Abstract
Acute gastrointestinal (GI) immune-related adverse events (irAE) are commonly reported by patients with cancer undergoing treatment with immune checkpoint inhibitors (CPI); however chronic irAEs are rare. We present a case of a 71-year-old woman with metastatic gastro-oesophageal junction (GOJ) adenocarcinoma who developed delayed-onset chronic intestinal pseudo-obstruction (CIPO) while receiving second-line pembrolizumab. Repeated CT scans of the abdomen/pelvis found no small bowel obstruction, and evaluations for bowel inflammation, infection and paraneoplastic syndrome were negative. Bowel rest and glucocorticoids were associated with transient symptom resolution; however, symptoms recurred within 1 month. The patient was ultimately supported with total parenteral nutrition and intestinal motility agents. After 4 months, the GOJ cancer remained stable with no signs of progression. As CPI use expands, the incidence of rare irAEs, such as CIPO, may increase.
Keywords: gastric cancer, immunology, stomach and duodenum, unwanted effects / adverse reactions
Background
Gastric cancer, together with oesophageal cancer, is the third most commonly diagnosed cancer worldwide,1 and the incidence of gastro-oesophageal junction (GOJ) adenocarcinoma has risen significantly in many economically developed countries in recent decades.2 In the USA, the incidence of GOJ adenocarcinoma has doubled from the 1970s through the early 2010s.3 Cytotoxic chemotherapies remain important in the treatment for metastatic gastric and GOJ cancers; however, there is a growing role for immunoncology therapies in the second line.
Recent advances in immunotherapy, specifically immune checkpoint inhibitors (CPI), have led to paradigmatic shifts in advanced and metastatic gastric/GOJ cancer treatment.4 5 These targeted therapies offer new opportunities for extending survival. Despite this, CPIs have also been associated with immune-related adverse events (irAE), with the luminal GI system notably affected.6 Diarrhoea and colitis are the most commonly reported GI irAEs, and the likelihood of occurrence varies depending on the particular pathway targeted by the CPI agent.6 We present here a rare irAE of pembrolizumab treatment resulting in chronic intestinal pseudo-obstruction (CIPO) that, to our knowledge, has not been documented before in GOJ adenocarcinoma.
Case presentation
A 71-year-old woman with GOJ adenocarcinoma metastatic to the supraclavicular lymph node receiving second-line systemic therapy with pembrolizumab presented to the emergency department with severe abdominal pain, decreased oral intake, and nausea and vomiting. Her prior medical history included hypertension, hyperlipidaemia, well-controlled asthma, macular degeneration, depression and a 35 pack-year smoking history (having quit nearly 20 years prior). Her family history was notable for a sister having died with large cell lung carcinoma, with no known family history of inflammatory bowel disease, autoimmune disease or other malignancies.
The patient was initially diagnosed with metastatic GOJ adenocarcinoma in 2017. She presented with dysphagia for solid food associated with an unintentional 4.5 kg weight loss. She was referred for oesophagogastroduodenoscopy (OGD), which identified a circumferential partially obstructing fungating and ulcerating mass in the lower third of the oesophagus and extending to the GOJ. Endoscopic ultrasound (EUS) showed a T3 lesion invading through the muscularis propria with three pathologically enlarged perigastric lymph nodes, N2. Fine-needle biopsy of one perigastric lymph node was positive for malignancy. Staging by positron emission tomography CT identified enlarged fluorodeoxyglucose (FDG)-avid supraclavicular and celiac lymph nodes. Fine-needle aspirate of the the left supraclavicular lymph node was performed under ultrasound guidance and also showed adenocarcinoma, providing a final tumor stage of T3N2M1. Molecular testing showed the tumour to be mismatch repair proficient, human epidermal growth factor receptor 2 (HER2) not overexpressed, and programmed cell death-ligand 1 (PD-L1)-positive (figure 1). She was treated in the first line with folinic acid–fluorouracil–oxaliplatin (FOLFOX) (mFOLFOX6) plus nivolumab as a participant on a clinical trial.
