in DRD, DRI, and DRV serotonergic neurons. While both voluntary and forced exercise increase stress resistance, these results suggest that repeated forced exercise, but not repeated voluntary exercise, increases activation of DRI serotonergic neurons, an effect that may contribute to the stress resistance effects of forced exercise. These results also suggest that mechanisms of exercise-induced stress resistance may differ depending on the controllability of the exercise.
Keywords: chronic exercise, c-Fos, FosB/ΔFosB, immunohistochemistry, raphe, serotonin
1. Introduction
Stress-related psychiatric disorders, including anxiety disorders and major depressive disorder (MDD), are prevalent and have major socioeconomic costs. For example, MDD is the second leading cause of disability-adjusted life years worldwide and a major contributor to the burden of suicide [1]. It is estimated that 16.2 million adults in the United States alone had at least one major depressive episode in 2016 [2]. Major depressive disorder also has a major economic cost, exceeding $83 billion in 2000 in the United States alone, a cost that continues to increase ([3]; for review, see [4]). Current treatments have limited efficacy and are associated with many adverse side effects. With current treatments, only 50% of depressed patients recover within six months [5]. Taken together, these findings indicate a need for more effective treatments with fewer negative side effects. Physical exercise is emerging as reliably efficacious in decreasing depressive symptoms [6], but the mechanisms involved are not known.
A major risk factor for development of MDD is stressful life events, which can precipitate and exacerbate psychiatric disorders, such as MDD and anxiety disorders [7-9]. An increase in stress resistance is one potential mechanism through which physical activity may mediate antidepressant and anxiolytic responses [10-13]. Stress resistance is defined as the ability of an organism to be protected from the negative consequences of future adverse events. This differs from stress resilience, which is the ability of an organism to be able to recover quickly from past adverse events [14]. Indeed, studies in rodents have found that voluntary access to running wheels for a period of six weeks, relative to a sedentary control condition, results in increased stress resistance [15-20].
Investigations into the neurochemical basis of the stress-protective effects of voluntary exercise have focused on the serotonergic (5-hydroxytryptamine; 5-HT) system [17,21,22]. Hyperactivation of 5-HT neurons in the dorsal part of the mid-rostrocaudal dorsal raphe nucleus (DRD) and caudal dorsal raphe nucleus (DRC) is sufficient to produce the behavioral consequences of inescapable stress (IS, a stress paradigm whereby the stress termination is independent of response from the subject), including depression- and anxiety-like behaviors, by sensitizing 5-HT neurons to subsequent mild stressors [23]. Excessive 5-HT efflux in projection sites of the DRD and DRC during mild stress leads to the behavioral consequences of IS, which resemble symptoms of human stress-related disorders, including social avoidance, exaggerated anxiety- and fear-related defensive behavioral responses, and shuttle box escape deficits, through a mechanism involving 5-HT2C receptors [24-26]. Six weeks of voluntary exercise prevents both the IS-induced hyperactivation [19] and subsequent sensitization [27] of 5-HT neurons in the DRD, perhaps through a mechanism involving upregulation of 5-HT1A autoreceptors [18]. Six weeks of voluntary wheel running also reduces mRNA coding for the 5-HT2C receptor within DR projection sites important for producing the behavioral effects of IS [28]. Together, these exercise adaptations in the 5-HT system could contribute to exercise-induced stress resistance.
The mechanisms by which wheel running changes the 5-HT system to enable stress resistance are still unknown. One possibility is that repeated recruitment of DR 5-HT neurons during chronic wheel running leads to the long-term, stable changes in gene expression within the 5-HT system that are thought to confer stress resistance. If this is the case, then wheel running should enhance DR 5-HT neuron activity. Although forced motor activity is associated with activation of DR 5-HT neurons [29-31], whether voluntary wheel running is associated with activation of DR 5-HT neurons is unknown [19]. The current study tests the hypothesis that wheel running elicits both acute and chronic activation of DR 5-HT neurons. Sedentary rats or rats allowed access to running wheels for 6 weeks were euthanized after sedentary conditions or after initiation of wheel running activity during the active phase of the diurnal activity rhythm. Activation of DR 5-HT neurons was quantified indirectly by immunolabeling for the acute and chronic activation markers c-Fos and FosB/ΔFosB within 5-HT neurons, identified with tryptophan hydroxylase, throughout the rostro-caudal extent of the DR. We investigated the effects of both voluntary and forced wheel running on DR c-Fos and FosB/ΔFosB, because both voluntary and forced wheel running prevent the behavioral consequences of IS [32].
2. Material and Methods
2.1. Animals
Adult male Fischer 344 rats (N = 41; F344/NHsd Harlan Laboratories, Indianapolis, IN, USA) arrived from the vendor weighing 161 ± 26 g. The rats were singly housed and randomly assigned to treatment groups. Both food (irradiated 18% protein, Cat. No. 2918.15, Harlan Laboratories) and tap water were provided ad libitum for the duration of the experiment. Rats were kept on a standard 12 h: 12 h light/dark cycle, with lights on at 3:45 AM. Room temperature was maintained at 22 °C. Rats were individually housed in polycarbonate cages (45 cm L × 25.2 cm W × 14.7 cm D; R20 series cage, Ancare, Bellmore, NY, USA). The room was organized by cage type, according to experiment, as follows: Experiment 1: 17 Mini-Mitter wheel (Model 640-0700-00, Mini-Mitter Company, Inc., Bend, OR, USA) cages and 24 standard-housing (no wheel) cages were located on one side of the room; Experiment 2: the opposing side of the room contained 24 Lafayette wheel cages (Model 80850a, Lafayette Instrument Company, Lafayette, IN, USA). Readers should refer to Greenwood et al. [32] for photos of the different wheel environments. The Mini-Mitter wheel environment is comprised of a standard housing cage with a wheel mounted to the inside of the cage, in which the rat lives for 24 hours per day for the entire experiment. The Mini-Mitter wheel environment is the typical housing environment for the majority of long-term voluntary exercise studies. The Lafayette wheel running condition is a wheel that lacks an associated cage; the rat is simply placed on the wheel and remains on the wheel for the duration of the active cycle. Rats in the Lafayette wheel running conditions do not live in this environment 24 hours a day, but rather are moved from a standard home cage into the wheel at the start of the active cycle and are removed from the wheel and placed back into their home cages at the end of the active cycle. Rats are not housed in the Lafayette wheels 24 hours a day, because the Lafayette wheels would be an uncomfortable place for the rats to sleep, since the wheels lack bedding. In Experiment 1, we used a sedentary control condition and a voluntary wheel running condition using the Mini-Mitter caging environment. In Experiment 2, we used a sedentary control condition and a forced wheel running condition using the Lafayette wheel condition. As the voluntary wheel running condition in the Mini-Mitter caging environment is not the optimal control group for the Lafayette forced wheel running group used in Experiment 2, Experiment 2 included the Lafayette voluntary wheel running condition in order to control for the differences in housing between the Mini-Mitter group in Experiment 1 and the Lafayette forced running in Experiment 2. Since we observed no differences between the Mini-Mitter and Lafayette voluntary running conditions, we can confidently conclude that differences observed in the Lafayette forced wheel running group were due to the forced nature of the exercise and not differences in the housing conditions. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, DC, 2011) and were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee. All possible efforts were made to minimize the number of rats used and their suffering.
2.2. Experimental design
2.2.1. Experiment 1. Repeated voluntary exercise for six weeks
The experimental design for Experiment 1 is illustrated in Fig. 1A, B. Upon arrival, rats were weighed and housed individually in cages containing a running wheel (Mini-Mitter), which was locked in order to prevent revolutions. All rats were housed with locked wheels for one week to allow acclimation to the colony. After one week, the rats were split into two treatment groups (N = 17 total): a Mini-Mitter wheel running condition (Voluntary MM, n = 9) and a sedentary, locked wheel control condition (Sedentary, n = 8). Wheels in the cages belonging to the Mini-Mitter wheel running condition were unlocked after acclimation, and these rats were allowed ad libitum access to the wheel 5 days per week. The wheels in the cages of the rats in the locked wheel condition remained locked throughout the experiment. See [32] for pictures of the different wheels and housing conditions.
Fig. 1.
Experimental timelines for Experiments 1 and 2. Abbreviations: LAF, Lafayette wheel running condition; FOR, forced wheel running condition, MM, Mini-Mitter wheel running condition; SED, sedentary; VOL, voluntary wheel running condition.
Each rat was handled twice daily, 4 days per week, and on two additional days during each week, the animals were handled once per day. Rats were weighed once per week during a handling session. Rats were lifted out of their cages for 5-8 seconds at the same times of day as the rats in Experiment 2 were shuttled between their home cage and the Lafayette wheel cage. The rats were handled within 30 minutes before or one hour after the onset of the dark and light cycle, respectively. This additional handling was performed to allow for comparison between the treatment groups of the different experiments by controlling for possible handling effects. In addition, to mimic the conditions of the treatments used in Experiment 2, rats in the wheel running group (Experiment 1) were allowed ad libitum access to running wheels only 5 days per week. On day 6, the rats were handled in the morning and the wheels were locked for 60 hours, at which point the week-long treatment cycle would repeat. After 7 days of acclimation, rats repeated the week-long exercise protocol for 37 days, during which time the rats were handled 10 times. Wheels were locked 2 nights/week (the same nights as for Experiment 2). Wheel revolutions were collected automatically by Vital View software (Mini Mitter Company, Inc.), and the distance traveled was calculated by multiplying wheel revolutions by wheel circumference.
2.2.2. Experiment 2. Repeated voluntary versus forced exercise for six weeks
The experimental design for Experiment 2 is illustrated in Fig. 1A, C. After the rats arrived, they were weighed and single-housed in a cage with the wheel locked for one week. Using a Lafayette wheel cage during the active phase of the diurnal activity rhythm (3:45 PM-3:45 AM) and the standard housing described above during the light phase, the rats were divided into 3 treatment groups (n = 8 per group). The three treatment groups consisted of: 1) ad libitum access to the wheel during the dark phase (Lafayette wheel running) 5 days per week (Voluntary LAF); 2) a forced run (forced running) condition (Forced LAF), where the rats were exposed to a motor-generated moving wheel during the dark phase 5 days per week; and 3) a Sedentary control group where the rats were moved to the Lafayette wheels 5 days per week, but the wheels were locked for the duration of the experiment. Motorized wheels belonging to rats in the Forced LAF group were driven by a motor controlled by the Activity Wheel Monitor software (Lafayette Instruments) according to a protocol preprogramed to closely approximate rats' natural voluntary running behavior based on analyses of prior experiments (Greenwood et al., 2013; Herrera et al., 2016). This pattern is characterized by brief bouts of running (average of 2.04 ± 1.95 min) at various speeds (range 4–17 m/min) interspersed with frequent periods of no running (range 0.33–30 min). Forced LAF rats were confined to the forced wheels for the entire active cycle and were returned to their home cages at the start of the sleep cycle. Pilot experiments revealed that rats forced to wheel run with no prior running experience were tumbled about in the wheel and hung onto the wheel rungs rather than running. Five days of prior experience with voluntary running minimized these non-running behaviors. For this reason, all rats in the Forced LAF condition were placed nightly into Voluntary LAF wheels for the first 5 nights of the experiment.
Each rat was weighed weekly starting on Day 5 of the experiment (Fig. 1). Rats were moved from their home cages 5 nights per week and placed in a Lafayette Instruments wheel environment (i.e., Voluntary LAF, Forced LAF, or Sedentary) just minutes before the beginning of the dark phase and returned to the individually housed polycarbonate cage within one hour after the start of the light phase. On Day 6, the rats were moved to their home cages where they remained for the next 60 hours, at which point the week-long treatment cycle was repeated. Wheel revolutions were collected automatically by Lafayette Activity Wheel Monitor software, and the distance traveled was calculated by multiplying wheel revolutions by wheel circumference. These exercise conditions provide stress resistance effects as measured by social exploration, fear conditioning, and a shuttle box escape task 24 h following inescapable tail shock stress [32].
