Significance
This work showed that a designed cyclic peptide ZY4 exhibits excellent activity against Pseudomonas aeruginosa and Acinetobacter baumannii. ZY4 not only inhibits planktonic growth and biofilm formation but also kills persister cells by permeabilizing bacterial membrane. ZY4 showed low propensity to induce resistance, high stability in vivo, and therapeutic potentials in MDR P. aeruginosa– and A. baumannii–induced infection. The present study provides a promising drug candidate in the war against multidrug resistance.
Keywords: antimicrobial peptides, multidrug resistance, nosocomial infections, inflammation
Abstract
The emergence of carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa raises fears of untreatable infections and poses the greatest health threats. Antimicrobial peptides (AMPs) are regarded as the most ideal solution to this menace. In this study, a set of peptides was designed based on our previously reported peptide cathelicidin-BF-15, and the lead peptide ZY4, a cyclic peptide stabilized by a disulfide bridge with high stability in vivo (the half-life is 1.8 h), showed excellent activity against P. aeruginosa and A. baumannii, including standard and clinical multidrug-resistant (MDR) strains. ZY4 killed bacteria by permeabilizing the bacterial membrane and showed low propensity to induce resistance, exhibited biofilm inhibition and eradication activities, and also killed persister cells. Notably, administration of ZY4 decreased susceptibility to lung infection by P. aeruginosa and suppressed dissemination of P. aeruginosa and A. baumannii to target organs in a mouse septicemia infection model. These findings identify ZY4 as an ideal candidate against MDR bacterial infections.
Multidrug resistance among nosocomial pathogens is a major threat to public health and poses a huge economic burden on global health care (1, 2). Antibiotic resistance kills about 700,000 people each year worldwide (3). Especially, the gram-negative carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa raise fears of untreatable infections. These 2 bacterial families have been listed as the first 2 critical pathogens in the threat list of drug-resistant bacteria that pose the greatest public health threats released by the World Health Organization (WHO) (3, 4). To address this global threat, research and funds toward the development of the most crucial antimicrobials are desperately needed. Despite an urgent need for new antimicrobial drugs, only a few new viable antibiotics are currently in clinical development (3). In this context, antimicrobial peptides (AMPs) represent promising candidates in the prevention and control of multidrug-resistant (MDR) bacterial infections (5–7).
AMPs play a pivotal role in the innate immunity, acting as the first line of defense and protecting the body against invading pathogens through their microbicidal activity. Natural and synthetic AMPs have earned much attention as the next-generation antibiotics for the treatment of MDR bugs due to their broad-spectrum activities, broad mechanisms of action, quick killing effect, and low tendency to induce resistance (8–11). Elucidation of the mechanisms of action of natural AMPs has enabled medicinal chemists to design and synthesize AMPs with more potent antimicrobial activities and high selectivity to a targeted pathogen (12–14).
Our previous studies have indicated that cathelicidin-BF, an antimicrobial peptide from snake venom of Bungarus fasciatus, exhibits strong and rapid antimicrobial activities (15, 16). After 10 y of efforts, cathelicidin-BF has been successfully used in the treatment of colpitis and was authorized to start clinical trials in 2018 (approval number CXHL1700235 from the Chinese National Medical Products Administration). Based on cathelicidin-BF, a panel of synthetic AMPs with enhanced antimicrobial activities but only about half the size (15 to 17 amino acids) of the parent peptide (30 amino acids) was designed (17, 18). The preclinical trials on 2 designed peptides are currently under way, and hopefully, both will be approved for medical application.
In the present study, we endeavored to develop synthetic peptides with improved antimicrobial activities against gram-negative pathogens, especially the critical pathogens in the threat list released by WHO. Thus, a set of peptides was designed, and their activities against P. aeruginosa and A. baumannii were tested in vitro and in vivo.
Results
Peptide Design and Functional Screening.