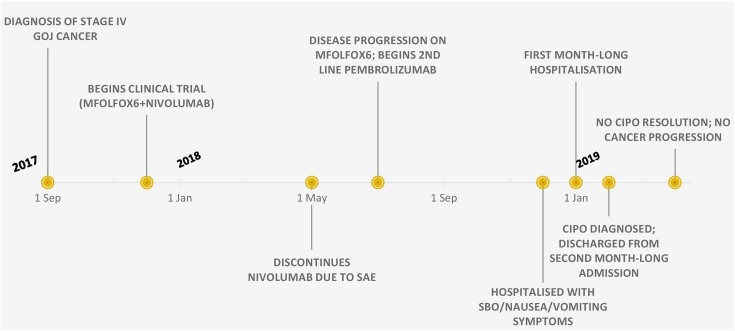
Figure 1.
Timeline of events. CIPO, chronic intestinal pseudo-obstruction; GOJ, gastro-oesophageal junction; SAE, serious adverse event; SBO, small bowel ileus.
Following cycle 9 of treatment, she developed a grade t3 rash consistent with nivolumab-induced dermatitis. The rash improved after prednisone administration but recurred with subsequent cycles, and nivolumab was ultimately discontinued after cycle 10. Following an additional three cycles of mFOLFOX6 alone, disease progression was identified by surveillance CT in the lung and hilar lymph nodes, and mFOLFOX6 was also discontinued. As her disease had not progressed on nivolumab and rash abated, the treating oncologist transitioned her to standard of care second-line pembrolizumab. She did not develop a rash. However, after completing cycle eight of pembrolizumab, the patient reported postprandial nausea and vomiting. She was referred to the emergency department and hospitalised with a diagnosis of small bowel obstruction (SBO). No anatomical abnormality to account for the SBO was found by CT or endoscopic evaluation. She was determined to have an ileus. Pembrolizumab was held at the time of hospitalisation. Initial conservative management led to symptomatic improvement, but oral rechallenge with food caused a recurrence of the suspected SBO. Surgical intervention was deemed not appropriate. While inpatient, she developed healthcare-acquired pneumonia and subsequent sepsis attributed to chronic aspiration of stomach contents. She was hospitalised for 1 month and ultimately discharged to home once tolerating an oral diet. She was rehospitalised 2 weeks later with similar symptoms and ultimately discharged on total parenteral nutrition (TPN). She has now remained out of the hospital for over 3 months. Surveillance CT has shown stable disease, and her performance status has improved.
Investigations
CT of the abdomen/pelvis showed a diffusely dilated and fluid-filled small bowel to the ileocecal valve, but with no focal transition point (figure 2). There was no free air, bowel wall thickening or pneumatosis. A kidney–ureter–bladder X-ray performed 6 hours postgastrografin administration to assess the structures of the GI tract showed oral contrast in the small bowel loops, though no contrast was seen in the colon. Multiple dilated small and large bowel loops to the level of the descending colon were found, which was consistent with prior examinations (figure 2). The patient underwent OGD and colonoscopy, which showed oedema and tight angulation of the sigmoid colon and grade B oesophagitis in the distal oesophagus. Biopsies of the fundic and antral mucosa showed chronic active gastritis; duodenal mucosa was within normal limits but showed decreased motility. Out of suspicion that this may represent a paraneoplastic ileus, an antibody panel was performed and was negative (table 1).
Figure 2.
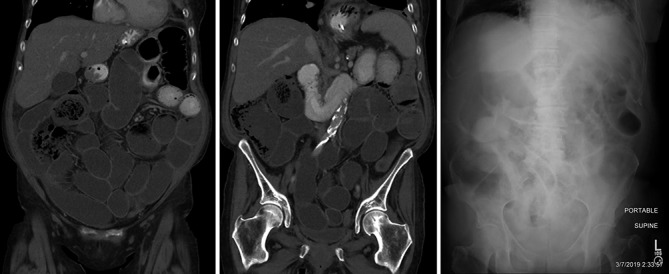
(A,B) Coronal reformations of an abdominopelvic CT show that the small bowel is fluid filled from duodenum through the ileocecal valve with many loops mildly dilated. Oral contrast that had begun being administered 1 hour prior to imaging had not progressed beyond the proximal jejunum. (C) Supine abdominal radiograph 1 month subsequent shows similarly mildly dilated loops of small bowel with air and fluid with stool having passed from the colon, which is now distended with gas in portions.
Table 1.
