Abstract
Insulin is now well-established as playing multiple roles within the brain, and specifically as regulating hippocampal cognitive processes and metabolism. Impairments to insulin signaling, such as those seen in type 2 diabetes and Alzheimer’s disease, are associated with brain hypometabolism and cognitive impairment but the mechanisms of insulin’s central effects are not determined. Several lines of research converge to suggest that the insulin - responsive glucose transporter GluT4 plays a central role in hippocampal memory processes, and that reduced activation of this transporter may underpin the cognitive impairments seen as a consequence of insulin resistance.
Background: glucose, insulin, and hippocampal memory.
Over the past two decades or so, insulin signalling within the brain has moved from being seen as non-existent (or, at most, a minor player in hypothalamic fuel-sensing1) to being recognised as an important scientific and clinical focus, central to the impact of both type 2 diabetes (T2DM) and Alzheimer’s disease (AD) - indeed, the latter has been characterised in some cases as ‘type 3 diabetes’ 2, 3. The fact that insulin is a key component of hippocampal memory processes, and the concept of systemic insulin resistance extending to the brain such that it impairs both hippocampal metabolism and cognitive function,4-7 are now well-established 6, 8-20.
Study of the metabolic regulation of cognition, and specifically of hippocampal memory processing, began with investigation of glucose, the brain’s primary fuel source (which field in turn emerged from earlier work on the mechanisms modulating the impact of stress on memory21). Reviews both in this issue and elsewhere have covered this history in detail: administration of exogenous glucose, either systemically or directly into the brain, is well established to acutely improve performance on challenging cognitive tests, both in laboratory animals and in a wide range of human populations 22-25. Importantly, glucose supply to the hippocampus was shown to be a constraint on performance of challenging memory tasks, so that cognitive demand depletes ECF glucose levels within brain regions involved in processing the specific task and to an extent that correlates with task difficulty 26, 27. This task-associated depletion was later directly confirmed to be correlated with both increased local lactate production 28 and caused by local glucose uptake and metabolism 29. Depletion is local rather than brain-wide, occurring only in the brain regions in processing a particular task 30-34. Glucose metabolism has become the primary marker for neural and cognitive activity in humans, forming the basis for techniques including fMRI and PET.
Conditions that impair brain glucose supply, such as aging, T2DM or AD, lead to more profound task-associated depletion of local brain glucose correlated with impaired cognitive performance 35-40. Details of the exact causes of task-associated increases in glucose demand remain to be precisely described and may vary, but there are a large number of candidate processes downstream of increased local glycolytic metabolism: for example, metabolic support for the energetically costly Na+/K+ ATPase and promotion of enhanced cellular excitability and synaptic plasticity 41-46.
Given the close relationship between local glucose metabolism and cognitive performance, interest in factors that might modulate brain glucose supply and metabolism increased: insulin was an early, and clinically-important, candidate and one whose receptors had already been shown to be altered, in the rat hippocampus, by water maze training 47. Indeed, insulin was confirmed to be a key player in hippocampal cognitive processes: specific blockade of endogenous intrahippocampal insulin markedly impairs spatial working memory, while physiological doses delivered to the hippocampus enhance it 4-6. Those studies also showed that diet-induced obesity and systemic insulin resistance (DIO; a rat model of lifestyle-induced type 2 diabetes) impairs both cognition and hippocampal metabolism in a manner resembling direct blockade of intrahippocampal insulin 5: the impairment seen resembles the cognitive and metabolic impairment seen in human patients with T2DM, who are especially prone to hippocampal dysfunction 48-57. Enhancement of memory by insulin has also been shown in several human studies 58-64, possibly in at least some cases by increasing insulin-mediated glucose transport through GluT4 65. In vivo studies using microdialysis directly confirmed that insulin acutely stimulates local hippocampal glycolysis 19, and other work has shown that insulin’s modulation of brain glucose metabolism is region-dependent, with cortex and hippocampus being most sensitive 18, 66-72. Nonetheless, the mechanisms by which insulin regulates memory processes are less understood 6, 13, 14, 19, 20, 59, 65, 73-78. Understanding the interplay of metabolic regulators is vital for understanding of both hippocampal function and the impact of e.g. T2DM and AD. The clinical fact that central insulin resistance causes cognitive impairment is now reasonably well-established, but not yet well understood at a cellular or molecular level.
In the periphery, insulin is thought of as a regulator of glucose. However, the converse is also true: release of insulin from the pancreas occurs in response to elevations in blood glucose. The molecular machinery that regulates such release is present in the brain, and has been shown to modulate hippocampal memory 41, 42, 79; moreover, there is increasing evidence for local synthesis and release of insulin in the brain 6, 80-86. This raises the possibility that one role of hippocampal insulin might be an effector of glucose’s procognitive actions, including via increased GluT4 translocation that permits increased glucose flux; this has been suggested 25 but remains untested. One potentially complicating factor both here and in consideration of the links between insulin resistance and cognitive impairment is that insulin transport across the blood-brain barrier may decrease with age87, so that reduced central insulin signaling might be seen even without diminished central insulin sensitivity.
Mechanisms by which insulin enhances memory
The focus of this paper is on GluT4 and insulin resistance - it is explicitly not intended to be a comprehensive review of mechanisms of brain aging - but it is important to note that regulation of GluT4 is far from the only mechanism by which insulin likely regulates cognitive processes: the brief discussion below is incomplete and omits major lines of research such as regulation of neurotransmission and neurotransmitter receptors (including NMDA, AMPA, and GABA receptors 78-88-90). However, it is worth discussing a couple of mechanisms that appear to regulate hippocampal cognition, especially in the context of this issue’s focus on central insulin resistance.
Equally important and conversely, GluT4 in the context of memory processes is not solely regulated by insulin: several other receptor tyrosine kinases (e.g. insulin-like growth factor-1 receptors and TrkB receptors) and other receptors involved in memory formation may regulate GluT4 91-94. Downstream of receptor activation, GluT4 translocation is regulated by several post-receptor signaling molecules critical for maintaining long term memory including insulin-like growth factor 2, brain-derived neurotrophic factor, Ca2+/calmodulin-dependent protein kinase II, phosphoinositide 3-kinase (PI3K), protein kinase A, MAPK, protein kinase-λ, and -protein kinase-ζ 91-101. Some non-receptor activation-dependent conditions are associated with GluT4 trafficking and activity, such as cellular depolarization via elevated intracellular [Ca2+] and elevated extracellular [K+] 102-106; as noted below, this includes recruitment of GluT4 to the synapse as a consequence of neuronal activity 107, a seminal recent finding that is consistent with prior work showing increased cerebellar GluT4 after prolonged exercise108. In general, although insulin’s CNS actions are likely to be mediated in significant partvia GluT4, the two are not synonymous. This is consistent with the fact that although GluT4 and insulin receptors (IR) show some degree of brain co-localization 17, 109, there is not a one-to-one correlation, in contrast to peripheral tissues where the two showtight co-localization.
Antagonism of beta-amyloid
One insight into likely mechanisms of insulin action in the brain comes from clinical studies which show a clear link between T2DM and development of AD, and that many AD patients have reduced hippocampal insulin signalling along with brain hypometabolism and accumulation of beta-amyloid (Aβ)54, 74, 110-117. Several studies including in vitro work suggest that insulin and Aβ oppose each other at a molecular level 118-126; recent work showed that acute administration of oligomeric Aβ to the hippocampus causes rapid cognitive impairment, reduced local glucose metabolism, and impaired translocation of the insulin-regulated glucose transporter GluT4 7, a pattern that closely resembles hippocampal insulin resistance. Related studies, discussed further below, showed that in DIO rats, specific antagonism of intrahippocampal oligomeric Aβ reversed cognitive impairment, suggesting a key interaction between insulin and Aβ in modulation of hippocampal cognitive processing. Insulin regulates Aβ processing and removal from the brain 127, 128, a large literature shows that the mutual antagonism between insulin and beta-amyloid mutually extends to e.g. glucose metabolism, glutamate signalling, and other pathways 60, 129-137, such that preventing impairment from accumulation of oligomeric Aβ is an important procognitive role of hippocampal insulin.
