Significance
Plants adapt their growth and development to ambient light conditions by modulating the activity of the COP1/SPA ubiquitin ligase. In Arabidopsis, photoactivated cryptochromes interact directly with COP1/SPA to suppress the activity of COP1 in blue light. The molecular mechanisms of CRY-mediated inhibition of COP1 are not fully understood. Here we show that CRY2 inhibits COP1 by displacing degradation substrates from COP1. This mechanism requires a valine-proline (VP) motif present in the C-terminal domain of CRY2. These amino acids are also present in interactors of Arabidopsis and human COP1 and have been previously shown to directly bind the WD repeat of Arabidopsis and human COP1. Thus, the evolution of the plant-specific, VP-containing C-terminal domain of CRY2 might have allowed for light control of COP1 activity.
Keywords: cryptochromes, COP1/SPA complex, WD40 repeat, VP motif, competition
Abstract
In plants, the cryptochrome photoreceptors suppress the activity of the COP1/SPA ubiquitin ligase to initiate photomorphogenesis in blue light. Both CRY1 and CRY2 interact with the COP1/SPA complex in a blue light-dependent manner. The mechanisms underlying the inhibition of COP1 activity through direct interactions with photoactivated CRYs are not fully understood. Here we tested the hypothesis that CRY2 inhibits COP1 by displacing the degradation substrates from COP1. To this end, we analyzed the role of a conserved valine-proline (VP) motif in the C-terminal domain of CRY2 (CCT2), which resembles the core COP1-WD40–binding sequences present in the substrates of COP1. We show that the VP motif in CRY2 is essential for the interaction of CRY2 with COP1 in yeast two-hybrid assays and in planta. Mutations in the VP motif of CRY2 abolished the CRY2 activity in photomorphogenesis, indicating the importance of VP. The interaction between COP1 and its VP-containing substrate PAP2 was prevented in the presence of coexpressed CRY2, but not in the presence of CRY2 carrying a VP mutation. Thus, since both PAP2 and CRY2 engage VP motifs to bind to COP1, these results demonstrate that CRY2 outcompetes PAP2 for binding to COP1. We further found that the previously unknown interaction between SPA1-WD and CCT2 occurs via the VP motif in CRY2, suggesting structural similarities in the VP-binding pockets of COP1-WD40 and SPA1-WD40 domains. A VP motif present in CRY1 is also essential for binding to COP1. Thus, CRY1 and CRY2 might share this mechanism of COP1 inactivation.
Light is an essential environmental cue that modulates growth and development of plants throughout their life cycles. Plants have evolved specialized photoreceptors that sense and respond to the changes in ambient light conditions. Photoreceptor-mediated light perception and signal transduction are keys to the major developmental transitions in plants, from seed germination to flowering (1–3). In dark-grown seedlings of Arabidopsis thaliana, the CONSTITUTIVE PHOTOMORPHOGENIC1/SUPPRESSOR OF PHYA-105 (COP1/SPA) complex acts as a master repressor of light signaling by ubiquitinating photomorphogenesis-promoting transcription factors, which subsequently are degraded via the 26S proteasome (4, 5). Both COP1 and SPA proteins (SPA1, SPA2, SPA3, and SPA4) contain central coiled-coil domains essential for homodimerization and heterodimerization as well as C-terminal WD40 repeat domains for substrate recognition, interaction with photoreceptors, and association with the higher-order E3 ligase subunits of the CULLIN4-DAMAGED DNA-BINDING 1 (CUL4-DDB1) complex. COP1 and SPA proteins differ in their N-termini; while COP1 has a RING-finger domain, SPA proteins contain kinase-like domains (4, 5). The COP1/SPA complex targets numerous transcription factors that regulate gene expression in response to light signals, including ELONGATED HYPOCOTYL5 (HY5) (6), its homolog HYH (7), LONG HYPOCOTYL IN FAR-RED1 (HFR1) (8, 9), SALT TOLERANCE (STO), its homolog STH (10), SHI-RELATED SEQUENCE5 (11), CONSTANS (CO) and other BBX family proteins (12–18) and PRODUCTION OF ANTHOCYANIN PIGMENT (PAP) proteins (19). On light exposure, the photoreceptors cryptochromes and phytochromes directly interact with the COP1/SPA complex to suppress the E3 ubiquitin ligase activity of COP1/SPA, thereby stabilizing the downstream transcription factors (2). This leads to a cascade of signaling events culminating in photomorphogenesis. Photoreceptors achieve this through several mechanisms, including nuclear exclusion of COP1 (20–22), degradation of SPA proteins (23, 24), and disruption of the COP1–SPA interaction (25–28).
The blue light receptor cryptochrome (CRY1 and CRY2) interact with the COP1/SPA complex in a blue light-specific manner (25, 28–30). Both CRY1 and CRY2 are structurally similar, containing a highly conserved N-terminal domain, the photolyase homology region (PHR), which evolved from DNA photolyases and a C-terminal domain known as cryptochrome carboxyl terminus (CCT) (31). PHR is the chromophore-binding region essential for blue light perception and the homodimerization of cryptochromes (32, 33). CCT is thought to be an intrinsically unstructured effector domain that relays the blue light signals perceived by PHR (34–37). Both CRY1 and CRY2 interact with the WD40 domain of COP1 via their CCT domains, CCT1 and CCT2 (38, 39). However, they differ in binding to SPA1; while CCT1 interacts with SPA1-WD (25, 28), CRY2 and SPA1 associate via their N-terminal domains (30). SPA proteins are required for CRY1–COP1 interactions in planta, but CRY2 associates with COP1 independently of SPA proteins (29). In addition, the presence of SPA1 enhances the CRY1–COP1 (29) as well as the CRY2–COP1 association in yeast cells (30). It has been shown that light-activated CRY1, but not CRY2, disrupts the COP1–SPA1 interaction (25, 28, 30). How an enhanced CRY2–COP1 association suppresses the activity of the COP1/SPA complex has not yet been further explored. Along with regulating COP1/SPA activity, CRY2 interacts with CIB transcription factors to regulate flowering time (40).
Many substrate proteins targeted by the COP1-SPA E3 ubiquitin ligase possess valine-proline–containing (VP) motifs (10, 41) which are essential for interacting with the WD40 domain of Arabidopsis and human COP1 (10, 42). The VP motif consists of an array of residues: VP(E/D)ɸG, in which ɸ represents a hydrophobic amino acid (10, 42). It has been shown that either deleting the VP-containing regions or mutating the VP to alanine residues (VPAA) in HY5 and STH abolishes the interaction with COP1 (10). The presence of a VP motif is not restricted to COP1 substrates but is also found in the UV-B photoreceptor UVR8, where it mediates the UV-B–induced interaction with COP1 (43). In addition, CRYs carry VP motifs in their CCT domains (44, 45), suggesting that this might be a general feature of COP1-WD–interacting proteins.
Here we analyzed the role of VP motifs in cryptochromes by performing yeast and in planta interaction assays to show that cryptochromes associate with the WD of COP1 and SPA1 via their VP motifs. In addition, based on colocalization and Förster resonant energy transfer-fluorescence lifetime imaging microscopy (FRET-FLIM) assays, we present compelling evidence for a VP-mediated competition between cryptochromes and COP1 substrates as a mechanism that suppresses the activity of the COP1/SPA complex in light-grown Arabidopsis.
