Abstract
The ventromedial prefrontal cortex (vmPFC) is consistently implicated in the cognitive and emotional symptoms of many psychiatric disorders, but the causal mechanisms of its involvement remain unknown. In part, this is because of the poor characterization of the disorders and their symptoms, and the focus of experimental studies in animals on subcortical (rather than cortical) dysregulation. Moreover, even in those experimental studies that have focused on the vmPFC, the preferred animal model for such research has been the rodent, in which there are marked differences in the organization of this region to that seen in humans, and thus the extent of functional homology is unclear. There is also a paucity of well-defined behavioral paradigms suitable for translating disorder-relevant findings across species. With these considerations in mind, we discuss the value of nonhuman primates (NHPs) in bridging the translational gap between human and rodent studies. We focus on recent investigations into the involvement in reward and threat processing of 2 major regions of the vmPFC, areas 25 and 32 in NHPs and their anatomical homologs, the infralimbic and prelimbic cortex, in rodents. We highlight potential similarities, but also differences between species, and consider them in light of the extent to which anatomical homology reflects functional homology, the expansion of the PFC in human and NHPs, and most importantly how they can guide future studies to improve the translatability of findings from preclinical animal studies into the clinic.
Keywords: anterior cingulate cortex, prelimbic–infralimbic, threat, reward, ventromedial PFC
The human brain, and in particular the expanded neocortex that is considered the seat of our intellectual and emotional competence, has long been considered the evolutionary feather in humanity’s enlarged cap. Since humans separated from the rodent line the neocortex has expanded to occupy 80% of the human brain, compared to just 28% in the rat. However, with this expansion has come added complexity and the potential for subtle and pervasive disruptions in function. Thus, the benefits endowed by our neocortex are tempered by the neurodegenerative and psychiatric disorders that are a consequence of its dysfunction and represent a considerable economic and individual burden.
The majority of the neocortical increase is composed of association cortices: those regions responsible for the complex mental processing that occurs outside the primary sensory and motoric areas. This expansion occurs in a way that is unique to humans and other primates who display different and additional sources of embryonic neurons, altered developmental and temporal trajectories of neuronal migration, differentiation of neuronal cell types not seen in rodents (particularly interneurons), and a complex fully developed neocortex comprising over 50 distinguishable areas, many of which are unknown in rodents (1). These anatomical distinctions are mirrored at the genetic and molecular level, with strong evidence that transcriptional and regulatory differences between distinct brain regions—particularly between the prefrontal and nonfrontal cortices—are an important driver of primate-specific neocortical evolution (2). As the largest area of association cortex, the prefrontal cortex (PFC) positioned in the anterior portion of the frontal lobes exemplifies many of these evolutionary differences, and crucially, is one of the regions that is most heavily implicated in the cognitive and emotional symptoms of many neuropsychiatric disorders. Consequently, this raises the question as to how appropriate it is for pharmacological research to use rodents as their preferred models when seeking to understand and develop therapeutics for these disorders. This question is particularly pertinent now since there has been little recent progress in the development of new treatments for common psychiatric disorders, such as depression and anxiety (3), and the failure rate for experimental CNS drugs is high (4).
There are many possible reasons for this impasse in treatments. First, there is the poor psychological and neurobiological characterization of both the disorders and their component symptoms (such as anxiety or anhedonia), and the recognition that the underlying neurobiological and psychological mechanisms responsible for them may differ between patients. Second, there is the focus, until recently, on subcortical mechanisms and structures, such as the amygdala, bed nucleus of the stria terminalis, and hypothalamus to investigate emotion and stress rather than cortical and—in particular—prefrontal mechanisms. Third, there is a paucity of behavioral paradigms suitable for translating findings from animals into the clinic. Fourth, there is an almost total reliance on findings from experimental studies of brain mechanisms of emotional-like symptoms in rodents, in which the organization of the PFC is markedly different from that seen in humans.
This review will focus on the fourth of these reasons by considering the comparability of prefrontal function between rodents and nonhuman primates (NHPs) as it relates to our understanding of the symptoms of anxiety and anhedonia. Clinical anxiety is not just restricted to the anxiety disorders but is also comorbid with many other neuropsychiatric disorders, including depression. Similarly, anhedonia, the loss of pleasure, is commonly reported in schizophrenia as well as being a major symptom of depression. Consequently, the focus of therapeutics has recently turned to the alleviation of a given symptom irrespective of the specific disorder in which it occurs, which depends on being able to link the underlying neurobiology to the specific cognitive and emotional processes that are disrupted.
A wealth of correlative human neuroimaging studies have implicated regions of the ventromedial (vm)PFC in the etiology and treatment of disorders of anxiety and depression (reviewed in ref. 5), but recent metaanalyses have proved inconclusive when attempting to identify the range of functions attributed to this region, including those relevant to disorders of negative emotion (6, 7). Similarly, patients with vmPFC damage display a range of behavioral deficits, including social cognition, decision making (including choosing between differently valued outcomes), personality, emotion, and memory (expertly reviewed by refs. 6 and 8); but the damage very often spans multiple different brain regions that affect both cell bodies and underlying fibers of passage (white matter) that hinder the allocation of structure to function (9). Consequently, we still lack a fundamental understanding of how particular symptoms relate to dysfunction within particular brain regions. A wealth of studies in rodents have also highlighted the critical role played by regions of the vmPFC in regulating responses to threat and reward (reviewed in refs. 10 and 11), of relevance to our understanding, respectively, of anxiety and anhedonia. However, as we shall see in Anatomical Considerations, below, it is unclear just how comparable regions of the vmPFC in rodents and humans are, highlighting the need for experimental studies in NHPs to bridge the gap. Only recently have studies begun to dissect out this region in monkeys and it is these studies that will be described here, focusing on areas 25 and 32, and their findings discussed in the context of how they compare to and inform the rodent and human literatures.
