Abstract
The advent of neuroimaging has provided foundational insights into the neural basis of psychiatric conditions, such as major depression. Across countless studies, dysfunction has been localized to distinct parts of the limbic system. Specific knowledge about affected locations has led to the development of circuit modulation therapies to correct dysfunction, notably deep brain stimulation (DBS). This and other emerging neuromodulation approaches have shown great promise, but their refinement has been slow and fundamental questions about their mechanisms of action remain. Here, we argue that their continued development requires reverse translation to animal models with close homology to humans, namely, nonhuman primates. With a particular focus on DBS approaches for depression, we highlight the parts of the brain that have been targeted by neuromodulation in humans, their efficacy, and why nonhuman primates are the most suitable model in which to conduct their refinement. We finish by highlighting key gaps in our knowledge that need to be filled to allow more rapid progress toward effective therapies in patients for whom all other treatment attempts have failed.
Keywords: deep brain stimulation, depression, subcallosal anterior cingulate cortex, nonhuman primate, psychiatric disorders
Mood and anxiety disorders impact the lives of millions worldwide. The effect on patients’ families, friends, and caregivers multiplies this impact, placing a massive burden on society. Despite the successes of modern therapies in managing these disorders, large treatment gaps exist. Indeed, for some individuals, none of the available treatments provide relief, leaving them in a chronic state of disability. For these “treatment-resistant” individuals, it is essential that novel therapeutic targets are identified and treatments developed.
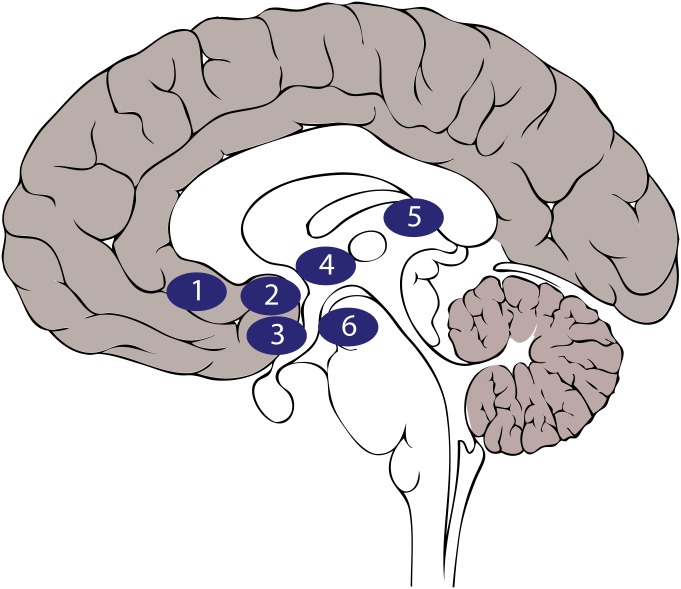
Our failure to comprehensively treat chronic depression and anxiety stems, in part, from the fact that the brain basis of affect is largely unknown. The development of functional neuroimaging technology in the early 1990s dramatically reduced this gap in our knowledge. Confirming what had been translated from basic science, neuroimaging studies revealed gross dysfunction within the limbic system of patients with major depression and anxiety disorders (1). Notably, the level of anatomical precision afforded by imaging techniques also identified distinct areas that could be targets for novel therapeutic interventions. Such was the case for the development of deep brain stimulation (DBS) for treatment-resistant depression (Fig. 1). Previously, DBS had proven highly effective in the treatment of Parkinson’s disease, where subthalamic nucleus (STN) had been targeted with high-frequency stimulation to correct pathological activity (2, 3). Targeting subcallosal anterior cingulate cortex (ACC) with the same DBS approach produced prolonged remission from depression for more than half of the patients in the first clinical trial (4). Rates of remission in subsequent trials have, however, varied considerably (5, 6). The dramatic improvement in some individuals previously unresponsive to standard treatments catalyzed interest in using DBS to treat psychiatric disorders and led to other brain areas being targeted based on specific hypotheses concerning the pathophysiology of depression (7, 8) (Fig. 1) or anxiety (9). These trials reported similar response rates to DBS targeting subcallosal ACC, but with different temporal response profiles and side effects. These studies highlight the fact that while circuit modulation therapies have shown great promise for treating psychiatric disorders, they are not always effective.
Fig. 1.
DBS targets for treatment-resistant depression. Approximate locations of DBS targets on a sagittal representation of the human brain are as follows: (1) subcallosal ACC, (2) ventral striatum/internal capsule, (3) nucleus accumbens, (4) bed nucleus of the stria terminalis, (5) lateral habenula, and (6) medial forebrain bundle.
Optimization of DBS for psychiatric disorders has been slowed by the fact that we lack detailed knowledge of how this intervention impacts neural circuits. DBS is not cell-specific and has differential effects on white and gray matter, meaning that it impacts a complex set of local and brain-wide interactions. As such, we have little understanding of why DBS fails to produce the desired effects in some cases, although patient heterogeneity likely plays a significant role. Ultimately, refinement of DBS and similar circuit-modulating treatments will accelerate greatly if we gain in-depth knowledge of how the limbic system controls human affect, what goes wrong when it fails, and how it is influenced by targeted stimulation.
Here, we argue that gaining these insights will require not just translation from animal models, but reverse translation from the clinic to species that share common features with humans. Nonhuman primates offer such a model. They share a close evolutionary history and ecological niche with humans and have highly differentiated limbic systems, as well as similar social structures and affective repertoires to humans. All of these features make them highly suitable for understanding function in the brain pathways that control human affect.