Insulin-regulated aminopeptidase (IRAP)
GluT4 translocates to the cell surface via complex machinery 138, 139 downstream of insulin, primarily through phosphatidyl inositol 3-kinase (PI3K), and Akt140. Activation of Akt inactivates glycogen synthase kinase 3 beta (GSK3β) and activates AS160 141-144. Activation of AS160, along with non-PI3K mechanisms, permits release of GluT4-containing glucose storage vesicles (GSVs) from their intracellular tethers 138, 139, 143, 145-147, allowing GluT4 to move to the cell surface. However, the GSVs contain proteins other than GluT4, including some involved in regulation of memory processes such as insulin-like growth factor 2 (IGF2) receptors, insulin-regulated aminopeptidase (IRAP), and the Ras GTPase-activating-like protein IQGAP1; 106, 148, 149-151. Hence, recruitment of GluT4 to the cell surface will concurrently recruit these proteins (and vice versa), so that increased plasma membrane GluT4 might in some cases be a correlate, rather than a cause, of insulin’s cognitive effects.).
IRAP, in particular, colocalises with GluT4 in the hippocampus 106 and is the receptor for angiotensin IV (AngIV), which enhances hippocampal memory 152, 153. The presence of IRAP in GSVs may both promote stability of the GLUT4 protein and regulate compartmentalization and recycling of GLUT4 from endosomes to GSVs following translocation and subsequent endocytosis 154. Interestingly, though, we have shown that glucose flux through GluT4 is required for cognitive enhancement by hippocampal AngIV 155, suggesting that IRAP is not an alternative to GluT4 as the transducer of insulin’s cognitive effects.
GluT4 and glucose metabolism
In the periphery, the primary role of insulin is to remove glucose from the blood via GluT4, which moves to the cell surface to permit increased glucose entry into cells when needed. GluT4 is heavily expressed in the hippocampus 156. Taken together with the facts that (i) hippocampal cognitive processes are limited by glucose supply32-34, (ii) administration of glucose to the hippocampus causes an increase in hippocampal metabolism that correlates with improved memory 5, 7, 26, 27, 39, 157-159, and (iii) intrahippocampal insulin acutely increases local hippocampal metabolism55, an attractive hypothesis is that cognitive enhancement by insulin requires GluT4 translocation as a mediator of on-demand glucose supply during memory processing 156, 160, 161. Researchers have speculated that because GluT4 exists in neuronal populations with high-energy demands, such as the hippocampus, it is likely that GluT4 supports GluT3, the principal neuronal glucose transporter, in meeting the demand for glucose supply to neurons during times of enhanced energy demand 161-163. This role of GluT4 is likely to be specific to neurons: in the hippocampus GluT4 appears to be abundant wherever there are insulin receptors, but the converse is not true 156, 164-166. Insulin receptors have been identified in many cell types (e.g. neurons, astrocytes, endothelia, microglia), whereas GluT4 shows neuron-specific localization 156, 167-169.
Several studies, from our lab and others, support this ‘on-demand energy supply’ hypothesis for hippocampal GluT4. In the hippocampus, insulin enhances GluT4 translocation in the brain in a time-course and kinase-dependent (i.e. PI3K) manner that is very consistent with insulin’s effects on peripheral GluT4 170. Physiologically-relevant increases in intrahippocampal insulin that enhance memory upregulate GluT4 translocation, and intrahippocampal Aβ at a dose that impairs cognition and hippocampal metabolism reduces GluT4 translocation 5, 7. More recently, we were able to show (using indinavir, see below) that GluT4 is a key player in hippocampal memory processes and specifically the impact of insulin. Increased glucose metabolism via GluT4 was required for enhancement of spatial memory by exogenous insulin; moreover, cognitive challenge using either of two hippocampally-mediated tasks in the absence of any treatment increased hippocampal GluT4 translocation after spatial working memory or fear-encoding (but not retrieval, interestingly; it appears that the role of GluT4 in hippocampal memory processes may be specific to encoding) 29, 171. Consistent with the ‘on-demand’ hypothesis, blockade of hippocampal GluT4 did not affect hippocampal metabolism at baseline, but prevented any increase in metabolism by insulin treatment29. Moreover, the role of GluT4 in memory appears to be at least somewhat region-specific: no effect of indinavir was seen on an amygdala-dependent task, in contrast to the marked effect on a very similar hippocampally-dependent task 171. These findings strongly support a key role for increased glucose metabolism, via GluT4, at times of increased glucose demand during hippocampally-mediated memory encoding.
In our 2016 paper 171, we noted as a potential inconsistency in this hypothesis that “Others have used high-resolution [14C]-2DG imaging to show that increased glucose utilization during upregulated neuronal activity occurs primarily in synaptic areas (the neuropil) rather than perikarya 172-174. Because GluT4 is expressed in perikarya rather than neuropil163, 175, which shows limited 2DG phosphorylation during hippocampally-dependent memory acquisition, increased neuronal glucose utilization seen during SA testing may not have been mediated entirely, or even primarily, through GluT4.” One possibility to resolve this apparent conflict was that insulin might undergo relatively bulk release in the hippocampus 176, and hence could conceiveably act simultaneously on both synapses to control glutamatergic neurotransmission and the cell body to enhance GluT4 translocation and glucose utilization. The apparent dissociation between the location of plasma membrane GluT4 and the site of energy demand was, though, potentially resolved in a seminal subsequent study which confirmed our finding of activity-dependent neuronal GluT4 translocation and showed that this occurs in large part at the synapse, rather than the cell body 107. This result expanded our understanding of the role of hippocampal GluT4, as potentially including both an insulin-dependent role (primarily at the neuronal cell body) and also a role in providing on-demand glycolytic support at the synapse (which is the location of the majority of activity-associated energy demand 45, 177, 178 and where glycolytic enzymes localize when energy demand exceeds supply179). Ashrafi et al.107 suggested that activation of AMP-kinase might mediate the synaptic recruitment of GluT4 during hippocampal cognitive processing, consistent with the discussion, above, of GluT4 being a target of multiple cognitive signalling pathways.
While acute blockade of GluT4 impaired memory encoding, chronic direct inhibition of hippocampal GluT4 impaired long-term memory, accompanied by e.g. reduced hippocampal BDNF, and alterations in task-associated hippocampal metabolism, but spared short-term memory 171. Further investigation of this dissociation is needed.
GluT4 and hippocampal insulin resistance
Peripheral vs. central regulation of GluT4
Outside the CNS, GluT4 translocation mediates the anabolic functions of insulin by increasing conversion of glucose into glycogen, protein, and fatty acids 180-183. Some of these effects are mechanistically impossible in the hippocampus, though; for instance, GluT4 is expressed in neurons 184, and glycogen conversely made only in astrocytes 185, 186, and thus insulin is unlikely to effect glucose conversion into glycogen through GluT4-mediated glucose uptake in the brain. In general, brain insulin signalling and regulation of GluT4 may be different from that in peripheral tissues. For example, brain IRs are structurally distinct from peripheral IRs 187. The neuronal IR is largely the -α isoform (IRα), which differs from the dominant -β (IRβ) isoform present in astrocytes, muscle, fat and liver due to alternative splicing of exon 11 188-191. Insulin and IGF2 bind IRα with high affinity 189, 192, although it is unknown whether IGF2-activation of IR promotes GluT4 translocation in the hippocampus. IRα is linked to mitogenic actions of insulin, and is somewhat less effective at increasing glucose metabolism than IRβ 189: in general, insulin’s effects in the hippocampus may not precisely match those seen elsewhere in the body.