Anatomical Considerations
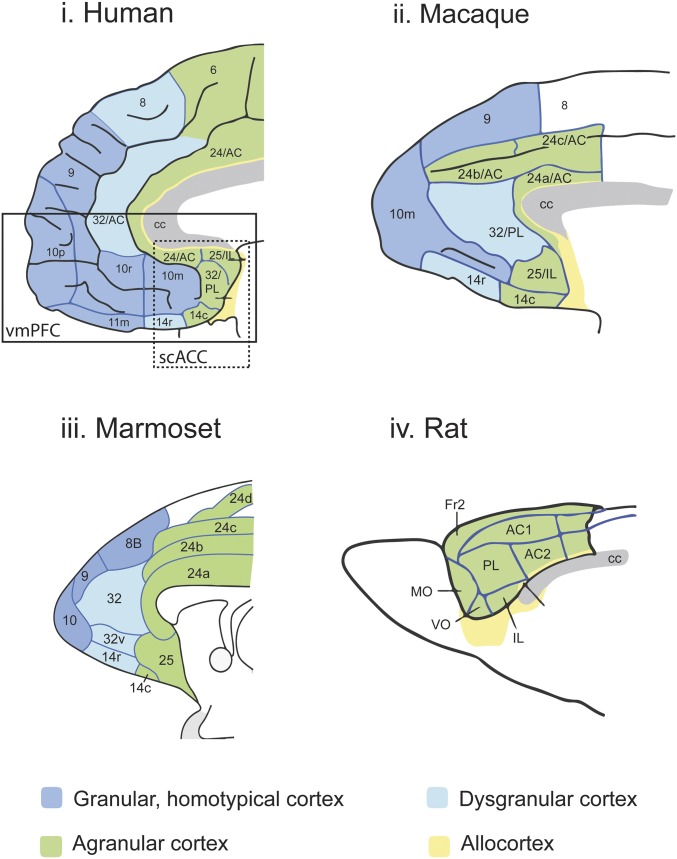
The PFC of humans and NHPs contains 3 types of cytoarchitectonically distinct cortices based on the presence or absence of the granule cell-containing layer 4. The granular, homotypical, neocortex, which is the most recent evolutionally, contains a prominent layer 4, and is found in BA 8, 9, 10, 11, and 47/12. The agranular cortex is the most ancient (posterior BA 13 and 14), has no layer 4, and is considered a transitional zone between the neocortex and more primitive allocortex, while the dysgranular cortex (anterior BA 13 and 14) has a sparse layer 4 (12). These regions are bordered by the anterior cingulate cortex (ACC), which wraps around the genu of the corpus callosum at the midline and comprises BA 24, 25, and 32 (13). Experimentally, these prefrontal and cingulate areas are often grouped according to their regional position. Hence, the dorsal and ventral regions of the lateral surface are referred to as the dorsolateral PFC (dlPFC; BA 8, 9, and 46) and ventrolateral PFC (vlPFC; BA 47/12 and 45), and those on the ventral surface, the orbitofrontal cortex (OFC; primarily BA 11 and 13 and orbital parts of 47/12 and 14). Of particular relevance to this review are those lying on the midline, ventral to the genu of the corpus callosum, which are variably called the vmPFC (BA 10 and 14, as well as ACC regions 25, 32, and ventral parts of 24a) or the subcallosal/subgenual ACC (sc/sgACC), the latter containing only those ventral regions caudal to the genu (Fig. 1). However, the vmPFC, particularly in respect to human lesion studies (6), often refers to a more extended region that includes areas lying on the medial orbital surface that variably include medial 11 and orbital 14, or 11m and 13a/b according to the maps, respectively, of refs. 14 and 15.
Fig. 1.
Cytoarchitectonic parcellation of the vmPFC and scACC in humans, monkeys, and rats. Mid sagittal views of the PFC and ACC in humans (i), macaque (ii), marmosets (iii), and rats (iv). (i, ii, and iv) Reprinted from ref. 12. Copyright (2008), with permission from Elsevier. Parcellation is based on Ongür et al. (15) for human and macaque but Paxinos et al. for marmoset (22) and rat (25, 104). There is general agreement regarding those regions that are agranular (green) and those regions in primates, including humans, which are homotypical granular cortex (dark blue). However, there is variation as to whether monkey and human area 32 is considered dysgranular as described by Paxinos et al. (25) and Zilles and colleagues (7) and depicted here in pale blue, or agranular, as described by Ongür et al. (15) in humans and macaques. It should also be noted that Ongür et al. suggest that 32/PL characterized in macaques is only a very small sector of area 32 in humans, lying very caudal in the subcallosal cortex, while the rest of area 32 (32/AC) they propose is unique to humans.
These regional subdivisions are frequently used when comparing primate and rodent brains, as rodent frontal areas are also subdivided into the dorsal, vmPFC, and OFC regions. However, one key difference between species is that rodents and other mammals only possess an agranular frontal cortex and lack both granular and dysgranular cortices (16). Thus, whether the highly granular and evolutionally recent regions of human and NHP PFC, such as the dlPFC and vlPFC, have correlates in rodents is unclear. In contrast, the OFC, appears more comparable across species, although the granular and dysgranular architecture of the human/NHP OFC (17, 18) still makes it difficult to compare with the agranular ventral, medial, lateral, and dorsolateral orbital regions (VO, MO, LO, and DLO, respectively) of the rodent. One interpretation states that agranular rat regions are homologous to the agranular primate OFC (12), while another suggests that the MO/VO, VLO/LO, and DLO may be similar to primate orbital areas 14, 13, and 47/12, respectively (19). However, the apparently greatest correspondence across species is within the ACC, large parts of which lie within the area often described as the vmPFC or scACC. Vogt et al. (13, 20), Rosa and colleagues (21, 22), and Petrides and colleagues (22) identify areas 25, 32, and 24 across humans, macaques, and marmosets, with the basic organization between the macaque and the marmoset being very similar. Differences between NHPs and humans do emerge, particularly with respect to area 32, where, according to Price and colleagues (15), a subsection of area 32 lying in front of the genu is not found in NHPs (Fig. 1). Similar regions to areas 25, 32, and 24 have been identified in rodents, which correspond roughly to infralimbic (IL), prelimbic (PL), and cingulate regions (Cg) (23–25), respectively, an alternative nomenclature frequently used to describe these regions in rodents. However, it should be noted that even when the same nomenclature is used across species, patterns of connectivity don’t always correspond. There is generally good correspondence between area 25/IL in rodents and area 25 in humans/NHPs on the basis of both cortical and subcortical connectivity patterns (13, 26, 27; but see exception in ref. 28). However, the areas homologous to area 32/PL and Cg/24 do show differences. For example, areas 24/Cg in rats and 24 in humans/NHPs show distinct patterns of striatal projections despite similar connections to the OFC and area 25/IL (26). Indeed, Haber and colleagues have identified portions of rodent PL that may be equivalent to rostral area 24 in NHPs rather than area 32 (26), consistent with the revised rodent atlas of Paxinos and Watson (29) that has renamed the anterior PL as area 32, while the posterior PL has been renamed 24.
These uncertainties and inconsistencies in terminology make it difficult to directly compare experimental findings across species (30). Even in areas where comparable anatomies suggest putative homology between humans, NHPs, and rodents, the primate PFC and ACC may have inherently different neurobiological processes to those of rodents because of the differences in cortical microstructure and the different networks of structures in which distinct prefrontal and cingulate regions are embedded. For example, although area 25/IL appears anatomically homologous across species, this area is integrated into a network that includes higher-order prefrontal areas, such as areas 9, 10, 46, and 47 in NHPs and humans, which are likely to have had additional impact on its function through primate evolution. Thus, for effective translation it is not only important to highlight the similarities between species but also to be aware of potential differences.