Nonhuman primate models were critical in developing DBS for Parkinson’s disease, a therapy now implemented in increasing numbers of people worldwide. We reason that the potential offered by nonhuman primate models for refining DBS treatments for psychiatric disorders will be no less important. This is particularly true given the expansion and differentiation of prefrontal cortex (PFC) in both species, a part of the brain heavily implicated in mood and anxiety disorders. In addition, unlike movement disorders, where the effects of DBS can be readily discerned (e.g., immediate reduction in tremor), the effects of DBS for psychiatric disorders only become fully apparent after months of stimulation. This slow time course, combined with the potential risks of surgery and a prolonged sham period in people suffering from treatment-resistant symptoms, presents a challenge for the traditional clinical trial design, where prospective, randomized control comparisons are typically used to establish efficacy. For instance, in DBS for depression, inclusion criteria generally require an episode duration of a minimum of 1 to 2 years and a minimum of 4 failed treatments, including electroconvulsive therapy (ECT). In this case, a control group that receives its usual non-DBS treatment may be difficult to justify, although it has been used successfully in a vagus nerve stimulation trial involving less resistant patients (10). To mitigate against false-positive results, most DBS trials for psychiatric disorders have used relatively short blinded control periods where stimulation may or may not delivered, after which open-label stimulation (i.e., all researchers and participants know who will receive stimulation) is tested (6). An alternative approach is blinded discontinuation once a stable response has been reached (11). Regardless of the brain target, DBS trials have not shown active stimulation to be more effective than sham stimulation during the blinded phase (5, 12, 13). However, these same studies consistently show clinically significant and sustained antidepressant effects with continued open-label intervention. Such contradictions may be resolved with the systematic characterization of factors impacting the chronology of these putative progressive, but delayed, antidepressant effects. Nonhuman primates therefore provide a potential means to conduct fully controlled studies of the temporal effects of DBS on affective processing that may strategically inform next-phase clinical trial design and implementation.
Our aim here is not to provide an exhaustive analysis of the current state of neuromodulation for psychiatric disorders. Instead, we will selectively review the current approaches to DBS for depression as an exemplar case, emphasizing the roadblocks to progress and why monkeys present the best model for reverse translation, as well as identifying where reverse translation to monkey models can provide important insights.
The Development of Current DBS Approaches for Depression
Clinically, major depression is characterized by a persistent state of sadness, lack of motivation, and loss of pleasure from previously rewarding experiences and situations (14). It is also associated with reduced ability to maintain attention; feelings of guilt; thoughts of suicide; and alterations in sleep, appetite, libido, and motor function. Many therapeutic options are available for depression, including psychotherapy, pharmacological therapies, and ECT. However, if a patient’s symptoms are not improved by multiple different treatments, they can meet the criteria for “treatment-resistant” depression (15). While precise definitions vary (16), in most cases, “treatment-resistant” patients have a long history of depression that does not respond to multiple attempts at treatment. In this case, they may become candidates for DBS.
The past 15 years have seen the testing of DBS in various brain areas to alleviate treatment-resistant depression (Fig. 1). These areas are broadly within the cortical and subcortical parts of the limbic system, and the reasons for targeting specific locations have varied widely. Here, we review 3 putative DBS targets for depression: 2 established and 1 emerging. Comprehensive reviews of other sites, including inferior thalamic peduncle, nucleus accumbens, vagus nerve, medial forebrain bundle, lateral habenula, and bed nucleus of the stria terminalis, can be found elsewhere (7, 8).
Subcallosal ACC.
Transient sadness or experimentally induced negative mood in healthy individuals is associated with increased activity within subcallosal ACC (17, 18). This increase is also linked to altered functional connectivity between subcallosal ACC and other parts of the brain during the processing of affective stimuli (18). Initial investigations of individuals with major depression reported hypoactivity within subcallosal ACC (19), but correction for gray matter loss in later studies revealed that there was actually an increase in activity (20). Subsequent studies corroborated this and went on to reveal that this hyperactivity is reduced following successful pharmacological therapy, suggesting a direct relationship between subcallosal ACC activity and depression (17, 21). Similarly, positron emission tomography imaging of patients undergoing cingulotomy for treatment-resistant depression also found that the greatest benefits from lesions were in individuals who had the highest levels of subcallosal ACC activity preoperatively (22). While additional studies have reported hyper- or even hypoactivity in depressed individuals and changes in activity of treatment responders (23), a common theme has remained: The function of subcallosal ACC is consistently linked to major depression.
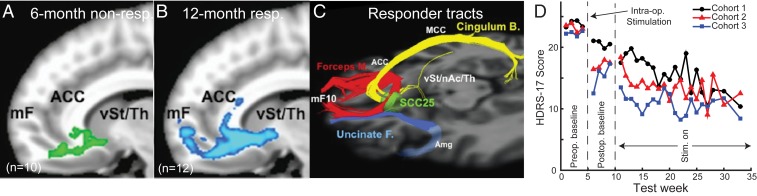
In 2005, the first clinical trial of subcallosal ACC stimulation in treatment-resistant depression was published (4). Using constant high-frequency (130 Hz) stimulation, remission was achieved in 4 of 6 patients. The efficacy of the approach has been further tested with varying degrees of success, including a multisite clinical trial that failed futility analysis (5, 24, 25). However, follow-up assessments in individuals who responded to stimulation have found beneficial effects persisting for years (26). Further investigation also found that stimulation contacts associated with the largest reduction in symptoms were not within the subcallosal ACC, but were instead in the adjacent white matter that projects to subcallosal ACC and nearby structures. Meticulous mapping of the white matter pathways with diffusion-weighted imaging tractography and finite element modeling revealed that the greatest reduction in symptoms was achieved when contacts were localized to the confluence of the forceps minor, uncinate fascicle, cingulum bundle, and subcortical fibers projecting to the ventral striatum, thalamus, and dorsal raphe (27) (Fig. 2 A–C, Right). A subsequent prospective study has shown the importance of specifically influencing these pathways. When this convergence point was targeted in a set of 11 patients, response rates were over 80%, with nearly half of the total participants (5 of 11) achieving remission within 6 months without any adjustments in the site of stimulation (6) (Fig. 2D, cohort 2). While not directly compared, this response exceeded that seen in a previous cohort where tractography-guided surgery was not utilized and where a comparable response rate required trial and error adjustments of the site of stimulation over 2 years (Fig. 2D, cohort 1). This tractography-guided implantation strategy is now standard and has been again implemented in a new cohort now under study (Fig. 2D, cohort 3).
Fig. 2.