As mentioned above, IR and GluT4 may have distinct spatial expression patterns within neurons, and these may alter at times of increased neuronal activity. Moreover, although rodent studies have identified GluT4 as primarily or exclusively neuronal, there is some evidence in the human brain that GluT4 may also be found on non-neuronal cells, such as microglia and endothelial cells 193, so that insulin’s effects in human brain may be more diverse than those observed in rodent studies. Several molecules and events necessary for hippocampal memory (e.g. CaMKII, DHA, PKC, PKA, BDNF, cellular depolarization, etc.) regulate GluT4 in adipocytes and muscle 91-106. It will be important to determine which of these effects also occur in neurons.
Impaired hippocampal GluT4 translocation in insulin resistance
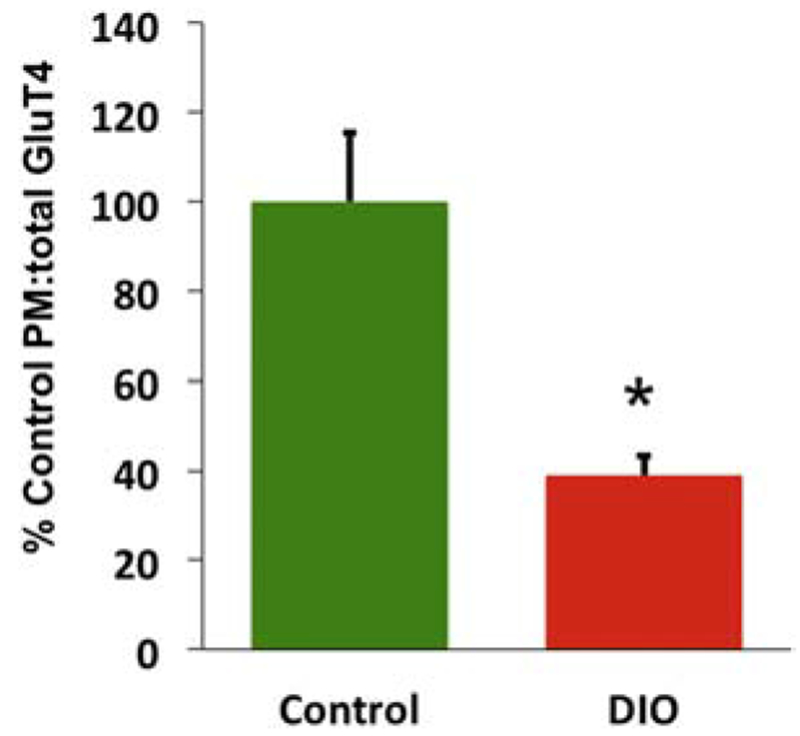
In peripheral tissues, deficits in GluT4 trafficking and intrinsic activity are linked to the adverse effects of insulin resistance 101, 183, 194-199. Because insulin resistance impairs hippocampal cognitive and metabolic processes 19, 134, 176, 200-207, identifying the molecular consequences of brain insulin resistance is important DIO animals show markedly reduced cognitive responsiveness to intrahippocampal insulin, accompanied by an absence of the increased local glucose metabolism seen in response to insulin in control animals 55. Perhaps surprisingly, although factors linked to T2DM such as elevated corticosterone impair hippocampal GluT4 translocation 168, there appear to be no data reported on hippocampal GluT4 in an animal T2DM model. As would be predicted, we found translocation of GluT4 to be impaired (Figure 1): after 12 weeks of a high-fat diet the proportion of hippocampal GluT4 found at the plasma membrane was less than half of that of control animals, consistent with impaired GluT4 translocation being a key factor in cognitive and metabolic impairments seen with hippocampal insulin resistance.
Figure 1.
DIO rats have reduced hippocampal plasma membrane (active) GluT4 compared to rats fed a control chow diet Y-axis shows the ratio of plasma membrane to total GluT4 in the hippocampus, normalised to the group mean for Control animals set at 100%. Data are mean + SEM. * = p<.05.
Interestingly, a second glucose transporter, GluT8, may be regulated by insulin208, 209 (although reports differ 210) and has been identified in the hippocampus, its primary location 184, 211. The impact of insulin resistance on hippocampal GluT8 is unknown.
Hippocampal beta-amyloid and GluT4.
T2DM and AD are linked mechanistically by insulin resistance: patients with AD have reduced brain insulin signalling 54, 74, 100, accompanied by hypometabolism, and both insulin and insulin sensitizers have proven effective at ameliorating AD 60, 74, 212, 213. Conversely, T2DM is a major risk factor for AD, and leads to both elevated brain Aβ and impaired clearance of Aβ 54, 74, 110-117. Abnormal accumulation of brain Aβ has been reported in transgenic rodent models, and we see elevated hippocampal Aβ in our DIO rat model also (unpublished data). In vivo, after as little as 10 min, oligomeric Aβ causes impaired hippocampal insulin signaling and reduced GluT4 translocation, accompanied by cognitive impairment and hippocampal hypometabolism but no effect on either GluT1 or GluT3 129. Moreover, cognitive impairment seen in the DIO rat model of diet-induced insulin resistance is completely reversed by blockade of intrahippocampal oligomeric Aβ 214, along with restoration to baseline of dysregulated hippocampal glutamate signalling that is a hallmark of amyloid’s hippocampal effects 215, 216, showing that Aβ is a key downstream effector of the cognitive impairment caused by insulin resistance. Further, GluT4 immunoreactivity colocalises with cholinergic markers 217, suggesting a role for GluT4 dysregulation in AD which is characterised by damage to cholinergic neurons 218, 219. Taken together, these findings strongly support the potential for (dys)regulation of GluT4 to be central to the pathological links between T2DM and AD. One possible caveat to this conclusion is offered by Nelson et al.220, who suggest that a direct T2DM-AD link is not certain and that cardiovascular pathology may be a better explanation for the link between T2DM and impaired cognition during aging.
Indinavir, HIV, and GluT4-related cognitive impairment
The GluT4 blocker indinavir was developed as an inhibitor of the HIV retroviral protease, and has no effect on mammalian proteases in either rats or humans 221, 222. Other than inhibition of HIV protease, the primary action of indinavir is to selectively inhibit GluT4 via competition at the cytoplasmic domain without affecting translocation of GSVs 223, 224, causing rapid and reversible peripheral insulin resistance 225-228. This dual activity offers a potential human model in which to assess the specific impact of GluT4 blockade: patients treated with GluT4-impairing protease inhibitors (PI; indinavir, nelfinavir) vs. those treated with the newer atazanavir, which has a low affinity for GluT4 227.