Regulation of Threat Processing in Areas 25/IL and 32/PL of Rodents, Humans, and NHPs
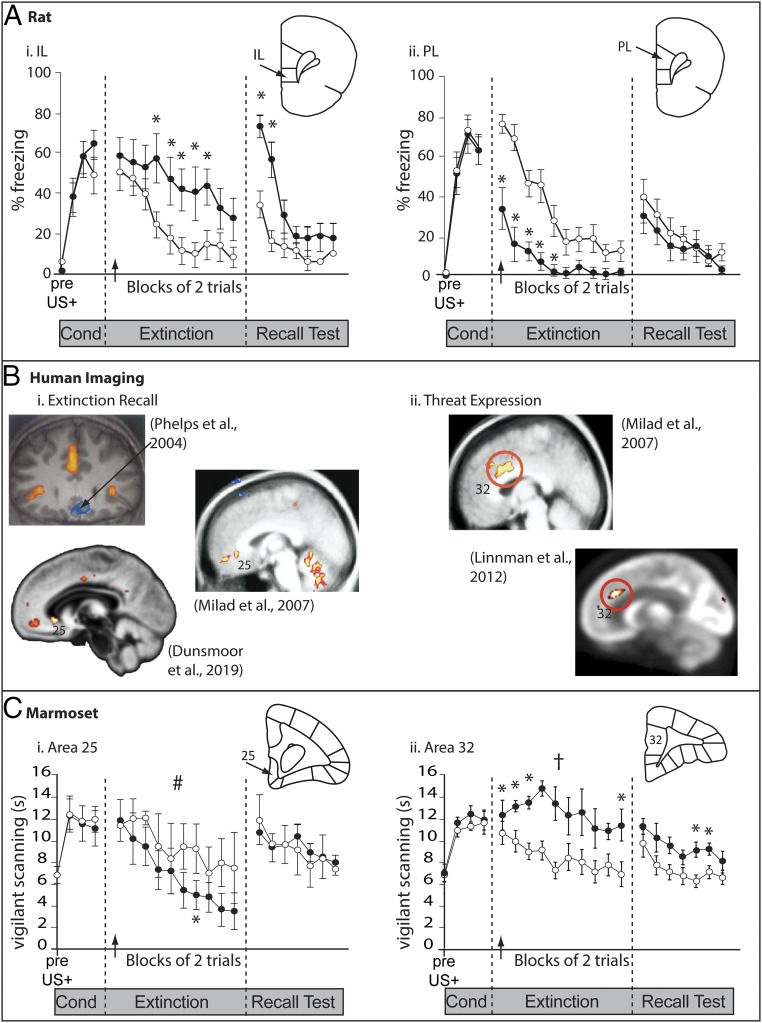
A wide range of behavioral tests have been developed to study the regulation of threat responses in animals that may ultimately provide insight into the neurobiological underpinnings of symptoms of anxiety. Many of these are species-specific, such as the elevated plus maze or open-field tests used to study anxiety-like behavior, which do not have obvious counterparts in studies of anxiety states in humans, as they focus on the natural tendencies of rodents to explore their environment, a behavior affected by many factors beyond those linked to anxiety per se (31, 32). One test, however, that has been successfully translated from rodents into NHPs and the clinic is Pavlovian threat conditioning, proposed to be particularly relevant to anxiety disorders, such as posttraumatic stress disorder (33). During conditioning, an initially neutral cue is repeatedly paired with a biologically relevant event (typically a footshock in rodents and loud noise or wrist shock in humans; unconditioned stimulus [US]), until it takes on the affective properties of the US and becomes a conditioned stimulus (CS). However, it should be noted that the measures of conditioning differ across species. A freezing response is most studied in rodents compared to an autonomic response (e.g., skin conductance or heart rate) in humans. Subcortically, such associative learning is known to depend on the CS and US information converging on the lateral nucleus of the amygdala across species, with amygdala projections to the brainstem, hypothalamus, and motor cortices mediating the relevant physiological and behavioral responses, and projections from the hippocampus providing contextual information (34, 35). These conditioned threat responses are regulated by the vmPFC. Typically, down-regulation of such responses is measured using an extinction protocol in which the CS, after a number of pairings with the US (acquisition), is presented in the absence of the US (extinction) and the conditioned response declines as the new association of CS and no US is learned. In rodents, early lesion studies implicated the IL and not PL in the recall of extinction. Additional lesion, electrophysiological, and pharmacological studies have since demonstrated that IL neurons are active during the recall of extinction, and lesions and temporary inactivations of the IL enhance conditioned threat and inhibit extinction recall (34, 36–38), leading to the hypothesis that the IL is associated with the recall of extinction memory, and critically down-regulates structures such as the amygdala during extinction learning and retention (34, 39, 40). In contrast, activity in the PL is associated with the expression of conditioned threat, as lesions and temporary inactivation of the PL facilitate extinction, while PL microstimulation increases the expression of conditioned threat and prevents extinction (34, 37, 38, 41) (Fig. 2A).
Fig. 2.
Threat extinction and the PFC in rats, marmosets, and humans. (A) Inactivation of the IL in rats at the start of extinction (muscimol; closed circles) hindered the behavioral extinction and extinction recall of an aversive (footshock) Pavlovian conditioned association (A, i), whereas inactivation of the PL hastened extinction (A, ii). Extinction, *P < 0.05; Recall, *P = 0.08. Reprinted by permission from ref. 34, Springer Nature: Neuropsychopharmacology, copyright (2010). (B) Human neuroimaging of threat extinction and recall (B, i) has identified regions of the scACC in which the deactivation induced by the CS+ is blocked following successful extinction recall (42, 43, 45). However, these regions of activity do not include area 25. (Top) Reprinted from ref. 42. Copyright (2004), with permission from Elsevier. (Middle) Reprinted from ref. 43. Copyright (2007), with permission from Elsevier. (Bottom) Reprinted with permission from ref. 45. Threat expression (B, ii) was associated with activity in the dorsal ACC highlighted in red (46, 47). (Top) Reprinted from ref. 46. Copyright (2007), with permission from Elsevier. (Bottom) Reprinted with permission from ref. 47 the American Journal of Psychiatry (Copyright 2012). American Psychiatric Association. All Rights Reserved. Finally, in marmosets (C) inactivation of area 25 (muscimol and baclofen, closed circles) during extinction hastened the extinction of an aversive (rubber snake) Pavlovian conditioned association (C, i), and area 32 inactivation enhanced fear expression during both extinction and recall (C, ii) (48), directly opposite to the findings in rats. See text for discussion of these apparent differences. Brain illustrations provide representative coronal sections to highlight the target area. *P < 0.05; #P < 0.05, manipulation × CS interaction; †P < 0.05, main effect of manipulation; error bars indicate SEM.