2.3. Euthanasia
After six weeks of treatment, rats from Experiments 1 and 2 were euthanized in time-matched groups of 3, 135 minutes-350 minutes after the onset of the dark phase, during which rats were exposed to exercise or control treatments as usual. The order was randomly selected for the five total groups, 2 groups from Experiment 1 and 3 groups from Experiment 2, for the first round of perfusions. Once a rat from each of the five treatment groups was euthanized and transcardially perfused the order was repeated until all rats had been euthanized.
2.4. Tissue processing
Brains were post-fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer overnight (12-16 hours), rinsed twice for 12 hours each time in 0.1 M sodium phosphate buffer, and finally placed into cryoprotectant (30% sucrose in 0.1 M sodium phosphate buffer, pH 7.4) until they sank. Brains were then blocked caudal to the mammillary bodies (approximately −5.0 mm with respect to bregma) into forebrain and hindbrain pieces using a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA), snap-frozen in isopentane cooled with dry ice, and stored at −80 °C until sectioning. Brains were sectioned using a Leica cryostat (Model CM1900, Leica GmbH, Wetzlar, Germany) at 30 microns and collected as 6 alternate sets of sections; sections were placed into 1.5 mL of cryoprotectant solution (30% ethylene glycol/ 20% glycerol/ 0.05 M sodium phosphate buffer, pH 7.4) in 24-well tissue culture plates and stored at −20 °C until processing for immunohistochemical staining. Sections were collected in separate sets of wells for the medulla (−15.84 mm bregma to the caudal border of the inferior colliculi at −9.86 mm bregma), for the isthmus/midbrain (−9.86 mm to −7.04 mm bregma), and for the ventral tegmental area (VTA; −7.04 to −5.00 mm bregma).
2.5. Antibodies
The brains were sectioned and processed for immunohistochemical staining of tryptophan hydroxylase (sheep anti-tryptophan hydroxylase (Tph) antibody (Cat. No. T8575, Sigma-Aldrich, St. Louis, MO, USA) as a marker of serotonergic neurons. This antibody has been characterized previously, and has been shown to bind both Tph1 and Tph2 isoforms [34]. The C-Fos antibody used as a marker of neuronal activation in the DR (i.e., rabbit anti-c-Fos antibody, Cat. No. PC-38, Lot No. D00119667; CalBiochem, San Diego, CA, USA) has previously been characterized and has been shown to bind specifically to amino acids 4-17 of the human c-Fos protein [35]. In addition, a second set of sections was stained for FosB/ΔFosB (Cat. No. sc-7203 Lot. C239, Santa Cruz Biotechnology, Santa Cruz, CA, USA; see [36-39]). This antibody recognizes both the transient FosB and the more stable, ΔFosB isoforms [40]. Importantly, the ΔFosB isoform accumulates in cells during repeated activity and thus can be used as a marker of chronic cellular activation.
2.6. Imnmnohistochemistry
Immunohistochemistry for c-Fos and FosB/ΔFosB was conducted on free-floating tissue in 12-well tissue culture plates in 1.0 mL of solution and gently shaken on an orbital shaker throughout the staining process. Unless stated otherwise, tissue was washed for 15 minutes in-between steps. Tissue was rinsed twice in a 0.05 M phosphate-buffered saline solution (PBS), then placed in 0.05 M PBS containing 1% hydrogen peroxide (H2O2) for 15 min. Tissue was then rinsed twice with 0.05 M PBS containing 0.3% Triton X-100 (PBST). Sections were incubated at 4 °C for 60 hours with a rabbit anti-c-Fos polyclonal antibody (1:1000) in PBST containing 0.1% bovine serum albumin (BSA) and 4% normal donkey serum (Cat. No. S30-100ML, Millipore Corporation, Billerica, MA USA). For FosB/ΔFosB immunostaining, tissue was incubated overnight with a rabbit anti-FosB polyclonal antibody (1:1000) in PBST containing 4% normal donkey serum. After primary antiserum incubation, tissue was washed twice in PBST, followed by incubation with 1:400 biotinylated donkey anti-rabbit IgG (Cat. No. 711-065-152, Lot No. 86149, Jackson Immunolabs, West Grove, PA, USA) in PBST for 90 min. Tissue was then washed twice in PBST, followed by incubation with an avidin-biotin-peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:500 avidin, 1:2000 biotin; Vector Laboratories, Burlingame, CA, USA) in 0.05 M PBS for 90 min. Tissue was then washed twice in PBST, then placed in a peroxidase substrate solution (SG substrate, Cat No. SK4700, Vector Laboratories, diluted as recommended by vendor) in 0.05 M PBS for 10 min. After the chromogen reaction, tissue was immediately washed twice in 0.05 M PBS, then placed in 0.05 M PBS containing 1% H2O2 for 15 min. Tissue was then washed twice in PBST, followed by a 60 h incubation in sheep anti-tryptophan hydroxylase antibody (1:2500) in PBST containing 4% normal donkey serum at 4 °C. After 4 nights, tissue was then washed twice in PBST, followed by a 90-min incubation in biotinylated rabbit anti-sheep IgG (1:200; Cat. No. PK-6016, Vectastain Elite, Vector Laboratories) in 0.05 M PBS. After incubation, tissue was washed twice in PBST, followed by incubation with an avidin-biotin-peroxidase complex (Elite ABC reagent; Cat. No. 6100, 1:250 avidin, 1:1000 biotin; Vector Laboratories) in PBST for 90 min. Tissue was washed twice in PBST, followed by an optimal incubation in 0.01% 3-3’-diaminobenzidine tetrahydrochloride (DAB, Cat. No. D9015; Sigma-Aldrich) in 0.05 M Tris-buffered saline (TBS) solution containing 0.0066% H2O2. Once optimal staining was achieved, the tissue was rinsed twice in 0.05 M PBS to stop the reaction. Brain sections were stored in 0.1 M sodium phosphate buffer containing 0.01% NaN3 at 4 °C until tissue mounting. Brain sections were then rinsed in 0.1 M sodium phosphate buffer and then rinsed in 0.15% gelatin diluted in distilled H2O, then mounted on glass microscope slides (VistaVision UniMark microscope slides, Cat. No. 16005-106; VWR International, Aurora, CO, USA), dehydrated through an alcohol series, and cleared with xylenes. Slides were mounted with cover slips using mounting medium (Entellen® Rapid Embedding Agent for Microscopy; Cat. No. 107961 Merck KGaA, Darmstadt, Germany).
2.7. Cell counting
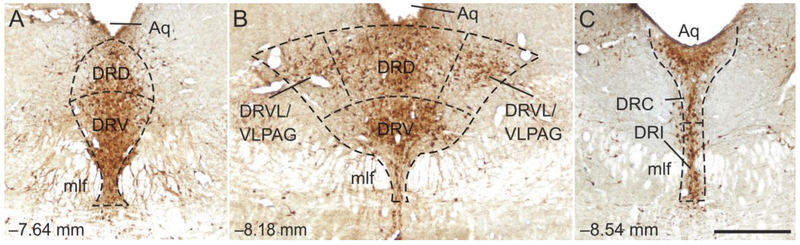
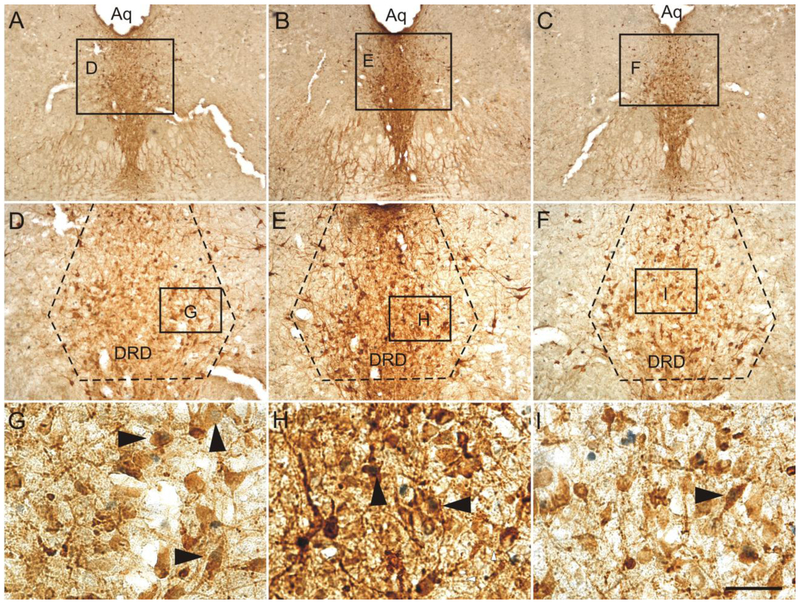
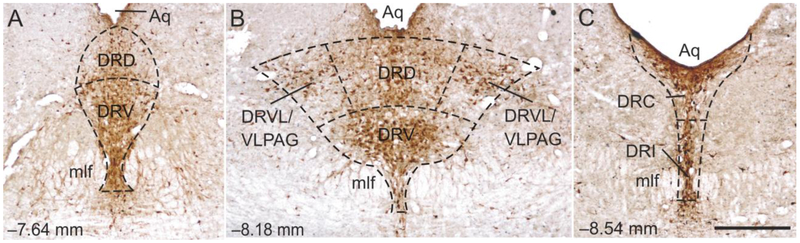
Three rostrocaudal levels of the DR were chosen for analysis (−7.64, −8.18, and −8.54 mm bregma). The subdivisions of the DR studied included the dorsal raphe nucleus, dorsal part (DRD), and dorsal raphe nucleus, ventral part (DRV) at −7.64 mm bregma, the DRD, DRV, and dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG) at −8.18 mm bregma, and the dorsal raphe nucleus, caudal part (DRC), and dorsal raphe nucleus, interfascicular part (DRI) at −8.54 mm bregma (Figs. 2-4). Rostrocaudal levels and anatomical divisions of the brainstem raphe complex were based on comparison of immunostained sections to a stereotaxic rat brain atlas (Paxinos and Watson, 1998) and an atlas of tryptophan hydroxylase immunostaining in the rat DR (Abrams et al., 2004). Cell counting of c-Fos (PC38)- or FosB/ΔFosB-immunoreactive (ir)/Tph-ir neurons, c-Fos (PC38)- or FosB/ΔFosB-ir/Tph-immunonegative cells, and total Tph-ir neurons was performed. Cells were counted from both left and right sides of the DRVL/VLPAG and summed to give a total number of cells. All remaining cell counts were from midline subdivisions. Cell counts were performed using brightfield microscopy at a total magnification of 100x. If necessary, a total magnification of 400x was used to verify double labeling. The experimenter was blind to the treatment groups throughout cell counting. (Figs. 2-4).
Fig. 2.