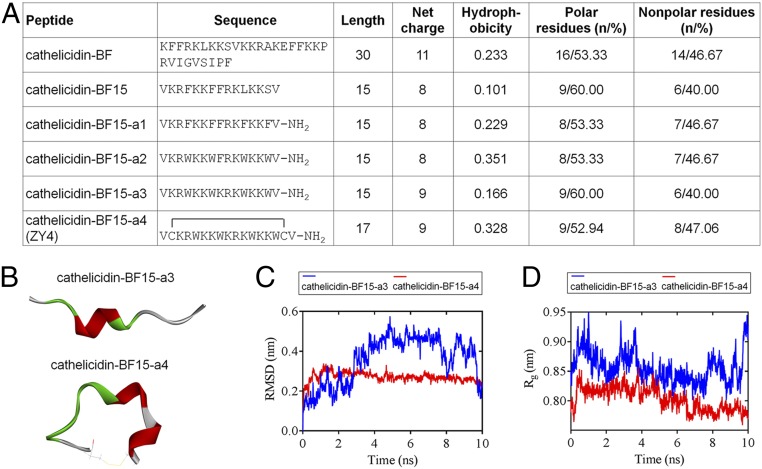
We reported cathelicidin-BF15, a 15–amino acid peptide, in our previous study (18), which showed higher antimicrobial activity against Escherichia coli than Bacillus subtilis and S. aureus, indicating this peptide displays a higher specificity in killing gram-negative bacteria. Based on cathelicidin-BF15, a set of linear and cyclic peptides was generated by amino acid substitutions. Aromatic residues play key roles in the biological activity of several antimicrobial and hemolytic peptides (6, 19, 20); thus, cathelicidin-BF15-a1 was first designed by substitution of Leu-11 and Ser-14 residues with Phe (Fig. 1A). Although cathelicidin-BF15-a1 exhibited more potent antimicrobial activity compared to cathelicidin-BF15 (SI Appendix, Table S1), it displayed higher hemolytic activity (SI Appendix, Table S2). Next, we investigated the effect of Phe substitution at positions 4, 7, 11, and 14 of cathelicidin-BF15-a1 by Trp, the importance of which has been described and proved (18, 19, 21, 22). The resulting peptide cathelicidin-BF15-a2 was more active against E. coli and B. subtilis (SI Appendix, Table S1), and its hemolysis was much lower (SI Appendix, Table S2). To obtain a more cationic peptide, we introduced a Lys residue by substitution of Phe-8 from cathelicidin-BF15-a2, resulting in cathelicidin-BF15-a3 (Fig. 1A), with potent antimicrobial activities (SI Appendix, Table S1) and greatly reduced hemolytic activity (SI Appendix, Table S2). The introduced Lys residue at position 8 was in the hydrophobic face of cathelicidin-BF15-a3, and its amphipathic structure was thus slightly diminished (SI Appendix, Fig. S1), which might further explain the decreased hemolytic activity, which was consistent with a previous report (23).
Fig. 1.
Primary sequence and structure prediction of ZY4 and its analogs. (A) Sequence and physicochemical properties of the designed peptides. (B) The 3-dimensional structures represent the last frames (t = 10 ns) of cathelicidin-BF15-a3 and cathelicidin-BF15-a4. The different colors represent various secondary structure types: green, turn; red, helix; white, coil. The yellow lines in cathelicidin-BF15-a4 indicate the disulfide bond. (C) The cathelicidin-BF15-a4 with lower and steady rmsd levels indicates that the structure of ZY4 is stabler than the structure of cathelicidin-BF15-a3. (D) The lower and steady Rg values of cathelicidin-BF15-a4 indicate that after introducing the disulfide bond, cathelicidin-BF15-a4 was stabler than cathelicidin-BF15-a3.
Cyclization through disulfide bonds occurs naturally in AMPs such as the human β-defensins family (24), and it has also been demonstrated that cyclization increases the antimicrobial activity and selectivity (25). For this purpose, we introduced a disulfide bridge by adding 2 Cys after the first and before the last Val of cathelicidin-BF15-a3, and a cyclic cathelicidin-BF15-a4 was thus obtained (Fig. 1 A and B and SI Appendix, Fig. S1). The 4 analogs of cathelicidin-BF15 were also amidated at the C terminus to improve their stability (Fig. 1A). Cathelicidin-BF15-a4 showed higher activity against the 4 tested strains and induced the lowest hemolytic activity. We performed in silico studies using 10 ns molecular dynamics (MD) simulations to evaluate peptide stability. We observed that cathelicidin-BF15-a4 time series show rmsd levels of ∼0.3 nm (3 Å), indicating that its structure is stabler than cathelicidin-BF15-a3 (Fig. 1C). Moreover, we can see from the reasonably invariant radius of gyration (Rg) values that cathelicidin-BF15-a4 is stabler than cathelicidin-BF15-a3 in its compact (folded) form over the course of 10 ns at 300 K (Fig. 1D). The cyclic peptide cathelicidin-BF15-a4 with the highest antimicrobial activity was named ZY4.
ZY4 Kills MDR P. aeruginosa and A. baumannii by Permeabilizing the Bacterial Membrane.
The in vitro antimicrobial properties of ZY4 were evaluated against MDR P. aeruginosa and A. baumannii. Remarkably, ZY4 exhibited potent antibacterial activity against several strains of P. aeruginosa (CICC21625, CMCC10104, C1, C2, C3, and C5) and A. baumannii (22933, CN40, 18C116, 18C132, 18C135, and 18C136) with minimal inhibitory concentration (MIC) values ranging between 2.0 and 4.5 µg/mL (0.8 to 1.9 µM) and 4.6 and 9.4 µg/mL (1.9 to 4.0 µM), respectively (SI Appendix, Table S3). Additionally, comparison of the MIC values of conventional antibiotics commonly used to treat P. aeruginosa infections, including colistin sulfate, tobramycin, levofloxacin, kanamycin, and carbenicillin disodium with ZY4, revealed that ZY4 has a significantly (P < 0.01) stronger antimicrobial activity against these microorganisms (SI Appendix, Table S3).