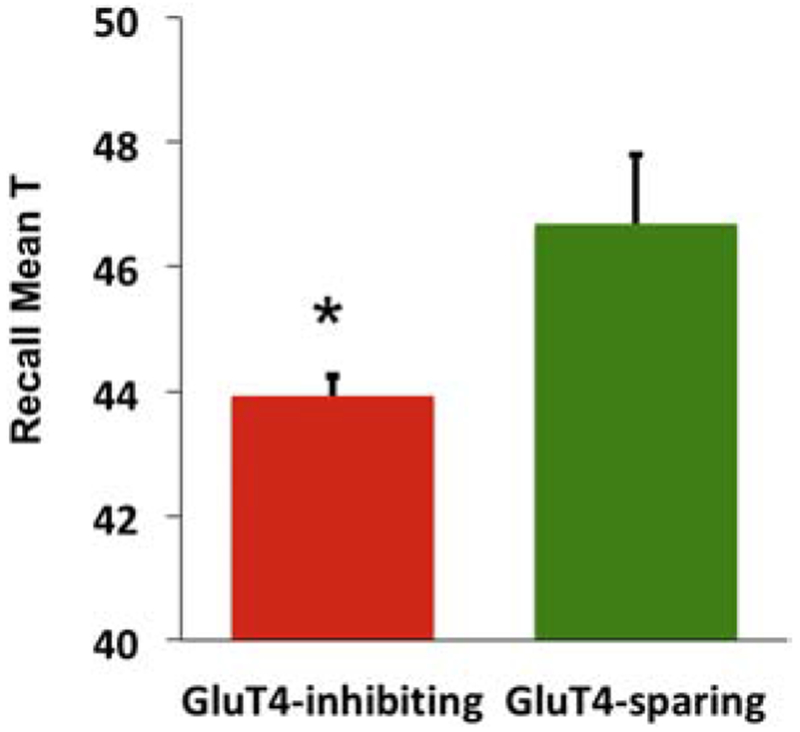
A common consequence of HIV infection is cognitive impairment including brain pathology, commonly referred to as HIV-associated neurocognitive disorder (HAND) 229; the prevalence of HAND has not decreased concomitantly with e.g. decreased viral load 230-235 and is roughly 30-50% among HIV-infected persons 236. Long-term PI use has been linked to HAND, with symptoms including hippocampal cognitive impairment and Aβ accumulation, resembling central insulin resistance 237. HIV impairs blood-brain barrier integrity, so that systemic drugs such as PI may have increased access to the brain 238; note that this may also conceivably alter transport of insulin and/or glucose from the periphery. This suggests the possibility that impaired hippocampal GluT4 activity may be a cause of HAND. As an initial test of this hypothesis, we analysed data from approximately 1100 patients in the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) dataset236. Consistent with this suggestion, we found that HIV patients with a history of GluT4-targeting PI use had impaired hippocampally-dependent recall memory relative to those who never took GluT4-targeting PI (Figure 2). This result is further support for GluT4 as a central regulator of hippocampal memory processes, and for impairment of glucose flux through GluT4 as a causal factor in cognitive impairments associated with central insulin resistance.
Figure 2.
CHARTER data showing that HIV patients on GluT4-inhibiting PI such as indinavir have impaired memory recall compared to patients taking PI that do not affect GluT4-mediated glucose uptake. Y-axis is demographically adjusted domain T-score for the recall domain, as defined in the CHARTER study236. Data are mean + SEM. * = p< .05.
Conclusion
Central, and more specifically hippocampal, insulin resistance appears to be a central element in the cognitive impairment seen in both T2DM and AD. Impairment of glucose supply to neurons subsequent to dysregulation of the insulin-sensitive glucose transporter GluT4 may be a unifying mechanism that explains, at least in part, the comorbidity of these two diseases. Additional disease states, such as HAND, where central GluT4 is impaired are also associated with cognitive impairment on hippocampal tasks. GluT4 is thus an attractive target for future therapeutic intervention: indeed, several treatments that increase the activity of GluT4 such as alpha lipoic acid, AICAR, insulin sensitizing drugs, and histone deacetylase inhibitors also increase memory 239-242. Moreover, GluT4 in the hippocampus of healthy subjects is a key component of memory processing and regulation, likely transducing on-demand glucose supply to neurons when needed to meet the metabolic needs of increased neuronal activity.
Acknowledgements
This work was supported by R01AG050598 to ECM. The CNS HIV Anti-Retroviral Therapy Effects Research was supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clegg D, Benoit S, Reed J, Sc W, Dunn-Meynell AA, Levin BE. Reduced anorexi effects of insulin in obesity-prone rats fed a moderate fat diet. American Journal of physiology 2005;288R981–986. [DOI] [PubMed] [Google Scholar]
- 2.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2008;2:1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - is this type 3 diabetes? Journal of Alzheimer’s Disease 2005;7:63–80. [DOI] [PubMed] [Google Scholar]
- 4.McNay EC. Insulin and ghrelin: peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol 2007;7:628–632. [DOI] [PubMed] [Google Scholar]
- 5.McNay E, Ong C, McCrimmon R, Cresswell J, Bogan J, Sherwin R. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of Learning and Memory 2010;93:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 2011;96:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-β1-42 oligomers acutely impairs spatial working memory, insulin signalling, and hippocampal metabolism. Journal of Alzheimer’s Disease 2012;29:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schechter R, Yanovitch T, Abboud M, Johnson G 3rd, Gaskins J Effects of brain endogenous insulin on neurofilament and MAPK in fetal rat neuron cell cultures. Brain Res 1998;808:270–278. [DOI] [PubMed] [Google Scholar]
- 9.Scherer T, O’Hare J, Diggs-Andrews K, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab 2011;13:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A 1978;75:5737–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978;272:827–829. [DOI] [PubMed] [Google Scholar]
- 12.Gerozissis K Brain insulin: regulation, mechanisms of action and functions. Cell Mol Neurobiol 2003;23:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 2005;12:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav 2006;50:748–752. [DOI] [PubMed] [Google Scholar]
- 15.Clarke DW, Mudd L, Boyd FT Jr., Fields M, Raizada MK. Insulin is released from rat brain neuronal cells in culture. J Neurochem 1986;47:831–836. [DOI] [PubMed] [Google Scholar]
- 16.Ma’nkovskii BN. [Insulin and the central nervous system]. Fiziol Zh 1989;35:110–117. [PubMed] [Google Scholar]
- 17.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 2008;58:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr. Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 1992;13:387–414. [DOI] [PubMed] [Google Scholar]
- 19.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of Learning and Memory 2010;93:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern SA, Alberini CM. Mechanisms of memory enhancement. Wiley interdisciplinary reviews Systems biology and medicine 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGaugh JL, Gold PE, Van Buskirk R, Haycock J. Modulating influences of hormones and catecholamines on memory storage processes. Progress in Brain Research 1975;42:151–162. [DOI] [PubMed] [Google Scholar]
- 22.Gold P Role of glucose in regulating the brain and cognition. American Journal of Clinical Nutrition 1995;61:987S–995S. [DOI] [PubMed] [Google Scholar]
- 23.Glucose Gold P. and age-related changes in memory. Neurobiology of Aging 2005;26S:S60–64. [DOI] [PubMed] [Google Scholar]
- 24.Korol DL, Gold PE. Glucose, memory, and aging. American Journal of Clinical Nutrition 1998;67:764S–771S. [DOI] [PubMed] [Google Scholar]
- 25.McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Cognitive and Behavioural Neuroscience Reviews 2002;1:264–280. [DOI] [PubMed] [Google Scholar]
- 26.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences of the United States of America 2000;97:2881–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiology of Learning & Memory 2001;75:325–337. [DOI] [PubMed] [Google Scholar]
- 28.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 2004;53:418–425. [DOI] [PubMed] [Google Scholar]
- 29.Pearson-Leary J, Jahagirdar V, Sage J, McNay EC. Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4. Behav Brain Res 2018;338:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandusky LA, Flint RW, McNay EC. Elevated glucose metabolism in the amygdala during an inhibitory avoidance task. Behav Brain Res 2013;245:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahagjrdar V, Ramcharitar J, Cotero VE, McNay EC. Moderate Recurrent Hypoglycemia Markedly Impairs Set-Shifting Ability in a Rodent Model: Cognitive and Neurochemical Effects. Open Diabetes J 2012;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 2001;75:325–337. [DOI] [PubMed] [Google Scholar]
- 33.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol A Biol Sci Med Sci 2001;56:B66–71. [DOI] [PubMed] [Google Scholar]
- 34.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A 2000;97:2881–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning CA, Honn VJ, Stone WS, Jane JS, Gold PE. Glucose effects on cognition in adults with Down’s syndrome. Neuropsychology 1998;12:479–484. [DOI] [PubMed] [Google Scholar]
- 36.Manning CA, Parsons MW, Gold PE. Anterograde and retrograde enhancement of 24-h memory by glucose in elderly humans. Behavioral & Neural Biology 1992;58:125–130. [DOI] [PubMed] [Google Scholar]
