Significance
EBNA1 is the only Epstein–Barr virus (EBV) latent protein responsible for viral genome maintenance and is expressed in all EBV-infected cells. Zn2+ is essential for oligomerization of the functional EBNA1. We constructed an EBNA1 binding peptide with a Zn2+ chelator to create an EBNA1-specific inhibitor (ZRL5P4). ZRL5P4 by itself is sufficient to reactivate EBV from its latent infection. ZRL5P4 is able to emit unique responsive fluorescent signals once it binds with EBNA1 and a Zn2+ ion. ZRL5P4 can selectively disrupt the EBNA1 oligomerization and cause nasopharyngeal carcinoma (NPC) tumor shrinkage, possibly due to EBV lytic induction. Dicer1 seems essential for this lytic reactivation. As can been seen, EBNA1 is likely to maintain NPC cell survival by suppressing viral reactivation.
Keywords: EBV-specific lytic inducer, EBNA1-targeting agent, dual-responsive fluorescent EBV probe
Abstract
Epstein–Barr nuclear antigen 1 (EBNA1) plays a vital role in the maintenance of the viral genome and is the only viral protein expressed in nearly all forms of Epstein–Barr virus (EBV) latency and EBV-associated diseases, including numerous cancer types. To our knowledge, no specific agent against EBV genes or proteins has been established to target EBV lytic reactivation. Here we report an EBNA1- and Zn2+-responsive probe (ZRL5P4) which alone could reactivate the EBV lytic cycle through specific disruption of EBNA1. We have utilized the Zn2+ chelator to further interfere with the higher order of EBNA1 self-association. The bioprobe ZRL5P4 can respond independently to its interactions with Zn2+ and EBNA1 with different fluorescence changes. It can selectively enter the nuclei of EBV-positive cells and disrupt the oligomerization and oriP-enhanced transactivation of EBNA1. ZRL5P4 can also specifically enhance Dicer1 and PML expression, molecular events which had been reported to occur after the depletion of EBNA1 expression. Importantly, we found that treatment with ZRL5P4 alone could reactivate EBV lytic induction by expressing the early and late EBV lytic genes/proteins. Lytic induction is likely mediated by disruption of EBNA1 oligomerization and the subsequent change of Dicer1 expression. Our probe ZRL5P4 is an EBV protein-specific agent that potently reactivates EBV from latency, leading to the shrinkage of EBV-positive tumors, and our study also suggests the association of EBNA1 oligomerization with the maintenance of EBV latency.
The Epstein–Barr virus (EBV) is a human herpesvirus which infects the vast majority (>90%) of humans worldwide and can establish life-long persistence in the host. This virus is causatively associated with the development and progression of many human malignancies of lymphocyte and epithelial origin, including Burkitt’s lymphoma, Hodgkin’s diseases, gastric carcinoma, and nasopharyngeal carcinoma (NPC) (1). In EBV-infected tumor cells, viral gene expression is limited to only a few latency-associated proteins which actively contribute to tumor cell growth, apoptosis resistance, and immune evasion (2). Under certain circumstances, the latent virus can be reactivated into its productive lytic phase after induction of immediate early and early phases, which will eventually result in the synthesis of new viral DNA, late structural proteins, and secretion of mature infectious virions with concomitant cell death.
Epstein–Barr nuclear antigen 1 (EBNA1) is the only viral protein expressed in nearly all forms of EBV latency and its associated cancers which plays a vital role in the maintenance of viral genome (3). EBNA1 is also important for the transcriptional activation of some other EBV latency genes (4). Homodimerization of EBNA1 is known to be critical for EBNA1–DNA binding and the subsequent functions of oriP (latent origin of replication), including viral DNA replication and segregation, maintenance of the EBV episomal genome, and transcriptional activation (5). Thus, EBNA1 not only serves as a potential marker for clinical imaging but also emerges as a molecular target for the treatment of conditions associated with EBV. Specific inhibition of EBNA1 by dominant-negative EBNA1 mutants (6), antisense oligonucleotides (7), oriP blocking agents, and small molecules/macromolecules (8–12) is shown to inhibit tumor cell growth. Furthermore, our recent study shows that the EBNA1-binding peptide P4 derived from the EBNA1 dimeric interface is able to interfere with the homodimerization of the EBNA1 monomer and suppress EBV-infected cell growth (13–16).
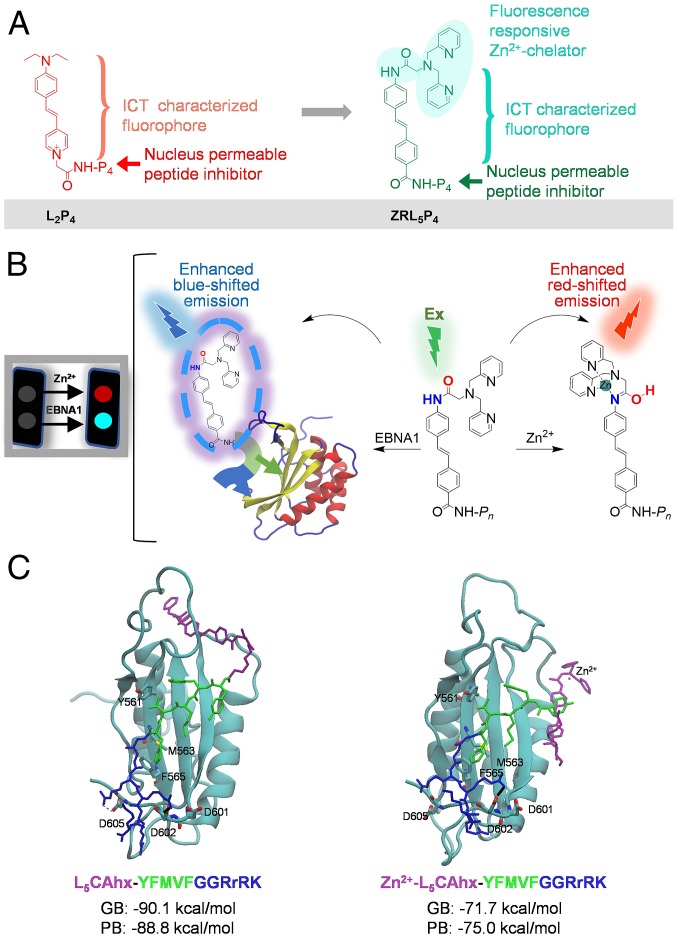
To further improve the activity of the previous peptide-based EBNA1-targeting probe L2P4, we have utilized the EBNA1 cofactor Zn2+ and constructed a dual-responsive fluorescent probe, ZRL5P4 (Fig. 1A), as a specific imaging and potent anticancer agent for EBV-associated malignancies. As previous studies have shown, Zn2+ is necessary for EBNA1 to dimerize and activate the oriP-enhanced transcription, and the unique region 1 (UR1) of EBNA1 contains a pair of essential cysteines which serve as donors to chelate the Zn2+ ion. This suggests that EBNA1 contains a second dimerization/oligomerization interface in its amino terminus, besides the one located within the DNA-binding domain (DBD) (4, 5). We therefore incorporated 1) an EBNA1 DBD-binding peptide P4 (14), 2) a Zn2+ chelator [amide-linked di-(2-picolyl)amine, DPA] to chelate Zn2+ adjacent to the EBNA1 protein, and 3) a dual-responsive fluorophore (ZRL5) independently reflecting its binding with Zn2+ and EBNA1 to construct a probe, ZRL5P4 (Fig. 1A). P4 (YFMVF-GG-RrRK) contains the pentapeptide P2 (YFMVF), which can occupy the first EBNA1 dimerization interface within the DBD (13, 17), and the nuclear localization sequence (NLS) tetrapeptide-RrRK (14). This NLS sequence can form salt bridges with the adjacent dimerization interface, which further enhances the interaction (14). The chosen Zn2+ chelator DPA upon binding with Zn2+, exhibits enhanced red-shifted emission of the probe (18). In addition, when the peptide P4 binds to EBNA1, the intramolecular charge transfer (ICT)-enabled fluorophore produces enhanced blue-shifted emission. Thus, the probe ZRL5P4 is able to emit 2 independent responsive emission signals when bound to a Zn2+ ion and the EBNA1 protein (Fig. 1B).
Fig. 1.
Chemical, fluorescent, and EBNA1-binding characteristics of the Zn2+ chelator EBNA1 probe ZRL5P4. (A) Chemical structures of L2P4 and ZRL5P4. (B) Schematic illustration of the emission response of ZRL5P4, binding to Zn2+ and EBNA1. (C) Representative conformations of ZRL5P4 and Zn2+-ZRL5P4 in the MD simulations. The calculated generalized Born (GB) and Poisson–Boltzmann (PB) values represent the binding free energy between EBNA1 and ZRL5P4 or Zn2+-ZRL5P4.