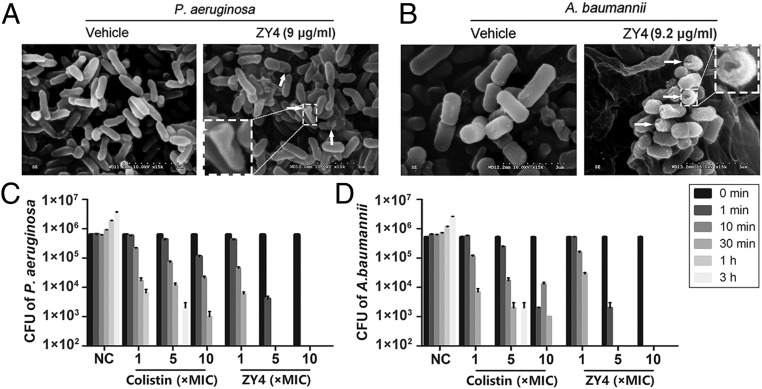
To assess the possible mechanism of ZY4 action on bacteria, membrane morphology was examined by scanning electron microscopy (SEM). As illustrated in Fig. 2A, comparison of treated versus untreated P. aeruginosa reveals a significant difference in membrane morphology. ZY4 caused disruption of the plasma membrane, whereas the plasma membrane of untreated cells remained intact (Fig. 2A). Similarly, after treatment with ZY4, the cells of A. baumannii also showed membrane disruption and pore formation in most cells (Fig. 2B). The permeabilization of bacterial membrane induced by ZY4 was further demonstrated by flow cytometric analysis of propidium iodide (PI) influx. As illustrated in SI Appendix, Fig. S2, PI influx was detected immediately after ZY4 was added to P. aeruginosa and A. baumannii cells, suggesting ZY4 permeabilized the membrane of bacteria in a time-dependent manner. Furthermore, ZY4 permeabilized the membrane of P. aeruginosa in a dose-dependent manner, proved by the percentage of PI-labeled cells positively correlated with the concentration of ZY4 (SI Appendix, Fig. S3).
Fig. 2.
ZY4 kills bacteria by permeabilizing the cell membrane. After treatment with or without ZY4 for 30 min, the membrane morphology of P. aeruginosa CICC21625 (A) and A. baumannii 22933 (B) was determined by SEM. The arrows indicate the typical damage to the plasma membranes of the bacteria. Killing kinetics of ZY4 against P. aeruginosa (C) and A. baumannii (D). Data represent means ± SD of 3 individual experiments.
Furthermore, ZY4 caused rapid killing effects in the tested strains, which completely killed P. aeruginosa and A. baumannii within 30 min at 1 × MIC (Fig. 2 C and D). Although the control drug colistin did not kill all of the bacteria within 30 min at 5 × MIC, the bactericidal effect caused by ZY4 was much faster and killed all these bacteria within 1 min at 5 and 10 × MIC. Collectively, these data suggested that ZY4 exhibits potent and quick antibacterial activity by permeabilizing the bacterial membrane.
ZY4 Maintains Its Antibacterial Activity in Plasma and Displays a Prolonged Half-Life.
We assessed the effect of plasma on ZY4’s antimicrobial property. Importantly, incubation of ZY4 in human plasma did not significantly affect its antibacterial activity against the tested P. aeruginosa and A. baumannii, even after 10 h of incubation (SI Appendix, Fig. S4), suggesting that ZY4 is stable in plasma. Indeed, 91% of the actual peptide remained after 10 h of plasma incubation in vitro (SI Appendix, Fig. S5A). Importantly, pharmacokinetic analysis of ZY4 demonstrated a half-life of 1.8 h after intravenous (i.v.) injection into mice (SI Appendix, Fig. S5 B and C), further indicating the protective effect of modifications to improve the stability of ZY4.
As an overture for experiments in vivo, we evaluated the potential toxicity of ZY4. As illustrated in SI Appendix, Fig. S6, ZY4 exerted a negligible effect on cell viability (less than 7.1%) even at a concentration as high as 80 μM. However, for human cathelicidin LL-37 as a control, it exhibited significant cytotoxic activity. In addition, in vivo acute toxicity of ZY4 to mice after i.v. injection showed only mild signs of acute toxicity at 40 mg/kg, while for colistin, it killed nearly all mice at 10 mg/kg (SI Appendix, Fig. S7), suggesting a lower toxicity of ZY4. Together, these properties make ZY4 an ideal candidate as a drug agent.