Given the proposed homology between primate areas 25/IL and 32/PL, it could be expected that the activity of areas 25 and 32 would correlate, respectively, with the recall of extinction and expression of conditioned threat in human neuroimaging studies, but this is where the story becomes more complex. With respect to the recall of extinction, similarities have been highlighted between rodents and humans based on the finding that in both species it involves the vmPFC. However, as described above, the vmPFC encompasses a large area in humans, containing multiple cytoarchitectonically defined regions and including areas in front of the subcallosal cortex. Close inspection reveals that the extinction recall-related area in humans does not map on to area 25, but instead, a more anterior region (42–45) at, or in front of the genu of the corpus callosum (Fig. 2 B, i). Similarly, threat acquisition, and threat expression during extinction recall in humans is more strongly correlated with cortical thickness and activity of the dorsal ACC (area 24), than with activity in area 32 (46, 47) (Fig. 2 B, ii), although this apparent discrepancy may be explained by appealing to the reclassification of these regions in the latest edition of the Paxinos and Watson rat atlas (29), in which the posterior PL is renamed area 24. Whether the effects of PL manipulations on threat expression in rodents can be attributed to altered activity in this posterior PL/area 24 region, however, remains to be determined.
These discrepancies between rodent and human studies concerning the role of the vmPFC in threat regulation highlight the importance of performing studies in NHPs in order to bridge the translational gap. Indeed, already new insight has been gained by manipulating the distinct regions of subcallosal cingulate in the marmoset, a New World monkey, using a similar Pavlovian conditioning paradigm to that used in both rodents and humans. These studies used a mildly aversive loud noise instead of foot/wrist shock, and to facilitate translation, cardiovascular indices were also measured, since many human studies measure autonomic/cardiovascular indices of negative emotion. Marmosets were therefore implanted with a wireless probe into the descending aorta in a single surgical operation such that, alongside behavior, blood pressure (BP) and heart rate (HR) could be measured remotely in freely moving animals. This approach allowed the selective manipulation of the NHP anatomical homologs (areas 25 and 32) of rodent IL and PL that have been identified in rodents as being important for threat extinction recall and threat expression, respectively. During discriminative threat-conditioning, selective temporary inactivation of area 25 with GABAA/B agonists abolished the expression of the conditioned increases in HR and orienting behavior associated with the CS period, while area 32 inactivation increased them (48), indicating that area 25 activity promotes such conditioned threat responses while area 32 activity inhibits them.
These results in marmosets are opposite to those expected from the enhanced and blunted conditioned freezing responses seen after IL and PL manipulations (lesions or inactivations), respectively, in rodents. Moreover, when the investigations in marmosets were extended to threat extinction and extinction recall tests designed to be as comparable as possible to those used in rodents, the effects remained opposite to those in rats. Specifically, area 25 inactivation induced on the day of extinction, hastened extinction and had no effect on extinction recall the following day (Fig. 2 C, i), while area 32 inactivations retarded extinction and extinction recall, and promoted the expression of threat responses (48) (Fig. 2 C, ii). Thus, these opposing functional effects between rodents and marmosets following inactivation in putatively anatomically homologous regions further highlight potential problems of cross-species translatability.
How comparable though are the findings in marmosets with those in humans? Such comparisons are currently limited as further studies in monkeys are required to investigate the causal involvement of those areas identified in human neuroimaging studies, namely the dACC and the more rostral subcallosal area (14/10/ventral 32), in emotion regulation, although a caudal region of the dACC has been implicated in conditioned threat responses in macaques (49). A role for area 25 in the expression of conditioned threat responses, as suggested by the findings in marmosets, has not so far been shown in human neuroimaging studies of Pavlovian threat conditioning (see ref. 50 for recent critical review of this literature). It should be noted, though, that comparison of conditioning with extinction does reveal activation in the ventral caudate and nucleus accumbens, which may well overlap into caudal area 25 (Fig. 2) (50). Moreover, the findings in marmosets are consistent with a recent neuroimaging metaanalysis of human vmPFC function, which concluded that negative affect in general is primarily associated with activity in the caudal scACC including area 25, while positive affect is associated with activity in more anterior regions that include area 32 (5). This is reinforced by the finding that area 25 neurons in macaques appear to preferentially signal negative outcomes (51, 52). In addition, area 25 overactivation induced by blockade of the excitatory amino acid transporter to reduce glutamate reuptake (using dihydrokainic acid; DHK), heightens anxiety-like behavioral responses in marmosets faced with an unknown human (53), the latter being a well-validated test of uncertainty-based negative affect in monkeys and children that has been successful at identifying those children with extreme anxious temperament at risk for developing pathological anxiety and depression in adolescence (54).
In addition to comparison between rodents, monkeys, and humans, of vmPFC involvement in threatening situations, it is also possible to compare this region’s involvement in the regulation of cardiovascular activity in nontask-related situations that are emotionally neutral. In a resting-like state, inactivation of marmoset area 25 reduced both basal HR and BP and also increased the parasympathetic component of heart rate variability (HRV) (48), the component of HRV that is reduced in anxiety and depression (55, 56). In contrast, corresponding manipulations of either the IL and PL in rats have no cardiovascular effects in emotionally neutral resting conditions (57, 58). Findings in humans are less clear. Although hypotensive responses are seen after electrophysiological stimulation of area 25 in humans (59) and macaques (60), the net effects of such stimulation (excitation or inhibition) and whether the effects are due to activation of adjacent fiber pathways are unclear.
Thus, in both threatening and emotionally neutral states there are marked differences in how these rodent and NHP areas contribute to threat conditioning and physiological processes. This may have important translational implications for disorders in which such emotional and physiological regulation is disrupted. Before considering potential explanations for these differences, we will compare the involvement of these same regions in 2 other symptoms of psychiatric disorders, namely anhedonia and deficits in cost–benefit decision making across rodents, NHPs and humans.
Regulation of Reward Processing in Areas 25/IL and 32/PL of Rodents, Humans, and NHPs
A loss of interest or pleasure in all or almost all activities characterizes clinical anhedonia and is a key diagnostic symptom for major depressive disorder. Indeed, anhedonia is not only a potential trait marker related to vulnerability to depression but is also a poor prognostic marker (61), often associated with treatment-resistant depression. Based on many years of preclinical studies on the brain circuits underlying the processing of reward, it is recognized that anhedonia may arise from dysregulation at multiple levels, including consummatory, anticipatory, and motivational, as well as in decision making, with patients primarily showing anticipatory, motivational, and decision-making deficits, rather than consummatory (reviewed in ref. 62). The vmPFC has been shown to encode internally elicited motivation processes (63), and in the few neuroimaging studies investigating anhedonia, anhedonia severity has been linked to enhanced activity in anterior regions of the vmPFC (areas 32 and 10/24) (64, 65), and context-specific deficits in connectivity in more caudal regions (66), although these studies did not differentiate between the subtypes of anhedonia, and whether changes were causal or compensatory is unknown. Indirect evidence implicating the vmPFC—and in particular, caudal subcallosal cingulate (area 25) overactivity—in symptoms of anhedonia, comes from studies of treatment-resistant depression in which activity in this region is reduced following successful treatment with deep brain stimulation (67).