Results
The VP Motif in CRY2 Is Important for the CRY2–COP1 Interaction.
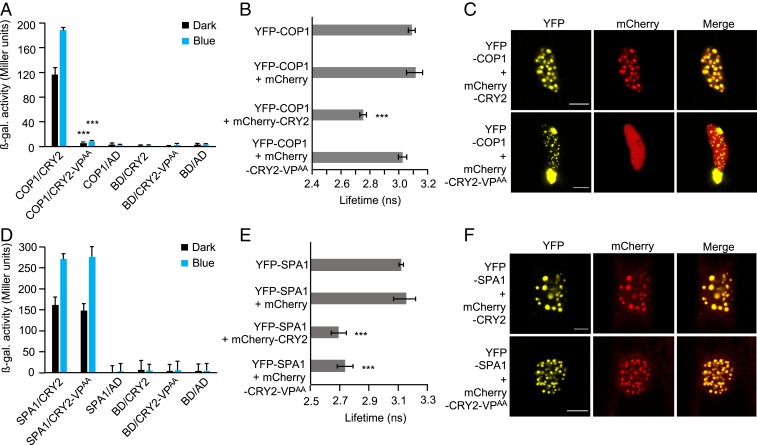
To investigate the role of the VP motif in CRY2, we mutated V531 and P532 to alanine residues (CRY2-VPAA) by site-directed mutagenesis and examined the interaction with COP1 in yeast two-hybrid (Y2H) assays (Fig. 1A). Full-length CRY2 interacted with COP1 both in darkness and on exposure to blue light, while showing an enhanced interaction in blue light (Fig. 1A). CRY2-VPAA was unable to interact with COP1 in darkness or in blue light (Fig. 1A), indicating that the VP motif is necessary for the CRY2–COP1 interaction.
Fig. 1.
Mutating the VP motif in CRY2 abolishes the interaction with COP1 but not with SPA1. (A and D) Y2H assays with COP1 (A) or SPA1 (D) as bait and CRY2 or CRY2-VPAA as prey. β-galactosidase activity was measured in cotransformed yeast cells after they were grown for 27 h in darkness (dark) or 24 h in darkness, followed by exposure to blue light (50 µmol m-2 s-1) (blue) for 3 h. Error bars indicate SEM. Asterisks indicate significant differences between CRY2 and CRY2-VPAA as prey, in darkness or blue light, respectively (***P < 0.001). AD, GAL4 activation domain; BD, GAL4-DNA–binding domain. (B and E) Lifetime of the donor fluorophore YFP-COP1 (B) or YFP-SPA1 (E) measured by FRET-FLIM inside the nuclear bodies of leek cells after particle bombardment. Twenty cells were evaluated. Error bars represent SEM. Asterisks indicate significant differences in lifetime compared with YFP-COP1 or YFP-SPA1 alone, respectively (***P < 0.001). (C and F) Representative confocal images of leek cells coexpressing YFP- and mCherry-tagged proteins. The indicated channels were subsequently merged to show colocalization. (Scale bars: 10 µm.) Confocal images for the combinations YFP-COP1 + mCherry and YFP-SPA1 + mCherry are shown in SI Appendix, Fig. S1.
To verify these results in vivo, we performed FRET-FLIM experiments after coexpressing YFP- and mCherry-tagged proteins in plant cells by particle bombardment (Fig. 1 B and C). The lifetime of the donor fluorophore (YFP) was significantly reduced when YFP-COP1 was coexpressed with mCherry-CRY2 compared with the expression of YFP-COP1 alone (Fig. 1B), confirming the COP1–CRY2 interaction in planta (29). No significant changes in the lifetime of YFP was observed when YFP-COP1 was coexpressed with mCherry-CRY2-VPAA compared with the lifetime of YFP-COP1 alone, indicating that the CRY2 protein with a mutated VP fails to interact with COP1. The lifetime of YFP also was not significantly changed when YFP-COP1 and mCherry were coexpressed. It is known that YFP-COP1 forms nuclear bodies (NBs) (20, 22, 46) in which the COP1-interacting proteins are colocalized (13). As is evident from the confocal microscopy images of cobombarded leek cells, YFP-COP1 was able to recruit mCherry-CRY2 into NBs (Fig. 1C and SI Appendix, Fig. S1). Strikingly, CRY2-VPAA failed to colocalize with COP1 and thus exhibited a diffused localization pattern throughout the nucleus. Taken together, these results demonstrate that the VP motif in CRY2 is necessary for the CRY2–COP1 interaction.
CRY2 with a Mutated VP Motif Retains an Interaction with SPA1.
The C-terminal part of both COP1 and SPA1 contains a WD40-repeat domain; therefore, the VP motif in CRY2 also might be important for the CRY2–SPA1 interaction. To test this, Y2H assays were performed using SPA1 as the bait and CRY2 or CRY2-VPAA as prey (Fig. 1D). We found that both CRY2 and CRY2-VPAA interacted with SPA1 at similar strengths in cotransformed yeast cells. This result indicates that the VP motif in CRY2 is not necessary for the CRY2–SPA1 interaction. We further tested these interactions using FRET-FLIM in cobombarded leek cells (Fig. 1 E and F). A significant decrease in the lifetime of YFP was observed when YFP-SPA1 was coexpressed either with mCherry-CRY2 or with mCherry-CRY2-VPAA, indicating that SPA1 interacts with CRY2 as well as with CRY2-VPAA in leek cells (Fig. 1E). Like YFP-COP1, YFP-SPA1 localizes in the nucleus and forms characteristic NBs, in which SPA1-interacting proteins are recruited (SI Appendix, Fig. S1) (17, 19, 26). Confocal microscopy images showed that YFP-SPA1 recruited both CRY2 and CRY2-VPAA into colocalizing NBs (Fig. 1F). Taken together, these results indicate that the VP motif in CRY2 is not necessary for the CRY2–SPA1 interaction and the SPA1-mediated recruitment of CRY2 into NBs.
The C-Terminal Domain of CRY2 Interacts with SPA1-WD, but Not with Full-Length SPA1.