ZY4 Exhibits Biofilm Inhibition and Eradication Activities and Kills Persister Cells.
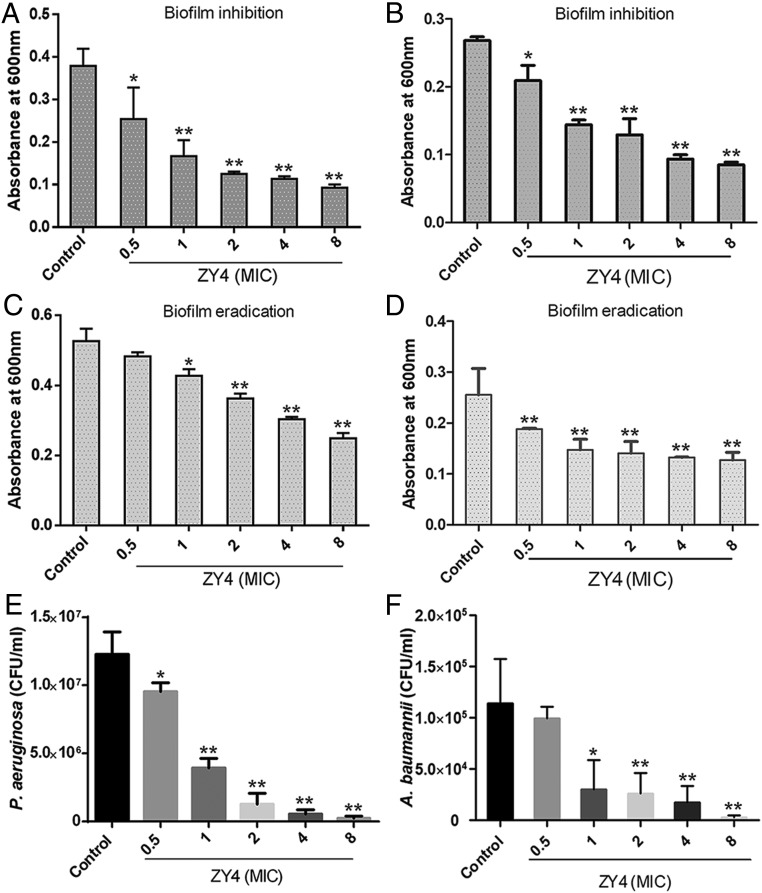
Biofilm production by bacteria complements disease pathogenicity and development of drug resistance (26, 27). Further efforts focused on investigating the effects of ZY4 on biofilm formation and eradication. Significantly, ZY4 dose dependently inhibited biofilm formation by P. aeruginosa (Fig. 3A) and A. baumannii (Fig. 3B). Furthermore, preformed biofilms of P. aeruginosa (Fig. 3C) and A. baumannii (Fig. 3D) were also eliminated by ZY4 in a dose-dependent manner.
Fig. 3.
ZY4 exhibits biofilm inhibition and eradication activities and kills persister cells. Inhibitory effects of ZY4 on P. aeruginosa (A) and A. baumannii (B) biofilm formation. Effects of ZY4 on established biofilm eradication of P. aeruginosa CICC21625 (C) and A. baumannii 22933 (D). Bactericidal activities of ZY4 against persister cells of P. aeruginosa CICC21625 (E) and A. baumannii 22933 (F). Data represent means ± SD of 3 individual experiments. *P < 0.05, **P < 0.01.
Chronic infections such as tuberculosis, cystic fibrosis–associated lung infections, and candidiasis are hard to treat because slow-growing bacteria termed persisters show strong drug tolerance (28–30). The effect of ZY4 on persisters was therefore tested. The persisters of P. aeruginosa (Fig. 3E) and A. baumannii (Fig. 3F) were dose dependently killed by ZY4; more than half of the persisters were eliminated by 1 × MIC of ZY4, indicating that the peptide is a potent candidate for killing persister cells. Together, the effects of ZY4 on the biofilm and persisters make it a promising antibiofilm and antipersister agent.
ZY4 Shows Low Propensity to Induce Resistance.
Development of resistance is a great challenge that hampers drug development and eradication of nosocomial pathogens. Therefore, we evaluated the selection of resistance through serial passaging in the presence of subinhibitory concentrations of ZY4 or colistin up to 60 passages. In contrast to colistin, P. aeruginosa strains (CICC21625, C1, and C2) did not easily select for resistance to ZY4 (SI Appendix, Fig. S8 A–C). Exposure to colistin resulted in a steady increase in MIC just only after the first 20 passages, resulting in a 16- to 25-fold increased MIC after 60 passages. However, no significant change was observed for ZY4, with only a 4.0- to 4.5-fold change after 60 passages (SI Appendix, Fig. S8 A–C). A similar phenomenon was observed for A. baumannii strains (SI Appendix, Fig. S8 D–F).