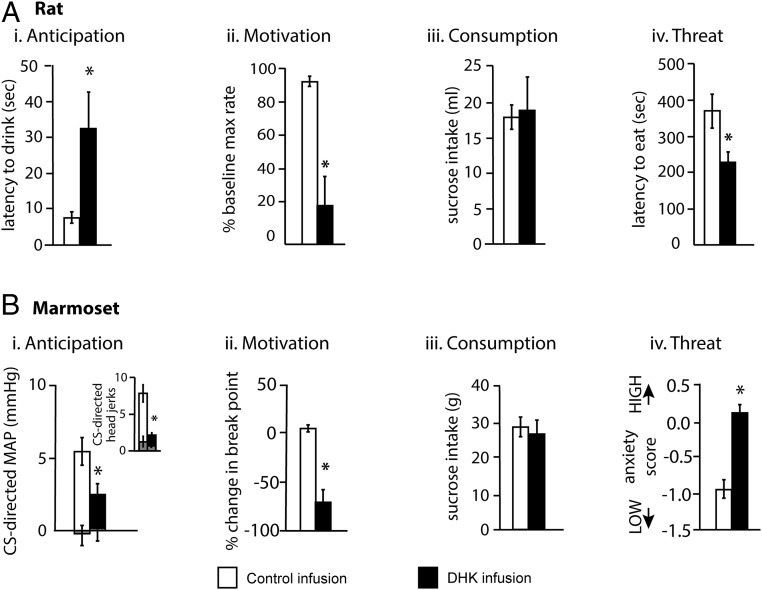
Alterations in activity in rodent IL and PL have been implicated in the regulation of reward-elicited behaviors, and in many cases a similar pattern of effects has been seen as for threat-elicited behaviors, with the PL promoting and IL reducing the expression of reward seeking. In many cases these effects are seen on spontaneous recall and reinstatement of previously extinguished Pavlovian and instrumental appetitive responses (68), implicating these regions in the anticipatory and motivational aspects of reward-elicited behaviors. It is less clear, though, whether IL and PL manipulations impact upon appetitive responses during initial acquisition and expression. Certainly, excitotoxic lesions of the IL have no effect on acquisition of appetitive Pavlovian-conditioned autoshaping (69) and NMDA antagonism of the IL has no effect on expression of instrumental conditioned responses at the start of extinction (70). Moreover, neither inactivation of the PL or IL affect the break point of a progressive ratio schedule (71). However, DHK-induced activation of IL, but not PL, increased the latency to approach, but had no effect on the consumption of, sucrose. It also reduced the threshold for lever pressing for electrical brain stimulation (72) (Fig. 3 A, i–iii). In contrast, there have been reports that GLAST/GLT-1 knockdown-induced sustained activation of the IL reduces overall sucrose consumption in a novelty-suppressed feeding test (73) (Fig. 3 A, iv). In summary, activation of the IL often has broader effects on rewarded behaviors than the effects of inactivation, and in general, activation tends to dampen reward-elicited responses.
Fig. 3.
Reward similarities but threat differences in IL/area 25 function following DHK-induced activation. Increased activation of the IL in rats with DHK slowed the latency to drink sucrose solution, a delay that could be interpreted as a deficit in the anticipation of sucrose reward (A, i). This is similar to the cardiovascular and behavioral (Inset) anticipatory reward deficits seen after area 25 DHK in marmosets (B, i; white [control]/black [DHK] histograms represent responses to the CS paired with reward, smaller gray bars represent responses to the unpaired CS). In both rats and marmosets increased activity in IL/area 25 reduced the motivation to work for reward (A, ii and B, ii); however, it should be noted that the task in rats required subjects to maintain the same level of responding for a steadily decreasing level of electrical brain stimulation, while in marmosets the task required an escalation of responding for the same reward. In neither rats nor marmosets did DHK-induced increased activity in area 25 alter the amount of sucrose consumed in a sucrose consumption test (A, iii and B, iii). In contrast to the similarities in reward-elicited responses in rats and marmosets, effects of DHK differed in threatening situations. Increased IL activity in rats reduced the latency to commence eating in a novelty-induced feeding task, suggesting reduced anxiety-like behavior (A, iv), while increased area 25 activity heightened anxiety-like responses to the threat of a human intruder in marmosets (B, iv). *P < 0.05. A, i–iii reprinted by permission from ref. 72, Springer Nature: Neuropsychopharmacology, copyright (2012); A, iv reprinted from ref. 73, which is licensed under CC BY 4.0; B data from ref. 53.
In contrast to the opposing effects of inactivation on threat regulation between rodents and marmosets, there appears more consistency with respect to the regulation of reward-elicited responses, in that overactivation of marmoset area 25, like the rat IL, dampens such responses. Specifically, DHK-induced overactivation of area 25, but not 32, blunted increases in behavioral and cardiovascular conditioned arousal in anticipation of food reward, and reduced the breakpoint on an instrumental progressive ratio paradigm (53), indicating reductions in the anticipation of, and motivation to work for, reward (Fig. 3 B, i and ii). There were no accompanying effects on the behavioral and cardiovascular indices of reward consumption, and increased activation of area 25 had no effects on sucrose consumption in a sucrose preference test (Fig. 3 B, iii). However, neither area 25 nor 32 are necessary for the expression of CS-triggered anticipatory appetitive arousal, as shown by the lack of effects of inactivation of either area. Direct comparison of these effects with rodent studies is currently difficult as appetitive Pavlovian studies of the PL/IL in rodents often measure conditioned discriminative approach responses to the US, rather than the CS-induced physiological and behavioral arousal measured in marmosets. However, it is the latter that is highly relevant to our understanding of the dysregulation of emotional arousal in human psychiatric disorders, and it is important for future studies to determine whether comparable effects are seen in rodents.
The finding that regionally specific increases in area 25 activity in marmosets not only blunts anticipatory and motivational aspects of reward-elicited behavior but also heightens threat-induced behaviors (on the human intruder test) is consistent with findings in depression, whereby symptoms of anhedonia—in particular, motivational and anticipatory, but not consummatory aspects—can be accompanied by comorbid anxiety. Such consistency of the marmoset studies with clinical observations is crucial to our ability to leverage neurobiological understanding into the translational development of new pharmacotherapeutics. Consequently, the strong clinical relevance of the monkey as a model organism and the validity of discriminative Pavlovian appetitive conditioning for measuring anhedonia-like responses has also allowed the efficacy of novel antidepressant drugs, such as ketamine, to be evaluated. Acute ketamine but not citalopram treatment ameliorated the blunted anticipatory arousal induced by increased activity of area 25 in marmosets, and also reversed the associated changes in activity seen throughout the PFC, dACC, and brainstem, revealing the network through which ketamine acts to ameliorate the anhedonia-like symptoms of increased area 25 activity (53).
Using both Positive and Negative Affective Cues to Guide Decision Making
The ability to weigh up learned costs and benefits is a crucial component of decision making and is impaired after damage to the vmPFC (74) and in psychiatric disorders in which vmPFC dysfunction is implicated. For example, patients suffering from anxiety make more decisions based on expected negative consequences, and patients suffering from depression make fewer decisions based on expected positive consequences (75, 76). Thus, the role of particular brain regions, such as area 25, which regulate both the positive and negative emotional information required for such decisions, and whose dysfunction is strongly associated with psychopathology, are of particular interest.