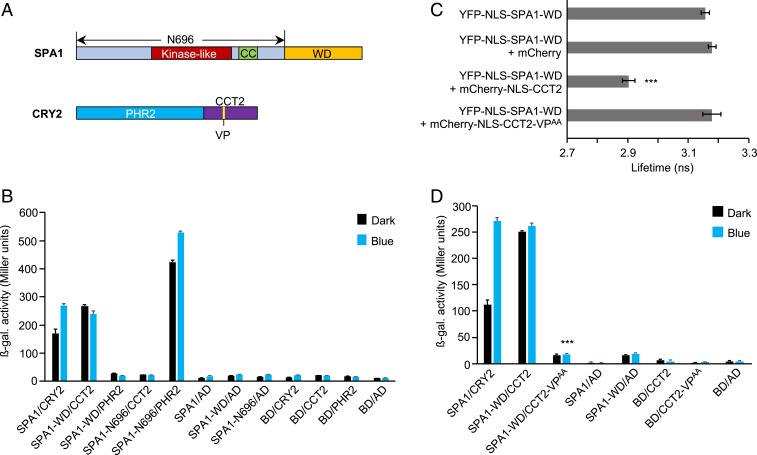
A previous study showed that the CRY2–SPA1 interaction in blue light is mediated by the respective N-terminal domains of CRY2 and SPA1 (30). Since the VP motif in CRY2 is situated in CCT2, there are 2 possible reasons why we did not observe a VP-mediated interaction between CRY2 and SPA1: either CCT2 is not involved in the SPA1–CRY2 interaction or the interaction between SPA1-WD and CCT2 via VP is prevented by the N terminus of SPA1, CRY2, or both. To investigate these possibilities, we first performed domain-specific interaction analyses between CRY2 and SPA1 via Y2H assays (Fig. 2B). In agreement with the previous study (30), SPA1-N696, which consists of the N-terminal kinase-like and coiled-coil domains (Fig. 2A), interacted very strongly with PHR2, whereas SPA1-WD failed to bind PHR2 (Fig. 2B). However, we found that SPA1-WD interacted with CCT2, which was not investigated previously (Fig. 2B).
Fig. 2.
The C-terminal domain of CRY2 interacts with SPA1-WD via its VP motif. (A) Domain structure of SPA1 and CRY2. The N-terminal 696 residues constitute SPA1-N696. SPA1-WD contains the C-terminal residues from 697 to 1,029. CRY2-PHR2 comprises the N-terminal residues from 1 to 489. C-terminal residues from 490 to 612 constitute CRY2-CCT2 with the VP motif (amino acids 531 to 532). (B and D) Y2H assays with SPA1 or N- and C-terminal deletions of SPA1 as bait and CRY2 and N- and C-terminal deletions of CRY2 or VP to AA mutation in CRY2 as prey. β-galactosidase activity was measured in cotransformed yeast cells after they were grown for 27 h in darkness (dark) or 24 h in darkness followed by exposure to blue light (50 µmol m-2 s-1) (blue) for 3 h. Error bars indicate SEM. In D, *** indicates a significant difference between the interaction strength of CCT2-VPAA when used as prey compared with CCT2 under blue light (P < 0.001). (C) Lifetime of donor (YFP-NLS-SPA1-WD) fluorophore measured by FRET-FLIM inside the nuclei of leek cells after particle bombardment. Twenty cells were measured. Error bars represent SEM. Asterisks indicate a significant difference in lifetime compared with YFP-NLS-SPA1-WD (***P < 0.001).
To examine this interaction in planta, we performed FRET-FLIM assays in cobombarded leek cells expressing YFP- and mCherry-tagged proteins (Fig. 2C and SI Appendix, Fig. S2). Since the predicted nuclear localization signals (NLSs) in SPA1 are located in the N-terminal domains (47), we fused an artificial NLS to SPA1-WD (YFP-NLS-SPA1-WD). An artificial NLS was also placed at the N terminus of CCT2 (mCherry-NLS-CCT2). The lifetime of YFP was significantly reduced in leek cells that coexpressed YFP-NLS-SPA1-WD and mCherry-NLS-CCT2 compared with those that expressed YFP-NLS-SPA1-WD alone (Fig. 2C), confirming the SPA1-WD-CCT2 interaction in planta.
To reinvestigate whether full-length SPA1 interacts with CCT2, we performed Y2H assays with SPA1 as bait and either CRY2 or CCT2 as prey (SI Appendix, Fig. S3). CCT2, unlike full-length CRY2, failed to interact with full-length SPA1, indicating that the N terminus of SPA1 prevents the interaction between SPA1-WD and CCT2, as noted above (Fig. 2B). In contrast, the COP1–CRY2 interaction was mediated only by the WD repeat domain of COP1. Ring+CC did not interact with CRY2 either in darkness or under blue light (SI Appendix, Fig. S4). To further confirm that the VP motif in CCT2 is indeed the only important motif for the interaction with COP1, we performed Y2H assays with COP1 as bait and either CRY2 or CCT2-VPAA as prey. CCT2-VPAA failed to interact with COP1, confirming that the CCT2–COP1 association occurs only through the VP motif (SI Appendix, Fig. S5).
The C-Terminal Domain of CRY2 Interacts with SPA1-WD via Its VP Motif.
Since the C-terminal domains of both COP1 and SPA1 contain 7 WD40 repeats with high sequence similarity (SI Appendix, Fig. S6), SPA1-WD might fold into a similar structure as COP1-WD. Thus, a similar VP-binding pocket may be formed in SPA1-WD to enable an interaction with CCT2. Indeed, CCT2-VPAA failed to interact with SPA1-WD in yeast cells (Fig. 2D), suggesting that CCT2 binds SPA1-WD via its VP motif. We further investigated this interaction through FRET-FLIM experiments. The lifetime of YFP in leek cells coexpressing YFP-NLS-SPA1-WD and mCherry-NLS-CCT2-VPAA was not significantly changed compared with those cells expressing YFP-NLS-SPA1-WD alone, indicating that the VP motif of CCT2 is essential for the SPA1–CCT2 association (Fig. 2C) in planta. Unlike full-length SPA1, which makes NBs when expressed alone, NLS-SPA1-WD and mCherry-NLS-CCT2 did not form any NBs and were seen diffused throughout the nucleus (SI Appendix, Figs. S1 and S2). Taken together, our results indicate that CRY2 associates with the WD40 repeat domains of COP1 and SPA1 using its VP peptide motif.
The VP Motif in CRY2 Is Essential for CRY2-Mediated De-Etiolation, Anthocyanin Accumulation, and HY5 Stabilization in Blue Light.
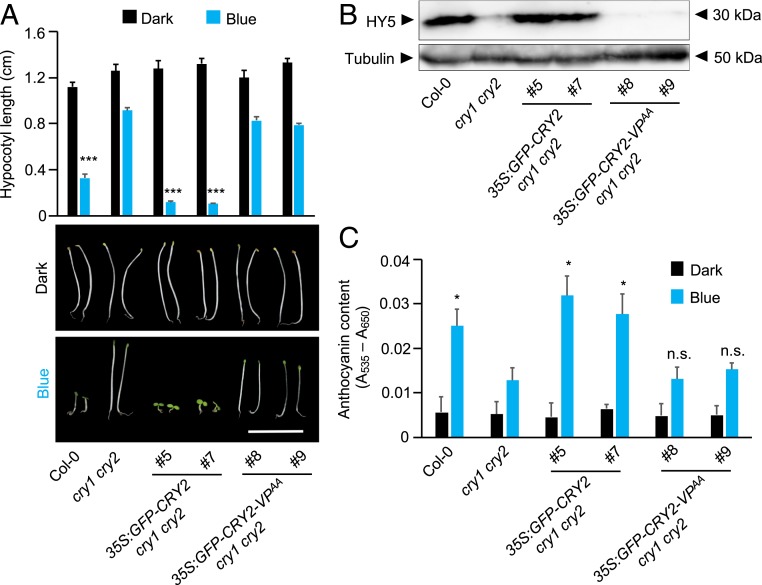
To investigate whether the VP motif of CRY2 is important for CRY2 function, we generated transgenic lines expressing either full-length CRY2 (35S:GFP-CRY2) or CRY2-VPAA (35S:GFP-CRY2-VPAA) in a cry1 cry2 double-mutant background (Fig. 3). The expression of similar levels of the GFP-tagged CRY variants in these lines was verified by immunoblot analysis using an anti-GFP antibody (SI Appendix, Fig. S7). Expression of GFP-CRY2 fully complemented the elongated hypocotyl phenotype of cry1 cry2 in blue light. In contrast, GFP-CRY2-VPAA–expressing seedlings had the same appearance as the cry1 cry2 mutant progenitor (Fig. 3A), showing that these lines exhibited no complementation of the cry1 cry2 mutant phenotype. Similarly, GFP-CRY2, but not GFP-CRY2-VPAA, caused an accumulation of HY5 protein in blue light-grown cry1 cry2 mutant seedlings (Fig. 3B).