Paraneoplastic panel evaluation
| Result name | Result | Unit | Reference value |
| ANNA-1, S | Negative | Titre | <1:240 |
| Reflex added | None. | ||
| ANNA-2, S | Negative | Titre | <1:240 |
| ANNA-3, S | Negative | Titre | <1:240 |
| AGNA-1, S | Negative | Titre | <1:240 |
| PCA-1, S | Negative | Titre | <1:240 |
| PCA-2, S | Negative | Titre | <1:240 |
| PCA-Tr, S | Negative | Titre | <1:240 |
| Amphiphysin Ab, S | Negative | Titre | <1:240 |
| CRMP-5-IgG, S | Negative | Titre | <1:240 |
| Striational (striated muscle) Ab, S | Negative | Titre | <1:120 |
| P/Q-type calcium channel Ab | 0.00 | nmol/L | ≤0.02 |
| N-type calcium channel Ab | 0.00 | nmol/L | ≤0.03 |
| ACh receptor (muscle) binding Ab | 0.00 | nmol/L | ≤0.02 |
| AChR ganglionic neuronal Ab, S | 0.00 | nmol/L | ≤0.02 |
| Neuronal (V-G) K+Channel Ab, S | 0.00 | nmol/L | ≤0.02 |
ACh, acetylcholine; AChR, acetylcholine receptor; AGNA-1, anti-glial nuclear antibody type 1; ANNA, anti-neuronal nuclear antibody; CRMP-5, collapsin-response mediator protein 5; PCA, Purkinje cell cytoplasmic antibody.
Differential diagnosis
CIPO is a rare condition that often mimics the symptoms of true mechanical obstruction in the small or large bowel, with half of the reported cases being associated with other diseases. Patients most commonly present with symptoms of abdominal pain, bloating and distension, as well as nausea, constipation and heartburn or regurgitation.7 8 Due to the recurrent and chronic nature of this event, we use the term ‘CIPO’. This is in contrast to ileus, which is typically acute and does not involve dilatation without a transition point7 as in the case presented here.
Testing for an infectious aetiology was negative. Concern for an underlying paraneoplastic syndrome activated by the CPI was considered, but after the panel returned negative (table 1), a rare CPI toxicity was considered the most likely culprit.
Treatment
Pembrolizumab was discontinued at the time of the patient’s first hospitalisation. Bowel rest was associated with transient symptom relief. Out of concern that this may represent an irAE, glucocorticoids were administered. There was no benefit, and they were subsequently tapered and discontinued. No other immunosuppressant medications were administered. Gut motility stimulators metoclopramide, neostigmine and pyridostigmine were also trialled under guidance from gastroenterology consultation. Ultimately, she was started on TPN to maintain nutrition status. Iron-deficiency anaemia was also treated concurrently with intravenous iron infusion.
Outcome and follow-up
The patient was discharged to home with continued TPN, metoclopramide and pyridostigmine. She has tolerated small sips of clear liquids. Surveillance CT of the chest/abdomen/pelvis showed no active malignancy. The patient’s cancer treatment remains on hold and will be resumed only if there is evidence of cancer progression.
Discussion
Immune-related adverse events are common with CPIs, with increased risk associated with their use compared with chemotherapeutic agents.9 Prior studies have found greater than 60% of patients taking CPIs report at least one irAE,10 with GI tract irAEs most commonly reported.6 The majority of these are attributed to bowel inflammation resulting in colitis. The case presented here is one of only two reported cases of paralytic ileus/chronic intestinal pseudo-obstruction occurring after treatment with a programmed cell death protein 1 (PD-1) inhibitor.
Fragulidis and colleagues recently reported a similar experience of acute small bowel ileus in a patient with non-small cell lung cancer undergoing treatment with another immune CPI, nivolumab.11 Nivolumab and pembrolizumab both operate by inhibiting the binding of PD-1 on effector T cells with PD-L1 on tumour cells. Similar to Fragulidis’s account, our patient also presented with CIPO symptoms well into her treatment duration with a PD-1 inhibitor (14 cycles and 8 cycles, respectively). Similarly, no radiographic cause of ileus was identified in either case. Despite the similarities mentioned, Fragulidis’s account had notable differences: response to treatment and resolution. They reported resolution of ileus following a course of glucocorticoids at the time of hospital discharge. In our case, the CIPO recurred after steroids and bowel rest.