Here we report how the EBNA1 probe ZRL5P4 can interfere the EBNA1 functions and inhibit EBV-positive cell and tumor growth. Indeed, we found that Zn2+ is essential for oligomerization but not for dimerization of the full-length EBNA1 protein, and this higher form of EBNA1 structure was specifically inhibited by ZRL5P4, but not by the old L2P4 probe. We observed further enhanced inhibition of oriP-mediated transactivation of EBNA1, as well as suppression of growth in EBV-positive tumor cells by ZRL5P4. Surprisingly, we also observed the induction of EBV lytic gene expression and the production of infectious EBV particles when examining the mechanism of how ZRL5P4 could inhibit tumor growth. These interesting results suggest that ZRL5P4 can reactivate EBV from its latent phase through the disruption of EBNA1. The strategy of targeted reactivation of the latent viral genome and the induction of cytotoxic effects in virus-driven tumors is known as cytolytic virus activation therapy, and some EBV lytic inducers have recently entered phase I/II clinical trials (2). Our dual-responsive fluorescent EBNA1 probe ZRL5P4 represents a specific agent to disrupt the EBNA1 protein and to potently reactivate EBV from latency, leading to tumor cell lysis and/or induction of viral proteins that can be targeted by immune cells and antiviral agents to eliminate EBV-infected tumor cells.
Results
Molecular Dynamics Simulations of Interactions between ZRL5P4 and EBNA1.
Before the actual synthesis, interactions between ZRL5P4 (with or without Zn2+) and EBNA1 DBD were initially determined by molecular dynamics (MD) simulations. As only the X-ray crystal structure of the dimeric EBNA1 DBD is available, we decided to use this partial structure of EBNA1 to check whether the presence of the Zn2+-chelating fluorophore ZRL5 (with or without binding to Zn2+) in ZRL5P4 would affect its P4 to bind to an EBNA1 monomer.
Our previous P4-EBNA1 complex model (14) was included as a starting point for a 500-ns simulation by using the flexible peptide docking tool CABS (SI Appendix, Figs. S1 and S2) (19). In line with our previous analysis (14), the pentapeptide YFMVF in P4 had occupied the dimerization interface through a hydrophobic interaction, and the NLS tetrapeptide-RrRK formed salt bridges with the adjacent negatively charged residues (D601, D602, and D605), which further enhanced the interaction. Next, 3-dimensional models of ZRL5 in both Zn2+-free and Zn2+-coordinated scenarios were built and optimized with the molecular mechanics analysis and conformational searching tool LowModeMD. The Zn2+-coordinated DPA chelator fragment in the Zn2+-ZRL5 molecule superposed very well with the crystal structure of a highly similar compound, Zn2+-ZTF (SI Appendix, Fig. S3) (18), suggesting that these 2 configurations converged at the global energy minimum. The various scenarios of Zn2+-ZRL5 dissolved in different solvents were also taken into consideration, and the Zn2+ ion formed 5 coordination sites where a solvent molecule occupies an additional site in DMSO (dimethyl sulfoxide)-Zn2+-ZRL5 and H2O-Zn2+-ZRL5 (SI Appendix, Fig. S4).
The tertiary interaction models of the Zn2+-unbound and -bound ZRL5P4 with EBNA1 (ZRL5P4-EBNA1 and Zn2+-ZRL5P4-EBNA1 complexes) were constructed based on the aforementioned P4-EBNA1 DBD and ZRL5/Zn2+-ZRL5 models (SI Appendix, Fig. S5). The 2 models were then subjected to 200-ns MD simulations for structure optimization and binding energy calculations (SI Appendix, Figs. S6–S9 and Tables S1 and S2). The final optimized models are shown in Fig. 1C (simulation 1); both complexes were simulated twice, and the second simulation model is shown in SI Appendix, Figs. S7, S9, and S10. Both interaction models showed stable probe–protein interactions. The hydrophobic interactions mediated by YFMVF and the salt bridges formed by RrRK were well conserved in the ZRL5P4-EBNA1 and the Zn2+-ZRL5P4-EBNA1 complexes, suggesting that the Zn2+ ion and ZRL5 do not affect P4 in a ZRL5P4 molecule to bind to an EBNA1 monomer. This observation was further supported by the calculated binding energies using MMPBSA (Fig. 1C and SI Appendix, Fig. S10 and Table S3) (20).
Synthesis of ZRL5P4 and Design/Synthesis of the Related EBNA1 Probes.
ZRL5P4 (Fig. 1A) was then synthesized, purified, and characterized. The synthetic route is shown in SI Appendix, Scheme S1, and the characterization processes were carried out (SI Appendix, Figs. S12–S14). Two other related probes, ZRL5P2 and ZRL5P6, were also synthesized to validate the EBNA1-blocking activity and to investigate the effects of the variation in a single amino acid in the blocking peptide. ZRL5P2 was used as a negative control, as it contains the EBNA1 binding motif (YFMVF) but lacks the NLS (RrRk) (14), and the rest of its structure is identical to ZRL5P4. On the other hand, ZRL5P6 contains the peptide P6 (YFIVF-GG-RrRK) which features the pentapeptide YFIVF to target the I563 in the common variant form of EBNA1 in various EBV strains (21). The purities of these 3 compounds were determined by high-performance liquid chromatography (HPLC) (SI Appendix, Fig. S11).
Dual-Responsive Emission by ZRL5P4 toward Their Binding to Zn2+ and EBNA1.
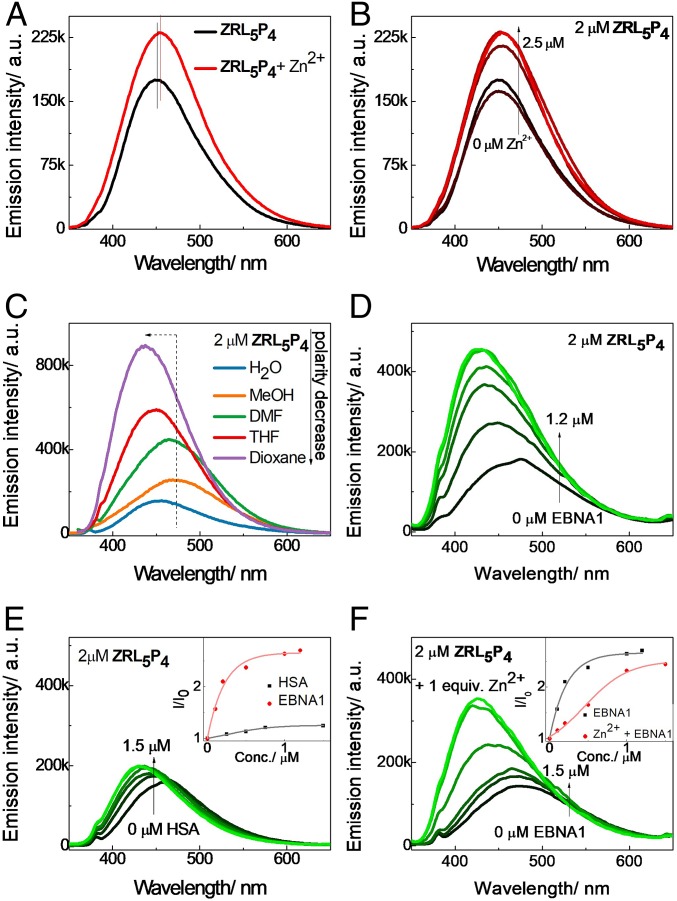
The fluorescent spectral changes of probes when bound to their targets are important indicators to reflect interactions (22). The absorption spectrum of ZRL5P4 in aqueous solution (Hepes) was first determined (SI Appendix, Fig. S15). The emission of ZRL5P2, ZRL5P4, and ZRL5P6 was then examined in the absence and presence of Zn2+ in aqueous solution (CH3CN/0.05 M Hepes [pH 7.4], 50:50) (Fig. 2A and SI Appendix, Fig. S16). Only ZRL5P4 showed a 1.3-fold emission enhancement with a 4-nm red-shifted emission (451 to 455 nm) upon the addition of Zn2+, while the other 2 probes showed either a slightly blue-shifted emission (ZRL5P2, 448 to 445 nm) or an unchanged maximum emission wavelength (ZRL5P6, 438 nm). We then focused on working out the detailed kinetics of the enhanced red-shifted emission mediated by ZRL5P4. We measured the luminescence of ZRL5P4 upon titration with various concentrations of Zn2+ to check their binding stoichiometry. A gradual red-shifted emission with a concomitant increase in the emission intensity was observed, and the change ceased when 1 equivalent Zn2+ had been added. This phenomenon indicates that ZRL5P4 and Zn2+ have a 1:1 stoichiometric ratio (Fig. 2B). In addition, the binding selectivity of ZRL5P4 toward Zn2+ versus other heavy transition metal ions was measured (SI Appendix, Fig. S17). Some of those metal ions decreased the emission of ZRL5P4 to different levels; while some showed blue-shifted emission others did not affect the emission. None of them showed a responsive emission similar to the one of Zn2+.
Fig. 2.
Dual-responsive emission of ZRL5P4 (2 µM; excitation 337 nm) toward Zn2+ and EBNA1. (A) Fluorescence spectral changes of ZRL5P4 in the presence of Zn2+ in aqueous solution (CH3CN/0.05 M Hepes [pH 7.4], 50:50). (B) Fluorescence spectral changes of ZRL5P4 upon gradual addition of Zn2+ in aqueous solution (CH3CN/0.05 M Hepes [pH 7.4], 50:50). (C) Solvation study of ZRL5P4 with a decrease of solvent polarity. Fluorescence spectral changes of ZRL5P4 in PBS upon addition of (D) EBNA1 and (E) HSA and upon addition of EBNA1 in the presence of (F) 1 equivalent Zn2+. (E, Inset) The selectivity of ZRL5P4 toward EBNA1 over HSA. (F, Inset) Interaction of ZRL5P4 with EBNA1 in the presence and absence of Zn2+. a.u., arbitrary units.