Additionally, the original and resistant P. aeruginosa strains were also tested for cross-resistance to other AMPs similar to ZY4, including ZY13 (18) and LZ1 (17), and other antibiotics, including tobramycin and levofloxacin. Few effects were observed on bacteria susceptibility to ZY4, ZY13, and LZ1; however, reduced bacteria susceptibilities to colistin, tobramycin, and levofloxacin were displayed (SI Appendix, Fig. S8 G–I). Therefore, our findings suggest that the use of ZY4 has a low likelihood to induce resistance and cross-resistance.
ZY4 Inhibits P. aeruginosa Lung Infection and Inflammation In Vivo.
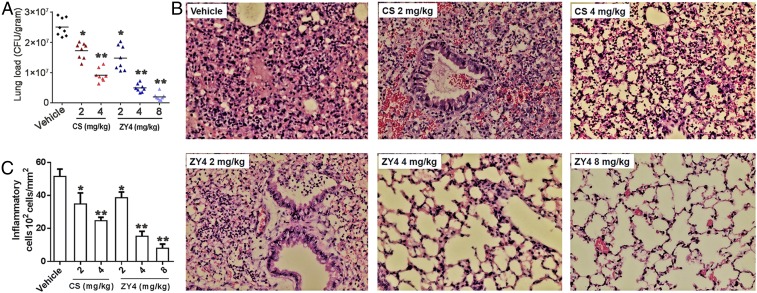
We next evaluated the therapeutic application of ZY4 through P. aeruginosa lung infection in the mouse model. Remarkably, administration of ZY4 significantly impacted lung bacteria load in a dose-dependent manner (Fig. 4A); the corresponding median colony forming units (CFU) were 2.51 × 107 CFU/g for the vehicle group and 1.45 × 107, 0.50 × 107, and 0.24 × 107 CFU/g for 2, 4, and 8 mg/kg of ZY4, representing a corresponding 42, 80, and 90% inhibition of lung colonization, respectively. Treatment with colistin also decreased the bacteria load, with a median of 1.72 × 107 and 0.86 × 107 CFU/g for 2 and 4 mg/kg of colistin, representing a 31% and 65% inhibition of lung colonization (Fig. 4A). Additionally, histopathological examination of fixed lung sections revealed ZY4 treatment significantly alleviated lung inflammation (Fig. 4B) and reduced infiltration of inflammatory cells into the lung in a dose-dependent manner (Fig. 4C). Moreover, evaluation of the cytokine level in supernatants of lung homogenates after P. aeruginosa inoculation revealed an up-regulation of cytokine levels (interleukin 6 [IL-6], tumor necrosis factor α [TNF-α], IL-1β, and IL-10). Remarkably, ZY4 dose dependently suppressed cytokine release (SI Appendix, Fig. S9), indicating potential anti-inflammatory effects of ZY4 in vivo, which was consistent with our in vitro tests showing that the peptide inhibits the release of IL-6 and TNF-α induced by lipopolysaccharide (LPS) in mouse RAW264.7 cells (SI Appendix, Fig. S10).
Fig. 4.
ZY4 inhibits P. aeruginosa lung infection and inflammation in vivo. (A) Following intranasal inoculation of mice (n = 8) with P. aeruginosa C1 and subsequent i.v. administration of ZY4 or colistin (CS) to examine their therapeutic effects, bacteria load in lung homogenate is shown. (B) Representative histopathological images of processed lung section with hematoxylin and eosin (H&E) staining. Images are presented at a magnification of 40×. (C) The number of counted infiltrated inflammatory cells within processed lung sections from B. Data represent the mean ± SD of 8 individual experiments. *P < 0.05, **P < 0.01.
ZY4 Suppresses Dissemination of Bacteria to Target Organs.