Fig. 3.
The VP motif in CRY2 is essential for seedling de-etiolation, anthocyanin biosynthesis, and HY5 accumulation in blue light. (A) Seedling phenotype of transgenic cry1 cry2 double mutants expressing GFP-CRY2 or GFP-CRY2-VPAA when grown in darkness (dark) or in blue light (blue) (2.5 µmol m−2 s−1) compared with WT (Col-0) and cry1 cry2. Hypocotyl length measurements are at the top (n = 20). Error bars indicate SEM. Asterisks indicate significant differences between cry1 cry2 and the respective genotype in blue light (***P < 0.001). (Scale bar: 1 cm.) (B) HY5 protein abundance in the transgenic lines expressing GFP-CRY2 or GFP-CRY2-VPAA in a cry1 cry2 double-mutant background, grown under blue light (2.5 µmol m−2 s−1), compared with Col-0 (WT) and cry1 cry2. HY5 protein was detected by an ⍺-HY5 antibody. Tubulin levels, detected by ⍺-TUB, are shown as loading controls. (C) Anthocyanin levels in the same genotypes as in B grown under blue light (blue) (2.5 µmol m−2 s−1) or in darkness (dark). Twenty seedlings were evaluated. Error bars represent SEM. The asterisk indicates significant differences in anthocyanin levels between cry1 cry2 and the respective genotype grown under blue light (*P < 0.05). n.s., not significant.
We further examined the anthocyanin levels in these transgenic lines. GFP-CRY2, but not GFP-CRY2-VPAA, restored blue light-induced anthocyanin accumulation in cry1 cry2 mutants (Fig. 3C). Taken together, these results indicate that the VP motif in CRY2 is essential for CRY2-mediated de-etiolation, HY5 stabilization, and anthocyanin biosynthesis in blue light.
The VP1 (VP548–549) Motif in CRY1 Is Essential for the CRY1–COP1 Interaction.
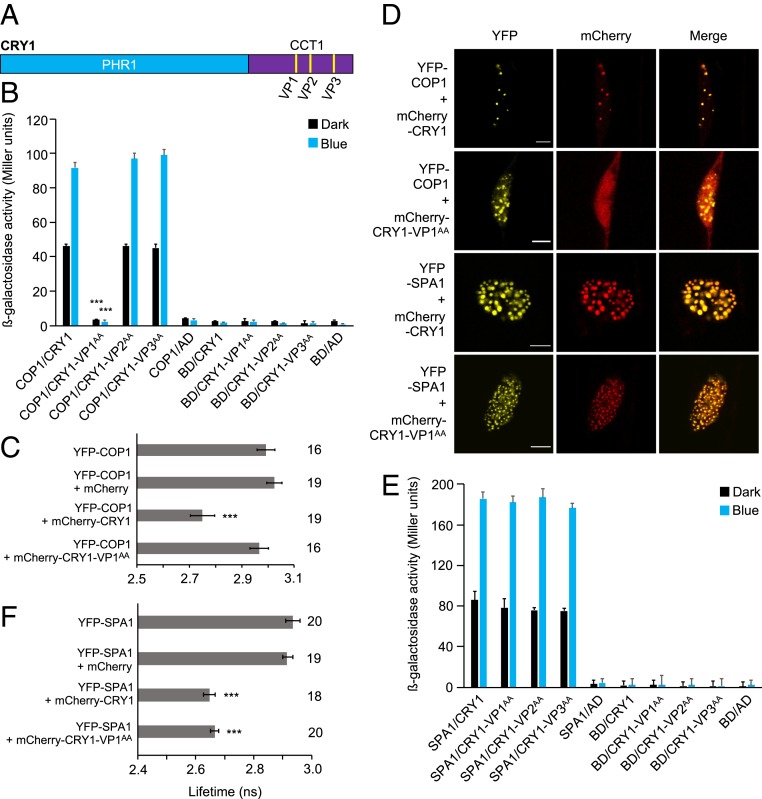
CRY1 and CRY2 share the same domain structure, but their C-terminal domains (CCT1 and CCT2) differ in size and sequence (3). CCT2 contains only one VP motif (VP531–532), while CCT1 has 3 instances of neighboring valine-proline residues (designated VP1 [VP548–549], VP2 [VP579–580], and VP3 [VP637–638]) (Fig. 4A), with VP1 showing the greatest sequence similarity with the VP motif in CRY2 (SI Appendix, Fig. S8A).
Fig. 4.
The VP1 (548 to 549) motif in CRY1 is essential for the interaction with COP1 but not with SPA1. (A) Domain structure of CRY1 depicting the 3 VP motifs, designated VP1 (548 to 549), VP2 (579 to 580), and VP3 (637 to 638). (B and E) Y2H assays with COP1 (B) or SPA1 (E) as bait and CRY1 or CRY1 with mutated VP motifs as prey. β-galactosidase activity was measured in cotransformed yeast cells after they were grown for 48 h in darkness (dark) or for 24 h in darkness followed by exposure to blue light (50 µmol m-2 s-1) (blue) for 24 h. Error bars indicate SEM. In B, asterisks indicate a significant difference between the interaction strength of CRY1-VP1AA and that of CRY1 when used as prey under blue light (***P < 0.001). (C and F) Lifetime of donor fluorophore YFP-COP1 (C) or YFP-SPA1 (F) measured by FRET-FLIM inside the nuclear bodies of leek cells after particle bombardment. The number adjacent to each bar represents the number of cells measured. Error bars represent SEM. Asterisks in C and F indicate a significant difference in lifetime compared with YFP-COP1 and YFP-SPA1, respectively (***P < 0.001). (D) Representative confocal images of leek cells coexpressing YFP- and mCherry-tagged proteins. The indicated channels were subsequently merged to show colocalization. (Scale bars: 10 µm.) A representative image for YFP-COP1 + mCherry is shown in SI Appendix, Fig. S1.