We next performed a solvation study to determine the ICT characteristic of each probe, as this mechanism contributes to the responsive signal emitted by the fluorophore in our EBNA1 probes when binding to EBNA1. The ICT-mediated emission is strongly solvent-dependent: The emission is enhanced and blue-shifted with decreasing solvent polarity. Also, the ICT emission is extremely sensitive to changes in the microenvironment and is generally increased when the microenvironment becomes hydrophobic, that is, when ZRL5P4 is bound to the EBNA1 monomer. Thus, this system can be utilized as a protein binding indicator (23). Emission changes of ZRL5P2, ZRL5P4, and ZRL5P6 were then measured as a function of solvent polarity. As can be seen in Fig. 2C and SI Appendix, Fig. S18, ZRL5P4 was shown to have the best ICT characteristics. Although the emission of ZRL5P6 was also enhanced, no blue shift was observed, however. ZRL5P4 was then evaluated for its binding activity toward the EBNA1 DBD protein. It was found that the addition of 1.2 µM EBNA1 DBD to ZRL5P4 caused a 2.7-fold emission enhancement, which was blue-shifted by 39 nm (466 to 427 nm) (Fig. 2D). When the irrelevant human serum albumin (HSA) was used as a negative control protein, the titration of HSA to ZRL5P4 showed only a slight increase in its emission intensity (Fig. 2E). Fig. 2E, Inset clearly shows that the binding of ZRL5P4 was highly selective for EBNA1 over HSA. In addition, the fluorescence response of ZRL5P4 was also measured in the presence of Zn2+ (Fig. 2F). A 2.4-fold enhancement with a 51 nm (477 to 426 nm) blue-shifted emission was observed, suggesting that the presence of Zn2+ did not significantly affect ZRL5P4-EBNA1 binding, as the same order of enhanced emission in the absence of Zn2+ was observed (Fig. 2D). This observation was further supported by the above MD simulation results (Fig. 1C).
Confirmation of the Interaction of ZRL5P4 with Zn2+ by NMR Study.
To characterize the mechanism of how ZRL5P4 interacts with Zn2+, 1H NMR titration analysis of ZRL5P4 with and without 1 equivalent Zn2+ were conducted. The amide–imidic acid tautomer-binding mode of a similar Zn2+ chelator was adopted to analyze if our Zn2+-chelating fluorophore ZRL5 interacts with Zn2+ in a similar fashion in different solvents (18). We found that the Zn2+ chelate complex is an amide tautomer in CH3CN, whereas it is an imidic tautomer in DMSO. The 1H NMR spectroscopy analysis of compound 5 (the precursor of ZRL5) with/without Zn2+ in CD3CN and DMSO-d6 was conducted. Compound 5 was used as a substitute for ZRL5P4 because the presence of its peptide moiety will hinder an accurate Zn2+ binding analysis. Consistent with the previous study (18), proton 8 showed a large upfield shift from 10.93 to 9.43 parts per million (ppm) in CD3CN but showed a down-field shift from 10.72 to 10.84 ppm in DMSO-d6 (SI Appendix, Fig. S19). These findings have confirmed the 2 binding modes of ZRL5P4 in MeCN and DMSO, indicating that the Zn2+-triggered amide tautomerization can also occur for ZRL5P4 (Fig. 1B).
ZRL5P4 Selectively Prevents Oligomerization of EBNA1 and Diminishes oriP-Enhanced Transactivation by EBNA1.
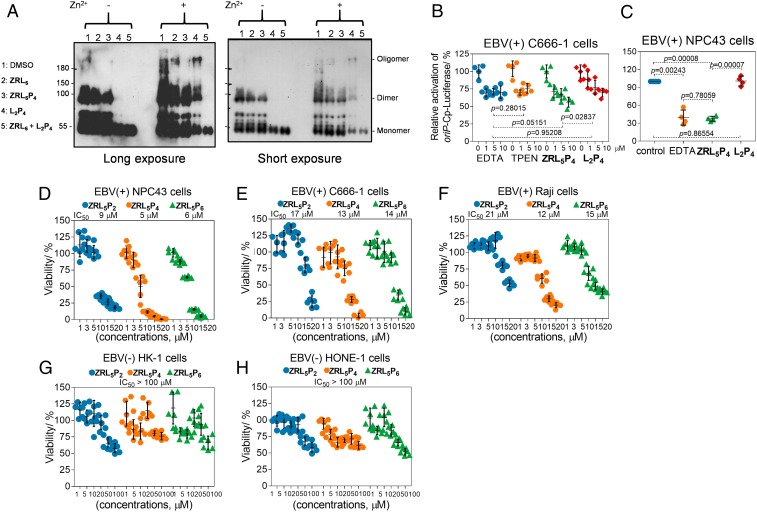
After the chemical and physical properties of ZRL5P4 were characterized, its ability to inhibit EBNA1 dimerization/oligomerization in the absence and presence of Zn2+ was assayed and was compared with the previous L2P4 EBNA1 probe. Zn2+ is necessary for the UR1-mediated self-association, and UR1 maps to the residues 64 to 89 of the amino terminus but not to the EBNA1 DBD. Thus, the full-length EBNA1 rather than the EBNA1 DBD was used. The Zn2+-chelating fluorophore, ZRL5, by itself was also included to determine the role of disruption of Zn2+ alone in the EBNA1 self-association. In the absence of Zn2+, the EBNA1 dimer formation was drastically prevented by L2P4 alone or in combination with ZRL5, whereas ZRL5P4 and ZRL5 had no obvious effect on the EBNA1 dimer (Fig. 3A). Interestingly, the EBNA1 oligomer could only be formed in the presence of Zn2+, and this oligomer was nearly completely abolished by ZRL5P4, but the dimer was not significantly affected by this probe. Whereas L2P4 could only partially inhibit the EBNA1 dimer but had no significant effect on the oligomer in the presence of Zn2+, ZRL5 had no obvious influence on both dimer and oligomer (Fig. 3A). These results indicate that the presence of Zn2+ can reinforce the dimerization and lead to oligomerization, and the exhaustion of Zn2+ ion in close proximity to EBNA1 by ZRL5P4 can eradicate the oligomerization. On the other hand, the combination treatment of L2P4 with ZRL5 could completely inhibit both dimer and oligomer formation. It is likely that these 2 compounds can work synergistically to eradicate the various forms of self-association of EBNA1. We then compared the effects of ZRL5P4 and L2P4 on the oriP-enhanced transactivation and investigated the role of the Zn2+ chelator in ZRL5P4 in disrupting the EBNA1 function. EBV-positive NPC C666-1 and NPC43 cells were exposed to ZRL5P4 and L2P4, and the luciferase reporter assay was performed. Two chelators known to have high specificity for Zn2+, ethylenediaminetetraacetic acid (EDTA) and N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), were included as positive control agents. ZRL5P4 diminished the transactivation by EBNA1 in both C666-1 and NPC43 cell lines, and L2P4 could be only effective in C666-1 (Fig. 3 B and C). The effect of ZRL5P4 was found to be more potent than L2P4 in both C666-1 (P = 0.02837) and NPC43 cell lines (P = 0.00007) (Fig. 3 B and C). These data suggest that the addition of Zn2+ is more effective in reducing the EBNA1 transactivation activity, and disrupting the EBNA1 oligomer seems more critical than its dimer.
Fig. 3.
ZRL5P4 inhibits EBNA1 oligomerization and transactivation and cell viability. (A) Full- length EBNA1 self-association in the absence/presence of Zn2+ by ZRL5/ZRL5P4/L2P4/ZRL5 + L2P4; 0.1% DMSO serves as the solvent control. Long and short exposure images of the same blot are shown for comparison of various forms of EBNA1 self-association. Analysis of the effects of EBNA1 probes (ZRL5P4, L2P4) on the oriP-enhanced transactivation in EBV-positive (B) C666-1 and (C) NPC43 cells. The transactivation activities were detected by the oriP-Cp-luciferase reporter. EDTA and TPEN are the known chelators of Zn2+. (D–H) Cytotoxic activities of the EBNA1 probes ZRL5P2, ZRL5P4, and ZRL5P6 in the EBV-positive and -negative cell lines. Cytotoxicity of EBV-positive (D) NPC43 cells, (E) C666-1 cells, and (F) Raji cells (concentrations 1, 3, 5, 10, 15, and 20 µM) and EBV-negative (G) HK-1 cells and (H) HONE-1 cells (concentrations 1, 5, 10, 20, 50, and 100 µM) were measured by the MTT assay. Cells were treated with different probes and then incubated for 5 d to test their cytotoxicity (half of the medium was replaced every 4 d with fresh medium containing the appropriate concentration of the probes). Data are expressed as the means ± SD.
ZRL5P4 Reduces the Viability of EBV-Positive Cells and Localizes to Their Nuclei.