One informative and translational approach to investigating such affective decision making in rats, monkeys, and humans is the use of instrumental approach–avoidance paradigms that simultaneously pit rewards and punishments against each other to influence decisions (77–80). However, even within such paradigms, there is significant task variation, which makes comparison both between- and within-species difficult (reviewed in ref. 81). Human neuroimaging studies typically study actions associated with learned reward and punishment and implicate activity within the hippocampus, OFC, dlPFC, and ACC (areas 24 and 32, but not 25), in decision-making conflict (82–84). In contrast, rodent studies typically use unlearned, innate cues to measure approach–avoidance behaviors in ethological situations in which there is no explicit reward or punishment, or use Pavlovian cues to guide responding that are often absent from human decision-making processes (85, 86), and have primarily focused on the role of the hippocampus, not the IL or PL. Furthermore, those studies that have investigated lesions or inactivations of the IL or PL have reported inconsistent results, with both anxiolytic and anxiogenic effects seen in a variety of paradigms (reviewed in ref. 81).
Consequently, because of the uncertainty over the roles of areas 25/IL and 32/PL in decision making, and the knowledge that they modulate threat conditioning differently across species, clarification of their role in decision making has been sought from monkeys. On a learned, action-based approach–avoidance task developed specifically for marmosets, area 25 inactivation rendered animals insensitive to punishment, and increased responding overall, while increased activation heightened punishment avoidance (87). This is consistent with the bidirectional effects of area 25 manipulation on the regulation of threat- and reward-elicited behaviors described above. Moreover, this increase in punishment sensitivity after area 25 activation can be compared to humans as it is similar to the increased sensitivity to negative feedback associated with depression, and by extension, scACC overactivity (82, 88). A similar punishment avoidance response has been induced by stimulation of a subset of negative encoding cells within area 32/24 in a macaque approach–avoidance paradigm (77), although the latter is difficult to compare to regional activations that impact both positive and negative coding cells. In the marmoset, inactivation of area 32 had no effect (87), while activation caused punishment insensitivity. Thus, the opposing roles of areas 25 and 32 demonstrated in the regulation of threat-elicited behaviors that were consistent with evidence from neuroimaging extend to the regulation of cost–benefit decision making, a finding that is difficult to reconcile with the increased punishment sensitivity seen after IL and PL excitotoxic lesions in rodent gambling (89, 90).
Reconciling the Differences
This review has highlighted both similarities and differences with respect to manipulations of area 25/IL and area 32/PL in marmosets and rats. Without using the exact same behavioral tests, the same pharmacological manipulations, and the same physiological measures, interpretation of differences between species will always be problematic. Despite such experimental differences, there was some correspondence between rats and marmosets with respect to reward processing. When area 25/IL activation did impact on Pavlovian and instrumental appetitive behaviors, it tended to reduce responding in both species. In contrast, inactivation of both areas 25 and 32 in marmosets, respectively, had opposing effects on threat-induced responding to the effects of IL and PL inactivation in rats. In the case of marmoset area 25, the fact that inactivation decreased threat behavior across multiple paradigms and different physiological indices reinforces the apparently marked differences between manipulations of area 25/IL in NHPs and rodents. The involvement of marmoset area 25 in negative processing did appear consistent, however, with the general consensus that activity in area 25 in humans is associated with negative affective states (see ref. 91 for review of area 25 function across species).
There are primarily 2 possible explanations for the discrepancies between rats and marmosets. The first is that despite their anatomical and cytoarchitectonic similarities, 25/IL and 32/PL are not functionally homologous in primates and rats. This may be a consequence of alterations in downstream connectivity, or a result of the different cognitive frameworks in which they reside, the latter a consequence of the more highly evolved PFC in which areas 25/IL and 32/PL are embedded within the primate as compared to the rodent brain. Alternatively, or perhaps in addition, there may be some functional homology between primates and rats but the nature of the regulation of threat-induced behaviors (i.e., whether responses are dampened or heightened) depends upon the context in which these behaviors take place (92), and these contextual associations may differ between the experimental studies undertaken. Given the varied effects that manipulations of the IL and PL in rats have on a wide range of behaviors—including habits and goal-directed actions (11, 93), attentional control of competing environmental cues (10, 94), and contextual processing (71)—it is likely these regions are involved in higher-order cognitive processes that, when dysregulated, result in complex phenotypes that don’t conform to a simple down- or up- regulation of affective responses.
Such higher-order psychological mechanisms have already been proposed to explain the involvement of the IL in rats in both the regulation of threat and the balance between habitual and goal-directed behaviors (10, 11). They suggest a role for the IL in promoting the performance of those responses that best reflect the animal’s experience with the current contingencies, be it a fear response or a habitual one (10), or IL’s role in suppressing established action–outcome relationships (11). Similarly, it has been proposed that the PL contributes to the expression of conditioned threat responses by virtue of its role in attentional control mechanisms, including the flexible shifting of attentional sets (95, 96), that allow the rat to attend selectively to those elements of the environment that best predict an outcome (10, 94). Support for the latter comes from the finding that the effects of PL lesions on conditioned threat responses are abolished if the rat is preexposed to the test apparatus prior to the start of conditioning, so that competition between the punctate and contextual cues within the apparatus at the time of conditioning is reduced. Whether dysregulation of such high-order processing could explain the anxiety-like and anhedonia-like effects seen in studies of area 25 and 32 in marmosets remains to be determined, with new experiments required to specifically address these hypotheses. However, it should be noted that attentional set-shifting in both humans and marmosets is associated with vlPFC function (97, 98), and specific deficits in shifting attention have been proposed to underlie the heightened anxiety-like (99) and avoidance responses (79) that accompany lesions or inactivation of the vlPFC (79, 99) because of a failure to shift attention away from highly salient aversive stimuli (100). Indeed, because PL lesions in rats disrupt attentional shifting in a task based on one originally developed in marmosets (95), it has been suggested that the PL may be functionally homologous to the vlPFC in monkeys. This does raise the possibility that more generalized processing modules reside in the rodent PL and IL, which, as a consequence of the expansion of the association cortex in humans and NHPs, have evolved into more specialized and regionally localized, cognitive processing units. Such a hypothesis could provide a more parsimonious explanation for the combination of functional overlaps and inconsistencies that are seen when comparing putative anatomical homologs of the PFC and ACC between rodents and primates.