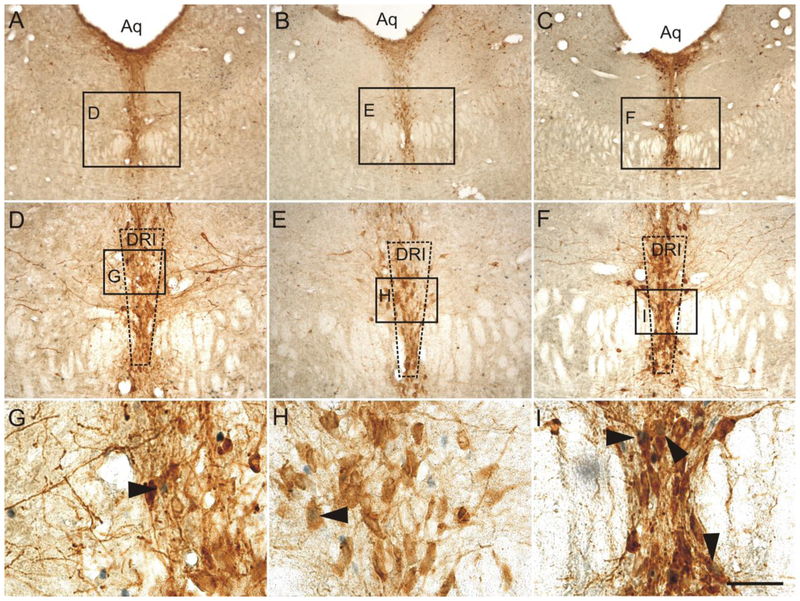
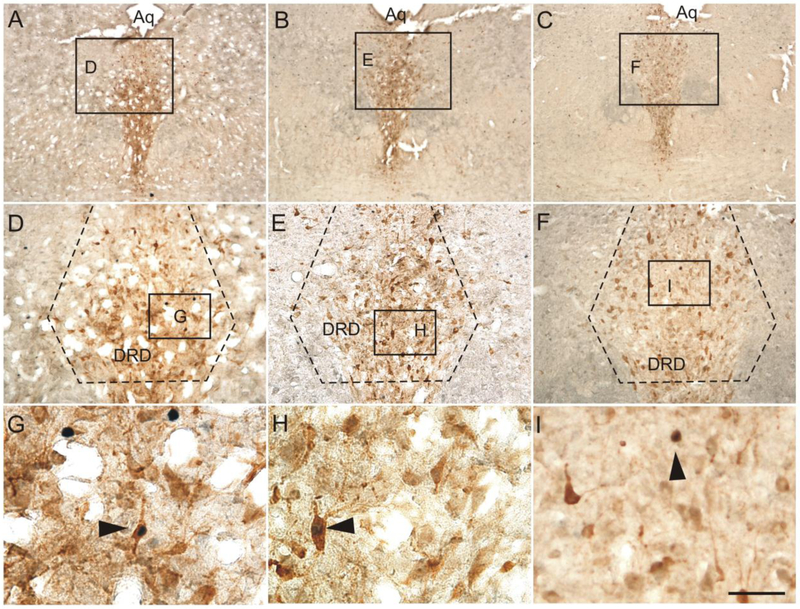
Low magnification photomicrographs illustrating c-Fos (PC38) and tryptophan hydroxylase (Tph) immunostaining from different rostrocaudal levels of the rat dorsal raphe nucleus (DR) in Experiment 1. C-Fos (PC38) immunostaining can be identified by a dark blue/black nuclear stain. Tryptophan hydroxylase-immunoreactive (ir) neurons and dendrites can be identified by a red/brown cytoplasmic stain. The rostrocaudal levels selected for analysis were: (A) −7.64 mm bregma, (B) −8.18 mm bregma, and (C) −8.54 mm bregma. The subdivisions of the DR that were analyzed at each rostrocaudal level are delineated by dashed lines. Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus. Scale bar, 500 μm.
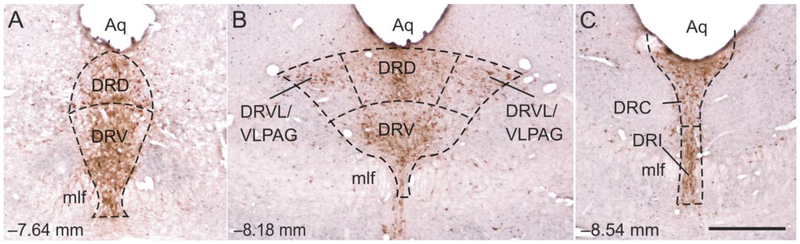
Fig. 4.
Low magnification photomicrographs illustrating FosB/ΔFosB and tryptophan hydroxylase (Tph) immunostaining from different rostrocaudal levels of the dorsal raphe nucleus (DR) in Experiment 2. FosB/ΔFosB immunostaining can be identified by a dark blue/black nuclear stain. Tryptophan hydroxylase-immunoreactive (ir) neurons and dendrites can be identified by a red/brown cytoplasmic stain. The rostrocaudal levels selected for analysis were (A) −7.64 mm bregma, (B) −8.18 mm bregma, and (C) −8.54 mm bregma. The subdivisions of the DR that were analyzed at each rostrocaudal level are delineated by dashed lines. Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus. Scale bar, 500 μm.
2.8. Statistical analysis
Statistical analyses were conducted using the software package IBM Statistical Package for the Social Sciences (version 24.0, SPSS Inc., Chicago, IL, USA). For analysis of body weight in Experiments 1 and 2, a linear mixed model analysis was used to calculate differences in bodyweight with exercise, week, and exercise by week used as the fixed effects. The SPSS syntax can be found in the supplemental material. If a two-tailed test with a level of significance of 0.05 was reached, post hoc analysis was carried out using Fisher’s least significant difference (LSD) test.
For analysis of distance run in Experiment 2 a linear mixed model analysis was used to calculate differences in distance with exercise, day, and exercise by day used as the fixed effects. The SPSS syntax can be found in the supplemental material. If a two-tailed test with a level of significance of 0.05 was reached, post hoc analysis was carried out using Fisher’s least significant difference (LSD) test to understand differences in wheel running by week. The SPSS syntax can be found in the supplemental material.
A two-tailed Fisher’s least significant difference (LSD) test was used to understand if there were differences in running distances the night of euthanasia between the Forced LAF and Voluntary LAF groups. The SPSS syntax can be found in the supplemental material.
For analysis of immunohistochemistry data, c-Fos- (PC38) immunoreactive (ir) cells and c-Fos (PC38)-ir/Tph-ir neurons and, additionally for Experiment 2, FosB/ΔFosB-ir cells and FosB/ΔFosB-ir/Tph-ir neurons in each rostrocaudal level of each DR subregion in each treatment group for each animal were generated. Extreme outliers (2.4%, c-Fos studies; 2.9%, FosB/ΔFosB study) were identified and removed using Grubbs’ test for single outliers using two-sided α = 0.05 [41]. All tests were two-tailed, with a level of significance of 0.05. We used a linear mixed model (LMM), modeling the number of double immunostained serotonergic neurons within each rostrocaudal level (units of mm bregma and included the values: −7.64, −8.18, and −8.54) of each raphe subregion (DRD, DRV, DRVL/VLPAG, DRC, DRI). In addition, 16 covariate structures were used, including: ARMA (1,1); compound symmetry; correlation compound symmetry; diagonal; first-order analytic; first-order ante-dependence; first-order autoregressive; first-order factor analytic; heterogeneous first-order autoregressive; heterogeneous compound symmetry; heterogeneous Toeplitz; Huynh-Feldt, identity; toeplitz; unstructured; and unstructured correlations. The SPSS syntax for the level one model can be found in the supplemental material. To determine the model of best fit, the surveyed covariance structures were compared to minimize −2 log-likelihood. Following this analysis, if a main effect or interaction between a main effect and any other factor reached p < 0.05, post hoc pairwise comparisons were made with Fisher’s least significant difference (LSD) test. The SPSS syntax for the post hoc analysis can be found in the supplemental material.
Furthermore, in the immunohistochemistry analysis, no post hoc analyses were conducted at specific points in the rostrocaudal extent of the DR if one of the group sample sizes was below 50% of the full sample size for its respective treatment group. Additionally, post hoc analyses were conducted only when the overall LMMs yielded significant effects of exercise treatment, raphe subregion, or exercise treatment within raphe subregion. Data are presented as means ± standard error of means (SEM). Two-tailed significance was set at p < 0.05.
3. Results
3.1. Experiment 1. Repeated voluntary exercise for six weeks
3.1.1. Body weight and distance run
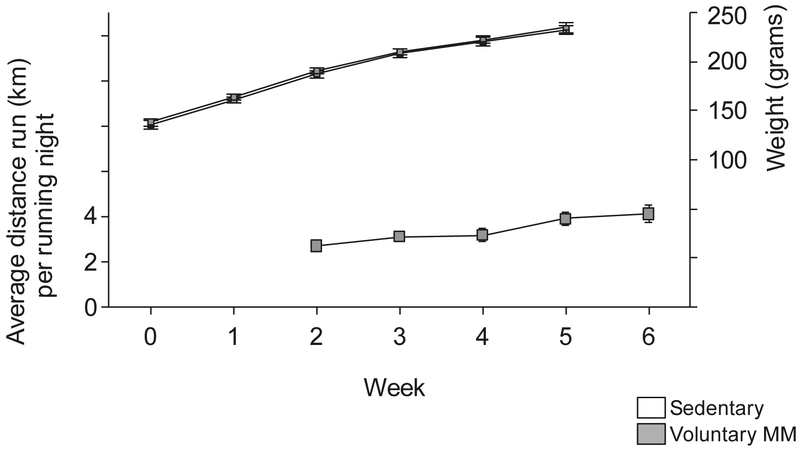
Analysis of body weight data revealed that there were no differences between the treatment groups (Fig. 5, Table S1). The first week of running data was lost due to a computer failure. The mean (SEM) running distance on the night of perfusion was 1729 (397) meters.
Fig. 5. Body weight and distance run.
The left y-axis depicts distance run per night by rats in Mini-Mitter wheels. Data represent mean ± SEM each week studied. The right y-axis depicts the body weight throughout the study.
3.1.3. c-Fos (PC38)-ir/Tph-ir neurons in the DR
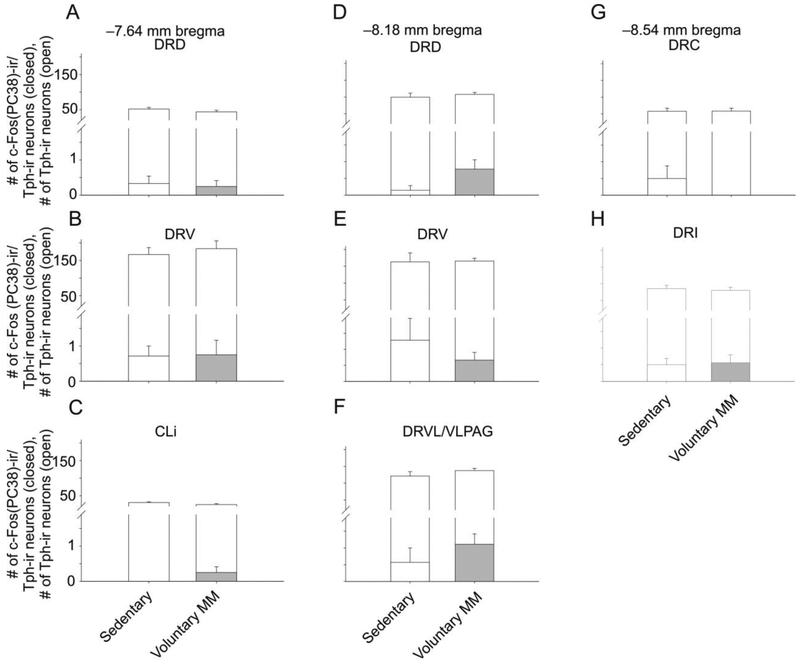
Analysis of c-Fos (PC38)-ir/Tph-ir and c-Fos (PC38)-ir/Tph-immunonegative neurons using LMM analysis revealed no significant effects of voluntary exercise versus sedentary condition in an overall model (Fig. 6, Tables 1 and S2).
Fig. 6.
Graphs illustrating numbers of c-Fos (PC38)-immunoreactive (ir)/tryptophan hydroxylase- (Tph-)ir cells from different rostrocaudal levels of the rat dorsal raphe nucleus (DR) in Experiment 1. Foreground bars represent the numbers of c-Fos (PC38)-ir/Tph-ir neurons. Background bars represent the total numbers of Tph-ir neurons (both c-Fos (PC38)-ir/Tph-ir neurons and c-Fos-immunonegative/Tph-ir neurons) within each subdivision. Graphs are arranged (A-H) according to rostrocaudal level in mm from bregma. Values indicate mean + standard error of the mean (SEM). Abbreviations: CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray.