We next measured the cell viability of a panel of EBV-positive (NPC43 and C666-1 NPC cells and Raji lymphoma cells) and EBV-negative (HK-1 and HONE-1 NPC cells) cells treated with ZRL5P2, ZRL5P4, and ZRL5P4 (Fig. 3 D–H). Both C666-1 and NPC43 have Y561F“M”VF565, while Raji has Y561F“I”VF565 at the DBD. Obvious cytotoxicity was observed in these 3 EBV-positive cells, even when treated with low dosages of 1 to 20 µM probes. ZRL5P4 and ZRL5P6 displayed very similar growth-inhibitory effects, with ZRL5P2 (lacking the NLS) being the least efficient one. In contrast, treatments with ZRL5P4 and ZRL5P6 in EBV-negative cells only showed <50% inhibition at a much higher dose (100 µM) (Fig. 3 G and H), indicating that the inhibitory activities are EBV-specific. When compared to the previous cytotoxicity study of L2P4 (median infectious dose; 23 µM in NPC43, 27 µM in C666-1, and 27 µM in Raji cells), ZRL5P4 is likely to be more potent, suggesting that the exploitation of the Zn2+ chelator enhances the cytotoxic activity, regardless of epithelial or lymphoid origin.
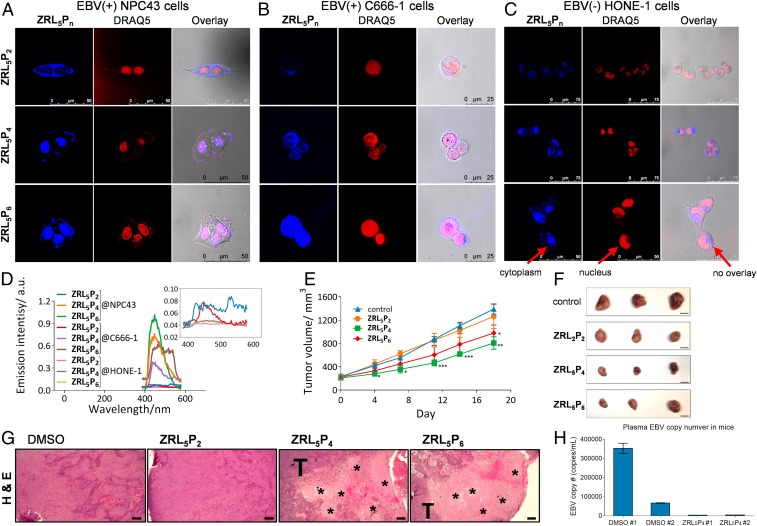
To show that entry into the nuclei is essential for the EBNA1 probes to inhibit tumor cell viability, subcellular localization of ZRL5P2, ZRL5P4, and ZRL5P6 were evaluated in both EBV-positive (C666-1 and NPC43) and EBV-negative (HONE-1) cell lines, using 2-photon excitation microscopy (λex: 700 nm). After incubation for 3 h, the fluorescence signals were collected in the blue channel. ZRL5P4 and ZRL5P6 were primarily located in the nuclei of EBV-positive cells (Fig. 4 A, B, and D) and this is in contrast to the imaging results of L2P4 we previously reported, which showed that the majority of L2P4 was localized to the cytoplasm, and only a small portion was found in the nuclei (14). On the other hand, ZRL5P2 was only found in the cytoplasm (Fig. 4 A, B, and D) and all 3 probes showed no nuclear localization in EBV-negative cells (Fig. 4 C and D). The EBNA1 expression detected by Western blot in these 3 cell lines correlates quite well with the signal intensities reflected by ZRL5P4 (SI Appendix, Fig. S20A). The cellular uptake of the new and old EBNA1 probes by EBV-positive cells was also compared and it was found that there the uptake of L2P4 was faster than ZRL5P4 into their nuclei; however, L2P4 also accumulated in the cytoplasm (SI Appendix, Fig. S20B). The inclusion of a Zn2+ chelator seems to decrease the uptake rate and increase the specificity of ZRL5P4. Taken together, all these imaging results indicated that the presence of both the Zn2+ chelator and NLS is critical for more specific nuclear localization of the new EBNA1 probes to selectively stain the EBV-positive cells.
Fig. 4.
Nuclear localization of the EBNA1 probes and their in vivo antitumor activities. (A–C) Two-photon fluorescence imaging of ZRL5P2, ZRL5P4, and ZRL5P6 in living EBV-positive (A) NPC43 cells and (B) C666-1 cells and EBV-negative (C) HONE-1 cells. ZRL5Pn, signal emitted from the respective EBNA1 probe. DRAQ5 is a fluorescent dye used to label the cell nuclei of the living cells as indicated. (D) In vitro emission spectra (from confocal microscopy) of ZRL5P2, ZRL5P4, and ZRL5P6 in the nucleus of EBV-positive NPC43 and C666-1 and EBV-negative HONE-1 cells. Emission intensity was much greater for ZRL5P4 and ZRL5P6 in EBV-positive cells. (E) In vivo antitumor activity of ZRL5P2, ZRL5P4, and ZRL5P4. Mice transplanted with C666-1–derived tumors were treated twice weekly with 4 µg per injection of the probes for 18 d. Throughout the treatment period, tumor volumes were measured. At the experimental endpoint, tumors were excised. Data are expressed as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control (0.1% DMSO). (Scale bars, 10 mm.) (F) Representative photographs of tumors. (G) Representative H&E staining images of tumor sections derived from the above in vivo animal study. Cell necrosis (acellular areas indicated by asterisk) was observed in the tumor nodules treated with ZRL5P4 and ZRL5P6. T, adjacent area with tumor cells. Magnification, 400×. (Scale bars, 20 µm.) (H) Response of plasma EBV DNA levels in mice transplanted with C666-1 cells after the treatment of ZRL5P4. The circulating EBV DNA level of each mouse (DMSO #1, #2; ZRL5P4 #1, #2) is shown.
ZRL5P4 Inhibits the Growth of EBV-Positive Tumors in BALB/c Nude Mice.
The in vivo effects of the 3 EBNA1 probes in the C666-1 xenograft were then examined. Treatment with ZRL5P2, ZRL5P4, and ZRL5P6 did not cause any significant changes in body or organ weights when compared to vehicle control (SI Appendix, Figs. S21 and S22), indicating that the 3 probes did not exhibit a toxic effect in vivo. Tumor growth was significantly inhibited by treatment with ZRL5P4 and ZRL5P6. Both the average tumor volume and tumor weight of mice treated with ZRL5P4 or ZRL5P6 was significantly decreased, compared to the control (Fig. 4 E and F and SI Appendix, Fig. S23). There was no significant difference in either tumor volume or tumor weight when comparing the effects of ZRL5P4 and ZRL5P6. ZRL5P2 without NLS, however, did not significantly affect tumor growth. For the control HeLa xenograft, there was no significant difference in either tumor volume or tumor weight between the control mice and those treated with the probes (SI Appendix, Fig. S24). When the tumor sections were stained with hematoxylin/eosin (H&E) to examine the tissue morphology, we found that cell necrosis was much more frequently observed in the tumor tissues with the treatments of ZRL5P4 or ZRL5P6 than with the ZRL5P2 and the solvent control (Fig. 4G). The cell death could be due to the cytotoxic activities of ZRL5P4 and ZRL5P6 observed in the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay for C666-1 cells (Fig. 3E), and that can also explain why the tumors shrank after these treatments. When performing an independent set of in vivo experiments with transplanted C666-1–derived tumors, the plasma EBV DNA dramatically dropped from ∼350,000 and ∼67,000 to ∼3,900 and ∼4,400 copies after treatment with ZRL5P4 (Fig. 4H). This circulating EBV DNA load was directly proportional to the viable cell areas in the tumors and inversely associated with body weight (SI Appendix, Fig. S25). Hence, the plasma EBV DNA is likely a biomarker for predicting the effectiveness of ZRL5P4.
Reactivation of the EBV Lytic Cycle by ZRL5P4.
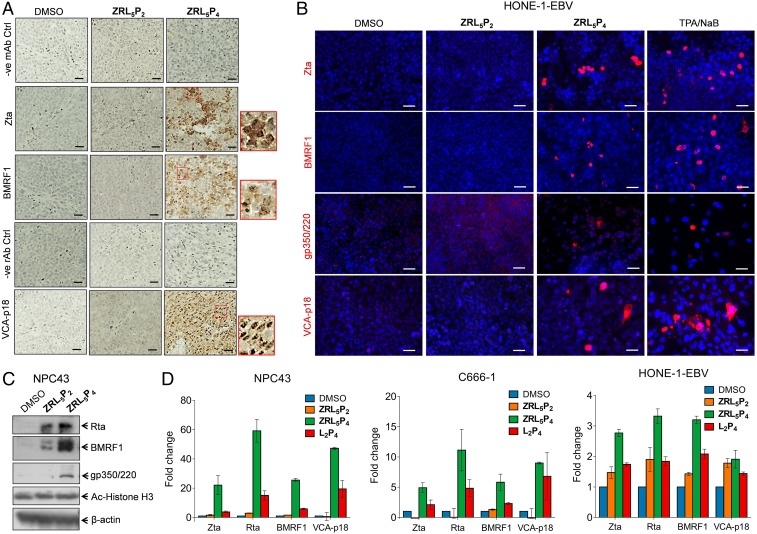
Interestingly, when tumor sections of Fig. 4G were analyzed with immunohistochemistry (IHC), the EBV immediate early, early, and late lytic proteins, Zta, BMRF1, and VCA-p18, were mainly detected in the tumors injected with ZRL5P4 (Fig. 5A). Nuclear and cytoplasmic staining of Zta, BMRF1, and VCA-p18 was observed in these tumor tissues. After treatment with ZRL5P4, ∼10 and 15% tumor areas were positive for Zta and BMRF1, respectively, and more than 80% was VCA-p18 positive. On the other hand, only negative staining was detected in the solvent control, and 0 to 2% was observed in ZRL5P2 for these 3 lytic proteins.