We mutated the individual VP motifs in CRY1 to alanine residues (CRY1-VP1AA, CRY1-VP2AA, and CRY1-VP3AA) and tested the interaction of these mutated CRY1 proteins with COP1 using Y2H assays. While full-length CRY1 interacted with COP1 in a blue light-enhanced fashion, CRY1-VP1AA failed to interact with COP1 in darkness and in blue light (Fig. 4B). The interaction between CRY1 and COP1 was not impaired when VP2 or VP3 was mutated, suggesting that only VP1 in CRY1 is essential for the CRY1–COP1 interaction (Fig. 4B). Mutating VP1 to AA in CCT1 was also sufficient to abolish the interaction of CCT1 with COP1 (SI Appendix, Fig. S9).
We then performed FRET-FLIM experiments to examine the role of VP1 in the CRY1–COP1 interaction in planta (Fig. 4 C and D). A significant decrease in the lifetime of YFP was observed when YFP-COP1 was coexpressed with mCherry-CRY1 compared with YFP-COP1 expressed alone, thus confirming a COP1–CRY1 interaction (Fig. 4C). In contrast, the lifetime of the donor fluorophore (YFP) did not change significantly in leek cells coexpressing YFP-COP1 and mCherry-CRY1-VP1AA compared with cells expressing YFP-COP1 alone, demonstrating that CRY1-VP1AA failed to interact with COP1 in planta. Similar to what was observed for CRY2 (Fig. 1C), COP1 recruited CRY1 into colocalizing NBs (Fig. 4D). In contrast, COP1 was not able to recruit CRY1-VP1AA into NBs (Fig. 4D). Taken together, these results indicate that the VP548–549 motif in CRY1 (VP1) is essential for the CRY1–COP1 interaction and also for the recruitment of CRY1 into colocalizing NBs by COP1.
Mutations of Individual VP Motifs in CRY1 do Not Abolish the CRY1–SPA1 Interaction.
Mutating the individual VP motifs in CRY1 did not impair the interaction with SPA1 in the cotransformed yeast cells in darkness or on exposure to blue light (Fig. 4E), suggesting that no individual VP motif in CRY1 per se is essential for the interaction with SPA1. In addition, in FRET-FLIM analyses, the lifetime of YFP was significantly reduced when YFP-SPA1 was coexpressed with mCherry-CRY1 or with mCherry-CRY1-VP1AA compared with YFP-SPA1 expressed alone or coexpressed with mCherry (Fig. 4F). mCherry-CRY1-VP1AA was also recruited into YFP-SPA1 NBs (Fig. 4D). Thus, both full-length CRY1 and CRY1-VP1AA interact with SPA1 in vivo. However, when we used SPA1-WD rather than full-length SPA1 as bait, mutating VP1 in CCT1 significantly reduced, but did not abolish, the SPA1-WD–CCT1 interaction (SI Appendix, Fig. S10). This result suggests that VP1 plays a significant role in the interaction between CCT1 and SPA1-WD.
CRY2 Abolishes the COP1–PAP2 Interaction in Blue Light.
It is evident that both cryptochromes and COP1 substrates interact with COP1 via their VP peptide motifs (Figs. 1 and 4). Thus, photoexcited cryptochromes might compete with substrates for binding to COP1-WD or SPA1-WD, resulting in displacement of the substrate proteins from the COP1/SPA complex.
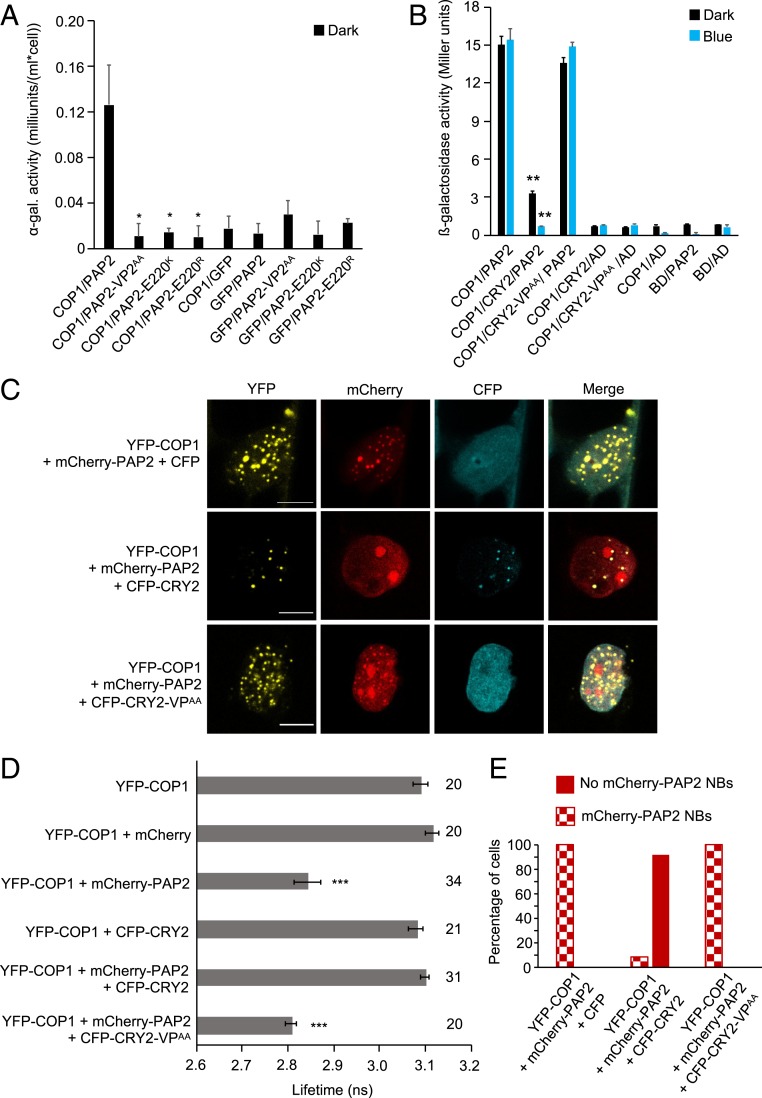
To test this hypothesis, we used an R2R3 MYB transcription factor, PRODUCTION OF ANTHOCYANIN PIGMENT2 (PAP2), which regulates anthocyanin biosynthesis in response to light (48). PAP2 is targeted by the COP1/SPA complex and is subsequently degraded in darkness (19). We first tested the COP1–PAP2 interaction in planta via FRET-FLIM experiments. The lifetime of YFP was significantly reduced in leek cells coexpressing YFP-COP1 and mCherry-PAP2 compared with cells expressing YFP-COP1 alone, confirming the COP1–PAP2 interaction (Fig. 5D) reported previously (19). PAP2 was also recruited into colocalizing NBs by COP1 (SI Appendix, Fig. S11). It was shown earlier that COP1-WD is involved in the COP1–PAP2 interaction (19). Of the 2 VP motifs (VP1 [VP36–37] and VP2 [VP218–219]) in PAP2, the VP2 core with the adjacent amino acids is more similar in sequence to the VP motifs present in other COP1 targets (SI Appendix, Fig. S8 B and C). Indeed, PAP2-VP2AA, PAP2-E220K, and PAP2-E220R (carrying a mutation in the glutamic acid next to VP) failed to interact with COP1 in yeast, suggesting that PAP2 uses its VP2 motif (including the adjacent E220) to bind to COP1 (Fig. 5A).