Diffusion tractography estimates of white matter pathways near to subcallosal ACC DBS targets and the time course of response to stimulation. (A) Diffusion tractography of 6-month nonresponders (non-resp, n = 10). Estimated tracts from active contact are shown in green. mF, medial frontal; Th, thalamus; vSt, ventral striatum. (B) Diffusion tractography of 2-year responders (n = 12, blue). Estimated tracts from active contact are shown in blue. (C) Pathways influenced in responders to DBS. Based on individual activation volume tract maps, all 12-month responders share bilateral pathways via the forceps minor (M.) and uncinate fasciculus (F.) to medial frontal cortex (Brodmann area 10); via the cingulum bundle to subgenual, rostral, and dorsal anterior and midcingulate; and via descending subcortical fibers to ventral striatum (nucleus accumbens [nAc] and ventral pallidum), putamen, hypothalamus, and anterior thalamus. Six-month nonresponders lack connections to both medial frontal and subcortical regions seen in the responder group. SCC25, subcallosal ACC. Reprinted from ref. 27. Copyright (2014), with permission from Elsevier. (D) Mean 17-item Hamilton Depression Rating Scale (HDRS) scores measured from 4 wk before surgery (preoperative [Preop.]) to 28 wk after surgery (postoperative [Postop.]) to implant DBS electrodes targeting white matter pathways close to subcallosal ACC. Postoperatively, participants undergo an initial 4-wk period where 130-Hz stimulation (Stim.) is not delivered, although all patients received acute DBS intraoperatively (Intra-op.) to assess side effects (all cohorts) and to record local field potentials (cohorts 2 and 3). Cohort 1 is shown in black (n = 17) (84), cohort 2 is shown in red (n = 11) (6), and cohort 3 is shown in blue (n = 10). Cohort 3 is from ongoing clinical trial NCT00367003.
Interestingly, use of this implantation strategy has further allowed intraoperative and laboratory testing of behavioral and electrophysiological change with acute stimulation at the optimized target (6, 28–30). While intraoperative stimulation at the optimized target may explain the observed acute behavioral effects and postoperative improvement and carryover even in the absence of active stimulation (Fig. 2D), it remains unclear what drives the progressive evolution of a full antidepressant response over the subsequent 6 months that occurs with chronic stimulation (Fig. 2D). Such slow changes could be due to network-level effects that could reflect functional reorganization, anatomical changes, or both. We take this point up later.
Internal Capsule/Ventral Striatum.
Basic research in humans and animal models identified the ventral striatum, especially the nucleus accumbens, as central to signaling rewarding stimuli as well as controlling reward seeking (31). In patients with depression, ventral striatum exhibits altered functional connectivity as measured by functional MRI, and ventral striatum blood oxygen level-dependent activation in response to pleasurable events is reduced in depressed individuals compared with controls (32). Previously, electrolytic lesions or DBS of ventral striatum had been used to treat anxiety disorders, with differing degrees of success (33). In a number of cases, however, reduction of depressive symptoms was also reported (34), indicating ventral striatum as a potential therapeutic target for treatment-resistant depression. In recent trials, DBS of ventral striatum or anterior limb of the internal capsule in patients with treatment-resistant depression has been associated with response rates of between 13% and 100%, as measured using either the Hamilton Depression Rating Scale or Montgomery–Asberg Depression Rating Scale (12, 35, 36). A nearby target within nucleus accumbens is also associated with 9 to 30% rates of responding (37, 38).
An open-label study targeting the anterior limb of the internal capsule reported that stimulation was associated with a significant reduction in depressive symptoms in 10 of 25 participants, with 5 of those participants achieving full remission from depression (11). However, 60% of participants did not see any improvement in symptoms despite identical surgical and testing procedures. The variability in response rates is likely caused by a number of different factors, including differences in which pathways are influenced by stimulation, similar to what has been seen for subcallosal ACC DBS. Diffusion tractography shows a clearly defined topographical organization of connections passing through the capsule with minute differences in dorsoventral position separating completely different projection pathways (39). Thus, it is possible that defining electrode placement individually for each participant based on pathway anatomy could be critical. Indeed, an emerging hypothesis is that influencing medial forebrain bundle may be essential to the antidepressant effects of this target, a finding supported by analyses of mood effects with DBS of the anterior limb of the internal capsule for obsessive-compulsive disorder (40, 41). An alternative explanation is that some patients do not respond to this therapy because they have a different subtype of depression, in which case the pathological brain circuits may not course through the internal capsule. In other words, there may be optimal targets for different depression subtypes. This highlights the importance of developing biomarkers that provide predictive validity for DBS targets.
Orbitofrontal Cortex.
Countless neuropsychological and neurophysiological investigations in both humans and animals have implicated the orbitofrontal cortex (OFC) in controlling affect (42), and lesions in humans are associated with marked changes in personality and emotional processing (43). Converging evidence from functional neuroimaging studies and single-neuron recording studies in human and nonhuman animals has similarly shown that the area is engaged when both aversive and pleasurable stimuli are evaluated and experienced (44). In depression, OFC has been found to be hyperactive (45) and, similar to subcallosal ACC, activity normalizes when individuals are in remission from depression (4, 46). There have been only a few attempts to directly manipulate the activity in the OFC of individuals with treatment-resistant depression, although these have mainly been limited to repetitive transcranial magnetic stimulation and have produced mixed clinical results (47).
Directly targeting the OFC with chronic DBS has not been attempted for treatment-resistant depression, but a recent study in epileptic patients indicates that it could be a potential therapeutic target (48). Prior to the implantation of intracranial electrodes to localize seizure foci in patients with drug-resistant epilepsy, Rao et al. (48) measured subjects’ mood. The patients’ mood was then repeatedly assessed over the course of a number of days as neural activity was recorded from all electrodes implanted in OFC as well as other parts of the limbic system. On a test day, either sham or acute high-frequency stimulation was delivered to a selected brain area, and effects on mood were measured. In patients who rated their mood as moderate to severely depressed prior to surgery, lateral OFC stimulation improved mood, as assessed by the Immediate Mood Scaler and measurements of speech content.