Fig. 5.
EBV lytic induction analysis of EBV-positive tumors and cells in response to the EBNA1 probes. (A) IHC analysis of lytic proteins Zta, BMRF1, and VCA-p18 in the transplanted C666-1–derived tumor tissues as described in Fig. 4 E–G. Representative results are shown. Nuclear and cytoplasmic staining of Zta, BMRF1, and VCA-p18 are observed in response to ZRL5P4. Insets (in the red boxes) are the enlarged images to indicate the cellular localization of the 3 proteins. Negative control staining images are included for the mouse antibodies (mAb) against Zta and BMRF1 and for the rat antibody (rAb) against VCA-p18. Magnification, 400×. (Scale bars, 20 µm.) (B) Representative images of immunofluorescent analysis of EBV lytic proteins, Zta, BMRF1, gp350/220, and VCA-p18 in HONE-1-EBV cells in response to 10 µM ZRL5P2 or ZRL5P4. TPA/NaB (20 ng/mL TPA, 3 mM NaB) serves as a positive control of lytic induction. The presence of these 4 lytic proteins is indicated by red signals. The nuclei were stained with DAPI. (Scale bars, 50 µm.) (C) Western blot analysis of Rta, BMRF1, and gp350/220 EBV lytic proteins in NPC43 cells cultured with or without 10 µM ZRL5P2 and ZRL5P4 for 7 d. Acetylated histone H3 (Ac-Histone H3) was also detected. β-actin serves as the loading control. (D) Gene expression analysis of EBV lytic genes, Zta, Rta, BMRF1, and VCA-p18 in NPC43, C666-1, and HONE-1-EBV cells cultured with or without 10 µM ZRL5P2, ZRL5P4, and L2P4, for 7 (NPC43 cells), 3 (C666-1 cells), or 5 (HONE-1-EBV) d. The gene expression was analyzed by qRT-PCR. The fold change of relative gene expression after each treatment was compared with the solvent control (DMSO). Data are expressed as the means ± CI.
Furthermore, other EBV-infected cell lines, NPC43 and HONE-1-EBV (the recombinant EBV-containing HONE-1), were included for the lytic phase analysis. The protein expression of the immediate early, early, and late lytic genes, Zta, BMRF1, gp350/220, and VCA-p18, was studied in NPC43, HONE-1-EBV, and C666-1 cell lines in response to 10 µM ZRL5P2 and ZRL5P4. Immunofluorescence (IF) staining was performed, and the conventional chemical inducer tetradecanoyl phorbol acetate (TPA) (20 ng/mL) with sodium butyrate (3 mM) (TPA/NaB) (2) was also included as a positive control. The positively stained cells for all 4 proteins were more frequently observed in ZRL5P4, and the response to ZRL5P2 was similar to that of the solvent control in HONE-1-EBV cells (Fig. 5B). Although the fold changes of the 4 lytic proteins in response to TPA/NaB were generally higher than ZRL5P4, the increased levels of the 2 late proteins gp350/220 and VCA-p18 were not statistically significant (SI Appendix, Fig. S26). A similar observation of lytic protein induction was obtained in the other 2 EBV-positive cell lines (SI Appendix, Figs. S27 and S28A).
The IF results in NPC43 and C666-1 cells were validated by Western blot analysis, which showed that the expression levels of a number of lytic proteins were dramatically induced by ZRL5P4 (Fig. 5C and SI Appendix, Fig. S28B). Although the expression of Rta and BMRF1 was also induced by ZRL5P2 in NPC43 cells, the increased levels of expression were much weaker than those of ZRL5P4 and the gp350/220 levels were similar to the solvent control. As some histone modifiers such as TPA/NaB can induce EBV lytic reactivation, we examined the effects of ZRL5P4 on the acetylation of histone H3 in 2 NPC cells lines. Our agent had no significant effect on both cell lines (Fig. 5C and SI Appendix, Fig. S28B), and it seems that histone modification might not be directly related to EBV reactivation induced by ZRL5P4.
We then compared the effects of our new ZRL5P4 versus the old L2P4 probes on the EBV lytic induction. The gene expression of EBV lytic genes including Zta, Rta, BMRF1, and VCA-p18, was studied in 3 EBV-positive cell lines (NPC43, HONE-1-EBV, and C666-1) in response to 10 µM ZRL5P2, ZRL5P4, or L2P4. The gene expression was quantified by the accurate qRT-PCR analysis. The expression of all 4 lytic genes was strikingly induced by ZRL5P4, whereas their expression in response to ZRL5P2 was similar to that of the solvent control (Fig. 5D). When compared with ZRL5P4, L2P4 could only induce the 4 lytic genes with much lower levels in NPC43 and C666-1 cells, and in HONE-1-EBV cells the changes of these genes were either comparable to or lower than the negative control ZRL5P2. As can be seen, the Zn2+ chelator in ZRL5P4 is critical in initiation of EBV reactivation.
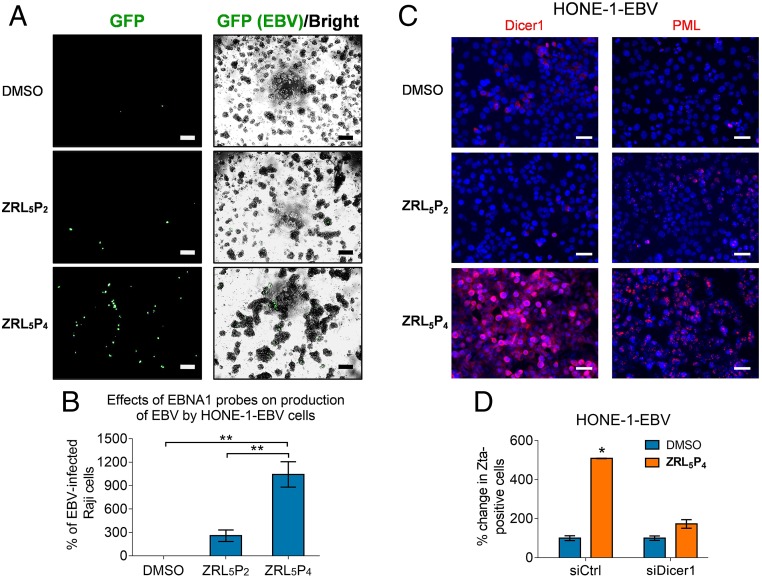
In order to verify if the EBV particles with infectious properties could actually be generated by ZRL5P4, HONE-1-EBV cells were used. The presence of the virions was detected by infecting Raji cells (an established B cell line), because the recombinant EBV genome in HONE-1-EBV encodes a green fluorescence protein (GFP), and the GFP-expressing Raji cells reflect the production of virions by the HONE-1-EBV cells. As can be seen, 10 µM ZRL5P4 could lead to the production of virions and which was 10.4-fold more than the DMSO control (P = 0.009) and was ∼4-fold more than the NLS-null version ZRL5P2 (P = 0.006) (Fig. 6 A and B). Although the viral titer of ZRL5P2 was 2.6-fold higher than the solvent control, the difference was not significant (P = 0.06). Taken together, the entry of ZRL5P4 into the nuclei of EBV-infected cells can induce the reactivation of EBV, which might mediate the shrinkage of the transplanted C666-1 tumors (Fig. 4 E–G).
Fig. 6.
Production of infectious EBV particle in response to ZRL5P4. The HONE-1-EBV cell line, which expresses GFP to indicate the presence of the EBV genome, was used. This cell line was treated with 10 µM ZRL5P2 or ZRL5P4 for 4 d, and the viral particles released in the culture medium were detected by the Raji cell assay. The culture medium was added to Raji cells for 3 d, and GFP expression reflects the reinfection by the HONE-1–released EBV particles. (A) Representative results are shown. The GFP signal was detected by ultraviolet light exposure, and the cell morphology was captured by phase-contrast light microscopy and the bright-field image was merged with the GFP image. Magnification, 400×. (Scale bars, 100 µm.) (B) Relative average viral titer in response to ZRL5P2 or ZRL5P4 was compared with the solvent control (DMSO). The Raji cell assay was performed in triplicate for each treatment. **P < 0.01, statistically significant difference. Data are expressed as the means ± SD. (C) Representative images of immunofluorescent analysis of Dicer1 and PML in HONE-1-EBV cells in response to 10 µM ZRL5P2 or ZRL5P4. The nuclei were counterstained with DAPI and indicated in blue. (Scale bars, 50 µm.) (D) Comparison of the number of Zta-positive cells after treatment with ZRL5P4 in the presence versus the absence of Dicer1. HONE-1-EBV and NPC43 were included. Gene silencing of Dicer1 was achieved by siRNA transfection. Zta was detected by immunofluorescent analysis. Control siRNA (siCTL) was used as a negative control. Relative percentage of Zta-positive was compared against the DMSO solvent control in the siRNA control cells. *P < 0.05.