Future Directions
Effective translation between rats, marmosets, and humans is especially important in the wake of emerging findings from studies into the development of emotion regulation and the differential onset of amygdala–vmPFC, vmPFC–amygdala, and vmPFC–cortical interactions in humans and rats (reviewed in ref. 101). Studies such as these will have important implications for our understanding of the etiology of emotion dysregulation in disorders, such as anxiety and depression, which very often have their onset during adolescence (102). They suggest that adversity, a known risk factor for the onset of mental health disturbance, may induce different symptomatology depending on the stage of development in which it occurs. Recently, structural MRI across development in marmosets has revealed that the most marked heterogeneity of developmental growth curves of all association cortices is within the PFC and ACC (103). Of particular relevance to the present discussion is the finding that area 25 and 32 showed a much earlier onset of gray matter decline (thought to reflect changes in synaptic pruning and myelination) compared to surrounding prefrontal regions, which may indicate earlier functional recruitment of these regions. However, that this decline was sustained across adolescence, with the maximum rate of decline occurring toward the end of adolescence, later than the maximum rate seen for all other prefrontal regions, may reflect their subsequent functional integration into these late-developing prefrontal networks. Selective intervention studies at distinct timepoints during the development of monkeys should test these hypotheses and contrast effects with similar studies in rodents.
Conclusion
To conclude, measuring behavioral and autonomic indices of threat- and reward-elicited behaviors in monkeys during the performance of comparable paradigms to those used in rodents and humans makes monkeys a unique investigative resource to bridge the gap between rodents and humans, facilitating forward translation into the clinic and back translation to rodents. Crucially, the ability to manipulate PFC/ACC regions that display markedly similar organization to humans allows an assessment of causality not possible in humans and allows translation to clinical states that may not always be possible with rodents. Moreover, in the case of putative anatomical homologs in the ACC of rodents and NHPs, such as areas 25/IL and 32/PL considered here, experimental studies in NHPs can determine the extent to which anatomical homology reflects functional homology. This will depend upon a more critical approach to cross-species comparisons and a greater willingness to recognize differences as well as similarities. Moreover, a concerted effort is required to develop not only more translationally relevant behavioral tasks but also translationally relevant physiological–behavioral outcomes for use across rodents, NHPs and humans.
Acknowledgments
We thank Dr. Nicole Horst for her artwork. The marmoset work described here was supported by Wellcome Trust Grant 108089/Z/15/ and Medical Research Council Grant MR/M023990/1 (to A.C.R.); and Medical Research Council career development Award RG62920 (to H.F.C.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission.
References
- 1.Rakic P., Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson M. B., et al. , Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62, 494–509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman S. E., Revolution stalled. Sci. Transl. Med. 4, 155cm11 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Gribkoff V. K., Kaczmarek L. K., The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120, 11–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers-Schulz B., Koenigs M., Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol. Psychiatry 17, 132–141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiser J., Koenigs M., The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatry 83, 638–647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomero-Gallagher N., et al. , Functional organization of human subgenual cortical areas: Relationship between architectonical segregation and connectional heterogeneity. Neuroimage 115, 177–190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider B., Koenigs M., Human lesion studies of ventromedial prefrontal cortex. Neuropsychologia 107, 84–93 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Rudebeck P. H., et al. , A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc. Natl. Acad. Sci. U.S.A. 111, 5391–5396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpe M. J., Killcross S., Modulation of attention and action in the medial prefrontal cortex of rats. Psychol. Rev. 125, 822–843 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Barker J. M., Taylor J. R., Chandler L. J., A unifying model of the role of the infralimbic cortex in extinction and habits. Learn. Mem. 21, 441–448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise S. P., Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 31, 599–608 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogt B. A., Paxinos G., Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 219, 185–192 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Mackey S., Petrides M., Architecture and morphology of the human ventromedial prefrontal cortex. Eur. J. Neurosci. 40, 2777–2796 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Ongür D., Ferry A. T., Price J. L., Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 460, 425–449 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Preuss T. M., Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J. Cogn. Neurosci. 7, 1–24 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Petrides M., Pandya D. N., “Comparative architectonic analysis of the human and the macaque frontal cortex” in Handbook of Neuropsychology, Boller F., Grafman J., Eds. (Elsevier Science B. V., Amsterdam, The Netherlands, 1994), pp. 17–58. [Google Scholar]
- 18.Semendeferi K., Damasio H., Frank R., Van Hoesen G. W., The evolution of the frontal lobes: A volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. J. Hum. Evol. 32, 375–388 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Price J. L., Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann. N. Y. Acad. Sci. 1121, 54–71 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Vogt B. A., et al. , Cingulate area 32 homologies in mouse, rat, macaque and human: Cytoarchitecture and receptor architecture. J. Comp. Neurol. 521, 4189–4204 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Burman K. J., Rosa M. G. P., Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J. Comp. Neurol. 514, 11–29 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G., Watson C., Petrides M., Rosa M., Tokuno H., The Marmoset Brain in Stereotaxic Coordinates (Academic Press, 2012). [Google Scholar]
- 23.Paxinos G., Watson C., The Rat Brain in Stereotaxic Coordinates (Academic Press, ed. 4, 1996). [Google Scholar]
- 24.Ongür D., Price J. L., The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G., et al. , The Rat Brain in Stereotaxic Coordinates (Academic Press, 1983). [Google Scholar]
- 26.Heilbronner S. R., Rodriguez-Romaguera J., Quirk G. J., Groenewegen H. J., Haber S. N., Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vertes R. P., Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Zikopoulos B., Höistad M., John Y., Barbas H., Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J. Neurosci. 37, 5051–5064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G., Watson C., The Rat Brain in Stereotaxic Coordinates (Elsevier Science, ed. 7, 2013). [Google Scholar]
- 30.Laubach M., Amarante L. M., Swanson K., White S. R., What, if anything, is rodent prefrontal cortex? eNeuro 5, ENEURO.0315-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos A., Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 29, 493–498 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Prut L., Belzung C., The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 463, 3–33 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Milad M. R., Quirk G. J., Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 63, 129–151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierra-Mercado D., Padilla-Coreano N., Quirk G. J., Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sotres-Bayon F., Sierra-Mercado D., Pardilla-Delgado E., Quirk G. J., Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldi E., Bucherelli C., Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci. Biobehav. Rev. 53, 160–190 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Vidal-Gonzalez I., Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 13, 728–733 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quirk G. J., Beer J. S., Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr. Opin. Neurobiol. 16, 723–727 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Do-Monte F. H., Manzano-Nieves G., Quiñones-Laracuente K., Ramos-Medina L., Quirk G. J., Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurent V., Westbrook R. F., Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn. Mem. 16, 520–529 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Courtin J., et al. , Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Phelps E. A., Delgado M. R., Nearing K. I., LeDoux J. E., Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43, 897–905 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Milad M. R., et al. , Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Kalisch R., et al. , Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J. Neurosci. 26, 9503–9511 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunsmoor J. E., et al. , Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. J. Neurosci. 