Of the sites outside of OFC, only stimulation within subcallosal ACC was associated with a slight improvement in mood. Notably, no mood changes were seen after lateral OFC stimulation in patients who only had minimal to mildly depressed mood at the outset of the study. In addition, depression state or the time course of mood changes was not evaluated, so it is not clear if these findings would be durable or safe with chronic high-frequency stimulation. Nonetheless, in patients with moderate to severe ratings, there was a correlation between the power in low-frequency bands (theta/alpha: 4–12 Hz) in lateral OFC and more negative mood assessments, and power in these bands was reduced when mood-changing stimulation was delivered. Finally, lateral OFC stimulation was associated with network-level reductions in low-frequency power across other sites implicated in controlling mood, including amygdala, insula, and hippocampus, suggesting that the effect of DBS on mood results from network-level changes. This final result emphasizes that refining DBS for treatment-resistant depression will require establishing how both acute and chronic stimulation of lateral OFC (and other sites) influences a broad network of areas.
To that end, research in epilepsy patients will be key to gaining traction on this problem, as it takes advantage of the unique situation where multiple indwelling electrodes are chronically monitored for days to weeks in a human. On the other hand, these patients often have pathological tissue in the putative depression networks under investigation, and it remains to be seen how this impacts studies of mood-relevant circuits and their response to stimulation. That said, such studies provide unprecedented insight and opportunity to learn about the responses of multiple nodes of a neural circuit during stimulation and to refine hypotheses for explicit testing in monkey models, where targets can be selected in advance and recordings conducted chronically.
Why Monkeys to Model Human Neuropsychiatric Disorders?
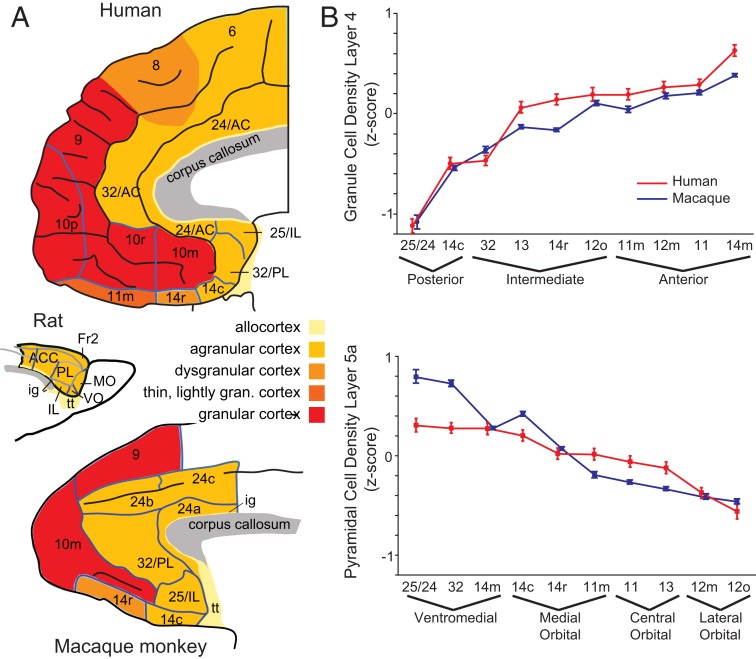
PFC is implicated in nearly every psychiatric disorder, and, as reviewed above, depression is no exception. Compared with other animals, the human PFC is enlarged and highly differentiated (Fig. 3 A, Top). This means that species with similarly enlarged and differentiated PFCs are essential to establishing its role in affective function and dysfunction. The macaque monkey is the most neuroanatomically characterized species of nonhuman primate. Based on cytoarchitecture (the distribution of neurons in each area), macaques have a highly differentiated PFC incorporating many, but not all, of the areas seen in the human PFC (Fig. 3 A, Bottom). For instance, macaque and human posterior PFC is composed of agranular cortex, which has no “granule” cells in layer IV. In contrast, anterior regions of PFC have densely packed granule cells in layer IV. A quantitative analysis of the density of neurons in human and macaque cortical layers 4 and 5a in both macaques and humans by Mackey and Petrides (49) confirms that macaque PFC shares many of the same features of human PFC with similar anterior posterior/medial-lateral gradients (Fig. 3B). The same gradients are not seen in rodents, and they do not have a true frontopolar cortex area 10, both of which limit their use as a model of human PFC (Fig. 3 A, Middle). An in-depth comparative analysis of rodent and primate PFC is provided by Preuss (50).
Fig. 3.
Comparative neuroanatomy of PFC. (A) Cytoarchitecture of the PFC in humans (Top), rats (Middle), and macaques (Bottom). Different shading represents different cortical layer structure. Brain sizes are not to scale. AC, anterior cingulate cortex; Fr2, second frontal region; gran., granular; ig, indusium griseum; IL, infralimbic; MO, medial orbital; PL, prelimbic; tt, tenia tecta; VO, ventral orbital. Reprinted with permission from ref. 85. (B) Comparative analysis of granule cell density in macaque and human ventromedial PFC. Mean density (SEM) of neurons in layers 4 (Top) and 5a (Bottom) in monkeys (blue) and humans (red) is shown in anterior, intermediate, and posterior areas. Reprinted with permission from ref. 49.