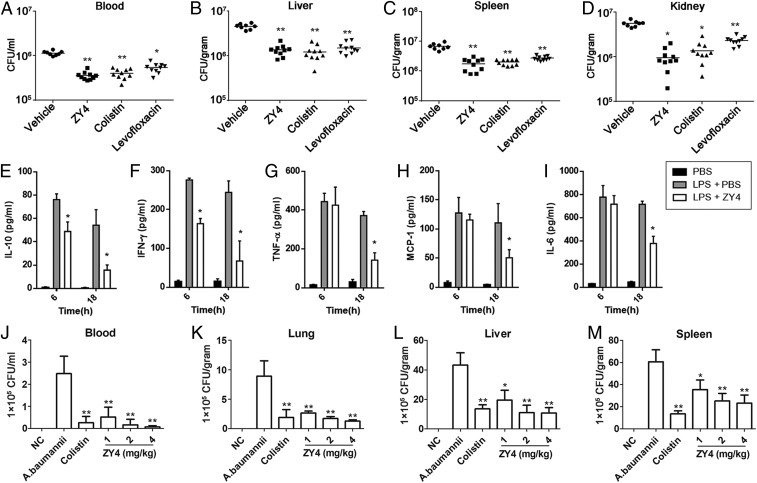
We evaluated the therapeutic potential of ZY4 as a candidate drug for bacteremia or endotoxemia by injecting mice with live bacteria cells or LPS. In the first set of experiments, significant dissemination of bacteria from the peritoneal cavity to the liver, spleen and kidney was observed 12 h after intraperitoneal (i.p.) inoculation of MDR P. aeruginosa C1 (2 × 107 CFU) in mice. Remarkably, 10 mg/kg ZY4 treatment (i.p.) significantly suppressed (6- to 8-fold) bacteria dissemination to these organs and blood (Fig. 5 A–D). Comparatively, the P. aeruginosa burden of the vehicle group was ∼106 CFU/mL in the blood and 106 to 107 CFU/g in the liver, spleen, and kidney 12 h after bacteria inoculation (Fig. 5 A–D). Although similar results were observed after treatment with colistin and levofloxacin, ZY4 showed higher effectivity against P. aeruginosa dissemination than those antibiotics (Fig. 5 A–D).
Fig. 5.
ZY4 suppresses dissemination of bacteria to target organs. (A–D) ZY4 suppresses dissemination of P. aeruginosa to target organs. Mice (n = 10) were i.p. injected with P. aeruginosa C1, followed by i.p injection of ZY4 (10 mg/kg), colistin (10 mg/kg), levofloxacin (100 mg/kg), or vehicle (PBS). Bacterial burden was determined in the blood (A), liver (B), spleen (C), and kidney (D). (E–I) ZY4 inhibits cytokine release in LPS shock mice model. Mice (n = 8) were i.p. injected with LPS (15 mg/kg) or PBS (control), followed by i.p. injection of ZY4 (10 mg/kg) or PBS 30 min after LPS injection. Levels of IL-10 (E), IFN-γ (F), TNF-α (G), MCP-1 (H), and IL-6 (I) in plasma were determined 6 and 18 h after LPS injection, respectively. (J–M) ZY4 suppresses dissemination of A. baumannii to target organs. A. baumannii was i.p. injected into female C57BL/6 mice (n = 8); 1 h after the injection, ZY4 (1, 2, 4 mg/kg), colistin (1 mg/kg), and vehicle (PBS) were i.p. applied. Bacterial burdens in blood (J), lung (K), liver (L), and spleen (M) are shown. NC, normal healthy mice. Data represent the mean ± SD of 8 individual experiments. *P < 0.05, **P < 0.01.
In the second set of experiments, female C57BL/6 mice were i.p. injected with a sublethal amount of E. coli LPS (15 mg/kg). Compared to control (phosphate-buffered saline [PBS]), LPS administration resulted in elevated levels of IL-10, interferon γ (IFN-γ), TNF-α, monocyte chemotactic protein 1 (MCP-1), and IL-6 in plasma at 6 and 18 h (Fig. 5 E–I). Remarkably, ZY4 treatment significantly reduced the plasma levels of these cytokines to a 2- to 4-fold change compared with the vehicle group at 18 h after LPS injection (Fig. 5 E–I), suggesting an anti-inflammatory potential of ZY4 in vivo.
Furthermore, the therapeutic potential of ZY4 was also determined in a MDR A. baumannii–induced bacteremia model. As illustrated in Fig. 5 J–M, treatment with ZY4 caused a dose-dependent decrease in bacterial load in the blood and the target organs, including the lung, liver, and spleen, compared to vehicle treatment. The same tendency was also observed in the plasma concentrations of IL-1β, IL-6, IL-10, and TNF-α after ZY4 treatment (SI Appendix, Fig. S11 A–D), further indicating the anti-inflammatory potential of ZY4. Taken collectively, these results indicated the roles of ZY4 in suppressing bacterial dissemination to target organs and inhibition of inflammation.
Discussion
In the present study, several approaches were employed to address these limitations of natural peptides. On the one hand, amino acid substitutions were used to improve antimicrobial activities while decreasing hemolytic and cytotoxic activity. On the other hand, C-terminal amidation and cyclization through disulfide bond were applied to improve stability. Furthermore, the ideal peptide ZY4 contained only 17 amino acids, thus greatly reducing the production cost due to its small size. Our data also indicated that cyclization through the disulfide bond for a small peptide may serve as a promising approach in the design of AMPs.