Table 1.
Mean ± SEM numbers of c-Fos (PC38)-ir/Tph-immunonegative cells in Experiment 1
| Rostrocaudal level (mm bregma) |
Subregion1 | Sedentary (mean ± SEM) |
Voluntary MM (mean ± SEM) |
|---|---|---|---|
| −7.64 | DRD | 1.8 ± 0.9 | 2.1 ±0.7 |
| DRV | 2.9 ± 1.1 | 2.0 ± 0.5 | |
| CLi | 0.3 ± 0.2 | 0.3 ± 0.2 | |
| −8.18 | DRD | 1.1 ± 0.5 | 1.6 ± 0.8 |
| DRV | 2.0 ± 1.1 | 1.2 ± 0.6 | |
| DRVL/VLPAG | 3.7 ± 1.2 | 3.2 ± 1.4 | |
| −8.54 | DRC | 1.3 ± 0.6 | 4.3 ± 1.5 |
| DRI | 2.8 ± 0.9 | 11.0 ± 3.4 |
The subregions used for Experiment 1 included: CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray.
3.2. Experiment 2. Repeated voluntary versus forced exercise for six weeks
3.2.1. Body weight and distance run
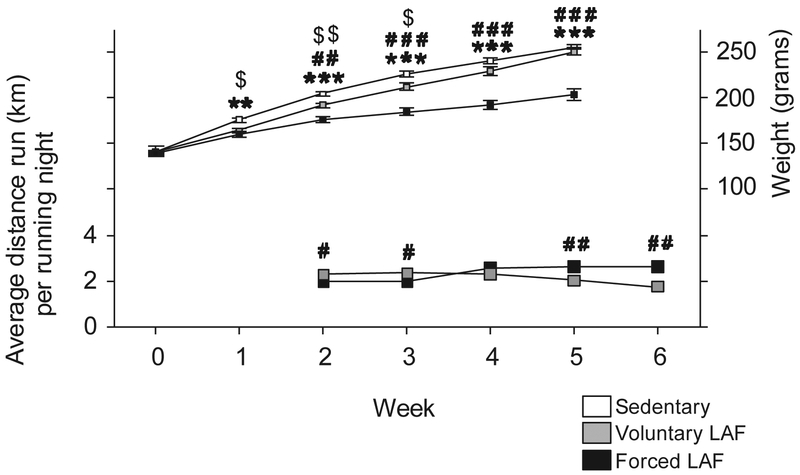
Analysis of group body weight and distance run over the 6-week time course using LMM revealed significant differences in both metrics. Forced LAF, relative to the sedentary condition, decreased body weight from week 1 through week 5. Forced LAF, relative to Voluntary LAF, decreased body weight from week 2 through week 5. Voluntary LAF, relative to sedentary condition, decreased body weight from week 1 through week 3 (Fig. 7, Table S3). The first week of running data was lost due to a computer failure. There was no difference in the distance run between the Voluntary (mean= 644 meters, SEM= 204) and Forced LAF (mean= 1057 meters, SEM= 156) groups on the night of euthanasia (t14 = 1.503, p = 0.15).
Fig. 7. Body weight and distance run of rats placed in LAF wheels.
The left y-axis depicts distance run per night by rats in LAF wheels. Data represent mean ± SEM each week studied. The right y-axis is the body weight throughout the study. **p < 0.01, ***p < 0.001, Sedentary versus Forced LAF exercise; #p < 0.05, ##p < 0.01, ###p < 0.001, Voluntary LAF exercise versus Forced LAF exercise,; $p < 0.05, $$p < 0.01, Sedentary versus Voluntary LAF exercise.
3.2.2. c-Fos (PC38)-ir/Tph-ir neurons in the DR
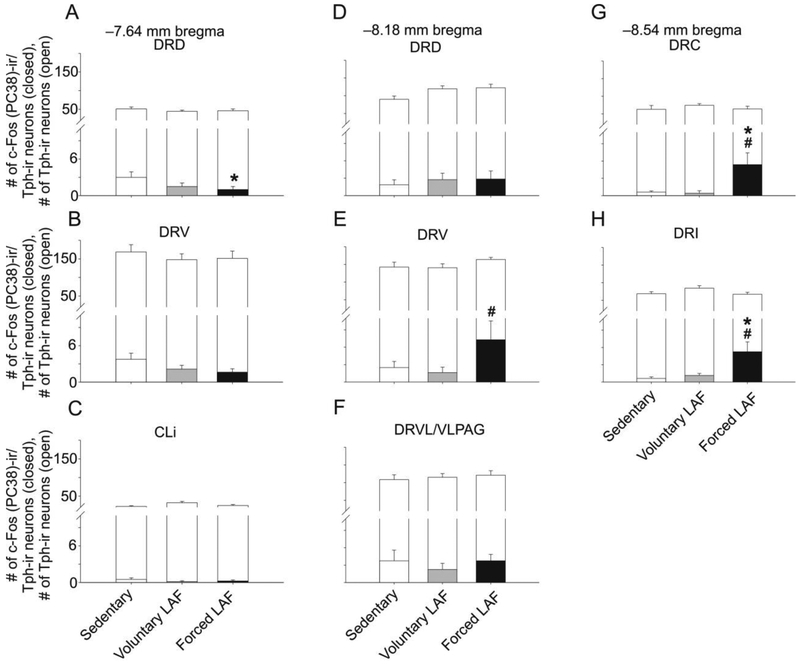
Analysis of c-Fos (PC38)-ir/Tph-ir neurons using LMM analysis revealed an exercise × raphe subregion interaction (F(14,107.4) = 4.263, p < 0.001) and a main effect of exercise (F(2.42.9) = 3.793, p < 0.01; Fig. 8, Table S4). Based on this finding, post hoc analysis was conducted on each of the raphe subregions.
Fig. 8.
Graphs illustrating numbers of c-Fos (PC38)-ir/tryptophan hydroxylase- (Tph-) immunostained cells from different rostrocaudal levels of the rat dorsal raphe nucleus (DR) in Experiment 2. Foreground bars represent the numbers of c-Fos (PC38)-ir/Tph-ir neurons. Background bars represent the total numbers of Tph-ir neurons (both c-Fos (PC38)-immunoreactive/Tph-immunoreactive neurons and c-Fos (PC38)-immunonegative/Tph-immunoreactive neurons) within each subdivision. Graphs are arranged (A-H) according to rostrocaudal level in mm from bregma. Abbreviations: CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray. *p < 0.05, Sedentary versus Forced LAF exercise. #p < 0.05, Voluntary LAF exercise versus Forced LAF exercise.
Exposure to forced exercise compared to the sedentary control condition decreased c-Fos (PC38)-ir/Tph-ir neurons within the DRD at −7.64 mm bregma. In contrast, at −8.18 mm bregma, forced exercise increased c-Fos (PC38)-ir/Tph-ir neurons within the DRV at −8.18 mm bregma, relative to the voluntary exercise condition. Finally, exposure to forced exercise, relative to the voluntary exercise condition as well as the sedentary condition, increased c-Fos (PC38)-ir/Tph-ir neurons within the DRC and the DRI at −8.54 mm bregma (Fig. 8, 9, 10).
Fig. 9.
Photomicrographs illustrate c-Fos (PC38)/tryptophan hydroxylase (Tph) immunostaining in the dorsal raphe nucleus (−7.64 mm bregma) in representative rats from each treatment group in Experiment 2. Photomicrographs illustrate immunostaining in rats exposed to (A, D, and G) sedentary conditions, (B, E, H) voluntary exercise, and (C, F, and I) forced exercise. Black boxes in A, B, and C indicate regions displayed at higher magnification in D, E, and F. Black boxes in D, E, and F indicate regions displayed at higher magnification in G, H, and I. Black arrowheads indicate c-Fos (PC38)-immunoreactive/Tph-immunoreactive neurons (brown cytoplasmic staining with blue/black nuclear staining). Abbreviations: DRD; dorsal raphe nucleus, dorsal part; Aq; cerebral aqueduct. Scale bar: A-C, 500 μm; D-F, 100 μm; G-I, 25 μm.
Fig. 10.
Photomicrographs illustrate c-Fos (PC38)/Tph immunostaining in the dorsal raphe nucleus (−8.54 mm bregma) in representative rats from each treatment group in Experiment 2. Photomicrographs illustrate immunostaining in rats exposed to (A, D, and G) sedentary conditions, (B, E, and H) voluntary exercise, and (C, F, and I) forced exercise. Black boxes in A, B, and C indicate regions displayed at higher magnification in D, E, and F. Black boxes in D, E, and F indicate regions displayed at higher magnification in G, H, and I. Black arrowheads indicate c-Fos (PC38)-immunoreactive/Tph-immunoreactive neurons (brown cytoplasmic staining with blue/black nuclear staining). Abbreviations: Aq; cerebral aqueduct; DRD; dorsal raphe nucleus, dorsal part. Scale bar: A-C, 500 μm; D-F, 100 μm; G-I, 25 μm.
Analysis of c-Fos (PC38)-ir/Tph2-immunonegative neurons using LMM analysis revealed an exercise × raphe subregion interaction (F(14,64.8) = 1.98 8, p < 0.05; Table S4). Table 2 illustrates the mean and standard error of the mean for each bregma level, subregion, and treatment group investigated. Exposure to forced exercise compared to the sedentary control condition revealed decreased c-Fos (PC38)-ir/Tph-immunonegative neurons within the DRV at −7.64 mm bregma. In contrast, at −8.18 mm bregma, forced exercise increased c-Fos (PC38)-ir/Tph-immunonegative neurons within the DRV, relative to the voluntary exercise or sedentary control condition. Additionally, at −8.54 mm bregma, voluntary exercise relative to the sedentary control condition increased c-Fos (PC38)-ir/Tph-immunonegative neurons within the DRC. Finally, exposure to forced exercise, relative to the sedentary control condition, increased c-Fos (PC38)-ir/Tph-immunonegative neurons within the DRI at −8.54 mm bregma.
Table 2.
c-Fos (PC38)-ir/Tph-immunonegative cells
| Rostrocaudal level (mm bregma) |
Subregion1 | Sedentary (mean ± SEM) |
Voluntary LAF (mean ± SEM) |
Forced LAF (mean ± SEM) |
|---|---|---|---|---|
| −7.64 | DRD | 9.0 ± 1.7 | 4.8 ± 0.9 | 7.9 ± 2.0 |
| DRV | 13.6 ± 2.6 | 8.1 ± 2.2 | 6.5 ± 1.7* | |
| CLi | 0.9 ± 0.2 | 0.6 ± 0.3 | 1.0 ± 0.4 | |
| −8.18 | DRD | 8.9 ± 4.3 | 10.1 ± 3.1 | 11.9 ± 2.9 |
| DRV | 4.6 ± 1.7 | 4.3 ± 1.1 | 16.8 ± 4.0**## | |
| DRVL/VLPAG | 25.1 ± 6.6 | 42.9 ± 10.5 | 42.9 ± 9.4 | |
| −8.54 | DRC | 1.7 ± 0.8 | 6.0 ± 2.2* | 3.9 ± 0.7 |
| DRI | 9.5 ± 2.5 | 16.9 ± 5.7 | 22.4 ± 4.8* |
The subregions used for Experiment 2 included: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray.
p < 0.05
p < 0.01, versus Sedentary group
p < 0.01, Voluntary LAF exercise versus Forced LAF exercise,.