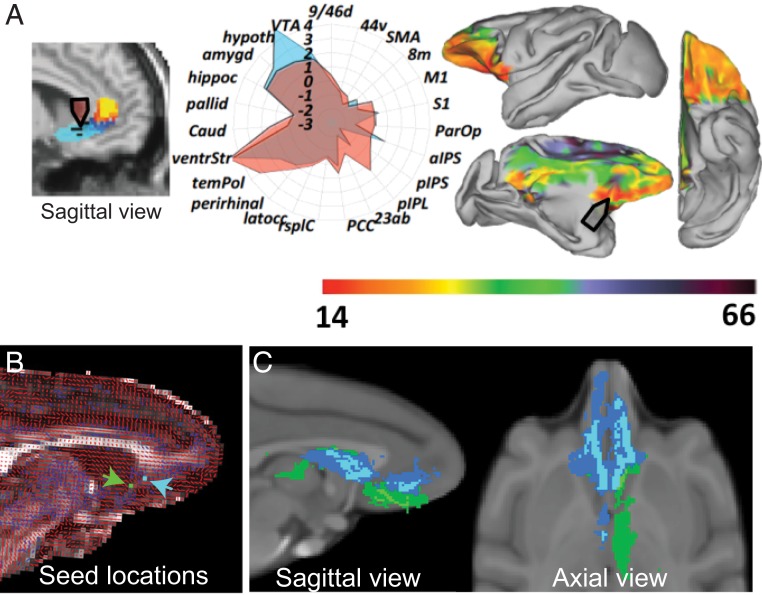
Knowing that human and macaque PFC is homologous based on cytoarchitecture is an important first step, but it is also critical that comparable regions of PFC are connected to other parts of the brain in a similar manner. This is especially important in the present context, given the observation that DBS appears to have the strongest effects on mood when targeting white matter pathways as opposed to cortex. In macaques, connections within PFC and to other parts of the limbic system have been extensively mapped using tracers injected directly into the brain (e.g., refs. 51–54), an approach that is not possible in humans. Thus, most of our assumptions about human brain connectivity originate from these animal models. Neuroimaging methods, such as resting state functional connectivity and diffusion tractography, can be applied to both humans and macaques and have been used to estimate and compare connections. Using such approaches, Rushworth and coworkers (55, 56) have shown that parts of the ventral and medial PFC show highly similar patterns of anatomical connectivity in humans and macaques. For instance, they compared the functional and diffusion-weighted imaging connectivity of subcallosal ACC in 25 macaques and 38 humans to other parts of the brain (Fig. 4A). This type of analysis allows a “connectional fingerprint” of the area to be determined in both species and compared. As can be seen in Fig. 4B, the connectional fingerprints of macaque and human subgenual ACC show a high degree of similarity, with overlapping connectivity profiles (compare blue and red shading on the polar plot). They repeated this type of analysis on other PFC regions, concluding that nearly all areas studied showed a high degree of similarity across humans and macaques (55). There were, however, 2 notable exceptions: 1) No analog of human anterior lateral PFC could be found in macaques, indicating that this portion of PFC is unique to humans, and 2) connectivity between auditory areas and PFC was markedly different between humans and macaques. Specifically, posterior auditory cortex showed preferential connectivity with parts of ventrolateral PFC in humans, such as Broca’s area, whereas these connections were more biased toward medial PFC in macaques. This distinction in auditory connectivity may be associated with the emergence of language in humans. We can only speculate on the uniquely human area in anterior lateral PFC, but it could be related to distinctive higher cognitive abilities in humans.
Fig. 4.
Comparative diffusion tractography of human and macaque subcallosal ACC for DBS. (A) Comparison of subcallosal ACC cortex in humans and macaques based on resting-state functional MRI and diffusion-weighted imaging. (Left) Human location of subcallosal ACC targeted in DBS for depression. (Center) Polar plot of estimated connections of human (red) and macaque (blue) subcallosal ACC. 23ab, area 23ab; 44v, ventral area 44; 8m, medial area 8; 9/46d, dorsal area 9/46; aIPS, aneterior intra parietal sulcus; amygd, amygdala; caud, caudate; hippoc, hippocampus; Hypoth, hypothalamus; latocc, lateral occipital cortex; M1, primary motor cortex; pallid, pallidum; ParOP, parietal operculum; PCC, posterior cingulate cortex; perirhinal, perirhinal cortex; pIPL, posterior inferior parietal lobule; pIPS, posterior intra parietal sulcus; rsplC, retrosplenial cortex; S1, primary sensory cortex; SMA, sensory motor area; temPol, temporal pole; ventrStr, ventral striatum; VTA, ventral tegmental area. (Right) Heat map superimposed on the macaque brain indicates parts of brain with the highest similarity to human subcallosal ACC. Reprinted from ref. 86, by permission of Oxford University Press. (B) Diffusion data from 1 subject. Primary (red) and secondary (blue) diffusion directions are shown. Candidate subcallosal ACC seeds (based on ref. 27) are shown (blue and green pixels). (C) Whole-brain probabilistic tractography of shared fiber tract maps of possible subcallosal ACC stimulation targets is shown on sagittal (Left) and axis (Right) views. Blue and green correspond to the seeds in B. An overlap figure for each of the seeds is shown (threshold: 4 of 6 subjects). The tracts from the blue seed location are qualitatively similar to those in the study by Riva-Posse et al. (27).
For macaques to be a model for reverse translation requires that the end points of the connections of PFC in macaques and humans are similar, but also that they obey the same organizing principles as they course from PFC to other parts of the brain. This point is especially important for establishing how DBS in humans might impact different white matter pathways in successfully treated individuals (27). Here, the work of Haber and coworkers (39, 57) has been especially illuminating. In a series of comparative studies of white matter projections using diffusion tractography and anatomical tracing, they show a high degree of similarity between humans and macaques in the organization of projection pathways near ventral PFC (57) and in the anterior limb of the internal capsule (39). In the ventral PFC, the position and organization of the uncinate fascicle and cingulum bundle, 2 of the white matter pathways implicated in the therapeutic effects of subcallosal ACC DBS, are highly conserved across the 2 species. In a separate analysis here, we confirmed and extended this work by focusing on subcallosal ACC (Fig. 4B). Taking anatomical locations of responders and nonresponders from the study by Riva-Posse et al. (27) as 2 points of origin, we estimated their anatomical connectivity in diffusion scans from 6 macaques. Mirroring what was seen in humans, these 2 locations are associated with distinct connectivity profiles, with the locations associated with responders exhibiting similar connectivity to those found in humans (compare Figs. 2 A and B and 4B). Thus, across analyses of cytoarchitecture, connections, and route of projections of PFC and limbic areas in macaques and humans, there is a high degree of similarity, making macaques a suitable neuroanatomical model for reverse translation.
By comparison to rodents, macaques are also more similar to humans in their higher cognitive abilities, exhibiting advanced planning (58), strategy use (59, 60), and categorization (61) to name just a few. Their social structures, use of facial expressions for social communication, and affective repertoire also closely mirror those of humans (62, 63). With relevance to depression in humans, macaques can also exhibit depressive-like phenotypes (an extensive review is provided in ref. 64). Depressive-like states in macaques can occur spontaneously or due to experimentally induced conditions, and these models have been extensively characterized both behaviorally (65, 66) and physiologically (67, 68). This close correspondence further emphasizes the importance of macaque models for understanding depression in humans.