To study the underlying mechanism(s) of how ZRL5P4 induces EBV lytic induction, the change in expression of Dicer and PML were examined, as previous studies indicate that these 2 proteins are associated with EBNA1-associated lytic induction (24, 25). The in situ protein expression of both Dicer1 and PML was consistently up-regulated in 2 NPC cell lines in response to ZRL5P4 (Fig. 6C and SI Appendix, Fig. S29A). The increased protein expression of Dicer1 and PML was validated by Western blot (SI Appendix, Fig. S29B). To show the specific induction of these 2 proteins by ZRL5P4, the expression was checked in the EBV-negative HONE-1 cells, and there was no induction of Dicer1 or PML in the ZRL5P4-treated cells (SI Appendix, Fig. S30A). Zta protein expression and cell viability were examined in both ZRL5P4-treated HONE-1 and HONE-1-EBV, to show that ZRL5P4 only had lytic-inducing and antitumor activities in the EBV-positive cells (SI Appendix, Fig. S30 A–C). Thus, the induction of Dicer1 and PML by ZRL5P4 is EBV-specific. To further demonstrate whether the lytic induction is mediated by Dicer1 and/or PML, we depleted their expression in EBV-positive cells and examined the expression of the key lytic protein Zta in response to ZRL5P4. When the expression of Dicer1 was depleted (SI Appendix, Figs. S29B and S31A), the ZRL5P4-induced Zta expression was almost completely attenuated in 2 EBV-positive cell lines (Fig. 6D), whereas the knockdown of PML itself had led to the induction of Zta expression in NPC43 cells, and ZRL5P4 could further enhance the Zta expression (SI Appendix, Fig. S31B). As can be seen, Dicer1 is likely a mediator which can be induced by ZRL5P4 to initiate the EBV reactivation.
Discussion
EBNA1 is known as a dimeric viral protein encoded by EBV; our previous work has confirmed P4 (CAhxYFMVFGGRrRK) is a physical blocker to hamper EBNA1 dimerization, and hence inhibit the functions of EBNA1 as well as the growth of EBV-positive cells. Interestingly, Zn2+ was also recently reported as a cofactor of EBNA1 self-association; it mediates interactions of the UR1 region between 2 EBNA1 monomers through the formation of “zinc finger” structure. We thereby constructed a probe, ZRL5P4, by taking advantages of its Zn2+-binding property. Unexpectedly, the Zn2+ chelator acts in close proximity to P4 to mainly disrupt the oligomerization rather than the dimerization of EBNA1 (Fig. 3A). Interestingly, the formation of EBNA1 oligomer was only observed in the presence of Zn2+ and could also stabilize the dimeric EBNA1. These results are in concordance with the previous studies that Zn2+ is required for self-association of EBNA1 at the UR1 domain where the Zn2+ coordination between 2 EBNA1 monomers occurs (4). The “self-association” can represent the dimer formation from 2 homomonomers of EBNA1, as well as the formation of higher-order EBNA1 complexes. This hypothesis is supported by the simulation model suggested by Hussain et al. (26) that a Zn2+ ion links 2 EBNA1 dimers to form an oligomer to bind to DNA. This EBNA1 complex structure could possibly represent a hexameric ring, as previously suggested (27). As the UR1 domain was reported to be essential for the transactivation activity of EBNA1 (4, 28), that can explain why ZRL5P4 was much more effective than L2P4 in disrupting the oriP-mediated transcription, growth inhibition, and lytic induction. The increased potency of ZRL5P4 on the various EBNA1 and cellular activities upon L2P4 is also reflected by the cellular imaging for the EBV-positive cell lines (Fig. 4). The imaging results explain that the stronger inhibition can be attributed to the nuclear localization of the probe, where EBNA1 is primarily located, and that nuclear EBNA1 is of critical importance for its dependent function. In vivo administration of ZRL5P4 was demonstrated to shrink subcutaneous tumors (Fig. 4). The plasma EBV DNA can act as a biomarker for prediction of the effectiveness of treatment with ZRL5P4 (Fig. 4H). Surprisingly, the subsequent IHC staining indicated that the necrotic tumor tissues were likely due to the induction of EBV lytic cycle (Figs. 4 and 5). The EBV immediate early, early, and late lytic gene/protein expression analyses and the in vitro Raji cell infection assay showed that ZRL5P4 can induce the EBV lytic cycle and produce infectious EBV particles (Figs. 5 and 6 A and B). When compared with the conventional chemical inducer (a common histone deacetylase inhibitor), the potency of ZRL5P4 seems not as strong as the TPA/NaB treatment (Fig. 5B and SI Appendix, Fig. S26). However, the micromolar ZRL5P4 concentration is sufficient to induce the late lytic proteins in a similar magnitude to the millimolar NaB treatment. The status of histone acetylation was not significantly affected by ZRL5P4, mechanisms other than histone modification might be involved.
In concordance with our findings, it has been reported that the depletion of EBNA1 gene expression by small interfering RNA (siRNA) in the EBV-infected epithelial cell lines can activate spontaneous lytic cycle induction, indicating that EBNA1 has a functional role in suppressing reactivation of EBV (24). Previous studies indicate that EBNA1 can disrupt PML and Dicer expression, and these 2 proteins are required for EBV reactivation (24, 25). Our results show that ZRL5P4 could specifically enhance the expression of these 2 proteins in the EBV-positive cells, but this was not observed in the EBV-negative cells. This observation further strengthens the evidence for the selectivity of ZRL5P4, which only functions in the presence of EBNA1. Importantly, the presence of Dicer1 seems essential for the lytic induction by ZRL5P4, while the change in PML expression levels is also associated with the ZRL5P4-induced EBV reactivation. Our study also suggests that the function of EBNA1 and the role of EBV latent infection is associated with the maintenance of tumor cell survival through suppressing lytic cycle reactivation. Substantial advancement has been observed toward development of novel viral-reactivating agents for induction of cytocidal effects in various EBV-associated tumor cells. This strategy has eventually entered clinical phase I/II trials, and significant improvements in clinical outcome were observed in at least a portion of the EBV-positive lymphoma patients (2). A recent study by Messick et al. (29) has described a small molecule that inhibits the DNA binding activity of EBNA1; this molecule can suppress the growth of NPC and other EBV-associated tumors but does not appear to induce the EBV lytic cycle. The lytic inactivation could be due to mechanisms other EBNA1-DNA binding, such as induction of Dicer1, PML, and so on, as reflected by the present study. Thus, to our knowledge, no specific lytic cycle inducers against EBV genes or proteins have been established. As EBNA1 is a foreign protein to the host, in theory the elimination of EBV-associated tumor cells by ZRL5P4 warrants absolute specificity over all other lytic inducers.
On the other hand, we initially thought that the genetic variation at the EBNA1 dimerization sequence (the amino acid 563) could be an important factor for the design of EBNA1 probes. ZRL5P6 was constructed to target the EBNA1 protein with an “I” residue at the variation position. However, the results of various biological assays show that both ZRL5P4 and ZRL5P6 were almost equally effective in all these assays, indicating that this amino acid residue is not critical for disrupting the self-association of the EBNA1 monomer. These findings suggest that ZRL5P4 and ZRL5P6 should be equally potent for suppressing the EBNA1 proteins in various EBV stains.
In conclusion, we have used Zn2+ as an important cofactor for EBNA1 function and have constructed a series of EBNA1 probes with a Zn2+ chelator and an EBNA1-binding peptide. The Zn2+ chelator can preferentially suppress the higher-order form of EBNA1 complex to further enhance the inhibitory activities of our new-generation compound ZRL5P4. Importantly, ZRL5P4 reactivates EBV lytic induction, which is associated with the shrinkage of EBV-positive tumors in the animal model. This study successfully targeted a single protein (EBNA1) to induce the EBV lytic cycle. As EBNA1 is a foreign protein to the host, in theory this strategy of elimination of EBV-associated tumor cells warrants absolute specificity over all other lytic induction therapies. The current lytic analysis results also suggest that the function of EBNA1 as well as the role of EBV latent infection is associated with the maintenance of tumor cell survival through the suppression of the lytic cycle reactivation. Exhaustion of Zn2+ in the microenvironment in protein molecules might represent a new strategy in designing inhibitory agents to target pathogenic proteins in cancer development and other diseases.
Materials and Methods
General.
Unless otherwise stated, all chemicals were used as purchased without further purification. Peptides were ordered from GL Biochem (Shanghai) Ltd. Full-length EBNA1 with N-terminal His tag was purchased from Abcam (ab138345). The siRNAs were supplied by Thermo Fisher. Solvents were dried using standard procedures. Purification of ZRL5P2, ZRL5P4, and ZRL5P6 was performed on a Waters semipreparative HPLC system. NMR spectra were recorded on a Bruker 400-MHz NMR spectrometer, and the chemical shifts were referenced internally to tetramethylsilane or the corresponding solvent residues in parts per million; coupling constants are reported in hertz. High-resolution mass spectra, reported as m/z, were obtained on either a Bruker Autoflex MALDI-TOF or an Agilent 6450 UHD Accurate-Mass Q-TOF spectrometer. UV-visible absorption spectra were recorded on a Cary 8454 spectrometer.
Syntheses.
All new compounds were characterized by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry. Characterization data are shown in SI Appendix.
MD Simulation.
All-atom unbiased MD simulations in AMBER 14 with aff99SBildn force field were used. The system preparation and simulation procedures were the same as those reported previously (14). In brief, a periodic boundary, cubic, TIP3 (30) explicit water box with a 20-Å buffer was used with charge neutralized by adding Cl− ions. The system was then minimized and equilibrated by sander using 3 stages: 1) heating from 100 to 300 K in 20 ps, 2) adjusting the solvent density to 1 g/mL in 20 ps, and 3) equilibrating in 200 ps with NPT ensemble. A subsequent 100-ns NPT simulation was performed with CUDA-accelerated PMEMD (31, 32). A 2-fs time step and SHAKE-enabled settings were used for all of the equilibration and production stages. A Berendsen thermostat was adopted for temperature control at all stages.