3.2.3. FosB/ΔFosB-ir/Tph-ir neurons in the DR
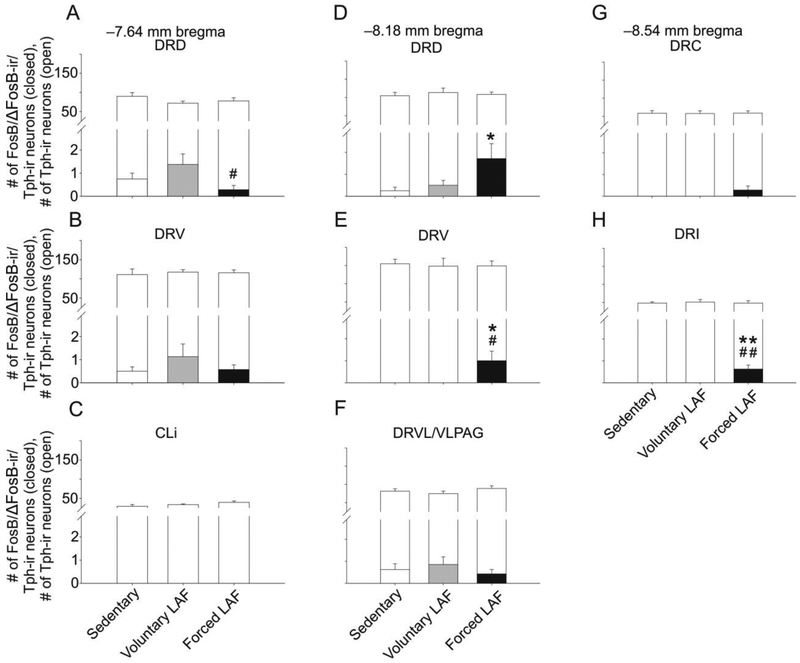
Analysis of FosB/ΔFosB-ir/Tph-ir neurons using LMM analysis revealed an exercise × raphe subregion interaction (F(14.66.2) = 2,454, p < 0.01) and a main effect of exercise (F(2,120.2) = 3.391, p < 0.05) (Fig. 11, Table S5). Based on this finding, post hoc analysis was conducted on each of the raphe subregions.
Fig 11.
Graphs illustrating numbers of FosB/ΔFosB-immunoreactive (ir)/tryptophan hydroxylase- (Tph-) ir cells from different rostrocaudal levels of the rat dorsal raphe nucleus (DR) in Experiment 2. Foreground bars represent the numbers of FosB/ΔFosB-ir/Tph-ir neurons. Background bars represent the total numbers of Tph-ir neurons (both FosB/ΔFosB-immunoreactive/Tph-immunoreactive neurons and FosB/ΔFosB-immunonegative/Tph-immunoreactive neurons) within each subdivision. Graphs are arranged (A-H) according to rostrocaudal level in mm from bregma. Abbreviations: CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; LAF, Lafayette. *p < 0.05. **p < 0.01. Sedentary versus Forced LAF exercise. #p < 0.05, ##p < 0.01 Voluntary LAF exercise versus Forced LAF exercise.
Exposure to forced exercise compared to the sedentary condition increased FosB/ΔFosB-ir/Tph-ir neurons within the DRD and DRV at −8.18 mm bregma and the DRI at −8.54 mm bregma. In contrast, forced exercise compared to voluntary exercise decreased FosB/ΔFosB-ir/Tph-ir neurons within the DRD at −7.46 mm bregma. Within the DRV at −8.18 mm bregma and the DRI at −8.54 mm bregma, forced exercise increased FosB/ΔFosB-ir/Tph-ir neurons compared to the voluntary exercise condition (Fig. 11, 12).
Fig. 12.
Photomicrographs illustrate FosB/ΔFosB/Tph immunostaining in the dorsal raphe nucleus (−7.64 mm bregma) in representative rats from each treatment group in Experiment 2. Photomicrographs illustrate immunostaining in rats exposed to (A, D, and G) sedentary conditions, (B, E, and H) voluntary exercise, and (C, F, and I) forced exercise. Black boxes in A, B, and C indicate regions displayed at higher magnification in D, E, and F. Black boxes in D, E, and F indicate regions displayed at higher magnification in G, H, and I. Black arrowheads indicate FosB/ΔFosB-immunoreactive/Tph-immunoreactive neurons (brown cytoplasmic staining with blue/black nuclear staining). Abbreviations: Aq; cerebral aqueduct; DRD; dorsal raphe nucleus, dorsal part. Scale bar: A-C, 500 μm; D-F, 100 μm; G-I, 25 μm.
Analysis of FosB/ΔFosB-ir/Tph-immunonegative neurons using LMM analysis revealed an exercise × raphe subregion interaction (F(14,59.3) = 3.050, p < 0.01) and a main effect of exercise treatment (F(2,124.2) = 21.731, p < 0.001; Table S5). Table 3 illustrates the mean and standard error of the mean for each bregma level, subregion, and treatment group investigated. Exposure to forced exercise compared to the voluntary exercise and sedentary control conditions increased FosB/ΔFosB-ir/Tph-immunonegative neurons within every subregion at −8.18 mm bregma and within the DRI at −8.54 mm bregma. Exposure to forced exercise also increased FosB/ΔFosB-ir/Tph-immunonegative expression relative to the sedentary control within the DRC at −8.54 mm bregma.
Table 3.
FosB/ΔFosB-ir/Tph-immunonegative neurons
| Rostrocaudal level (mm bregma) |
Subregion1 | Sedentary (mean ± SEM) |
Voluntary LAF (mean ± SEM) |
Forced LAF (mean ± SEM) |
|---|---|---|---|---|
| −7.64 | DRD | 5.3 ± 1.3 | 5.9 ± 1.8 | 7.3 ± 1.7 |
| DRV | 1.6 ± 0.4 | 3.1 ± 1.1 | 1.6 ± 0.8 | |
| CLi | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | |
| −8.18 | DRD | 3.4 ± 1.0 | 3.6 ± 1.0 | 7.3 ± 1.7 *# |
| DRV | 1.4 ± 0.4 | 3.0 ± 0.9 | 7.6 ± 1.7**# | |
| DRVL/VLPAG | 10.1 ± 2.0 | 12.6± 3.1 | 21.8 ± 2.6**# | |
| −8.54 | DRC | 1.3 ± 0.5 | 2.7 ± 0.5 | 3.6 ± 1.1* |
| DRI | 1.0 ± 0.3 | 7.1 ± 1.5* | 11.0 ± 2.3*** |
The subregions used in Experiment 2 included: CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray.
p < 0.05
p < 0.01
p < 0.001, versus Sedentary group
p < 0.05 Voluntary LAF exercise versus Forced LAF exercise.
4. Discussion
Here we report the novel finding that forced exercise modulates expression of activation markers in a subregion-specific manner within serotonergic neurons of the DR. Relative to sedentary conditions, forced exercise—but not voluntary exercise—increased c-Fos and FosB/ΔFosB expression in serotonergic neurons within the DRI. Forced exercise also increased FosB/ΔFosB expression in the mid-rostrocaudal DRD and DRV and decreased c-Fos and FosB/ΔFosB expression in the rostral DRD, relative to sedentary and voluntary exercise conditions, respectively. In addition, forced exercise, relative to sedentary control conditions, decreased body weight throughout the experimental protocol. These observations suggest that repeated activation of specific populations of DR neurons could contribute to the stress-protective effects of forced, but not voluntary, exercise. The pattern of c-Fos expression within nonserotonergic neurons in the DR followed the same general pattern as that observed for c-Fos and FosB/ΔFosB expression within DR serotonergic neurons.
It has been reported previously that both voluntary and forced exercise provide protection against anxiety- and depression-like consequences of stress [32]. Given the differential activation of c-Fos and FosB/ΔFosB in serotonergic neurons within subregions of the DR between the voluntary and forced exercise groups found in the current study, it is likely that these protective effects are the product of activation of different neural circuitry. It is possible that the activation of distinct subsets of serotonergic neurons following forced exercise, but not voluntary exercise, may provide some insight into how the forced exercise condition, specifically, provides stress-protective effects. Within the dorsal raphe nucleus, activation of serotonergic neurons within the DRD, DRV, and DRC subsets (i.e., regions with increased c-Fos and/or FosB/ΔFosB expression in serotonergic neurons in the forced exercise condition) have been shown to be stress-promoting, while activation of the DRVL/VLPAG and DRI subsets have been shown to be stress-protective (for a review within the context of exercise, please see [20,42]). In the current study, the forced exercise condition was associated with increased c-Fos and/or FosB/ΔFosB expression in serotonergic neurons within both stress-promoting regions (i.e., DRD, DRV, and DRC), and stress-protective regions (i.e., DRI). Although these findings are paradoxical, a likely possibility is that forced exercise is both acutely stressful and can confer long-term stress-protective effects. An analogous situation, albeit one that involves different neurobiological mechanisms, is that escapable tail shock, while clearly acutely stressful, is protective against negative consequences of future stressors [14].
The lack of effects of voluntary exercise on c-Fos expression in DR serotonergic neurons is surprising, given the longstanding association of behavioral arousal and locomotor activity with activation of DR serotonergic neuronal firing [29,30,43-47]. It should be noted, however, that all animals in the study, regardless of the exercise condition, would have responded to the transition from the light phase to the dark phase of the light cycle with increases in behavioral arousal. Indeed, there is greater c-Fos expression within DR 5-HT neurons during the active cycle compared to the inactive cycle [48]. Furthermore, it has been shown that some serotonergic neurons, specifically those in the raphe obscurus, show increases in neuronal firing that are highly correlated with the speed of running on a treadmill [49,50]. It is, therefore, possible that both voluntary and forced exercise could increase c-Fos in the raphe obscurus, relative to sedentary control conditions, but we did not include the raphe obscurus in our analysis [51-54]. Additionally, Cunha et al. (2012) [55] report that depletion of 5-HT with p-chlorophenylalanine (PCPA), a selective and irreversible inhibitor of Tph, prevents the antidepressant effects of voluntary exercise in the forced swim and tail-suspension tests, two animal models of depression. It is possible that previously observed effects of voluntary exercise on the 5-HT system and resulting stress resistance could be due to local modulation of terminal 5-HT release, rather than modulation of cell firing. Indeed, voluntary exercise reduces mRNA coding for the terminal 5-HT1B autoreceptor and the serotonin transporter [18], changes that could increase extracellular 5-HT without necessarily impacting neural firing. Additional experiments will be required to understand the mechanisms by which voluntary exercise changes the 5-HT system.
The lack of effect of voluntary wheel running on c-Fos expression in serotonergic neurons in the DR could be because voluntary wheel running does not recruit DR 5-HT neurons to an extent sufficient to induce c-Fos. Alternatively, voluntary exercise might induce c-Fos in DR serotonergic neurons, but the c-Fos response to voluntary exercise might have habituated over the 6 weeks of the study, so that by the time rats were euthanized after an acute bout of wheel running, voluntary exercise no longer induced c-Fos. Indeed, c-Fos responds robustly to acute stimuli, but quickly habituates when stimuli become repeated or chronic. In contrast, FosB family proteins, especially ΔFosB, accumulate with repeated stimuli and remain stably expressed even following chronic exposure to the same stimulus. The lack of an effect of voluntary exercise on FosB/ΔFosB expression in serotonergic neurons in Experiment 2 is, therefore, further evidence that voluntary exercise does not activate serotonergic neurons in the DR. It should be noted, however, that, although voluntary exercise had no effect on c-Fos expression in serotonergic neurons in the DR, it increased c-Fos expression in non-serotonergic neurons in the DRC, and increased FosB/ΔFosB in non-serotonergic neurons in the DRI. Future studies should explore the neurochemical phenotype of these non-serotonergic cells affected by voluntary exercise, and their potential role in the stress-protective effects of voluntary exercise.