Key Questions for Reverse Translation from Humans to Monkeys
The review of current DBS targets for treatment-resistant depression lays bare the variability in response rates within and across brain areas. Even in the most well-studied location, subcallosal ACC, remission is not achieved in all DBS patients and questions about mechanism persist. Monkeys present the best available animal model for reverse translation, but what questions need to be addressed to enable DBS approaches for depression to advance? Here, we propose a set of issues we believe are particularly pressing and amenable to investigation in nonhuman primates.
Do We Have the Best Stimulation Parameters for Treatment-Resistant Depression?
In nearly all clinical trials, high-frequency stimulation (100 to 130 Hz) has been chronically delivered to alleviate the symptoms of depression. How high-frequency stimulation impacts local and distributed circuits in highly differentiated brains is unclear. In vitro and in vivo studies suggest that it either blocks (3, 69) or desynchronizes neural activity (70), or potentially both (71). By contrast, the impact of DBS for Parkinson’s disease has been well characterized. Here, high-frequency stimulation of STN alleviates rigidity, tremors, and bradykinesia and is associated with reductions in the power of the beta oscillations (∼13 to 30 Hz) (72). Based on these and other observations, one hypothesis is that DBS decreases symptoms by blocking or desynchronizing activity in STN, and thus uncouples pathologically synchronized oscillators in larger motor control circuits (3, 73).
Extending this hypothesized mechanism to depression, it could be that stimulation of white matter tracts adjacent to subcallosal ACC desynchronizes multiple areas connecting through those tracts. This would fit with a preliminary report that DBS near to subcallosal ACC reduces the power of beta and gamma oscillations around the DBS electrode (29). In addition, similar effects on low-frequency oscillations locally and in other areas were reported with acute OFC stimulation (48). This account could explain the mechanisms through which DBS acutely affects the brain of depressed patients, but it is important to note that unlike Parkinson’s disease, depression does not have a pathognomonic oscillatory abnormality due to a known neurodegenerative process. Thus, deductions of abnormal functioning are best made through analyses of changes with therapeutic stimulation.
In contrast to Parkinson’s disease, successful treatments for depression likely involve resetting and rebalancing a set of brain circuits, as opposed to acutely compensating for the loss of a specific population of neurons (4). This leads to the question of whether high-frequency stimulation applied continuously is an optimal approach for depression, or whether refined regimens after an initial phase of high-frequency stimulation might be more beneficial. To this end, the next-generation DBS devices that allow ongoing monitoring and recording of local field potentials from the stimulating electrode will be critical. They will allow researchers to characterize activity changes over time, as a function of the magnitude of clinical improvement (or not). Using nonhuman primates to explore the parameter space of stimulation in limbic targets, and the short- and long-term effects on highly differentiated brains, could provide new avenues to DBS therapy with the potential to reduce side effects and/or enhance recovery (74).
What Are the Network-Level Effects of DBS for Treatment-Resistant Depression?
Reduction in depression symptoms following DBS appears in stages, with immediate change in negative mood and attention predictably evoked by initial stimulation in the operating room (4). Thereafter, more gradual changes in cognitive and vegetative symptoms occur with ongoing chronic stimulation (26), and the full clinical response generally further evolves over months (Fig. 2D). One interpretation of the slow time course is that the therapeutic effects of DBS may occur in different stages with distinct mechanisms, some rapid and some slow (6, 28–30).
If we understood the mechanisms of the plasticity behind these changes, they could potentially be harnessed in tandem with DBS to speed the onset of remission from depression. One possibility is that chronic stimulation delivered to both gray matter and white matter in and around subcallosal ACC causes rapid changes in neural firing or synaptic integrity, as well as direct stimulation-induced changes in myelination. From this view, stimulating myelination of pathologically impacted tracts, possibly forceps minor, uncinate fascicle, and cingulum bundle, could be the biological basis for symptom reduction and development of resilience over time. Such a hypothesis is supported by the changes in estimated connections near to the DBS electrodes of patients who progress from being nonresponders to responders (27). This hypothesis is also supported by the time course of metabolic and blood flow changes in regions with direct connections to and from subcallosal ACC over the course of months of DBS therapy (4). It also fits with the effect of electrical or optogenetic stimulation of white matter or cortex in rodents, which is associated with increased oligodendrocyte proliferation and myelination (75, 76). Of course, the opposite could also be true. High-frequency stimulation of axons blocks neural activity (69), meaning it is possible that the effect of DBS could be to reduce myelination and functional connectivity through activity-dependent myelination mechanisms (77). In this case, the therapeutic effects of DBS could be indirect, enabling other circuits to dominate function. Arbitrating between these 2 possibilities requires micro- and macroscopic study of the effects of DBS on white matter in the limbic system of animal models with white matter organization similar to humans.
Instead of impacting white matter, the therapeutic effects of DBS in treatment-resistant depression could be driven by neurophysiological remodeling at the level of synapses, with changes in white matter occurring later. If we understood the physiological remodeling after DBS, it might be possible to improve or speed up this process by optimizing stimulation regimens, for example, using multiple stimulation devices to improve the rate of remission. The neurophysiological impact of DBS for depression is not well established on other limbic structures as few reports are available. This is because the primary areas of the human brain impacted in depression are minimally accessible with noninvasive methods such as EEG and electrode placement in epilepsy patients is often sparse. Of the available studies, acute stimulation of lateral OFC in epilepsy patients caused network-wide change in low-frequency power in the theta and alpha bands, which is associated with improved mood (48), while stimulation of white matter near to subcallosal ACC is associated with broad power spectrum changes in subcallosal ACC in the same hemisphere (29). What is needed is a comprehensive analysis of how DBS impacts single neurons and local field potential activity in multiple brain areas simultaneously in both healthy and pathological states. Indeed, developing animal models of pathological network activity is one of the key challenges for reverse translation, as DBS applied to healthy or less severely depressed individuals has minimal effect (48). In order to determine how DBS normalizes pathological activity, such aberrant activity will first have to be induced in nonhuman primate models.