Collins et al describe a small cohort of patients referred for GI irAEs assessment after anti-PD-1 therapy. Of the 20 patients confirmed to have a GI irAE, one patient with a poorly differentiated metastatic neuroendocrine tumour receiving pembrolizumab developed a pseudo-obstruction.12 Similar to our and Fragulidis’s cases, the patient experienced delayed-onset of irAE (after 11 cycles). Further akin to our case, the patient’s pseudo-obstruction was resistant to glucocorticoid therapy, and the patient was eventually placed on TPN. Despite these similarities, the presence of chronic colonic inflammation (including crypt disarray), lymphocytic and plasma cell infiltration of the lamina propria and apoptosis of the epithelial cells reported is dissimilar from both our and Fragulidis’s cases.
Treatment with anti-PD-1 therapies have resulted in delayed onset adverse events, even after therapy has been discontinued.13 14 Pembrolizumab in particular has been found to have a greater median time to reported irAE compared with other CPIs.14 As use of these agents expands, clinicians must be mindful of the potential for delayed onset irAEs. Additionally, educating other providers who are likely to encounter and treat irAEs for cancer patients may be a critical step in stemming the impact of such events.
We report here a case of CIPO following prolonged CPI use as treatment of advanced GOJ adenocarcinoma, a rare complication of immune-mediated antineoplastic therapy. This is one of the first cases of non-inflammatory luminal GI tract irAEs reported. This case report highlights the importance of recognising a suspected irAE associated with the use of immunotherapy as delayed diagnosis of CIPO can have substantial impact on a patient’s quality of life.
Learning points.
When using immune checkpoint inhibitors (CPI), physicians should be concerned with gastrointestinal adverse events such as colitis; however, they should be mindful that rarer adverse events, such as chronic intestinal pseudo-obstruction, are also possible.
Physicians should remain diligent and attentive of immune-related adverse events with delayed onset presenting in later treatment cycles or even after treatment has been discontinued.
As the CPI therapeutic class is more widely used in various cancer types, rare adverse events may become more commonplace, providing additional challenges in oncological treatment.
Footnotes
Contributors: MPS, JAZ and AJB contributed to care of this patient and acquisition of data reported. RJB, MPS and AJB composed the manuscript and figures. All authors (RJB, JAZ, MPS and AJB) provided edits and final approval of the manuscript. There are four authors (RJB, JAZ, MPS and AJB). All are included in this contributorship statement.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. De Martel C, Parsonnet J. Stomach cancer : Thun M, Linet MS, Cerhan JR, Schottenfeld and fraumeni cancer epidemiology and prevention. 4th edn Oxford: Oxford University Press, 2017. [Google Scholar]
- 3. Bartel M, Brahmbhatt B, Bhurwal A. Incidence of gastroesophageal junction cancer continues to rise: analysis of surveillance, epidemiology, and end results (SEER) database. Journal of Clinical Oncology 2019;37:40 10.1200/JCO.2019.37.4_suppl.40 [DOI] [Google Scholar]
- 4. Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 5. Fashoyin-Aje L, Donoghue M, Chen H, et al. Fda approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist 2019;24:103–9. 10.1634/theoncologist.2018-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cramer P, Bresalier RS. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep 2017;19:3 10.1007/s11894-017-0540-6 [DOI] [PubMed] [Google Scholar]
- 7. De Giorgio R, Cogliandro RF, Barbara G, et al. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am 2011;40:787–807. 10.1016/j.gtc.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 8. Stanghellini V, Cogliandro RF, de Giorgio R, et al. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil 2007;19:440–52. 10.1111/j.1365-2982.2007.00902.x [DOI] [PubMed] [Google Scholar]
- 9. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fragulidis G, Pantiora E, Michalaki V, et al. Immune-related intestinal pseudo-obstruction associated with nivolumab treatment in a lung cancer patient. J Oncol Pharm Pract 2019;25:487–91. 10.1177/1078155217738325 [DOI] [PubMed] [Google Scholar]
- 12. Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol 2017;28:2860–5. 10.1093/annonc/mdx403 [DOI] [PubMed] [Google Scholar]
- 13. Wang LL, Patel G, Chiesa-Fuxench ZC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol 2018;154:1057–61. 10.1001/jamadermatol.2018.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7–18. 10.1016/j.ctrv.2016.02.003 [DOI] [PubMed] [Google Scholar]