Luminescence Measurements.
Luminescent spectra were recorded on a Horiba Fluorolog-3 spectrofluorometer equipped with a xenon lamp. Specifically, the selectivity assay of ZRL5P4 toward Zn2+ among many heavy metal ions was performed in Hepes buffer (0.05 M, pH 7.4)/CH3CN (50:50) using perchlorate salts as the metal source [Zn(ClO4)2, Cd(ClO4)2, Cu(ClO4)2, Co(ClO4)2, Ni(ClO4)2, Hg(ClO4)2 and Mg(ClO4)2]. The selectivity assay of ZRL5P4 toward EBNA1 was measured in phosphate-buffered saline (PBS) buffer. Luminescence titration experiments were performed by gradually increasing the concentration of the analytes in the aqueous solution of ZRL5P4, including Zn2+, EBNA1, and HSA; the titration was stopped when the emission change of ZRL5P4 ceased.
Dimerization/Oligomerization Assay.
Full-length EBNA1 (residues 1 to 641) with an N-terminal His tag (ab138345; Abcam) was used in this assay. Each 0.875 µg of EBNA1 was incubated without or with 50 µM Zn2+ at room temperature in the presence of 8 ng oriP DNA and 100 µM probe (buffer/L2P4/ZRL5P4) for 1 h at 37 °C to allow self-association to occur. After incubation, sodium dodecyl sulfate (SDS) loading buffer was added to each system, which was then separated using denaturing SDS/polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and blotted with an antibody against the His tag (GeneTex); the obtained protein bands provided information of dimerization/oligomerization inhibition.
Luciferase Reporter Assay for EBNA1 oriPI-Dependent Transactivation.
To study EBNA1-dependent transactivation, the luciferase vector J988F containing the EBV C promoter and oriPI (family of repeats) was constructed. The EBV C promoter and oriPI (nucleotides 7447 to 11412) regions were subcloned from the previously described plasmid pgCp(-3889)CAT (33, 34) as a HindIII fragment into the pGL3Basic luciferase vector (Promega). Correct sequences were ascertained by Sanger sequencing using the ABI PRISM Big Dye terminator cycle sequencing kit (Applied Biosystems). EBV-positive C666-1 and NPC43 cells were then transiently transfected with the J988F reporter plasmid. Cells were seeded in 12-well plates and cotransfected with the J988F plasmid (2 µg per well) and a pRL Renilla luciferase control reporter (500 ng per well) (Promega) using Lipofectamine 2000 (Invitrogen). After 24 h, the cells were treated with ZRL5P4, L2P4, EDTA, or TPEN (10 µM) for another 8 h. Cells were lysed with Passive Lysis Buffer (Promega), and the lysate was then transferred onto a white, opaque, 96-well plate. The luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega) with the GloMax 96 Microplate Luminometer (Promega). The pRL Renilla luciferase reporter was used as an internal control to normalize the transfection efficiency among the samples.
Cell Culture.
Six cell lines were used in this work: the EBV-negative HK-1 and HONE-1 lines and the EBV-positive NPC43, C666-1, HONE-1-EBV, and Raji lines. HK-1, HONE-1, HONE-1-EBV, C666-1, and Raji cells were grown in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin at 37 °C and 5% CO2. NPC43 cells were maintained in RPMI 1640 with 10% FBS and 4 µM Y27362 (inhibitor of Rho-associated, coiled-coil-containing protein kinase; Enzo Life Sciences). C666-1, HK-1, and HONE-1 cells were obtained from the Hong Kong NPC AoE Cell Line Repository, NPC43 and HONE-1-EBV were supplied by S.W.T., and Raji was supplied by ATCC. All of the cell lines were authenticated by using the AmpFℓSTR Identifier PCR Amplification kit (Life Technologies) (SI Appendix, Table S5) and were tested to be mycoplasma-negative by PCR.
MTT Assay.
All cells were subcultured in 96-well plates at the optimal growth density (HK-1, 1 × 104 cells/100 µL per well; HONE-1, 8 × 103 cells/100 µL per well; NPC43, 8 × 103 cells/100 µL per well; C666-1, 3 × 104 cells/100 µL per well; Raji, 1 × 104 cells/100 µL per well) for 24 h. The growth medium was then replaced by solutions of ZRL5P2, ZRL5P4, or ZRL5P6 at concentrations of 1, 5, 10, 20, 50, and 100 µM for EBV-negative cells or at concentrations of 1, 3, 5, 10, 15, and 20 µM for EBV-positive cells (for the suspensions of C666-1 and Raji cells, the 96-well plate was centrifuged at 1,000 rpm for 3 min before each replacement/withdrawal of medium and operated with care during the assay). After culturing for a further 5 d (half of the volume of medium was replaced every 4 d with fresh medium containing the appropriate drug concentration), the cells were rinsed with PBS and then incubated with a solution of MTT (0.5 mg/mL, 50 µL) in PBS at 37 °C for 3 h. Then, 70% of the medium was carefully removed, DMSO (100 µL) was added, and the plate was shaken for 30 min to solubilize the formazan produced by living cells. The optical densities were measured with a dual-wavelength Labsystem Multiskan microplate reader (Merck Eurolab) at wavelengths of 540 and 690 nm and expressed as a percentage relative to control cells (cells without drug treatment served as the control). Measurements were performed in triplicate and repeated twice. Cell viability (percent) was calculated according to Eq. 1:
| [1] |
where ODi and ODc are the optical densities of the surviving cells treated with or without drug, respectively.
Confocal Microscopy and Costaining.
Cells were incubated with ZRL5P2/ZRL5P4/ZRL5P6 (10 µM) for 3 h and then costained with DRAQ5 (5 µM) for 30 min. Images were acquired using a Leica TCS SP8 confocal laser-scanning microscope equipped with a coherent femtosecond laser (680 to 1,050 nm), argon laser (432, 457, and 488 nm), He-Ne laser (632 nm), ultraviolet lamp, and a controlled CO2-content stage-top tissue culture chamber (37 °C, 2 to 7% CO2). In vitro images of ZRL5P2/ZRL5P4/ZRL5P6 were obtained under 2-photon excitation (λex = 700 nm), whereas images of DRAQ5 were acquired under single-photon excitation (λex = 638 nm). The real-time live imaging was performed with a Nikon Eclipse Ti2 confocal laser-scanning microscope.
Nude Mice Xenograft and Intratumoral Injection.
C666-1 cells (8 × 106) suspended in 100 µL of serum-free RPMI medium were injected into the right flanks of 6- to 8-wk-old male BALB/c nude mice. After 21 d of inoculation, when tumors had grown to an average volume of ∼220 mm3, mice were assigned to treatment groups (n = 5 per group) such that the average tumor volumes varied between groups by no more than 10%. Twice weekly, mice received 4 µg per tumor intratumoural injections of ZRL5P2, ZRL5P4, or ZRL5P6 in 0.1% DMSO using a 29-gauge syringe. Mice that received an equivalent volume of 0.1% DMSO alone served as controls. The treatment period lasted for 18 d. Body weight and tumor volumes were measured twice weekly, and tumor volumes were calculated as (length × width2)/2. At the end of the treatment period, mice were killed and their tumors and major organs were harvested and weighed. The investigators were blinded to treatment grouping during the experiments and analysis of data. All animal experiments were approved by the Department of Health of the Hong Kong Government and the Animal Subjects Ethics Sub-Committee of Hong Kong Polytechnic University. No power analyses were used to calculate the sample size for the animal studies.
Quantitative Analysis of Cell-Free Plasma EBV DNA.
The plasma EBV DNA was detected as previously described (35). In brief, the plasma DNA was extracted using the QIAamp DNA Blood mini kit. The PCR primers and probe and the reaction condition strictly followed the same study. The EBV-positive cell line Namalwa was used as a standard, and the EBV copy number was calculated.
qPCR Gene Expression Analysis.
qPCR was performed as reported (36). The primers used in this study were described in a previous publication (36, 37).
IF Staining.
IF staining was performed as previously described (36). Primary antibodies against Zta were supplied from Argene (Verniolle). Antibodies against BMRF1, gp350/220, and VCA-p18 were generated by J.M.M. These antibodies were produced and characterized as previously described (38–40). The Dicer1 and PML antibodies were supplied by Abcam and Bethyl Laboratories, respectively. Images were captured by the Eclipse Ti System (Nikon).
Western Blot Analysis.
Western blot analysis of Zta, Rta, BMRF1, and gp350/220 was performed as reported (41). The Rta antibody was supplied from Argene (Verniolle) and the β-actin antibody for loading control was supplied from Cell Signaling Technology. Antibodies against Zta, BMRF1, gp350/220, Dicer1, and PML were the ones used for IF staining. A 1:400 dilution of the primary antibodies was used.
Preparation of Formalin-Fixed, Paraffin-Embedded Tumor Tissues, H&E Stain, and IHC.
The transplanted tumor tissues were fixed with formalin and embedded in paraffin accordingly to the general practice. The histologic sections were prepared and stained with H&E. The lytic protein markers were stained with IHC antibodies against Zta and BMRF1. The Zta and the BMRF1 antibody were the ones used for Western blot analysis. The slides were incubated with the primary antibodies (1:100 dilution) for IHC as previously described (41).