Are There Biomarkers for Subcallosal ACC Hyperactivity?
In Parkinson’s disease, aberrant oscillations within the basal ganglia and associated motor system precede the onset of rigidity and bradykinesia (78). These neural signals have been harnessed as biomarkers of a pathological network state to adaptively control stimulation of the STN using closed-loop devices (79). For depression, we lack such network-level biomarkers, but their discovery could usher in a new era of adaptive stimulation for treatment-resistant depression. This could have the benefit of reducing side effects as well as potentially improving treatment efficacy by adaptively changing stimulation delivery or frequencies. Three recent insights potentially provide such markers, although additional research is required. First, recordings within the subcallosal ACC of patients undergoing DBS electrode implantation reported a relationship between beta band power (13 to 30 Hz) and depression severity, suggesting that this could be a neurophysiological marker of dysfunction (80). Second, using a combined stimulation and recording DBS system, a study reported that specific patterns of local field potential activity were associated with mood state (81). Third, a recent study in marmosets shows that acutely inducing neural hyperactivity within subcallosal ACC is associated with activity changes in dorsal ACC, insula, and brainstem structures (82). Similar to the effects of lesions of subcallosal ACC (83), inducing hyperactivity in subcallosal ACC is associated with an anhedonic-like behavioral phenotype. How chronically induced hyper- or hypoactivity of subcallosal ACC changes activity in interconnected brain regions is still to be determined, but doing so could reveal biomarkers that could be used to adaptively control DBS stimulation.
Summary
DBS for depression and other psychiatric disorders is a promising therapeutic approach for patients who do not respond to first-line behavioral therapies and pharmacotherapies. As we have detailed, these emerging approaches have yet to reach their full potential. To do so will require fluid cross-talk between clinical and basic researchers working with nonhuman primates. Here, we have highlighted some of the most pressing questions regarding the therapeutic mechanisms of DBS. There are doubtless many more. By addressing these, it may be possible to realize the full potential of DBS for psychiatric disorders, bringing comprehensive treatments to individuals who were previously untreatable.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01MH110822, to P.H.R.; UH3NS103550, to H.S.M.), National Institute of Drug Abuse (K08DA039351, to E.L.R.), and Hope for Depression Research Foundation. Human studies performed at Emory were carried out in accordance with the Emory University Institutional Review Board with written informed consent from all subjects under Food and Drug Administration ID nos. G060028 and G130107, and Clinicaltrials.gov ID nos. NCT00367003 and NCT01984710.
Footnotes
Conflict of interest statement: H.S.M. is a paid consultant of and licensor of intellectual property to Abbott Labs.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Using Monkey Models to Understand and Develop Treatments for Human Brain Disorders,” held January 7–8, 2019, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. NAS colloquia began in 1991 and have been published in PNAS since 1995. From February 2001 through May 2019 colloquia were supported by a generous gift from The Dame Jillian and Dr. Arthur M. Sackler Foundation for the Arts, Sciences, & Humanities, in memory of Dame Sackler’s husband, Arthur M. Sackler. The complete program and video recordings of most presentations are available on the NAS website at http://www.nasonline.org/using-monkey-models.
This article is a PNAS Direct Submission.
References
- 1.Mayberg H. S., Limbic-cortical dysregulation: A proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9, 471–481 (1997). [DOI] [PubMed] [Google Scholar]
- 2.McIntyre C. C., Grill W. M., Sherman D. L., Thakor N. V., Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. J. Neurophysiol. 91, 1457–1469 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Beurrier C., Bioulac B., Audin J., Hammond C., High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J. Neurophysiol. 85, 1351–1356 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Mayberg H. S., et al. , Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Holtzheimer P. E., et al. , Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 4, 839–849 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Riva-Posse P., et al. , A connectomic approach for subcallosal cingulate deep brain stimulation surgery: Prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drobisz D., Damborská A., Deep brain stimulation targets for treating depression. Behav. Brain Res. 359, 266–273 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Holtzheimer P. E., Mayberg H. S., Deep brain stimulation for psychiatric disorders. Annu. Rev. Neurosci. 34, 289–307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman W. K., Alterman R. L., Deep brain stimulation for intractable psychiatric disorders. Annu. Rev. Med. 63, 511–524 (2012). [DOI] [PubMed] [Google Scholar]
- 10.George M. S., et al. , A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry 58, 364–373 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Bergfeld I. O., et al. , Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 73, 456–464 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Dougherty D. D., et al. , A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78, 240–248 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Coenen V. A., et al. , Superolateral medial forebrain bundle deep brain stimulation in major depression: A gateway trial. Neuropsychopharmacology 44, 1224–1232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association , Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Washington, DC, ed. 5, 2013). [Google Scholar]
- 15.Fava M., Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53, 649–659 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Sackeim H. A., The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry 62 (suppl. 16), 10–17 (2001). [PubMed] [Google Scholar]
- 17.Mayberg H. S., et al. , Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Harrison N. A., et al. , Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drevets W. C., et al. , Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Mayberg H. S., et al. , Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8, 1057–1061 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Mayberg H. S., et al. , Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol. Psychiatry 48, 830–843 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Dougherty D. D., et al. , Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J. Neurosurg. 99, 1010–1017 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Siegle G. J., et al. , Toward clinically useful neuroimaging in depression treatment: Prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch. Gen. Psychiatry 69, 913–924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozano A. M., et al. , A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J. Neurosurg. 116, 315–322 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Lozano A. M., et al. , Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 64, 461–467 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Kennedy S. H., et al. , Deep brain stimulation for treatment-resistant depression: Follow-up after 3 to 6 years. Am. J. Psychiatry 168, 502–510 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Riva-Posse P., et al. , Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 76, 963–969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters A. C., et al. , Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum. Brain Mapp. 39, 4844–4856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smart O., et al. , Initial unilateral exposure to deep brain stimulation in treatment-resistant depression patients alters spectral power in the subcallosal cingulate. Front. Comput. Neurosci. 12, 43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi K. S., Riva-Posse P., Gross R. E., Mayberg H. S., Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72, 1252–1260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haber S. N., Behrens T. E., The neural network underlying incentive-based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron 83, 1019–1039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein J., et al. , Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry 163, 1784–1790 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Pepper J., Hariz M., Zrinzo L., Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: A review of the literature. J. Neurosurg. 