39, 3264–3276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milad M. R., et al. , A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry 62, 1191–1194 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Linnman C., et al. , Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am. J. Psychiatry 169, 415–423 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallis C. U., Cardinal R. N., Alexander L., Roberts A. C., Clarke H. F., Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc. Natl. Acad. Sci. U.S.A. 114, E4075–E4084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klavir O., Genud-Gabai R., Paz R., Low-frequency stimulation depresses the primate anterior-cingulate-cortex and prevents spontaneous recovery of aversive memories. J. Neurosci. 32, 8589–8597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fullana M. A., et al. , Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 88, 16–25 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Monosov I. E., Hikosaka O., Regionally distinct processing of rewards and punishments by the primate ventromedial prefrontal cortex. J. Neurosci. 32, 10318–10330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azab H., Hayden B. Y., Correlates of economic decisions in the dorsal and subgenual anterior cingulate cortices. Eur. J. Neurosci. 47, 979–993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander L., et al. , Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron 101, 307–320.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalin N. H., Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. Eur. Neuropsychopharmacol. 27, 543–553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemp A. H., et al. , Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 67, 1067–1074 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Miu A. C., Heilman R. M., Miclea M., Reduced heart rate variability and vagal tone in anxiety: Trait versus state, and the effects of autogenic training. Auton. Neurosci. 145, 99–103 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Müller-Ribeiro F. C. D. F., et al. , Contribution of infralimbic cortex in the cardiovascular response to acute stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R639–R650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tavares R. F., Corrêa F. M., Resstel L. B., Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J. Neurosci. Res. 87, 2601–2607 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Lacuey N., et al. , Cortical structures associated with human blood pressure control. JAMA Neurol. 75, 194–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaada B. R., Pribram K. H., Epstein J. A., Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J. Neurophysiol. 12, 347–356 (1949). [DOI] [PubMed] [Google Scholar]
- 61.Pizzagalli D. A., Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10, 393–423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treadway M. T., Zald D. H., Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 35, 537–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouret S., Richmond B. J., Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J. Neurosci. 30, 8591–8601 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keedwell P. A., Andrew C., Williams S. C. R., Brammer M. J., Phillips M. L., The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry 58, 843–853 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Dunn R. T., et al. , Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol. Psychiatry 51, 387–399 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Young C. B., et al. , Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl. Psychiatry 6, e810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayberg H. S., et al. , Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Warren B. L., et al. , Distinct fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J. Neurosci. 36, 6691–6703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chudasama Y., Robbins T. W., Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 23, 8771–8780 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters J., De Vries T. J., D-cycloserine administered directly to infralimbic medial prefrontal cortex enhances extinction memory in sucrose-seeking animals. Neuroscience 230, 24–30 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Riaz S., et al. , Prelimbic and infralimbic cortical inactivations attenuate contextually driven discriminative responding for reward. Sci. Rep. 9, 3982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.John C. S., et al. , Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 37, 2467–2475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gasull-Camós J., Tarrés-Gatius M., Artigas F., Castañé A., Glial GLT-1 blockade in infralimbic cortex as a new strategy to evoke rapid antidepressant-like effects in rats. Transl. Psychiatry 7, e1038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bechara A., Damasio A. R., Damasio H., Anderson S. W., Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Dickson J. M., Perceived consequences underlying approach goals and avoidance goals in relation to anxiety. Pers. Individ. Dif. 41, 1527–1538 (2006). [Google Scholar]
- 76.Dickson J. M., MacLeod A. K., Approach and avoidance goals and plans: Their relationship to anxiety and depression. Cognit. Ther. Res. 28, 415–432 (2004). [Google Scholar]
- 77.Amemori K., Graybiel A. M., Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat. Neurosci. 15, 776–785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bach D. R., et al. , Human hippocampus arbitrates approach-avoidance conflict. Curr. Biol. 24, 541–547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke H. F., Horst N. K., Roberts A. C., Regional inactivations of primate ventral prefrontal cortex reveal two distinct mechanisms underlying negative bias in decision making. Proc. Natl. Acad. Sci. U.S.A. 112, 4176–4181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito R., Lee A. C. H., The role of the hippocampus in approach-avoidance conflict decision-making: Evidence from rodent and human studies. Behav. Brain Res. 313, 345–357 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Kirlic N., Young J., Aupperle R. L., Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behav. Res. Ther. 96, 14–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aupperle R. L., Melrose A. J., Francisco A., Paulus M. P., Stein M. B., Neural substrates of approach-avoidance conflict decision-making. Hum. Brain Mapp. 36, 449–462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlund M. W., et al. , The tipping point: Value differences and parallel dorsal-ventral frontal circuits gating human approach-avoidance behavior. Neuroimage 136, 94–105 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Croxson P. L., Walton M. E., O’Reilly J. X., Behrens T. E. J., Rushworth M. F. S., Effort-based cost-benefit valuation and the human brain. J. Neurosci. 29, 4531–4541 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schumacher A., Vlassov E., Ito R., The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus 26, 530–542 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Friedman A., et al. , A corticostriatal path targeting striosomes controls decision-making under conflict. Cell 161, 1320–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallis C. U., Cockcroft G. J., Cardinal R. N., Roberts A. C., Clarke H. F., Hippocampal interaction with area 25, but not area 32, regulates marmoset approach-avoidance behaviour. Cereb Cortex, bhz015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roiser J. P., Sahakian B. J., Hot and cold cognition in depression. CNS Spectr. 18, 139–149 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Paine T. A., Asinof S. K., Diehl G. W., Frackman A., Leffler J., Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: Reversal by D1 receptor antagonist administration. Behav. Brain Res. 243, 247–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeeb F. D., Baarendse P. J. J., Vanderschuren L. J. M. J., Winstanley C. A., Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology (Berl.) 232, 4481–4491 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Alexander L., Clarke H. F., Roberts A. C., A focus on the functions of area 25. Brain Sci. 9, 129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pennington Z. T., Anderson A. S., Fanselow M. S., The ventromedial prefrontal cortex in a model of traumatic stress: Fear inhibition or contextual processing? Learn. Mem. 24, 400–406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balleine B. W., O’Doherty J. P., Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharpe M. J., Killcross S., The prelimbic cortex contributes to the down-regulation of attention toward redundant cues. Cereb. Cortex 24, 1066–1074 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Birrell J. M., Brown V. J., Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 20, 4320–4324 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bissonette G. B., et al. , Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 28, 11124–11130 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hampshire A., Owen A. M., Fractionating attentional control using event-related fMRI. Cereb. Cortex 16, 1679–1689 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Dias R., Robbins T. W., Roberts A. C., Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380, 69–72 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Agustín-Pavón C., et al. , Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol. Psychiatry 72, 266–272 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Shiba Y., Santangelo A. M., Roberts A. C., Beyond the medial regions of prefrontal cortex in the regulation of fear and anxiety. Front. Syst. Neurosci. 10, 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casey B. J., Heller A. S., Gee D. G., Cohen A. O., Development of the emotional brain. Neurosci. Lett. 693, 29–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kessler R. C., et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Sawiak S. J., et al. , Trajectories and milestones of cortical and subcortical development of the marmoset brain from infancy to adulthood. Cereb. Cortex 28, 4440–4453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palomero-Gallagher N., Zilles K., “Isocortex” in The Rat Nervous System, Paxinos G., Ed. (Elsevier, ed. 4, 2015), pp. 601–625. [Google Scholar]