EBV Infection Assay.
The HONE-1-EBV cell line was used to produce the infectious EBV particles for the lytic analysis; this cell line was generated by introducing a GFP open reading frame in the recombinant Akata EBV genome into the EBV-negative NPC cell line HONE-1 (42). The procedures of production of viral particles and quantitation of virus titers were followed as previously described (43, 44). In brief, after incubation with various EBNA1 probes for 96 h, the supernatants were filtered through 0.45-µm-pore filters and the viral particles were enriched by centrifugation at 20,000 × g for 2 h. The relative virus titers were determined by the Raji cell assay and were quantified with the GFP expression of Raji cells infected with the virus stocks to be analyzed. The Raji cells (1 × 105) were incubated in 96-well plates and cultivated for 3 d at 37 °C to allow the expression of GFP. The number of GFP-positive cells was counted by ultraviolet microscopy.
Supplementary Material
Acknowledgments
This work was funded by the Hong Kong Baptist University (RC-IRMS/16-17/CHE, RC-ICRS/16-17/02A-BOL, RC-IRMS/16-17/01, and MPCF-002-2018/19), Hong Kong Polytechnic University (HKPolyU 153021/18P), Hong Kong Research Grants Council (HKBU 20301615 and 12300117, the NPC Area of Excellence, AoE/M 06/08 Center for Nasopharyngeal Carcinoma Research, and Research Grants Council Collaborative Research Fund Scheme C4001-18GF).
Footnotes
Competing interest statement: K.-L.W., N.K.M., and L.J. are listed as inventors on a filed US nonprovisional patent titled “Zinc-binder based EBNA1-specific compounds” with the application number 16/249,987.
This article is a PNAS Direct Submission. R.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915372116/-/DCSupplemental.
References
- 1.Cohen J. I., Epstein-Barr virus infection. N. Engl. J. Med. 343, 481–492 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Novalić Z., Rossen T. M. v., Greijer A. E., Middeldorp J. M., Agents and approaches for lytic induction therapy of Epstein-Barr virus associated malignancies. Med. Chem. 6, 449–466 (2016). [Google Scholar]
- 3.Frappier L., Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses 4, 1537–1547 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aras S., Singh G., Johnston K., Foster T., Aiyar A., Zinc coordination is required for and regulates transcription activation by Epstein-Barr nuclear antigen 1. PLoS Pathog. 5, e1000469 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westhoff Smith D., Sugden B., Potential cellular functions of Epstein-Barr nuclear antigen 1 (EBNA1) of Epstein-Barr virus. Viruses 5, 226–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy G., Komano J., Sugden B., Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc. Natl. Acad. Sci. U.S.A. 100, 14269–14274 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth G., Curiel T., Lacy J., Epstein-Barr viral nuclear antigen 1 antisense oligodeoxynucleotide inhibits proliferation of Epstein-Barr virus-immortalized B cells. Blood 84, 582–587 (1994). [PubMed] [Google Scholar]
- 8.Thompson S., Messick T., Schultz D. C., Reichman M., Lieberman P. M., Development of a high-throughput screen for inhibitors of Epstein-Barr virus EBNA1. J. Biomol. Screen. 15, 1107–1115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N., et al. , Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS One 5, e10126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda A., et al. , DNA ligand designed to antagonize EBNA1 represses Epstein-Barr virus-induced immortalization. Cancer Sci. 102, 2221–2230 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Sun X., et al. , Hsp90 inhibitors block outgrowth of EBV-infected malignant cells in vitro and in vivo through an EBNA1-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 107, 3146–3151 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z., et al. , Novel pyrrole-imidazole polyamide Hoechst conjugate suppresses Epstein-Barr virus replication and virus-positive tumor growth. J. Med. Chem. 61, 6674–6684 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Jiang L., et al. , EBNA1-specific luminescent small molecules for the imaging and inhibition of latent EBV-infected tumor cells. Chem. Commun. (Camb.) 50, 6517–6519 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Jiang L., et al. , EBNA1-targeted probe for the imaging and growth inhibition of tumours associated with the Epstein–Barr virus. Nat. Biomed. Eng. 1, 0042 (2017). [Google Scholar]
- 15.Jiang L., et al. , EBNA1-targeted inhibitors: Novel approaches for the treatment of Epstein-Barr virus-associated cancers. Theranostics 8, 5307–5319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zha S., et al. , Responsive upconversion nanoprobe for monitoring and inhibition of EBV-associated cancers via targeting EBNA1. Nanoscale 10, 15632–15640 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kim S. Y., Song K. A., Kieff E., Kang M. S., Small molecule and peptide-mediated inhibition of Epstein-Barr virus nuclear antigen 1 dimerization. Biochem. Biophys. Res. Commun. 424, 251–256 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Xu Z., et al. , Zn2+-triggered amide tautomerization produces a highly Zn2+-selective, cell-permeable, and ratiometric fluorescent sensor. J. Am. Chem. Soc. 132, 601–610 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Kurcinski M., Jamroz M., Blaszczyk M., Kolinski A., Kmiecik S., CABS-dock web server for the flexible docking of peptides to proteins without prior knowledge of the binding site. Nucleic Acids Res. 43, W419–W424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller B. R., 3rd, et al. , MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 8, 3314–3321 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Correia S., et al. , Natural variation of Epstein-Barr virus genes, proteins, and primary microRNA. J. Virol. 91, e00375-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolanowski J. L., Liu F., New E. J., Fluorescent probes for the simultaneous detection of multiple analytes in biology. Chem. Soc. Rev. 47, 195–208 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y., Yokoyama K., Design and synthesis of intramolecular charge transfer-based fluorescent reagents for the highly-sensitive detection of proteins. J. Am. Chem. Soc. 127, 17799–17802 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Sivachandran N., Wang X., Frappier L., Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. J. Virol. 86, 6146–6158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansouri S., Pan Q., Blencowe B. J., Claycomb J. M., Frappier L., Epstein-Barr virus EBNA1 protein regulates viral latency through effects on let-7 microRNA and dicer. J. Virol. 88, 11166–11177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain M., Gatherer D., Wilson J. B., Modelling the structure of full-length Epstein-Barr virus nuclear antigen 1. Virus Genes 49, 358–372 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Deakyne J. S., Malecka K. A., Messick T. E., Lieberman P. M., Structural and functional basis for an EBNA1 hexameric ring in Epstein-Barr virus episome maintenance. J. Virol. 91, e01046-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy G., Sugden B., EBNA-1, a bifunctional transcriptional activator. Mol. Cell. Biol. 23, 6901–6908 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messick T. E., et al. , Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein-Barr virus latent infection and tumor growth. Sci. Transl. Med. 11, eaau5612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindorff-Larsen K., et al. , Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Götz A. W., et al. , Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized born. J. Chem. Theory Comput. 8, 1542–1555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon-Ferrer R., Götz A. W., Poole D., Le Grand S., Walker R. C., Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Nilsson T., Sjöblom A., Masucci M. G., Rymo L., Viral and cellular factors influence the activity of the Epstein-Barr virus BCR2 and BWR1 promoters in cells of different phenotype. Virology 193, 774–785 (1993). [DOI] [PubMed] [Google Scholar]
- 34.Nilsson T., Zetterberg H., Wang Y. C., Rymo L., Promoter-proximal regulatory elements involved in oriP-EBNA1-independent and -dependent activation of the Epstein-Barr virus C promoter in B-lymphoid cell lines. J. Virol. 75, 5796–5811 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo Y. M., et al. , Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 59, 1188–1191 (1999). [PubMed] [Google Scholar]
- 36.Lung H. L., et al. , SAA1 polymorphisms are associated with variation in antiangiogenic and tumor-suppressive activities in nasopharyngeal carcinoma. Oncogene 34, 878–889 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Ramayanti O., et al. , Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer 140, 149–162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hessing M., van Schijndel H. B., van Grunsven W. M., Wolf H., Middeldorp J. M., Purification and quantification of recombinant Epstein-Barr viral glycoproteins gp350/220 from Chinese hamster ovary cells. J. Chromatogr. A 599, 267–272 (1992). [DOI] [PubMed] [Google Scholar]
- 39.Zeng Y., Middeldorp J., Madjar J. J., Ooka T., A major DNA binding protein encoded by BALF2 open reading frame of Epstein-Barr virus (EBV) forms a complex with other EBV DNA-binding proteins: DNAase, EA-D, and DNA polymerase. Virology 239, 285–295 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Zhang J. X., et al. , Epstein-Barr virus expression within keratinizing nasopharyngeal carcinoma. J. Med. Virol. 55, 227–233 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Lung H. L., et al. , THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene 24, 6525–6532 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Hui K. F., et al. , Activation of lytic cycle of Epstein-Barr virus by suberoylanilide hydroxamic acid leads to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int. J. Cancer 131, 1930–1940 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Dirmeier U., et al. , Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein-Barr virus. Cancer Res. 63, 2982–2989 (2003). [PubMed] [Google Scholar]
- 44.Lan K., Verma S. C., Murakami M., Bajaj B., Robertson E. S., Epstein-Barr Virus (EBV): Infection, propagation, quantitation, and storage. Curr. Protoc. Microbiol. Chap. 14, Unit 14E.2.1–Unit 14E.2.21 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.