- 37.Manning CA, Ragozzino ME, Gold PE. Glucose enhancement of memory inpatients with probable senile dementia of the Alzheimer’s type. Neurobiology of Aging 1993;14:523–528. [DOI] [PubMed] [Google Scholar]
- 38.Manning CA, Stone WS, Korol DL, Gold PE. Glucose enhancement of 24-h memory retrieval in healthy elderly humans. Behavioural Brain Research 1998;93:71–76. [DOI] [PubMed] [Google Scholar]
- 39.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. Journals of Gerontology Series A-Biological Sciences & Medical Sciences 2001;56B66–71. [DOI] [PubMed] [Google Scholar]
- 40.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and Neural Hippocampal Effects of Long-Term Moderate Recurrent Hypoglycemia. Diabetes 2006;55:1088–1095. [DOI] [PubMed] [Google Scholar]
- 41.Stefani MR, Nicholson GM, Gold PE. AFP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience 1999;93:557–563. [DOI] [PubMed] [Google Scholar]
- 42.Stefani MR, Gold PE. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Res 1998;813:50–56. [DOI] [PubMed] [Google Scholar]
- 43.Gjedde A, Marrett S, Vafaee M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cereb Blood Flow Metab 2002;22:1–14. [DOI] [PubMed] [Google Scholar]
- 44.Hall CN, Klein-Flugge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 2012;32:8940–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 2012;32:1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cogn Neurosci Rev 2002;1:264–280. [DOI] [PubMed] [Google Scholar]
- 47.Zhao W-Q, Chen H, Xu H, et al. Brain insulin receptors and spatial memory: correlated changes in gene expression, tyrosine phosphorylation, and signalling molecules in the hippocampus of water maze trained rats. Journal of Biological Chemistry 1999;274:34893–34902. [DOI] [PubMed] [Google Scholar]
- 48.Akisaki T, Sakurai T, Takata T, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT). Diabetes Metab Res Rev 2006;22:376–384. [DOI] [PubMed] [Google Scholar]
- 49.Awad N, Gagnon M, Messier C.The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol 2004;26:1044–1080. [DOI] [PubMed] [Google Scholar]
- 50.Brands AM, Biessels GJ, Kappelle LJ, et al. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord 2007;23:343–350. [DOI] [PubMed] [Google Scholar]
- 51.Cosway R, Strachan M, Dougall A, Frier B, Deary I. Cognitive function and information processing in type 2 diabetes. Diabetic Medicine 2001;18:803–810. [DOI] [PubMed] [Google Scholar]
- 52.den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604–1610. [DOI] [PubMed] [Google Scholar]
- 53.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007;50:711–719. [DOI] [PubMed] [Google Scholar]
- 54.Hassing L, Grant M, Hofer S, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. Journal of the International Neuropsychological Society 2004;10:599–607. [DOI] [PubMed] [Google Scholar]
- 55.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem 2010;93:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winocur G, Greenwood CE, Piroli GG, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behavioral Neuroscience 2005;119:1389–1395. [DOI] [PubMed] [Google Scholar]
- 57.Benedict C, Brooks SJ, Kullberg J, et al. Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care 2012;35:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kem W, Peters A, Fruehwald-Schultes B, Deininger E, Bom J, Fehm HL. Improving Influence of Insulin on Cognitive Functions in Humans. Neuroendocrinology 2001;74:270–280. [DOI] [PubMed] [Google Scholar]
- 59.Reger MA, Watson GS, Frey Ii WH, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiology of Aging 2006;27:451–458. [DOI] [PubMed] [Google Scholar]
- 60.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates {beta}-amyloid in early AD. Neurology 2008;70:440–448. [DOI] [PubMed] [Google Scholar]
- 61.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004;29:1326–1334. [DOI] [PubMed] [Google Scholar]
- 62.Benedict L, Nelson C, Schunk E, Sullwold K, Seaquist E. Effect of insulin on the brain activity obtained during visual and memory tasks in healthy human subjects. Neuroendocrinology 2006;83:20–26. [DOI] [PubMed] [Google Scholar]
- 63.Benedict C, Hallschmid M, Schmitz K, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 2007;32:239–243. [DOI] [PubMed] [Google Scholar]
- 64.Seaquist ER, Chen W, Benedict LE, et al. Insulin reduces the BOLD response but is without effect on the VEP during presentation of a visual task in humans. J Cereb Blood Flow Metab 2007;27:154–160. [DOI] [PubMed] [Google Scholar]
- 65.Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiology of Agjng 1996;17:123–130. [DOI] [PubMed] [Google Scholar]
- 66.Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Khiri H, Halfon P. Impact of immune interventions on proviral HIV-1 DNA decay in patients receiving highly active antiretroviral therapy. HIV Med 2001;2:189–194. [DOI] [PubMed] [Google Scholar]
- 67.Hirvonen J, Virtanen KA, Nummenmaa L, et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 2011;60:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gelling RW, Morton GJ, Morrison CD, et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 2006;3:67–73. [DOI] [PubMed] [Google Scholar]
- 69.Hoyer S, Henneberg N, Knapp S, Lannert H, Martin E. Brain glucose metabolism is controlled by amplification and desensitization of the neuronal insulin receptor. Ann N Y Acad Sci 1996;777:374–379. [DOI] [PubMed] [Google Scholar]
- 70.Hoyer S, Prem L, Sorbi S, Amaducci L. Stimulation of glycolytic key enzymes in cerebral cortex by insulin. Neuroreport 1993;4:991–993. [DOI] [PubMed] [Google Scholar]
- 71.Bingham EM, Hopkins D, Smith D, et al. The Role of Insulin in Human Brain Glucose Metabolism. Diabetes 2002;51:3384–3390. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer S Memory function and brain glucose metabolism. Pharmacopsychiatry 2003;36 Suppl ES62–67. [DOI] [PubMed] [Google Scholar]
- 73.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiology and Behavior 2000;68:509–514. [DOI] [PubMed] [Google Scholar]
- 74.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. European Journal of Pharmacology 2004;490:97–113. [DOI] [PubMed] [Google Scholar]
- 75.Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides 2007;28:1029–1034. [DOI] [PubMed] [Google Scholar]
- 76.Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. Insulin protects against stress-induced impairments in water maze performance. Behav Brain Res 2007;176:230–236. [DOI] [PubMed] [Google Scholar]
- 77.Zhao W, Chen H, Xu H, et al. Brain Insulin Receptors and Spatial Memory. Journal of Biological Chemistry 1999;274:34893–34902. [DOI] [PubMed] [Google Scholar]
- 78.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 2001;177:125–134. [DOI] [PubMed] [Google Scholar]
- 79.Stefani MR, Gold PE. Intrahippocampal infusions of k-atp channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. Journal of Neuroscience 2001;21:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dom A, Rinne A, Bernstein HG, Hahn HJ, Ziegler M. Insulin and C-peptide in human brain neurons (insulin/C-peptide/brain peptides/immunohistochemistry/radioimmunoassay). Journal fur Hirnforschung 1983;24:495–499. [PubMed] [Google Scholar]
- 81.Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 1998;105:423–438. [DOI] [PubMed] [Google Scholar]
- 82.Gerozissis K Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol 2008;585:38–49. [DOI] [PubMed] [Google Scholar]
- 83.Orosco M, Gerozissis K, Rouch C, Nicolaidis S. Feeding-related immunoreactive insulin changes in the PVN-VMH revealed by microdialysis. Brain Research 1995;671:149–158. [DOI] [PubMed] [Google Scholar]
- 84.Csajbok EA, Kocsis AK, Farago N, et al. Expression of GLP-1 receptors in insulin-containing interneurons of rat cerebral cortex. Diabetologia 2019;62:717–725. [DOI] [PubMed] [Google Scholar]
- 85.Csajbok EA, Tamas G. Cerebral cortex: a target and source of insulin? Diabetologia 2016;59:1609–1615. [DOI] [PubMed] [Google Scholar]