Fig. 5.
CRY2 competes with PAP2 for interaction with COP1. (A) Y2H assays with COP1 as bait and PAP2, PAP2-VP2AA, PAP2-E220K, or PAP2-E220R as prey. GFP served as a negative control. ⍺-galactosidase activity was measured in dark-grown yeast cells coexpressing bait and prey proteins. The asterisk indicates significant difference in COP1-interaction strength of PAP2VP2AA, PAP2-E220K, or PAP2-E220R when used as prey proteins compared to when full-length PAP2 was used (*P < 0.05). (B) Y3H assay with COP1 as bait and PAP2 as prey in the presence or absence of CRY2 or CRY2-VPAA. β-galactosidase activity was measured in cotransformed yeast cells after they were grown for 4 d in darkness (dark) or for 4 d in darkness followed by exposure to blue light (50 µmol m-2 s-1) (blue) for 4 h. Error bars indicate SEM. Asterisks indicate a significant difference in the interaction between COP1 and PAP2 in the presence of CRY2 compared with the absence of CRY2, under blue light (**P < 0.01). (C) Representative confocal images of leek cells coexpressing the indicated proteins. The indicated channels were subsequently merged to show colocalization. (Scale bars: 10 µm.) (D) Lifetime of donor (YFP-COP1) fluorophore measured by FRET-FLIM inside the nuclear bodies of leek cells after particle bombardment. The number adjacent to each bar represents the number of cells measured. Error bars represent SEM. Asterisks indicate a significant difference in lifetime compared with YFP-COP1 (***P < 0.001). The lifetime of YFP-COP1 was measured without exciting the CFP-tagged proteins. (E) Percentage of cells in which mCherry-PAP2 NBs were formed when YFP-COP1 and mCherry-PAP2 were coexpressed with CFP, CFP-CRY2, or CFP-CRY2-VPAA. Sixty cells were evaluated for each combination.
To investigate the possibility of competition between CRY2 and PAP2 to occupy the COP1-WD40 domain, we performed yeast three-hybrid (Y3H) assays in which the interaction strength between COP1 and PAP2 was compared in the presence or absence of CRY2 (Fig. 5B). Expressing CRY2 as a bridge protein strongly reduced the interaction between COP1 and PAP2, with a stronger effect in blue light than in darkness (Fig. 5B). Indeed, in blue light, no interaction between COP1 and PAP2 was observed in the presence of coexpressed CRY2. This suggests that CRY2 can disrupt the COP1–PAP2 interaction in a blue light-enhanced manner in yeast. Strikingly, the interaction between COP1 and PAP2 was not affected by the coexpression of CRY2-VPAA (Fig. 5B). This result is consistent with the idea that CRY2 uses its VP motif to interact with COP1, thereby disrupting the association between COP1 and PAP2.
To test the competition hypothesis in planta, a FRET-FLIM–based colocalization assay was designed in which leek cells were cobombarded with 3 separate constructs to express COP1, PAP2, and CRY2 or CRY2-VPAA fused to 3 different fluorescent tags: YFP, mCherry, and CFP, respectively. The purpose of attaching a CFP tag to CRY2 and CRY2-VPAA was to confirm the successful expression of the respective CFP-tagged proteins in each cell considered via visualization of the CFP signal under the microscope. We observed that YFP-COP1 was able to recruit mCherry-PAP2 into colocalizing NBs in cobombarded leek cells (Fig. 5C and SI Appendix, Fig. S11). Coexpressing CFP along with YFP-COP1 and mCherry-PAP2 did not affect the colocalization of mCherry-PAP2 with YFP-COP1 (Fig. 5C). In contrast, coexpressing CFP-CRY2 disrupted the formation of colocalizing COP1-PAP2 NBs in a large majority of the cobombarded leek cells (Fig. 5 C and E). YFP-COP1 recruited CFP-CRY2 into NBs in these cells. In contrast, coexpression of CFP-CRY2-VPAA did not alter the colocalization of mCherry-PAP2 and YFP-COP1(Fig. 5C). This demonstrates that CFP-CRY2-VPAA failed to affect the recruitment of mCherry-PAP2 into NBs by YFP-COP1. Taken together, these colocalization assays suggest that the VP motif of CRY2 competes with PAP2 for binding to COP1 and thus prevents the COP1-mediated recruitment of PAP2 into NBs.
We then subjected the cobombarded leek cells to FRET-FLIM (Fig. 5D). To exclude the possibility that the coexpressed CFP-CRY2 affects the lifetime of the excited YFP-COP1, we measured the lifetime of YFP-COP1 in the presence and absence of CFP-CRY2. No significant change in the lifetime of YFP was observed when expressed alone or coexpressed with CFP-CRY2 (Fig. 5D), indicating that the CFP-tagged proteins can be effectively used to ensure the expression of CRY2 or CRY2-VPAA in further experiments without affecting the lifetime of YFP, the donor fluorophore. The lifetime of YFP was not significantly changed in cells that coexpressed YFP-COP1, mCherry-PAP2, and CFP-CRY2 and did not show mCherry-PAP2 NBs (Fig. 5D). These results indicate that the presence of CFP-CRY2 affected the interaction between YFP-COP1 and mCherry-PAP2. Strikingly, the lifetime of YFP was significantly reduced in the cells coexpressing CFP-CRY2-VPAA together with YFP-COP1 and mCherry-PAP2, indicating that the VPAA mutation in CRY2 abolished the effect of CRY2 on the COP1–PAP2 interaction. Taken together, our results suggest that CRY2 via its VP motif competes with PAP2 and thus prevents the COP1–PAP2 interaction.
The ability of CRY2 to displace PAP2 from COP1 correlated with the abundance of CFP-CRY2. In leek cells coexpressing CFP-CRY2, YFP-COP1, and mCherry-PAP2, mCherry-PAP2 colocalized with YFP-COP1 NBs in cells in which CFP-CRY2 was highly expressed (SI Appendix, Fig. S12A). To examine whether exposure to blue light influences the competitive ability of CFP-CRY2, we coexpressed the fusion proteins in hypocotyls of etiolated white mustard (Sinapis alba) seedlings by particle bombardment. S. alba is more closely related to Arabidopsis than leek and is widely used as a model for plant photobiology (49–51). When seedlings were incubated in darkness following bombardment, the majority of the transfected cells displayed mCherry-PAP2 colocalization with YFP-COP1 NBs, indicating that CRY2 was not very efficient in displacing PAP2 from COP1 in darkness (SI Appendix, Fig. S12 C and D). In contrast, in blue light-incubated S. alba seedlings, most transfected cells showed a diffuse localization of mCherry-PAP2 that did not colocalize with YFP-COP1 NBs. This result demonstrates that the blue light activation of CRY2 enhances the ability of CRY2 to compete with PAP2, in agreement with the blue light-induced interaction between CRY2 and the COP1/SPA complex in planta (29).