In contrast to voluntary exercise, forced exercise increased expression of both acute (c-Fos) and chronic (FosB/ΔFosB) activity markers in several DR subregions. Interestingly, the pattern of c-Fos and FosB/ΔFosB expression in DR serotonergic neurons following exposure to forced exercise was similar to that observed following exposure to a number of conditions previously associated with antidepressant-like behavioral responses. For example, Hale and colleagues showed that exposure to warm ambient temperature, relative to room temperature conditions, increased c-Fos expression in serotonergic neurons within subregions of the DR, including the DRI. Open and randomized, double blind, sham-controlled clinical trials have shown antidepressant effects following exposure to infrared whole-body heating [56,57]. Consistent with the hypothesis that DRI serotonergic neurons are thermosensitive, c-Fos expression in DRI serotonergic neurons correlates with change in core body temperature in rats exposed to home cage control conditions, or forced swimming at 19 °C, 25 °C, and 35 °C [58]. Other conditions associated with antidepressant-like behavioral responses that activate DRI serotonergic neurons include either intratracheal or subcutaneous administration of a heat-killed preparation of Mycobacterium vaccae [59], as well as intraperitoneal administration of lipopolysaccharide [60]. Subcutaneous administration of M. vaccae has antidepressant-like behavioral effects in the forced swim test [59] and has long-term stress resistance effects [58,61-67].
If activation of DRI serotonergic neurons is common to several stress-protective and antidepressant strategies, including forced exercise, it is important to elucidate the mechanisms through which DRI activation elicits these effects. The DRI projects to a distributed system previously implicated in the etiology and pathophysiology of major depressive disorder [20,59,68-72]. This includes the mediodorsal thalamic nucleus, medial orbital cortex, anterior cingulate cortex, dorsolateral prefrontal cortex, ventral pallidum [73], nucleus accumbens [74], and the dorsal and ventral hippocampus [20,75]. The mediodorsal thalamic nucleus, medial orbital cortex, nucleus accumbens, and ventral pallidum have been identified as a neural circuit mediating components of hedonic responses, including liking, wanting, and learning [76-80], and therefore may be particularly relevant to symptoms of anhedonia in MDD. Consistent with DRI activation leading to functional changes in these circuits is the observation that administration of heat-killed M. vaccae increases c-Fos in the DRI and also increases serotonin and serotonin metabolism in the medial prefrontal cortex [60,81], a common effect of antidepressant drugs.
Another explanation for why forced, but not voluntary, exercise increased cFos and FosB/ΔFosB within 5-HT neurons of the DR could be simply that the Forced LAF rats ran more than the Voluntary LAF rats. Indeed, the Forced LAF rats ran more than the Voluntary LAF rats during the last two weeks of the study. However, the voluntary Mini-Mitter rats used in Experiment 1 ran distances very similar to the Forced LAF rats used in Experiment 2, and yet, voluntary wheel running also failed to increase activation markers within DR 5-HT neurons in Experiment 1. Moreover, Forced LAF and Voluntary LAF rats ran equal distances prior to perfusion on the last day of Experiment 2. These observations suggest that the differences in the effects of exercise on activation markers within DR 5-HT neurons is due to the controllability of the exercise, rather than differences in distance run between exercise types.
In contrast to the observed increase in c-Fos expression in DRI serotonergic neurons produced by forced exercise, forced exercise decreased c-Fos expression in serotonergic neurons within the rostral DRD. Greenwood et al. have shown that 6 weeks of voluntary exercise increases htr1a mRNA expression in the rostral DR [16-19]. If this also occurs following forced exercise, an increase in 5-HT1A autoreceptor-mediated inhibition would be expected to decrease the excitability of rostral DRD serotonergic neurons, as observed in our study. In this case, the excitability could be induced by the behavioral activation that occurs during the transition from the inactive to active cycle, the time when the rats were euthanized. Increases in htr1a may be stress protective by preventing hyperactivation of DRD serotonergic neurons, which is thought to be responsible for the negative consequences of inescapable stress [82].
Forced exercise also increased FosB/ΔFosB expression in serotonergic neurons within the mid-rostrocaudal dorsal DR region (DRD, dorsomedial DR). Based on previous studies, activity of the dorsomedial DR is associated with increased anxiety-like defensive behavioral responses. The dorsomedial DR has been shown to be activated by a number of anxiogenic stimuli including anxiogenic drugs [83], anxiety-related neuropeptides (such as urocortin 2 [84-87]), social defeat [88], and inescapable stress [89]. The increase in FosB/ΔFosB in this region could, therefore, reflect some degree of anticipatory anxiety prior to the daily forced exercise, or even in-between exercise bouts during the dark phase, which, for the rats in the forced exercise condition, is associated with uncertainty or unpredictability regarding the onset of each bout of exercise. Consistent with this possibility, we previously observed that forced wheel running elicits some classic hallmarks of chronic stress, including adrenal hypertrophy and attenuation of weight gain [32]. In support of interactions among forced exercise, the hypothalamic-pituitary-adrenal (HPA) axis, and FosB/ΔFosB expression in serotonergic neurons within the mid-rostrocaudal DRD and DRV, chronic treatment of rats with corticosterone in the drinking water (40 μg/ml or 100 μg/ml) for 21 days increases tph2 mRNA expression [90], as well as Tph2 protein expression, and Tph2 activity [91] within the DRD and DRV subregions when assessed 2 h into the inactive, light phase (tph2 mRNA) or 10 h into the light phase (Tph2 protein and activity). The changes observed in the DRD may be particularly relevant to behavioral outcomes of stress-exposure as exposure to inescapable stress increases tph2 mRNA expression in the DRD, but not the DRV subregion, 4 h following stress exposure in a model of learned helplessness [92]. Forced wheel running can, therefore, enable stress resistance despite potential stress and anxiety elicited by forced exercise. Interestingly, this is in stark contrast to forced treadmill training, which also produces signs of chronic stress in rats, but fails to prevent the behavioral consequences of inescapable stress [32]. For reviews of the presumed role of serotonergic neurons within the dorsomedial DR in control of anxiety-like defensive behavioral responses, see [42,93-97].
One limitation of the current study is the use of the Fos-family transcription factors as an index of neural activity. The lack of c-Fos expression is not conclusive evidence for a lack of neuronal activation [98]. Furthermore, it has been well documented that c-Fos induction does not detect cells with a net inhibitory synaptic or transcriptional drive, and c-Fos induction, as a generic marker of transcriptional activation, does not provide evidence for transcriptional activation of specific target genes in a certain cell type of interest [98]. Furthermore, the maximal level of c-Fos protein expression typically occurs between 1 and 3 h following an acute challenge; c-Fos then gradually disappears from the cell nucleus by 4–6 h after challenge, and thus, the timing of assessment of c-Fos expression is also important [98]. Complementary approaches, including in vivo recording in behaving animals, are required in order to gain a complete understanding of the effects of voluntary and forced exercise on brainstem serotonergic neuronal function.
Convergence among high intensity forced exercise, M. vaccae NCTC 11659, anti-inflammatory and immunoregulatory signaling, DRI serotonergic activation, and stress resistance Of potential relevance to neurobiological mechanisms through which forced exercise induces stress resistance, both forced exercise and peripheral immune activation using a heat-killed preparation of the immunomodulatory bacterium, Mycobacterium vaccae NCTC 11659, acutely activate DRI serotonergic neurons [68,81]. Similar to forced exercise, immunization withM vaccae NCTC 11659 has antidepressant-like behavioral effects [59,68] and confers long-lasting stress resistance effects [61-65,99]. Both immunization with M. vaccae NCTC 11659 [100] and forced high intensity exercise (but not low, or moderate intensity forced exercise) for six weeks [101] in mice increase anti-inflammatory cytokines (i.e., interleukin (IL)-10 and transforming growth factor beta (TGFβ)) and regulatory T cells (Treg). Conversely, depletion of Treg selectively increases Tph2 mRNA expression in DRI serotonergic neurons (without affecting Tph2 mRNA expression in any other DR subregion) and prevents the stress resistance effects of M. vaccae NCTC 11659 [65]. In line with these findings, depletion of Treg induces anxiety- and depressive-like behavioral responses [102]. Together, these data suggest a close association between anti-inflammatory and immunoregulatory signaling, DRI serotonergic function, and stress resistance. Interestingly, 6 weeks of voluntary wheel running fails to modulate the balance of circulating pro- and anti-inflammatory cytokines, including tumor necrosis factor (TNF), IL-1β, IL-6, and IL-10, both under basal conditions and following uncontrollable stress, although voluntary exercise can promote an anti-inflammatory state within specific tissues, primarily by increasing IL-10 [103]. Although further studies are needed to clarify effects of different exercise conditions on anti-inflammatory cytokines (i.e. IL-10 and TGFβ) and immunoregulation (i.e. Treg), these data suggest that some forms of forced exercise would be expected to induce anti-inflammatory and immunoregulatory signaling, and promote stress resistance through a circuit involving activation of DRI serotonergic neurons. Despite the clear parallels between immunization with M. vaccae NCTC 11659 and forced exercise on anti-inflammatory and immunoregulatory signaling, activation of DRI serotonergic neurons, and stress resistance, the degree to which immunization with M. vaccae NCTC 11659 and forced exercise mediate stress resistance through similar neurobiological mechanisms will require further study.
5. Conclusions
Forced exercise increased both c-Fos and FosB/ΔFosB in DRI serotonergic neurons, relative to sedentary control conditions. These findings are consistent with a number of studies showing that stimuli that have antidepressant-like and stress-protective behavioral effects, including peripheral immune stimulation and whole-body heating, activate DRI serotonergic neurons. The finding that forced exercise, but not voluntary exercise, increased activation of DRI serotonergic neurons suggests that forced and voluntary exercise may act through different mechanism to enable stress resistance. Future studies are required to determine if DRI serotonergic neurons are indeed causal in the stress-protective effects of forced exercise.
Supplementary Material
Fig. 3.
Low magnification photomicrographs illustrating c-Fos (PC38) and tryptophan hydroxylase (Tph) immunostaining from different rostrocaudal levels of the rat dorsal raphe nucleus (DR) in Experiment 2. C-Fos (PC38) immunostaining can be identified by a dark blue/black nuclear stain. Tryptophan hydroxylase-immunoreactive (ir) neurons and dendrites can be identified by a red/brown cytoplasmic stain. The rostrocaudal levels selected for analysis were (A) −7.64 mm bregma, (B) −8.18 mm bregma, and (C) −8.54 mm bregma. The subdivisions of the DR that were analyzed at each rostrocaudal level are delineated by dashed lines. Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; mlf, medial longitudinal fasciculus. Scale bar, 500 μm.
Highlights.
Forced exercise increased c-Fos expression in DRC, DRI, and DRV serotonergic neurons
Forced exercise increased FosB/ΔFosB expression in DRD, DRI, and DRV serotonergic neurons
Results suggest that the mechanisms of exercise-induced stress resistance may differ depending on exercise controllability.
Acknowledgements
Dr. Christopher A. Lowry is supported by the National Institute of Mental Health (grant number 1R21MH116263), Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (grant number 1 I21 RX002232-01), the Colorado Department of Public Health and Environment (CDPHE; grant number DCEED-3510), and the Alfred P. Sloan Foundation (grant number G-2016-7077). Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd.
We would like to thank Zachary D. Barger for proofreading the manuscript.
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- 5-HT1A
serotonin receptor type 1A
- 5-HT2C
serotonin receptor type 2C
- Aq
cerebral aqueduct
- CLi
caudal linear nucleus
- DRC
dorsal raphe nucleus, caudal part
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL/VLPAG
dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray
- FOR
forced wheel running condition
- -ir
immunoreactive
- LAF
Lafayette
- LMM
linear mixed model
- mlf
medial longitudinal fasciculus
- DR
dorsal raphe nucleus
- MM
Mini-Mitier
- MDD
major depressive disorder
- PBS
phosphate-buffered saline
- SED
sedentary
- Tph
tryptophan hydroxylase
- VOL
voluntary wheel running condition
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferrari AJ, Charlson FJ, Norman RE. Patten SB Freedman G Murray CJL, Vos T, Whiteford HA, Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010, PLoS Med. 10 (2013) e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].NIMH » Major Depression, (n.d.). https://www.nimh.nih.gov/health/statistics/major-depression.shtml (accessed November 17, 2018).