- 86.Molnar G, Farago N, Kocsis AK, et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci 2014;34:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sartorius T, Peter A, Heni M, et al. The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PLoS One 2015;10:e0126804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang CC, You JL, Lee CC, Hsu KS. Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol Cell Neurosci 2003;24:831–841. [DOI] [PubMed] [Google Scholar]
- 89.Ma XH, Zhong P, Gu Z, Feng J, Yan Z. Muscarinic potentiation of GABA(A) receptor currents is gated by insulin signaling in the prefrontal cortex. J Neurosci 2003;23:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MY Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A 2001;98:3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suwa M, Yamamoto KI, Nakano H, Sasaki H, Radak Z, Kumagai S. Brain-derived neurotrophic factor treatment increases the skeletal muscle glucose transporter 4 protein expression in mice. Physiological research / Academia Scientiarum Bohemoslovaca 2010;59:619–623. [DOI] [PubMed] [Google Scholar]
- 92.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med 2006;12:425–431. [DOI] [PubMed] [Google Scholar]
- 93.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A 2006;103:1605–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mora S, Kaliman P, Chillaron J, Testar X, Palacin M, Zorzano A. Insulin and insulin-like growth factor I (IGF-I) stimulate GLUT4 glucose transporter translocation in Xenopus oocytes. Biochem J 1995;311 (Pt l):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dehvari N, Hutchinson DS, Nevzorova J, et al. beta(2)-Adrenoceptors increase translocation of GLUT4 via GPCRkinase sites in the receptor C-terminal tail. Br J Pharmacol 2012;165:1442–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab 2008;295:E698–704. [DOI] [PubMed] [Google Scholar]
- 97.Mukwevho E, Kohn TA, Lang D, Nyatia E, Smith J, Ojuka EO. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am J Physiol Endocrinol Metab 2008;294:E582–588. [DOI] [PubMed] [Google Scholar]
- 98.Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO. Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab 2007;292:E413–420. [DOI] [PubMed] [Google Scholar]
- 99.Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J Biol Chem 1998;273:14285–14292. [DOI] [PubMed] [Google Scholar]
- 100.Zhang JF, Yang JP, Wang GH, Xia Z, Duan SZ, Wu Y Role of PKCzeta translocation in the development of type 2 diabetes in rats following continuous glucose infusion. Diabetes Metab Res Rev 2010;26:59–70. [DOI] [PubMed] [Google Scholar]
- 101.Li P, Koike T, Qin B, et al. A high-fructose diet impairs Akt and PKCzeta phosphorylation and GLUT4 translocation in rat skeletal muscle. Horm Metab Res 2008;40:528–532. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Wang P, Xu J, Desir GV Voltage-gated potassium channel Kvl.3 regulates GLUT4 trafficking to the plasma membrane via a Ca2+-dependent mechanism. Am J Physiol Cell Physiol 2006;290:C345–351. [DOI] [PubMed] [Google Scholar]
- 103.Xu J, Wang P, Li Y, et al. The voltage-gated potassium channel Kvl.3 regulates peripheral insulin sensitivity. Proc Natl Acad Sci U S A 2004;101:3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wijesekara N, Tung A, Thong F, Klip A. Muscle cell depolarization induces again in surface GLUT4 via reduced endocytosis independently of AMPK. Am J Physiol Endocrinol Metab 2006;290E1276–1286. [DOI] [PubMed] [Google Scholar]
- 105.Yu B, Poirier LA, Nagy LE. Mobilization of GLUT-4 from intracellular vesicles by insulin and K(+) depolarization in cultured H9c2 myotubes. Am J Physiol 1999;277E259–267. [DOI] [PubMed] [Google Scholar]
- 106.Fernando RN, Albiston AL, Chai SY The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus—potential role in modulation of glucose uptake in neurones? Eur J Neurosci 2008;28:588–598. [DOI] [PubMed] [Google Scholar]
- 107.Ashrafi G, Wu Z, Farrell RJ, Ryan TA. GLUT4 Mobilization Supports Energetic Demands of Active Synapses. Neuron 2017;93:606–615 e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bakirtzi K, Belfort G, Lopez-Coviella I, et al. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J Neurosci 2009;29:5193–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim B, Sullivan KA, Backus C, Feldman EL. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid Redox Signal 2011;14:1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. LancetNneurology 2004;3:169–178. [DOI] [PubMed] [Google Scholar]
- 111.Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nature clinical practice Neurology 2006;2:159–166. [DOI] [PubMed] [Google Scholar]
- 112.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004;53:474–481. [DOI] [PubMed] [Google Scholar]
- 113.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. American journal of epidemiology 1997;145:301–308. [DOI] [PubMed] [Google Scholar]
- 114.Mayeux R, Stem Y. Epidemiology of Alzheimer disease. Cold Spring Harbor perspectives in medicine 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 2007;56:384–402. [DOI] [PubMed] [Google Scholar]
- 116.Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci 2010;299:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis 2010;20:723–736. [DOI] [PubMed] [Google Scholar]
- 118.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America 1998;95:6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klein WL. A[beta] toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochemistry International 2002;41:345–352. [DOI] [PubMed] [Google Scholar]
- 120.Gong Y, Chang L, Viola KL, et al. Alzheimer’s disease-affected brain: Presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences of the United States of America 2003;100:10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lacor PN, Buniel MC, Chang L, et al. Synaptic Targeting by Alzheimer’s-Related Amyloid {beta} Oligomers. J Neurosci 2004;24:10191–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao W-Q, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 2008;22:246–260. [DOI] [PubMed] [Google Scholar]
- 123.De Felice FG, Vieira MNN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Aβ oligomers. Proceedings of the National Academy of Sciences 2009;106:1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao W-Q, Lacor PN, Chen H, et al. Insulin Receptor Dysfunction Impairs Cellular Clearance of Neurotoxic Oligomeric Aβ. Journal of Biological Chemistry 2009;284:18742–18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. [see comment]. Nature 2002;416:535–539. [DOI] [PubMed] [Google Scholar]
- 126.Miichi Y, Sakurai T, Akisaki T, Yokono K. Effects of insulin and amyloid beta(l-42) oligomers on glucose incorporation and mitochondrial function in cultured rat hippocampal neurons. Geriatr Gerontol Int 2011;11:517–524. [DOI] [PubMed] [Google Scholar]
- 127.Gasparini L, Netzer W, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends in Pharmacological Sciences 2002;23:288–293. [DOI] [PubMed] [Google Scholar]
- 128.Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. Journal of Neuroscience 2001;21:2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-beta(l-42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimers Dis 2012;30:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HK, Kumar P, Fu Q, Rosen KM, Querlurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell 2009;20:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee C-C, Kuo Y-M, Huang C-C, Hsu K-S. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiology of Aging 2009;30:377–387. [DOI] [PubMed] [Google Scholar]
- 132.Zhao WQ, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 2008;22:246–260. [DOI] [PubMed] [Google Scholar]
- 133.Wei Qiao Q, Marshal FF. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: review and hypothesis. Neurobiology of Aging 2006;27:190–198. [DOI] [PubMed] [Google Scholar]
- 134.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. The FASEB Journal 2004. [DOI] [PubMed] [Google Scholar]