Discussion
On light absorption, photoreceptors suppress the activity of the COP1/SPA E3 ubiquitin ligase, which marks the onset of photomorphogenesis in plants. Different photoreceptors have evolved distinct mechanisms that operate interdependently in various tissues and at various developmental stages to orchestrate plant responses toward changing ambient light conditions. In blue light, cryptochromes likely undergo intramolecular conformational changes that allow their CCT domains to interact with the WD repeat domain of COP1 (38, 39). Here we investigated the function of VP motifs in CCTs, which resemble the WD-binding VP motifs present in COP1 substrates (10, 41). VP motifs are a characteristic feature of COP1-interacting proteins in animal and plant systems (42).
As revealed by Y2H and FRET-FLIM assays, both CRY1 and CRY2 required VP motifs in their respective CCT domains to bind to COP1. Mutating the relevant VP to alanine residues in cryptochromes was sufficient to disrupt their interaction with COP1. These results are consistent with a recent cocrystallization study showing that peptides within CRY1-CCT and CRY2-CCT domains involve their respective VP motifs in binding to the WD repeat of COP1 (52). The VP motif in CRY2 is located within the NC80 domain that was previously found to be sufficient to induce photomorphogenesis (36). In line with this, transgenic plants expressing CRY2-VPAA did not complement the mutant phenotype of cry1 cry2 in blue light, suggesting that CRY2-VPAA failed to suppress the activity of the COP1/SPA complex in these plants. In agreement with this interpretation, lines expressing CRY2-VPAA failed to accumulate the COP1/SPA target HY5 in blue light. A P-to-L missense mutation within the CRY1 VP1 motif is found in a cry1 loss-of-function EMS-induced allele (53). Although this mutation did not abolish the interaction with COP1 in a Y2H (38), it still might weaken it in planta.
Our analysis shows that CRYs, as well as COP1 substrates, engage VP motifs for binding to the WD repeat domain of COP1. This implies that CRYs and substrates might compete for binding to COP1, as has been hypothesized in a previous theoretical analysis (45). Indeed, here we showed that CRY2 effectively outcompetes the transcription factor PAP2 for binding to COP1, thereby displacing PAP2 from COP1. Thus, the CCT domain of CRY2 appears to have a greater affinity than PAP2 for the VP-binding domain of COP1. The previously reported SPA1-mediated enhancement of the CRY2–COP1 interaction in blue light (30) might contribute to the highly competitive activity of CRY2 in blue light. In line with our hypothesis, a similar observation was made by Lau et al. (52) using cocrystallization of COP1-WD in complex with VP-containing peptides of photoreceptors and COP1 substrates. Besides CRY1 and CRY2, the UV-B–activated photoreceptor UVR8 binds COP1-WD via a VP motif, thereby competing with COP1 substrates. This suggests that diverse photoreceptors utilize the VP-mediated competition mechanism to prevent COP1–substrate associations.
While one molecular mechanism underlying immediate, CRY1-mediated COP1/SPA inactivation has been resolved, how CRY2 inactivates the COP1/SPA complex has remained enigmatic. It has been shown that photoactivated CRY1 leads to a rapid dissociation of the COP1–SPA interaction (25, 28). In contrast, photoactivated CRY2 does not disturb the COP1–SPA1 interaction, suggesting that an alternative mechanism exists that allows CRY2-mediated suppression of COP1/SPA activity. Thus, the competitive displacement of transcription factors from COP1 is likely the major immediate mechanism by which the photoreceptor CRY2 inactivates the COP1/SPA E3 ligase. Since CRY1 also interacts with COP1-WD and SPA1-WD via a VP motif, it is possible that in addition to dissociating COP1-SPA1, CRY1 competes with COP1 substrates and prevents their interaction with COP1-WD and SPA1-WD. How these mechanisms cooperate with the light-induced nuclear exclusion of COP1 (20–22) remains to be determined.
The WD repeat domain of COP1 has been reported to interact with the CCT domains of CRY1 and CRY2 (38, 39), a result that we have confirmed here. Similarly, SPA1-WD interacts with CCT1 of CRY1 (25, 28). In contrast, the interaction between SPA1 and CRY2 occurs via the respective N-terminal domains (30), that is, the kinase-like domain in SPA1 and the PHR domain in CRY2. Here our interaction domain-mapping revealed an additional, previously unknown interaction between SPA1-WD and CCT2. This interaction was absent in full-length SPA1, suggesting that the N-terminal domain of SPA1 inhibits the interaction between SPA1-WD and CRY2. Mutating the VP motif in CCT2 abolished this interaction, indicating that SPA1-WD might use a similar VP-binding pocket as COP1-WD. Consistent with this finding, the amino acids involved in the interaction between COP1-WD and the VP motif in TRIB1 (42) are conserved in SPA1-WD (SI Appendix, Fig. S6). Thus, SPA1-WD might fold into a VP-binding conformation similar to that of COP1-WD.
In line with this observation, a previous study reported that SPA1 carrying mutations in the conserved amino acids K767 (to E), W812 (to A), and G869 (to E) (which correspond to K422 in WD-2, W467 in WD-3, and G524 in WD-4 of COP1, respectively; SI Appendix, Fig. S6) failed to rescue the spa1-3 mutant phenotype in transgenic plants (54). On the other hand, the VPAA mutation in CCT1 abolished the interaction with COP1, while it only weakened the interaction with SPA1-WD, suggesting that there might be differences in the SPA1-WD and COP1-WD interactions with CRY1.
Structural information on SPA1 and the COP1/SPA complex in association with CRYs is needed to unequivocally resolve similarities and differences in the respective interactions with CRYs and to determine whether the SPA1 N terminus acts as structural barrier to prevent SPA1-WD and CCT2 association.
Methods
All Arabidopsis lines used in this study were in the Col-0 accession. The cry1 cry2 mutant has been described previously (55). The generation of transgenic plants expressing GFP-CRY2 andGFP-CRY2-VPAA is described in SI Appendix. LED light sources for the blue light experiments have been described previously (56). Transgenic plants expressing GFP-CRY2, GFP-CRY2-VPAA, cry1 cry2, and Col-0 were grown in blue light at a fluence rate of 2.5 μmol m−2 s−1 for 6 d and then analyzed for hypocotyl elongation as described previously (56). For detection of GFP-tagged proteins, protein extraction using Lämmli buffer and immunoblot analysis were performed according to previously published protocols (19). Proteins were detected by α-GFP antibody (Roche Diagnostics) and α-tubulin (Sigma-Aldrich).
Plasmid construction, Y2H, Y3H, confocal microscopy, particle bombardment, colocalization, and FRET-FLIM analyses are described in detail in SI Appendix. The plasmids used for this study are listed in SI Appendix, Table S1. Oligonucleotides used for cloning are listed in SI Appendix, Table S2. The materials used in this study are available from the corresponding author on request.
Supplementary Material
Acknowledgments
We thank Christian Jüngst (Cluster of Excellence in Cellular Stress Responses in Aging-Associated Diseases Imaging Facility) for assisting with confocal microscopy and FRET-FLIM analyses, Christina Philipp (Max Planck Institute for Plant Breeding Research) for the yeast strain EGY48 (p8op-LacZ), Xing Wang Deng (Peking University) for the ⍺-HY5 antibody, Stefan Kircher (University of Freiburg) for the S. alba transfection protocol, and Emanuel Bruckisch, Lisa Morguet, Shalima Ganesan, Bastian Welter, and Christian Schenkel for laboratory assistance. We also thank Martin Hülskamp for hosting A.S. and the greenhouse staff for the expert care of our plants. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (HO 2793-3, to U.H.) and Germany’s Excellence Strategy–EXC 2048/1 (Project 390686111, to U.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909181116/-/DCSupplemental.