- [3].Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK, The economic burden of depression in the United States: how did it change between 1990 and 2000?, J. Clin. Psychiatry 64 (2003) 1465–75. http://www.ncbi.nlm.nih.gov/pubmed/14728109 (accessed November 18, 2018). [DOI] [PubMed] [Google Scholar]
- [4].Mathers CD, Loncar D, Projections of Global Mortality and Burden of Disease from 2002 to 2030, PLoS Med. 3 (2006) e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirsclifeld RM, Shea T, Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects., Arch. Gen. Psychiatry 49 (1992) 809–16. http://www.ncbi.nlm.nih.gov/pubmed/1417434 (accessed November 18, 2018). [DOI] [PubMed] [Google Scholar]
- [6].Rethorst CD, Wipfli BM, Landers DM, The Antidepressive Effects of Exercise, Sport. Med 39 (2009) 491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- [7].Chorpita BF, Barlow DH, The development of anxiety: the role of control in the early environment., Psychol. Bull 124 (1998) 3–21. http://www.ncbi.nlm.nih.gov/pubmed/9670819 (accessed November 17, 2018). [DOI] [PubMed] [Google Scholar]
- [8].Kendler KS, Karkowski LM, Prescott CA, Causal Relationship Between Stressful Life Events and the Onset of Major Depression, Am. J. Psychiatry 156 (1999) 837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- [9].Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, Alloy LB, Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity., J. Clin. Psychol 70 (2014) 209–23. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR, Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months., Psychosom. Med 62 (n.d.) 633–8. http://www.ncbi.nlm.nih.gov/pubmed/11020092 (accessed November 17, 2018). [DOI] [PubMed] [Google Scholar]
- [11].Taylor AH, Physical activity, anxiety, and stress, in: Phys. Act. Psychol. Well-Being, Routledge, 2001: pp. 10–45. https://www.cabdirect.org/cabdirect/abstract/20013166837 (accessed November 17, 2018). [Google Scholar]
- [12].Salmon P, Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory., Clin. Psychol. Rev 21 (2001) 33–61. http://www.ncbi.nlm.nih.gov/pubmed/11148895 (accessed November 18, 2018). [DOI] [PubMed] [Google Scholar]
- [13].Brosse AL, Sheets ES, Lett HS, Blumenthal JA, Exercise and the Treatment of Clinical Depression in Adults, Sport. Med 32 (2002) 741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- [14].Maier SF, Behavioral control blunts reactions to contemporaneous and future adverse events: Medial prefrontal cortex plasticity and a corticostriatal network, Neurobiol. Stress 1 (2015) 12–22. doi: 10.1016/J.YNSTR.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moraska A, Fleshner M, Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression, Am. J. Physiol. Integr. Comp. Physiol 281 (2001) R484–R489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- [16].Greenwood BN, Fleshner M, Exercise, Learned Helplessness, and the Stress-Resistant Brain, NeuroMolecular Med. 10 (2008) 81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- [17].Greenwood BN, Fleshner M, Exercise, Stress Resistance, and Central Serotonergic Systems, Exerc. Sport Sci. Rev 39 (2011) 140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Greenwood BN, Foley TE, Day HEW, Burhans D, Brooks L, Campeau S, Fleshner M, Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha1b-adrenergic receptor mRNA in the rat raphe nuclei, Biol. Psychiatry 57 (2005) 559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- [19].Greenwood BN, Foley TE, Day HEW, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M, Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons., J. Neurosci 23 (2003) 2889–98. http://www.ncbi.nlm.nih.gov/pubmed/12684476 (accessed July 13, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nicastro TM, Greenwood BN, Central monoaminergic systems are a site of convergence of signals conveying the experience of exercise to brain circuits involved in cognition and emotional behavior, Curr. Zool 62 (2016) 293–306. doi: 10.1093/cz/zow027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Christianson JP. Greenwood BN, Stress-protective neural circuits: not all roads lead through the prefrontal cortex, Stress. 17 (2014) 1–12. doi: 10.3109/10253890.2013.794450. [DOI] [PubMed] [Google Scholar]
- [22].Nicastro TM, Greenwood BN, Central monoaminergic systems are a site of convergence of signals conveying the experience of exercise to brain circuits involved in cognition and emotional behavior., Curr. Zool 62 (2016) 293–306. doi: 10.1093/cz/zow027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rozeske RR, Evans a. K., Frank MG, Watkins LR, Lowry C. a., Maier SF, Uncontrollable, But Not Controllable, Stress Desensitizes 5-HT1A Receptors in the Dorsal Raphe Nucleus, J. Neurosci 31 (2011) 14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Strong PV, Greenwood BN, Fleshner M, The effects of the selective 5-HT2C receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats, Psychopharmacology (Berl). 203 (2009) 665–675. doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- [25].Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF, 5-Hydroxytryptamine 2C Receptors in the Basolateral Amygdala Are Involved in the Expression of Anxiety After Uncontrollable Traumatic Stress, Biol. Psychiatry 67 (2010) 339–345. doi: 10.1016/j.biopsych.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].V Strong P, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN, 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior., Neuroscience. 197 (2011) 132–44. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clark PJ, Amat J, McConnell SO, Ghasem PR, Greenwood BN, Maier SF, Fleshner M, Running Reduces Uncontrollable Stress-Evoked Serotonin and Potentiates Stress-Evoked Dopamine Concentrations in the Rat Dorsal Striatum, PLoS One. 10 (2015) e0141898. doi: 10.1371/journal.pone.0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Greenwood BN, Strong PV, Loughridge AB, Day HEW, Clark PJ, Mika A, Hellwinkel JE, Spence KG, Fleshner M, 5-HT2C Receptors in the Basolateral Amygdala and Dorsal Striatum Are a Novel Target for the Anxiolytic and Antidepressant Effects of Exercise, PLoS One. 7 (2012) e46118. doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jacobs BL, Fomal CA, 5-HT and motor control: a hypothesis, Trends Neurosci. 16 (1993) 346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- [30].Jacobs B, Fornal CA, Activity of Serotonergic Neurons in Behaving Animals, Neuropsychopharmacology. 21 (1999) 9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- [31].Jacobs BL, Fomal CA, Serotonin and motor activity., Curr. Opin. Neurobiol 7 (1997) 820–5. http://www.ncbi.nlm.nih.gov/pubmed/9464975 (accessed November 18, 2018). [DOI] [PubMed] [Google Scholar]
- [32].Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC, Fleshner M, Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex., Eur. J. Neurosci 37 (2013) 469–78. doi: 10.1111/ejn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, Loetz E, Campeau S, Fleshner M, Greenwood BN, Neurochemical and behavioural indices of exercise reward are independent of exercise controllability, Eur. J. Neurosci 43 (2016) 1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hale MW, Dady KF, Evans AK, Lowry C. a., Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling, Exp. Neurol 227 (2011) 264–278. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- [35].Rinaman L, Strieker EM, Hoffman GE, Verbalis JG, Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline.. Neuroscience. 79 (1997) 1165–75. doi: 10.1016/S0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- [36].Grande C, Zhu H, Martin AB, Lee M, Ortiz O, Hiroi N, Moratalla R, Chronic treatment with atypical neuroleptics induces striosomal FosB/ΔFosB expression in rats, Biol. Psychiatry 55 (2004) 457–463. doi: 10.1016/J.BIOPSYCH.2003.08.008. [DOI] [PubMed] [Google Scholar]
- [37].Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ, FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects., Proc. Natl. Acad. Sci. U. S. A 94 (1997) 10397–402. http://www.ncbi.nlm.nih.gov/pubmed/9294222 (accessed December 10, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hiroi N, Martín AB, Grande C, Alberti I, Rivera A, Moratalla R, Molecular dissection of dopamine receptor signaling, J. Chem. Neuroanat 23 (2002) 237–242. doi: 10.1016/S0891-0618(02)00010-8. [DOI] [PubMed] [Google Scholar]
- [39].Hiroi N, Graybiel AM, Atypical and typical neuroleptic treatments induce distinct programs of transcription factor expression in the striatum., J. Comp. Neurol 374 (1996) 70–83. doi: . [DOI] [PubMed] [Google Scholar]
- [40].Berton O, Covington HE, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ, Induction of ΔFosB in the Periaqueductal Gray by Stress Promotes Active Coping Responses, Neuron. 55 (2007) 289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- [41].Grubbs FE, Procedures for Detecting Outlying Observations in Samples, Teclmometrics. 11 (1969) 1–21. [Google Scholar]
- [42].Hassell JE, Yamashita PSM, Johnson PL, Zangrossi H, Shekhar A, Lowry CA, Stress, Panic, and Central Serotonergic Inhibition, in: Stress Neuroendocrinol. Neurobiol, Elsevier, 2017: pp. 153–164. doi: 10.1016/B978-0-12-802175-0.00015-2. [DOI] [Google Scholar]
- [43].Jacobs B, Activation of 5-HT neuronal activity during motor behavior, Semin. Neurosci 7 (1995) 401–408. doi: 10.1016/1044-5765(95)90004-7. [DOI] [Google Scholar]
- [44].Jacobs BL, Azmitia EC, Structure and function of the brain serotonin system.. Physiol. Rev 72 (1992) 165–229. [DOI] [PubMed] [Google Scholar]
- [45].Rasmussen K, Heym J, Jacobs BL, Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats, Exp. Neurol 83 (1984) 302–317. doi: 10.1016/S0014-4886(84)90100-6. [DOI] [PubMed] [Google Scholar]
- [46].Rueter LE, Fomal CA, Jacobs BL, A Critical Review of 5-HT Brain Microdialysis and Behavior, Rev. Neurosci 8 (1997) 117–138. doi: 10.1515/REVNEURO.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- [47].Jacobs BL, Fomal CA, Activity of brain serotonergic neurons in the behaving animal., Pharmacol. Rev 43 (1991) 563–78. http://www.ncbi.nlm.nih.gov/pubmed/1775508 (accessed October 28, 2018). [PubMed] [Google Scholar]
- [48].Januš Onis S, Fite KV, Diurnal Variation of c-Fos Expression in Subdivisions of the Dorsal Raphe Nucleus of the Mongolian Gerbil (Meriones unguiculatus), 2001. https://labs.psych.ucsb.edu/janusonis/skirmantas/janusonis2001.pdf (accessed December 20, 2018). [DOI] [PubMed]
- [49].Veasey SC, Fomal CA, Metzler CW, Jacobs BL, Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats.. Neuroscience. 79 (1997) 161–9. http://www.ncbi.nlm.nih.gov/pubmed/9178872 (accessed February 21, 2018). [DOI] [PubMed] [Google Scholar]
- [50].Veasey SC, Fomal CA, Metzler CW, Jacobs BL, Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats., J. Neurosci 15 (1995) 5346–59. http://www.ncbi.nlm.nih.gov/pubmed/7623157 (accessed February 21, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Richerson GB, Serotonergic neurons as carbon dioxide sensors that maintain ph homeostasis, Nat. Rev. Neurosci 5 (2004) 449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- [52].Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB, Midbrain serotonergic neurons are central pH chemoreceptors, Nat. Neurosci 6 (2003) 1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- [53].Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB, Acidosis-Stimulated Neurons of the Medullary Raphe Are Serotonergic, J. Neurophysiol 85 (2001) 2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- [54].Wang W, Pizzonia JH, Richerson GB, Chemosensitivity of rat medullary raphe neurones in primary tissue culture., J. Physiol 511 (Pt 2) (1998) 433–50. doi: 10.1111/J.1469-7793.1998.433BH.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cunlia MP, Machado DG, Capra JC, Jacinto J, Bettio LE, Rodrigues ALS, Antidepressant-like effect of creatine in mice involves dopaminergic activation, J. Psychophannacol 26 (2012) 1489–1501. doi: 10.1177/0269881112447989. [DOI] [PubMed] [Google Scholar]