The high tendency for resistance development to antibiotics is partly due to their single primary target or a single mode of action. The innate host defense peptides have evolved for billions of years, acting as the first line of protection against invading pathogens; a way for them to kill bacteria might represent the most effective model of action and the lowest propensity to induce resistance. As demonstrated in our study, ZY4 permeabilized and disrupted the bacteria membrane of P. aeruginosa and A. baumannii, resulting in a rapid killing effect and low propensity to induce resistance. These results were consistent with several other studies that suggest that the bactericidal action of most AMPs is by targeting the bacterial membrane and disrupting the lipid bilayer structure (12, 31).
Biofilm-related infections such as those disseminated through medical devices result in increased risks of infection and patient mortality; therefore, finding biofilm-targeting therapies is a top priority (32). P. aeruginosa and A. baumannii are major biofilm-producing bacteria; biofilm production by bacteria complements disease pathogenicity and development of drug resistance and has been implicated in chronic infections (26, 27, 33). Recent studies have highlighted the possible use of AMPs to prevent biofilm formation or to treat established biofilms (12, 34, 35). In this study, ZY4 was demonstrated to inhibit planktonic growth and biofilm formation by P. aeruginosa and A. baumannii. ZY4 also exhibited biofilm eradication activity. Although some antibiotics have been proved to penetrate the biofilm matrix (36), they show no antibacterial activity against these slowly growing cells, especially the dormant subpopulation known as persister cells (37–39). The reason might be that since ZY4 targets the cell membrane, it therefore proved to be highly effective against these dormant persister cells. Increasing evidence also suggests that several Trp/Arg-containing AMPs were able to kill persister cells (12, 33, 40). Such AMPs with antibiofilm and antipersister activities might represent promising candidates for the development of new antimicrobials.
During P. aeruginosa lung infections, inflammation is orchestrated by the pronounced secretion of cytokines locally and massive infiltration of inflammatory cells. In line with this, histological examination of lung sections after bacterial inoculation revealed a pronounced presence of infiltrated inflammatory cells. Remarkably, we observed that i.v. administration of ZY4 not only reduced bacterial burden but also alleviated lung inflammation. A major cause of nosocomial infections is gram-negative P. aeruginosa and A. baumannii causing both localized and systemic infections with high mortality rates (41–43). In this study, using live bacteria and LPS, we induced bacteremia and endotoxemia in mice to evaluate the potential of ZY4 as a drug candidate. Remarkably, injection of ZY4 significantly suppressed bacteria growth and cytokine release in target organs, including the liver, spleen, and kidney, and in blood, suggesting antibacterial and anti-inflammatory potentials of ZY4 in vivo. Moreover, the therapeutic potential of ZY4 was also determined in an A. baumannii–induced bacteremia model which further demonstrated that ZY4 suppressed bacterial dissemination to target organs and inflammation.
Taken collectively, our results demonstrated the potentials of ZY4, a designed cyclic peptide, which as a possible drug candidate not only inhibits planktonic growth and biofilm formation but kills the persisters of MDR P. aeruginosa and A. baumannii by permeabilizing bacterial membrane. ZY4 showed low propensity to induce resistance, high stability in plasma, and therapeutic potentials in MDR P. aeruginosa– and A. baumannii–induced infection in vivo. The present study provided a promising drug candidate in the battle against antibiotic resistance.
Materials and Methods
Mice experiments were approved, and handling of animals followed guidelines set by the Animal Care and Use Committee of the Kunming Institute of Zoology, Chinese Academy of Sciences (SMKX-2018017).
Data Availability.
The helix wheel structures were constructed by HeliQuest (http://heliquest.ipmc.cnrs.fr/). Bacteria preparation, MD simulations, antibacterial properties, toxicity assays, plasma stability, pharmacokinetic analysis, membrane permeabilization, killing kinetic assay, biofilm assays, and resistance were performed by using standard approaches. Statistical analysis was performed with GraphPad Prism 6 software or Discovery Studio 3.1. A detailed description of materials and methods and all of the necessary data can be found in the SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Lin Zeng for technical advice and assistance with liquid chromatography with tandem mass spectrometry (LC/MS/MS) spectrum analysis. This work was supported by a National Natural Science Foundation of China (NSFC) grant (21761142002), Chinese Academy of Science grants (XDB31000000, SAJC201606, KGFZD-135-17-011, and KFJ-BRP-008), and a Yunnan Province grant (2015HA023) to R.L. and a NSFC grant (81770464), Chinese Academy of Science grants (XDA12040221 and Youth Innovation Promotion Association [2017432]), and a National Key R&D Program of China grant (2018YFC2000400) to Z.Z.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909585117/-/DCSupplemental.