References
- 1.Kami C., Lorrain S., Hornitschek P., Fankhauser C., Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91, 29–66 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Podolec R., Ulm R., Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 45, 18–25 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Yang Z., et al. , Cryptochromes orchestrate transcription regulation of diverse blue light responses in plants. Photochem. Photobiol. 93, 112–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon C., Sheerin D. J., Hiltbrunner A., SPA proteins: SPAnning the gap between visible light and gene expression. Planta 244, 297–312 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Hoecker U., The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 37, 63–69 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Osterlund M. T., Hardtke C. S., Wei N., Deng X. W., Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Holm M., Ma L. G., Qu L. J., Deng X. W., Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 16, 1247–1259 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., et al. , Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17, 804–821 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang I. C., Yang J. Y., Seo H. S., Chua N. H., HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19, 593–602 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm M., Hardtke C. S., Gaudet R., Deng X.-W., Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 20, 118–127 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan T. T., Xu H. H., Zhang Q., Zhang L. Y., Lu Y. T., The COP1 target SHI-RELATED SEQUENCE5 directly activates photomorphogenesis-promoting genes. Plant Cell 30, 2368–2382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laubinger S., et al. , Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133, 3213–3222 (2006). Erratum in: Development. 133, 4608 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Liu L. J., et al. , COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20, 292–306 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna R., et al. , The Arabidopsis B-box zinc finger family. Plant Cell 21, 3416–3420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D., et al. , BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 113, 7655–7660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F., et al. , B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell 30, 2006–2019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordoñez-Herrera N., et al. , The transcription factor COL12 is a substrate of the COP1/SPA E3 ligase and regulates flowering time and plant architecture. Plant Physiol. 176, 1327–1340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishak K. P., et al. , The B-box bridge between light and hormones in plants. J. Photochem. Photobiol. B 191, 164–174 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Maier A., et al. , Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 74, 638–651 (2013). [DOI] [PubMed] [Google Scholar]
- 20.von Arnim A. G., Deng X.-W., Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79, 1035–1045 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Pacín M., Legris M., Casal J. J., Rapid decline in nuclear costitutive photomorphogenesis1 abundance anticipates the stabilization of its target elongated hypocotyl5 in the light. Plant Physiol. 164, 1134–1138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcerowicz M., Kerner K., Schenkel C., Hoecker U., SPA proteins affect the subcellular localization of COP1 in the COP1/SPA ubiquitin ligase complex during photomorphogenesis. Plant Physiol. 174, 1314–1321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balcerowicz M., et al. , Light exposure of Arabidopsis seedlings causes rapid de-stabilization as well as selective post-translational inactivation of the repressor of photomorphogenesis SPA2. Plant J. 65, 712–723 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Chen S., Lory N., Stauber J., Hoecker U., Photoreceptor specificity in the light-induced and COP1-mediated rapid degradation of the repressor of photomorphogenesis SPA2 in Arabidopsis. PLoS Genet. 11, e1005516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B., Zuo Z., Liu H., Liu X., Lin C., Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 25, 1029–1034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X. D., et al. , Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol. Plant 8, 467–478 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Sheerin D. J., et al. , Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27, 189–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lian H.-L., et al. , Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 25, 1023–1028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtkotte X., Ponnu J., Ahmad M., Hoecker U., The blue light-induced interaction of cryptochrome 1 with COP1 requires SPA proteins during Arabidopsis light signaling. PLoS Genet. 13, e1007044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo Z., Liu H., Liu B., Liu X., Lin C., Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 21, 841–847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B., et al. , Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J. Plant Res. 129, 137–148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad M., Photocycle and signaling mechanisms of plant cryptochromes. Curr. Opin. Plant Biol. 33, 108–115 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Sang Y., et al. , N-terminal domain-mediated homodimerization is required for photoreceptor activity of Arabidopsis CRYPTOCHROME 1. Plant Cell 17, 1569–1584 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalitin D., Yu X., Maymon M., Mockler T., Lin C., Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15, 2421–2429 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shalitin D., et al. , Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417, 763–767 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Yu X., et al. , Derepression of the NC80 motif is critical for the photoactivation of Arabidopsis CRY2. Proc. Natl. Acad. Sci. U.S.A. 104, 7289–7294 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H.-Q., et al. , The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103, 815–827 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Yang H.-Q., Tang R.-H., Cashmore A. R., The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13, 2573–2587 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H., Ma L.-G., Li J.-M., Zhao H.-Y., Deng X. W., Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Liu H., et al. , Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1539 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Datta S., Hettiarachchi G. H. C. M., Deng X.-W., Holm M., Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18, 70–84 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uljon S., et al. , Structural basis for substrate selectivity of the E3 ligase COP1. Structure 24, 687–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin R., Arongaus A. B., Binkert M., Ulm R., Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell 27, 202–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin C., Shalitin D., Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 54, 469–496 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Müller P., Bouly J.-P., Searching for the mechanism of signalling by plant photoreceptor cryptochrome. FEBS Lett. 589, 189–192 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Stacey M. G., Hicks S. N., von Arnim A. G., Discrete domains mediate the light-responsive nuclear and cytoplasmic localization of Arabidopsis COP1. Plant Cell 11, 349–364 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoecker U., Tepperman J. M., Quail P. H., SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 284, 496–499 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez A., Zhao M., Leavitt J. M., Lloyd A. M., Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 53, 814–827 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Mohr H., Lectures on photomorphogenesis. For. Sci. 19, 262 (1973). [Google Scholar]
- 50.Wenng A., Ehmann B., Schäfer E., The 23-kDa polypeptide of the photosynthetic oxygen-evolving complex from mustard seedlings (Sinapis alba L.). Nucleotide sequence of cDNA and evidence for phytochrome control of its mRNA abundance. FEBS Lett. 246, 140–144 (1989). [DOI] [PubMed] [Google Scholar]
- 51.Stolpe T., et al. , In planta analysis of protein-protein interactions related to light signaling by bimolecular fluorescence complementation. Protoplasma 226, 137–146 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Lau K., Podolec R., Chappuis R., Ulm R., Hothorn M., Plant photoreceptors and their signaling components compete for COP1 binding via VP peptide motifs. EMBO J. 38, e102140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad M., Lin C., Cashmore A. R., Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8, 653–658 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Yang J., Wang H., The central coiled-coil domain and carboxyl-terminal WD-repeat domain of Arabidopsis SPA1 are responsible for mediating repression of light signaling. Plant J. 47, 564–576 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Guo H., Duong H., Ma N., Lin C., The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light-dependent post-transcriptional mechanism. Plant J. 19, 279–287 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Laubinger S., Fittinghoff K., Hoecker U., The SPA quartet: A family of WD-repeat proteins with a central role in suppression of photomorphogenesis in arabidopsis. Plant Cell 16, 2293–2306 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.