- 135.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proceedings of the National Academy of Sciences of the United States of America 2003;100:4162–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie L, Helmerhorst E, Taddei K, Plewright B, van Bronswijk W, Martins R. Alzheimer’s β-Amyloid Peptides Compete for Insulin Binding to the Insulin Receptor. The Journal of Neuroscience 2002;22:RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kurochkin IV, Goto S. Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett 1994;345:33–37. [DOI] [PubMed] [Google Scholar]
- 138.Bogan JS, Kandor KV Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Current opinion in cell biology 2010;22:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bogan JS, Rubin BR, Yu C, et al. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J Biol Chem 2012;287:23932–23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clodfelder-Miller B, De Samo P, Zmijewska AA, Song L, Jope RS. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. The Journal of biological chemistry 2005;280:39723–39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Molecular biology of the cell 2004;15:4406–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Larance M, Ramm G, Stockli J, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. The Journal of biological chemistry 2005;280:37803–37813. [DOI] [PubMed] [Google Scholar]
- 143.Peck GR, Ye S, Pham V, et al. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol Endocrinol 2006;20:2576–2583. [DOI] [PubMed] [Google Scholar]
- 144.Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS 160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 2007;56:414–423. [DOI] [PubMed] [Google Scholar]
- 145.Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annual review of biochemistry 2012;81:507–532. [DOI] [PubMed] [Google Scholar]
- 146.Keller SR. The insulin-regulated aminopeptidase: A companion and regulator of GLUT4. Frontiers in Bioscience 2003;8:410–420. [DOI] [PubMed] [Google Scholar]
- 147.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005;20:271–284. [DOI] [PubMed] [Google Scholar]
- 148.Schrick C, Fischer A, Srivastava DP, Tronson NC, Penzes P, Radulovic J. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron 2007;55:786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao C, Frausto SF, Guedea AL, et al. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J Neurosci 2011;31:8533–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agjs-Balboa RC, Arcos-Diaz D, Wittnam J, et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J 2011;30:4071–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen DY, Stem SA, Garcia-Osta A, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature 2011;469:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Bundel D, Smolders I, Yang R, Albiston AL, Michotte Y, Chai SY. Angiotensin IV and LW-haemorphin 7 enhance spatial working memory in rats: effects on hippocampal glucose levels and blood flow. Neurobiol Leam Mem 2009;92:19–26. [DOI] [PubMed] [Google Scholar]
- 153.Albiston AL, Morton CJ, Ng HL, et al. Identification and characterization of a new cognitive enhancer based on inhibition of insulin-regulated aminopeptidase. FASEB J 2008;22:4209–4217. [DOI] [PubMed] [Google Scholar]
- 154.Jordens I, Molle D, Xiong W, Keller SR, McGraw TE. Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles. Mol Biol Cell 2010;21:2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sandusky LA, McNay EC. Dorsal hippocampus is a site of action for cognitive enhancement by Angiotensin IV, which involves GluT4. Society for Neuroscience annual meeting. San Diego, CA2013. [Google Scholar]
- 156.El Messari S, Aït-Ikhlef A, Ambroise D-H, Penicaud L, Arluison M. Expression of insulin-responsive glucose transporter GLUT4 mRNA in the rat brain and spinal cord: An in situ hybridization study. Journal of Chemical Neuroanatomy 2002;24:225–242. [DOI] [PubMed] [Google Scholar]
- 157.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes 2006;55:1088–1095. [DOI] [PubMed] [Google Scholar]
- 158.Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus 2013;23:1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 2011;6:e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leloup C, Arluison M, Kassis N, et al. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Molecular Brain Research 1996;38:45–53. [DOI] [PubMed] [Google Scholar]
- 161.Alquier T, Leloup C, Lorsignol A, Penicaud L. Translocable Glucose Transporters in the Brain. Diabetes 2006;55:S131–S138. [Google Scholar]
- 162.El Messari S, Leloup C, Quignon M, Brisorgueil MJ, Penicaud L, Arluison M. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol 1998;399:492–512. [DOI] [PubMed] [Google Scholar]
- 163.Leloup C, Arluison M, Kassis N, et al. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res Mol Brain Res 1996;38:45–53. [DOI] [PubMed] [Google Scholar]
- 164.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 2007;9:316–323. [DOI] [PubMed] [Google Scholar]
- 165.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem 2005;280:18543–18550. [DOI] [PubMed] [Google Scholar]
- 166.van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 2005;94:1158–1166. [DOI] [PubMed] [Google Scholar]
- 167.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res 2009;1296:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Piroli GG, Grillo CA, Reznikov LR, et al. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology 2007;85:71–80. [DOI] [PubMed] [Google Scholar]
- 169.Apelt J, Mehlhom G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. Journal of Neuroscience Research 1999;57:693–705. [PubMed] [Google Scholar]
- 170.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Research 2009;1296:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pearson-Leary J, McNay EC. Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. J Neurosci 2016;36:11851–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sokoloff L Mapping of local cerebral funcitonal activity by measurement of of local cerebral glucose utilization with (14) deoxyglucose. Brain 1979;102:653–668. [DOI] [PubMed] [Google Scholar]
- 173.Sokoloff L Localization of Functional Activity in the Central Nervous System by Measurement of Glucose Utilization with Radioactive Deoxyglucose. J Cereb Blood Flow Metab 1981;1:7–36. [DOI] [PubMed] [Google Scholar]
- 174.Sokoloff L Modeling metabolic processes in the brain in vivo. Ann Neurol 1984; 15 Suppl:S1–11. [DOI] [PubMed] [Google Scholar]
- 175.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Research 1998;797:1–11. [DOI] [PubMed] [Google Scholar]
- 176.Al Duarte, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res 2012;2012:384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci 2012;32:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001;21:1133–1145. [DOI] [PubMed] [Google Scholar]
- 179.Jang S, Nelson JC, Bend EG, et al. Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron 2016;90:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GFP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol 1992;117:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic 2011;12:672–681. [DOI] [PubMed] [Google Scholar]
- 182.Thong FSL, Dugani CB, Klip A. Turning Signals On and Off: GLUT4 Traffic in the Insulin-Signaling Highway. Physiology 2005;20:271–284. [DOI] [PubMed] [Google Scholar]
- 183.Wallberg-Henriksson H, Zierath JR. GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice (review). Mol Membr Biol 2001;18:205–211. [DOI] [PubMed] [Google Scholar]
- 184.McEwen BS, Reagan LR Glucose transporter expression in the central nervous system: relationship to synaptic function. European Journal of Pharmacology 2004;490:13–24. [DOI] [PubMed] [Google Scholar]
- 185.DiNuzzo M, Maraviglia B, Giove F. Why does the brain (not) have glycogen? Bioessays 2011;33:319–326. [DOI] [PubMed] [Google Scholar]
- 186.Heni M, Hennige AM, Peter A, et al. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One 2011;6:e21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heidenreich KA, Zahniser NR, Berhanu P, Brandenburg D, Olefsky JM. Structural differences between insulin receptors in the brain and peripheral target tissues. J Biol Chem 1983;258:8527–8530. [PubMed] [Google Scholar]
- 188.Kenner KA, Kusari J, Heidenreich KA. cDNA sequence analysis of the human brain insulin receptor. Biochem Biophys Res Commun 1995;217:304–312. [DOI] [PubMed] [Google Scholar]