References
- 1.Li X. Z., Plésiat P., Nikaido H., The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin. Microbiol. Rev. 28, 337–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathwani D., Raman G., Sulham K., Gavaghan M., Menon V., Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 3, 32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willyard C., The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Tomczyk S., et al. , Control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: A systematic review and reanalysis of quasi-experimental studies. Clin. Infect. Dis. 68, 873–884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahar A. A., Ren D., Antimicrobial peptides. Pharmaceuticals (Basel) 6, 1543–1575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock R. E., Sahl H. G., Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Mwangi J., Hao X., Lai R., Zhang Z. Y., Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 40, 488–505 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van’t Hof W., Veerman E. C., Helmerhorst E. J., Amerongen A. V., Antimicrobial peptides: Properties and applicability. Biol. Chem. 382, 597–619 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Deslouches B., Di Y. P., Antimicrobial peptides: A potential therapeutic option for surgical site infections. Clin. Surg. 2, 1740 (2017). [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S., Sharma G., Dang S., Gupta S., Gabrani R., Antimicrobial peptides as anti-infectives against Staphylococcus epidermidis. Med. Princ. Pract. 25, 301–308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeffler J. M., Nelson D., Fischetti V. A., Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294, 2170–2172 (2001). [DOI] [PubMed] [Google Scholar]
- 12.de Breij A., et al. , The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 10, eaan4044 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Ilić N., et al. , Selective antimicrobial activity and mode of action of adepantins, glycine-rich peptide antibiotics based on anuran antimicrobial peptide sequences. Biochim. Biophys. Acta 1828, 1004–1012 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Vaudry H., Tonon M. C., Vaudry D., Editorial: Trends in regulatory peptides. Front. Endocrinol. (Lausanne) 9, 125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., et al. , Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS One 3, e3217 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., et al. , Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS One 6, e22120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., et al. , A small peptide with therapeutic potential for inflammatory acne vulgaris. PLoS One 8, e72923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L., et al. , A designed tryptophan- and lysine/arginine-rich antimicrobial peptide with therapeutic potential for clinical antibiotic-resistant Candida albicans vaginitis. J. Med. Chem. 59, 1791–1799 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Haug B. E., Skar M. L., Svendsen J. S., Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Pept. Sci. 7, 425–432 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Bell A., Antimalarial peptides: The long and the short of it. Curr. Pharm. Des. 17, 2719–2731 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Chan D. I., Prenner E. J., Vogel H. J., Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 1758, 1184–1202 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Deslouches B., et al. , De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 49, 316–322 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihajlovic M., Lazaridis T., Charge distribution and imperfect amphipathicity affect pore formation by antimicrobial peptides. Biochim. Biophys. Acta 1818, 1274–1283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz T., Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Dathe M., Nikolenko H., Klose J., Bienert M., Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 43, 9140–9150 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M., The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 759348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overhage J., et al. , Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 76, 4176–4182 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu Y., et al. , Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol. Cell 62, 284–294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucher R. C., Pathogenesis of cystic fibrosis airways disease. Trans. Am. Clin. Climatol. Assoc. 112, 99–107 (2001). [PMC free article] [PubMed] [Google Scholar]
- 30.Fauvart M., De Groote V. N., Michiels J., Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 60, 699–709 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Rozek A., Hancock R. E., Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 276, 35714–35722 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Percival S. L., Suleman L., Vuotto C., Donelli G., Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 64, 323–334 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Grassi L., et al. , Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 8, 1917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batoni G., Maisetta G., Esin S., Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 1858, 1044–1060 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran G., Kim E. Y., Shin S. Y., LL-37-derived membrane-active FK-13 analogs possessing cell selectivity, anti-biofilm activity and synergy with chloramphenicol and anti-inflammatory activity. Biochim. Biophys. Acta Biomembr. 1859, 722–733 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Dunne W. M. Jr, Mason E. O. Jr, Kaplan S. L., Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 37, 2522–2526 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K., Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230, 13–18 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Stewart P. S., Costerton J. W., Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Yao Y., Sturdevant D. E., Otto M., Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: Insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J. Infect. Dis. 191, 289–298 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Zhang M., Zhou C., Kallenbach N. R., Ren D., Control of bacterial persister cells by Trp/Arg-containing antimicrobial peptides. Appl. Environ. Microbiol. 77, 4878–4885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Delden C., Pseudomonas aeruginosa bloodstream infections: How should we treat them? Int. J. Antimicrob. Agents 30 (suppl. 1), S71–S75 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Geisinger E., Isberg R. R., Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 11, e1004691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peleg A. Y., Seifert H., Paterson D. L., Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 21, 538–582 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The helix wheel structures were constructed by HeliQuest (http://heliquest.ipmc.cnrs.fr/). Bacteria preparation, MD simulations, antibacterial properties, toxicity assays, plasma stability, pharmacokinetic analysis, membrane permeabilization, killing kinetic assay, biofilm assays, and resistance were performed by using standard approaches. Statistical analysis was performed with GraphPad Prism 6 software or Discovery Studio 3.1. A detailed description of materials and methods and all of the necessary data can be found in the SI Appendix.