122, 1028–1037 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Denys D., et al. , Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry 67, 1061–1068 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Strong D. R., et al. , Reversible increase in smoking after withdrawal of ventral capsule/ventral striatum deep brain stimulation in a depressed smoker. J. Addict. Med. 6, 94–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malone D. A., Jr et al. , Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 65, 267–275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bewernick B. H., Kayser S., Sturm V., Schlaepfer T. E., Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology 37, 1975–1985 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millet B., et al. , Limbic versus cognitive target for deep brain stimulation in treatment-resistant depression: Accumbens more promising than caudate. Eur. Neuropsychopharmacol. 24, 1229–1239 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Safadi Z., et al. , Functional segmentation of the anterior limb of the internal capsule: Linking white matter abnormalities to specific connections. J. Neurosci. 38, 2106–2117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebrand L. C., et al. , Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimul. 12, 353–360 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Baldermann J. C., et al. , Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol. Psychiatry 85, 735–743 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Bechara A., Damasio H., Damasio A. R., Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Hornak J., et al. , Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126, 1691–1712 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Rudebeck P. H., Murray E. A., The orbitofrontal oracle: Cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84, 1143–1156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drevets W. C., et al. , A functional anatomical study of unipolar depression. J. Neurosci. 12, 3628–3641 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brody A. L., et al. , Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 50, 171–178 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Nauczyciel C., et al. , Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: A double-blind, crossover study. Transl. Psychiatry 4, e436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao V. R., et al. , Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr. Biol. 28, 3893–3902.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Mackey S., Petrides M., Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur. J. Neurosci. 32, 1940–1950 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Preuss T. M., Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J. Cogn. Neurosci. 7, 1–24 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Carmichael S. T., Price J. L., Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Barbas H., Blatt G. J., Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5, 511–533 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Vogt B. A., Pandya D. N., Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 262, 271–289 (1987). [DOI] [PubMed] [Google Scholar]
- 54.Haber S. N., Kunishio K., Mizobuchi M., Lynd-Balta E., The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 15, 4851–4867 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neubert F. X., Mars R. B., Sallet J., Rushworth M. F., Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. U.S.A. 112, E2695–E2704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neubert F. X., Mars R. B., Thomas A. G., Sallet J., Rushworth M. F., Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron 81, 700–713 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Jbabdi S., Lehman J. F., Haber S. N., Behrens T. E., Human and monkey ventral prefrontal fibers use the same organizational principles to reach their targets: Tracing versus tractography. J. Neurosci. 33, 3190–3201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernádi I., Grabenhorst F., Schultz W., Planning activity for internally generated reward goals in monkey amygdala neurons. Nat. Neurosci. 18, 461–469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genovesio A., Brasted P. J., Mitz A. R., Wise S. P., Prefrontal cortex activity related to abstract response strategies. Neuron 47, 307–320 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiang F.-K., Wallis J. D., Spatiotemporal encoding of search strategies by prefrontal neurons. Proc. Natl. Acad. Sci. U.S.A. 115, 5010–5015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller E. K., Freedman D. J., Wallis J. D., The prefrontal cortex: Categories, concepts and cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1123–1136 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grueter C. C., Chapais B., Zinner D., Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 33, 1002–1037 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parr L. A., The evolution of face processing in primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1764–1777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worlein J. M., Nonhuman primate models of depression: Effects of early experience and stress. ILAR J. 55, 259–273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shively C. A., et al. , Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol. Psychol. 69, 67–84 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Hennessy M. B., McCowan B., Jiang J., Capitanio J. P., Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front. Behav. Neurosci. 8, 309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willard S. L., Riddle D. R., Forbes M. E., Shively C. A., Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol. Psychiatry 74, 890–897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jarczok M. N., Koenig J., Shively C. A., Thayer J. F., Behavioral depression is associated with increased vagally mediated heart rate variability in adult female cynomolgus monkeys (Macaca fascicularis). Int. J. Psychophysiol. 131, 139–143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen A. L., Durand D. M., High frequency stimulation can block axonal conduction. Exp. Neurol. 220, 57–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson C. J., Beverlin B. 2nd, Netoff T., Chaotic desynchronization as the therapeutic mechanism of deep brain stimulation. Front. Syst. Neurosci. 5, 50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson D., Moehlis J., Clustered desynchronization from high-frequency deep brain stimulation. PLOS Comput. Biol. 11, e1004673 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kühn A. A., et al. , High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 28, 6165–6173 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cassidy M., et al. , Movement-related changes in synchronization in the human basal ganglia. Brain 125, 1235–1246 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Smart O. L., Tiruvadi V. R., Mayberg H. S., Multimodal approaches to define network oscillations in depression. Biol. Psychiatry 77, 1061–1070 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q., Brus-Ramer M., Martin J. H., McDonald J. W., Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 479, 128–133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibson E. M., et al. , Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demerens C., et al. , Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. U.S.A. 93, 9887–9892 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hammond C., Bergman H., Brown P., Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 30, 357–364 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Little S., et al. , Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark D. L., Brown E. C., Ramasubbu R., Kiss Z. H. T., Intrinsic local beta oscillations in the subgenual cingulate relate to depressive symptoms in treatment-resistant depression. Biol. Psychiatry 80, e93–e94 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Veerakumar A., et al. , Field potential 1/f activity in the subcallosal cingulate region as a candidate signal for monitoring deep brain stimulation for treatment resistant depression. J. Neurophysiol. 10.1152/jn.00875 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alexander L., et al. , Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron 101, 307–320.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudebeck P. H., et al. , A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc. Natl. Acad. Sci. U.S.A. 111, 5391–5396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holtzheimer P. E., et al. , Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ongür D., Ferry A. T., Price J. L., Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 460, 425–449 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Johansen-Berg H., et al. , Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex 18, 1374–1383 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]