- [56].Hanusch K-U, Janssen CH, Billheimer D, Jenkins I, Spurgeon E, Lowry CA, Raison CL, Whole-Body Hyperthermia for the Treatment of Major Depression: Associations With Thermoregulatory Cooling, Am. J. Psychiatry 170 (2013) 802–804. doi: 10.1176/appi.ajp.2013.12111395. [DOI] [PubMed] [Google Scholar]
- [57].Janssen CW, Lowry CA, Mehl MR, Allen JJB, Kelly KL, Gartner DE, Medrano A, Begay TK, Rentscher K, White JJ, Fridman A, Roberts LJ, Robbins ML, Hanusch K, Cole SP, Raison CL, Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder, JAMA Psychiatry. 73 (2016) 789. doi: 10.1001/jamapsychiatry.2016.1031. [DOI] [PubMed] [Google Scholar]
- [58].Kelly KJ, Donner NC, Hale MW, Lowry CA, Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner, Neuroscience. 197 (2011) 251–268. doi: 10.1016/J.NEUROSCIENCE.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lowry CA, Hollis JH, de Vries A, Pan B, Brunet LR, Hunt JRF, Paton JFR, vanKampen E, Knight DM, Evans AK, Rook GAW, Lightman SL, Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior., Neuroscience. 146 (2007) 756–72. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hollis JH, Evans AK, Bruce KPE, Lightman SL, Lowry CA, Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons, Brain. Behav. Immun 20 (2006) 569–577. doi: 10.1016/J.BBI.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [61].Fonken LK, Frank MG, D’Angelo HM, Heinze JD, Watkins LR, Lowry CA, Maier SF, Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction, Nemobiol. Aging 71 (2018) 105–114. doi: 10.1016/j.neurobiolaging.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fox JH, Hassell JE, Siebler PH, Arnold MR, Lamb AK, Smith DG, Day HEW, Smith TM, Simmennan EM, Outzen AA, Holmes KS, Brazell CJ, Lowry CA, Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm, Brain. Behav. Immun 66 (2017) 70–84. doi: 10.1016/j.bbi.2017.08.014. [DOI] [PubMed] [Google Scholar]
- [63].Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, Watkins LR, Maier SF, Lowry CA, Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior, Brain. Behav. Immun 73 (2018) 352–363. doi: 10.1016/j.bbi.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Frank MG, Fonken LK. Watkins LR, Maier SF, Lowry CA, Could Probiotics Be Used to Mitigate Neuroinflammation?, ACS Chem. Neurosci (2018) acschemneuro.8b00386. doi: 10.1021/acschemneuro.8b00386. [DOI] [PubMed] [Google Scholar]
- [65].Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Wheeler KJ, Fox JH, Hassell JE, Greenwood BN, Jansch C, Leclmer A, Schmidt D, Uschold-Schmidt N, Füchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, González A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA, Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice, Proc. Natl. Acad. Sci 113 (2016) E3130–E3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Coimnons KG, Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system, Eur. J. Neurosci 27 (2008) 2611–2618. doi: 10.1111/j.1460-9568.2008.06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Payet JM, Burnie E, Sathananthan NJ, Russo AM, Lawther AJ, Kent S, Lowry CA, Hale MW, Exposure to Acute and Chronic Fluoxetine has Differential Effects on Sociability and Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus of Juvenile Male BALB/c Mice, Neuroscience. 386 (2018) 1–15. doi: 10.1016/j.neuroscience.2018.06.022. [DOI] [PubMed] [Google Scholar]
- [68].Siebler PH, Heinze JD, Kienzle DM, Hale MW, Lukkes JL, Donner NC, Kopelman JM, Rodriguez OA, Lowry CA, Acute Administration of the Nonpathogenic, Saprophytic Bacterium, Mycobacterium vaccae, Induces Activation of Serotonergic Neurons in the Dorsal Raphe Nucleus and Antidepressant-Like Behavior in Association with Mild Hypothermia, Cell. Mol. Nemobiol 38 (2018) 289–304. doi: 10.1007/s10571-017-0564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hale MW, Raison CL. Lowry CA, Integrative physiology of depression and antidepressant drag action: Implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression, Pharmacol. Ther 137 (2013) 108–118. doi: 10.1016/j.pharmthera.2012.09.005. [DOI] [PubMed] [Google Scholar]
- [70].Lowry CA, Lightman SL. Nutt DJ, That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion., J. Psychopharmacol 23 (2009) 392–400. doi: 10.1177/0269881108099956. [DOI] [PubMed] [Google Scholar]
- [71].Raison CL. Hale MW, Williams LE, Wager TD, Lowry CA, Somatic influences on subjective well-being and affective disorders: the convergence of thermosensory and central serotonergic systems, Front. Psychol 5 (2015) 1580. doi: 10.3389/fpsyg.2014.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Drevets WC, Price JL, Furey ML, Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression, Brain Struct. Funct 213 (2008) 93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jones BE, Cuello AC, Afferents to the basal forebrain cholinergic cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine--neurons., Neuroscience. 31 (1989) 37–61. http://www.ncbi.nlm.nih.gov/pubmed/2475819 (accessed November 18, 2018). [DOI] [PubMed] [Google Scholar]
- [74].Van Bockstaele EJ, Biswas A, Pickel VM, Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens.. Brain Res. 624 (1993) 188–98. http://www.ncbi.nlm.nih.gov/pubmed/8252391 (accessed March 1, 2018). [DOI] [PubMed] [Google Scholar]
- [75].Knowland D, Lilascharoen V, Pacia CP, Shin S. Wang EH-J, Lim BK, Distinct Ventral Pallidal Neural Populations Mediate Separate Symptoms of Depression., Cell. 170 (2017) 284–297.el8. doi: 10.1016/j.cell.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Berridge KC, Kringelbach ML, Affective neuroscience of pleasure: reward in humans and animals.. Psychopharmacology (Berl). 199 (2008) 457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Berridge KC, Kringelbach ML, Building a neuroscience of pleasure and well-being., Psychol. Well. Being 1 (2011) 1–3. doi: 10.1186/2211-1522-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kringelbach ML, Berridge KC, The functional neuroanatomy of pleasure and happiness., Discov. Med 9 (2010) 579–87. http://www.ncbi.nlm.nih.gov/pubmed/20587348 (accessed November 18, 2018). [PMC free article] [PubMed] [Google Scholar]
- [79].Peciña S, Smith KS, Berridge KC, Hedonic Hot Spots in the Brain, Neurosci. 12 (2006) 500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- [80].Smith KS, Tindell AJ, Aldridge JW, Berridge KC, Ventral pallidum roles in reward and motivation, Behav. Brain Res 196 (2009) 155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lowry C. a., Hollis JH, de Vries a., Pan B, Brunet LR, Hunt JRF, Paton JFR, vanKampen E, Knight DM, Evans a. K., Rook G. a W., Lightman SL, Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior, Neuroscience. 146 (2007) 756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Maier SF, Watkins LR, Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor, Neurosci. Biobehav. Rev 29 (2005) 829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- [83].Abrams JK, Johnson PL, Hollis JH, Lowry C. a., Anatomic and functional topography of the dorsal raphe nucleus, Ann. N. Y. Acad. Sci 1018 (2004) 46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- [84].Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF, Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala, Neuroscience. 129 (2004) 509–519. doi: 10.1016/J.NEUROSCIENCE.2004.07.052. [DOI] [PubMed] [Google Scholar]
- [85].Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, Lowry C. a., Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GAB Aergic interneurons in the basolateral amygdala, Prog. Neuro-Psychopharmacology Biol. Psychiatry 34 (2010) 1285–1293. doi: 10.1016/j.pnpbp.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Staub DR, Spiga F, Lowry CA, Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus, Brain Res. 1044 (2005) 176–189. doi: 10.1016/J.BRAINRES.2005.02.080. [DOI] [PubMed] [Google Scholar]
- [87].Staub DR, Evans AK, Lowry CA, Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons, Brain Res. 1070 (2006) 77–89. doi: 10.1016/J.BRAINRES.2005.10.096. [DOI] [PubMed] [Google Scholar]
- [88].Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA, Early life experience alters behavior during social defeat: Focus on serotonergic systems, Neuroscience. 136 (2005) 181–191. doi: 10.1016/J.NEUROSCIENCE.2005.07.042. [DOI] [PubMed] [Google Scholar]
- [89].Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF, Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor, Brain Res. 826 (1999) 35–43. doi: 10.1016/S0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- [90].Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA, Elevated tph2 mRNA expression in a rat model of chronic anxiety.. Depress. Anxiety 29 (2012) 307–19. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Donner NC, Siebler PH, Johnson DT, Villarreal MD, Mani S, Matti AJ, Lowry CA, Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis, Psychoneuroendocrinology. 63 (2016) 178–190. doi: 10.1016/j.psyneuen.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Donner NC, Kubala KH, Hassell JE, Lieb MW, Nguyen KT, Heinze JD, Drugan RC, Maier SF, Lowry CA, Two models of inescapable stress increase tph2 mRNA expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus, Neurobiol. Stress 8 (2018) 68–81. doi: 10.1016/j.ynstr.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hale MW, Lowry CA, Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits, Psychopharmacology (Berl). 213 (2011) 243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- [94].Hale MW, Shekhar A, Lowry CA, Stress-related Serotonergic Systems: Implications for Symptomatology of Anxiety and Affective Disorders, Cell. Mol. Neurobiol 32 (2012) 695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lowry C. a., Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A, Serotonergic systems, anxiety, and affective disorder: Focus on the dorsomedial part of the dorsal raphe nucleus, Ann. N. Y. Acad. Sci 1148 (2008) 86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- [96].Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A, Modulation of anxiety circuits by serotonergic systems, Stress. 8 (2005) 233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- [97].Paul ED, Johnson PL, Shekhar A, Lowry C. a., The Deakin/Graeff hypothesis: Focus on serotonergic inhibition of panic, Neurosci. Biobehav. Rev 46 (2014) 379–396. doi: 10.1016/j.neubiorev.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kovács KJ, c-Fos as a transcription factor: a stressful (re)view from a functional map., Neurochem. Int 33 (1998) 287–97. http://www.ncbi.nlm.nih.gov/pubmed/9840219 (accessed May 1, 2019). [DOI] [PubMed] [Google Scholar]
- [99].Loupy KM, Arnold MR, Hassell JE, Lieb MW, Milton LN, Cler KE, Fox JH, Siebler PH, Schmidt D, Noronha SISR, Day HEW, Lowry CA, Evidence that preimmunization with a heat-killed preparation of Mycobacterium vaccae reduces corticotropin-releasing hormone mRNA expression in the extended amygdala in a fear-potentiated startle paradigm, Brain. Behav. Immun 77 (2019) 127–140. doi: 10.1016/j.bbi.2018.12.015. [DOI] [PubMed] [Google Scholar]
- [100].Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C, Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells, Nat. Med 8 (2002) 625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- [101].Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y, Effect of exercise training intensity on murine T-regulatory cells and vaccination response, Scand. J. Med. Sci. Sports 22 (2012) 643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- [102].Kim S-J, Lee H, Lee G, Oh S-J, Shin M-K, Shim I, Bae H, CD4+CD25+ Regulatory T Cell Depletion Modulates Anxiety and Depression-Like Behaviors in Mice, PLoS One. 7 (2012) e42054. doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M, Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats, Brain. Behav. Immun 39 (2014) 87–98. doi: 10.1016/j.bbi.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.