- 189.Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 1999;19:3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hernandez-Sanchez C, Mansilla A, de Pablo F, Zardoya R. Evolution of the insulin receptor family and receptor isoform expression invertebrates. Mol Biol Evol 2008;25:1043–1053. [DOI] [PubMed] [Google Scholar]
- 191.Beffiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 2009;30:586–623. [DOI] [PubMed] [Google Scholar]
- 192.Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem Int 1993;22:1–10. [DOI] [PubMed] [Google Scholar]
- 193.Nijland PG, Michailidou I, Witte ME, et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 2014;62:1125–1141. [DOI] [PubMed] [Google Scholar]
- 194.Mueckler M Insulin resistance and the disruption of Glut4 trafficking in skeletal muscle. J Clin Invest 2001;107:1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tsao TS, Li J, Chang KS, et al. Metabolic adaptations in skeletal muscle overexpressing GLUT4: effects on muscle and physical activity. FASEB J 2001;15:958–969. [DOI] [PubMed] [Google Scholar]
- 196.Govers R, Coster AC, James DE. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol Cell Biol 2004;24:6456–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.IVY JL. Muscle Insulin Resistance Amended with Exercise Training: Role of GLUT4 Expression. Medicine & Science in Sports & Exercise 2004;36:1207–1211. [PubMed] [Google Scholar]
- 198.Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: from physiology to pathology. American Journal of Physiology - Endocrinology And Metabolism 2008;295E38–E45. [DOI] [PubMed] [Google Scholar]
- 199.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 2008;413:201–215. [DOI] [PubMed] [Google Scholar]
- 200.Heininger K A unifying hypothesis of Alzheimer’s disease. IV Causation and sequence of events. Rev Neurosci 2000;11 Spec No:213–328. [DOI] [PubMed] [Google Scholar]
- 201.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res 2012;9:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Luchsinger JA, Tang M-X, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with wtroke in a multiethnic cohort. Am J Epidemiol 2001;154:635–641. [DOI] [PubMed] [Google Scholar]
- 203.Peila R, Rodriguez BL, Launer LJ. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies. Diabetes 2002;51:1256–1262. [DOI] [PubMed] [Google Scholar]
- 204.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased Risk of Type 2 Diabetes in Alzheimer Disease. Diabetes 2004;53:474–481. [DOI] [PubMed] [Google Scholar]
- 205.Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol 2008;65:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Launer LJ. Diabetes: Vascular or Neurodegenerative: An Epidemiologic Perspective. Stroke 2009;40:S53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Julien C, Tremblay C, Phivilay A, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiology of Aging 2010;31:1516–1531. [DOI] [PubMed] [Google Scholar]
- 208.Carayannopoulos MO, Chi MM, Cui Y, et al. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A 2000;97:7313–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem 2000;275:4607–4612. [DOI] [PubMed] [Google Scholar]
- 210.Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res 2004;75:835–844. [DOI] [PubMed] [Google Scholar]
- 211.Reagan LP, Rosell DR, Alves SE, et al. GLUT8 glucose transporter is localized to excitatory and inhibitory neurons in the rat hippocampus. Brain Res 2002;932:129–134. [DOI] [PubMed] [Google Scholar]
- 212.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol 2006;199:265–273. [DOI] [PubMed] [Google Scholar]
- 213.Searcy JL, Phelps JT, Pancani T, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2012;30:943–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Osborne DM, Fitzgerald DP, O’Leary KE, et al. Intrahippocampal administration of a domain antibody that binds aggregated amyloid-beta reverses cognitive deficits produced by diet-induced obesity. Biochim Biophys Acta 2016;1860:1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci 2011;31:6627–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009;62:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience 2002;111:19–34. [DOI] [PubMed] [Google Scholar]
- 218.Selkoe DJ. Alzheimer’s Disease Is a Synaptic Failure. Science 2002;298:789–791. [DOI] [PubMed] [Google Scholar]
- 219.Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer’s disease The Lancet 1976;308:1403–1403. [DOI] [PubMed] [Google Scholar]
- 220.Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not ‘brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011;121:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kadas J, Weber IT, Bagossi P, et al. Narrow substrate specificity and sensitivity toward ligand-binding site mutations of human T-cell Leukemia virus type 1 protease. J Biol Chem 2004;279:27148–27157. [DOI] [PubMed] [Google Scholar]
- 222.Darke PL, Nutt RF, Brady SF, et al. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun 1988;156:297–303. [DOI] [PubMed] [Google Scholar]
- 223.Rudich A, Konrad D, Torok D, et al. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 2003;46:649–658. [DOI] [PubMed] [Google Scholar]
- 224.Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS 2002;16:859–863. [DOI] [PubMed] [Google Scholar]
- 225.Hertel J, Struthers H, Horj CB, Hruz PW. A Structural Basis for the Acute Effects of HIV Protease Inhibitors on GLUT4 Intrinsic Activity. Journal of Biological Chemistry 2004;279:55147–55152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hresko RC, Hruz PW. HIV Protease Inhibitors Act as Competitive Inhibitors of the Cytoplasmic Glucose Binding Site of GLUTs with Differing Affinities for GLUT1 and GLUT4. PLoS One 2011;6:e25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hruz PW, Yan Q, Struthers H, Jay PY. HIV protease inhibitors that block GLUT4 precipitate acute, decompensated heart failure in a mouse model of dilated cardiomyopathy. FASEB J 2008;22:2161–2167. [DOI] [PubMed] [Google Scholar]
- 228.Vyas AK, Koster JC, Tzekov A, Hruz PW. Effects of the HIV Protease Inhibitor Ritonavir on GLUT4 Knock-out Mice. Journal of Biological Chemistry 2010;285:36395–36400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. The Lancet Infectious diseases 2013;13:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Calmy A, Hirschel B, Cooper DA, Carr A. Clinical update: adverse effects of antiretroviral therapy. Lancet 2007;370:12–14. [DOI] [PubMed] [Google Scholar]
- 231.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel ΠΙ criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007;30:113–119. [DOI] [PubMed] [Google Scholar]
- 232.Carr A Toxicity of antiretroviral therapy and implications for drug development. Nat Rev Drug Discov 2003;2:624–634. [DOI] [PubMed] [Google Scholar]
- 233.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol 2010;5:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Winston A, Duncombe C, Li PC, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis 2010;50:920–929. [DOI] [PubMed] [Google Scholar]
- 235.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol 2011;24:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Heaton RK, Clifford DB, Franklin DR Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005;19:407–411. [DOI] [PubMed] [Google Scholar]
- 238.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci 2011;31:9456–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sandouk T, Reda D, Hofmann C. The antidiabetic agent pioglitazone increases expression of glucose transporters in 3T3-F442A cells by increasing messenger ribonucleic acid transcript stability. Endocrinology 1993;133:352–359. [DOI] [PubMed] [Google Scholar]
- 240.Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 2003;84:1173–1183. [DOI] [PubMed] [Google Scholar]
- 241.Christensen DP, Dahllof M, Lundh M, et al. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med 2011;17:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem 2011;18:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]